Abstract
Producing biofuels directly from cellulose, known as consolidated bioprocessing, is believed to reduce costs substantially compared to a process in which cellulose degradation and fermentation to fuel are accomplished in separate steps. Here we present a metabolic engineering example for the development of a Clostridium cellulolyticum strain for isobutanol synthesis directly from cellulose. This strategy exploits the host's natural cellulolytic activity and the amino acid biosynthesis pathway and diverts its 2-keto acid intermediates toward alcohol synthesis. Specifically, we have demonstrated the first production of isobutanol to approximately 660 mg/liter from crystalline cellulose by using this microorganism.
INTRODUCTION
Relative to biofuels produced from corn (starch rich) and sugar cane (sucrose rich), biofuels obtained from cellulosic materials could result in lower fuel costs (16), greater petroleum displacement (13), and lower greenhouse gas emissions (16). To meet its potential, technological advances to improve the conversion efficiency of the recalcitrant lignocellulose to fermentable sugars are needed (29). So far, improvements in cellulase production (17, 20) and pretreatment techniques (1) have aided in increasing cellulose degradation efficiency in a cost-effective manner.
Another approach which has generated much interest is consolidated bioprocessing (CBP). This process utilizes microorganisms to perform biomass hydrolysis and the fermentation of the sugars into biofuel within a single process (16). Research in this area has taken one of two approaches. In one approach, referred to as the “recombinant cellulolytic strategy” (14), microorganisms that have previously demonstrated high biofuel yields are engineered to utilize cellulose and/or the sugars resulting from cellulose degradation. These organisms have been genetically engineered to expand their substrate range to include cellulose or the sugars freed from cellulose or hemicellulose degradation, as in the case of ethanologenic organisms such as Escherichia coli (23, 33), Zymomonas mobilis (4, 18), and Saccharomyces cerevisiae (14, 27). Research efforts continue to improve the strains' cellulolytic abilities to industrially relevant levels. For the “native cellulolytic strategy” (14), research has focused mainly on microorganisms that possess cellulosomes, which are extracellular multienzyme complexes that aid in the digestion of cellulose. While these microorganisms are capable of efficiently hydrolyzing cellulose, their biofuel productivities are significantly lower than those of existing industrial strains. In addition to improving biofuel productivity (22), research efforts are also focused on increasing ethanol yields (31), eliminating competing pathways (26), and improving ethanol tolerance (30).
Most studies employing the native cellulolytic strategy have been conducted with the thermophilic, cellulolytic Clostridium thermocellum. This strain is particularly attractive because it is able to thrive in high-temperature fermentations, which are conducive to high-level substrate conversion, low contamination risk, and high-level product recovery (15). Although C. thermocellum has potential to be a CBP organism, issues such as low transformation efficiency (28) and the lack of publications demonstrating successful overexpression of foreign proteins in C. thermocellum significantly impede the engineering progress of this organism to produce synthetic biofuels, such as isobutanol. One way to hasten this progress is to first establish and optimize the desired metabolic pathways in a closely related, more amenable organism. Once the specifics, such as identifying which genes to overexpress, mutate, and/or delete, have been determined, the same strategy can then be adapted to C. thermocellum. Clostridium cellulolyticum, which was originally isolated from decayed grass (21), is a useful candidate for this initial metabolic engineering work because, like C. thermocellum, it belongs to Clostridium group III, based on 16S rRNA phylogenetic analysis (7), and because it is a mesophile, many problems that are associated with the heterologous expression of proteins in thermophiles are circumvented. In addition, C. cellulolyticum has a sequenced genome (GenBank accession NC_011898.1) and there exist well-established DNA transfer techniques (24) and gene overexpression methods (10) for it. As a potential CBP organism in its own right, C. cellulolyticum can not only utilize cellulose similar to C. thermocellum but also utilize additional sugars freed from hemicellulose degradation, including xylose, arabinose, fructose, galactose, mannose, and ribose (9).
Previously, C. cellulolyticum has been genetically engineered for improved ethanol production (10). Similarly, most of the research concerning the construction of a CBP organism has focused on ethanol production. Despite this, it has been asserted that higher alcohols (i.e., alcohols with more than two carbons), such as isobutanol, are better candidates for gasoline replacement because they have energy density, octane value, and Reid vapor pressure that are more similar to those of gasoline (5). Unlike ethanol, isobutanol can also be blended at any ratio with gasoline or used directly in current engines without modification (8). In this study, we have metabolically engineered C. cellulolyticum to produce isobutanol. By expressing enzymes that direct the conversion of pyruvate to isobutanol by using an engineered valine biosynthesis pathway, we were able to produce up to 660 mg/liter of isobutanol by using C. cellulolyticum growing on crystalline cellulose.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Strains and plasmids used in this study are listed in Table 1. Restriction enzymes, phosphatase (New England BioLabs, Ipswich, MA), ligase (rapid DNA ligation kit; Roche, Mannheim, Germany), and KOD DNA polymerase (EMD Chemicals, San Diego, CA) were used for cloning. Oligonucleotides were synthesized by Eurofins MWG Operon (Huntsville, AL).
