Abstract
Enterohemorrhagic Escherichia coli O157:H7 (EHEC) is a significant human pathogen that resides in healthy cattle. It is thought that a reduction in the prevalence and numbers of EHEC in cattle will reduce the load of EHEC entering the food chain. To this end, an intervention strategy involving the addition of chitosan microparticles (CM) to feed in order to reduce the carriage of this pathogen in cattle was evaluated. Experiments with individual Holstein calves and a crossover study found that the addition of CM to feed decreased E. coli O157:H7 shedding. In the crossover study, CM resulted in statistically significant reductions in the numbers recovered from rectal swab samples (P < 0.05) and the duration of shedding (P < 0.05). The effects of feeding CM to calves differed, indicating that the optimal levels of CM may differ between animals or that other factors are involved in the interaction between CM and E. coli O157:H7. In vitro studies demonstrated that E. coli O157:H7 binds to CM, suggesting that the reduction in shedding may result at least in part from the binding of positively charged CM to negatively charged E. coli cells. Additional studies are needed to determine the impact of CM feeding on animal production, but the results from this study indicate that supplementing feed with CM reduces the shedding of E. coli O157:H7 in cattle.
INTRODUCTION
Escherichia coli O157:H7 remains a significant cause of food recalls and human illness despite the implementation of government regulations and process interventions to reduce transmission by contaminated foods. Ground beef remains a primary vehicle of food-borne dissemination, but a variety of foods have been involved in outbreaks (21). Cattle are considered a primary source in outbreaks involving nonbeef foods which become contaminated by environmental or waterborne E. coli O157:H7. The low infectious dose of this pathogen (12, 28) requires new or additional intervention strategies to further reduce its prevalence and numbers entering the food chain.
Practices to prevent or reduce E. coli O157:H7 contamination of beef carcasses are primarily applied at the processing level, with the most common treatments being hide washes, trimming of contaminated carcass parts, steam vacuuming, hot water and acid washes, and steam treatment (1, 2, 4, 5). Despite the implementation of these practices, there continue to be significant numbers of recalls and beef-linked illness caused by this pathogen. The prevalence of cattle shedding E. coli O157:H7 prior to processing ranges from 2% to 42%, which correlates with the frequency of carcass contamination (7). One of the challenges to the development of preharvest interventions is the transmission of E. coli O157:H7 between animals and by environmental sources, like contaminated water (8, 27). Competitive exclusion and vaccination have been evaluated as preharvest interventions, and both have been reported to reduce but not eliminate E. coli O157:H7 from cattle (3, 9, 16, 19).
Chitosan has been used to make microparticles (i.e., chitosan microparticles [CM]) and used as an agent of drug and vaccine delivery to the lower intestinal tract (24, 29, 30). As part of a study testing an intervention strategy, control animals receiving CM alone had a pronounced decrease in the shedding of E. coli O157:H7 in cattle. Based upon these observations, follow-up experiments with individual calves and a crossover study found statistically significant reductions in E. coli O157:H7 numbers in rectal swab samples as well as in the duration of shedding. The physical binding of E. coli O157:H7 to CM in vitro may explain in part the mechanism of decreased shedding in cattle.
MATERIALS AND METHODS
Bacterial strains and inoculum preparation.
E. coli O157:H7 strain Food Research Institute-Kaspar culture collection number 47 (FRIK47; original strain designation, EDL933W) was used for cattle inoculations. The strain was cultured overnight (18 h) in Trypticase soy broth (Difco, Detroit, MI) at 37°C with shaking (150 rpm) and diluted 1:1,000 in sterile tap water. The diluted cell suspension, approximately 106 CFU/ml, was used to inoculate calves by the addition of 1.0 ml to the drinking water of each animal (27). In experiments assessing CM binding, E. coli O157:H7 strains FRIK2803 (FRIK47 containing pEYFP) and FRIK2802 (E. coli K-12 containing pEYFP) were used. pEYFP (Clontech) encodes enhanced yellow fluorescent protein (YFP) under the control of the lac promoter (18).
CM preparation.
CM were prepared as described previously (30), with minor modifications. A 1% (wt/vol) chitosan solution was prepared from chitin (crab shells; Sigma Chemical Co., St. Louis, MO) in 2% acetic acid and 1% Tween 80. To facilitate cross-linking, the chitosan solution was stirred and sonicated with the addition of 2 ml of sodium sulfate (10% [wt/vol]). Mixing and sonication were continued for 20 min. The CM were collected by centrifugation (3,000 × g) for 20 min, washed three times with sterile water, and freeze-dried.
Maintenance and inoculation of cattle.
