Abstract
Background and Purpose
Statin pretreatment has been associated with improved outcomes in patients with ischemic stroke. Although several mechanisms have been examined in animal models, few have been examined in patients. We hypothesized that patients using statins before stroke onset may have greater reperfusion than patients not using statins.
Methods
Acute ischemic stroke patients underwent two MR scans: within 4.5 (tp1) and at 6 hours (tp2) after stroke onset. Regions of reperfusion were defined by prolonged MTT at tp1 which normalized at tp2. Four MTT thresholds were assessed to ensure that results were not spuriously based on an arbitrary threshold. Baseline characteristics, relative reperfusion, and change in NIH Stroke Scale between tp1 and 1-month follow-up (ΔNIHSS) were compared between patients who were taking statins at stroke onset and those who were not.
Results
Thirty-one stroke patients were prospectively enrolled; 12 were taking statins, while 19 were not. Baseline characteristics did not differ between the two groups except the statin group had greater coronary artery disease (p=0.03). Patients using statins showed significantly greater reperfusion compared to untreated patients across all MTT thresholds. For MTT=4 seconds, median relative reperfusion was 50% (IQR 30%,56%) in the pre-existing statin group vs. 13% (IQR=5%,36%) in the untreated group, p=0.014. The statin group had greater ΔNIHSS (8.8±4.0 points) compared to the untreated group (4.4±5.7 points), p=0.028.
Conclusion
Statin use prior to ischemic stroke onset was associated with greater early reperfusion and NIHSS improvement. Further studies in larger populations are required to confirm our preliminary findings.
Keywords: ischemic stroke, statin, reperfusion
Introduction
3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors (statins) are widely prescribed for the prevention of vascular events. The Stroke Prevention with Aggressive Reduction of Cholesterol Level (SPARCL) trial demonstrated that atorvastatin reduced risk of recurrent stroke by 16% compared to placebo1. In addition to having fewer events, patients taking atorvastatin showed a trend towards improved neurological outcomes at 90 days after their stroke outcome event2. Several additional studies have found that patients taking statins at the time of stroke onset had improved outcomes and lower mortality than those who were not taking statins3–6. Moreover, withdrawal of statins in patients admitted for ischemic stroke has been associated with worse neurological outcomes, greater early neurological deterioration, and larger infarct sizes7.
While the neuroprotective mechanisms of statins in ischemia have not been fully elucidated, several potential roles in both animals and humans have been studied. In rodent models, statins have been shown to promote fibrinolysis, inhibit prothrombotic pathways, and limit infarct size8–11. In models of ischemia-reperfusion, statins have protected multiple organ systems against reperfusion injury12, 13 and have augmented cerebral blood flow (CBF) secondary to upregulation of endothelial nitric oxide synthase (eNOS)14, although such a CBF effect was not reproduced in humans with atherosclerotic carotid occlusion15. In other clinical studies, statin use was associated with greater recanalization in a cohort of ischemic stroke patients undergoing acute intervention16, and in aneurysmal subarachnoid hemorrhage, patients treated with statins were less likely to develop vasospasm17. In several studies, the neuroprotective effects have been independent of cholesterol lowering10, 11. Given the potential vascular effects of statins in the setting of ischemia, we hypothesized that improved neurological outcomes observed with statin pretreatment may be related to greater reperfusion during the hyper-acute phase of ischemic stroke. Thus, we measured early reperfusion (within 6 hours of stroke onset) in acute ischemic stroke patients, comparing patients with pre-existing statin use to untreated patients.
Methods
Patients and Inclusion Criteria
This was a retrospective analysis of data from a prospectively collected observational study of serial MRI’s performed in acute ischemic stroke patients at a large urban tertiary care referral center, admitting over 800 ischemic strokes per year. Data from the first 31 patients in this study (with planned enrollment of 75 patients) were used for a retrospective analysis of the effect of statins on reperfusion. After Institutional Review Board approval, the study enrolled consecutive patients within 4.5 hours of stroke onset based on the following pre-specified inclusion criteria: clinically-suspected acute cortical ischemic stroke; age ≥ 18 years; NIHSS ≥ 5; and patient or patient’s next of kin capable of informed consent. Exclusion criteria included bilateral strokes or any acute endovascular or surgical intervention. Both tPA-treated and untreated patients were included. For patients receiving tPA, the study imposed no delay in time-to-tPA-treatment and no deviation from standard practices. The NIHSS was collected prospectively by a stroke neurologist on admission, at both imaging time points, and at one month follow-up. Clinical data including demographic data, past medical history, and home medications were obtained by the research coordinator prospectively during enrollment.
Magnetic Resonance Imaging Protocol
Patients were scanned with MRI within 4.5 hours (tp1) and at 6 hours (tp2) after stroke onset, on a 3T Siemens whole body Trio system with a circular polarized head coil. For patients receiving tPA, the tp1 scan was performed as soon as possible after tPA bolus. The protocol included diffusion-weighted images, FLAIR images [TR/TE=10000/115 ms; inversion time = 2500 ms; matrix=512×416; 20 slices, slice thickness (TH)=5mm], and dynamic susceptibility contrast (DSC) perfusion images with Gadolinium contrast injected at 5 ml/s with a power injector (a T2*-weighted gradient echo EPI sequence; TR/TE=1500/43 ms; 14 slices, TH=5mm, zero interslice gap; matrix=128×128). Magnetic resonance angiography (MRA) was not performed.
Data analysis
Mean transit time [MTT=cerebral blood volume (CBV)/CBF18] was chosen to define the perfusion deficit because MTT is uniform across gray and white matter (unlike CBF or CBV), allowing for use of a single threshold across both tissue types19, 20. Voxels within the middle cerebral artery (MCA) territory of the contralateral hemisphere were manually chosen to obtain an arterial input function. Six parameter rigid image registration was performed to align all images across both scans for each patient using FSL 3.2 (FMRIB, Oxford, UK)21. Accuracy of image registration was evaluated manually.