Table 1.
List of plasmids and strains used in this study
| Strain or plasmid | Phenotype, genotype, or construct descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| E. coli XL10-Gold | Tetr Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173endA1supE44thi-1recA1gyrA96relA1lac Hte [F′ proABlacIqZΔM15 Tn10 (Tetr) Amy Camr] | Stratagene |
| C. cellulolyticum H10 | ATCC 35319 | ATCC |
| Plasmids | ||
| pAT187 | Kmr; broad-host-range plasmid | 24 |
| pWH159 | Kmr; 5.5-kb EcoRI fragment of pAT187 was ligated with the EcoRI fragment of the PCR product of pAT187; For oligo WH177, Rev oligo WH178 | This study |
| pWH168 | Emr Kmr; ermC was cloned into pWH159 by ligating the AatII-PstI fragment of PCR product; For oligo WH248, Rev oligo WH249, with pECN2 (11) as the template | This study |
| pWH199 | Emr Kmr; the ferredoxin promoter and multiple-cloning site (see Fig. S1 in the supplemental material) were cloned into pWH168 with the BssHII-AgeI fragment of PCR product; For oligo WH194, Rev oligo WH195; the template was synthesized by PCR assembly, using 18 primers (FD1 to FD18) | This study |
| pWH203 (*alsS-ilvC-ilvD) | Emr Kmr; 0.8-kb BamHI-XbaI fragment of pWH315 was cloned into the same sites of pWH277 | This study |
| pWH277 (alsS-ilvC-ilvD) | Emr Kmr; alsS from B. subtilis and ilvCD from E. coli were amplified from pSA69 (2); For oligo WH301, Rev oligo WH302; BamHI-NotI fragment of PCR product ligated into the BamHI and NotI sites of pWH199 | This study |
| pWH278 (alsS) | Emr Kmr; alsS from B. subtilis was amplified from pSA69 (2); For oligo WH261, Rev oligo WH262; BamHI-NotI fragment of PCR product ligated into the same restriction sites as pWH199 | This study |
| pWH314 (alsS-ilvCD-kivd-adhA) | Emr Kmr; kivd from L. lactis and adhA from E. coli were amplified by PCR amplification using pSA65 (2) as a template; For oligo WH886, Rev oligo WH885; NotI-BamHI fragment of PCR product ligated into NotI and BglII sites of pWH203 | This study |
| pWH315 (*alsS-ilvC-ilvD-klvD-adhA) | Emr Kmr; spontaneous mutation in alsS | This study |
| pWH318 (kivd-yqhD) | Emr Kmr; kivd from L. lactis and yqhD from E. coli were amplified by PCR from pCS97 (C. Shen, unpublished); For oligo WH888, Rev oligo WH887; Acc65I-BamHI fragment of PCR product ligated into the Acc65I and BamHI sites of pWH199 | This study |
| pWH320 (kivd-yqhD-alsS-ilvC-ilvD) | Emr Kmr; alsS from B. subtilis and ilvCD from E. coli were amplified from pSA69 (2); For oligo WH900, Rev oligo WH901; SpeI-NotI fragment of PCR product ligated into the XbaI and NotI sites of pWH318 | This study |
Abbreviations: Amy, amylase; Camr, chloramphenicol resistance; For, forward primer; Rev, reverse primer; oligo, oligonucleotide.
Chemicals.
Unless indicated otherwise, commercial reagents, enzymes, and coenzymes were supplied by Sigma Chemical Company (St. Louis, MO).
Media and cultivation.
C. cellulolyticum was grown at 34°C in VM medium (11) that had been modified to reduce precipitation. The medium contained the following components: KH2PO4 (1.0 g/liter), K2HPO4 (3.4 g/liter), urea (2.14 g/liter), MgCl2·6H2O (1.0 g/liter), CaCl2·2H2O (0.15 g/liter), FeSO4·6H2O (1.25 mg/liter), 3-(N-morpholino)propanesulfonic acid (MOPS; 10.0 g/liter), resazurin (2.0 mg/liter), vitamin solution (10 ml/liter), yeast extract (2.0 g/liter), oligoelement solution (1 ml/liter), cysteine-HCl (1.0 g/liter), and cellobiose (5.1345 g/liter). The vitamin solution (100×) contained biotin (0.08 μM), pyridoxamine (0.02 μM), cyanocobalamin (0.001 μM), ρ-aminobenzoic acid (0.15 μM), thiamine (0.9 μM), and l-alanine (0.22 μM). The 1,000× oligoelement solution contained FeSO4·7H2O (5.0 g liter−1), ZnSO4·7H2O (1.44 g liter−1), MnSO4·7H2O (1.12 g liter−1), CuSO4·5H2O (0.25 g liter−1), Na2B4O7 (0.20 g liter−1), (Mo)7(NH4)6O24·4H2O (1.00 g liter−1), NiCl2 (0.04 g liter−1), CoCl2 (0.02 g liter−1), HBO3 (0.03 g liter−1), Na2SeO3 (0.02 g liter−1), and HCl (50 ml of a 10 M concentration).
