Abstract
ATP-sensitive (KATP) channels are present in the surface and internal membranes of cardiac, skeletal and smooth muscle cell, and provide a unique feedback between muscle cell metabolism and electrical activity. In so doing, they can play an important role in the control of contractility, particularly when cellular energetics are compromised, protecting the tissue against calcium overload and fiber damage, but the cost of this protection may be enhanced arrhythmic activity. Generated as complexes of Kir6.1 or Kir6.2 pore-forming subunits with regulatory sulfonylurea receptor subunits, SUR1 or SUR2, the differential assembly of KATP channels in different tissues gives rise to tissue-specific physiological and pharmacological regulation, and hence to the tissue-specific pharmacological control of contractility. The last ten years have provided insights to the regulation and role of muscle KATP channels, in large part driven by studies of mice in which the protein determinants of channel activity have been deleted or modified. As yet, few human diseases have been correlated with altered muscle KATP activity, but genetically modified animals give important insights to likely pathological roles of aberrant channel activity in different muscle types.
A. INTRODUCTION
It is now a quarter century since Akinori Noma (305) first described ATP-sensitive K+-currents in cardiac myocytes. In the intervening years, details of the signature inhibition of KATP by ATP, activation by MgADP, and pharmacological modulation by inhibitory sulfonylureas, and potassium channel opening (KCO) drugs, have been clarified, and are described in earlier reviews (13, 295, 299, 385). The cloning of genes encoding the underlying subunits, in the mid 1990’s (2, 56, 168, 171) led to a detailed molecular dissection of channel function and regulation, and insights to the tissue-specific nature and regulation of these channels. Here we review these advances in understanding of the molecular structure and physiological function of cardiac, skeletal and smooth muscle KATP, with a particular focus on the insights that have been gained over the last ten years, driven in large part by the generation of knockout mouse models for each of the KATP genes (KCNJ8, KCNJ11, ABCC8, ABCC9) (55, 275, 360, 362) and transgenic mice with altered KATP function (114, 219, 260). These studies have pointed to muscle-specific roles of KATP channels, including ischemic protection in the heart, protection against fiber damage in skeletal muscle, and control of vasomotor tone in smooth muscle. Unlike the pancreatic β-cell, where human disorders clarify the physiological role of KATP in glucose-dependent insulin secretion (126, 221, 379), no consistent picture has yet emerged of human myopathic disease resulting from altered KATP activity. However, there are compelling findings and correlations that implicate not only an important physiologic role for KATP, but potential pathophysiologic roles in disease states.
Molecular basis of KATP channel activity
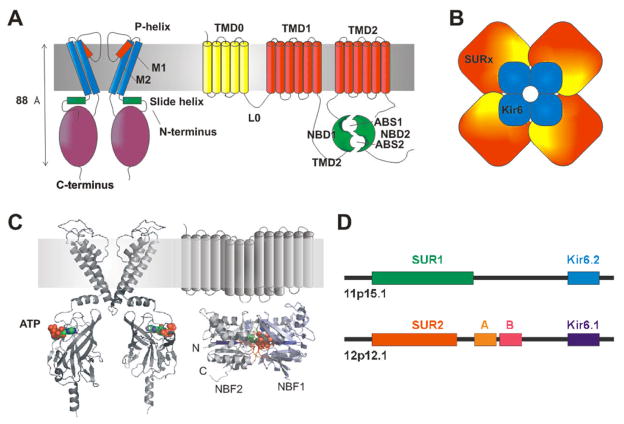
KATP channels are heterooctameric complexes of pore-forming Kir channel subunits together with members of the ATP binding cassette (ABC) family of membrane proteins (Fig. 1). Two genes, KCNJ8 (Kir6.1) and KCNJ11 (Kir6.2) encode KATP pore-forming subunits (167, 168), while two SUR genes, ABCC8 (SUR1) and ABCC9 (SUR2), generate regulatory subunits (2, 56, 168). Alternative RNA splicing gives rise to several SUR protein variants (e.g. SUR2A and SUR2B) that confer distinct physiological and pharmacological properties on the channel complex (53, 365). Interestingly (Fig. 1D), the genes for Kir6.2 and SUR1 are located next to each other on human chromosome 11p15.1 (168) suggesting an as yet unconsidered co-regulation at the gene level. In addition, the genes for Kir6.1 and SUR2 are also adjacent to one another on chromosome 12p12.1 (56, 170), implicating an evolutionary duplication event. In order to recapitulate KATP channel activity in a heterologous expression system, both Kir6 and SUR subunits must be co-expressed (168), and biophysical and biochemical studies show that Kir6.2 and SUR1 subunits combine in a 4:4 stoichiometry (Fig. 1) to generate the functional KATP channel (60, 169, 373). Similarly, biochemical studies demonstrate that the SUR2 protein variants, SUR2A and SUR2B, can also coassemble with Kir6 subunits (20, 167, 311, 442), presumably in a similar octameric arrangement.
Fig. 1. The structural basis of KATP channel activity.
(A) KATP channels are formed from Kir6 (left) and SUR (right) subunits. Kir6 consists of a tetrameric arrangement of subunits, each consisting of two transmembrane helioces (M1, M2) a pore-forming region (including the p-helix and selectivity fitler) and cytoplasmic N-temrinus (including the amphipathic slide helix) and C-terminus. SUR consists of the TM0 and L0 regions that interact with and mosulate gating of Kir6, followed by two additional 6-helix TM regions, each followed by a nucleotide binding fold (NBF). The two nucleotide binding folds come together to generate two nucleotide interacting sites at their interface. (B) The KATP channel is an octameric structure of Kir6 and SUR subunits. (C) Kir6 and SUR can be modeled on the structure of other prokaryotic and eukaryotic proteins. Such structures predict one ATP binding site at each of the Kir6 interfaces, and Mg-nucleotide binding sites in the dimeric NBF structures of SUR. (D) Human SUR and Kir6 gene structures indicate that each pair are located adjacent to each other on two chromosomes.
The Kir6 subunit
Kir6.1 and Kir6.2 are typical inward rectifier channel proteins consisting of two transmembrane helical domains, TM1 and TM2, and cytoplasmic N- and C-termini (Fig. 1C). The two transmembrane helices and pore domain of Kir6.1 and Kir6.2 (Fig. 1C) share significant structural similarity to the pore-forming S5–S6 membrane segment of voltage-gated K+ channels. Both Kir subunits contain a highly conserved sequence of residues called the K+ channel signature sequence (TVGY/FG) (152), that confers K+ selectivity and is found throughout the K+ channel family. While the same general pore structure is predicted for both Kir6.1 and Kir6.2, the two subunits exhibit distinct single channel conductances of ~35 and ~80 pS, respectively in 150 mM K+ solutions (167, 168, 178, 216, 442), conferred by specific residues in the TM1-TM2 regions (341).
The signature property of KATP is inhibition by intracellular ATP with ATP-binding occurring on the Kir6 subunit (92, 239, 374, 410, 411). Heterologously expressed KATP channels comprised of Kir6.2 plus SUR1 or SUR2A close in the presence of ATP with an average K1/2 for inhibition of ~10 μM (298). Although initially reported to be insensitive to ATP (442), Kir6.1-containing channels have since been shown to exhibit ATP inhibition comparable to Kir6.2 (18, 356). Both non-hydrolyzable ATP analogues and ATP inhibit KATP channels, confirming that inhibition is not a consequence of phosphorylation, but of direct binding to the channel (110, 197, 237). In the absence of Mg2+, ADP also blocks channel activity (197, 237, 305), albeit with lower affinity, suggesting that electrostatic interactions with the phosphate moieties on the ATP molecule, particulary the γ-phosphate, are important for binding (187).
Following the publication of the structure of the tetrameric cytoplasmic domains of eukaryotic Kir3.1 (304), and of the full length bacterial homolog KirBac1.1 (231), modeling of the tetrameric Kir6 pore revealed that three-dimensional folding brings several residues in the cytoplasmic N-and C-termini together (R50, R201, G334, II82) to form a binding pocket for ATP (Fig. 1C) (8, 103, 188, 342, 406), for a total of four binding sites per channel (i.e. each Kir subunit in the tetrameric pore binds one molecule of ATP) (8, 103, 136, 406). In addition, the N-terminus contains an amphipathic “interfacial” or “slide“ helix, predicted to lie parallel to the inner surface of the lipid membrane (Fig. 1A, C) (102). This “slide” helix is physically coupled to the cytoplasmic end of the pore and may provide the physical link between ATP binding and the gate of the channel, which is likely to be located at the crossing point of the inner helix bundle (230, 231, 321, 343). More detailed homology models of Kir6.2 continue to follow the appearance of more refined bacterial channel structures (KirBac3.1) and eukaryotic channel cytoplasmic domains (Kir3.1, Kir2.1) (230, 231, 273, 304, 321). Mikhailov et al. have successfully purified a Kir6.2/SUR1 complex and provided a low-resolution structure using single-particle electron microscopy (273), but direct crystallographic analysis of the KATP channel pore structure has thus far been unattainable.
The SUR subunit
In the absence of Mg2+, nucleotides inhibit KATP channel activity, but in the presence of Mg2+, both ATP and ADP stimulate channel activity (95, 160, 196, 237), through interaction with the SUR subunit (132, 303, 372). The unifying structural feature of all ABC proteins are the cytoplasmic nucleotide binding folds, NBF1 and NBF2, comprised of Walker A and Walker B nucleotide-binding motifs and other conserved sequences (154) (Fig. 1C). The NBF domains contain the binding sites for Mg2+-adenosine nucleotides that serve to stimulate KATP function (48, 264). In the ABC superfamily, the transmembrane domains (TMD1,2) each comprised of 6 transmembrane helices typically carry out transmembrane transport function. However, SUR1 and SUR2 are atypical in that no such transport function has been identified, and these subunits contain an additional transmembrane domain at the N-terminus, TMD0, that is joined to TMD1 by a cytoplasmic linker L0 (Fig. 1). Physical interaction between the cytoplasmic N-terminus of Kir6.2 and the TMD0 domain of SUR, including the cytoplasmic L0 linker, is demonstrated to be important in regulation of Kir6.2 gating by SUR (19).
In bacterial ABC proteins, dimerization of NBFs is favoured by the presence of nucleotides, and ATP hydrolysis requires co-operative interactions of the two NBFs (233, 380), leading to the hypothesis that the nucleotide-bound dimer is the catalytically active species. By analogy, it is suggested that Mg2+-dependent ATP hydrolysis at the dimeric SUR NBFs provides the ‘power stroke’ that overcomes the inhibitory effect of ATP on Kir6. The Mg2+ dependence of α32P- and γ32P-azido-ATP labelling of SUR NBFs suggests that SUR NBFs also hydrolyse ATP (266, 412). Trapping of channels in the activated state by vanadate (a transition-state analogue that mimics a post-hydrolytic, ADP-bound state) and in an inactivated state by beryllium fluoride (a transition-state analogue that mimics the pre-hydrolytic, ATP-bound state) (460) further support the hypothesis that ATP hydrolysis underlies channel activation in intact cells, where ATP concentrations will always be at millimolar levels, even when metabolism is substantially inhibited (98). However, direct measurements of nucleotide hydrolysis by SUR NBFs are sparse (37, 263, 273, 460), and SUR-NBF mutations analogous to those that have drastic effects on ATP hydrolysis in bacterial NBFs have only modest effects on SUR-mediated hydrolysis (37). In membrane patch-clamp experiments, channel activation by exogenous MgADP is much more effective than activation by MgATP, and the same ‘hydrolysis’ mutations lead to decreased MgADP stimulation of KATP channels (37, 132, 372, 462). Why MgADP should so strongly activate the channel in excised patches if a hydrolytic cycle is required for activation is unclear. This would require that the ‘activated’ state resulting from hydrolysis persists after MgADP dissociation, at least long enough for rebinding of exogenous MgADP to maintain the state. Just how reasonable this notion is has not been addressed and it remains conceivable that hydrolysis, if it occurs, is an epiphenomenon and that preferential binding of MgADP at the second nucleotide binding site is actually the physiological activator of the channel.
Additional subunits of KATP and macromolecular complexes
In addition to different gene products, SUR splicing may also be important in determining channel function (53, 142, 350, 365). Expression of SUR1 splice variants with deletions of TM16/17 (133), in NBF1 (142) and in NBF2 (350) have been reported in the heart. Alternative splicing of SUR2 exons 14 and 17, leading to deletion of segments of NBF1 are also expressed in the heart (53). The functional significance of these variants is not known, although channels containing SUR2A Δexon17 are reportedly less sensitive to ATP inhibition (53). In addition, the recent identification of short-form or partial SUR2 gene products (328) has arisen from studies of the SUR2−/− mouse. The targeting construct used in the generation of these animals contained a replacement of exons 12–16, coding for regions of NBF1 (55). Naturally transcribed short-form SUR2 subunits lacking the NBF1 domain have been identified using antibodies raised against distal parts of the protein (329, 388) and are reported to coassemble with Kir6.1 and Kir6.2 to form glibenclamide-insensitive, ATP-sensitive, currents in ventricular myocytes (328). A further recent study provides evidence that these short forms are present in mitochondria, and therefore may actually be a component of ‘mitoKATP’ (449).
While the coassembly of Kir6x and SURx are sufficient to reiterate hallmark properties of KATP channels, additional proteins may also contribute to a macromolecular channel complex and fine-tune channel function. Recent studies have implicated an elaborate β-cell KATP channel macromolecular structure (318, 368), and there is mounting evidence that metabolic enzymes, including adenylate kinase (AK) (49), creatine kinase (CK) (73) and lactate dehydrogenase (LDH) (71), can physically associate with, and regulate, the cardiac KATP channel complex. Formation of a multi-protein complex (Fig. 2) that includes both phosphotransfer and glycolytic enzymes (89, 191) may explain early studies of the native sarcolemmal channel, in which direct application of glycolytic substrates to excised membrane patches inhibited KATP channel activity (426, 427). Recent studies further demonstrate the importance of a local phosphotransfer network in regulating KATP channel activity (363): by amplifying small changes in cytoplasmic ATP concentration, adenylate kinase and creatine kinase may play an integral role in regulating the nucleotide concentration in the localized membrane environment (Fig. 2), thereby regulating the KATP response to metabolic events. In the cell, glycolysis can oscillate periodically, driven by feedback loops in regulation of key glycolytic enzymes by free ADP and other metabolites (155, 309, 444). Several studies have shown that when the capacity to buffer cellular ATP and ADP levels is suppressed by metabolic inhibition, oscillations in glycolysis can cause concurrent oscillations in ventricular action potential duration, due to oscillatory activation of KATP (9, 309, 310, 444), which may promote arrhythmias during acute metabolic stresses, such as myocardial ischemia.
Fig. 2. Complexities of KATP channel regulation in striated muscle.
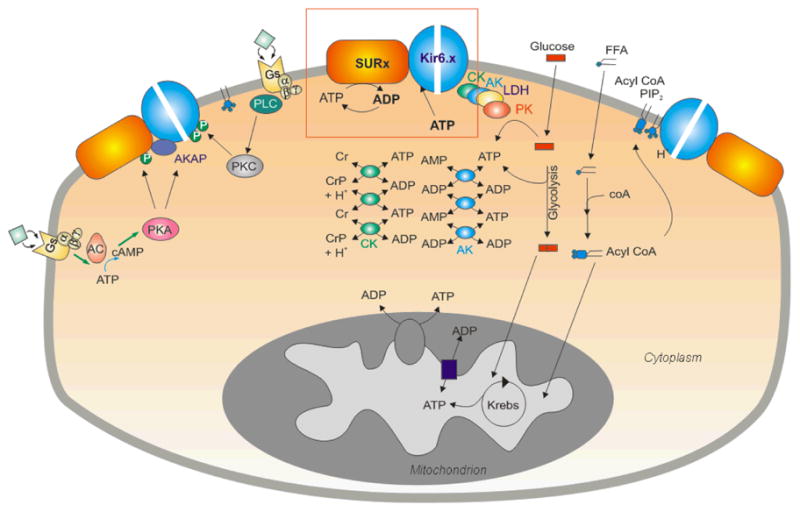
The balance of ATP synthesis and usage, reflected by ATP and ADP levels, is the major direct determinant of channel activity (red box). Metabolic enzymes, including adenylate kinase (AK), creatine kinase (CK), and lactate dehydrogenase (LDH) in the cytoplasm and physically associated with the channel may serve to amplify metabolic changes, or locally buffer and control ATP/ADP levels, thereby fine tuning channel activity. Non-nucleotide ligands, including PIP2, acyl-coA and H+ may also play a key role. PIP2 and acyl CoAs have powerful stimulatory effects that are antagonistic to ATP inhibition. In addition, hormone receptor activation can lead to protein phosphorylation (P) with both stimulatory and inhibitory effects on the channel. However, none of these molecules acts in isolation and the resultant KATP channel activity is an integrated response to a myriad of interrelated metabolic signals.
Modulation by cellular lipids, metabolites and protein kinases
Nucleotide regulation is the signature property of KATP, although other regulatory ligands modulate channel activity through direct interaction with the Kir6 subunit, the SUR subunit or both. Membrane phospholipids, phosphoinositides, in particular (e.g. phosphatidylinosital-4,5-bisphosphate (PIP2), potently stimulate KATP activity by binding the Kir6.2 subunit (29, 107, 344, 375, 439). Application of PIP2 directly to the intracellular side of excised membrane patches leads to significant increase in KATP channel activity and restores channel activity after channel ‘run-down’ (107, 156). Conversely, treatment of a patch with phospholipase C (PLC), to hydrolyze PIP2, reduces channel activity (107, 156). Similarly, receptor–mediated activation of phospholipase C (PLC) modulates KATP activity in recombinant cell systems where receptor and channel proteins are overexpressed (29, 438), although direct support for a dynamic regulation in native muscle cells is lacking. Importantly, there exists a negative coupling between PIP2 activation and ATP sensitivity of KATP channels such that as PIP2 increases, channel open probability increases and ATP-sensitivity decreases (103). Physiologically, this means that ATP sensitivity is not a fixed parameter, and may change dynamically with changes in membrane composition. Residues involved in PIP2 binding and activation overlap with the ATP binding site on Kir6.2, consistent with their competitive effects observed in binding assays (103, 258, 344). The activation of channels by PIP2 requires the presence of both the headgroup and the phospholipid tail, since the cleavage products of PIP2 hydrolysis (IP3 and di-acyl glycerol), have little effect on channel activity. The negative charge of the head group is also critical, since phosphatidyl serine (net negative charge) can activate KATP but phosphatidyl choline (net positive charge) cannot (107). Consistent with an electrostatic interaction between the negatively charged head group and a region of the Kir6.2 protein close to the membrane, a number of positively charged amino acid residues in the slide helix region directly interact with PIP2 in the membrane (79, 80, 358, 374). While PIP2 interaction with Kir6.1 has been less extensively studied, Kir6.1 channels are activated by PIP2 (335), suggesting conservation of the fundamental determinants of phospholipid binding and gating (164, 347, 455).
Long chain acyl-coA molecules (LC-CoA), intermediates of β-oxidation of fatty acids, have also been shown to modulate KATP channel activity in an analogous manner to membrane phosphoinositides (249). The same residues on Kir6.2 that mediate PIP2 activation, also contribute to the stimulatory effect of LC-CoA (262, 358), suggesting that Kir6.2 is the major site of LC-CoA action. The effect of LC-CoA on ATP sensitivity is greater in ventricular myocytes than in pancreatic β-cells (43, 44, 117, 235), and the degree of acyl-coA activation increases with chain length for cardiac ventricular channels, while in pancreatic β-cells medium chain fatty acyl moieties seem to be less effective. PIP2 and lipid modulation of ATP sensitivity is a very powerful modulator of channel activity, and changes of lipids in pathophysiological conditions may be an important regulator. Since cardiac ventricular KATP is predominantly SUR2A-dependent, whereas the pancreatic KATP is predominantly SUR1-dependent (see below), the distinct effects of acyl-coA on cardiac ventricular and pancreatic channels suggest that there is also a role for differential SUR involvement in determining the activation properties of the lipid metabolites, although this needs further work and the implications remain unclear.
Acidic intracellular pH stimulates KATP channel activity (87, 88, 440), and intracellular acidification concomitant with anaerobic metabolism provides another potential physiological stimulus of KATP activity. The mechanistic basis of pH regulation is still not completely established, although there is a general consensus that protons act to decrease sensitivity to inhibitory ATP. Mutagenesis studies of recombinant Kir6.2 subunit have revealed two amino acids (Thr71 and His175) that appear to be critical determinants of pH sensing (76, 437), but it remains unclear whether these residues are directly protonated and how they regulate the effect of pH on ATP-dependent gating.
Agonist-dependent, protein kinase A (PKA) phosphorylation is an important regulator of smooth muscle KATP channels and of pancreatic KATP, although the importance of channel phosphorylation is not entirely clear in striated muscle. The Kir6.2 subunit has two consensus PKA phosphorylation sites (33) which when phosphorylated increase channel open probability (33, 247). In human SUR1, a unique, constitutively phosphorylated PKA site acts to both increase the surface expression of the channel and decrease channel open probability, although this residue is not conserved in rodent SUR1 (33). The specific effects of PKA on Kir6.2/SUR2A combinations have not been examined, and it is unknown whether the potential PKA sites of SUR2A are phosphorylated and what effect, if any, this might have on channel activity in striated muscle, where these subunits are predominant. In contrast, the physiological role of PKA-dependent activation of KATP channels in vascular smooth muscle is now established, and phosphorylation of both Kir6.1 and SUR2B appear to be critical in regulating channel activity, and hence vascular tone (see section D).
Protein kinase C has mixed actions on native ventricular KATP channels, inhibiting at low micromolar ATP concentrations (242), but activating at high ATP concentrations (246), through phosphorylation of highly conserved T180 residue in the Kir6.2 subunit (243). Chronic PKC activation also stimulates the retrieval of Kir6.2 from the surface membrane through a dynamin-dependent mechanism (162). Kir6.1/SUR2B channels are inhibited (335) by acute PKC treatment, due to phosphorylation of residues in Kir6.1 (see section D), whereas Kir6.2/SUR2B channel activity is reportedly unaffected by PKC (366, 402), highlighting the specificity of KATP channel subunit combinations in physiological regulation.
Structural basis of KATP pharmacology
The SUR subunit also determines the sensitivity of the channel to a huge range of pharmacological KATP channel openers (KCOs) and blockers (2, 17, 21, 22, 37, 82, 139, 282, 292, 413). All SUR isoforms are inhibited by the sulfonylurea glibenclamide, with sulfonylurea inhibition being dependent on nucleotide concentrations, and hence on metabolic state (223, 339). SUR1 is typically more sensitive to sulfonylureas than SUR2 (131), a difference that has been exploited with SUR1/2 chimeric constructs to implicate a binding site for tolbutamide in TMD2 of SUR1 (17, 22). Mutation of residue S1237 in SUR1 to tyrosine, the analogous residue in SUR2, reduces the affinity for tolbutamide and gliblenclamide (17), while the reverse mutation (Y1206S in SUR2) increases affinity (141). The SUR subunits confer also sensitivity to KCO’s such as diazoxide, cromakalim, and pinacidil (298). Diazoxide is an effective activator of SUR1 and SUR2B, but not SUR2A, whereas pinacidil and cromakalim are effective activators of SUR2A and SUR2B, but not SUR1 (112, 333), highlighting the potential for tissue specificity, which remains an important pharmaceutical strategy (15). The channel openers typically contain one or more nitrogen-substituted aromatic rings, and include derivatives of cyanogaunidines (e.g. pinacidil), benzopyrans (e.g. cromakalim), but also many others, and a common pharmacophore has proven elusive (61, 261). Regions important for KCO action are spread throughout the SUR subunits, and include TMD1 and NBD1 for diazoxide (21), and TMD2 for cromakalim and pinacidil (140, 282, 413). These openers all require the presence of hydrolysable ATP (90, 140, 359), which suggests that they act to stabilize or enhance ATP hydrolysis at the NBFs.
B. KATP IN CARDIAC MUSCLE
Under normal physiological conditions, voltage-gated K+ channels and IK1, the strong inward rectifier current, provide the major repolarizing current and stabilize the cardiac resting potential (297), while KATP channels seem to play little or no role in regulating heart rhythm or contraction, despite the fact that they are the most densely expressed K+ channel and if fully activated can lead to complete cessation of cardiac electrical activity and contractile failure (237, 238). Recent studies, taking advantage of genetically altered mice, are providing unique insight into the molecular mechanisms of cardioprotection and surprising revelations about the molecular basis of KATP channel activity.
Cardiac muscle KATP structure
Ventricular sarcolemmal KATP structure
Several splice variants of SUR1 and SUR2, as well as Kir6.1 and Kir6.2, are expressed in the heart (147, 167, 276, 284, 285). Given that any pair of SURx:Kir6.x tetramers can co-assemble when heterologously expressed (167, 168), and that even within a single channel more than one SUR isotype or Kir6 isotype can coexist (50, 51, 77, 218, 325, 431), determining the molecular makeup of the channel in different tissues has been challenging. A number of observations support the conclusion that KATP channels in ventricular myocytes are composed primarily of SUR2A and Kir6.2 subunits. First, SUR1 mRNA is relatively low in the heart, whereas SUR2 is abundant (168). Second, while both SUR2A and SUR2B splice variants are expressed in the heart, only the properties of recombinant Kir6.2/SUR2A channels closely match those of the native ventricular myocyte channel (20, 167), with high sensitivity to pinacidil and cromakalim, and insensitivity to diazoxide. Kir6.2 and SUR2A physically interact in vitro to form functional channels (255), and, most significantly, KATP channel activity is essentially absent in hearts of either Kir6.2−/− mice (391) or SUR2−/− mice (55) (Table 1), providing unequivocal evidence that Kir6.2 and SUR2A are at least essential components of the KATP channel in ventricular sarcolemma. Expression of dominant-negative Kir6.1 subunits can inhibit KATP currents in ventricular myocytes (361, 405, 414), although this could be due to heterologous assembly with endogenous Kir6.2 subunits during channel turnover, and KATP current in ventricular myocytes is apparnetly unaffected in Kir6.1−/− mice, further arguing against a role for Kir6.1 in native sarcolemmal KATP (276).
Table 1.