Eight Holstein calves (steers) were screened for O157 shedding, and three (calves 5, 15, and 75) were found to be persistent shedders and used in an initial chitosan feeding study. For the crossover study, weaned Holstein bull calves were purchased from a local farmer and transported to the Livestock Laboratory at the University of Wisconsin—Madison. Calves were treated with BoviShield (Pfizer Animal Health, Exton, PA), tilmicosin phosphate (Micotil 300; Elanco Animal Health, Indianapolis, IN), and/or florfenicol (Nuflor; Schering-Plough Animal Health, Kenilworth, NJ) as needed. Inoculation of the calves did not occur for 24 days following the last injection to allow for clearance of the drugs and to allow the cattle to acclimate to the feed and environment. At the end of the study, cattle were humanely euthanized. The Animal Care and Use Committee of the University of Wisconsin—Madison approved the animal housing and procedures used in this study.
Calves were housed in individual pens within a climate-controlled containment facility (65°F with 50% relative humidity). The pens had grated floors to minimize manure accumulation and facilitate cleaning. Metal gates separated the pens but allowed some contact between calves in adjacent pens. The pens and central walkway were cleaned daily, and feed and water were provided outside the pens with access through headgates in order to minimize fecal contamination and direct human-calf contact. Water containers were rinsed daily to remove residual feed and filled with fresh tap water. Water was provided ad libitum except during the 12 h preceding inoculation. Calves were fed an alfalfa-grain blend that consisted of whole corn (36%), whole oats (18%), oat groats (18%), whole roasted soybeans (14%), alfalfa meal pellets (7.8%), liquid molasses (4%), calcium carbonate (1%), Bovatec premix (0.7%; Hoffman-LaRoche, Inc., Nutley, NJ), salt and trace minerals (0.3%), and vitamins A and D (0.2%). At least three fecal samples were collected from calves prior to inoculation and tested for E. coli O157:H7 to ensure that calves were not shedding this organism. After overnight withdrawal of water, water containers were inoculated with strain FRIK47 at a dose of 106 CFU.
Sample collection.
Shedding was monitored by testing feces and the rectal-anal junction. Fecal samples (ca. 10 g) were obtained by digital rectal retrieval or from a freshly passed fecal pat and placed in a sterile receptacle. A sample of the rectal-anal mucosa was collected with a sterile swab as previously described (6, 23). Feed and water samples were collected in sterile containers (Whirlpack bags or specimen cups). Samples were transported to the laboratory and tested within 2 h of collection to minimize bacterial growth.
Detection and enumeration of E. coli O157:H7 bacteria.
The presence or absence of E. coli O157:H7 in 10 g of feces and swabs of the rectal-anal junction was determined as previously described (11). Fecal samples were resuspended in (1:10-diluted) modified EC (mEC) broth (Difco), and swabs were suspended in 3 ml of mEC broth. Samples were serially diluted in 0.1% (wt/vol) peptone and spread plated on duplicate plates of MacConkey sorbitol agar (Difco) supplemented with cefixime (50 μg/liter; Lederle Labs, Pearl River, NY) and potassium tellurite (2.5 mg/liter; Sigma) (CT-SMAC) (31) to determine the number of E. coli O157:H7 bacteria. Plates were incubated at 42°C for 18 to 24 h, and typical O157 colonies (i.e., sorbitol-negative colonies) that were O157 agglutination positive were enumerated. The minimum detection limit of the direct plating procedure was approximately 100 CFU/g of feces and 30 CFU/swab. Enrichment combined with immunomagnetic separation (IMS) was used for presence or absence determinations on samples that did not yield E. coli O157 by direct plating. Samples were enriched in mEC broth supplemented with novobiocin (20 μg/ml; Sigma) for 18 to 24 h at 37°C with shaking, and E. coli O157:H7 was detected by using immunomagnetic anti-O157 beads as described by the manufacturer (Dynal, Carlsbad, CA). The beads were streaked on CT-SMAC plates and incubated at 42°C for 18 to 24 h. Sorbitol-negative colonies were confirmed for the O157 antigen by latex agglutination (Remel, Lenexa, KS).
Crossover study and statistical analysis.
A randomized, controlled, crossover study was conducted to evaluate the influence of CM-supplemented feed on E. coli O157:H7 shedding in dairy cattle. The measured parameters for each group were compared for two-tailed P values by an unpaired t test. Seven Holstein calves were randomly assigned to receive feed with or without CM. When cattle were treated with CM, 8 g of CM were mixed with 8 lb of feed administered daily for 6 days, starting 2 or 3 days postinfection. Cattle began receiving CM-supplemented or nonsupplemented feed when FRIK47 was detected on two consecutive days in fecal and/or swab samples. Calves were considered free of FRIK47 when both fecal and rectal swab samples tested negative for at least three consecutive days. Once the calves had cleared FRIK47 following the first inoculation, the animals were switched (crossed over) to feed either with or without CM depending upon which feed group they were in for the first inoculation. Shedding of FRIK47 was monitored in daily fecal and rectal swab samples. The duration of shedding was the number of days of a positive fecal or rectal swab sample until three consecutive fecal and rectal swab samples tested negative for FRIK47 by the IMS method. In addition, the numbers of FRIK47 strains in fecal and rectal swab samples were determined during shedding.