For agar plates, 0.8% (wt/vol) agar (Difco Laboratories, Detroit, MI) was added to the media. To make competent cells, to prepare cell lysates for enzyme assays and daily maintenance, and to determine isobutanol production on cellobiose, the strains were grown in modified VM medium. To examine isobutanol, lactate, acetate, and ethanol production on cellulose, the strains were grown in modified VM medium, in which cellobiose and yeast extract were replaced with 10 g/liter of crystalline cellulose (50 μm; Sigma type 50).
Stock cultures of C. cellulolyticum were maintained at −80°C in 15% (vol/vol) glycerol and were grown for one transfer in cellobiose medium before the initiation of growth experiments.
Transformation.
Cell transformation was conducted as described previously (11) with some modifications. Cells were grown for 17 to 24 h in 10-ml cultures of modified VM medium to late exponential phase (optical density at 600 nm [OD600], 0.5 to 1.0; 5 × 106 CFU/ml). The following steps were all performed with anoxic solutions under anaerobic conditions at 4°C. The cells were washed twice with cold electroporation buffer (270 mM sucrose, 1 mM MgCl2, 5 mM sodium phosphate buffer, pH 7.4). The cells were resuspended in 600 μl of electroporation buffer. For each transformation, 200 μl of the cells was mixed with 2 μg of MspI-methylated plasmid DNA. The DNA was methylated overnight with 5 units of MspI methyltransferase (New England BioLabs, Ipswich, MA) and then purified with the DNA Clean and Concentrator kit (Zymo Research Inc., Orange, CA). In 2-mm-gap electroporation cuvettes (Molecular BioProducts, San Diego, CA), the cells and plasmid DNA were electroporated (1.5 kV, 25 μF, and 48 Ω) with a Bio-Rad gene pulser apparatus (Bio-Rad Laboratories, Richmond, CA). The electroporated cells were transferred to 10 ml of fresh modified VM medium. The cells were recovered for 24 h at 34°C, then the cells were collected by centrifugation, and cell pellet was spread on modified VM cellobiose agar plates supplemented with 10 μg/ml erythromycin. The plates were incubated at 34°C anaerobically for 5 to 7 days. Single colonies were transferred to 10 ml VM cellobiose medium supplemented with 10 μg/ml erythromycin.
Analytical procedures.
Bacterial growth was measured spectrophotometrically at 600 nm. For cultures containing cellulose, the cellulose was allowed to settle for at least 2 h before samples were taken for measurement.
The produced alcohol compounds were quantified by a gas chromatograph (GC) with a flame ionization detector. The system consisted of a model 5890A GC (Hewlett-Packard, Avondale, PA) and a model 7673A automatic injector, sampler, and controller (Hewlett-Packard). The separation of alcohol compounds was carried out using a DB-WAX capillary column (30 m; 0.32-mm inside diameter; 0.50-μm film thickness) purchased from Agilent Technologies (Santa Clara, CA). The GC oven temperature was initially held at 40°C for 5 min and raised with a gradient of 15°C/min until it reached 120°C. It was then raised with a gradient of 50°C/min until it reached 230°C and held for 4 min. Helium was used as the carrier gas, with 9.3-lb/in2 inlet pressure. The injector and detector were maintained at 225°C. Supernatant of culture broth (0.5 μl) was injected in split injection mode with a 1:15 split ratio. Pentanol was used as the internal standard.
Enzyme assays.
The cells were grown for 17 to 24 h in 50-ml cultures of modified VM medium to late exponential phase (OD600, 0.5 to 1.0; 5 × 106 CFU/ml). The cells were harvested, washed in 50 mM potassium phosphate buffer, pH 7.5, and resuspended in 0.5 ml of the same buffer. Crude extract was prepared under aerobic conditions with 0.1-mm glass beads and a Mini Bead Beater 8 (BioSpec Products, Inc., Bartlesville, OK). Total protein measurements were made with the Bradford protein assay kit from Bio-Rad (Hercules, CA).
The AlsS assay was performed as described previously (32), with the exception that the reaction mixture contained 20 mM sodium pyruvate, 100 mM MOPS buffer, pH 7.0, 1 mM MgCl2, and 100 μM cocarboxylase. The concentration of acetoin produced was determined by a standard curve created using pure acetoin. One specific unit of AlsS activity corresponds to the formation of 1 nmol of acetoin per min per mg of soluble protein at 34°C.