Genotype-Phenotype Correlation in Genetically-Modified Mice with Altered KATP channel Function
| Mouse genotype | KATP function | Early Death | Coronary spasm | Hypertension | Heart rate | Arhythmias | Major phenotypes | Citation |
|---|---|---|---|---|---|---|---|---|
| Whole-animal | ||||||||
| Kir6.2−/− | Loss-of-function in striated muscle, neurons and β-cell | No | No | ↔ | ↔ | No | Insulin secretion impaired, Impaired cardiac and skeletal muscle stress response. Increased glucose uptake in sk. muscle, Increased exercise-induced fiber damage | (240, 250, 274, 275, 391, 392, 393, 400, 461) |
| Kir6.1−/− | Loss-of-function in vascular smooth muscle | Yes | Yes | ↔ | ↔ | Yes | Coronary-vasospasm and premature death | (276) |
| SUR2−/− | Loss-of-function in all muscle | Yes | Yes | ↑ | ↔ | Yes | Coronary-vasospasm and premature death, resistant to ischemia-reperfusion damage. Increased glucose uptake in sk. Muscle. | (54, 55) |
| SUR1−/− | Loss-of-function in atrial muscle, neurons, and β-cell | No | No | ↔ | n.d. | No | Insulin secretion impaired, Loss of atrial KATP channels, Resistant to ischemia-reperfusion damage | (112, 371) |
| CMV-SUR2A | Gain-of-function in cardiac muscle | No | No | n.d. | n.d. | n.d. | Resistant to ischemic damage | (93) |
| Cardiac-specific transgene | ||||||||
| αMHC-Kir6.2[ΔN30,K185Q] | Reduced ATP-sensitivity and current density in cardiac muscle | No | n.d. | n.d. | ↓ | No | Remodeling of E-C coupling, ↑ ICa,, ischemic preconditioning abolished | (111, 219) |
| αMHC-FLAG-SUR1 | Reduced current density in heart | No | n.d. | n.d. | n.d. | Yes | PR prolongation | (113, 114) |
| αMHC-FLAG-SUR2A | Reduced current density in heart | No | n.d. | n.d. | n.d. | No | No significant physiological effect | (114) |
| αMHC-Kir6.2[ΔN30, K185Q] × αMHC-FLAG-SUR1 | Reduced ATP-sensitivity and current density in cardiac muscle | Yes | n.d. | n.d. | n.d | Yes | Constellation of arrhythmias and premature death | (113) |
| αMHC- Kir6.2[ΔN30, K185Q] × αMHC-FLAG-SUR2A | Reduced ATP-sensitivity and current density in cardiac muscle | No | n.d. | n.d. | n.d | No | No significant physiological effect | (113) |
| αMHC-Kir6.1-AAA | Dominant-negative suppression in cardiac muscle | Yes | n.d. | n.d. | ↑ | No | APD prolongation, Premature death | (405) |
| αMHC-Kir6.2-AAA | Dominant-negative suppression in cardiac muscle | Yes | n.d. | n.d. | ↔ | No | APD prolongation, Premature death | (405) |
| Vascular-specific transgene | ||||||||
| SM22-SUR2B/SUR2−/− | Smooth muscle-restored expression in SUR2−/− mice | Yes | Yes | n.d. | ↔ | Yes | Coronary-vasospasm and premature death | (199) |
| Tek-Kir6.1-AAA | Dominant-negative suppression of endothelial KATP | No | Yes | n.d. | n.d. | n.d. | (260) | |
SUR1 and SUR2 subunits can also coassemble within the same channel complex (50, 51, 431), and both SUR1 mRNA and protein have been detected in the hearts of various species (177, 284, 285, 322, 350, 381, 450). KATP channel activity is unaffected in ventricular myocytes from SUR1−/− mice (100, 112), yet antisense oligonucleotides specifically directed at SUR1 markedly reduce rat neonatal ventricular KATP current density (450). It is important to recognize that the molecular architecture need not be static and may change in response to metabolism or other signals. For example, hypoxia increases SUR2A subunit expression in cultured rat heart H9C2 cells (72), and SUR1 expression and diazoxide-sensitive current are both increased in infarcted heart regions (177). In addition, Kir6.1 mRNA expression is significantly elevated following myocardial ischemia or hypoxia, raising the possibility that Kir6.1 protein may be upregulated in myocytes in response to stress (177, 271). At this juncture, whether the increase in Kir6.1 in the heart during cardiac disease reflects increased expression specifically in cardiac myocyte or other cell types remains uncertain.
Atrial sarcolemmal KATP structure
Comparatively few studies have examined the biophysical and pharmacological properties of KATP channel in atrial myocytes but the work that has been done suggests that the structure of atrial and ventricular KATP are distinct. We have recently demonstrated that SUR1 protein is present in wild type mouse atrial myocytes and that KATP currents are undetectable in atrial myocytes from SUR1−/− mice (112), providing unequivocal evidence for an essential role of SUR1 in the atrial sarcolemmal KATP channel in mice (Table 1). In agreement with this conclusion, native atrial KATP is more sensitive to diazoxide than pinacidil, similar to recombinant SUR1/Kir6.2, while the ventricular channel exhibits the opposite specificity (112, 127), similar to recombinant SUR2A/Kir6.2. Although genetic proof by knockout of specific subunits is not possible in other species, there is some evidence for chamber-specific KATP pharmacology in other species. For instance, KATP channels in neonatal rat atrial myocytes are significantly more sensitive to activation by metabolic inhibition and diazoxide than are ventricular KATP channels, as would be expected for SUR1-containing channels (26, 324). The functional implication of disparate structure of atrial and ventricular KATP is unclear, but conceivably, SUR1-based channels are necessary for an atrial specific function, such as coupling atrial stretch with the secretion of atrial natriuretic peptide (186, 206, 348, 441). Interestingly, atrial KATP channels have been reported to be activated by stretch (214, 326, 416), although the data is sparse, and the finding that atrial Kir3.4 channels are also stretch-sensitive (173) may provide an alternative explanation.
Nodal and Purkinje cell KATP structure
KATP channel currents have been detected in sino-atrial (SA) node (143), atrio-ventricular (AV) node (198), and Purkinje fiber cells (245), although the molecular structure in these specialized conducting cells has not been extensively tested. The observed KATP single channel conductance in rabbit SA node cells is reported to be smaller than that in ventricular myocytes (52 versus 80 pS) (143), which may suggest a role for Kir6.1 in the channel, but sarcolemmal KATP is abolished in Kir6.2−/− SA node cells (Table 1) indicating a requirement for the Kir6.2 pore-forming subunit (119). The AV node and Purkinje cells from Kir6.2−/− animals have not been similarly studied. Additionally, the identity of the SUR component is uncertain, although the channels in these cell types do respond to the relatively SUR2A-specific opener cromakalim, suggesting a major role for SUR2A in nodal KATP channels (143, 198, 245).
The molecular basis of ‘mitoKATP‘
The major focus of this review is on sarcolemmal membrane KATP channels, but it is necessary to also consider the mitochondrial KATP (‘mitoKATP’) channel that has also been significantly implicated in cardiac pathophysiology. A K+-selective, small conductance channel was first identified in fused giant mitoplasts from rat liver mitochondria (174), and reported to be reversibly inhibited by application of ATP, glibenclamide, and 4-aminopyridine (4-AP). Further examination revealed a number of properties that distinguish these ‘mitoKATP’ from sarcolemmal KATP channels. First, ‘mitoKATP’ channels are reportedly inhibited by acyl-coA and activated by GTP and GDP when ATP is also present, and second, ‘mitoKATP’ channels reconstituted into liposomes exhibited increased diazoxide sensitivity relative to reconstituted Kir6.2/SUR2A channels (123, 319). Collectively, these observations implicate an ATP-sensitive K+ conductance in the mitochondria that is distinct from sarcolemmal channels.
The pharmacology of heterologously expressed SUR1/Kir6.1 complexes appears to most closely resemble the reported ‘mitoKATP’ properties (161, 252), most particularly in being sensitivie to activation by diazoxide. However, studies to date indicate that ‘mitoKATP’ channel activity is unaffected in Kir6.1−/− and Kir6.2−/− animals (276, 393), arguing that neither of these gene products form the channel. Other biochemical and physiological efforts to determine whether specific SUR or Kir6 subunits are normally present in mitochondria have yielded inconsistent results (81, 116, 161, 232, 378, 390, 459). This may in part reflect the difficulty of isolating pure mitochondria. Contamination of mitochondrial preparations with sarcolemmal proteins could yield erroneous results, particularly given the abundance of KATP channels in the sarcolemma. In addition, the biochemical and molecular analysis may be hindered by lack of specific antibodies for KATP subunits. For example, a recent study by Foster, et al. (116), which analyzed the proteins precipitated by anti-Kir6.1 antibodies from mitochondrial extracts, found that no Kir6.1 protein was detectable by mass spectrometry. Instead, NADH flavoprotein I and mitochondrial isocitrate dehydrogenase were detected in high abundance indicating non-specificity of the antibodies. As discussed further below, short-form SUR2 subunits lacking the NBF1 domain have been identified in cardiac cells (328), and specifically localized to mitochondria (449), using antibodies raised against distal parts of the protein (329, 388). Although further studies are needed, these short forms may actually be a component of ‘mitoKATP’ (449).
Lack of confirmed presence of canonical SUR or Kir6 subunits in mitochondria has led to alternative hypotheses regarding ‘mitoKATP’ structure. Diazoxide, an activator of ‘mitoKATP’, has been shown to inhibit succinate dehydrogenase (145). Consistent with the idea that this key enzyme of both the Krebs cycle and electron transport chain might be a component of the ‘mitoKATP’ channel, Ardehali and colleagues identified a macromolecular complex by co-immunoprecipitation that included succinate dehydrogenase, mitochondrial ATP-binding cassette protein-1 (mABC-1), ATP synthase, adenine nucleotide translocase, and phosphate carrier proteins, that recapitulated ‘mitoKATP’ activity including diazoxide activation and 5-hydroxydecanoate inhibition (10, 11). In this case, it is not clear which component should be forming the channel pore, although given the chloride channel function of the ABC protein CFTR (31), conceivably the mABC could form the pore, but the reported records show only single channel activity over brief periods, and follow-up studies have not yet emerged.
There is now a large body of work on the pathophysiological involvement of ‘mitoKATP’ in cardiac pathophysiology and the phenomenon of preconditioning (144, 308). The above discussion reflects our own skepticism regarding the nature of the ‘mitoKATP’, but since the interpretations of such studies lean heavily on the attendant pharmacology, the finding that diazoxide-sensitive KATP channels are present in the sarcolemma, as well as the reconfirmation that 5-hydroxydecanoate can inhibit sarcolemmal KATP channels (241), warrants at the least a careful reassessment of the interpretations.
Regulation of cardiac KATP function and implications for heart function
The multi-level regulation by membrane phospholipids (PIP2), fatty acids (LC-Acyl-CoA), protein kinases (PKA, PKC), pH, and intracellular nucleotides (ATP, MgADP) ensures complexity of metabolic sensing by KATP channels (Fig. 2). ATP inhibits native channel activity in excised atrial or ventricular membrane patches with an average K1/2 of approximately 50–100 μM (110, 237, 305, 306). Several studies have demonstrated that when channels are opened by metabolic inhibition, injection of ATP into the cytosol closes channels, implicating the fall in [ATP] as a contributor to channel opening under these extreme conditions (238, 306, 395). Nevertheless, ATP probably never falls below millimolar levels in a living tissue (97), and the activating effect of Mg2+-bound nucleotide diphosphates will be important physiologically (237) so it is probably most appropriate to consider both the fall in ATP and accompanying rise in ADP as the major physiological regulatory parameters (Fig. 2).
Additional cytoplasmic compartmentation may modify the nucleotide-sensitivity in vivo. In open-cell-attached patch-clamp experiments, where the cytoplasmic milieu is largely intact, channel activity is apparently less sensitive to global changes in ATP, presumably as a result of the phosphotransfer enzymes and ATP buffering capacity (300). Compartmentalization of ATP may also be important; there is a close functional co-localization of the KATP channel and the Na-K-ATPase, such that changes in Na-K-ATP-ase activity can result in very local changes in ATP levels that are sensed by the KATP channel (148, 193, 327).
The role of intracellular pH, membrane phospholipids, channel phosphorylation and fatty acyl-coA in the regulation of native KATP channels in cardiac muscle are less well understood. Although recombinant Kir6.2/SUR2A channels are sensitive to a fall in intracellular pH (76, 437) as can occur during myocardial ischemia (418), native cardiac KATP channels are resistant to intracellular acidification by as much as 1 pH unit (75, 225, 237) and insensitive to lactate (237). Adenosine, a metabolite released from metabolically challenged myocytes, stimulates KATP channel activity in ventricular myocytes (179, 207) through a G-protein signaling cascade. PKC-dependent cardiac preconditioning by adenosine (see below) indicates an important role for adenosine-mediated KATP channel activity in vivo, but it remains unclear whether sarcolemmal or mitochondrial KATP or both are direct targets (63, 251, 355).
Importantly, intracellular elements other than ATP must act to keep cardiac KATP channels closed under normal conditions in order to prevent AP shortening and to maintain contractility. This is made quite clear by studies of transgenic mice that express ATP-insensitive Kir6.2 subunits in the heart, such that sarcolemmal channels are ~100 fold less sensitive to inhibition by intracellular ATP (111, 219). In these animals, KATP channels remain largely closed and the action potential is not shortened under normal conditions. Conversely, expression of similar ATP-insensitive Kir6.2 transgenes in pancreatic β-cells suppresses electrical activity and can completely shut down insulin secretion (125, 220, 340). Experimental studies (212, 302, 428) have consistently demonstrated that activation of only 1% of the total sarcolemmal KATP population is sufficient to shorten the action potential by 50%, and significantly reduce contractility. Computer models of the action potential, in which KATP activity is controlled only by ATP/ADP ratios (300, 364), indicate that this would happen at low millimolar ATP and micromolar ADP levels in hearts with normal KATP channels, and that in hearts expressing the above ATP-insensitive transgenes, dramatic AP shortening should occur at any physiological ATP levels. It thus appears that additional factors are acting to keep these channels closed in cardiac myocytes. What combination of regulatory factors that control KATP channel opening in cardiac myocytes remains a key question.
KATP in physiology and pathophysiology of cardiac muscle: Insight from genetically modified mice
Ischemia-Reperfusion and Myocardial Infarction in KATP null mice
Sarcolemmal KATP channels seem to be predominantly closed and do not contribute significantly to the process of excitation-contraction coupling in physiological conditions (except perhaps under adrenergic stimulation, see below), since application of sulfonylureas generally has little or no effect on the cardiac action potential (105), and ventricular action potential duration (APD) and contractile function are unaffected in isolated ventricular myocytes from Kir6.2−/− animals (Table 1) (240, 391, 393). However, when metabolism is inhibited chemically, or with profound anoxia, the action potential shortens dramatically and contraction fails as a result of KATP activation (66, 238, 419), both effects being absent when KATP channels are ablated either by gene knockout of Kir6.2 (393) or treatment with a KATP channel blocker (153, 419).
Activation of KATP during ischemia is likely to be cardioprotective in vivo, since reduction of the APD and reduction of contraction may preserve ATP stores that would otherwise be consumed during the contractile cycle. In support of this idea, treatment with the KATP opener pinacidil during ischemia increased the levels of cellular ATP and energy stored as creatine phosphate (269), and in Kir6.2−/− hearts, the time to contractile failure is prolonged but the time to onset of contracture, which likely reflects the depletion of ATP (301), is reduced (393). In addition to preservation of ATP, KATP activation can reduce excessive Ca2+ entry and its destructive sequellae, including arrhythmia, contractile dysfunction and cell death (36). Increase in intracellular Ca2+ in response to metabolic inhibition-reperfusion is exacerbated in both cardiac myocytes from Kir6.2−/− animals relative to wild type myocytes, and in untransfected COS-7 cells (relative to cells expressing recombinant KATP channels) (24, 30, 189, 190). The increase in Ca2+ during metabolic inhibition-reperfusion is correlated with cell death and membrane depolarization, and is significantly blunted by activation of KATP channels (23, 24, 189, 190). Ca2+ entry by reverse-mode Na+-Ca2+ exchanger activity is a major contributor to Ca2+ overload during ischemia-reperfusion (357) and, by reducing membrane depolarization, it is reasonable to predict that KATP activation will minimize reverse-mode exchanger activity (23). Diastolic Ca2+ overload, myocardial damage, and increased mortality are also observed in isoproterenol challenged Kir6.2−/− myocytes (461). In this case, the L-type Ca channel inhibitor verapamil, blocks the increased mortality and is consistent with the model that KATP-dependent hyperpolarization also suppresses Ca2+ entry through voltage dependent Ca2+ channels in wild type animals. Finally, evidence of ‘contractile dysfunctions’ in skeletal muscle in the absence of KATP (57, 58) gives further support to the conclusion that a major function of KATP activation during metabolic stress is to reduce Ca2+ entry by minimizing cell depolarization.
Although there is a generally accepted consensus that activation of KATP within ventricular myocytes will be protective, there are some unexplained and unexpected findings that may require adaptation of the current model. For example, SUR1−/− mice actually exhibit reduced infarct size and greater functional recovery following ischemia-reperfusion than WT mice (100). As discussed above, SUR1 is not normally a major component of mouse ventricular KATP channel (100, 112, 127), suggesting that extra-ventricular KATP channels, perhaps in neurons or in atria, contribute to increased infarct size through an as yet to be defined mechanism. Similarly, infarct size resulting from global ischemia is reduced in SUR2−/− mice when compared to WT mice (387). Since KATP channel activity in ventricular myocytes is essentially absent in SUR2−/− mice, an extra-ventricular origin of protection is again postulated. Because SUR2−/− mice also lack vascular smooth muscle KATP, it is intriguing to speculate that persistant coronary vasospasm in these mice might generate a kind of preconditioning that helps protect the heart (see section D). Consistent with this notion, the Ca channel blocker nifedipine reversed the cardioprotection evident in SUR2−/− mice (387), potentially by blocking spontaneous vasospasm.
Cardiac KATP channels and arrhythmia
Common ECG findings during myocardial ischemia are a peaked T wave and elevation or depression of the ST-segment, reflecting dispersion of repolarization. Consistent with activation of sarcolemmal KATP underlying these features of the ECG during ischemia, both are blocked by the sulfonylureas glibenclamide and HMR1098 (40, 217, 229), and the KATP channel opener pinacidil induces ST-segment elevation in the absence of ischemia (229). Moreover, ischemia-induced changes in the ECG that are reminiscent of ST-elevation are absent in Kir6.2−/− mice (Table 1) (240), arguing strongly that KATP channel activation causes ST-segment elevation. It should be pointed out, however, that ST-segment elevation also occurs in SUR2−/− mice (see below) (54). This is surprising, since the absence of SUR2A leads to loss of ventricular KATP, and suggests that it may be the absence of SUR2 in vascular smooth muscle cells or elsewhere that is responsible.
Arrhythmias frequently accompany myocardial ischemia and reperfusion. Increased potassium conductance can stabilize the resting membrane potential, which may suppress ectopic pacemaker activity, but because KATP channel activation also accelerates repolarization, shortening of the QT-interval and reduced refractory period may actually predispose the heart to re-entrant arrhythmias. Most studies (39, 52, 66, 369, 436), but not all (369), indicate that pharmacological activation of KATP promotes ventricular tachycardia and fibrillation, while channel blockers during ischemia-reperfusion generally decrease the incidence of sustained tachycardia and ventricular fibrillation (205, 436), but again not in all studies (369). Pharmacological treatments used to tease out the effects of KATP channels on cardiac rhythm have thus yielded equivocal results but in general, KATP activation should probably be considered pro-arrhythmic in the setting of ischemia.
Cardiac hypertrophy and heart failure in KATP null mice
In response to a chronic stress such as hypertension, the complex pathological phenomena of cardiac hypertrophy and heart failure ensue. There is typically a progression of the heart from a compensated state characterized by myocardial growth and maintained cardiac output (i.e. cardiac hypertrophy), to a decompensated state in which cardiac contractility is depressed (i.e. dilated cardiomyopathy). Elevated intracellular Ca2+ is a key component in the development of cardiac hypertrophy (433); activation of the calcineurin-NFAT signal transduction cascade by Ca2+ drives the expression of hypertrophy genes. By virtue of their responsivity to changes in energy supply and their ability to regulate APD, Ca2+ entry and contraction, KATP channels should tend to abrogate the disease process. Kir6.2−/− animals do exhibit increased mortality and exaggerated hypertrophy in response to both pressure overload (163, 443), and to mineralocorticoid/salt challenge (202). Moreover, elevated intracellular Ca2+ and increased calcineurin-NFAT activation are evident in Kir6.2−/− myocytes compared with WT following stress, consistent with the greater degree of hypertrophy (202, 443). Cardiac function is also dramatically impaired in Kir6.2−/− animals following hypertensive stress (i.e TAC, mineralocorticoid/DOCA salt treatment): APD (443) and QT interval (202) are prolonged, indicating that KATP may normally act to normalize the APD. Progression to congestive heart failure also appears to be more rapid in the absence of KATP channels. Both animals with trans-aortic constriction (TAC) and Kir6.2−/− animals treated with mineralocorticoid/DOCA salt exhibit increased pulmonary congestion and reduced fractional shortening (202, 443), Taken together these data suggest that KATP may normally impede the myocardial death that is associated with the transition from hypertrophy to overt heart failure (91), such that in the absence of KATP, heart failure is accelerated.
Importantly, KATP channels respond differently to metabolic signals in the hypertrophied or failing heart as compared to the normal heart. KATP channels are less sensitive to inhibitory ATP in hypertrophied or heart failure myocytes (47, 224, 452) and in a transgenic TNFα mouse model of heart failure, KATP channels responded poorly to metabolic inhibition (159) suggesting impaired metabolic control of channel activity. Reduced creatine kinase flux, a feature of the hypertrophic and failing heart (172), is observed in transgenic TNFα mouse model (159), and in open-cell attached patches, KATP channels are less responsive to creatine phosphate (159), suggesting that disruption of this phosphate shuttling pathway impairs ATP generation and hence channel activity in the TNFα mouse model. Consistent with the hypothesis that impaired metabolic sensing by KATP can affect the progression of heart failure, mutations (F1524S and A1513T) within the SUR2 gene locus that reduce the catalytic activity of NBF2, and should therefore reduce physiological activation of the channel, are associated with human dilated cardiomyopathy (38).
Finally, altered KATP channels may also contribute to the metabolic phenotype of the failing heart. In a pressure overload model of heart failure, reduced KATP channel activity impairs expression of the transcription coactivator PGC-1α (163), which controls expression of a number of metabolic enzymes (109). A recent proteomic comparison of wild-type and Kir6.2−/− animals reveals a KATP-dependent subproteome (i.e. proteins that are differentially expressed in WT and Kir6.2−/− myocardium), ~60% of which consists of proteins directly related to cellular metabolism (12, 463). These early studies suggest that feedback from KATP activity to metabolism may turn out to be an important process in the chronic cardiac response to altered demands, and associated disease processes.
Ischemic preconditioning and cardiac KATP channels
Because KATP channel activation can minimize the cell damage that results from ischemic or metabolic challenge, substantial effort has been devoted to exploiting the KATP channel either pharmacologically or physiologically to improve cardiac function in disease states. Paradoxically, “preconditioning” of the heart by brief ischemic periods improves the recovery of contractile function and reduces the infarct size that results from a subsequent prolonged metabolic insult (290). That this effect can be blocked by glibenclamide, has generated much interest in the cardioprotective role of KATP channels (134). Preconditioning of the heart can be mimicked by a number of humoral factors, including adenosine (248, 445) and acetylcholine (447). Blockade of adenosine-(445) and acetylcholine-induced preconditioning (447) with the KATP channel blocker glibenclamide, coupled with the observation that the cardioprotective effects can be reproduced with KATP channel openers (448) seemingly confirms a role for KATP channels in preconditioning. However, a number of subsequent observations suggest an internal site of action in the mitochondria, leading to an ongoing debate over the distinct roles of sarcolemmal KATP and ‘mitoKATP’ in protection from ischemia.
The first line of evidence that questioned a role for sarcolemmal KATP channels in preconditioning was that cardioprotection by KATP channel openers bimakalim, cromakalim or pinacidil did not require shortening of the cardiac APD (138, 446). An additional line of argument arose from the observation that the channel opener diazoxide mimics ischemic preconditioning (122). Because diazoxide is relatively ineffective at opening ventricular sarcolemmal KATP channels, but effective in stimulating ‘mitoKATP’ activity, the latter became a primary candidate cardioprotective factor. Finally, 5-hydroxydecanoic acid (5-HD), reported to block ‘mitoKATP’ (although initially described as a sarcolemmal KATP blocker (307)) effectively abolishes ischemic preconditioning (253). These observations have been central to the argument that ‘mitoKATP’, and not sarcolemmal KATP, plays the major role in ischemic preconditioning.
However, one key assumption, that diazoxide is a specific opener for ‘mitoKATP’ and does not act on the sarcolemmal KATP is incorrect: diazoxide has been shown to open sarcolemmal KATP channels in ventricular myocytes at high concentrations (122) or when ADP concentrations are elevated (83) and it is now clear that sarcolemmal KATP channels in the mouse atrium are exclusively diazoxide sensitive (112, 127). In light of these findings, the experimental conditions in preconditioning studies should be carefully re-examined to ensure that diazoxide is not activating sarcolemmal channels in the ventricle. Diazoxide has also been shown to inhibit succinate dehydrogenase (145), a key enzyme in both the electron transport chain and citric acid cycle and F1F0 ATPase function (67, 69), raising the possibility that diazoxide speeds ATP depletion during ischemia, providing cardioprotection by indirectly activating sarcolemmal KATP channels.
Similarly, the idea that 5-HD is a specific blocker of ‘mitoKATP’ channels must also be carefully evaluated. An increase in flavoprotein oxidation occurs as a result of mitochondrial uncoupling and 5-HD blocks the increase in flavoprotein oxidation during ischemia (161, 252). This finding, coupled with observations that 5-HD failed to block cromakalim-induced sarcolemmal KATP activation and resultant APD shortening (268), has led to the assertion that 5-HD is a‘mitoKATP’ specific blocker. However, 5-HD was initially described as a blocker of sarcolemmal KATP channels in guinea-pig ventricular myocytes (307), and has also been shown to both reduce extracellular K+ accumulation and to block APD shortening in the ischemic heart (283), all consistent with blockade of sarcolemmal KATP channels (349).
While there is certainly much work required to sort out the molecular details of ischemic preconditioning, it is important to note that both ischemic- and diazoxide-induced preconditioning are abolished in hearts from Kir6.2−/− animals (392, 393) as well as in hearts with transgenic overexpression of ATP-insensitive Kir6.2 subunits (337)(Table 1). Because the prevailing data indicate that Kir6.2 is not a subunit of ‘mitoKATP’ (see above), these findings provide further challenge to the primacy of ‘mitoKATP’ in preconditioning, and argue that Kir6.2, and thus the sarcolemmal KATP channel, is a critical player in cardiac preconditioning.