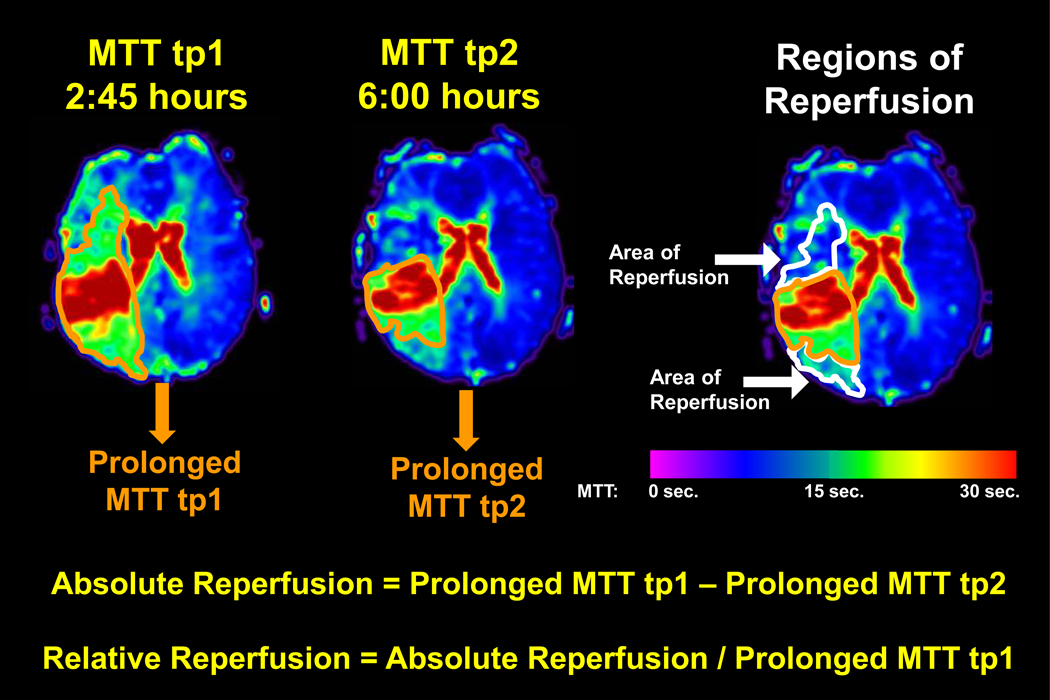
MTT prolongation for each voxel within the ischemic hemisphere was calculated as: [MTT of ischemic voxel] – [median MTT of contralateral hemisphere]. A voxel was defined as “prolonged” if MTT was ≥ a pre-defined threshold. Because the perfusion deficit volume varies depending on the MTT threshold chosen22, 23, four MTT prolongation thresholds (3, 4, 5, and 6 seconds) were tested to determine if results were consistent across a range of putative thresholds. Prolonged MTT volumes provided measures of perfusion deficit volumes at tp1 and tp2. The absolute volume of reperfusion was calculated as: [prolonged MTT volume at tp1] – [prolonged MTT volume at tp2]. Subsequently, the relative volume of reperfusion was calculated as: [prolonged MTT at tp1 – prolonged MTT at tp2] / [prolonged MTT at tp1] (Figure 1). The individuals performing the reperfusion calculations were blinded to the treatment status of the patient. Isolated regions of abnormal perfusion < 1 ml were removed from the analysis to minimize inclusion of misregistered regions or noise-induced variations.
Figure 1. Calculation of Relative Reperfusion.
MTT maps were prepared for each time-point. For each MTT threshold, all voxels with values > the chosen MTT threshold were defined as “prolonged”. Prolonged MTT volumes provided a measure of the perfusion deficit volume at tp1 and tp2. Absolute and relative reperfusion volumes were calculated as shown. Warm colors represent maximal hypoperfusion with longer MTT and cool colors represent no hypoperfusion with short MTT, as shown on the color bar. The orange outline delineates a perfusion deficit defined by an MTT threshold > 4 seconds longer than the median MTT of the contralateral hemisphere. At tp2, the perfusion deficit shrinks; thus, the white outline delineates regions of reperfusion using this threshold (> 4 seconds).
Statistical Analysis
Pre-existing statin use and untreated groups were compared using the Wilcoxon Rank Sum test for continuous variables except when data was Gaussian (confirmed by the Kolmogorov-Smirnov and D'Agostino-Pearson omnibus tests), where the t-test was used. Fisher’s Exact test compared categorical baseline characteristics between the two groups. Alpha ≤ 0.05 was required for statistical significance. Statistical analyses were performed using STATA 10.1.
Statins and Relative Reperfusion
Relative reperfusion for each MTT threshold (3, 4, 5, and 6 seconds) was compared between the untreated and statin groups. In addition to the total group, the tPA-treated subgroup was analyzed to ensure that statin effects were not confounded by the higher proportion of statin patients receiving tPA. A linear regression model was developed as a prediction model for relative reperfusion based on the MTT=4 second threshold (as this is a commonly used threshold of ischemia in acute stroke literature). Predictors were selected with a forward stepwise procedure in which a p-value ≤ .20 was required for entry and p-value ≤ .05 was required to be retained in the model. At each step, regression diagnostics evaluated distributional assumptions of the residuals and functional form of the covariates. Ten potential covariates were considered: pre-existing statin use, tPA treatment, volume of tp1 perfusion deficit (MTT=4sec), age, gender, admission NIHSS, admission mean arterial pressure (MAP), admission glucose, LDL, and time between tp1 and tp2 scans. Diffusion weighted imaging (DWI) volume at tp1 was considered for inclusion in the model, however, DWI volume demonstrated strong covariance (r=0.74, p<0.0001) with volume of tp1 perfusion deficit, leading to multi-collinearity. Therefore, we chose to include only volume of tp1 perfusion deficit in the model to control for baseline volume of initial ischemia. Model goodness-of-fit was assessed by Akaike's information criterion. Potential interactions between statin use and tPA treatment or MAP were assessed.
Statins and Neurological Improvement
Neurological improvement, as assessed by ΔNIHSS (NIHSS on admission - NIHSS at 1 month), was compared in the untreated and pre-existing statin use groups. This comparison was performed in all patients and in the tPA-treated subgroup. In the same way as described for relative reperfusion, a forward stepwise procedure for a linear regression model was created to identify which, of 10, baseline clinical variables predicted neurological improvement as measured by ΔNIHSS. Potential interactions between statin use and tPA treatment or age were assessed.