In vitro CM binding assay.
Twenty microliters of chitosan microparticles (0.1% [wt/vol]) in phosphate-buffered saline (PBS) and poly-l-lysine (0.1% [wt/vol]; Sigma) was transferred to the wells of a glass slide (hydrophobic Teflon wells, 6-mm diameter; Electron Microscopy Sciences, Hatfield, PA) and air dried. FRIK2802 (E. coli K-12 carrying pEYFP) and FRIK2803 (FRIK47 carrying pEYFP) were grown to exponential growth phase and induced with IPTG (isopropyl-β-d-thiogalactopyranoside; 100 μM) for 3 h prior to harvest. The harvested cells were washed with PBS, and about 4 × 106 cells were added to wells coated with CM, poly-l-lysine, and PBS and incubated in a humid chamber to prevent drying and nonspecific binding of bacteria to the glass slide. Slides were washed 3 times with PBS to remove unbound cells and air dried. For microscopic observation, a drop of antifade reagent (ProLong Gold, Invitrogen) was added to each well and a coverslip (22 by 60 mm; Gold Seal) was placed over the wells. The YFP-expressing cells were observed and enumerated by fluorescence microscopy (BH2 microscope; Olympus, Japan).
RESULTS
CM decreased shedding of E. coli O157:H7 in cattle.
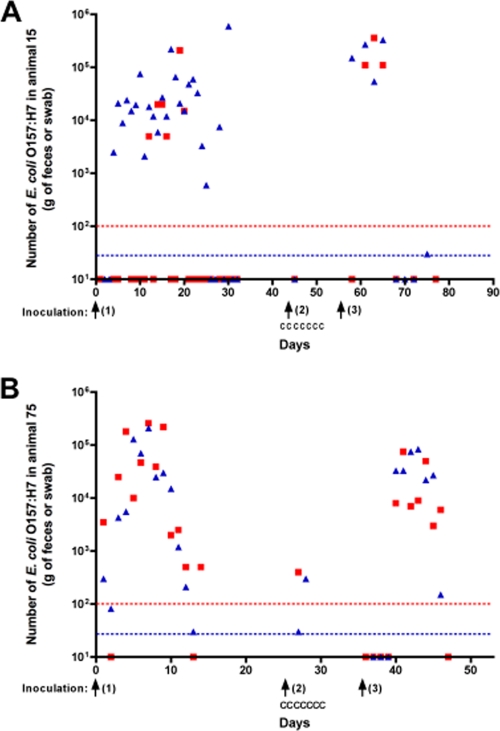
Prior to the start of experiments, the steers were visibly healthy and tested negative for E. coli O157:H7 by IMS. Because variation in the shedding of E. coli O157:H7 in cattle is typical (13, 17) and some cattle do not persistently shed E. coli O157:H7 (26, 27), we screened and selected three calves (calves 5, 15, and 75) that were found to be persistent shedders. These calves were orally inoculated with 106 CFU of FRIK47, and shedding was confirmed at 2 days postinoculation. Calf number 5 was then provided feed (∼8 lb [3,632 g]) supplemented with 8 g of CM. The calf was fed the CM-supplemented feed for 8 days. The remaining two calves (15 and 75) served as positive controls and were provided feed without CM. Fecal and swab samples were collected and analyzed daily until FRIK47 had cleared from the animal (three consecutive negative IMS tests for fecal and rectal swab samples). The positive-control animals, calves 15 and 75, shed FRIK47 for 32 and 14 days, respectively (inoculation 1; Fig. 1), while calf 5 shed FRIK47 for 2 days (data not shown). Necropsy samples of the reticulum, rumen, jejunum, cecum, colon, rectum, and gallbladder also tested negative by IMS, indicating that FRIK47 bacteria were absent or significantly reduced in number in calf 5.
Fig. 1.