To measure the reduction of 2-acetolactate to 2,3-dihydroxy-isovalerate, the oxidation of NADPH was monitored by a decrease in absorbance at 340 nm. The substrate, 2-acetolactate, was first produced in a separate reaction as described for the Als assay using purified, heterogeneously expressed Bacillus subtilis AlsS in E. coli strain BL21. From this reaction, 180 μl was added to 200 mM potassium phosphate buffer, pH 7.5, 4 mM MgCl2, and 0.1 mM NADPH. The samples were incubated at 34°C for 5 min, and then the reaction was initiated with the addition of cell extracts. The consumption of NADPH was monitored at 340 nm (extinction coefficient, 6.22 mM−1 cm−1). IlvC activity is expressed as nmol of NADPH oxidized per min per mg of soluble protein at 34°C.
The IlvD assay was performed as described previously (12). The 500-μl reaction mixture contained 5 mM MgSO4, 50 mM Tris-Cl, pH 8.0, cell extract, and 10 mM 2,3-dihydroxy-isovalerate. The substrate, 2,3-dihydroxy-isovalerate, was synthesized as described previously (6). After the reaction mixture was preincubated for 5 min at 34°C, the substrate was added to initiate the reaction. The samples were incubated for 15 min at 34°C. The reaction was terminated by the addition of 125 μl of 10% (wt/vol) trichloroacetic acid, and then 250 μl of saturated 2,4-dinitrophenylhydrazine in 2 N HCl was added to the samples. After 20 min at room temperature, 875 μl of 2.5 N NaOH was added and then the samples were incubated for another 30 min at room temperature. The samples were then spun down for 1 min to remove coagulated protein. Sample absorbances were measured at 550 nm. Standard curves based on known amounts of 2-ketoisovalerate were generated. The specific activities were calculated as 1 nmol of 2-ketoisovalerate synthesized per min per mg of soluble protein at 34°C.
The decarboxylation activity of Kivd was assayed as described previously (34), with some modifications. Kivd activity was measured at 34°C by using a coupled enzymatic assay method. ADH6 was isolated as previously described (34). Excess ADH6 was used to reduce aldehyde into alcohol, and concomitantly, cofactor NADPH was oxidized to NADP+. The assay mixture contained 0.2 mM NADPH, 0.1 μM ADH6, and 20 mM 2-ketoisovalerate in assay buffer (50 mM potassium phosphate buffer, pH 7.0, 1 mM MgSO4, 0.5 mM thiamine diphosphate [ThDP]) with a total volume of 0.2 ml. The reactions were started by the addition of the 2-ketoisovalerate. The consumption of NADPH was monitored at 340 nm (extinction coefficient, 6.22 mM−1 cm−1). One specific unit of Kivd activity corresponds to the oxidation of 1 nmol of NADPH per min per mg of soluble protein at 34°C.
To measure the alcohol dehydrogenase (ADH) activities of YqhD and AdhA, the oxidation of NADPH was monitored by a decrease in absorbance at 340 nm. The assay mixture contained 50 mM MOPS, pH 7.0, 25 mM isobutyraldehyde, and 0.2 mM NAD(P)H. The samples were incubated at 34°C for 5 min, and then the reaction was initiated with the addition of cell extracts. The consumption of NAD(P)H was monitored at 340 nm (extinction coefficient, 6.22 mM−1 cm−1). One specific unit of ADH activity corresponds to the oxidation of 1 nmol of NAD(P)H per min per mg of soluble protein at 34°C.
RESULTS
A single base pair insertion in alsS enables expression in C. cellulolyticum.
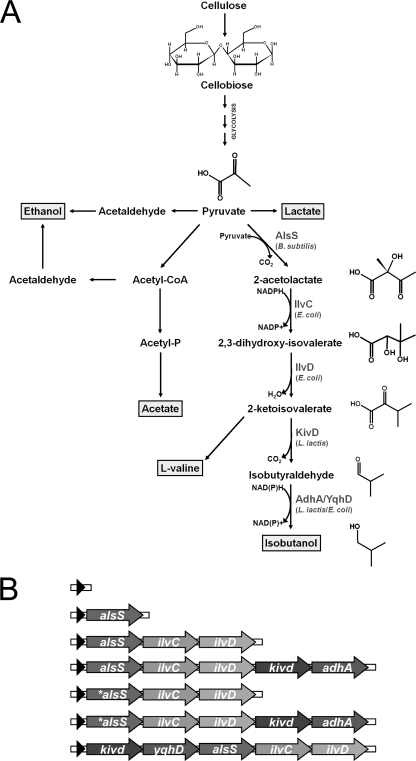
In order to achieve direct isobutanol production from pyruvate, the genes encoding B. subtilis α-acetolactate synthase, E. coli acetohydroxyacid isomeroreductase, E. coli dihydroxy acid dehydratase, Lactococcus lactis ketoacid decarboxylase, and E. coli and L. lactis alcohol dehydrogenases (Fig. 1A) were cloned into a pAT187 derivative plasmid (25). These specific genes were chosen because they were the same genes utilized for isobutanol production in E. coli (2) and Synechococcus elongatus (3). The different combinations of the genes (Fig. 1B) were cloned as single synthetic operons driven by the constitutive ferredoxin (Fd) promoter from Clostridium pasteurianum.