Results
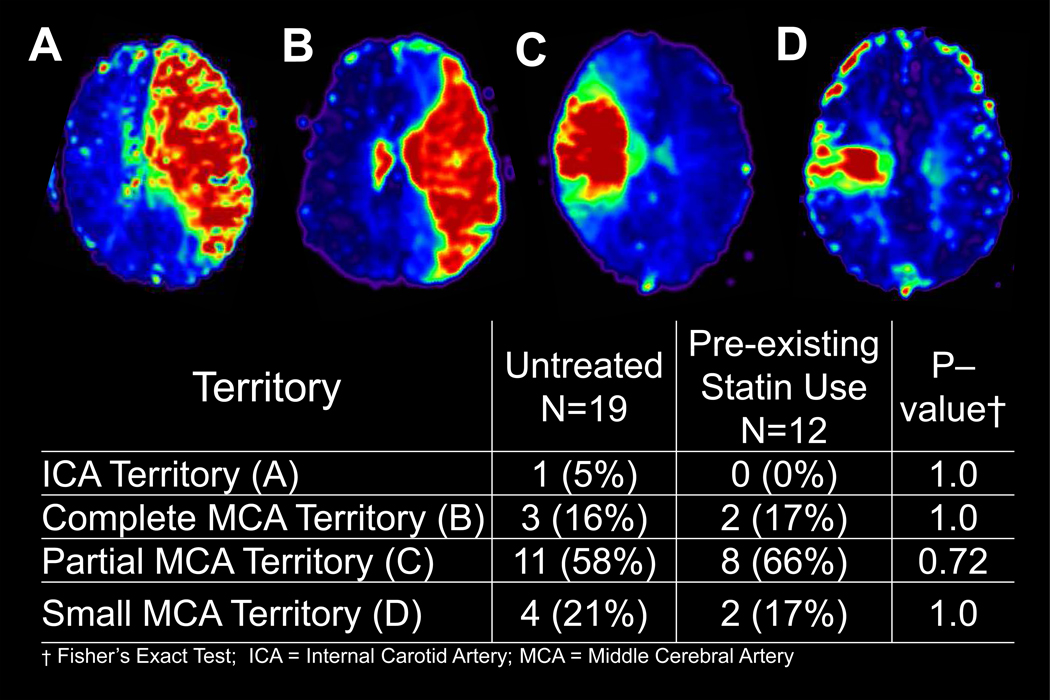
Of 31 acute ischemic stroke patients enrolled, 12 were taking statins at stroke onset, while 19 were not. Patient demographics and baseline characteristics were not significantly different between the two groups except that the statin group had significantly more patients with a history of coronary artery disease (CAD) (p=0.03) (Table 1). Patients were imaged with MRI at 3.0±0.8 hours (tp1) and 6.4±0.4 hours (tp2) after onset. The co-registered images were well-aligned with the template images, yielding inaccuracies < three voxels in any direction. In order to assess whether the location of perfusion deficit territories were balanced between the untreated and pre-existing statin use groups, perfusion deficit territories based on tp1 MTT maps were assigned to one of four groups: 1) internal carotid artery (ICA), (2) complete MCA, (3) partial MCA, and (4) small MCA (Figure 2). There were no significant differences between the two groups.
Table 1.
Baseline characteristics of the untreated and pre-existing statin use groups
| Untreated N=19 |
Pre-existing Statin Use N=12 |
P–value† | |
|---|---|---|---|
| Female, n (%)* | 11 (58%) | 3 (25%) | 0.14 |
| Age (years), median (IQR)‡ | 60 (54, 72) | 62 (59, 66) | 0.72 |
| Admission NIHSS, median (IQR) | 16 (10, 18) | 18 (13, 20) | 0.60 |
| tPA treatment, n (%)* | 12 (63%) | 11 (92%) | 0.11 |
| Volume tp1 perfusion deficit (ml), median (IQR) | 62 (23,116) | 50 (17,88) | 0.73 |
| Time between tp1 and tp2 (min), median (IQR) | 215 (187, 239) | 195 (159, 223) | 0.19 |
| Admission MAP (mmHg), median (IQR) | 110 (94, 145) | 112 (108, 116) | 0.86 |
| Admission Glucose (mg/dl), median (IQR) | 128 (101, 146) | 145 (121, 165) | 0.26 |
| LDL (mg/dl), median (IQR) | 100 (73, 119) | 72 (59, 103) | 0.16 |
| African-American, n (%)* | 6 (32%) | 4 (33%) | 1.0 |
| Time from tPA-bolus to tp1 (min), median (IQR) | 44 (23, 54) | 50 (41, 60) | 0.32 |
| Absolute Volume Reperfusion (ml), median (IQR) | 6 (3, 13) | 12 (7,31) | 0.06 |
| Volume DWI lesion tp1 (ml), median (IQR) | 32 (7, 55) | 15 (8, 30) | 0.51 |
| Onset-to-needle time (min), median (IQR) | 110 (99, 121) | 110 (101, 133) | 0.65 |
| History of Hypertension, n (%)* | 15 (79%) | 10 (83%) | 1.0 |
| History of Diabetes, n (%)* | 7 (37%) | 6 (50%) | 0.71 |
| History of Congestive Heart Failure, n (%)* | 5 (26%) | 1 (8%) | 0.36 |
| Current Tobacco Use, n (%)* | 7 (37%) | 5 (42%) | 1.0 |
| History of Coronary Artery Disease, n (%)* | 2 (10%) | 6 (50%) | 0.03 |
| History of Stroke or TIA, n (%)* | 2 (10%) | 4 (33%) | 0.17 |
Wilcoxon Rank Sum Test unless indicated by (*).
Fisher’s Exact Test was used for assessing difference in frequencies between two groups.
Due to small sample size, results are expressed as median and inter-quartile range (IQR).
NIHSS = NIH Stroke Scale; MAP = Mean Arterial Pressure; LDL=Low Density Lipoprotein; DWI = Diffusion Weighted Imaging; TIA = Transient Ischemic Attack
Figure 2. Distribution of Perfusion Deficits at tp1 (based on MTT).