Shedding of E. coli O157:H7 (FRIK47) by two Holstein calves and the impact of feed supplemented with chitosan microparticles (CM). Two calves, animal 15 (A) and animal 75 (B), were test negative for E. coli O157:H7 on three consecutive sample days prior to inoculation. CM were fed to the cows for 6 days starting 1 day before inoculation. The numbers of E. coli O157:H7 bacteria were determined by direct plating. Samples negative by direct plating were tested by immunomagnetic separation (IMS) specific for E. coli O157. Numbers of FRIK47 bacteria in feces (red squares) and swabs of the rectal-anal junction (blue triangles) are plotted. The dashed lines indicate the minimum detection levels of direct plating for fecal (red) and rectal swab (blue) samples. Symbols on the x axis indicate a positive IMS test on the respective days. Arrows indicate inoculation with FRIK47. C designates a day that CM were added to feed.
After the clearance of FRIK47 following inoculation 1 (11 consecutive IMS-negative samples [Fig. 1]), calves 15 and 75 were inoculated with strain FRIK47 (inoculation 2). Calf 15 was fed CM-supplemented feed for 6 days starting the day before inoculation. As shown in Fig. 1A, fecal and swab samples from calf 15 tested positive by enrichment on the day following inoculation but tested negative for the next 9 days (Fig. 1A). To ensure that the observed decrease in O157 shedding was not a result of immunity or other responses resulting from inoculation with the same O157 strain, the calf was inoculated again (inoculation 3) and provided feed without CM. Calf 15 shed FRIK47 for 23 days after inoculation 3, demonstrating that the animal was still capable of persistent shedding (Fig. 1A). The numbers of FRIK47 bacteria detected in fecal and swab samples following inoculation 3 ranged from 101 to 3.6 × 105 CFU. In comparison, the numbers in fecal and swab samples following inoculation 1 ranged from 101 to 6.0 × 105 CFU. These results demonstrated that multiple inoculations with FRIK47 had little or no impact on shedding.
Results with calf 75 were similar to those obtained from calf 15. Following inoculation 1, calf 75 shed for 14 days at 101 to 2.2 × 105 CFU per gram or swab when fed unsupplemented feed (Fig. 1B). When calf 75 was provided CM-supplemented feed for 7 days starting the day before inoculation, FRIK47 was isolated from samples on the 2 days after inoculation, and then the samples tested negative for the next 8 days. After inoculation 3, calf 75 shed FRIK47 for 12 days at numbers from 101 to 7.5 × 104 CFU per gram or swab, similar to the results following inoculation 1. Data from these calves suggested that CM reduced shedding of E. coli O157:H7 and provided support for conducting a study with additional cattle.
Crossover study.
A crossover study using eight cattle inoculated with FRIK47 to evaluate if CM feeding had a significant impact on E. coli O157:H7 shedding in cattle was conducted. The use of a randomized and controlled crossover study allowed each calf to act as its own control and therefore has the advantage of eliminating individual differences from treatment effects that are inherent to shedding studies with this organism in cattle (11, 27).
Eight Holstein calves were housed in individual pens in a common room at the Livestock Laboratory at the University of Wisconsin—Madison. The calves were inoculated with approximately 106 CFU of FRIK47 and were randomly grouped to receive feed with or without CM. Cattle began receiving CM-supplemented or nonsupplemented feed after detection of FRIK47 on two consecutive days following inoculation. Animal 16 did not shed FRIK47, indicating that this calf was recalcitrant to FRIK47 colonization. Thus, seven animals were used in the crossover study to evaluate CM feeding. The duration of shedding was the number of days from the start to the end of shedding. The end of shedding was defined as three consecutive days of test-negative samples by IMS. Once the calves had cleared FRIK47 following inoculation 1, they were reinoculated (inoculation 2) and switched to the other feed group (control feed [no CM] or feed with CM).
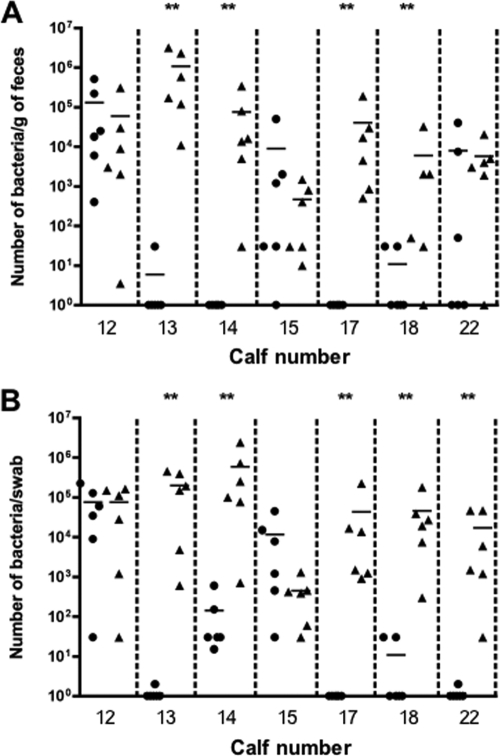
As shown in Table 1, calves shed FRIK47 following inoculation, but the durations of shedding differed. There was a statistically significant reduction in the duration of shedding, as determined by fecal samples (P < 0.005) and swab samples of the rectal-anal junction (P < 0.05), for calves fed the CM-supplemented feed. The total number of days of fecal shedding of FRIK47 decreased for all 7 animals when fed CM, from a mean of 13.80 ± 2.0 days for calves fed the control feed to 3.85 ± 4.2 days for calves fed the CM-supplemented feed. The mean numbers of days of positive rectal swab samples were 12.7 ± 2.7 days for calves fed the control feed and 6.4 ± 6.5 days for calves fed the CM-supplemented feed. These results demonstrated that CM feeding significantly reduced shedding of FRIK47 in calves, although the influence of CM feeding varied between animals. For example, CM feeding had a pronounced effect in calves 13, 14 (fecal samples only), 15, 17, and 18 but had little or no effect in calf 12 and a subtle effect in calf 22. In calf 14, there was no reduction in the duration of shedding as determined by rectal swabs, but all fecal samples tested negative, and this may provide insight into the possible mechanism of action. The variability in results with CM feeding on E. coli O157:H7 shedding is probably a consequence of animal factors.