Catecholamine challenge and exercise in KATP null mice
Most studies of KATP channel regulation in vivo have focused on severe metabolic events (e.g. global ischemia or complete metabolic inhibition), but none have clearly identified the normal physiologic events, or minimal metabolic stress, that cause channel opening. During exercise or catecholamine (eg. isoproterenol)-stimulation, increases in heart rate and contractility support the metabolic demands of skeletal muscle. Kir6.2−/− mice exhibit reduced tolerance to treadmill stress and impaired survival in response to isoproterenol-treatment (Table 1), suggesting that cardiac KATP normally activated during vigorous exercise, and that it is required to support the increased workload (461). The signal driving KATP activation under these conditions could be decreased ATP, and elevated ADP or agonist-dependent channel phosphorylation. The deleterious phenotype of the Kir6.2−/− animals seems to result from two major outcomes. First, isoproterenol-induced action potential shortening, is absent in the Kir6.2−/− heart, implicating KATP channels as key regulators of exercise- or stress-induced action potential shortening (461). Second, histological evidence of contraction bands, indicative of Ca2+ overload, in hearts from isoproterenol-treated Kir6.2−/− mice are consistent with enhanced Ca2+ entry, and Ca2+ channel blockade with verapamil improves survival of Kir6.2−/− mice during isoproterenol treatment (461). Evidence of focal myocardial necrosis, ventricular hypertrophy and increased mortality in Kir6.2−/− mice subjected to a four week swim training regimen (203) corroborates the idea that exercise-induced activation of KATP is a physiologically important cardioprotective mechanism.
SUR2−/− animals do exhibit reduced exercise (both treadmill and swim training) capacity (388), similar to Kir6.2−/− animals (203). Importantly, however, no exercise-induced decrement in cardiac function is reported in SUR2−/− mice (388). Isoproterenol challenge does not affect survival and actually ameliorates the arrhythmias that accompany spontaneous coronary vasospasm in SUR2−/− animals (388), with no ST-segment depression that is characteristic in the isoproterenol response of wild type mice. It is thus not entirely clear that it is the lack of cardiac KATP function that underlies the reduced exercise capacity in these animal models. Additional non-cardiac benefits of exercise training which might contribute to increased exercise tolerance include a decrease in body weight and in fasting blood glucose. Interestingly these features are observed in both wild-type mice and SUR2−/− mice, but are absent in Kir6.2−/− animals (203) (Table 1). Since the latter additionally lack KATP in the pancreas, altered pancreatic function may be the primary factor in this case, and might ultimately underlie the exercise-induced cardiac dysfunction in Kir6.2−/− animals.
C. KATP IN SKELETAL MUSCLE
KATP channels are also among the most densely expressed K+ channels in the skeletal muscle sarcolemma (382, 383). Our understanding of the physiological role of the skeletal muscle channel has lagged behind that of its cardiac counterpart and some counterintuitive observations using pharmacological agents to modulate skeletal KATP activity were initially hard to reconcile. Nevertheless, recent studies of genetically altered mice reveal the important myoprotective role of sarcolemmal KATP in skeletal muscle physiology.
Molecular structure and regulation of skeletal muscle KATP
Higher levels of SUR2 expression than SUR1 in skeletal muscle (167, 168), together with observations that cromakalim and pinacidil (SUR2-selective openers) (4, 331), typically stimulate skeletal muscle KATP channels better than diazoxide (SUR1- or SUR2B-selective opener) (28, 35, 424) have led to the general assumption that skeletal muscle KATP channels are formed of Kir6.2 plus SUR2A subunits. The conclusion is further supported by loss of KATP activity in skeletal muscles from Kir6.2−/− (274) and SUR2−/− (55) animals. However, more recent studies reveal that, as in the heart, skeletal muscle KATP channel structure is more heterogeneous. Using a combination of electrophysiological and mRNA expression analyses, a recent study provided evidence that KATP channel density and channel composition vary in different types of skeletal muscle (407): KATP channel density is higher in fast-twitch fibers than in slow type and this is correlated with an increased expression of the Kir6.2 subunit. In addition, SUR1 expression is significantly higher in fast-twitch muscles, and these KATP channels exhibit a characteristic diazoxide-sensitivity, indicative of a functional role for SUR1. Thus, as in cardiac muscle (112), it appears that while Kir6.2 is the predominant Kir6x isoform, both SUR1 and SUR2A are likely to play a role in the generation of functional channels, depending on the fiber type.
As in cardiac muscle, skeletal muscle KATP channels remain predominantly closed at rest and do not contribute to electrical activity unless the muscle is stressed (165). Consistent with this idea, glibenclamide treatment has no effect on muscle excitability or contractility under normal resting conditions (265). Channel regulation by intracellular ATP and MgADP is similar to that in cardiac muscle (3, 382, 383, 420), and metabolic inhibition similarly leads to activation of KATP channels in the intact myocyte (5). Adenosine, which is released from metabolically compromised muscle, also stimulates skeletal muscle KATP as it does in cardiac KATP (27). One notable distinction between cardiac and skeletal muscle KATP channels is their regulation by intracellular pH. Cardiac channels are relatively insensitive to decreases in pH (75, 225, 237), while intracellular acidification is a potent activator of skeletal muscle KATP (87, 88, 384).
KATP in physiology and pathophysiology of skeletal muscle: Insight from genetically modified mice
Fatigue and exercise in KATP null mice
Muscle fatigue is the decline in force production during prolonged and repetitive stimulation and many biochemical mechanisms have been proposed to contribute to the fatigue process (6). One possible mechanism is that activation of KATP channels, in response to reduction of ATP/ADP ratios, might underlie a decrease in action potential duration and hence twitch force. Consistent with this idea, application of cromakalim or pinacidil opens KATP channels in skeletal muscle and accelerates the force failure in anoxic or fatiguing conditions (128, 129, 265, 430). However, glibenclamide, which will block KATP channels activity does not slow the decline of force during fatigue, instead, glibenclamide has no effect or may even accelerate the decline in tetanic force during fatigue, similar to channel openers (68, 244, 265, 415). Thus, paradoxically, both openers and blockers of KATP can accelerate skeletal muscle fatigue.
Myographic examination of muscles from Kir6.2−/− and SUR2−/− animals has helped to resolve this paradox. These studies reveal that the initial decline in force during fatigue is not typically associated with KATP activation directly (hence glibenclamide does not slow fatigue development in wild type skeletal muscle), but the activation of KATP channels after fatigue has developed still helps to preserve a polarized membrane potential, protecting against voltage-dependent Ca entry and rise of (non-stimulated) tension that is observed in Kir6.2−/− muscles (129) or glibenclamide-treated wild type muscles (265), that are exposed to fatiguing stimuli (57, 58, 128, 130). This model accounts for the observation that the rate and extent of post-fatigue recovery is decreased in both Kir6.2−/− (57, 128, 129) and glibenclamide treated muscle (130). Finally, the observation that there is extensive fiber damage in Kir6.2−/− (203, 400) subjected to treadmill or swim training protocols reinforces the conclusion that KATP channel activation is a physiologically relevant myoprotective mechanism in vivo, even though KATP activation does not itself underlie the gradual loss of contractile activity in fatigue.
It remains to be seen whether these same phenotypes are found in SUR2−/− mice, which also lack sarcolemmal KATP but initial studies suggest that this will be the case: extensive fiber damage, as in Kir6.2−/− mice, is observed in trained SUR2−/− mice (388). It is not clear whether mitoKATP channels have a role in myoprotection. As in the heart, skeletal muscle can be preconditioned by ischemia or adenosine and again this process has been linked to mitoKATP (286, 287, 314, 315), but detailed studies of skeletal muscle preconditioning have yet to be carried out on KATP null mice, and these will be necessary to confirm a role for ‘mitoKATP’.
Glucose uptake in KATP null mice
A potentially interesting, but poorly understood, role of skeletal muscle KATP is regulation of glucose uptake. It has been recognized for some time that treatment with sulfonylureas improves glucose homeostasis, independent of its effect on insulin secretion, by improving peripheral insulin sensitivity (86, 346, 386). Skeletal muscle is a major site for insulin-stimulated glucose uptake and sulfonylureas can enhance insulin-stimulated and basal glucose uptake into isolated skeletal muscle or L6 myotubes (330, 409, 423). The conclusion that blockade of skeletal muscle KATP channels increases basal or insulin-dependent glucose uptake is supported by studies of KATP-deficient Kir6.2−/− and SUR2−/− animals (Table 1) (55, 274). In these mice there is enhanced glucose uptake, consistent with an inhibitory effect of skeletal muscle KATP activity on glucose uptake. It remains unclear precisely how KATP regulates glucose uptake, but appears to be independent of the classical IRS-1/PI3-K signaling pathway (277).
A negative relationship between KATP channel activity and glucose uptake potentially provides a negative feedback from electrical activity to the metabolic state of the cell and its substrate usage. It is intriguing to speculate that with increased KATP channel activity (e.g. as will occur with KATP mutations associated with neonatal diabetes (298)), not only will insulin secretion from β–cells be reduced, but glucose uptake into skeletal muscle may also be limited, thereby exacerbating the hyperglycemic phenotype. Similarly, we may also speculate that in obesity an accumulation of fatty acyl coA intermediates, by activating KATP (249), will exacerbate insulin resistance (422).
D. KATP CHANNELS IN SMOOTH MUSCLE
Multiple K+-channel types control electrical activity of the smooth muscle cell, including members of i) the voltage-dependent K+-channel family (Kv); ii) large-conductance Ca2+-activated K+-channels (BKCa); iii) two-pore domain K+-channel (K2P); and iv) inward rectifier K+-channels (Kir), including the ATP-sensitive K+-channels (KATP) (182, 213, 295, 425). The membrane potential of the smooth muscle myocyte (approximately-50 mV at rest in arteries and arterioles) is determined primarily by the K+-conductance which, in turn, controls diameter of the blood vessels and, thereby, the vascular tone. According to the paradigm, inhibition of K+-channel activity will cause depolarization of the membrane potential, activation of L-type voltage-sensitive Ca2+-channels, Ca2+-entry and vasoconstriction (295), although, unlike cardiac and skeletal muscle, most smooth muscle cells do not generate action potentials (157, 158). Conversely, activation of K+-channels, because of the K+ electrochemical gradient, will lead to K+-efflux, membrane hyperpolarization, decrease in voltage-dependent Ca2+-entry and vasodilation (295). The relationship between membrane potential and Ca2+-influx is especially steep in smooth muscle, with membrane depolarization or hyperpolarization of only a few millivolts causing several fold increases or decreases in [Ca2+]i respectively (294, 296). Thus, modulation of membrane potential by both metabolic and humoral vasoactivators that act on K+-channels can have profound effects on smooth muscle tone. The primary focus of this section is on the role of KATP in maintaining and regulating vessel tone in the prototypical smooth muscle, the vascular smooth muscle (VSM), and the possible cardiovascular consequences of altered VSM KATP.
Vascular smooth muscle KATP structure
Deciphering the molecular composition of the KATP subtypes in VSM has proven challenging. This is due to a number of factors, including the low density of KATP expression in the vasculature (~300 KATP channels/cell in VSM versus ~50,000 channels/cell in cardiac myocytes) (84, 300); the experimental difficulties in isolating and analyzing the electrophysiological properties of vascular smooth muscle cells (295, 312), and, most notably, the complex and variable biophysical and pharmacological properties of native VSM KATP, which likely reflect differential expression of multiple KATP subtypes in vascular beds (e.g. cororany arteries coexpress KATP subtypes with varying conductances) (32, 41, 78, 195, 201, 281, 313, 385). Early studies on native VSM KATP from various sources demonstrated considerable variations in single channel conductances, even after accounting for different experimental conditions (65, 120, 280, 281, 313, 421, 456). Low-conductance channels (unitary conductances from 20–50 pS) represent the predominant KATP channel subtype; whereas medium- and high conductance KATP channels exhibit a more limited distribution in the vasculature (50–70 pS and >200 pS, respectively) (64). Adding to the molecular complexity, KATP channels from coronary arteries are reported to exhibit multiple sub-conductance states (313). Unlike classic KATP channels of the heart (1, 167) and pancreas (16, 168), the predominant VSM KATP subtype is inactive in isolated membrane patches, and requires nucleoside diphosphates (ADP, UDP, GDP) in the presence of Mg 2+ to open, leading to their functional designation as ‘nucleoside-dependent’ K+-channels, or KNDP channels (32, 195, 456)
Heterologously expressed Kir6.1/SUR2B channels recapitulate many of the salient biophysical properties of the native VSM KATP/KNDP including: i) a lack of spontaneous channel activity upon membrane patch excision; ii) an obligatory requirement for di-, and triphosphate nucleosides in the presence of Mg 2+ for activity; iii) a high sensitivity to the potassium channel openers (KCOs), pinacidil and nicorandil, and to the inhibitory sulfonylurea, glibenclamide; iv) a low unitary conductance of ~33 pS (145 mM external [K+]); and v) a complex regulation by ATP in which micromolar [MgATP]i activates the channel; whereas, millimolar [ATP]i inhibits channel activity (18, 108, 171, 178, 356, 442). Electrophysiological studies of Kir6.1−/− and SUR2−/− mice confirm an essential requirement for both Kir6.1 and SUR2 subunits in formation of VSM KATP: in aortic smooth muscle isolated from Kir6.1−/− and SUR2−/− mice pinacidil- and glibenclamide-sensitive KATP currents are absent, whereas normal KATP currents are observed in smooth muscle myocytes from both Kir6.2−/− mice (54, 276, 391).
Again, however, the conclusion that the Kir6.1/SUR2B heteromultimer exclusively forms the ATP-sensitive K+-channel in VSM, requires qualification. In addition to variations in the reported unitary conductances, a subpopulation of VSM KATP in portal vein exhibits spontaneous activity in excised membrane patches, and displays high sensitivity to inhibitory ATP (K1/2 ATP = ~20 μM), and a unitary conductance (49 pS in external K+ 60 mM) that is reminiscent of the cardiac KATP channel (Kir6.2/SUR2A) (65, 305, 408, 456). Thus it is reasonable to conclude based on the current dataset that while the Kir6.1/SUR2B channel represents the predominant VSM KATP, other subtypes are also likely to be expressed in specific vascular beds, separately or in combination with Kir6.1/SUR2B subunits (456), and contribute to overall contractility. As discussed below, the VSM KATP represents a clinically important target in the pharmacological control of vascular disease and diversity of subunit makeup provides scope for vascular bed-specific agents. Diazoxide, an activator of SUR2B-containing channels (267), has long been recognized as a potent hypotensive agent in treatment of both acute (7, 401, 417), and pregnancy-induced hypertension (257), and understanding the molecular diversity and differential expression of KATP subtypes will be important in further refining pharmacological approaches to target specific vascular beds and to treat hypertensive disorders (e.g. angina, hypertension, asthma)
Regulation of VSM KATP and implications for vascular tone
KATP channels are tonically active in many vascular beds, including the coronary (94, 96, 166, 351), and mesenteric circulation (121, 293), and hence play a role in establishing basal tone in these vessels. This conclusion is based on the potent property of glibenclamide to depolarize and constrict isolated arteries (96, 121, 293, 351) and to increase coronary vascular resistance in perfused vascular beds under resting conditions in vivo (94, 175). Inhibition of the VSM KATP channel will cause membrane depolarization, a sustained rise in intracellular [Ca2+] (through both Ca2+ influx and Ca2+ release from intracellular stores), a decrease in arteriole diameter and increase in vascular resistance (280, 281, 421). Conversely, activation of KATP channels leads to a lowering of intracellular Ca2+ levels, an increase in arteriole diameter and a decrease in vascular resistance (385, 435). Not surprisingly, the VSM KATP represents an important mediator in the vascular response of various pharmacological and endogenous factors (45).
Effects of endogenous vasodilators and vasoconstrictors on VSM KATP
Vasoactive factors modulate VSM KATP currents through activation of serine/threonine protein kinase pathways, including cAMP-dependent protein kinase A (PKA), Ca2+- and phospholipid-dependent protein kinase C (PKC) (45), and possibly the cGMP-dependent protein kinase G (PKG) (334). Adenosine, released from cardiac myocytes during hypoxia/ischemia, was the first vasoactive factor shown to directly activate VSM KATP and to cause vasodilation through adenosine (A2) receptor activation (34, 84, 85, 181, 210, 211, 272). Other endogenous vasodilators of note (Fig. 3) include the calcitonin-generated peptide (CGRP) released from sensory nerve endings (279, 293, 332, 429), β-adrenoreceptor agonists (208, 278, 291), vasoactive intestinal peptide (VIP) released from perivascular nerves, as well as nitric oxide (NO) (288) and the eicosanoid, prostacyclin (PGl2), both released from endothelial cells (183, 234)(Fig. 3). Vasodilators that bind to G-protein (Gs)-coupled receptors (e.g. adenosine (A2) receptor) activate intracellular adenylate cyclase (AC) leading to elevated cAMP levels, increased PKA activity, and direct phosphorylation and activation of the SUR2B-containing channel complex (336, 367). PKA-mediated phosphorylation of SUR2B occurs at three key residues (Thr633, Ser1387, and Ser1465) in the nucleotide-binding folds (NBF1, NBF2) and these modifications are postulated to enhance binding of stimulatory MgADP to the NBFs (336, 367). Biochemical studies on isolated basilar (brain) and coronary arterial smooth muscle utilizing membrane permanent cAMP analogues and PKA-specific modulators confirm a central role of the PKA-KATP axis in mediating the effect of endogenous vasodilators (208, 332, 429), although the physiologic action of vasoactive factors is complex and involves additional KATP-independent pathways. For example, vasorelaxation of basilar and mesenteric arterial smooth muscle by CGRP is only partially reversed by glibenclamide (~12–60% of the total current is glibenclamide-insensitive) (209, 259, 293, 332), and a separate study reports virtually no effect of glibenclamide on CGRP-induced vasodilation in coronary arteries (194). Nitric oxide (NO), which mediates its effect through the cGMP-PKG signaling pathway may cause activation of KATP in multicellular or intact systems (226, 288), although direct application of NO-donors to smooth muscle cells has no obvious effect (334), and direct evidence for phosphorylation of KATP by PKG is lacking.
Fig. 3. Signaling pathways involved in regulation and modulation of the smooth muscle KATP channel.
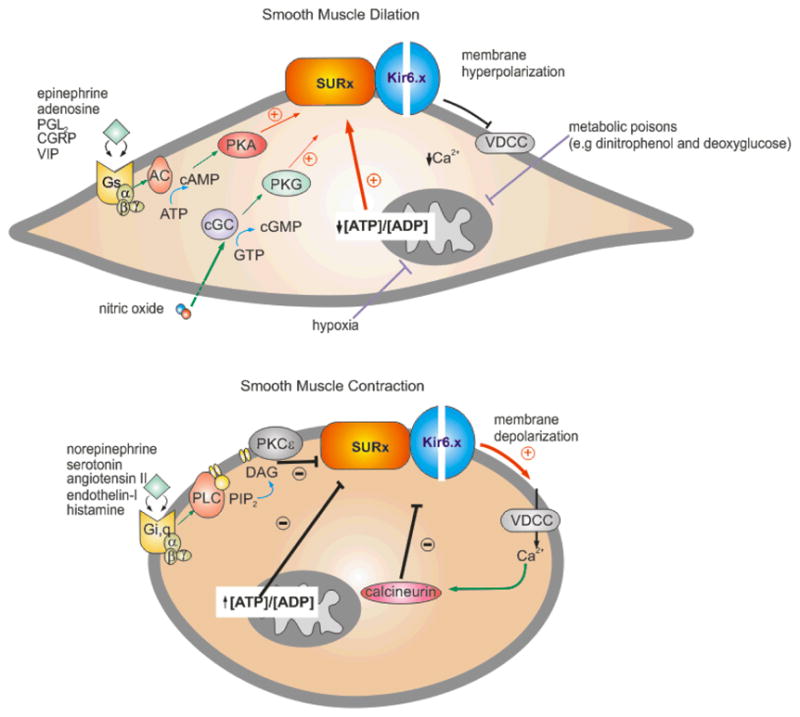
The KATP channel determines, in part, the membrane potential of the smooth muscle cell and, hence, the contractile state. Vasoactive factors that inhibit KATP activity cause membrane depolarization, activation of voltage-dependent Ca2+-channels (VDCC), a rise in intracellular [Ca2+] and smooth muscle contraction. Conversely, factors that activate KATP prevent the depolarization-dependent rise in [Ca2+] and promote smooth muscle dilation. (A) Endogenous vasodilators, including epinephrine, adenosine, prostacyclin (PGl2), calcitonin-generated peptide (CGRP), and the vasoactive intestinal peptide (VIP), stimulate KATP activity through the classic G-protein (Gs)/adenylate cyclase (AC)/protein kinase A (PKA) signaling pathway. Serine/threonine phosphorylation at several key residue in SUR2B (Thr633, Ser1387, and Ser1465) is thought to underlie channel activation. Conversely, nitric oxide activates KATP through the protein kinase G (PKG) signaling pathway. Hypoxia and metabolic poisons (dinitrophenol and deoxygluxose) indirectly activate KATP by suppressing oxidative phosphorylation, resulting in a decrease in the ATP/ADP levels. The K+-channel opener drugs (KCOs) (eg. pinacidil and diazoxide) promote their anti-hypertensive effects by opening vascular smooth KATP channels via interaction with the SUR2B subunit. (B) Vasoconstrictor agonists that target VSM KATP to promote contraction include the neurotransmitter serotonin (5-HT), angiotensin-II, endothelin-I, and the pro-inflammatory histamine. Binding of vasoconstrictors to the G-protein receptor subtypes, Gi or Gq, indirectly activates the PKC-ε isoform. In turn, PKCε-mediated phosphorylation of a serine-rich motif in the C-terminus of Kir6.1 results in a decrease in the frequency of channel openings. Ca2+-activated calcineurin (phosphatase type 2B) inhibits VSM KATP although the mechanism is unclear. Sulfonylurea compounds (e.g glibenclamide) induce vasoconstriction by binding to the SUR2B subunit causing KATP channel closure.
Conversely, endogenous vasoconstrictors act, in part, by inhibiting KATP currents via the PKC signaling pathway (Fig. 3), as evidenced by the ability of vasoconstrictors to reduce pre-activated KATP currents, but not in the presence of PKC inhibitors (64). Vasoconstrictor agonists that target VSM KATP to promote contraction include the neurotransmitter serotonin (5-HT) (42, 211), the renal-derived angiotensin-II (150, 227, 280), endothelin-I from endothelium (124, 281, 288, 316, 317), and pro-inflammatory histamine released from mast cells (42, 211), among others (Fig. 3). Binding of vasoconstrictors to the G-protein coupled receptor subtypes, Gi or Gq, activates the PKC-ε isoform (150, 192, 352). In turn, PKCε-mediated phosphorylation of a serine-rich motif in the C-terminus of Kir6.1 (S354, S379, S385, S391, and S397) results in both decreased frequency of channel openings (366) and a caveolin-dependent internalization of the Kir6.1/SUR2B channel complex from the plasma membrane (185, 353). The net effect of PKC-mediated decrease in KATP current (Fig. 3) is membrane depolarization and vasoconstriction (65, 335, 366, 402).
Notably, the inhibitory action of angiotensin-II on VSM KATP involves not only activation of PKC signaling, but also suppression of steady-state PKA activity (150), implying a potential crosstalk between the positive (PKA) and negative (PKC) branches of the protein kinase signaling pathways in net regulation of VSM KATP (Fig. 3). Finally, alternative signaling pathways can potentially regulate VSM KATP activity and thereby, modulate vascular tone. For example, the Ca2+-dependent phosphatase type 2B (PP-2B), also known as calcineurin, inhibits KATP in isolated aortic smooth muscle under conditions of elevated intracellular Ca2+ (434). The exact mechanism of PP-2B action is unclear, but may involve dephosphorylation of PKA-mediated phosphorylation sites on the channel (228, 457). In addition, KATP in the retinal microvasculature is sensitive to the redox state of the cell, with oxidants from polyamine catabolism increasing KATP activity, and causing local vasodilation (176).
It is of course important to note that the integrated activity of all K+-channels types (Kv, BKCa, Kir) ultimately establishes the membrane potential of smooth muscle cells and, hence, the contractile state. Since the activity of other K+-channels is modulated by endogenous vasoactive factors through PK-signaling pathways (e.g. Kv currents are inhibited by endothelin-I and angiotensin-II (PKC) (151, 213, 370), whereas BKCa channels are activated by NO (PKG) (345) and bradykinin (PKC) (118)), the net effect of PK modulation on VSM electrical activity will be complex.
Adenosine triphosphate; a passive or dynamic regulator of VSM KATP activity?
The hallmark of the archetypal KATP channel is inhibition by ATP and stimulation by MgADP through direct interactions with the Kir6.X and SUR subunits, respectively (298). With respect to the VSM KATP, intracellular nucleotides are clearly important in establishing the magnitude of KATP/KNDP currents, since induction of hypoxia activates KATP currents and induces a pronounced vasodilatation of coronary arteries (85, 338, 394). The hypoxic effect is presumably a consequence of a lowered ATP/ADP ratio in the myocyte, and not necessarily secondary to release of vasoactive adenosine from the damaged cardiac tissue (85). Moreover, inhibition of metabolism with metabolic poisons (e.g dinitrophenol, cyanide and deoxyglucose), which lowers the ATP/ADP ratio, also activates whole-cell KATP currents in various smooth muscle cell-types as well as in transfected cells expressing reconstituted Kir6.1/SUR2B (32, 70, 85, 108, 376, 454).
Nevertheless, it remains unclear what the adenosine nucleotide levels are in the VSM myocyte and whether cellular metabolism changes sufficiently to dynamically regulate VSM KATP activity under physiological condition. We may speculate that ambient ATP and ADP levels establish a tonic level of VSM KATP activity upon which vasoactive factors act (295). In this way, the KATP channel will determine the basal tone by providing a steady ‘brake’ on contractility, the strength of which is determined by the ambient ATP/ADP levels (Fig. 3) (108). Vasodilators and vasoconstrictors will then act to either increase or decrease the strength of the KATP ‘brake’, and hence the contractile state. The net contractility at any time will therefore be a summation of both vasodilatator and vasoconstrictor inputs. In metabolically inhibited states, such as hypoxia or ischemia, a fall in the intracellular ATP/ADP ratio will cause a resetting of the KATP ‘brake’ to favor vasodilatation (this pathology would apply to renal (256), cerebral (46, 338), and pulmonary arteries (432) where KATP may not contribute to the basal tone but rather, the channel may serve a protective role by opening to promote vasodilation during severe hypoxia (59, 394, 432)). Thus, while the ambient [ATP]/[ADP] ratio may establish a background level of KATP activity, it may be the vasoactive cues that dynamically regulate the magnitude of VSM KATP and, hence, the contractile state.