In order to assess whether the location of perfusion deficit territories were balanced between the untreated and pre-existing statin use groups, tp1 perfusion deficit territories were assigned to one of four groups: 1) internal carotid artery (ICA), (2) complete middle cerebral artery (MCA), (3) partial MCA, and (4) small MCA (Figure 2). There were no significant differences between the two groups. Example of MTT maps for each of the four groups are shown in the upper panel, labeled A, B, C, and D for ICA, complete MCA, partial MCA, and small MCA, respectively.
To assess how representative our study population (N=31) was relative to our hospital stroke population (N=2057) over the 2.5 year enrollment time period, we compared characteristics of both groups. While age, gender, and race were similar, the mean NIHSS score was higher in the study cohort for non-tPA-treated patients. In addition, for tPA-treated patients, the mean door-to-needle time in the study cohort was shorter compared to the hospital tPA-treated patients (51 vs. 60 min, p=0.04). There was no difference is rate of symptomatic intracerebral hemorrhage (Online Supplementary Data).
Statin Use is Associated with Increased Relative Reperfusion
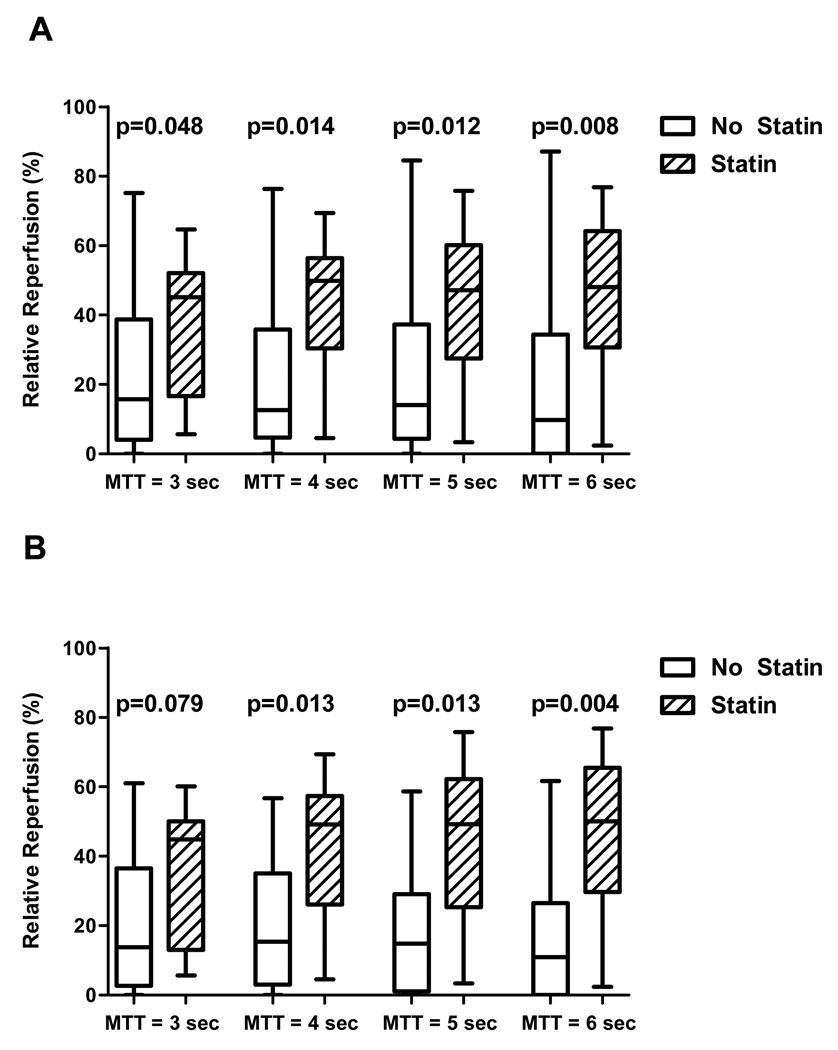
We compared relative reperfusion in the untreated group and statin group for each of four MTT thresholds to determine if findings were consistent across all MTT thresholds. Relative reperfusion was greater in the statin group compared with the non-statin group for all MTT thresholds (Figure 3a). Median reperfusion across the four MTT thresholds for the statin group was 48% (IQR 28%, 64%) and for the untreated group was 13% (IQR 4%, 35%).
Figure 3. Statin use and relative reperfusion.
(A) Entire sample, n=31: Boxplots (median, IQR) for relative reperfusion at each MTT threshold are shown for all 31patients. For each MTT threshold, there was significantly greater reperfusion in the statin group compared to the untreated group. (B) tPA-treated patients only, n=23: Boxplots for relative reperfusion at each MTT threshold are shown for tPA-treated patients only. For each MTT threshold except MTT=3 seconds, there was significantly greater reperfusion in the statin group compared to the untreated group.
Because tPA treatment was more common in the statin group, we performed the same analysis as above in the subset of 23 patients treated with tPA to determine if the statin effect persisted in this subgroup. We continued to observe greater relative reperfusion in the statin group in three of the four MTT thresholds, with a strong trend for MTT=3 seconds (Figure 3b). For tPA-treated patients, median reperfusion across the four MTT thresholds for the statin group was 48% (IQR 26%, 56%) and for the untreated group, was 15% (IQR 2%, 30%).
A forward selection stepwise procedure determined which clinical variables best predicted early reperfusion after stroke. In addition to statin use, nine baseline variables which were hypothesized to possibly affect reperfusion were included (Table 1, top nine variables). Clinical variables which remained in the final model included: (1) statin use, β (SE) [regression coefficient (standard error)]=17.9(7.3), p=0.021; (2) volume tp1 perfusion deficit, β(SE) =−0.156(0.06), p=0.024; and (3) admission MAP, β(SE)=−0.318(0.15), p=0.040. The final model explained 41.1% of the variance in relative reperfusion (Table 2). Therefore, at stroke onset, statin use, smaller perfusion deficit, and lower blood pressure best predicted relative reperfusion.
Table 2.