Table 1.
Crossover study evaluating the effect of CM feeding on the shedding of E. coli O157:H7 in inoculated calvesa
| Calf | Duration of shedding (days)b |
|||
|---|---|---|---|---|
| Without CM feeding |
With CM feeding |
|||
| Fecal samples | Swab samples | Fecal samples | Swab samples | |
| 12 | 15 | 13 | 11 | 14 |
| 13 | 12 | 12 | 1 | 0 |
| 14 | 15 | 15 | 0 | 15 |
| 15 | 13 | 14 | 5 | 8 |
| 17 | 11 | 13 | 0 | 0 |
| 18 | 14 | 15 | 2 | 0 |
| 22 | 17 | 7 | 8 | 8 |
| Mean ± SEc | 13.8 ± 2.0 | 12.7 ± 2.7 | 3.85 ± 4.2 | 6.4 ± 6.5 |
Cattle were inoculated with FRIK47 and shedding was confirmed. Fecal and swab samples were examined for the presence of FRIK47 by direct plating and IMS. CM were fed to the treatment group for 6 days after shedding was established.
The duration of shedding was the number of days of shedding (positive fecal or swab sample) from the first positive sample until FRIK47 was not detected in either fecal or swab samples for three consecutive days.
Data are the mean values for duration of shedding (days) ± standard errors (SE) of the means.
The numbers of FRIK47 bacteria shed during the 6 days of CM feeding was determined by direct plating on CT-SMAC (Fig. 2). There was a statistically significant reduction in the numbers of FRIK47 bacteria in feces of calves 13, 14, 17, and 18 (P < 0.05) (Fig. 2A). In these animals, the numbers were at or below the detection level of the direct plating method (100 CFU/g), but the samples also tested negative by IMS (<1 CFU/g or swab). As a group, the numbers of FRIK47 bacteria in fecal samples were not significantly lower in CM-fed animals. However, there was a significant (P < 0.05) decrease in the numbers of FRIK47 bacteria recovered from swab samples of CM-fed calves when analyzed as a group. For individual calves, significant reductions were observed for calves 13, 14, 17, 18, and 22 (Fig. 2B). There was no effect of CM on fecal and swab samples from calves 12 and 15 (Fig. 2A and B).
Fig. 2.
Results from a crossover study examining the effect of CM addition to feed on shedding of E. coli O157:H7 (FRIK47) in cattle. Calves were inoculated with 106 CFU of FRIK47, and shedding was monitored by direct plating and IMS of fecal (A) and swab (B) samples. CM were added to the feed of half of the animals for 6 days, and the numbers of FRIK47 bacteria in the fecal and swab samples were enumerated. Once shedding had stopped, calves were switched to CM-supplemented feed or the control feed depending upon which group they were assigned to after inoculation 1. The shedding of FRIK47 was monitored daily. Each symbol represents a different sample day (CM-supplemented feed, circles; control feed, triangles). Horizontal bars correspond to the median E. coli O157:H7 CFU/g of feces or swab for an individual animal. Symbols on the x axis represent samples that were test negative by IMS. Asterisks (**) above the column corresponding to a particular animal denote statistically significant differences (P < 0.05) as determined by a t test.
Calves fed a CM-supplemented diet did not exhibit weight loss, decreased feed consumption, abnormal behaviors, or diarrhea. An examination of the intestinal tract and organs of calves during necropsy found no signs of pathology, although additional studies are needed to evaluate growth efficiency or other possible adverse effects from CM feeding. For example, CM may disrupt the normal E. coli populations and other flora that may require that CM feeding be restricted to a few days before harvest.