Fig. 1.
The pathway for isobutanol production in C. cellulolyticum (A) and the ferredoxin promoter (black arrow)-driven operons used in this study (B). The asterisk indicates the adenine insertion in the alsS gene sequence.
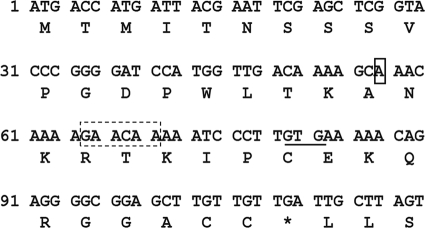
The activities of the first three enzymes in the isobutanol pathway were examined by transforming plasmids expressing alsS or alsS ilvCD into C. cellulolyticum. While C. cellulolyticum was successfully transformed with the empty vector, no C. cellulolyticum alsS or alsS ilvCD transformants were obtained. The same results were observed after repeated transformation efforts. Due to the fact that alsS and alsS ilvCD transformants could not be obtained, the complete isobutanol pathway was then examined. C. cellulolyticum was transformed with a plasmid expressing alsS ilvCD kivd adhA. While transformants were obtained, sequence confirmation of the plasmid revealed that a single adenine insertion, which is not found in the wild-type alsS sequence, was present 54 bp downstream of the start ATG. This single insertion, by shifting the reading frame, results in a downstream premature stop codon (TGA) and, subsequently, a truncated 37-amino-acid protein (Fig. 2). This spontaneous mutation in alsS (*alsS) was found to have originated in the E. coli strain used for cloning.
Fig. 2.
The first 120 bp of the alsS sequence with the adenine insertion mutation. The adenine insertion (solid box), the putative start GTG, which restores the alsS reading frame (underline), the premature stop codon (*), and the putative Shine-Dalgarno sequence (dashed box) are indicated.
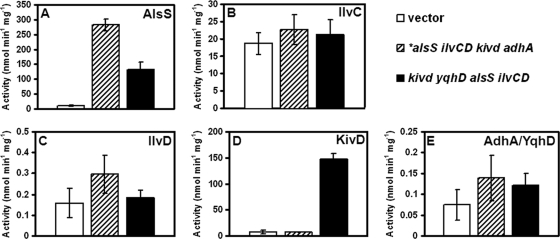
The frameshift mutation in the alsS sequence was a cause for great concern because of the effect it could have on AlsS activity. Thus, to determine the activities of AlsS and the other enzymes expressed from the synthetic operon, enzymatic assays were performed on lysates of the C. cellulolyticum strain expressing *alsS ilvCD kivd adhA (Fig. 3 A). Surprisingly, for the AlsS assay, the *alsS ilvCD kivd adhA lysates were found to demonstrate an activity of 282 nmol min−1 mg−1, which was significantly higher than the 11 nmol min−1 mg−1 demonstrated by the strain transformed with the vector (Fig. 3A). Thus, despite the insertion mutation, the mutant retained a significant level of activity. However, unlike the case for AlsS, we were not able to detect enzymatic activity for IlvC (Fig. 3B), IlvD (Fig. 3C), Kivd (Fig. 3D), or AdhA (Fig. 3E). There were no statistically significant differences between the activities of these enzymes in the lysates of the *alsS ilvCD kivd adhA strain and the vector control strain.
Fig. 3.
Activity assays of isobutanol pathway enzymes for C. cellulolyticum strains expressing the empty vector, *alsS ilvCD kivd adhA, and kivd yqhD alsS ilvCD, determining the activity for AlsS (one specific unit of Als activity corresponds to the formation of 1 nmol of acetoin per min per mg of soluble protein at 34°C) (A), IlvC (one specific unit of IlvC activity corresponds to the oxidation of 1 nmol of NADPH per min per mg of soluble protein at 34°C) (B), IlvD (one specific unit of IlvD activity corresponds to the formation of 1 nmol of 2-ketoisovalerate per min per mg of soluble protein at 34°C) (C), Kivd (one specific unit of Kivd activity corresponds to the oxidation of 1 nmol of NADPH per min per mg of soluble protein at 34°C) (D), and AdhA and YqhD activity [one specific unit of ADH activity corresponds to the oxidation of 1 nmol of NAD(P)H per min per mg of soluble protein at 34°C] (E).