KATP in physiology and pathophysiology of vascular smooth muscle: Insight from genetically modified mice
Hypertension in KATP null mice
Mouse models in which the Kir6.1 and SUR2 genes have been ‘knocked-out’ highlight the critical role of KATP in the cardiovascular system, particulary in the coronary circulation (54, 276). The cardiovascular phenotypes of Kir6.1−/− and SUR2−/− mice are similar (Table 1) and include baseline hypertension, coronary artery vasospasm and sudden cardiac death, with 50% of Kir6.1−/− and SUR2−/− mice reportedly dying by approximately 6 and 27 weeks of age, respectively. Electrocardiograms from both animals show marked ST segment elevation and atrioventricular (AV) block, and which may account for the sudden death. Importantly, SUR2−/− mice treated with nifedipine (which inhibits the voltage-sensitive Ca2+-currents downstream of KATP) exhibit a reduction in coronary artery vasospasm, implicating abnormally elevated [Ca2+]i due to loss of hyperpolarizing KATP current as causal in the hypercontractility (54). Collectively, these KATP-null mice recapitulate the clinical features of the human disorder of Prinzmetal (or variant) angina, and predict that loss-of-function mutations in the VSM KATP may be disease-causing in humans (see below).
Vascular response to sepsis in KATP null mice
During infection, the normal host immune response includes cytokine-induced systemic inflammation, or sepsis (62, 389). To meet the increased energetic demands during septic shock, the cardiovascular system responds through coronary artery vasodilatation to increase coronary circulation and oxygen delivery to the heart. The altered response of Kir6.1−/− mice to induced endotoxemia (204) implicates the VSM KATP in this adaptive vascular response. When immuno-stimulated with the endotoxin lipopolysaccharide (LPS)(Table 1), Kir6.1−/− mice show a marked and persistent ST elevation (indicative of myocardial ischemic injury) a decrease in cardiac performance, and increased mortality, indicating that loss of VSM KATP predisposes Kir6.1−/− mice to a significant survival disadvantage. Importantly, administration of the KATP opener pinacidil (236) improves survivability in endotoxin-treated wild-type mice presumably by increasing coronary circulation, but has no effect on the survival rate in Kir6.1−/− mice.
A recent, unbiased screen of randomly ethyl-N-nitrosourea (ENU)-mutagenized mice for increased susceptibility to immunostimulatory agents has independently confirmed this critical role of Kir6.1 in mediating the vascular response to metabolic stress (74). Mutagenized mice with a loss-of-function mutation in Kir6.1 were identified based on their decreased survivability when challenged with mouse cytomegalovirus (MCMV) infection (74). In this same study, specific suppression of the SUR ortholog in Drosophila melanogaster by RNA interference similarly causes hypersensitivity to viral infection (74). It is intriguing to speculate that the baseline coronary vasospasm and sudden cardiac death in the initial studies of Kir6.1−/− and SUR2−/− mice (54, 276) may also reflect an impaired adaptive response; in this case, to metabolic stress in the course of normal pathogen exposure.
Role of extra-smooth muscle KATP in the control of vascular tone
While the hypertensive phenotype of both Kir6.1−/− and SUR2−/− mice seemingly underscores the critical role of the VSM KATP in controlling vascular tone, particularly in the coronary arteries and arterioles, this conclusion requires qualification. In addition to smooth muscle, Kir6.1 transcripts are detected in heart, lung, brain, pancreas, and endothelium (451). Similarly, RNA transcripts for the SUR2 gene (ABCC9), which encodes the SUR2A and SUR2B splice variants, are found in multiple tissues, including cardiac and skeletal muscle (SUR2A) (56, 167), brain (SUR2A) and endothelium (SUR2B), (178). Thus, the possibility exists that the cardiovascular phenotypes of Kir6.1−/− and SUR2−/− mice reflect loss of KATP in both smooth muscle and non-smooth muscle tissues (124). A role for non-smooth muscle KATP in cardiovascular homeostasis is supported by the finding that targeted suppression of endothelial KATP (Kir6.1/SUR2B) by transgenesis results in an increase in coronary perfusion pressure and a decrease in coronary blood flow (184, 270, 451), a similar phenotype to that observed in Kir6.1−/− mice (276). Interestingly, release of the vasoconstrictor endothelin-1 is increased by transgenic suppression of endothelial KATP, potentially implicating an elevated level of circulating endothelin-1 as causal in the vasoconstriction. These studies raise the possibility of KATP-dependent paracrine signaling between endothelial cells and overlying vascular smooth myocytes, with the endothelial KATP regulating the release of endothelin-1, which then acts to inhibit the VSM KATP. At present, the mechanism by which endothelial KATP inhibits endothelin-1 release is unknown, although immunolocalization of the endothelial KATP (Kir6.1) to intracellular organelles may indicate a role in the exocytotic machinery on endothelin-containing vesicles (260).
Further support for a potential role of non-VSM KATP in regulation of vascular tone comes from additional mouse studies. Transgenic restoration of VSM KATP currents by specifically expressing the SUR2B isoform in VSM of SUR2−/− mice, does not resolve the coronary artery vasospasm, atrioventricular (AV) heart block, or sudden cardiac death exhibited by SUR2−/− animals (199). Although this result suggests the importance of a smooth muscle-extrinsic process, the conclusion must be tempered by inability to confirm the extent of the restored KATP activity, particularly in the microcirculation.
Taken together, the transgenic mouse studies implicate VSM KATP (Kir6.1/SUR2) as central in control of vascular tone, although KATP in other cells types, such as the brain and endothelium, may also contribute. Generation of additional mouse models of altered KATP in specific tissues will further help to define the interaction of non-vascular with vascular KATP in regulating vascular tone.
Cardiovascular disease and VSM KATP: Where are the KATP mutations?
Mutations in the pancreatic β-cell KATP channel (both the Kir6.2 and SUR1 subunits) underlie insulin secretory disorders in mice and humans (222). Heterozygous, activating mutations suppress glucose-induced insulin secretion from theβ-cell and give rise to neonatal diabetes, whereas inactivating mutations result in unregulated secretion and underlie the corollary disorder of congenital hyperinsulinism (CHI) (14, 135). This realization has led to a significant improvement in therapy options to treat insulin secretory disorders secondary to β-cell KATP mutations (320, 323). Given the high sequence and structural homology between Kir6.1 and Kir6.2 (75% amino acid identity), and assuming similar mutation rates at the respective gene loci (Kir6.2 on 11p15.1 (168) and Kir6.1 on 12p12 (104)), it is reasonable to predict that homologous mutations in Kir6.1 will occur naturally and cause pathology in humans. Specifically, based on the coronary artery hypertension and coronary vasospasm described for Kir6.1−/− and SUR2−/− mice (54, 276), loss-of function VSM KATP is predicted to underlie a Prinzmetal angina or similar phenotype in humans. Two recent genetic screens of Italian and Japanese cohorts diagnosed with impaired coronary vasoreactivity and coronary spastic angina (CSA), respectively, have failed to uncover associated Kir6.1 mutations (101, 404). However, the impact of the studies was limited due to the small sample size in each case (n=18–19 patients) and failure to screen SUR2 (ABCC8) for associated variants. The Kir6.1 and SUR2 genes are located on human chromosome 12p (104), and chromosome 12p recombination has been shown to underlie autosomal-dominant hypertension (25) and postural hypotension is linked to chromosome 12 (146). Again however, our recent analysis of Kir6.1 and SUR2 genes in a cohort of such patients failed to reveal any associate variants (99).
Nevertheless, there is increasing biochemical and genetic evidence linking the Kir6.1 and SUR2B isoforms with cardiovascular abnormalities, including i) association of a Kir6.1 variant (S422L) with ventricular fibrillation with prominent early depolarization (137), ii) a growth hormone-induced hypotension is associated with upregulation of Kir6.1 and SUR2B transcript levels in rat aorta (403), iii) both Kir6.1 and SUR2B protein expression is reduced in aortas from obese hypertensive (106) and spontaneously hypertensive rats (41), which may contribute to the hypercontractility. Comprehensive assessment of VSM KATP as a risk or causal factor in cardiovascular disease will require both larger control-based studies with increasing power, as well as additional animal models of KATP-induced vascular disease in order to identify potentially affected cohorts.
KATP in visceral smooth muscle
KATP channels are also expressed in a variety of visceral (non-vascular) smooth muscle tissues, including gallbladder (458), stomach (180), esophagus (149), bladder (115), intestine (215), and uterus (254, 354). Although less well studied, the biophysical properties and physiology of visceral KATP generally parallel those of VSM KATP. As in the vasculature there is significant heterogeneity of KATP expression, with the predominant non-vascular KATP subtyperecapitulating the salient properties of the KNDP channel, including a low unitary conductance (~40 pS), a requirement for NDPs for activation, and similar sensitivity to KCOs (pinacidil and diazoxide) (192, 215, 377, 397, 398, 453). In addition to Kir6.1 and SUR2B, urethral myocytes express the Kir6.2 and SUR1 isoforms (453), and there is evidence for heteromeric assembly of Kir6.1 with the Kir6.2 in a (Kir6.1)3-(Kir6.2)1 arrangement with channels exhibiting intermediate channel behavior (399). Colonic smooth muscle actually expresses Kir6.2 and SUR2B mRNA with no evidence for Kir6.1 transcript expression. A role for KATP in regulating basal tone in visceral smooth muscle is evidenced by the ability of glibenclamide to increase tone and cause depolarization in multiple tissues including trachea (289), airway (200), and urethra (396). Surprisingly, perhaps, there are no reports of non-vascular smooth muscle dysfunction (e.g. altered myometrial contractility during parturition, or nutrition deficits due to impaired gastrointestinal function) in either Kir6.1−/− or SUR2−/− mice (54, 276). Whether the lack of a phenotype reflects compensatory K+-channel expression in non-vascular smooth muscle, or more subtle physiological consequences of KATP ablation, remains unclear.
Acknowledgments
In reviewing this broad topic of KATP in muscle, we have tried to comprehensively acknowledge recent advances, and to draw attention to major earlier studies, and to point to relevant earlier reviews. We would like to acknowledge the support of the National Institutes of Health (grants HL45742 and HL95010 to CGN), and American Heart Association (grant-in-aid to JCK), which have supported our own efforts in this field.
Prior to final acceptance of this manuscript, our co-author, colleague and friend, Joseph C. “Bo” Koster passed away, suddenly, unexpectedly and prematurely. In this final collaborative review effort, we wish to acknowledge his contribution to our lives, both professional and personal. Through his kindness, humor, and wisdom, Bo enriched our lives every day and he will be sorely missed.
Contributor Information
Thomas P. Flagg, Email: Thomas.Flagg@usuhs.mil.
Decha Enkvetchakul, Email: denkvetc@slu.edu.
F. REFERENCES
- 1.Aguilar-Bryan L, Nichols CG, Rajan AS, Parker C, Bryan J. Co-expression of sulfonylurea receptors and KATP channels in hamster insulinoma tumor (HIT) cells. Evidence for direct association of the receptor with the channel. J Biol Chem. 1992;267:14934–14940. [PubMed] [Google Scholar]
- 2.Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JPt, Boyd AE, 3rd, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 3.Allard B, Lazdunski M. Nucleotide diphosphates activate the ATP-sensitive potassium channel in mouse skeletal muscle. Pflugers Arch. 1992;422:185–192. doi: 10.1007/BF00370419. [DOI] [PubMed] [Google Scholar]
- 4.Allard B, Lazdunski M. Pharmacological properties of ATP-sensitive K+ channels in mammalian skeletal muscle cells. Eur J Pharmacol. 1993;236:419–426. doi: 10.1016/0014-2999(93)90480-6. [DOI] [PubMed] [Google Scholar]
- 5.Allard B, Lazdunski M, Rougier O. Activation of ATP-dependent K+ channels by metabolic poisoning in adult mouse skeletal muscle: role of intracellular Mg(2+) and pH. J Physiol. 1995;485 (Pt 2):283–296. doi: 10.1113/jphysiol.1995.sp020730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 7.Andersson KE. Clinical pharmacology of potassium channel openers. Pharmacol Toxicol. 1992;70:244–254. doi: 10.1111/j.1600-0773.1992.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 8.Antcliff JF, Haider S, Proks P, Sansom MS, Ashcroft FM. Functional analysis of a structural model of the ATP-binding site of the KATP channel Kir6.2 subunit. EMBO Journal. 2005;24:229–239. doi: 10.1038/sj.emboj.7600487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aon MA, Cortassa S, Marban E, O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 10.Ardehali H, Chen Z, Ko Y, Mejia-Alvarez R, Marban E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proc Natl Acad Sci U S A. 2004;101:11880–11885. doi: 10.1073/pnas.0401703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ardehali H, O’Rourke B, Marban E. Cardioprotective role of the mitochondrial ATP-binding cassette protein 1. Circulation Research. 2005;97:740–742. doi: 10.1161/01.RES.0000186277.12336.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arrell DK, Zlatkovic J, Kane GC, Yamada S, Terzic A. ATP-sensitive K+ channel knockout induces cardiac proteome remodeling predictive of heart disease susceptibility. J Proteome Res. 2009;8:4823–4834. doi: 10.1021/pr900561g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- 14.Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. Diabetes. 2005;54:2503–2513. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashcroft FM, Gribble FM. New windows on the mechanism of action of K(ATP) channel openers. Trends Pharmacol Sci. 2000;21:439–445. doi: 10.1016/s0165-6147(00)01563-7. [DOI] [PubMed] [Google Scholar]
- 16.Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 17.Ashfield R, Gribble FM, Ashcroft SJ, Ashcroft FM. Identification of the high-affinity tolbutamide site on the SUR1 subunit of the K(ATP) channel. Diabetes. 1999;48:1341–1347. doi: 10.2337/diabetes.48.6.1341. [DOI] [PubMed] [Google Scholar]
- 18.Babenko AP, Bryan J. A conserved inhibitory and differential stimulatory action of nucleotides on K(IR)6.0/SUR complexes is essential for excitation-metabolism coupling by K(ATP) channels. J Biol Chem. 2001;276:49083–49092. doi: 10.1074/jbc.M108763200. [DOI] [PubMed] [Google Scholar]
- 19.Babenko AP, Bryan J. Sur domains that associate with and gate KATP pores define a novel gatekeeper. J Biol Chem. 2003;278:41577–41580. doi: 10.1074/jbc.C300363200. [DOI] [PubMed] [Google Scholar]
- 20.Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Reconstituted human cardiac KATP channels: functional identity with the native channels from the sarcolemma of human ventricular cells. Circ Res. 1998;83:1132–1143. doi: 10.1161/01.res.83.11.1132. [DOI] [PubMed] [Google Scholar]
- 21.Babenko AP, Gonzalez G, Bryan J. Pharmaco-topology of sulfonylurea receptors. Separate domains of the regulatory subunits of K(ATP) channel isoforms are required for selective interaction with K(+) channel openers. J Biol Chem. 2000;275:717–720. doi: 10.1074/jbc.275.2.717. [DOI] [PubMed] [Google Scholar]
- 22.Babenko AP, Gonzalez G, Bryan J. The tolbutamide site of SUR1 and a mechanism for its functional coupling to K(ATP) channel closure. FEBS Lett. 1999;459:367–376. doi: 10.1016/s0014-5793(99)01215-6. [DOI] [PubMed] [Google Scholar]
- 23.Baczko I, Giles WR, Light PE. Pharmacological activation of plasma-membrane KATP channels reduces reoxygenation-induced Ca(2+) overload in cardiac myocytes via modulation of the diastolic membrane potential. Br J Pharmacol. 2004;141:1059–1067. doi: 10.1038/sj.bjp.0705702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baczko I, Jones L, McGuigan CF, Manning Fox JE, Gandhi M, Giles WR, Clanachan AS, Light PE. Plasma membrane KATP channel-mediated cardioprotection involves posthypoxic reductions in calcium overload and contractile dysfunction: mechanistic insights into cardioplegia. FASEB J. 2005;19:980–982. doi: 10.1096/fj.04-3008fje. [DOI] [PubMed] [Google Scholar]
- 25.Bahring S, Rauch A, Toka O, Schroeder C, Hesse C, Siedler H, Fesus G, Haefeli WE, Busjahn A, Aydin A, Neuenfeld Y, Muhl A, Toka HR, Gollasch M, Jordan J, Luft FC. Autosomal-dominant hypertension with type E brachydactyly is caused by rearrangement on the short arm of chromosome 12. Hypertension. 2004;43:471–476. doi: 10.1161/01.HYP.0000111808.08715.ec. [DOI] [PubMed] [Google Scholar]
- 26.Baron A, van BL, Monnier D, Roatti A, Baertschi AJ. A novel K(ATP) current in cultured neonatal rat atrial appendage cardiomyocytes. Circ Res. 1999;85:707–715. doi: 10.1161/01.res.85.8.707. [DOI] [PubMed] [Google Scholar]
- 27.Barrett-Jolley R, Davies NW. Kinetic analysis of the inhibitory effect of glibenclamide on KATP channels of mammalian skeletal muscle. J Memb Biol. 1997;155:257–262. doi: 10.1007/s002329900178. [DOI] [PubMed] [Google Scholar]
- 28.Barrett-Jolley R, McPherson GA. Characterization of K(ATP) channels in intact mammalian skeletal muscle fibres. Br J Pharmacol. 1998;123:1103–1110. doi: 10.1038/sj.bjp.0701727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- 30.Baumann P, Poitry S, Roatti A, Baertschi AJ. Plasmalemmal KATP channels shape triggered calcium transients in metabolically impaired rat atrial myocytes. Am J Physiol. 2002;283:H2296–2305. doi: 10.1152/ajpheart.00393.2002. [DOI] [PubMed] [Google Scholar]
- 31.Bear CE, Duguay F, Naismith AL, Kartner N, Hanrahan JW, Riordan JR. Cl- channel activity in Xenopus oocytes expressing the cystic fibrosis gene. J Biol Chem. 1991;266:19142–19145. [PubMed] [Google Scholar]
- 32.Beech DJ, Zhang H, Nakao K, Bolton TB. K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br J Pharmacol. 1993;110:573–582. doi: 10.1111/j.1476-5381.1993.tb13849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beguin P, Nagashima K, Nishimura M, Gonoi T, Seino S. PKA-mediated phosphorylation of the human K(ATP) channel: separate roles of Kir6.2 and SUR1 subunit phosphorylation. EMBO J. 1999;18:4722–4732. doi: 10.1093/emboj/18.17.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belloni FL, Hintze TH. Glibenclamide attenuates adenosine-induced bradycardia and coronary vasodilatation. Am J Physiol. 1991;261 doi: 10.1152/ajpheart.1991.261.3.H720. [DOI] [PubMed] [Google Scholar]
- 35.Benton DC, Haylett DG. Effects of cromakalim on the membrane potassium permeability of frog skeletal muscle in vitro. Br J Pharmacol. 1992;107:152–155. doi: 10.1111/j.1476-5381.1992.tb14478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 37.Bienengraeber M, Alekseev AE, Abraham MR, Carrasco AJ, Moreau C, Vivaudou M, Dzeja PP, Terzic A. ATPase activity of the sulfonylurea receptor: a catalytic function for the KATP channel complex. Faseb J. 2000;14:1943–1952. doi: 10.1096/fj.00-0027com. [DOI] [PubMed] [Google Scholar]
- 38.Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O’Cochlain F, Gao F, Karger AB, Ballew JD, Hodgson DM, Zingman LV, Pang YP, Alekseev AE, Terzic A. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nature Genetics. 2004;36:382–387. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Billman GE. The cardiac sarcolemmal ATP-sensitive potassium channel as a novel target for anti-arrhythmic therapy. Pharmacology & Therapeutics. 2008;120:54–70. doi: 10.1016/j.pharmthera.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Billman GE, Englert HC, Scholkens BA. HMR 1883, a Novel Cardioselective Inhibitor of the ATP-Sensitive Potassium Channel. Part II: Effects on Susceptibility to Ventricular Fibrillation Induced by Myocardial Ischemia in Conscious Dogs. J Pharmacol Exp Ther. 1998;286:1465–1473. [PubMed] [Google Scholar]
- 41.Blanco-Rivero J, Gamallo C, Aras-Lopez R, Cobeno L, Cogolludo A, Perez-Vizcaino F, Ferrer M, Balfagon G. Decreased expression of aortic KIR6.1 and SUR2B in hypertension does not correlate with changes in the functional role of K(ATP) channels. Eur J Pharmacol. 2008;587:204–208. doi: 10.1016/j.ejphar.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 42.Bonev AD, Nelson MT. Vasoconstrictors inhibit ATP-sensitive K+ channels in arterial smooth muscle through protein kinase C. J Gen Physiol. 1996;108:315–323. doi: 10.1085/jgp.108.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Branstrom R, Corkey BE, Berggren PO, Larsson O. Evidence for a unique long chain acyl-CoA ester binding site on the ATP-regulated potassium channel in mouse pancreatic beta cells. J Biol Chem. 1997;272:17390–17394. doi: 10.1074/jbc.272.28.17390. [DOI] [PubMed] [Google Scholar]
- 44.Branstrom R, Leibiger IB, Leibiger B, Corkey BE, Berggren PO, Larsson O. Long chain coenzyme A esters activate the pore-forming subunit (Kir6. 2) of the ATP-regulated potassium channel. J Biol Chem. 1998;273:31395–31400. doi: 10.1074/jbc.273.47.31395. [DOI] [PubMed] [Google Scholar]
- 45.Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharm Physiol. 2002;29:312–316. doi: 10.1046/j.1440-1681.2002.03650.x. [DOI] [PubMed] [Google Scholar]
- 46.Brayden JE. Membrane hyperpolarization is a mechanism of endothelium-dependent cerebral vasodilation. Am J Physiol. 1990;259:H668–673. doi: 10.1152/ajpheart.1990.259.3.H668. [DOI] [PubMed] [Google Scholar]
- 47.Cameron JS, Kimura S, Jackson-Burns DA, Smith DB, Bassett AL. ATP-sensitive K+ channels are altered in hypertrophied ventricular myocytes. Am J Physiol. 1988;255:H1254–1258. doi: 10.1152/ajpheart.1988.255.5.H1254. [DOI] [PubMed] [Google Scholar]
- 48.Campbell JD, Proks P, Lippiat JD, Sansom MS, Ashcroft FM. Identification of a functionally important negatively charged residue within the second catalytic site of the SUR1 nucleotide-binding domains. Diabetes. 2004;53 (Suppl 3):123–127. doi: 10.2337/diabetes.53.suppl_3.s123. [DOI] [PubMed] [Google Scholar]
- 49.Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, Hodgson D, Bienengraeber M, Puceat M, Janssen E, Wieringa B, Terzic A. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci U S A. 2001;98:7623–7628. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan KW, Wheeler A, Csanady L. Sulfonylurea Receptors Type 1 and 2A Randomly Assemble to Form Heteromeric KATP Channels of Mixed Subunit Composition. J Gen Physiol. 2007;131:43–58. doi: 10.1085/jgp.200709894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng WW, Tong A, Flagg TP, Nichols CG. Random assembly of SUR subunits in K(ATP) channel complexes. Channels. 2008;2:34–38. doi: 10.4161/chan.2.1.6046. [DOI] [PubMed] [Google Scholar]
- 52.Chi L, Uprichard AC, Lucchesi BR. Profibrillatory actions of pinacidil in a conscious canine model of sudden coronary death. J Cardiovasc Pharm. 1990;15:452–464. doi: 10.1097/00005344-199003000-00016. [DOI] [PubMed] [Google Scholar]
- 53.Chutkow WA, Makielski JC, Nelson DJ, Burant CF, Fan Z. Alternative splicing of sur2 Exon 17 regulates nucleotide sensitivity of the ATP-sensitive potassium channel. J Biol Chem. 1999;274:13656–13665. doi: 10.1074/jbc.274.19.13656. [DOI] [PubMed] [Google Scholar]
- 54.Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 K(ATP) channels. [see comment] J Clin Inv. 2002;110:203–208. doi: 10.1172/JCI15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chutkow WA, Samuel V, Hansen PA, Pu J, Valdivia CR, Makielski JC, Burant CF. Disruption of Sur2-containing KATP channels enhances insulin-stimulated glucose uptake in skeletal muscle. Proc Natl Acad Sci U S A. 2001;98:11760–11764. doi: 10.1073/pnas.201390398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chutkow WA, Simon MC, Le Beau MM, Burant CF. Cloning, tissue expression, and chromosomal localization of SUR2, the putative drug-binding subunit of cardiac, skeletal muscle, and vascular KATP channels. Diabetes. 1996;45:1439–1445. doi: 10.2337/diab.45.10.1439. [DOI] [PubMed] [Google Scholar]