E. coli O157:H7 binding to CM.
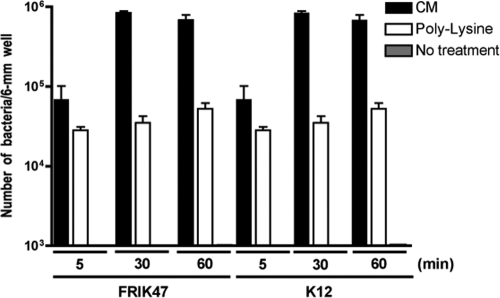
To begin to understand the mechanism of CM-mediated reduction in E. coli O157:H7 shedding, the binding of E. coli O157:H7 to CM was tested. To facilitate cell counting, FRIK47 was transformed with pEYFP, which encodes enhanced YFP under the control of the lac promoter. Exponentially growing cells were induced with IPTG and transferred to the surface of a CM-coated glass slide. After unbound bacteria were removed by washing, the CM-coated slide was examined by using a fluorescence microscope, and the total numbers of bound cells were calculated. As shown in Fig. 3, FRIK2803 (FRIK47 containing pEYFP) bound to the CM-coated slide, while few cells attached to the untreated control slide. The attachment efficiency of the CM-coated slide was about 10 times better than that of the slides coated with poly-l-lysine, which were used as a positive control (10). These results suggest that CM may bind to E. coli O157:H7 in the lumen of cattle and interfere with colonization, including that at the rectal-anal junction. Similar numbers of E. coli K-12 (H48) bacteria also attached to CM-coated slides, indicating that CM do not specifically bind to E. coli O157:H7. The interaction between CM and E. coli is under investigation and may provide insights to maximize the effect of CM feeding on E. coli O157:H7 shedding.
Fig. 3.
Binding of E. coli O157:H7 to CM. FRIK47 bacteria carrying pEYFP grown in the presence of IPTG were harvested and incubated on uncoated glass slides (negative control), slides coated with CM, or slides coated with poly-l-lysine (positive control). Total numbers of bacteria in 6-mm wells were counted by fluorescence microscopy. Results are from three independent experiments. The error bars represent the standard deviations.
DISCUSSION
Current intervention and control practices fail to completely eliminate E. coli O157:H7 from its bovine host and the food supply (19, 21); therefore, new or additional interventions are needed. Results from this study demonstrated that supplementing feed with CM reduced significantly the duration of shedding of E. coli O157:H7 in inoculated cattle. The reduction may be a result of CM binding to E. coli and interfering with colonization or acting as a “scrubbing agent.”
The variation in shedding of E. coli O157:H7 in cattle is typical (13, 17), and some cattle never become persistent shedders even when inoculated or housed with cohorts that are shedding (26, 27). This animal-to-animal variation in shedding of this organism was a primary reason for the use of a crossover study to minimize individual differences from treatment effects. Employing this study design requires that multiple oral inoculations do not induce immunity or change the numbers and duration of shedding of this organism. As shown in Fig. 1, both the duration of shedding and the numbers of FRIK47 bacteria in fecal and swab samples following the first and third inoculations were similar and demonstrated that multiple oral inoculations of FRIK47 had no impact on shedding. Consistent with our observations, previous studies have found that oral inoculation and colonization with E. coli O157:H7 does not trigger a significant immune system response (15, 25).
A significant reduction in the duration of FRIK47 shedding in inoculated calves was observed by examining both rectal swab and fecal samples when cattle were fed CM-supplemented feed. Additionally, CM feeding significantly reduced the numbers of FRIK47 bacteria in rectal swab samples, while the numbers of FRIK47 detected in fecal samples (as a group) were not statistically reduced, although they were lower for four calves. One possible explanation is that E. coli O157:H7 colonizes sites that are inaccessible to CM, like the gallbladder (11), and is periodically released into the lumen. It is also possible that at an optimal level of CM in feed, an effect would have been observed for calves that did not exhibit a significant reduction in fecal shedding (i.e., calves 12, 15, and 22). To support this possibility, calf 22 was still shedding at the end of the crossover study and was fed 16 g CM per day for 2 days (i.e., twice the normal amount added to feed), and both fecal and swab samples tested negative within 3 days of feeding (data not shown). This observation suggests that additional studies to optimize the quantity of CM added to feed are warranted.