Multivariable model of clinical variables predicting relative reperfusion and ΔNIHSS
| Predictors of Relative Reperfusion* | ||
|---|---|---|
| Clinical Variable | β (SE)† | P-value‡ |
| Pre-existing Statin Use | 17.9 (7.3) | 0.021 |
| Volume Prolonged MTT at tp1 | −0.156 (0.06) | 0.024 |
| Mean Arterial Pressure | −0.318 (0.15) | 0.040 |
| Predictors of ΔNIHSS from Admission to One Month§ | ||
| Clinical Variable | β (SE)† | P-value‡ |
| Pre-existing Statin Use | 4.82 (1.7) | 0.010 |
| Age | −0.163 (0.06) | 0.015 |
β(SE)=regression coefficient(standard error)
P < 0.05 was considered statistically significant.
Model including pre-existing statin use, volume of prolonged MTT at tp1, and admission mean arterial pressure explained 41.1% of the variance in relative reperfusion.
Model including the pre-existing statin use and age explained 31.9% of the variance in ΔNIHSS.
Statin Use Predicts NIHSS Improvement
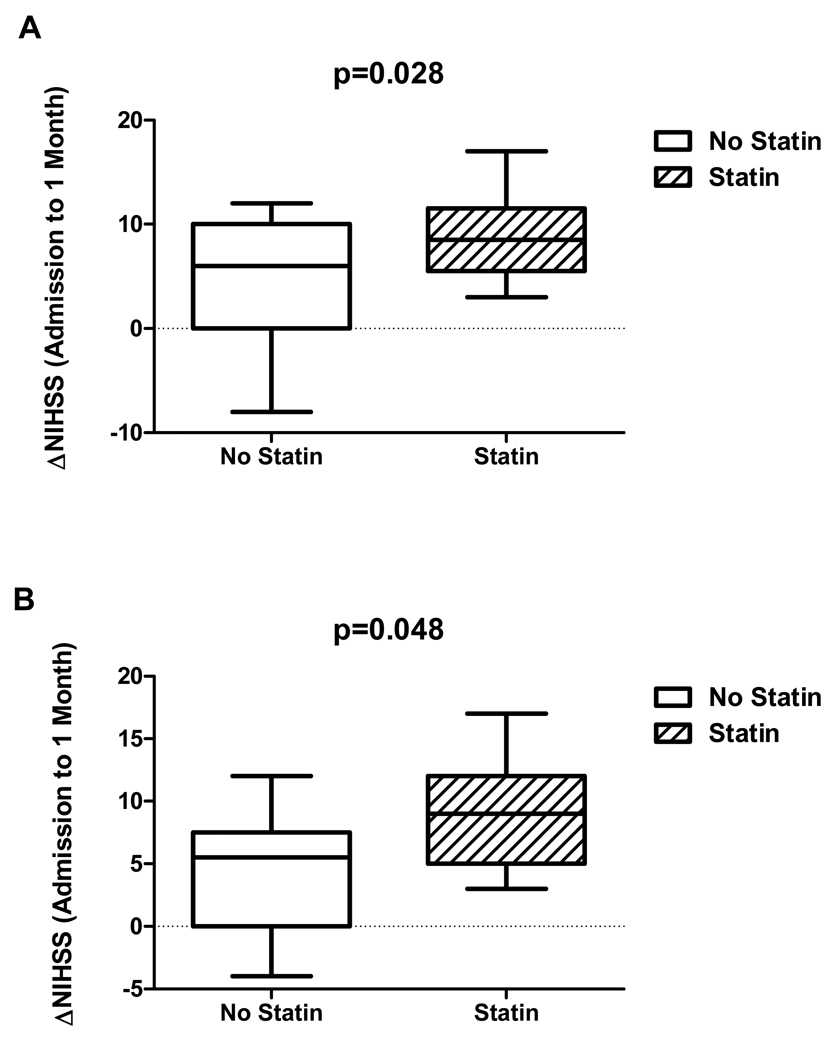
After assessing variables associated with reperfusion, we evaluated how pre-existing statin use may predict neurological improvement, defined by the absolute change in NIHSS from admission to one month follow-up (ΔNIHSS). ΔNIHSS was greater in the statin group, 8.8 ±4.0 (mean +/− SD) points, compared to the untreated group, 4.4±5.7 points, p=0.028 (Figure 4a). When, the tPA-treated subgroup (n=23) were evaluated in a separate analysis, ΔNIHSS continued to be greater in patients taking statins at stroke onset, 8.9±4.2 points, compared to those who were not 4.4±4.9 points, p=0.048 (Figure 4b).
Figure 4. Statin use and neurological improvement.
(A) Entire sample, n=31: Boxplots (median, IQR) for improvement in NIHSS from admission to 1 month follow-up (ΔNIHSS) are shown for all 31 patients. There was greater NIHSS improvement in the statin group compared to the untreated group. (B) tPA-treated patients only, n=23: Boxplots for ΔNIHSS are shown for tPA-treated patients only. There was significantly greater improvement in NIHSS in the statin group.
A forward selection stepwise procedure was utilized to develop a prediction model of ΔNIHSS. Clinical variables which remained in the final model included: (1) pre-existing statin use, β(SE)=4.82(1.7), p=0.010 and (2) age, β(SE)= −0.163(0.06), p=0.015. In this model, statin use and younger age were associated with NIHSS improvement, together explaining 31.9% of the variance in ΔNIHSS (Table 2).
Discussion
Statins and Early Reperfusion
In this retrospective study of prospectively collected MRI data, patients using statins prior to stroke onset had 2-3-fold greater early reperfusion than patients not taking statins. This difference persisted across all MTT thresholds examined, indicating that regardless of how the perfusion deficit was defined (conservatively or liberally), statin use was associated with greater reperfusion. Given the increased frequency of tPA treatment in the statin group, we analyzed this group separately and found that statin use continued to be associated with greater relative reperfusion.