- 57.Cifelli C, Boudreault L, Gong B, Bercier JP, Renaud JM. Contractile dysfunctions in ATP-dependent K+ channel-deficient mouse muscle during fatigue involve excessive depolarization and Ca2+ influx through L-type Ca2+ channels. Exp Physiol. 2008;93:1126–1138. doi: 10.1113/expphysiol.2008.042572. [DOI] [PubMed] [Google Scholar]
- 58.Cifelli C, Bourassa F, Gariepy L, Banas K, Benkhalti M, Renaud JM. KATP channel deficiency in mouse flexor digitorum brevis causes fibre damage and impairs Ca2+ release and force development during fatigue in vitro. J Physiol. 2007;582:843–857. doi: 10.1113/jphysiol.2007.130955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clapp LH, Gurney AM. ATP-sensitive K+ channels regulate resting potential of pulmonary arterial smooth muscle cells. Am J Physiol. 1992;262:H916–920. doi: 10.1152/ajpheart.1992.262.3.H916. [DOI] [PubMed] [Google Scholar]
- 60.Clement JP, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, Bryan J. Association and stoichiometry of K(ATP) channel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- 61.Coghlan MJ, Carroll WA, Gopalakrishnan M. Recent developments in the biology and medicinal chemistry of potassium channel modulators: update from a decade of progress. J Med Chem. 2001;44:1627–1653. doi: 10.1021/jm000484+. [DOI] [PubMed] [Google Scholar]
- 62.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 63.Cohen MV, Yang XM, Downey JM. Acidosis, oxygen, and interference with mitochondrial permeability transition pore formation in the early minutes of reperfusion are critical to postconditioning’s success. Basic Res Cardiol. 2008;103:464–471. doi: 10.1007/s00395-008-0737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cole WC, Clement-Chomienne O. ATP-sensitive K+ channels of vascular smooth muscle cells. J Cardiovasc Electrophysiol. 2003;14:94–103. doi: 10.1046/j.1540-8167.2003.02376.x. [DOI] [PubMed] [Google Scholar]
- 65.Cole WC, Malcolm T, Walsh MP, Light PE. Inhibition by protein kinase C of the K(NDP) subtype of vascular smooth muscle ATP-sensitive potassium channel. Circ Res. 2000;87:112–117. doi: 10.1161/01.res.87.2.112. [DOI] [PubMed] [Google Scholar]
- 66.Cole WC, McPherson CD, Sontag D. ATP-regulated K+ channels protect the myocardium against ischemia/reperfusion damage. Circ Res. 1991;69:571–581. doi: 10.1161/01.res.69.3.571. [DOI] [PubMed] [Google Scholar]
- 67.Comelli M, Metelli G, Mavelli I. Downmodulation of mitochondrial F0F1 ATP synthase by diazoxide in cardiac myoblasts: a dual effect of the drug. Am J Physiol. 2007;292:H820–829. doi: 10.1152/ajpheart.00366.2006. [DOI] [PubMed] [Google Scholar]
- 68.Comtois A, Light P, Renaud J. Effect of tolbutamide on the rate of fatigue and recovery in frog sartorius muscle. J Pharm Exp Ther. 1995;274:1061–1066. [PubMed] [Google Scholar]
- 69.Contessi S, Metelli G, Mavelli I, Lippe G. Diazoxide affects the IF1 inhibitor protein binding to F1 sector of beef heart F0F1ATPsynthase. Biochem Pharmacol. 2004;67:1843–1851. doi: 10.1016/j.bcp.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 70.Conway MA, Nelson MT, Brayden JE. 2-Deoxyglucose-induced vasodilation and hyperpolarization in rat coronary artery are reversed by glibenclamide. Am J Physiol. 1994;266:H1322–1326. doi: 10.1152/ajpheart.1994.266.4.H1322. [DOI] [PubMed] [Google Scholar]
- 71.Crawford RM, Budas GR, Jovanovic S, Ranki HJ, Wilson TJ, Davies AM, Jovanovic A. M-LDH serves as a sarcolemmal K(ATP) channel subunit essential for cell protection against ischemia. EMBO J. 2002;21:3936–3948. doi: 10.1093/emboj/cdf388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crawford RM, Jovanovic S, Budas GR, Davies AM, Lad H, Wenger RH, Robertson KA, Roy DJ, Ranki HJ, Jovanovic A. Chronic Mild Hypoxia Protects Heart-derived H9c2 Cells against Acute Hypoxia/Reoxygenation by Regulating Expression of the SUR2A Subunit of the ATP-sensitive K+ Channel. J Biol Chem. 2003;278:31444–31455. doi: 10.1074/jbc.M303051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crawford RM, Ranki HJ, Botting CH, Budas GR, Jovanovic A. Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. FASEB J. 2002;16:102–104. doi: 10.1096/fj.01-0466fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Croker B, Crozat K, Berger M, Xia Y, Sovath S, Schaffer L, Eleftherianos I, Imler JL, Beutler B. ATP-sensitive potassium channels mediate survival during infection in mammals and insects. Nat Genet. 2007;39:1453–1460. doi: 10.1038/ng.2007.25. [DOI] [PubMed] [Google Scholar]
- 75.Cuevas J, Bassett AL, Cameron JS, Furukawa T, Myerburg RJ, Kimura S. Effect of H+ on ATP-regulated K+ channels in feline ventricular myocytes. Am J Physiol. 1991;261:H755–761. doi: 10.1152/ajpheart.1991.261.3.H755. [DOI] [PubMed] [Google Scholar]
- 76.Cui N, Li L, Wang X, Shi Y, Shi W, Jiang C. Elimination of allosteric modulation of myocardial KATP channels by ATP and protons in two Kir6.2 polymorphisms found in sudden cardiac death. Physiol Genomics. 2006;25:105–115. doi: 10.1152/physiolgenomics.00106.2005. [DOI] [PubMed] [Google Scholar]
- 77.Cui Y, Giblin JP, Clapp LH, Tinker A. A mechanism for ATP-sensitive potassium channel diversity: Functional coassembly of two pore-forming subunits. Proc Natl Acad Sci U S A. 2001;98:729–734. doi: 10.1073/pnas.011370498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cui Y, Tran S, Tinker A, Clapp LH. The molecular composition of K(ATP) channels in human pulmonary artery smooth muscle cells and their modulation by growth. Am J Respir Cell Mol Biol. 2002;26:135–143. doi: 10.1165/ajrcmb.26.1.4622. [DOI] [PubMed] [Google Scholar]
- 79.Cukras CA, Jeliazkova I, Nichols CG. The role of NH(2)-terminal positive charges in the activity of inward rectifier K(ATP) channels. J Gen Physiol. 2002;120:437–446. doi: 10.1085/jgp.20028621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cukras CA, Jeliazkova I, Nichols CG. Structural and functional determinants of conserved lipid interaction domains of inward rectifying kir6.2 channels. J Gen Physiol. 2002;119:581–591. doi: 10.1085/jgp.20028562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cuong DV, Kim N, Joo H, Youm JB, Chung J-Y, Lee Y, Park WS, Kim E, Park YS, Han J. Subunit composition of ATP-sensitive potassium channels in mitochondria of rat hearts. Mitochondrion. 2005;5:121–133. doi: 10.1016/j.mito.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 82.D’Hahan N, Jacquet H, Moreau C, Catty P, Vivaudou M. A transmembrane domain of the sulfonylurea receptor mediates activation of ATP-sensitive K(+) channels by K(+) channel openers. Mol Pharmacol. 1999;56:308–315. doi: 10.1124/mol.56.2.308. [DOI] [PubMed] [Google Scholar]
- 83.D’Hahan N, Moreau C, Prost AL, Jacquet H, Alekseev AE, Terzic A, Vivaudou M. Pharmacological plasticity of cardiac ATP-sensitive potassium channels toward diazoxide revealed by ADP. Proc Natl Acad Sci U S A. 1999;96:12162–12167. doi: 10.1073/pnas.96.21.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dart C, Standen NB. Adenosine-activated potassium current in smooth muscle cells isolated from the pig coronary artery. J Physiol. 1993;471:767–786. doi: 10.1113/jphysiol.1993.sp019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Daut J, Maier-Rudolph W, von Beckerath N, Mehrke G, Gunther K, Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990;247:1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- 86.Davidson MB, Molnar IG, Furman A, Yamaguchi D. Glyburide-stimulated glucose transport in cultured muscle cells via protein kinase C-mediated pathway requiring new protein synthesis. Diabetes. 1991;40:1531–1538. doi: 10.2337/diab.40.11.1531. [DOI] [PubMed] [Google Scholar]
- 87.Davies NW. Modulation of ATP-sensitive K+ channels in skeletal muscle by intracellular protons. Nature. 1990;343:375–377. doi: 10.1038/343375a0. [DOI] [PubMed] [Google Scholar]
- 88.Davies NW, Standen NB, Stanfield PR. The effect of intracellular pH on ATP-dependent potassium channels of frog skeletal muscle. J Physiol. 1992;445:549–568. doi: 10.1113/jphysiol.1992.sp018939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dhar-Chowdhury P, Harrell MD, Han SY, Jankowska D, Parachuru L, Morrissey A, Srivastava S, Liu W, Malester B, Yoshida H, Coetzee WA. The glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase, triose-phosphate isomerase, and pyruvate kinase are components of the K(ATP) channel macromolecular complex and regulate its function. J Biol Chem. 2005;280:38464–38470. doi: 10.1074/jbc.M508744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dickinson KE, Bryson CC, Cohen RB, Rogers L, Green DW, Atwal KS. Nucleotide regulation and characteristics of potassium channel opener binding to skeletal muscle membranes. Mol Pharmacol. 1997;52:473–481. doi: 10.1124/mol.52.3.473. [DOI] [PubMed] [Google Scholar]
- 91.Diwan A, Dorn GW., 2nd Decompensation of cardiac hypertrophy: cellular mechanisms and novel therapeutic targets. Physiology (Bethesda) 2007;22:56–64. doi: 10.1152/physiol.00033.2006. [DOI] [PubMed] [Google Scholar]
- 92.Drain P, Li L, Wang J. KATP channel inhibition by ATP requires distinct functional domains of the cytoplasmic C terminus of the pore-forming subunit. Proc Natl Acad Sci U S A. 1998;95:13953–13958. doi: 10.1073/pnas.95.23.13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Du Q, Jovanovic S, Clelland A, Sukhodub A, Budas G, Phelan K, Murray-Tait V, Malone L, Jovanovic A. Overexpression of SUR2A generates a cardiac phenotype resistant to ischemia. FASEB J. 2006;20:1131–1141. doi: 10.1096/fj.05-5483com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duncker DJ, Van Zon NS, Altman JD, Pavek TJ, Bache RJ. Role of K+ATP channels in coronary vasodilation during exercise. Circulation. 1993;88:1245–1253. doi: 10.1161/01.cir.88.3.1245. [DOI] [PubMed] [Google Scholar]
- 95.Dunne MJ, Petersen OH. Intracellular ADP activates K+ channels that are inhibited by ATP in an insulin-secreting cell line. FEBS Letters. 1986;208:59–62. doi: 10.1016/0014-5793(86)81532-0. [DOI] [PubMed] [Google Scholar]
- 96.Eckman DM, Frankovich JD, Keef KD. Comparison of the actions of acetylcholine and BRL 38227 in the guinea-pig coronary artery. Br J Pharmacol. 1992;106:9–16. doi: 10.1111/j.1476-5381.1992.tb14285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elliott AC, Smith GL, Allen DG. Simultaneous measurements of action potential duration and intracellular ATP in isolated ferret hearts exposed to cyanide. Circ Res. 1989;64:583–591. doi: 10.1161/01.res.64.3.583. [DOI] [PubMed] [Google Scholar]
- 98.Elliott AC, Smith GL, Eisner DA, Allen DG. Metabolic changes during ischaemia and their role in contractile failure in isolated ferret hearts. J Physiol. 1992;454:467–490. doi: 10.1113/jphysiol.1992.sp019274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ellis JA, Lamantia A, Chavez R, Scurrah KJ, Nichols CG, Harrap SB. Genes Controlling Postural Changes in Blood Pressure: Comprehensive association analysis of ATP-Sensitive Potassium Channel Genes KCNJ8 and ABCC9. Physiol Genomics. 2009 doi: 10.1152/physiolgenomics.00173.2009. [DOI] [PubMed] [Google Scholar]
- 100.Elrod JW, Harrell M, Flagg TP, Gundewar S, Magnuson MA, Nichols CG, Coetzee WA, Lefer DJ. Role of sulfonylurea receptor type 1 subunits of ATP-sensitive potassium channels in myocardial ischemia/reperfusion injury. Circulation. 2008;117:1405–1413. doi: 10.1161/CIRCULATIONAHA.107.745539. [DOI] [PubMed] [Google Scholar]
- 101.Emanuele E, Falcone C, Carabela M, Minoretti P, D’Angelo A, Montagna L, Geroldi D. Absence of Kir6.1/KCNJ8 mutations in Italian patients with abnormal coronary vasomotion. Int J Mol Med. 2003;12:509–512. [PubMed] [Google Scholar]
- 102.Enkvetchakul D, Jeliazkova I, Bhattacharyya J, Nichols CG. Control of inward rectifier K channel activity by lipid tethering of cytoplasmic domains. J GenPhysiol. 2007;130:329–334. doi: 10.1085/jgp.200709764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Enkvetchakul D, Nichols CG. Gating mechanism of KATP channels: function fits form. J Gen Physiol. 2003;122:471–480. doi: 10.1085/jgp.200308878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Erginel-Unaltuna N, Yang WP, Blanar MA. Genomic organization and expression of KCNJ8/Kir6.1, a gene encoding a subunit of an ATP-sensitive potassium channel. Gene. 1998;211:71–78. doi: 10.1016/s0378-1119(98)00086-9. [DOI] [PubMed] [Google Scholar]
- 105.Faivre JF, Findlay I. Effects of tolbutamide, glibenclamide and diazoxide upon action potentials recorded from rat ventricular muscle. Biochim Biophys Acta. 1989;984:1–5. doi: 10.1016/0005-2736(89)90334-9. [DOI] [PubMed] [Google Scholar]
- 106.Fan LH, Tian HY, Wang J, Huo JH, Hu Z, Ma AQ, Cao YX. Downregulation of Kir6.1/SUR2B channels in the obese rat aorta. Nutrition. 2008 doi: 10.1016/j.nut.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 107.Fan Z, Makielski JC. Anionic phospholipids activate ATP-sensitive potassium channels. J Biol Chem. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- 108.Farzaneh T, Tinker A. Differences in the mechanism of metabolic regulation of ATP-sensitive K+ channels containing Kir6.1 and Kir6.2 subunits. Cardiovasc Res. 2008;79:621–631. doi: 10.1093/cvr/cvn138. [DOI] [PubMed] [Google Scholar]
- 109.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Findlay I. ATP4- and ATP.Mg inhibit the ATP-sensitive K+ channel of rat ventricular myocytes. Pflugers Arch. 1988;412:37–41. doi: 10.1007/BF00583729. [DOI] [PubMed] [Google Scholar]
- 111.Flagg TP, Charpentier F, Manning-Fox J, Remedi MS, Enkvetchakul D, Lopatin A, Koster J, Nichols C. Remodeling of excitation-contraction coupling in transgenic mice expressing ATP-insensitive sarcolemmal KATP channels. Am J Physiol. 2004;286:H1361–1369. doi: 10.1152/ajpheart.00676.2003. [DOI] [PubMed] [Google Scholar]
- 112.Flagg TP, Kurata HT, Masia R, Caputa G, Magnuson MA, Lefer DJ, Coetzee WA, Nichols CG. Differential structure of atrial and ventricular KATP: atrial KATP channels require SUR1. Circ Res. 2008;103:1458–1465. doi: 10.1161/CIRCRESAHA.108.178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Flagg TP, Patton B, Masia R, Mansfield C, Lopatin AN, Yamada KA, Nichols CG. Arrhythmia susceptibility and premature death in transgenic mice overexpressing both SUR1 and Kir6.2[DeltaN30, K185Q] in the heart. Am J Physiol. 2007;293:H836–845. doi: 10.1152/ajpheart.00011.2007. [DOI] [PubMed] [Google Scholar]
- 114.Flagg TP, Remedi MS, Masia R, McLerie M, Lopatin A, Nichols C. Transgenic overexpression of SUR1 in the heart exerts dominant negative effects on sarcolemmal K ATP. J Mol Cell Cardiol. 2005;39:647–656. doi: 10.1016/j.yjmcc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 115.Foster CD, Speakman MJ, Fujii K, Brading AF. The effects of cromakalim on the detrusor muscle of human and pig urinary bladder. Br J Urol. 1989;63:284–294. doi: 10.1111/j.1464-410x.1989.tb05191.x. [DOI] [PubMed] [Google Scholar]
- 116.Foster DB, Rucker JJ, Marban E. Is Kir6.1 a subunit of mitoK(ATP)? Biochem Biophys Res Commun. 2008;366:649–656. doi: 10.1016/j.bbrc.2007.11.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fox JEM, Magga J, Giles WR, Light PE. Acyl coenzyme A esters differentially activate cardiac and [beta]-cell adenosine triphosphate-sensitive potassium channels in a side-chain length-specific manner. Metabolism. 2003;52:1313–1319. doi: 10.1016/s0026-0495(03)00199-9. [DOI] [PubMed] [Google Scholar]
- 118.Frieden M, Sollini M, Beny J. Substance P and bradykinin activate different types of KCa currents to hyperpolarize cultured porcine coronary artery endothelial cells. J Physiol. 1999;519(Pt 2):361–371. doi: 10.1111/j.1469-7793.1999.0361m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fukuzaki K, Sato T, Miki T, Seino S, Nakaya H. Role of sarcolemmal ATP-sensitive K+ channels in the regulation of sinoatrial node automaticity: an evaluation using Kir6.2-deficient mice. J Physiol. 2008;586:2767–2778. doi: 10.1113/jphysiol.2007.148932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Furspan PB, Webb RC. Decreased ATP sensitivity of a K+ channel and enhanced vascular smooth muscle relaxation in genetically hypertensive rats. J Hypertens. 1993;11:1067–1072. doi: 10.1097/00004872-199310000-00010. [DOI] [PubMed] [Google Scholar]
- 121.Garland JG, McPherson GA. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992;105:429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D’Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 123.Garlid KD, Paucek P, Yarov-Yarovoy V, Sun X, Schindler PA. The Mitochondrial K[IMAGE] Channel as a Receptor for Potassium Channel Openers. J Biol Chem. 1996;271:8796–8799. doi: 10.1074/jbc.271.15.8796. [DOI] [PubMed] [Google Scholar]
- 124.Gendron ME, Thorin E, Perrault LP. Loss of endothelial KATP channel-dependent, NO-mediated dilation of endocardial resistance coronary arteries in pigs with left ventricular hypertrophy. Br J Pharmacol. 2004;143:285–291. doi: 10.1038/sj.bjp.0705937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Girard CA, Wunderlich FT, Shimomura K, Collins S, Kaizik S, Proks P, Abdulkader F, Clark A, Ball V, Zubcevic L, Bentley L, Clark R, Church C, Hugill A, Galvanovskis J, Cox R, Rorsman P, Bruning JC, Ashcroft FM. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J Clin Invest. 2009;119:80–90. doi: 10.1172/JCI35772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, Edghill EL, Frayling TM, Temple IK, Mackay D, Shield JP, Sumnik Z, van Rhijn A, Wales JK, Clark P, Gorman S, Aisenberg J, Ellard S, Njolstad PR, Ashcroft FM, Hattersley AT. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. New Engl J Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 127.Glukhov AV, Flagg TP, Fedorov VV, Efimov IR, Nichols CG. Differential K(ATP) channel pharmacology in intact mouse heart. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gong B, Legault D, Miki T, Seino S, Renaud JM. KATP channels depress force by reducing action potential amplitude in mouse EDL and soleus muscle. Am J Physiol. 2003;285:C1464–1474. doi: 10.1152/ajpcell.00278.2003. [DOI] [PubMed] [Google Scholar]
- 129.Gong B, Miki T, Seino S, Renaud JM. A K(ATP) channel deficiency affects resting tension, not contractile force, during fatigue in skeletal muscle. Am J Physiol Cell Physiol. 2000;279:C1351–1358. doi: 10.1152/ajpcell.2000.279.5.C1351. [DOI] [PubMed] [Google Scholar]
- 130.Gramolini A, Renaud JM. Blocking ATP-sensitive K+ channel during metabolic inhibition impairs muscle contractility. Am J Physiol. 1997;272:C1936–1946. doi: 10.1152/ajpcell.1997.272.6.C1936. [DOI] [PubMed] [Google Scholar]
- 131.Gribble FM, Ashcroft FM. Differential sensitivity of beta-cell and extrapancreatic K(ATP) channels to gliclazide. Diabetologia. 1999;42:845–848. doi: 10.1007/s001250051236. [DOI] [PubMed] [Google Scholar]
- 132.Gribble FM, Tucker SJ, Ashcroft FM. The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by Mg-ADP and diazoxide. EMBO J. 1997;16:1145–1152. doi: 10.1093/emboj/16.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gros L, Trapp S, Dabrowski M, Ashcroft FM, Bataille D, Blache P. Characterization of two novel forms of the rat sulphonylurea receptor SUR1A2 and SUR1BDelta31. Br J Pharmacol. 2002;137:98–106. doi: 10.1038/sj.bjp.0704836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gross GJ, Auchampach JA. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res. 1992;70:223–233. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- 135.Haider S, Antcliff JF, Proks P, Sansom MS, Ashcroft FM. Focus on Kir6.2: a key component of the ATP-sensitive potassium channel. J Mol Cell Cardiol. 2005;38:927–936. doi: 10.1016/j.yjmcc.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 136.Haider S, Grottesi A, Hall BA, Ashcroft FM, Sansom MS. Conformational dynamics of the ligand-binding domain of inward rectifier K channels as revealed by molecular dynamics simulations: toward an understanding of Kir channel gating. Biophys J. 2005;88:3310–3320. doi: 10.1529/biophysj.104.052019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Haissaguerre M, Chatel S, Sacher F, Weerasooriya R, Probst V, Loussouarn G, Horlitz M, Liersch R, Schulze-Bahr E, Wilde A, Kaab S, Koster J, Rudy Y, Le Marec H, Schott JJ. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 2009;20:93–98. doi: 10.1111/j.1540-8167.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 138.Hamada K, Yamazaki J, Nagao T. Shortening of Action Potential Duration is not Prerequisite for Cardiac Protection by Ischemic Preconditioning or a KATPChannel Opener. J Mol Cell Cardiol. 1998;30:1369–1379. doi: 10.1006/jmcc.1998.0701. [DOI] [PubMed] [Google Scholar]
- 139.Hambrock A, Kayar T, Stumpp D, Osswald H. Effect of two amino acids in TM17 of Sulfonylurea receptor SUR1 on the binding of ATP-sensitive K+ channel modulators. Diabetes. 2004;53 (Suppl 3):S128–134. doi: 10.2337/diabetes.53.suppl_3.s128. [DOI] [PubMed] [Google Scholar]
- 140.Hambrock A, Loffler-Walz C, Kurachi Y, Quast U. Mg2+ and ATP dependence of K(ATP) channel modulator binding to the recombinant sulphonylurea receptor, SUR2B. Br J Pharmacol. 1998;125:577–583. doi: 10.1038/sj.bjp.0702109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hambrock A, Loffler-Walz C, Russ U, Lange U, Quast U. Characterization of a mutant sulfonylurea receptor SUR2B with high affinity for sulfonylureas and openers: differences in the coupling to Kir6.x subtypes. Mol Pharmacol. 2001;60:190–199. doi: 10.1124/mol.60.1.190. [DOI] [PubMed] [Google Scholar]
- 142.Hambrock A, Preisig-Muller R, Russ U, Piehl A, Hanley PJ, Ray J, Daut J, Quast U, Derst C. Four novel splice variants of sulfonylurea receptor 1. Am J Physiol. 2002;283:587–598. doi: 10.1152/ajpcell.00083.2002. [DOI] [PubMed] [Google Scholar]
- 143.Han X, Light PE, Giles WR, French RJ. Identification and properties of an ATP-sensitive K+ current in rabbit sino-atrial node pacemaker cells. J Physiol. 1996;490:337–350. doi: 10.1113/jphysiol.1996.sp021148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hanley PJ, Daut J. K(ATP) channels and preconditioning: a re-examination of the role of mitochondrial K(ATP) channels and an overview of alternative mechanisms. J Mol Cell Cardiol. 2005;39:17–50. doi: 10.1016/j.yjmcc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 145.Hanley PJ, Mickel M, Loffler M, Brandt U, Daut J. K(ATP) channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Harrap SB, Cui JS, Wong ZY, Hopper JL. Familial and genomic analyses of postural changes in systolic and diastolic blood pressure. Hypertension. 2004;43:586–591. doi: 10.1161/01.HYP.0000118044.84189.44. [DOI] [PubMed] [Google Scholar]
- 147.Harrell MD, Harbi S, Hoffman JF, Zavadil J, Coetzee WA. Large-scale analysis of ion channel gene expression in the mouse heart during perinatal development. Physiol Genomics. 2007;28:273–283. doi: 10.1152/physiolgenomics.00163.2006. [DOI] [PubMed] [Google Scholar]
- 148.Haruna T, Horie M, Kouchi I, Nawada R, Tsuchiya K, Akao M, Otani H, Murakami T, Sasayama S. Coordinate interaction between ATP-sensitive K+ channel and Na+, K+-ATPase modulates ischemic preconditioning. Circulation. 1998;98:2905–2910. doi: 10.1161/01.cir.98.25.2905. [DOI] [PubMed] [Google Scholar]
- 149.Hatakeyama N, Wang Q, Goyal RK, Akbarali HI. Muscarinic suppression of ATP-sensitive K+ channel in rabbit esophageal smooth muscle. Am J Physiol. 1995;268:C877–885. doi: 10.1152/ajpcell.1995.268.4.C877. [DOI] [PubMed] [Google Scholar]
- 150.Hayabuchi Y, Davies NW, Standen NB. Angiotensin II inhibits rat arterial KATP channels by inhibiting steady-state protein kinase A activity and activating protein kinase Ce. J Physiol. 2001;530:193–205. doi: 10.1111/j.1469-7793.2001.0193l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hayabuchi Y, Standen NB, Davies NW. Angiotensin II inhibits and alters kinetics of voltage-gated K(+) channels of rat arterial smooth muscle. Am J Physiol. 2001;281:H2480–2489. doi: 10.1152/ajpheart.2001.281.6.H2480. [DOI] [PubMed] [Google Scholar]
- 152.Heginbotham L, Lu Z, Abramson T, MacKinnon R. Mutations in the K+ channel signature sequence. Biophys J. 1994;66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hescheler J, Belles B, Trube G. ATP-dependent potassium channels in the cardiac cell. Biomedica Biochimica Acta. 1987;46:S677–681. [PubMed] [Google Scholar]