Chitosan is derived from chitin and is a linear polymer composed of β-1,4-linked N-acetyl-d-glucosamine and d-glucosamine that is positively charged (24, 29, 30). In vitro binding of FRIK47 to CM may explain how CM feeding reduces shedding in cattle. The binding of CM to E. coli is not surprising, since it is thought that positively charged chitosan molecules bind to negatively charged bacteria. Flagella (H7) have been shown to participate in the attachment and tropism to rectal epithelium cells in vitro (14). However, it is unlikely that H7 flagella are specifically involved in binding to CM, because an E. coli K-12 strain with H48 flagella bound to CM at numbers similar to those obtained with E. coli O157:H7. It is possible that flagella participate in binding to CM in a nonspecific manner, but it is plausible that other surface molecules, probably glycoproteins and pili (22) of both commensal and pathogenic E. coli, contribute to interactions with CM. Although CM were used in these studies, chitosan has antimicrobial activity against a variety of bacteria, yeasts, and molds (20, 24, 29, 30). Preliminary studies of the antibacterial activity of soluble chitosan (in dilute acetic acid) found that chitosan was bacteriostatic to E. coli O157:H7 (data not shown) and bactericidal to E. coli K-12. It is possible that CM influences E. coli O157:H7 shedding by a combination of binding activity and antimicrobial activity of soluble chitosan.
This study identified a possible new intervention strategy to reduce E. coli O157:H7 shedding in cattle. Future studies will determine the optimal level of CM in cattle feed and investigate the mechanism of action of CM on the reduction of E. coli O157:H7 shedding in cattle. Additionally, the possible impact of CM feeding on the shedding of other Gram-negative pathogens like Salmonella spp. will also be investigated.
ACKNOWLEDGMENTS
This work was supported by the American Meat Institute Foundation and in part by the Food Research Institute, College of Agricultural and Life Sciences, University of Wisconsin—Madison.
The assistance of Peter Crump in study design and statistical analyses is greatly appreciated. We thank Merike Seaman for technical support on studies evaluating the antimicrobial activity of chitosan and the staff of the Livestock Laboratory, University of Wisconsin, particularly Terry Jobsis and Kim Trumble. We thank Dongin Park and Thomas Mand for critical discussion and review of the manuscript.
Footnotes
Published ahead of print on 18 February 2011.
REFERENCES
- 1. Algino R. J., Ingham S. C., Zhu J. 2007. Survey of antimicrobial effects of beef carcass intervention treatments in very small state-inspected slaughter plants. J. Food Sci. 72:M173–M179 [DOI] [PubMed] [Google Scholar]
- 2. Bosilevac J. M., Nou X., Barkocy-Gallagher G. A., Arthur T. M., Koohmaraie M. 2006. Treatments using hot water instead of lactic acid reduce levels of aerobic bacteria and Enterobacteriaceae and reduce the prevalence of Escherichia coli O157:H7 on preevisceration beef carcasses. J. Food Prot. 69:1808–1813 [DOI] [PubMed] [Google Scholar]
- 3. Callaway T. R., et al. 2004. What are we doing about Escherichia coli O157:H7 in cattle? J. Anim. Sci. 82(E-Suppl.):E93–E99 [DOI] [PubMed] [Google Scholar]
- 4. Castillo A., Lucia L. M., Goodson K. J., Savell J. W., Acuff G. R. 1998. Comparison of water wash, trimming, and combined hot water and lactic acid treatments for reducing bacteria of fecal origin on beef carcasses. J. Food Prot. 61:823–828 [DOI] [PubMed] [Google Scholar]
- 5. Castillo A., et al. 2001. Lactic acid sprays reduce bacterial pathogens on cold beef carcass surfaces and in subsequently produced ground beef. J. Food Prot. 64:58–62 [DOI] [PubMed] [Google Scholar]
- 6. Davis M. A., et al. 2006. Comparison of cultures from rectoanal-junction mucosal swabs and feces for detection of Escherichia coli O157 in dairy heifers. Appl. Environ. Microbiol. 72:3766–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elder R. O., et al. 2000. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc. Natl. Acad. Sci. U. S. A. 97:2999–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Faith N. G., et al. 1996. Prevalence and clonal nature of Escherichia coli O157:H7 on dairy farms in Wisconsin. Appl. Environ. Microbiol. 62:1519–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fox J. T., et al. 2009. Efficacy of Escherichia coli O157:H7 siderophore receptor/porin proteins-based vaccine in feedlot cattle naturally shedding E. coli O157. Foodborne Pathog. Dis. 6:893–899 [DOI] [PubMed] [Google Scholar]
- 10. Hiraga S., Ichinose C., Niki H., Yamazoe M. 1998. Cell cycle-dependent duplication and bidirectional migration of SeqA-associated DNA-protein complexes in E. coli. Mol. Cell 1:381–387 [DOI] [PubMed] [Google Scholar]