The presence of AlsS activity, despite the stop codon introduced by the frameshift mutation, suggests that the 37-amino-acid truncated protein is not the only translation product. It is likely that an alternate Shine-Dalgarno (SD) sequence and start site are present downstream of the insertion. After examining the sequence, we identified likely candidates for the alternative SD sequence and start site (Fig. 2), which are approximately 8 and 23 bp, respectively, downstream from the adenine insertion. This would result in an AlsS that is 25 amino acids shorter than the wild-type AlsS and explain the activity in the transformants.
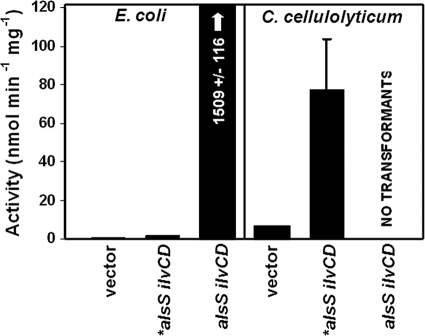
To further analyze the effect of the alsS mutation, we compared the AlsS and *AlsS activities in E. coli because we were unable to obtain a C. cellulolyticum transformant expressing the wild-type alsS. Figure 4 compares the AlsS activities of E. coli strains expressing the *alsS ilvCD and alsS ilvCD constructs. While the *alsS mutant presented no significant activity in E. coli, the wild-type alsS variant demonstrated activity that was approximately 1,000-fold greater than that of the empty vector. This result strongly suggests that the mutation significantly reduces the activity of AlsS. This difference in activity may explain why C. cellulolyticum cannot be transformed with constructs that contain alsS as the first gene in the operon, which was the case for the alsS, alsS ilvCD, and alsS ilvC ilvD kivd adhA strains (Fig. 1B).
Fig. 4.
AlsS activity of E. coli and C. cellulolyticum expressing the vector, the *alsS ilvCD construct, or the alsS ilvCD construct. One specific unit of AlsS activity corresponds to the formation of 1 nmol of acetoin per min per mg of soluble protein at 34°C.
Production of isobutanol from cellobiose and cellulose.
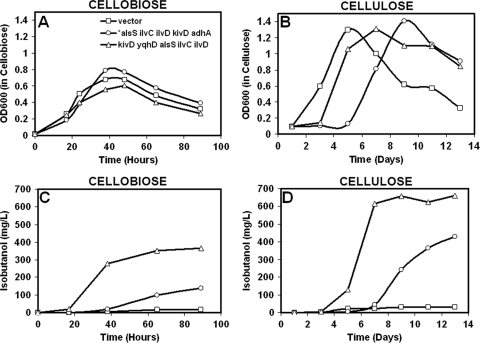
Despite the mutation in alsS, the *alsS ilvCD kivd adhA strain was found to produce isobutanol titers of 140 mg/liter from cellobiose over a period of 90 h (Fig. 5 C) and 420 mg/liter (Fig. 5D) on cellulose over a period of 13 days. These titers are significantly higher than the 17 mg/liter and 30 mg/liter of isobutanol that are produced by the strains transformed with the empty vector on cellobiose and cellulose, respectively (Fig. 5C and D).
Fig. 5.
Growth of C. cellulolyticum strains on cellobiose (A) and cellulose (B) and the isobutanol production (mg/liter) on cellobiose (C) and cellulose (D). The figure shows one data set representative of three independent experiments, with all three showing comparable results.
In order to test our hypothesis of the wild-type AlsS's toxic effect on C. cellulolyticum growth during transformation and to obtain transformants with the wild-type AlsS, it was necessary to decrease the activity of the wild-type AlsS. To achieve this, a kivd yqhD alsS ilvCD strain in which alsS was the third gene in the operon was constructed. Previously, it has been shown that mRNA abundance decreases with increasing distance of the gene from the promoter, irrespective of gene content (19). Specifically, for the operons that were studied, the researchers found that mRNA abundance decreased by approximately 50% from one gene to the next (19). Thus, as alsS was the third gene in the operon, it would be expected that the alsS mRNA abundance would be less than that if alsS was the first gene in the operon. After successful transformation of the kivd yqhD alsS ilvCD strain, the resulting transformants were found to produce up to 364 mg/liter of isobutanol on cellobiose over a period of 90 h (Fig. 5C) and 660 mg/liter of isobutanol on cellulose within 7 to 9 days (Fig. 5D).
Although successful transformation suggested that AlsS activity had been successfully attenuated, enzyme assays were performed to quantify the activity of AlsS and the products of the other genes in the operon. As seen in Fig. 3A, AlsS activity for the strain expressing kivd yqhD alsS ilvCD resulted in a level of AlsS activity approximately 10-fold higher than that of the vector control, with activities of 133 and 11 nmol min−1 mg−1, respectively (Fig. 3A). For Kivd activity, the kivd yqhD alsS ilvCD strain had 19-fold higher Kivd activity than the strain expressing the vector alone, with activities of 147.1 and 7.9 nmol min−1 mg−1, respectively (Fig. 3D). Unlike the cases for AlsS and Kivd, no activity could be detected for IlvC (Fig. 3B), IlvD (Fig. 3C), and YqhD (Fig. 3E) when kivd yqhD alsS ilvCD was expressed. There were no statistically significant differences between the activities of these enzymes when comparing the kivd yqhD alsS ilvCD strain and the vector control strain.