- 154.Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 155.Higgins J. A Chemical Mechanism for Oscillation of Glycolytic Intermediates in Yeast Cells. Proc Natl Acad Sci U S A. 1964;51:989–994. doi: 10.1073/pnas.51.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hilgemann DW, Ball R. Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- 157.Hirst GD, Edwards FR. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989;69:546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- 158.Hirst GD, Silverberg GD, van Helden DF. The action potential and underlying ionic currents in proximal rat middle cerebral arterioles. J Physiol. 1986;371:289–304. doi: 10.1113/jphysiol.1986.sp015975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hodgson DM, Zingman LV, Kane GC, Perez-Terzic C, Bienengraeber M, Ozcan C, Gumina RJ, Pucar D, O’Coclain F, Mann DL, Alekseev AE, Terzic A. Cellular remodeling in heart failure disrupts K(ATP) channel-dependent stress tolerance. EMBO J. 2003;22:1732–1742. doi: 10.1093/emboj/cdg192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hopkins WF, Fatherazi S, Peter-Riesch B, Corkey BE, Cook DL. Two sites for adenine-nucleotide regulation of ATP-sensitive potassium channels in mouse pancreatic beta-cells and HIT cells. J Membr Biol. 1992;129:287–295. doi: 10.1007/BF00232910. [DOI] [PubMed] [Google Scholar]
- 161.Hu H, Sato T, Seharaseyon J, Liu Y, Johns DC, O’Rourke B, Marban E. Pharmacological and histochemical distinctions between molecularly defined sarcolemmal KATP channels and native cardiac mitochondrial KATP channels. Mol Pharmacol. 1999;55:1000–1005. [PubMed] [Google Scholar]
- 162.Hu K, Huang CS, Jan YN, Jan LY. ATP-Sensitive Potassium Channel Traffic Regulation by Adenosine and Protein Kinase C. Neuron. 2003;38:417–432. doi: 10.1016/s0896-6273(03)00256-3. [DOI] [PubMed] [Google Scholar]
- 163.Hu X, Xu X, Huang Y, Fassett J, Flagg TP, Zhang Y, Nichols CG, Bache RJ, Chen Y. Disruption of Sarcolemmal ATP-Sensitive Potassium Channel Activity Impairs the Cardiac Response to Systolic Overload. Circ Res. 2008;103:1009–1017. doi: 10.1161/CIRCRESAHA.107.170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 165.Hussain M, Wareham AC, Head SI. Mechanism of action of a K+ channel activator BRL 38227 on ATP-sensitive K+ channels in mouse skeletal muscle fibres. J Physiol. 1994;478:523–532. doi: 10.1113/jphysiol.1994.sp020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Imamura Y, Tomoike H, Narishige T, Takahashi T, Kasuya H, Takeshita A. Glibenclamide decreases basal coronary blood flow in anesthetized dogs. Am J Physiol. 1992;263:H399–404. doi: 10.1152/ajpheart.1992.263.2.H399. [DOI] [PubMed] [Google Scholar]
- 167.Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 168.Inagaki N, Gonoi T, Clement JPt, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor [see comments] Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 169.Inagaki N, Gonoi T, Seino S. Subunit stoichiometry of the pancreatic beta-cell ATP-sensitive K+ channel. FEBS Lett. 1997;409:232–236. doi: 10.1016/s0014-5793(97)00488-2. [DOI] [PubMed] [Google Scholar]
- 170.Inagaki N, Inazawa J, Seino S. cDNA sequence, gene structure, and chromosomal localization of the human ATP-sensitive potassium channel, uKATP-1, gene (KCNJ8) Genomics. 1995;30:102–104. doi: 10.1006/geno.1995.0018. [DOI] [PubMed] [Google Scholar]
- 171.Inagaki N, Tsuura Y, Namba N, Masuda K, Gonoi T, Horie M, Seino Y, Mizuta M, Seino S. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J Biol Chem. 1995;270:5691–5694. doi: 10.1074/jbc.270.11.5691. [DOI] [PubMed] [Google Scholar]
- 172.Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res. 2009;81:412–419. doi: 10.1093/cvr/cvn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 174.Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- 175.Inui J, Imamura H. Effects of acetylcholine on calcium-dependent electrical and mechanical responses in the guinea-pig papillary muscle partially depolarized by potassium. Naunyn Schmiedebergs Arch Pharmacol. 1977;299:1–7. doi: 10.1007/BF00508630. [DOI] [PubMed] [Google Scholar]
- 176.Ishizaki E, Fukumoto M, Puro DG. Functional K(ATP) channels in the rat retinal microvasculature: topographical distribution, redox regulation, spermine modulation and diabetic alteration. J Physiol. 2009;587:2233–2253. doi: 10.1113/jphysiol.2009.169003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Isidoro Tavares N, Philip-Couderc P, Papageorgiou I, Baertschi AJ, Lerch R, Montessuit C. Expression and function of ATP-dependent potassium channels in late post-infarction remodeling. J Mol Cell Cardiol. 2007;42:1016–1025. doi: 10.1016/j.yjmcc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 178.Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- 179.Ito H, Vereecke J, Carmeliet E. Mode of regulation by G protein of the ATP-sensitive K+ channel in guinea-pig ventricular cell membrane. J Physiol. 1994;478:101–107. doi: 10.1113/jphysiol.1994.sp020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ito K, Kanno T, Suzuki K, Masuzawa-Ito K, Takewaki T, Ohashi H, Asano M, Suzuki H. Effects of cromakalim on the contraction and the membrane potential of the circular smooth muscle of guinea-pig stomach. Br J Pharmacol. 1992;105:335–340. doi: 10.1111/j.1476-5381.1992.tb14255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jackson WF. Arteriolar tone is determined by activity of ATP-sensitive potassium channels. Am J Physiol. 1993;265:H1797–1803. doi: 10.1152/ajpheart.1993.265.5.H1797. [DOI] [PubMed] [Google Scholar]
- 182.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jackson WF, Konig A, Dambacher T, Busse R. Prostacyclin-induced vasodilation in rabbit heart is mediated by ATP-sensitive potassium channels. Am J Physiol. 1993;264:H238–243. doi: 10.1152/ajpheart.1993.264.1.H238. [DOI] [PubMed] [Google Scholar]
- 184.Janigro D, West GA, Gordon EL, Winn HR. ATP-sensitive K+ channels in rat aorta and brain microvascular endothelial cells. Am J Physiol. 1993 doi: 10.1152/ajpcell.1993.265.3.C812. [DOI] [PubMed] [Google Scholar]
- 185.Jiao J, Garg V, Yang B, Elton TS, Hu K. Protein kinase C-epsilon induces caveolin-dependent internalization of vascular adenosine 5′-triphosphate-sensitive K+ channels. Hypertension. 2008;52:499–506. doi: 10.1161/HYPERTENSIONAHA.108.110817. [DOI] [PubMed] [Google Scholar]
- 186.Jiao JH, Baertschi AJ. Synergistic regulation of ANF in isolated rat hearts. Am J Physiol. 1995;268:H1405–1411. doi: 10.1152/ajpheart.1995.268.4.H1405. [DOI] [PubMed] [Google Scholar]
- 187.John SA, Weiss JN, Ribalet B. ATP sensitivity of ATP-sensitive K+ channels: role of the gamma phosphate group of ATP and the R50 residue of mouse Kir6.2. J Physiol. 2005;568:931–940. doi: 10.1113/jphysiol.2005.095638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.John SA, Weiss JN, Xie LH, Ribalet B. Molecular mechanism for ATP-dependent closure of the K+ channel Kir6.2. J Physiol. 2003;552:23–34. doi: 10.1113/jphysiol.2003.048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jovanovic A, Jovanovic S, Lorenz E, Terzic A. Recombinant cardiac ATP-sensitive K+ channel subunits confer chemical hypoxia-reoxygenation injury. Circulation. 1998;98:1548–1555. doi: 10.1161/01.cir.98.15.1548. [DOI] [PubMed] [Google Scholar]
- 190.Jovanovic N, Jovanovic S, Jovanovic A, Terzic A. Gene delivery of Kir6.2/SUR2A in conjunction with pinacidil handles intracellular Ca2+ homeostasis under metabolic stress. FASEB J. 1999;13:923–929. doi: 10.1096/fasebj.13.8.923. [DOI] [PubMed] [Google Scholar]
- 191.Jovanovic S, Du Q, Crawford RM, Budas GR, Stagljar I, Jovanovic A. Glyceraldehyde 3-phosphate dehydrogenase serves as an accessory protein of the cardiac sarcolemmal K(ATP) channel. EMBO Rep. 2005;6:848–852. doi: 10.1038/sj.embor.7400489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jun JY, Kong ID, Koh SD, Wang XY, Perrino BA, Ward SM, Sanders KM. Regulation of ATP-sensitive K(+) channels by protein kinase C in murine colonic myocytes. Am J Physiol. 2001;281:C857–864. doi: 10.1152/ajpcell.2001.281.3.C857. [DOI] [PubMed] [Google Scholar]
- 193.Kabakov AY. Activation of KATP channels by Na/K pump in isolated cardiac myocytes and giant membrane patches. Biophys J. 1998;75:2858–2867. doi: 10.1016/S0006-3495(98)77728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kageyama M, Yanagisawa T, Taira N. Calcitonin gene-related peptide relaxes porcine coronary arteries via cyclic AMP-dependent mechanisms, but not activation of ATP-sensitive potassium channels. J Pharmacol Exp Ther. 1993;265:490–497. [PubMed] [Google Scholar]
- 195.Kajioka S, Kitamura K, Kuriyama H. Guanosine diphosphate activates an adenosine 5′-triphosphate-sensitive K+ channel in the rabbit portal vein. J Physiol. 1991;444:397–418. doi: 10.1113/jphysiol.1991.sp018885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kakei M, Kelly RP, Ashcroft SJ, Ashcroft FM. The ATP-sensitivity of K+ channels in rat pancreatic B-cells is modulated by ADP. FEBS Lett. 1986;208:63–66. doi: 10.1016/0014-5793(86)81533-2. [DOI] [PubMed] [Google Scholar]
- 197.Kakei MNA, Shibasaki T. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985;363:441–462. doi: 10.1113/jphysiol.1985.sp015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kakei M, Noma A. Adenosine-5′-triphosphate-sensitive single potassium channel in the atrioventricular node cell of the rabbit heart. J Physiol. 1984;352:265–284. doi: 10.1113/jphysiol.1984.sp015290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kakkar R, Ye B, Stoller DA, Smelley M, Shi NQ, Galles K, Hadhazy M, Makielski JC, McNally EM. Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circ Res. 2006;98:682–689. doi: 10.1161/01.RES.0000207498.40005.e7. [DOI] [PubMed] [Google Scholar]
- 200.Kamei K, Yoshida S, Imagawa J, Nabata H, Kuriyama H. Regional and species differences in glyburide-sensitive K+ channels in airway smooth muscles as estimated from actions of KC 128 and levcromakalim. Br J Pharmacol. 1994;113:889–897. doi: 10.1111/j.1476-5381.1994.tb17076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kamouchi M, Kitamura K. Regulation of ATP-sensitive K+ channels by ATP and nucleotide diphosphate in rabbit portal vein. Am J Physiol. 1994;266:H1687–1698. doi: 10.1152/ajpheart.1994.266.5.H1687. [DOI] [PubMed] [Google Scholar]
- 202.Kane GC, Behfar A, Dyer RB, O’Cochlain DF, Liu XK, Hodgson DM, Reyes S, Miki T, Seino S, Terzic A. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet. 2006;15:2285–2297. doi: 10.1093/hmg/ddl154. [DOI] [PubMed] [Google Scholar]
- 203.Kane GC, Behfar A, Yamada S, Perez-Terzic C, O’Cochlain F, Reyes S, Dzeja PP, Miki T, Seino S, Terzic A. ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. Diabetes. 2004;53 (Suppl 3):169–175. doi: 10.2337/diabetes.53.suppl_3.s169. [DOI] [PubMed] [Google Scholar]
- 204.Kane GC, Lam CF, O’Cochlain F, Hodgson DM, Reyes S, Liu XK, Miki T, Seino S, Katusic ZS, Terzic A. Gene knockout of the KCNJ8-encoded Kir6.1 K(ATP) channel imparts fatal susceptibility to endotoxemia. FASEB J. 2006;20:2271–2280. doi: 10.1096/fj.06-6349com. [DOI] [PubMed] [Google Scholar]
- 205.Kantor PF, Coetzee WA, Carmeliet EE, Dennis SC, Opie LH. Reduction of ischemic K+ loss and arrhythmias in rat hearts. Effect of glibenclamide, a sulfonylurea. Circ Res. 1990;66:478–485. doi: 10.1161/01.res.66.2.478. [DOI] [PubMed] [Google Scholar]
- 206.Kim SH, Cho KW, Chang SH, Kim SZ, Chae SW. Glibenclamide suppresses stretch-activated ANP secretion: involvements of K+ATP channels and L-type Ca2+ channel modulation. Pflugers Arch. 1997;434:362–372. doi: 10.1007/s004240050409. [DOI] [PubMed] [Google Scholar]
- 207.Kirsch GE, Codina J, Birnbaumer L, Brown AM. Coupling of ATP-sensitive K+ channels to A1 receptors by G proteins in rat ventricular myocytes. Am J Physiol. 1990;259 doi: 10.1152/ajpheart.1990.259.3.H820. [DOI] [PubMed] [Google Scholar]
- 208.Kitazono T, Faraci FM, Heistad DD. Effect of norepinephrine on rat basilar artery in vivo. Am J Physiol. 1993;264:H178–182. doi: 10.1152/ajpheart.1993.264.1.H178. [DOI] [PubMed] [Google Scholar]
- 209.Kitazono T, Heistad DD, Faraci FM. Role of ATP-sensitive K+ channels in CGRP-induced dilatation of basilar artery in vivo. Am J Physiol. 1993;265:H581–585. doi: 10.1152/ajpheart.1993.265.2.H581. [DOI] [PubMed] [Google Scholar]
- 210.Kleppisch T, Nelson MT. Adenosine activates ATP-sensitive potassium channels in arterial myocytes via A2 receptors and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1995;92:12441–12445. doi: 10.1073/pnas.92.26.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kleppisch T, Nelson MT. ATP-sensitive K+ currents in cerebral arterial smooth muscle: pharmacological and hormonal modulation. Am J Physiol. 1995;269:H1634–1640. doi: 10.1152/ajpheart.1995.269.5.H1634. [DOI] [PubMed] [Google Scholar]
- 212.Knopp A, Thierfelder S, Koopmann R, Biskup C, Bohle T, Benndorf K. Anoxia generates rapid and massive opening of KATP channels in ventricular cardiac myocytes. Cardiovasc Res. 1999;41:629–640. doi: 10.1016/s0008-6363(98)00238-7. [DOI] [PubMed] [Google Scholar]
- 213.Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008;44:65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]
- 214.Kocic I, Hirano Y, Hiraoka M. The effects of K+ channels modulators terikalant and glibenclamide on membrane potential changes induced by hypotonic challenge of guinea pig ventricular myocytes. J Pharmacol Sci. 2004;95:27–32. doi: 10.1254/jphs.95.27. [DOI] [PubMed] [Google Scholar]
- 215.Koh SD, Bradley KK, Rae MG, Keef KD, Horowitz B, Sanders KM. Basal activation of ATP-sensitive potassium channels in murine colonic smooth muscle cell. Biophys J. 1998;75:1793–1800. doi: 10.1016/S0006-3495(98)77621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kondo C, Repunte VP, Satoh E, Yamada M, Horio Y, Matsuzawa Y, Pott L, Kurachi Y. Chimeras of Kir6.1 and Kir6.2 reveal structural elements involved in spontaneous opening and unitary conductance of the ATP-sensitive K+ channels. Receptors & Channels. 1998;6:129–140. [PubMed] [Google Scholar]
- 217.Kondo T, Kubota I, Tachibana H, Yamaki M, Tomoike H. Glibenclamide attenuates peaked T wave in early phase of myocardial ischemia. Cardiovasc Res. 1996;31:683–687. doi: 10.1016/0008-6363(96)00016-8. [DOI] [PubMed] [Google Scholar]
- 218.Kono Y, Horie M, Takano M, Otani H, Xie LH, Akao M, Tsuji K, Sasayama S. The properties of the Kir6.1–6.2 tandem channel co-expressed with SUR2A. Pflugers Arch. 2000;440:692–698. doi: 10.1007/s004240000315. [DOI] [PubMed] [Google Scholar]
- 219.Koster JC, Knopp A, Flagg TP, Markova KP, Sha Q, Enkvetchakul D, Betsuyaku T, Yamada K, Nichols CG. Tolerance for ATP-Insensitive KATP Channels in Transgenic Mice. Circ Res. 2001;89:1022–1029. doi: 10.1161/hh2301.100342. [DOI] [PubMed] [Google Scholar]
- 220.Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG. Targeted overactivity of beta cell K(ATP) channels induces profound neonatal diabetes. Cell. 2000;100:645–654. doi: 10.1016/s0092-8674(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 221.Koster JC, Permutt A, Nichols CG. Diabetes and insulin secretion: the KATP connection. Diabetes. 2005;54:3065–3030. doi: 10.2337/diabetes.54.11.3065. [DOI] [PubMed] [Google Scholar]
- 222.Koster JC, Permutt MA, Nichols CG. Diabetes and insulin secretion: the ATP-sensitive K+ channel (K ATP) connection. Diabetes. 2005;54:3065–3072. doi: 10.2337/diabetes.54.11.3065. [DOI] [PubMed] [Google Scholar]
- 223.Koster JC, Sha Q, Nichols CG. Sulfonylurea and K(+)-channel opener sensitivity of K(ATP) channels. Functional coupling of Kir6.2 and SUR1 subunits. J Gen Physiol. 1999;114:203–213. doi: 10.1085/jgp.114.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Koumi SI, Martin RL, Sato R. Alterations in ATP-sensitive potassium channel sensitivity to ATP in failing human hearts. Am J Physiol. 1997;272:1656–1665. doi: 10.1152/ajpheart.1997.272.4.H1656. [DOI] [PubMed] [Google Scholar]
- 225.Koyano T, Kakei M, Nakashima H, Yoshinaga M, Matsuoka T, Tanaka H. ATP-regulated K+ channels are modulated by intracellular H+ in guinea-pig ventricular cells. J Physiol. 1993;463:747–766. doi: 10.1113/jphysiol.1993.sp019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kubo M, Nakaya Y, Matsuoka S, Saito K, Kuroda Y. Atrial natriuretic factor and isosorbide dinitrate modulate the gating of ATP-sensitive K+ channels in cultured vascular smooth muscle cells. Circ Res. 1994;74:471–476. doi: 10.1161/01.res.74.3.471. [DOI] [PubMed] [Google Scholar]
- 227.Kubo M, Quayle JM, Standen NB. Angiotensin II inhibition of ATP-sensitive K+ currents in rat arterial smooth muscle cells through protein kinase C. J Physiol. 1997;503:489–496. doi: 10.1111/j.1469-7793.1997.489bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kubokawa M, McNicholas CM, Higgins MA, Wang W, Giebisch G. Regulation of ATP-sensitive K+ channel by membrane-bound protein phosphatases in rat principal tubule cell. Am J Physiol. 1995;269:F355–362. doi: 10.1152/ajprenal.1995.269.3.F355. [DOI] [PubMed] [Google Scholar]
- 229.Kubota I, Yamaki M, Shibata T, Ikeno E, Hosoya Y, Tomoike H. Role of ATP-sensitive K+ channel on ECG ST segment elevation during a bout of myocardial ischemia. A study on epicardial mapping in dogs. Circulation. 1993;88:1845–1851. doi: 10.1161/01.cir.88.4.1845. [DOI] [PubMed] [Google Scholar]
- 230.Kuo A, Domene C, Johnson LN, Doyle DA, Venien-Bryan C. Two different conformational states of the KirBac3.1 potassium channel revealed by electron crystallography. Structure. 2005;13:1463–1472. doi: 10.1016/j.str.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 231.Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–1926. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- 232.Lacza Z, Snipes JA, Kis B, Szabó C, Grover G, Busija DW. Investigation of the subunit composition and the pharmacology of the mitochondrial ATP-dependent K+ channel in the brain. Brain Research. 2003;994:27–36. doi: 10.1016/j.brainres.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 233.Lammens A, Schele A, Hopfner KP. Structural biochemistry of ATP-driven dimerization and DNA-stimulated activation of SMC ATPases. Current Biology. 2004;14:1778–1782. doi: 10.1016/j.cub.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 234.Lamontagne D, Konig A, Bassenge E, Busse R. Prostacyclin and nitric oxide contribute to the vasodilator action of acetylcholine and bradykinin in the intact rabbit coronary bed. J Cardiovasc Pharmacol. 1992;20:652–657. doi: 10.1097/00005344-199210000-00020. [DOI] [PubMed] [Google Scholar]
- 235.Larsson O, Deeney JT, Branstrom R, Berggren PO, Corkey BE. Activation of the ATP-sensitive K+ channel by long chain acyl-CoA. A role in modulation of pancreatic beta-cell glucose sensitivity. J Biol Chem. 1996;271:10623–10626. doi: 10.1074/jbc.271.18.10623. [DOI] [PubMed] [Google Scholar]
- 236.Lawson K. Is there a role for potassium channel openers in neuronal ion channel disorders? Expert Opin Investig Drugs. 2000;9:2269–2280. doi: 10.1517/13543784.9.10.2269. [DOI] [PubMed] [Google Scholar]
- 237.Lederer WJ, Nichols CG. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol. 1989;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lederer WJ, Nichols CG, Smith GL. The mechanism of early contractile failure of isolated rat ventricular myocytes subjected to complete metabolic inhibition. J Physiol. 1989;413:329–349. doi: 10.1113/jphysiol.1989.sp017657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li L, Wang J, Drain P. The I182 region of k(ir)6.2 is closely associated with ligand binding in K(ATP) channel inhibition by ATP. Biophys J. 2000;79:841–852. doi: 10.1016/S0006-3495(00)76340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Li RA, Leppo M, Miki T, Seino S, Marban E. Molecular basis of electrocardiographic ST-segment elevation. Circ Res. 2000;87:837–839. doi: 10.1161/01.res.87.10.837. [DOI] [PubMed] [Google Scholar]
- 241.Li X, Rapedius M, Baukrowitz T, Liu GX, Srivastava DK, Daut J, Hanley PJ. 5-Hydroxydecanoate and coenzyme A are inhibitors of native sarcolemmal KATP channels in inside-out patches. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbagen.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 242.Light PE, Allen BG, Walsh MP, French RJ. Regulation of adenosine triphosphate-sensitive potassium channels from rabbit ventricular myocytes by protein kinase C and type 2A protein phosphatase. Biochemistry. 1995;34:7252–7257. doi: 10.1021/bi00021a041. [DOI] [PubMed] [Google Scholar]
- 243.Light PE, Bladen C, Winkfein RJ, Walsh MP, French RJ. Molecular basis of protein kinase C-induced activation of ATP-sensitive potassium channels. Proc Natl Acad Sci U S A. 2000;97:9058–9063. doi: 10.1073/pnas.160068997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Light PE, Comtois AS, Renaud JM. The effect of glibenclamide on frog skeletal muscle: evidence for K+ATP channel activation during fatigue. J Physiol. 1994;475:495–507. doi: 10.1113/jphysiol.1994.sp020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Light PE, Cordeiro JM, French RJ. Identification and properties of ATP-sensitive potassium channels in myocytes from rabbit Purkinje fibres. Cardiovasc Res. 1999;44:356–369. doi: 10.1016/s0008-6363(99)00218-7. [DOI] [PubMed] [Google Scholar]
- 246.Light PE, Sabir AA, Allen BG, Walsh MP, French RJ. Protein kinase C-induced changes in the stoichiometry of ATP binding activate cardiac ATP-sensitive K+ channels. A possible mechanistic link to ischemic preconditioning. Circ Res. 1996;79:399–406. doi: 10.1161/01.res.79.3.399. [DOI] [PubMed] [Google Scholar]
- 247.Lin YF, Jan YN, Jan LY. Regulation of ATP-sensitive potassium channel function by protein kinase A-mediated phosphorylation in transfected HEK293 cells. EMBO J. 2000;19:942–955. doi: 10.1093/emboj/19.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Liu G, Thornton J, Van Winkle D, Stanley A, Olsson R, Downey J. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–356. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- 249.Liu GX, Hanley PJ, Ray J, Daut J. Long-chain acyl-coenzyme A esters and fatty acids directly link metabolism to K(ATP) channels in the heart. Circ Res. 2001;88:918–924. doi: 10.1161/hh0901.089881. [DOI] [PubMed] [Google Scholar]
- 250.Liu XK, Yamada S, Kane GC, Alekseev AE, Hodgson DM, O’Cochlain F, Jahangir A, Miki T, Seino S, Terzic A. Genetic disruption of Kir6.2, the pore-forming subunit of ATP-sensitive K+ channel, predisposes to catecholamine-induced ventricular dysrhythmia. Diabetes. 2004;53 (Suppl 3):165–168. doi: 10.2337/diabetes.53.suppl_3.s165. [DOI] [PubMed] [Google Scholar]
- 251.Liu Y, Gao WD, O’Rourke B, Marban E. Cell-type specificity of preconditioning in an in vitro model. Bas Res Cardiol. 1996;91:450–457. doi: 10.1007/BF00788726. [DOI] [PubMed] [Google Scholar]
- 252.Liu Y, Ren G, O’Rourke B, Marban E, Seharaseyon J. Pharmacological Comparison of Native Mitochondrial KATP Channels with Molecularly Defined Surface KATP Channels. Mol Pharmacol. 2001;59:225–230. [PubMed] [Google Scholar]
- 253.Liu Y, Sato T, O’Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- 254.Longo M, Jain V, Vedernikov YP, Hankins GD, Garfield RE, Saade GR. Effects of L-type Ca(2+)-channel blockade, K(+)(ATP)-channel opening and nitric oxide on human uterine contractility in relation to gestational age and labour. Mol Hum Reprod. 2003;9:159–164. doi: 10.1093/molehr/gag023. [DOI] [PubMed] [Google Scholar]
- 255.Lorenz E, Terzic A. Physical association between recombinant cardiac ATP-sensitive K+ channel subunits Kir6.2 and SUR2A. J Mol Cell Cardiol. 1999;31:425–434. doi: 10.1006/jmcc.1998.0876. [DOI] [PubMed] [Google Scholar]
- 256.Loutzenhiser RD, Parker MJ. Hypoxia inhibits myogenic reactivity of renal afferent arterioles by activating ATP-sensitive K+ channels. Circ Res. 1994;74:861–869. doi: 10.1161/01.res.74.5.861. [DOI] [PubMed] [Google Scholar]
- 257.Lowe SA, Rubin PC. The pharmacological management of hypertension in pregnancy. J Hypertens. 1992;10:201–207. doi: 10.1097/00004872-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 258.MacGregor GG, Dong K, Vanoye CG, Tang L, Giebisch G, Hebert SC. Nucleotides and phospholipids compete for binding to the C terminus of KATP channels. Proc Natl Acad Sci U S A. 2002;99:2726–2731. doi: 10.1073/pnas.042688899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Maggi CA, Giuliani S, Santicioli P. Multiple mechanisms in the smooth muscle relaxant action of calcitonin gene-related peptide (CGRP) in the guinea-pig ureter. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:537–547. doi: 10.1007/BF00173024. [DOI] [PubMed] [Google Scholar]