- 11. Jeong K. C., et al. 2007. Isolation of Escherichia coli O157:H7 from the gall bladder of inoculated and naturally-infected cattle. Vet. Microbiol. 119:339–345 [DOI] [PubMed] [Google Scholar]
- 12. Kaper J. B., Nataro J. P., Mobley H. L. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123–140 [DOI] [PubMed] [Google Scholar]
- 13. Low J. C., et al. 2005. Rectal carriage of enterohemorrhagic Escherichia coli O157 in slaughtered cattle. Appl. Environ. Microbiol. 71:93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mahajan A., et al. 2009. An investigation of the expression and adhesin function of H7 flagella in the interaction of Escherichia coli O157 : H7 with bovine intestinal epithelium. Cell. Microbiol. 11:121–137 [DOI] [PubMed] [Google Scholar]
- 15. Matthews L., et al. 2006. Heterogeneous shedding of Escherichia coli O157 in cattle and its implications for control. Proc. Natl. Acad. Sci. U. S. A. 103:547–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McNeilly T. N., et al. 2010. Immunization of cattle with a combination of purified intimin-531, EspA and Tir significantly reduces shedding of Escherichia coli O157:H7 following oral challenge. Vaccine 28:1422–1428 [DOI] [PubMed] [Google Scholar]
- 17. McNeilly T. N., et al. 2008. Escherichia coli O157:H7 colonization in cattle following systemic and mucosal immunization with purified H7 flagellin. Infect. Immun. 76:2594–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ormö M., et al. 1996. Crystal structure of the Aequorea victoria green fluorescent protein. Science 273:1392–1395 [DOI] [PubMed] [Google Scholar]
- 19. Potter A. A., et al. 2004. Decreased shedding of Escherichia coli O157:H7 by cattle following vaccination with type III secreted proteins. Vaccine 22:362–369 [DOI] [PubMed] [Google Scholar]
- 20. Rabea E. I., Badawy M. E., Stevens C. V., Smagghe G., Steurbaut W. 2003. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4:1457–1465 [DOI] [PubMed] [Google Scholar]
- 21. Rangel J. M., Sparling P. H., Crowe C., Griffin P. M., Swerdlow D. L. 2005. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982-2002. Emerg. Infect. Dis. 11:603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rendón M. A., et al. 2007. Commensal and pathogenic Escherichia coli use a common pilus adherence factor for epithelial cell colonization. Proc. Natl. Acad. Sci. U. S. A. 104:10637–10642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rice D. H., Sheng H. Q., Wynia S. A., Hovde C. J. 2003. Rectoanal mucosal swab culture is more sensitive than fecal culture and distinguishes Escherichia coli O157:H7-colonized cattle and those transiently shedding the same organism. J. Clin. Microbiol. 41:4924–4929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sadeghi A. M., et al. 2008. Preparation, characterization and antibacterial activities of chitosan, N-trimethyl chitosan (TMC) and N-diethylmethyl chitosan (DEMC) nanoparticles loaded with insulin using both the ionotropic gelation and polyelectrolyte complexation methods. Int. J. Pharm. 355:299–306 [DOI] [PubMed] [Google Scholar]
- 25. Sanderson M. W., Besser T. E., Gay J. M., Gay C. C., Hancock D. D. 1999. Fecal Escherichia coli O157:H7 shedding patterns of orally inoculated calves. Vet. Microbiol. 69:199–205 [DOI] [PubMed] [Google Scholar]
- 26. Shere J. A., Bartlett K. J., Kaspar C. W. 1998. Longitudinal study of Escherichia coli O157:H7 dissemination on four dairy farms in Wisconsin. Appl. Environ. Microbiol. 64:1390–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shere J. A., et al. 2002. Shedding of Escherichia coli O157:H7 in dairy cattle housed in a confined environment following waterborne inoculation. Appl. Environ. Microbiol. 68:1947–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tilden J., Jr., et al. 1996. A new route of transmission for Escherichia coli: infection from dry fermented salami. Am. J. Public Health 86:1142–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van der Lubben I. M., et al. 2003. Chitosan microparticles for mucosal vaccination against diphtheria: oral and nasal efficacy studies in mice. Vaccine 21:1400–1408 [DOI] [PubMed] [Google Scholar]
- 30. van der Lubben I. M., Verhoef J. C., van Aelst A. C., Borchard G., Junginger H. E. 2001. Chitosan microparticles for oral vaccination: preparation, characterization and preliminary in vivo uptake studies in murine Peyer's patches. Biomaterials 22:687–694 [DOI] [PubMed] [Google Scholar]
- 31. Zadik P. M., Chapman P. A., Siddons C. A. 1993. Use of tellurite for the selection of verocytotoxigenic Escherichia coli O157. J. Med. Microbiol. 39:155–158 [DOI] [PubMed] [Google Scholar]