DISCUSSION
Previously, we successfully showed that E. coli can be metabolically engineered to produce isobutanol by manipulating E. coli's amino acid biosynthesis pathway by diverting the 2-keto acid intermediates toward biofuel production (2). Using the same metabolic engineering strategy, we were able to achieve an isobutanol titer of 660 mg/liter by the cellulolytic mesophile C. cellulolyticum by expressing kivd yqhD alsS ilvCD. To our knowledge, this is the first demonstration of isobutanol production directly from cellulose.
We encountered several difficulties with C. cellulolyticum that we did not meet with E. coli regarding the expression of the isobutanol pathway. One of these difficulties arose from the lack of an inducible expression system in C. cellulolyticum. As we were without the ability to control gene expression, the toxicity of some of the genes had a greater effect on the microorganism's growth than they would have otherwise. Specifically, the expression of the gene that encodes acetolactate synthase, alsS, appears to have a toxic effect in C. cellulolyticum, an effect which is evidenced by the lack of alsS, alsS ilvCD, and alsS ilvCD kivd adhA transformants. Moreover, this problem with transformation is alleviated when the amount of alsS mRNA is decreased, as in the case for *alsS, *alsS ilvCD, *alsS ilvCD kivd adhA, and kivd yqhD alsS ilvCD constructs. It is likely that the control conferred by an inducible system would aid in tempering the expression level of AlsS and, subsequently, its inhibitory growth effects.
Another difficulty we encountered was the lack of detectable activity for IlvC, IlvD, and the alcohol dehydrogenases (ADHs) AdhA and YqhD. From the enzyme activity assays (Fig. 3), we were unable to detect activities that were significantly greater than that found for the vector control. However, despite the results of the enzyme assays, it appears that some activity is present. For example, although no Kivd and AdhA activity in C. cellulolyticum transformed with *alsS ilvCD kivd adhA was detected, it appears that there is in vivo activity, as the *alsS ilvCD kivd adhA transformants were found to produce an isobutanol titer of 428 mg/liter, while *alsS ilvCD transformants had a titer of 278 mg/liter. It is not surprising that the lack of these enzyme activities did not preclude isobutanol production, because C. cellulolyticum possesses native enzymes that can perform the same functions. Homologues of ilvC and ilvD are part of C. cellulolyticum's valine biosynthesis pathway, and C. cellulolyticum possesses ADHs for ethanol fermentation. Still, additional IlvC, IlvD, and ADH activity would most likely lead to higher isobutanol titers. Differences between GC contents and codon usage frequencies of C. cellulolyticum and E. coli may explain the lack of expression of the E. coli genes in the host C. cellulolyticum. The utilization of C. cellulolyticum ilvC, ilvD, and ADH genes or the codon optimization of the E. coli genes may resolve this problem.
A significant amount of research has been dedicated to engineering organisms that are capable of consolidated bioprocessing. These CBP organisms are anticipated to have the ability to efficiently degrade cellulose and to convert the resulting sugars to biofuels at high productivities. Toward this goal, the production of isobutanol from cellulose has been shown to be feasible in the mesophilic C. cellulolyticum. Both the successes and problems encountered in establishing this pathway in C. cellulolyticum will aid in the adaptation of this strategy in related cellulolytic thermophiles, such as C. thermocellum and Caldicellulosiruptor bescii.
ACKNOWLEDGMENTS
This work was supported in part by the BioEnergy Science Center (BESC) at Oak Ridge National Laboratory, a Department of Energy Bioenergy Research Center, and by the UCLA-DOE Institute for Genomics and Proteomics.
We thank P. Courvalin from the Institut Pasteur for kindly providing pAT187, and we thank M. Young from the University of Wales for pECN2.
Footnotes
Published ahead of print on 4 March 2011.