- 260.Malester B, Tong X, Ghiu I, Kontogeorgis A, Gutstein DE, Xu J, Hendricks-Munoz KD, Coetzee WA. Transgenic expression of a dominant negative K(ATP) channel subunit in the mouse endothelium: effects on coronary flow and endothelin-1 secretion. FASEB J. 2007;21:2162–2172. doi: 10.1096/fj.06-7821com. [DOI] [PubMed] [Google Scholar]
- 261.Mannhold R. KATP channel openers: structure-activity relationships and therapeutic potential. Med Res Rev. 2004;24:213–266. doi: 10.1002/med.10060. [DOI] [PubMed] [Google Scholar]
- 262.Manning Fox JE, Nichols CG, Light PE. Activation of adenosine triphosphate-sensitive potassium channels by acyl coenzyme A esters involves multiple phosphatidylinositol 4,5-bisphosphate-interacting residues. Mol Endocrinol. 2004;18:679–686. doi: 10.1210/me.2003-0431. [DOI] [PubMed] [Google Scholar]
- 263.Masia R, Enkvetchakul D, Nichols CG. Differential nucleotide regulation of KATP channels by SUR1 and SUR2A. J Mol Cell Cardiol. 2005;39:491–501. doi: 10.1016/j.yjmcc.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 264.Masia R, Nichols CG. Functional clustering of mutations in the dimer interface of the nucleotide binding folds of the sulfonylurea receptor. J Biol Chem. 2008;283:30322–30329. doi: 10.1074/jbc.M804318200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Matar W, Nosek TM, Wong D, Renaud J-M. Pinacidil suppresses contractility and preserves energy but glibenclamide has no effect during muscle fatigue. Am J Physiol. 2000;278:C404–416. doi: 10.1152/ajpcell.2000.278.2.C404. [DOI] [PubMed] [Google Scholar]
- 266.Matsuo M, Tanabe K, Kioka N, Amachi T, Ueda K. Different binding properties and affinities for ATP and ADP among sulfonylurea receptor subtypes, SUR1, SUR2A, and SUR2B. J Biol Chem. 2000;275:28757–28763. doi: 10.1074/jbc.M004818200. [DOI] [PubMed] [Google Scholar]
- 267.Matsuoka T, Matsushita K, Katayama Y, Fujita A, Inageda K, Tanemoto M, Inanobe A, Yamashita S, Matsuzawa Y, Kurachi Y. C-Terminal tails of sulfonylurea receptors control ADP-induced activation and diazoxide modulation of ATP-sensitive K(+) channels. Circ Res. 2000;87:873–880. doi: 10.1161/01.res.87.10.873. [DOI] [PubMed] [Google Scholar]
- 268.McCullough JR, Normandin DE, Conder ML, Sleph PG, Dzwonczyk S, Grover GJ. Specific block of the anti-ischemic actions of cromakalim by sodium 5-hydroxydecanoate. Circ Res. 1991;69:949–958. doi: 10.1161/01.res.69.4.949. [DOI] [PubMed] [Google Scholar]
- 269.McPherson CD, Pierce GN, Cole WC. Ischemic cardioprotection by ATP-sensitive K+ channels involves high-energy phosphate preservation. Am J Physiol. 1993;265:H1809–1818. doi: 10.1152/ajpheart.1993.265.5.H1809. [DOI] [PubMed] [Google Scholar]
- 270.Mederos y Schnitzler M, Derst C, Daut J, Preisig-Muller R. ATP-sensitive potassium channels in capillaries isolated from guinea-pig heart. J Physiol. 2000;525(Pt 2):307–317. doi: 10.1111/j.1469-7793.2000.t01-1-00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Melamed-Frank M, Terzic A, Carrasco AJ, Nevo E, Avivi A, Levy AP. Reciprocal regulation of expression of pore-forming KATP channel genes by hypoxia. Mol Cell Biochem. 2001;225:145–150. doi: 10.1023/a:1012286624993. [DOI] [PubMed] [Google Scholar]
- 272.Merkel LA, Lappe RW, Rivera LM, Cox BF, Perrone MH. Demonstration of vasorelaxant activity with an A1-selective adenosine agonist in porcine coronary artery: involvement of potassium channels. J Pharm Exp Ther. 1992;260:437–443. [PubMed] [Google Scholar]
- 273.Mikhailov MV, Campbell JD, de Wet H, Shimomura K, Zadek B, Collins RF, Sansom MS, Ford RC, Ashcroft FM. 3-D structural and functional characterization of the purified KATP channel complex Kir6.2-SUR1. EMBO J. 2005;24:4166–4175. doi: 10.1038/sj.emboj.7600877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Miki T, Minami K, Zhang L, Morita M, Gonoi T, Shiuchi T, Minokoshi Y, Renaud JM, Seino S. ATP-sensitive potassium channels participate in glucose uptake in skeletal muscle and adipose tissue. Am J Physiol. 2002;283:E1178–1184. doi: 10.1152/ajpendo.00313.2002. [DOI] [PubMed] [Google Scholar]
- 275.Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J, Seino S. Defective insulin secretion and enhanced insulin action in KATP channel- deficient mice. Proc Natl Acad Sci U S A. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- 277.Minami K, Morita M, Saraya A, Yano H, Terauchi Y, Miki T, Kuriyama T, Kadowaki T, Seino S. ATP-sensitive K+ channel-mediated glucose uptake is independent of IRS-1/phosphatidylinositol 3-kinase signaling. Am J Physiol. 2003;285:E1289–1296. doi: 10.1152/ajpendo.00278.2003. [DOI] [PubMed] [Google Scholar]
- 278.Miyoshi H, Nakaya Y. Activation of ATP-sensitive K+ channels by cyclic AMP-dependent protein kinase in cultured smooth muscle cells of porcine coronary artery. Biochem Biophys Res Commun. 1993;193:240–247. doi: 10.1006/bbrc.1993.1615. [DOI] [PubMed] [Google Scholar]
- 279.Miyoshi H, Nakaya Y. Calcitonin gene-related peptide activates the K+ channels of vascular smooth muscle cells via adenylate cyclase. Basic Res Cardiol. 1995;90:332–336. doi: 10.1007/BF00797911. [DOI] [PubMed] [Google Scholar]
- 280.Miyoshi Y, Nakaya Y. Angiotensin II blocks ATP-sensitive K+ channels in porcine coronary artery smooth muscle cells. Biochem Biophys Res Commun. 1991;181:700–706. doi: 10.1016/0006-291x(91)91247-a. [DOI] [PubMed] [Google Scholar]
- 281.Miyoshi Y, Nakaya Y, Wakatsuki T, Nakaya S, Fujino K, Saito K, Inoue I. Endothelin blocks ATP-sensitive K+ channels and depolarizes smooth muscle cells of porcine coronary artery. Circ Res. 1992;70:612–616. doi: 10.1161/01.res.70.3.612. [DOI] [PubMed] [Google Scholar]
- 282.Moreau C, Jacquet H, Prost AL, D’Hahan N, Vivaudou M. The molecular basis of the specificity of action of K(ATP) channel openers. EMBO J. 2000;19:6644–6651. doi: 10.1093/emboj/19.24.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Moritani K, Miyazaki T, Miyoshi S, Asanagi M, Zhao LS, Mitamura H, Ogawa S. Blockade of ATP-sensitive potassium channels by 5-hydroxydecanoate suppresses monophasic action potential shortening during regional myocardial ischemia. Cardiovasc Drugs Ther. 1994;8:749–756. doi: 10.1007/BF00877122. [DOI] [PubMed] [Google Scholar]
- 284.Morrissey A, Parachuru L, Leung M, Lopez G, Nakamura TY, Tong X, Yoshida H, Srivastava S, Chowdhury PD, Artman M, Coetzee WA. Expression of ATP-sensitive K+ channel subunits during perinatal maturation in the mouse heart. Pediatric Research. 2005;58:185–192. doi: 10.1203/01.PDR.0000169967.83576.CB. [DOI] [PubMed] [Google Scholar]
- 285.Morrissey A, Rosner E, Lanning J, Parachuru L, Chowdhury PD, Han S, Lopez G, Tong X, Yoshida H, Nakamura TY, Artman M, Giblin JP, Tinker A, Coetzee WA. Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiology. 2005;5:1. doi: 10.1186/1472-6793-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Moses MA, Addison PD, Neligan PC, Ashrafpour H, Huang N, McAllister SE, Lipa JE, Forrest CR, Pang CY. Inducing late phase of infarct protection in skeletal muscle by remote preconditioning: efficacy and mechanism. Am J Physiol. 2005;289:R1609–1617. doi: 10.1152/ajpregu.00395.2005. [DOI] [PubMed] [Google Scholar]
- 287.Moses MA, Addison PD, Neligan PC, Ashrafpour H, Huang N, Zair M, Rassuli A, Forrest CR, Grover GJ, Pang CY. Mitochondrial KATP channels in hindlimb remote ischemic preconditioning of skeletal muscle against infarction. Am J Physiol. 2005;288:H559–567. doi: 10.1152/ajpheart.00845.2004. [DOI] [PubMed] [Google Scholar]
- 288.Murphy ME, Brayden JE. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitive potassium channels. J Physiol. 1995;486:47–58. doi: 10.1113/jphysiol.1995.sp020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Murray MA, Boyle JP, Small RC. Cromakalim-induced relaxation of guinea-pig isolated trachealis: antagonism by glibenclamide and by phentolamine. Br J Pharmacol. 1989;98:865–874. doi: 10.1111/j.1476-5381.1989.tb14615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Murry C, Jennings R, Reimer K. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 291.Narishige T, Egashira K, Akatsuka Y, Imamura Y, Takahashi T, Kasuya H, Takeshita A. Glibenclamide prevents coronary vasodilation induced by beta 1-adrenoceptor stimulation in dogs. Am J Physiol. 1994;266:H84–92. doi: 10.1152/ajpheart.1994.266.1.H84. [DOI] [PubMed] [Google Scholar]
- 292.Nelson DA, Aguilar BL, Bryan J. Specificity of photolabeling of beta-cell membrane proteins with an 125I-labeled glyburide analog. J Biol Chem. 1992;267:14928–14933. [PubMed] [Google Scholar]
- 293.Nelson MT, Huang Y, Brayden JE, Hescheler J, Standen NB. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990;344:770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- 294.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 295.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 296.Nelson MT, Standen NB, Brayden JE, Worley JFd. Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature. 1988;336:382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- 297.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 298.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 299.Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991;261:H1675–1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- 300.Nichols CG, Lederer WJ. The regulation of ATP-sensitive K+ channel activity in intact and permeabilized rat ventricular myocytes. J Physiol. 1990;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Nichols CG, Lederer WJ. The role of ATP in energy-deprivation contractures in unloaded rat ventricular myocytes. Can J Physiol Pharmacol. 1990;68:183–194. doi: 10.1139/y90-029. [DOI] [PubMed] [Google Scholar]
- 302.Nichols CG, Ripoll C, Lederer WJ. ATP-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circulation Research. 1991;68:280–287. doi: 10.1161/01.res.68.1.280. [DOI] [PubMed] [Google Scholar]
- 303.Nichols CG, Shyng SL, Nestorowicz A, Glaser B, Clement JP, Gonzalez G, Aguilarbryan L, Permutt MA, Bryan J. Adenosine Diphosphate As an Intracellular Regulator Of Insulin Secretion. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- 304.Nishida M, MacKinnon R. Structural Basis of Inward Rectification. Cytoplasmic Pore of the G Protein-Gated Inward Rectifier GIRK1 at 1.8 A Resolution. Cell. 2002;111:957–965. doi: 10.1016/s0092-8674(02)01227-8. [DOI] [PubMed] [Google Scholar]
- 305.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 306.Noma A, Shibasaki T. Membrane current through adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985;363:463–480. doi: 10.1113/jphysiol.1985.sp015722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Notsu T, Tanaka I, Takano M, Noma A. Blockade of the ATP-sensitive K+ channel by 5-hydroxydecanoate in guinea pig ventricular myocytes. J Pharmacol Exp Ther. 1992;260:702–708. [PubMed] [Google Scholar]
- 308.O’Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res. 2004;94:420–432. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.O’Rourke B, Ramza BM, Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science. 1994;265:962–966. doi: 10.1126/science.8052856. [DOI] [PubMed] [Google Scholar]
- 310.O’Rourke B, Ramza BM, Romashko DN, Marban E. Metabolic oscillations in heart cells. Adv Exp Med Biol. 1995;382:165–174. doi: 10.1007/978-1-4615-1893-8_17. [DOI] [PubMed] [Google Scholar]
- 311.Okuyama Y, Yamada M, Kondo C, Satoh E, Isomoto S, Shindo T, Horio Y, Kitakaze M, Hori M, Kurachi Y. The effects of nucleotides and potassium channel openers on the SUR2A/Kir6.2 complex K+ channel expressed in a mammalian cell line, HEK293T cells. Pflugers Archiv. 1998;435:595–603. doi: 10.1007/s004240050559. [DOI] [PubMed] [Google Scholar]
- 312.Ottolia M, Platano D, Qin N, Noceti F, Birnbaumer M, Toro L, Birnbaumer L, Stefani E, Olcese R. Functional coupling between human E-type Ca2+ channels and mu opioid receptors expressed in Xenopus oocytes. FEBS Lett. 1998;427:96–102. doi: 10.1016/s0014-5793(98)00401-3. [DOI] [PubMed] [Google Scholar]
- 313.Ottolia M, Toro L. Reconstitution in lipid bilayers of an ATP-sensitive K+ channel from pig coronary smooth muscle. J Membr Biol. 1996;153:203–209. doi: 10.1007/s002329900123. [DOI] [PubMed] [Google Scholar]
- 314.Pang CY, Neligan P, Xu H, He W, Zhong A, Hopper R, Forrest CR. Role of ATP-sensitive K+ channels in ischemic preconditioning of skeletal muscle against infarction. Am J Physiol. 1997;273:H44–51. doi: 10.1152/ajpheart.1997.273.1.H44. [DOI] [PubMed] [Google Scholar]
- 315.Pang CY, Yang RZ, Zhong A, Xu N, Boyd B, Forrest CR. Acute ischaemic preconditioning protects against skeletal muscle infarction in the pig. Cardiovasc Res. 1995;29:782–788. [PubMed] [Google Scholar]
- 316.Park WS, Han J, Kim N, Youm JB, Joo H, Kim HK, Ko JH, Earm YE. Endothelin-1 inhibits inward rectifier K+ channels in rabbit coronary arterial smooth muscle cells through protein kinase C. J Cardiovasc Pharmacol. 2005;46:681–689. doi: 10.1097/01.fjc.0000182846.08357.ed. [DOI] [PubMed] [Google Scholar]
- 317.Park WS, Ko EA, Han J, Kim N, Earm YE. Endothelin-1 acts via protein kinase C to block KATP channels in rabbit coronary and pulmonary arterial smooth muscle cells. J Cardiovasc Pharmacol. 2005;45:99–108. doi: 10.1097/01.fjc.0000150442.49051.f7. [DOI] [PubMed] [Google Scholar]
- 318.Pasyk EA, Kang Y, Huang X, Cui N, Sheu L, Gaisano HY. Syntaxin-1A binds the nucleotide-binding folds of sulphonylurea receptor 1 to regulate the KATP channel. J Biol Chem. 2004;279:4234–4240. doi: 10.1074/jbc.M309667200. [DOI] [PubMed] [Google Scholar]
- 319.Paucek P, Yarov-Yarovoy V, Sun X, Garlid KD. Inhibition of the Mitochondrial KATP Channel by Long-chain Acyl-CoA Esters and Activation by Guanine Nucleotides. J Biol Chem. 1996;271:32084–32088. doi: 10.1074/jbc.271.50.32084. [DOI] [PubMed] [Google Scholar]
- 320.Pearson ER, Flechtner I, Njolstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, Shield J, Robert JJ, Holst JJ, Clark PM, Ellard S, Sovik O, Polak M, Hattersley AT Neonatal Diabetes International Collaborative G. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. New Engl J Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 321.Pegan S, Arrabit C, Zhou W, Kwiatkowski W, Collins A, Slesinger PA, Choe S. Cytoplasmic domain structures of Kir2.1 and Kir3.1 show sites for modulating gating and rectification. Nature Neuroscience. 2005;8:279–278. doi: 10.1038/nn1411. [DOI] [PubMed] [Google Scholar]
- 322.Philip-Couderc P, Tavares NI, Roatti A, Lerch R, Montessuit C, Baertschi AJ. Forkhead Transcription Factors Coordinate Expression of Myocardial KATP Channel Subunits and Energy Metabolism. Circ Res. 2008;102:e20–35. doi: 10.1161/CIRCRESAHA.107.166744. [DOI] [PubMed] [Google Scholar]
- 323.Pinney SE, MacMullen C, Becker S, Lin YW, Hanna C, Thornton P, Ganguly A, Shyng SL, Stanley CA. Clinical characteristics and biochemical mechanisms of congenital hyperinsulinism associated with dominant KATP channel mutations. J Clin Invest. 2008;118:2877–2886. doi: 10.1172/JCI35414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Poitry S, van Bever L, Coppex F, Roatti A, Baertschi AJ. Differential sensitivity of atrial and ventricular K(ATP) channels to metabolic inhibition. Cardiovasc Res. 2003;57:468–476. doi: 10.1016/s0008-6363(02)00715-0. [DOI] [PubMed] [Google Scholar]
- 325.Pountney DJ, Sun ZQ, Porter LM, Nitabach MN, Nakamura TY, Holmes D, Rosner E, Kaneko M, Manaris T, Holmes TC, Coetzee WA. Is the molecular composition of K(ATP) channels more complex than originally thought? J Mol Cell Cardiol. 2001;33:1541–1546. doi: 10.1006/jmcc.2001.1407. [DOI] [PubMed] [Google Scholar]
- 326.Priebe L, Beuckelmann DJ. Cell swelling causes the action potential duration to shorten in guinea-pig ventricular myocytes by activating IKATP. Pflugers Arch. 1998;436:894–898. doi: 10.1007/pl00008087. [DOI] [PubMed] [Google Scholar]
- 327.Priebe L, Friedrich M, Benndorf K. Functional interaction between K(ATP) channels and the Na(+)-K(+) pump in metabolically inhibited heart cells of the guinea-pig. J Physiol. 1996;492:405–417. doi: 10.1113/jphysiol.1996.sp021317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Pu J-L, Ye B, Kroboth SL, McNally EM, Makielski JC, Shi N-Q. Cardiac sulfonylurea receptor short form-based channels confer a glibenclamide-insensitive KATP activity. J Mol Cell Cardiol. 2008;44:188–200. doi: 10.1016/j.yjmcc.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Pu JL, Ye B, Kroboth SL, McNally EM, Makielski JC, Shi NQ. Cardiac sulfonylurea receptor short form-based channels confer a glibenclamide-insensitive KATP activity. J Mol Cell Cardiol. 2008;44:188–200. doi: 10.1016/j.yjmcc.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Pulido N, Casla A, Suarez A, Casanova B, Arrieta FJ, Rovira A. Sulphonylurea stimulates glucose uptake in rats through an ATP-sensitive K+ channel dependent mechanism. Diabetologia. 1996;39:22–27. doi: 10.1007/BF00400409. [DOI] [PubMed] [Google Scholar]
- 331.Quasthoff S, Spuler A, Lehmann HF, Grafe P. Cromakalim, pinacidil and RP 49356 activate a tolbutamide-sensitive K+ conductance in human skeletal muscle fibres. Pflugers Archiv. 1989 doi: 10.1007/BF00582294. [DOI] [PubMed] [Google Scholar]
- 332.Quayle JM, Bonev AD, Brayden JE, Nelson MT. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J Physiol. 1994;475:9–13. doi: 10.1113/jphysiol.1994.sp020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Quayle JM, Bonev AD, Brayden JE, Nelson MT. Pharmacology of ATP-sensitive K+ currents in smooth muscle cells from rabbit mesenteric artery. Am J Physiol. 1995;269:C1112–1118. doi: 10.1152/ajpcell.1995.269.5.C1112. [DOI] [PubMed] [Google Scholar]
- 334.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- 335.Quinn KV, Cui Y, Giblin JP, Clapp LH, Tinker A. Do anionic phospholipids serve as cofactors or second messengers for the regulation of activity of cloned ATP-sensitive K+ channels? Circ Res. 2003;93:646–655. doi: 10.1161/01.RES.0000095247.81449.8E. [DOI] [PubMed] [Google Scholar]
- 336.Quinn KV, Giblin JP, Tinker A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ Res. 2004;94:1359–1366. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- 337.Rajashree R, Koster JC, Markova KP, Nichols CG, Hofmann PA. Contractility and ischemic response of hearts from transgenic mice with altered sarcolemmal K(ATP) channels. A J Physiol. 2002;283:584–590. doi: 10.1152/ajpheart.00107.2002. [DOI] [PubMed] [Google Scholar]
- 338.Reid JM, Paterson DJ, Ashcroft FM, Bergel DH. The effect of tolbutamide on cerebral blood flow during hypoxia and hypercapnia in the anaesthetized rat. Pflugers Arch. 1993;425:362–364. doi: 10.1007/BF00374187. [DOI] [PubMed] [Google Scholar]
- 339.Reimann F, Tucker SJ, Proks P, Ashcroft FM. Involvement of the n-terminus of Kir6.2 in coupling to the sulphonylurea receptor. J Physiol. 1999;518:325–336. doi: 10.1111/j.1469-7793.1999.0325p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Remedi MS, Kurata HTASFTWER, Kleinridders A, Tong AJCB, Koster JC, Nichols CG. Secondary consequences of beta-cell inexcitability: Identification and prevention in a murine model of KATP-induced Neonatal Diabetes Mellitus. Cell Metabolism. 2009;9:140–151. doi: 10.1016/j.cmet.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Repunte VP, Nakamura H, Fujita A, Horio Y, Findlay I, Pott L, Kurachi Y. Extracellular links in Kir subunits control the unitary conductance of SUR/Kir6.0 ion channels. EMBO J. 1999;18:3317–3324. doi: 10.1093/emboj/18.12.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Ribalet B, John SA, Weiss JN. Molecular basis for Kir6.2 channel inhibition by adenine nucleotides. Biophys J. 2003;84:266–276. doi: 10.1016/S0006-3495(03)74847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Ribalet B, John SA, Xie LH, Weiss JN. ATP-sensitive K+ channels: regulation of bursting by the sulphonylurea receptor, PIP2 and regions of Kir6.2. J Physiol. 2006;571:303–317. doi: 10.1113/jphysiol.2005.100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Ribalet B, John SA, Xie LH, Weiss JN. Regulation of the ATP-sensitive K channel Kir6.2 by ATP and PIP(2) J Mol Cell Cardiol. 2005;39:71–77. doi: 10.1016/j.yjmcc.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 345.Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol. 1993 doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- 346.Rogers BJ, Standaert ML, Pollet RJ. Direct effects of sulfonylurea agents on glucose transport in the BC3H-1 myocyte. Diabetes. 1987;36:1292–1296. doi: 10.2337/diab.36.11.1292. [DOI] [PubMed] [Google Scholar]
- 347.Rohacs T, Chen J, Prestwich GD, Logothetis DE. Distinct specificities of inwardly rectifying K(+) channels for phosphoinositides. J Biol Chem. 1999;274:36065–36072. doi: 10.1074/jbc.274.51.36065. [DOI] [PubMed] [Google Scholar]
- 348.Saegusa N, Sato T, Saito T, Tamagawa M, Komuro I, Nakaya H. Kir6.2-deficient mice are susceptible to stimulated ANP secretion: KATP channel acts as a negative feedback mechanism? Cardiovasc Res. 2005;67:60–68. doi: 10.1016/j.cardiores.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 349.Sakamoto K, Yamazaki J, Nagao T. 5-Hydroxydecanoate selectively reduces the initial increase in extracellular K+ in ischemic guinea-pig heart. Eur J Pharmacol. 1998;348:31–35. doi: 10.1016/s0014-2999(98)00238-6. [DOI] [PubMed] [Google Scholar]
- 350.Sakura H, Trapp S, Liss B, Ashcroft FM. Altered functional properties of KATP channel conferred by a novel splice variant of SUR1. J Physiol. 1999;521(Pt 2):337–350. doi: 10.1111/j.1469-7793.1999.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Samaha FF, Heineman FW, Ince C, Fleming J, Balaban RS. ATP-sensitive potassium channel is essential to maintain basal coronary vascular tone in vivo. Am J Physiol. 1992;262:C1220–1227. doi: 10.1152/ajpcell.1992.262.5.C1220. [DOI] [PubMed] [Google Scholar]
- 352.Sampson LJ, Davies LM, Barrett-Jolley R, Standen NB, Dart C. Angiotensin II-activated protein kinase C targets caveolae to inhibit aortic ATP-sensitive potassium channels. Cardiovasc Res. 2007;76:61–70. doi: 10.1016/j.cardiores.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 353.Sampson LJ, Hayabuchi Y, Standen NB, Dart C. Caveolae localize protein kinase A signaling to arterial ATP-sensitive potassium channels. Circ Res. 2004;95:1012–1018. doi: 10.1161/01.RES.0000148634.47095.ab. [DOI] [PubMed] [Google Scholar]
- 354.Sanborn BM. Ion channels and the control of myometrial electrical activity. Semin Perinatol. 1995;19:31–40. doi: 10.1016/s0146-0005(95)80045-x. [DOI] [PubMed] [Google Scholar]
- 355.Sato T, Sasaki N, Seharaseyon J, O’Rourke B, Marban E. Selective pharmacological agents implicate mitochondrial but not sarcolemmal K(ATP) channels in ischemic cardioprotection. Circulation. 2000;101:2418–2423. doi: 10.1161/01.cir.101.20.2418. [DOI] [PubMed] [Google Scholar]
- 356.Satoh E, Yamada M, Kondo C, Repunte VP, Horio Y, Iijima T, Kurachi Y. Intracellular nucleotide-mediated gating of SUR/Kir6.0 complex potassium channels expressed in a mammalian cell line and its modification by pinacidil. J Physiol. 1998;511:663–674. doi: 10.1111/j.1469-7793.1998.663bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Schafer C, Ladilov Y, Inserte J, Schafer M, Haffner S, Garcia-Dorado D, Piper HM. Role of the reverse mode of the Na+/Ca2+ exchanger in reoxygenation-induced cardiomyocyte injury. Cardiovasc Res. 2001;51:241–250. doi: 10.1016/s0008-6363(01)00282-6. [DOI] [PubMed] [Google Scholar]
- 358.Schulze D, Krauter T, Fritzenschaft H, Soom M, Baukrowitz T. Phosphatidylinositol 4,5-bisphosphate (PIP2) modulation of ATP and pH sensitivity in Kir channels. A tale of an active and a silent PIP2 site in the N terminus. J Biol Chem. 2003;278:10500–10505. doi: 10.1074/jbc.M208413200. [DOI] [PubMed] [Google Scholar]
- 359.Schwanstecher M, Sieverding C, Dorschner H, Gross I, Aguilar-Bryan L, Schwanstecher C, Bryan J. Potassium channel openers require ATP to bind to and act through sulfonylurea receptors. EMBO Journal. 1998;17:5529–5535. doi: 10.1093/emboj/17.19.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, Bryan J. Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J Biol Chem. 2000;275:9270–9277. doi: 10.1074/jbc.275.13.9270. [DOI] [PubMed] [Google Scholar]
- 361.Seharaseyon J, Sasaki N, Ohler A, Sato T, Fraser H, Johns DC, O’Rourke B, Marban E. Evidence against functional heteromultimerization of the KATP channel subunits Kir6.1 and Kir6.2. J Biol Chem. 2000;275:17561–17565. doi: 10.1074/jbc.275.23.17561. [DOI] [PubMed] [Google Scholar]
- 362.Seino S, Iwanaga T, Nagashima K, Miki T. Diverse roles of K(ATP) channels learned from Kir6.2 genetically engineered mice. Diabetes. 2000;49:311–318. doi: 10.2337/diabetes.49.3.311. [DOI] [PubMed] [Google Scholar]