Based on vasculoprotective effects of statins, we hypothesized that early reperfusion may be responsible, in part, for the improved clinical outcomes seen in statin pretreated populations in large clinical trials. Regardless of statin type, animal studies have found decreased infarct size with statin pretreatment10, 24; however, pathways underlying this neuroprotection continue to be investigated. Potential mechanisms leading to early reperfusion fall into three broad categories: (1) improved blood flow / vasomotor reactivity, (2) anti-thrombotic effects, and (3) anti-inflammatory effects. Before cholesterol-lowering properties take effect, statins exert pleiotropic effects on the vascular wall, including the upregulation of endothelial nitric oxide synthase (eNOS), increasing nitric oxide production with resultant increased CBF13, 14, 25. Consistent with this mechanism, statins decreased perfusion deficits following rodent middle cerebral artery occlusion (MCAO)26 and improved cerebral vasomotor reactivity in stroke patients undergoing SPECT imaging27. The anti-thrombotic effects of statins act via increasing endogenous tPA levels9 as well as by inhibiting plasminogen activator-18. Moreover, increased nitric oxide leads to decreased platelet activation and aggregation10. Finally, the anti-inflammatory effect of statins was demonstrated in large clinical trials showing prevention of vascular events in patients with concurrent lowering of C-reactive protein28, 29. By inhibition of leukocyte and cytokine activation, statins mitigate ischemia-reperfusion injury and accordingly may prevent ‘no-reflow’, a phenomenon in which tissue reperfusion is not restored despite vessel recanalization30, 31.
In a linear regression model, we identified three baseline variables which explained 41% of the variance in relative reperfusion: statin use, perfusion deficit volume at tp1, and baseline MAP. Our data suggests that larger perfusion deficits have relatively less reperfusion, a finding supported by a recent report in a smaller cohort of tPA-treated patients32. A smaller initial perfusion deficit may signify less clot burden, facilitating recanalization either spontaneously or in the presence of thrombolytics. Clinical studies demonstrated that larger perfusion lesions, baseline stroke severity, and large-vessel occlusion predicted infarct growth, stroke evolution, and early neurological deterioration33, 34.
Our model revealed that higher blood pressure (BP) was significantly associated with less reperfusion. While several observational studies have associated elevated BP early after stroke onset with increased disability, several of these studies, including the International Stroke Trial, found a U-shaped curve in which low BP was also associated with poor outcomes35. Moreover, this negative relationship of BP and reperfusion opposes a common approach of stroke physicians to avoid BP lowering, especially in chronically hypertensive patients. In our sample, three (of seven) patients did not receive tPA due to elevated BP, suggesting the possibility of less reperfusion due to no tPA treatment; however, tPA was not a significant predictor of reperfusion in our sample; thus, the relationship between BP and reperfusion warrants further study.
We chose to use reperfusion determined by MTT as an outcome measure rather than recanalization (using MRA) because reperfusion is a more sensitive and quantitative measure than recanalization which relies on crude measures of stenosis. Studies have shown that recanalization may occur without tissue reperfusion due to proximal clot breakdown with distal embolization, and reperfusion may occur in the absence of recanalization likely due to collateral flow36–38. Moreover, a recent study comparing computed topography (CT) perfusion to CT angiography suggested that reperfusion more accurately predicted infarct volumes than recanalization39.
Statins and Neurological Improvement
Pre-existing statin use predicted greater neurological improvement as measured by ΔNIHSS in the entire sample as well as in tPA-treated patients. In a multivariable model, besides statin use, lower age also predicted improved neurological status, a finding that was not surprising since age is one of the strongest predictors of disability after stroke40, 41.
Our finding that statin pretreatment was associated with greater neurological improvement in NIHSS may be consistent with findings from several large clinical studies which found improved clinical outcomes in statin pretreated patients2–6. Besides vasculoprotective effects, statins have been reported to stimulate production of anti-apoptotic protein, Bcl-2, in neurons and human neuroblastoma cells42, 43 and prevent glutamate-induced excitotoxicity in cortical neurons44. Statins administered within one day of stroke were found to improve synaptogenesis, neurogenesis, and angiogenesis in a rodent MCAO model45.
Our study has several limitations. Limited sample size may result in type II errors due to insufficient power. Although the study was prospective, there was no randomization of treatment, which may lead to selection bias. The treatment groups were unblinded; however, the investigators performing the reperfusion analysis were blinded to the clinical data. We obtained information on statin use from patients and family without independent confirmation. Patients using statins received more tPA than the untreated patients (though not statistically significant), so we performed the analysis in the tPA-treated subgroup to eliminate tPA as an independent variable, and found similar results. Ideally, the study would be repeated with a larger sample of non-tPA treated patients. We identified greater CAD in the statin group, as might be expected since CAD is a major indication for statin use. This imbalance may have introduced bias favoring reperfusion due to better medical care prior to stroke onset or limiting reperfusion due to greater medical comorbidities in CAD patients. Our study cohort included strokes of greater severity compared to the hospital stroke patients admitted to our institution; therefore, our results cannot be directly applied to patients with low stroke severity. Furthermore, our study did not include MRA which would have given information about site of occlusion and degree of recanalization. However, by evaluating the location of the tp1 perfusion deficit territories, we did not find any significant imbalance between the two groups (Figure 2), suggesting that bias due to unequal distribution of occlusion sites is less likely.
Conclusions
Pre-existing statin use was associated with greater reperfusion and neurological improvement, raising the hypothesis that statin pretreatment may improve clinical outcomes after stroke by enhancing early reperfusion. Due to the small patient sample with non-randomized treatment, further studies are required to confirm these findings.
Supplementary Material
Acknowledgements
Funding:
This study was supported by a grant from National Institute of Health (NIH 5P50NS055977). This publication was also supported by Grant Numbers 1 UL1 RR024992-01 and 1 KL2 RR 024994-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose.
References
- 1.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein LB, Amarenco P, Zivin J, Messig M, Altafullah I, Callahan A, Hennerici M, MacLeod MJ, Sillesen H, Zweifler R, Michael K, Welch A. Statin treatment and stroke outcome in the stroke prevention by aggressive reduction in cholesterol levels (sparcl) trial. Stroke. 2009;40:3526–3531. doi: 10.1161/STROKEAHA.109.557330. [DOI] [PubMed] [Google Scholar]
- 3.Greisenegger S, Mullner M, Tentschert S, Lang W, Lalouschek W. Effect of pretreatment with statins on the severity of acute ischemic cerebrovascular events. J Neurol Sci. 2004;221:5–10. doi: 10.1016/j.jns.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Marti-Fabregas J, Gomis M, Arboix A, Aleu A, Pagonabarraga J, Belvis R, Cocho D, Roquer J, Rodriguez A, Garcia MD, Molina-Porcel L, Diaz-Manera J, Marti-Vilalta JL. Favorable outcome of ischemic stroke in patients pretreated with statins. Stroke. 2004;35:1117–1121. doi: 10.1161/01.STR.0000125863.93921.3f. [DOI] [PubMed] [Google Scholar]
- 5.Reeves MJ, Gargano JW, Luo Z, Mullard AJ, Jacobs BS, Majid A. Effect of pretreatment with statins on ischemic stroke outcomes. Stroke. 2008;39:1779–1785. doi: 10.1161/STROKEAHA.107.501700. [DOI] [PubMed] [Google Scholar]
- 6.Yoon SS, Dambrosia J, Chalela J, Ezzeddine M, Warach S, Haymore J, Davis L, Baird AE. Rising statin use and effect on ischemic stroke outcome. BMC Med. 2004;2:4. doi: 10.1186/1741-7015-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanco M, Nombela F, Castellanos M, Rodriguez-Yanez M, Garcia-Gil M, Leira R, Lizasoain I, Serena J, Vivancos J, Moro MA, Davalos A, Castillo J. Statin treatment withdrawal in ischemic stroke: A controlled randomized study. Neurology. 2007;69:904–910. doi: 10.1212/01.wnl.0000269789.09277.47. [DOI] [PubMed] [Google Scholar]
- 8.Bourcier T, Libby P. Hmg coa reductase inhibitors reduce plasminogen activator inhibitor-1 expression by human vascular smooth muscle and endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:556–562. doi: 10.1161/01.atv.20.2.556. [DOI] [PubMed] [Google Scholar]
- 9.Asahi M, Huang Z, Thomas S, Yoshimura S, Sumii T, Mori T, Qiu J, Amin-Hanjani S, Huang PL, Liao JK, Lo EH, Moskowitz MA. Protective effects of statins involving both enos and tpa in focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:722–729. doi: 10.1038/sj.jcbfm.9600070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laufs U, Gertz K, Huang P, Nickenig G, Bohm M, Dirnagl U, Endres M. Atorvastatin upregulates type iii nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke. 2000;31:2442–2449. doi: 10.1161/01.str.31.10.2442. [DOI] [PubMed] [Google Scholar]
- 11.Amin-Hanjani S, Stagliano NE, Yamada M, Huang PL, Liao JK, Moskowitz MA. Mevastatin, an hmg-coa reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice. Stroke. 2001;32:980–986. doi: 10.1161/01.str.32.4.980. [DOI] [PubMed] [Google Scholar]
- 12.Nagaraja TN, Knight RA, Croxen RL, Konda KP, Fenstermacher JD. Acute neurovascular unit protection by simvastatin in transient cerebral ischemia. Neurol Res. 2006;28:826–830. doi: 10.1179/174313206X153914. [DOI] [PubMed] [Google Scholar]
- 13.Filusch A, Buss S, Hardt S, Katus HA, Kuecherer HF, Hansen A. Evaluation cardioprotective effects of atorvastatin in rats by real time myocardial contrast echocardiography. Echocardiography. 2008;25:974–981. doi: 10.1111/j.1540-8175.2008.00724.x. [DOI] [PubMed] [Google Scholar]
- 14.Yamada M, Huang Z, Dalkara T, Endres M, Laufs U, Waeber C, Huang PL, Liao JK, Moskowitz MA. Endothelial nitric oxide synthase-dependent cerebral blood flow augmentation by l-arginine after chronic statin treatment. J Cereb Blood Flow Metab. 2000;20:709–717. doi: 10.1097/00004647-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Derdeyn CP, Carpenter DA, Videen TO, Grubb RL, Jr, Powers WJ. No effect of low-dose statins treatment on cerebral blood flow in humans with atherosclerotic cerebrovascular disease. J Cereb Blood Flow Metab. 2007;27:1643–1648. doi: 10.1038/sj.jcbfm.9600469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Georgiadis AL, Hussein HM, Vazquez G, Shah Q, Suri MF, Ezzeddine MA, Qureshi AI. Premorbid use of statins is associated with higher recanalization rates in patients with acute ischemic stroke undergoing endovascular treatment. J Neuroimaging. 2009;19:19–22. doi: 10.1111/j.1552-6569.2008.00319.x. [DOI] [PubMed] [Google Scholar]
- 17.Sillberg VA, Wells GA, Perry JJ. Do statins improve outcomes and reduce the incidence of vasospasm after aneurysmal subarachnoid hemorrhage: A meta-analysis. Stroke. 2008;39:2622–2626. doi: 10.1161/STROKEAHA.107.508341. [DOI] [PubMed] [Google Scholar]
- 18.Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part i: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36:715–725. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- 19.Kuppusamy K, Lin W, Cizek GR, Haacke EM. In vivo regional cerebral blood volume: Quantitative assessment with 3d t1-weighted pre- and postcontrast mr imaging. Radiology. 1996;201:106–112. doi: 10.1148/radiology.201.1.8816529. [DOI] [PubMed] [Google Scholar]
- 20.Ostergaard L, Sorensen AG, Kwong KK, Weisskoff RM, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part ii: Experimental comparison and preliminary results. Magn Reson Med. 1996;36:726–736. doi: 10.1002/mrm.1910360511. [DOI] [PubMed] [Google Scholar]
- 21.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 22.Parsons MW, Yang Q, Barber PA, Darby DG, Desmond PM, Gerraty RP, Tress BM, Davis SM. Perfusion magnetic resonance imaging maps in hyperacute stroke: Relative cerebral blood flow most accurately identifies tissue destined to infarct. Stroke. 2001;32:1581–1587. doi: 10.1161/01.str.32.7.1581. [DOI] [PubMed] [Google Scholar]