- 363.Selivanov VA, Alekseev AE, Hodgson DM, Dzeja PP, Terzic A. Nucleotide-gated KATP channels integrated with creatine and adenylate kinases: amplification, tuning and sensing of energetic signals in the compartmentalized cellular environment. Mol Cell Biochem. 2004;256–257:243–256. doi: 10.1023/b:mcbi.0000009872.35940.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Shaw RM, Rudy Y. Electrophysiologic effects of acute myocardial ischemia: a theoretical study of altered cell excitability and action potential duration [see comments] Cardiovasc Res. 1997;35:256–272. doi: 10.1016/s0008-6363(97)00093-x. [DOI] [PubMed] [Google Scholar]
- 365.Shi N-Q, Ye B, Makielski JC. Function and distribution of the SUR isoforms and splice variants. J Mol Cell Cardiol. 2005;39:51–60. doi: 10.1016/j.yjmcc.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 366.Shi Y, Cui N, Shi W, Jiang C. A short motif in Kir6.1 consisting of four phosphorylation repeats underlies the vascular KATP channel inhibition by protein kinase C. J Biol Chem. 2008;283:2488–2494. doi: 10.1074/jbc.M708769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, Rojas A, Ha BT, Jiang C. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol. 2007;293:R1205–1214. doi: 10.1152/ajpregu.00337.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y, Seino S. Interaction of ATP Sensor, cAMP Sensor, Ca2+ Sensor, and Voltage-dependent Ca2+ Channel in Insulin Granule Exocytosis. J Biol Chem. 2004;279:7956–7961. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- 369.Shigematsu S, Sato T, Abe T, Saikawa T, Sakata T, Arita M. Pharmacological Evidence for the Persistent Activation of ATP-Sensitive K+ Channels in Early Phase of Reperfusion and Its Protective Role Against Myocardial Stunning. Circulation. 1995;92:2266–2275. doi: 10.1161/01.cir.92.8.2266. [DOI] [PubMed] [Google Scholar]
- 370.Shimoda LA, Sylvester JT, Sham JS. Inhibition of voltage-gated K+ current in rat intrapulmonary arterial myocytes by endothelin-1. Am J Physiol. 1998;274:L842–853. doi: 10.1152/ajplung.1998.274.5.L842. [DOI] [PubMed] [Google Scholar]
- 371.Shiota C, Larsson O, Shelton KD, Shiota M, Efanov AM, Hoy M, Lindner J, Kooptiwut S, Juntti-Berggren L, Gromada J, Berggren PO, Magnuson MA. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J Biol Chem. 2002;277:37176–37183. doi: 10.1074/jbc.M206757200. [DOI] [PubMed] [Google Scholar]
- 372.Shyng S, Ferrigni T, Nichols CG. Regulation of KATP channel activity by diazoxide and MgADP. Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J Gen Physiol. 1997;110:643–654. doi: 10.1085/jgp.110.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Shyng S, Nichols CG. Octameric stoichiometry of the KATP channel complex. J Gen Physiol. 1997;110:655–664. doi: 10.1085/jgp.110.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Shyng SL, Cukras CA, Harwood J, Nichols CG. Structural determinants of PIP(2) regulation of inward rectifier K(ATP) channels [In Process Citation] J Gen Physiol. 2000;116:599–608. doi: 10.1085/jgp.116.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- 376.Silberberg SD, van Breemen C. A potassium current activated by lemakalim and metabolic inhibition in rabbit mesenteric artery. Pflugers Arch. 1992;420:118–120. doi: 10.1007/BF00378653. [DOI] [PubMed] [Google Scholar]
- 377.Sim JH, Yang DK, Kim YC, Park SJ, Kang TM, So I, Kim KW. ATP-sensitive K(+) channels composed of Kir6.1 and SUR2B subunits in guinea pig gastric myocytes. Am J Physiol. 2002;282:G137–144. doi: 10.1152/ajpgi.00057x.2002. [DOI] [PubMed] [Google Scholar]
- 378.Singh H, Hudman D, Lawrence CL, Rainbow RD, Lodwick D, Norman RI. Distribution of Kir6.0 and SUR2 ATP-sensitive potassium channel subunits in isolated ventricular myocytes. J Mol Cell Cardiol. 2003;35:445–459. doi: 10.1016/s0022-2828(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 379.Slingerland AS, Hattersley AT. Mutations in the Kir6.2 subunit of the KATP channel and permanent neonatal diabetes: new insights and new treatment. Annals of Medicine. 2005;37:186–195. doi: 10.1080/07853890510007287. [DOI] [PubMed] [Google Scholar]
- 380.Smith PC, Karpowich N, Millen L, Moody JE, Rosen J, Thomas PJ, Hunt JF. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Molecular Cell. 2002;10:139–149. doi: 10.1016/s1097-2765(02)00576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Soltysinska E, Olesen SP, Christ T, Wettwer E, Varro A, Grunnet M, Jespersen T. Transmural expression of ion channels and transporters in human nondiseased and end-stage failing hearts. Pflugers Arch. 2009;459:11–23. doi: 10.1007/s00424-009-0718-3. [DOI] [PubMed] [Google Scholar]
- 382.Spruce AE, Standen NB, Stanfield PR. Studies of the unitary properties of adenosine-5′-triphosphate-regulated potassium channels of frog skeletal muscle. J Physiol. 1987;382:213–236. doi: 10.1113/jphysiol.1987.sp016364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Spruce AE, Standen NB, Stanfield PR. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985;316:736–738. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- 384.Standen NB, Pettit AI, Davies NW, Stanfield PR. Activation of ATP-dependent K+ currents in intact skeletal muscle fibres by reduced intracellular pH. Proc Biol Sci. 1992;247:195–198. doi: 10.1098/rspb.1992.0028. [DOI] [PubMed] [Google Scholar]
- 385.Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- 386.Standing VF, Foy JM. The effect of glibenclamide on glucose uptake in the isolated rat diaphragm. Postgrad Med J. 1970;(Suppl):16–20. [PubMed] [Google Scholar]
- 387.Stoller D, Kakkar R, Smelley M, Chalupsky K, Earley JU, Shi NQ, Makielski JC, McNally EM. Mice lacking sulfonylurea receptor 2 (SUR2) ATP-sensitive potassium channels are resistant to acute cardiovascular stress. J Mol Cell Cardiol. 2007;43:445–454. doi: 10.1016/j.yjmcc.2007.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Stoller D, Pytel P, Katz S, Earley JU, Collins K, Metcalfe J, Lang RM, McNally EM. Impaired exercise tolerance and skeletal muscle myopathy in sulfonylurea receptor-2 mutant mice. Am J Physiol. 2009;297:R1144–1153. doi: 10.1152/ajpregu.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Suffredini AF, Fromm RE, Parker MM, Brenner M, Kovacs JA, Wesley RA, Parrillo JE. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989;321:280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- 390.Suzuki M, Kotake K, Fujikura K, Inagaki N, Suzuki T, Gonoi T, Seino S, Takata K. Kir6.1: a possible subunit of ATP-sensitive K+ channels in mitochondria. Biochem Biophys Res Comm. 1997;241:693–697. doi: 10.1006/bbrc.1997.7891. [DOI] [PubMed] [Google Scholar]
- 391.Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Ogura T, Seino S, Marban E, Nakaya H. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res. 2001;88:570–577. doi: 10.1161/01.res.88.6.570. [DOI] [PubMed] [Google Scholar]
- 392.Suzuki M, Saito T, Sato T, Tamagawa M, Miki T, Seino S, Nakaya H. Cardioprotective Effect of Diazoxide Is Mediated by Activation of Sarcolemmal but Not Mitochondrial ATP-Sensitive Potassium Channels in Mice. Circulation. 2003;107:682–685. doi: 10.1161/01.cir.0000055187.67365.81. [DOI] [PubMed] [Google Scholar]
- 393.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Taguchi H, Heistad DD, Kitazono T, Faraci FM. ATP-sensitive K+ channels mediate dilatation of cerebral arterioles during hypoxia. Circ Res. 1994;74:1005–1008. doi: 10.1161/01.res.74.5.1005. [DOI] [PubMed] [Google Scholar]
- 395.Taniguchi J, Noma A, Irisawa H. Modification of the cardiac action potential by intracellular injection of adenosine triphosphate and related substances in guinea pig single ventricular cells. Circ Res. 1983;53:131–139. doi: 10.1161/01.res.53.2.131. [DOI] [PubMed] [Google Scholar]
- 396.Teramoto N, Creed KE, Brading AF. Activity of glibenclamide-sensitive K+ channels under unstimulated conditions in smooth muscle cells of pig proximal urethra. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:418–424. doi: 10.1007/pl00005071. [DOI] [PubMed] [Google Scholar]
- 397.Teramoto N, McMurray G, Brading AF. Effects of levcromakalim and nucleoside diphosphates on glibenclamide-sensitive K+ channels in pig urethral myocytes. Br J Pharmacol. 1997;120:1229–1240. doi: 10.1038/sj.bjp.0701033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Teramoto N, Yunoki T, Tanaka K, Takano M, Masaki I, Yonemitsu Y, Sueishi K, Ito Y. The effects of caffeine on ATP-sensitive K(+) channels in smooth muscle cells from pig urethra. Br J Pharmacol. 2000;131:505–513. doi: 10.1038/sj.bjp.0703586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Teramoto N, Zhu HL, Shibata A, Aishima M, Walsh EJ, Nagao M, Cole WC. ATP-sensitive K+ channels in pig urethral smooth muscle cells are heteromultimers of Kir6.1 and Kir6.2. Am J Physiol. 2009;296:F107–117. doi: 10.1152/ajprenal.90440.2008. [DOI] [PubMed] [Google Scholar]
- 400.Thabet M, Miki T, Seino S, Renaud J-M. Treadmill running causes significant fiber damage in skeletal muscle of KATP channel-deficient mice. Physiol Genomics. 2005;22:204–212. doi: 10.1152/physiolgenomics.00064.2005. [DOI] [PubMed] [Google Scholar]
- 401.Thien TA, Huysmans FT, Gerlag PG, Koene RA, Wijdeveld PG. Diazoxide infusion in severe hypertension and hypertensive crisis. Clin Pharmacol Ther. 1979;25:795–799. doi: 10.1002/cpt1979256795. [DOI] [PubMed] [Google Scholar]
- 402.Thorneloe KS, Maruyama Y, Malcolm AT, Light PE, Walsh MP, Cole WC. Protein kinase C modulation of recombinant ATP-sensitive K(+) channels composed of Kir6.1 and/or Kir6.2 expressed with SUR2B. J Physiol. 2002;541:65–80. doi: 10.1113/jphysiol.2002.018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Tivesten A, Barlind A, Caidahl K, Klintland N, Cittadini A, Ohlsson C, Isgaard J. Growth hormone-induced blood pressure decrease is associated with increased mRNA levels of the vascular smooth muscle KATP channel. J Endocrinol. 2004;183:195–202. doi: 10.1677/joe.1.05726. [DOI] [PubMed] [Google Scholar]
- 404.Tomita H, Sasaki S, Osanai T, Nakano T, Higuma T, Yokoyama J, Hanada H, Yasujima M, Okumura K. Mutational analysis of Kir6.1 in Japanese patients with coronary spastic angina. Int J Mol Med. 2006;18:589–591. [PubMed] [Google Scholar]
- 405.Tong X, Porter LM, Liu G, Dhar-Chowdhury P, Srivastava S, Pountney DJ, Yoshida H, Artman M, Fishman GI, Yu C, Iyer R, Morley GE, Gutstein DE, Coetzee WA. Consequences of cardiac myocyte-specific ablation of KATP channels in transgenic mice expressing dominant negative Kir6 subunits. Am J Physiol. 2006;291:H543–551. doi: 10.1152/ajpheart.00051.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Trapp S, Haider S, Jones P, Sansom MS, Ashcroft FM. Identification of residues contributing to the ATP binding site of Kir6.2. EMBO J. 2003;22:2903–2912. doi: 10.1093/emboj/cdg282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Tricarico D, Mele A, Lundquist AL, Desai RR, George AL, Camerino DC. Hybrid assemblies of ATP-sensitive K+ channels determine their muscle-type-dependent biophysical and pharmacological properties. Proc Natl Acad Sci U S A. 2006;103:1118–1123. doi: 10.1073/pnas.0505974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Trube G, Hescheler J. Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflugers Archiv. 1984;401:178–184. doi: 10.1007/BF00583879. [DOI] [PubMed] [Google Scholar]
- 409.Tsiani E, Ramlal T, Leiter L, Klip A, Fantus I. Stimulation of glucose uptake and increased plasma membrane content of glucose transporters in L6 skeletal muscle cells by the sulfonylureas gliclazide and glyburide. Endocrinology. 1995;136:2505–2512. doi: 10.1210/endo.136.6.7750472. [DOI] [PubMed] [Google Scholar]
- 410.Tucker SJ, Gribble FM, Proks P, Trapp S, Ryder TJ, Haug T, Reimann F, Ashcroft FM. Molecular determinants of KATP channel inhibition by ATP. EMBO J. 1998;17:3290–3296. doi: 10.1093/emboj/17.12.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- 412.Ueda K, Komine J, Matsuo M, Seino S, Amachi T. Cooperative binding of ATP and MgADP in the sulfonylurea receptor is modulated by glibenclamide. Proc Natl Acad Sci U S A. 1999;96:1268–1272. doi: 10.1073/pnas.96.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Uhde I, Toman A, Gross I, Schwanstecher C, Schwanstecher M. Identification of the potassium channel opener site on sulfonylurea receptors. J Biol Chem. 1999;274:28079–28082. doi: 10.1074/jbc.274.40.28079. [DOI] [PubMed] [Google Scholar]
- 414.van Bever L, Poitry S, Faure C, Norman RI, Roatti A, Baertschi AJ. Pore loop-mutated rat KIR6.1 and KIR6.2 suppress KATP current in rat cardiomyocytes. Am J Physiol. 2004;287:850–859. doi: 10.1152/ajpheart.00054.2004. [DOI] [PubMed] [Google Scholar]
- 415.Van Lunteren E, Moyer M, Torres A. ATP-sensitive K+ channel blocker glibenclamide and diaphragm fatigue during normoxia and hypoxia. J Appl Physiol. 1998;85:601–608. doi: 10.1152/jappl.1998.85.2.601. [DOI] [PubMed] [Google Scholar]
- 416.Van Wagoner D. Mechanosensitive gating of atrial ATP-sensitive potassium channels. Circ Res. 1993;72:973–983. doi: 10.1161/01.res.72.5.973. [DOI] [PubMed] [Google Scholar]
- 417.Varon J, Marik PE. The diagnosis and management of hypertensive crises. Chest. 2000;118:214–227. doi: 10.1378/chest.118.1.214. [DOI] [PubMed] [Google Scholar]
- 418.Vaughan-Jones RD, Spitzer KW, Swietach P. Intracellular pH regulation in heart. J Mol Cell Cardiol. 2009;46:318–331. doi: 10.1016/j.yjmcc.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 419.Venkatesh N, Lamp ST, Weiss JN. Sulfonylureas, ATP-sensitive K+ channels, and cellular K+ loss during hypoxia, ischemia, and metabolic inhibition in mammalian ventricle. Circ Res. 1991;69:623–637. doi: 10.1161/01.res.69.3.623. [DOI] [PubMed] [Google Scholar]
- 420.Vivaudou MB, Arnoult C, Villaz M. Skeletal muscle ATP-sensitive K+ channels recorded from sarcolemmal blebs of split fibers: ATP inhibition is reduced by magnesium and ADP. J Memb Biol. 1991;122:165–175. doi: 10.1007/BF01872639. [DOI] [PubMed] [Google Scholar]
- 421.Wakatsuki T, Nakaya Y, Inoue I. Vasopressin modulates K(+)-channel activities of cultured smooth muscle cells from porcine coronary artery. Am J Physiol. 1992;263:H491–496. doi: 10.1152/ajpheart.1992.263.2.H491. [DOI] [PubMed] [Google Scholar]
- 422.Wasada T. Adenosine triphosphate-sensitive potassium (K(ATP)) channel activity is coupled with insulin resistance in obesity and type 2 diabetes mellitus. Intern Med. 2002;41:84–90. doi: 10.2169/internalmedicine.41.84. [DOI] [PubMed] [Google Scholar]
- 423.Wasada T, Yano T, Ohta M, Yui N, Iwamoto Y. ATP-Sensitive potassium channels modulate glucose transport in cultured human skeletal muscle cells. Endocr J. 2001;48:369–375. doi: 10.1507/endocrj.48.369. [DOI] [PubMed] [Google Scholar]
- 424.Weik R, Neumcke B. Effects of potassium channel openers on single potassium channels in mouse skeletal muscle. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:258–263. doi: 10.1007/BF00169435. [DOI] [PubMed] [Google Scholar]
- 425.Weir EK, Olschewski A. Role of ion channels in acute and chronic responses of the pulmonary vasculature to hypoxia. Cardiovasc Res. 2006;71:630–641. doi: 10.1016/j.cardiores.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 426.Weiss JN, Lamp ST. Cardiac ATP-sensitive K+ channels. Evidence for preferential regulation by glycolysis. J Gen Physiol. 1989;94:911–935. doi: 10.1085/jgp.94.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Weiss JN, Lamp ST. Glycolysis preferentially inhibits ATP-sensitive K+ channels in isolated guinea pig cardiac myocytes. Science. 1987;238:67–69. doi: 10.1126/science.2443972. [DOI] [PubMed] [Google Scholar]
- 428.Weiss JN, Venkatesh N, Lamp ST. ATP-sensitive K+ channels and cellular K+ loss in hypoxic and ischaemic mammalian ventricle. J Physiol. 1992;447:649–673. doi: 10.1113/jphysiol.1992.sp019022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Wellman GC, Quayle JM, Standen NB. ATP-sensitive K+ channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. J Physiol. 1998;507:117–129. doi: 10.1111/j.1469-7793.1998.117bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Weselcouch E, Sargent C, Wilde M, Smith M. ATP-sensitive potassium channels and skeletal muscle function in vitro. J Pharmacol Exp Ther. 1993;267:410–416. [PubMed] [Google Scholar]
- 431.Wheeler A, Wang C, Yang K, Fang K, Davis K, Styer AM, Mirshahi U, Moreau C, Revilloud J, Vivaudou M, Liu S, Mirshahi T, Chan KW. Coassembly of Different Sulfonylurea Receptor Subtypes Extends the Phenotypic Diversity of ATP-sensitive Potassium (KATP) Channels. Mol Pharmacol. 2008;74:1333–1344. doi: 10.1124/mol.108.048355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Wiener CM, Dunn A, Sylvester JT. ATP-dependent K+ channels modulate vasoconstrictor responses to severe hypoxia in isolated ferret lungs. J Clin Invest. 1991;88:500–504. doi: 10.1172/JCI115331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun. 2004;322:1178–1191. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 434.Wilson AJ, Jabr RI, Clapp LH. Calcium modulation of vascular smooth muscle ATP-sensitive K(+) channels: role of protein phosphatase-2B. Circ Res. 2000;87:1019–1025. doi: 10.1161/01.res.87.11.1019. [DOI] [PubMed] [Google Scholar]
- 435.Winquist RJ, Heaney LA, Wallace AA, Baskin EP, Stein RB, Garcia ML, Kaczorowski GJ. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989;248:149–156. [PubMed] [Google Scholar]
- 436.Wolleben CD, Sanguinetti MC, Siegl PK. Influence of ATP-sensitive potassium channel modulators on ischemia-induced fibrillation in isolated rat hearts. J Mol Cell Cardiol. 1989;21:783–788. doi: 10.1016/0022-2828(89)90717-7. [DOI] [PubMed] [Google Scholar]
- 437.Wu J, Cui N, Piao H, Wang Y, Xu H, Mao J, Jiang C. Allosteric modulation of the mouse Kir6.2 channel by intracellular H+ and ATP. J Physiol. 2002;543:495–504. doi: 10.1113/jphysiol.2002.025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Xie LH, Horie M, Takano M. Phospholipase C-linked receptors regulate the ATP-sensitive potassium channel by means of phosphatidylinositol 4,5-bisphosphate metabolism. Proc Natl Acad Scie U S A. 1999;96:15292–15297. doi: 10.1073/pnas.96.26.15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Xie LH, John SA, Ribalet B, Weiss JN. Phosphatidylinositol-4,5-bisphosphate (PIP2) regulation of strong inward rectifier Kir2.1 channels: multilevel positive cooperativity. J Physiol. 2008;586:1833–1848. doi: 10.1113/jphysiol.2007.147868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Xu H, Wu J, Cui N, Abdulkadir L, Wang R, Mao J, Giwa LR, Chanchevalap S, Jiang C. Distinct histidine residues control the acid-induced activation and inhibition of the cloned katp channel. J Biol Chem. 2001;276:38690–38696. doi: 10.1074/jbc.M106595200. [DOI] [PubMed] [Google Scholar]
- 441.Xu T, Jiao JH, Pence RA, Baertschi AJ. ATP-sensitive potassium channels regulate stimulated ANF secretion in isolated rat heart. Am J Physiol. 1996;271:H2339–2345. doi: 10.1152/ajpheart.1996.271.6.H2339. [DOI] [PubMed] [Google Scholar]
- 442.Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J Physiol. 1997;499:715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Yamada S, Kane GC, Behfar A, Liu X-K, Dyer RB, Faustino RS, Miki T, Seino S, Terzic A. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J Physiol. 2006;577:1053–1065. doi: 10.1113/jphysiol.2006.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Yang JH, Yang L, Qu Z, Weiss JN. Glycolytic oscillations in isolated rabbit ventricular myocytes. J Biol Chem. 2008;283:36321–36327. doi: 10.1074/jbc.M804794200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Yao Z, Gross G. A comparison of adenosine-induced cardioprotection and ischemic preconditioning in dogs. Efficacy, time course, and role of KATP channels. Circulation. 1994;89:1229–1236. doi: 10.1161/01.cir.89.3.1229. [DOI] [PubMed] [Google Scholar]
- 446.Yao Z, Gross G. Effects of the KATP channel opener bimakalim on coronary blood flow, monophasic action potential duration, and infarct size in dogs. Circulation. 1994;89:1769–1775. doi: 10.1161/01.cir.89.4.1769. [DOI] [PubMed] [Google Scholar]
- 447.Yao Z, Gross GJ. Acetylcholine mimics ischemic preconditioning via a glibenclamide-sensitive mechanism in dogs. Am J Physiol. 1993;264:H2221–H2225. doi: 10.1152/ajpheart.1993.264.6.H2221. [DOI] [PubMed] [Google Scholar]
- 448.Yao Z, Gross GJ. Activation of ATP-sensitive potassium channels lowers threshold for ischemic preconditioning in dogs. Am J Physiol. 1994;267:H1888–1894. doi: 10.1152/ajpheart.1994.267.5.H1888. [DOI] [PubMed] [Google Scholar]
- 449.Ye B, Kroboth SL, Pu JL, Sims JJ, Aggarwal NT, McNally EM, Makielski JC, Shi NQ. Molecular identification and functional characterization of a mitochondrial sulfonylurea receptor 2 splice variant generated by intraexonic splicing. Circ Res. 2009;105:1083–1093. doi: 10.1161/CIRCRESAHA.109.195040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. Antisense oligodeoxynucleotides of sulfonylurea receptors inhibit ATP-sensitive K+ channels in cultured neonatal rat ventricular cells. Pflugers Archiv. 1999;437:400–408. doi: 10.1007/s004240050794. [DOI] [PubMed] [Google Scholar]
- 451.Yoshida H, Feig JE, Morrissey A, Ghiu IA, Artman M, Coetzee WA. K ATP channels of primary human coronary artery endothelial cells consist of a heteromultimeric complex of Kir6.1, Kir6.2, and SUR2B subunits. J Mol Cell Cardiol. 2004;37:857–869. doi: 10.1016/j.yjmcc.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 452.Yuan F, Brandt NR, Pinto JMB, Wasserlauf BJ, Myerburg RJ, Bassett AL. Hypertrophy Decreases Cardiac KATPChannel Responsiveness to Exogenous and Locally Generated (Glycolytic) ATP. J Mol Cell Cardiol. 1997;29:2837–2848. doi: 10.1006/jmcc.1997.0518. [DOI] [PubMed] [Google Scholar]
- 453.Yunoki T, Teramoto N, Ito Y. Functional involvement of sulphonylurea receptor (SUR) type 1 and 2B in the activity of pig urethral ATP-sensitive K+ channels. Br J Pharmacol. 2003;139:652–660. doi: 10.1038/sj.bjp.0705268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Zhang H, Bolton TB. Activation by intracellular GDP, metabolic inhibition and pinacidil of a glibenclamide-sensitive K-channel in smooth muscle cells of rat mesenteric artery. Br J Pharmacol. 1995;114:662–672. doi: 10.1111/j.1476-5381.1995.tb17190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Zhang H, He C, Yan X, Mirshahi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat Cell Biol. 1999;1:183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]
- 456.Zhang HL, Bolton TB. Two types of ATP-sensitive potassium channels in rat portal vein smooth muscle cells. Br J Pharmacol. 1996;118:105–114. doi: 10.1111/j.1476-5381.1996.tb15372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Zhang L, Bonev AD, Mawe GM, Nelson MT. Protein kinase A mediates activation of ATP-sensitive K+ currents by CGRP in gallbladder smooth muscle. Am J Physiol. 1994;267:G494–499. doi: 10.1152/ajpgi.1994.267.3.G494. [DOI] [PubMed] [Google Scholar]
- 458.Zhang L, Bonev AD, Nelson MT, Mawe GM. Activation of ATP-sensitive potassium currents in guinea-pig gall-bladder smooth muscle by the neuropeptide CGRP. J Physiol. 1994;478:483–491. doi: 10.1113/jphysiol.1994.sp020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Zhou M, Tanaka O, Sekiguchi M, He HJ, Yasuoka Y, Itoh H, Kawahara K, Abe H. ATP-sensitive K(+)-channel subunits on the mitochondria and endoplasmic reticulum of rat cardiomyocytes. J Histochem Cytochem. 2005;53:1491–1500. doi: 10.1369/jhc.5A6736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Zingman LV, Alekseev AE, Bienengraeber M, Hodgson D, Karger AB, Dzeja PP, Terzic A. Signaling in channel/enzyme multimers: ATPase transitions in SUR module gate ATP-sensitive K+ conductance. Neuron. 2001;31:233–245. doi: 10.1016/s0896-6273(01)00356-7. [DOI] [PubMed] [Google Scholar]
- 461.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Zingman LV, Hodgson DM, Bienengraeber M, Karger AB, Kathmann EC, Alekseev AE, Terzic A. Tandem function of nucleotide binding domains confers competence to SUR in gating KATP channels. J Biol Chem. 2002;1:1. doi: 10.1074/jbc.M109452200. [DOI] [PubMed] [Google Scholar]
- 463.Zlatkovic J, Arrell DK, Kane GC, Miki T, Seino S, Terzic A. Proteomic profiling of KATP channel-deficient hypertensive heart maps risk for maladaptive cardiomyopathic outcome. PROTEOMICS. 2009;9:1314–1325. doi: 10.1002/pmic.200800718. [DOI] [PMC free article] [PubMed] [Google Scholar]