- 23.Thijs VN, Adami A, Neumann-Haefelin T, Moseley ME, Marks MP, Albers GW. Relationship between severity of mr perfusion deficit and dwi lesion evolution. Neurology. 2001;57:1205–1211. doi: 10.1212/wnl.57.7.1205. [DOI] [PubMed] [Google Scholar]
- 24.Endres M. Statins and stroke. J Cereb Blood Flow Metab. 2005;25:1093–1110. doi: 10.1038/sj.jcbfm.9600116. [DOI] [PubMed] [Google Scholar]
- 25.Laufs U, Endres M, Stagliano N, Amin-Hanjani S, Chui DS, Yang SX, Simoncini T, Yamada M, Rabkin E, Allen PG, Huang PL, Bohm M, Schoen FJ, Moskowitz MA, Liao JK. Neuroprotection mediated by changes in the endothelial actin cytoskeleton. J Clin Invest. 2000;106:15–24. doi: 10.1172/JCI9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shabanzadeh AP, Shuaib A, Wang CX. Simvastatin reduced ischemic brain injury and perfusion deficits in an embolic model of stroke. Brain Res. 2005;1042:1–5. doi: 10.1016/j.brainres.2005.01.105. [DOI] [PubMed] [Google Scholar]
- 27.Murakami M, Fujioka S, Hirata Y, Kuratsu J. Low-dose of statin treatment improves cerebrovascular reactivity in patients with ischemic stroke: Single photon emission computed tomography analysis. J Stroke Cerebrovasc Dis. 2008;17:16–22. doi: 10.1016/j.jstrokecerebrovasdis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on c-reactive protein levels: The pravastatin inflammation/crp evaluation (prince): A randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Reduction in c-reactive protein and ldl cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the jupiter trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 30.Pahan K, Sheikh FG, Namboodiri AM, Singh I. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest. 1997;100:2671–2679. doi: 10.1172/JCI119812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marino F, Guasti L, Cosentino M, Rasini E, Ferrari M, Maio RC, Loraschi A, Cimpanelli MG, Schembri L, Legnaro M, Molteni E, Crespi C, Crema F, Venco A, Lecchini S. Simvastatin treatment in subjects at high cardiovascular risk modulates at1r expression on circulating monocytes and t lymphocytes. J Hypertens. 2008;26:1147–1155. doi: 10.1097/HJH.0b013e3282f97dde. [DOI] [PubMed] [Google Scholar]
- 32.An HFA, Vo K, Eldeniz C, Ponisio R, Zhu H, Li Y, Chen Y, Powers WJ, Lee JM, Lin W. Early changes of tissue perfusion after tpa in hyperacute ischemic stroke. Stroke. 2010 doi: 10.1161/STROKEAHA.110.590323. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bang OY, Kim GM, Chung CS, Kim SJ, Kim KH, Jeon P, Saver JL, Liebeskind DS, Lee KH. Differential pathophysiological mechanisms of stroke evolution between new lesions and lesion growth: Perfusion-weighted imaging study. Cerebrovasc Dis. 29:328–335. doi: 10.1159/000278928. [DOI] [PubMed] [Google Scholar]
- 34.Thanvi B, Treadwell S, Robinson T. Early neurological deterioration in acute ischaemic stroke: Predictors, mechanisms and management. Postgrad Med J. 2008;84:412–417. doi: 10.1136/pgmj.2007.066118. [DOI] [PubMed] [Google Scholar]
- 35.Leonardi-Bee J, Bath PM, Phillips SJ, Sandercock PA. Blood pressure and clinical outcomes in the international stroke trial. Stroke. 2002;33:1315–1320. doi: 10.1161/01.str.0000014509.11540.66. [DOI] [PubMed] [Google Scholar]
- 36.Janjua N, Alkawi A, Suri MF, Qureshi AI. Impact of arterial reocclusion and distal fragmentation during thrombolysis among patients with acute ischemic stroke. AJNR Am J Neuroradiol. 2008;29:253–258. doi: 10.3174/ajnr.A0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali LK, Saver JL. The ischemic stroke patient who worsens: New assessment and management approaches. Rev Neurol Dis. 2007;4:85–91. [PubMed] [Google Scholar]
- 38.Liebeskind DS. Collaterals in acute stroke: Beyond the clot. Neuroimaging Clin N Am. 2005;15:553–573. doi: 10.1016/j.nic.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Soares BP, Tong E, Hom J, Cheng SC, Bredno J, Boussel L, Smith WS, Wintermark M. Reperfusion is a more accurate predictor of follow-up infarct volume than recanalization: A proof of concept using ct in acute ischemic stroke patients. Stroke. 41:e34–e40. doi: 10.1161/STROKEAHA.109.568766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Anderson CS. Long-term disability after first-ever stroke and related prognostic factors in the perth community stroke study, 1989–1990. Stroke. 2002;33:1034–1040. doi: 10.1161/01.str.0000012515.66889.24. [DOI] [PubMed] [Google Scholar]
- 41.Counsell C, Dennis M, McDowall M, Warlow C. Predicting outcome after acute and subacute stroke: Development and validation of new prognostic models. Stroke. 2002;33:1041–1047. doi: 10.1161/hs0402.105909. [DOI] [PubMed] [Google Scholar]
- 42.Johnson-Anuna LN, Eckert GP, Franke C, Igbavboa U, Muller WE, Wood WG. Simvastatin protects neurons from cytotoxicity by up-regulating bcl-2 mrna and protein. J Neurochem. 2007;101:77–86. doi: 10.1111/j.1471-4159.2006.04375.x. [DOI] [PubMed] [Google Scholar]
- 43.Butterick TA, Igbavboa U, Eckert GP, Sun GY, Weisman GA, Muller WE, Wood WG. Simvastatin stimulates production of the antiapoptotic protein bcl-2 via endothelin-1 and nfatc3 in sh-sy5y cells. Mol Neurobiol. 2010;41:384–391. doi: 10.1007/s12035-010-8122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosel J, Gandor F, Harms C, Synowitz M, Harms U, Djoufack PC, Megow D, Dirnagl U, Hortnagl H, Fink KB, Endres M. Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurones. J Neurochem. 2005;92:1386–1398. doi: 10.1111/j.1471-4159.2004.02980.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.