REFERENCES
- 1. Alvira P., Tomas-Pejo E., Ballesteros M., Negro M. J. 2010. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour. Technol. 101:4851–4861 [DOI] [PubMed] [Google Scholar]
- 2. Atsumi S., Hanai T., Liao J. C. 2008. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451:86–89 [DOI] [PubMed] [Google Scholar]
- 3. Atsumi S., Higashide W., Liao J. C. 2009. Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat. Biotechnol. 27:1177–1180 [DOI] [PubMed] [Google Scholar]
- 4. Brestic-Goachet N., Gunasekaran P., Cami B., Baratti J. C. 1989. Transfer and expression of an Erwinia chrysanthemi cellulase gene in Zymomonas mobilis. J. Gen. Microbiol. 135:893–902 [Google Scholar]
- 5. Cascone R. 2008. Biobutanol—a replacement for bioethanol? Chem. Eng. Prog. 104:S4–S9 [Google Scholar]
- 6. Cioffi E. A., Shaw K. J., Bailey W. F., Berg C. M. 1980. Improved synthesis of the sodium salt of DL-alpha, beta-dihydroxyisovaleric acid. Anal. Biochem. 104:485–488 [DOI] [PubMed] [Google Scholar]
- 7. Collins M. D., et al. 1994. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int. J. Syst. Bacteriol. 44:812–826 [DOI] [PubMed] [Google Scholar]
- 8. Dürre P. 2007. Biobutanol: an attractive biofuel. Biotechnol. J. 2:1525–1534 [DOI] [PubMed] [Google Scholar]
- 9. Gowen C. M., Fong S. S. 2010. Exploring biodiversity for cellulosic biofuel production. Chem. Biodivers. 7:1086–1097 [DOI] [PubMed] [Google Scholar]
- 10. Guedon E., Desvaux M., Petitdemange H. 2002. Improvement of cellulolytic properties of Clostridium cellulolyticum by metabolic engineering. Appl. Environ. Microbiol. 68:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jennert K. C., Tardif C., Young D. I., Young M. 2000. Gene transfer to Clostridium cellulolyticum ATCC 35319. Microbiology 146(Part 12):3071–3080 [DOI] [PubMed] [Google Scholar]
- 12. Kiritani K., Narise S., Wagner R. P. 1966. The dihydroxy acid dehydratase of Neurospora crassa. J. Biol. Chem. 241:2042–2046 [PubMed] [Google Scholar]
- 13. Kojima M., Johnson T. 2005. Potential for biofuels for transport in developing countries. Energy Sector Management Assistance Programme (ESMAP), Energy and Water Department, the World Bank Group, Washington, DC [Google Scholar]
- 14. la Grange D. C., den Haan R., van Zyl W. H. 2010. Engineering cellulolytic ability into bioprocessing organisms. Appl. Microbiol. Biotechnol. 87:1195–1208 [DOI] [PubMed] [Google Scholar]
- 15. Lynd L. 1989. Production of ethanol from lignocellulosic materials using thermophilic bacteria: critical evaluation of potential and review, p. 1–52In Lignocellulosic materials, vol. 38 Springer Berlin, Heidelberg, Germany [Google Scholar]
- 16. Lynd L. R., van Zyl W. H., McBride J. E., Laser M. 2005. Consolidated bioprocessing of cellulosic biomass: an update. Curr. Opin. Biotechnol. 16:577–583 [DOI] [PubMed] [Google Scholar]
- 17. Merino S. T., Cherry J. 2007. Progress and challenges in enzyme development for biomass utilization. Adv. Biochem. Eng. Biotechnol. 108:95–120 [DOI] [PubMed] [Google Scholar]
- 18. Moniruzzaman M., et al. 1997. Fermentation of corn fibre sugars by an engineered xylose utilizing Saccharomyces yeast strain. World J. Microbiol. Biotechnol. 13:341–346 [Google Scholar]
- 19. Nishizaki T., Tsuge K., Itaya M., Doi N., Yanagawa H. 2007. Metabolic engineering of carotenoid biosynthesis in Escherichia coli by ordered gene assembly in Bacillus subtilis. Appl. Environ. Microbiol. 73:1355–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Percival Zhang Y. H., Himmel M. E., Mielenz J. R. 2006. Outlook for cellulase improvement: screening and selection strategies. Biotechnol. Adv. 24:452–481 [DOI] [PubMed] [Google Scholar]
- 21. Petitdemange E., Caillet F., Giallo J., Gaudin C. 1984. Clostridium cellulolyticum sp. nov., a cellulolytic, mesophilic species from decayed grass. Int. J. Syst. Bacteriol. 34:155–159 [Google Scholar]
- 22. Roberts S. B., Gowen C. M., Brooks J. P., Fong S. S. 2010. Genome-scale metabolic analysis of Clostridium thermocellum for bioethanol production. BMC Syst. Biol. 4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Srivastava R., Kumar G. P., Srivastava K. K. 1995. Construction of a recombinant cellulolytic Escherichia coli. Gene 164:185–186 [DOI] [PubMed] [Google Scholar]
- 24. Tardif C., Maamar H., Balfin M., Belaich J. P. 2001. Electrotransformation studies in Clostridium cellulolyticum. J. Ind. Microbiol. Biotechnol. 27:271–274 [DOI] [PubMed] [Google Scholar]
- 25. Trieu-Cuot P., Carlier C., Martin P., Courvalin P. 1987. Plasmid transfer by conjugation from Escherichia coli to Gram-positive bacteria. FEMS Microbiol. Lett. 48:289–294 [Google Scholar]
- 26. Tripathi S. A., et al. 6 August 2010. Development of pyrF-based genetic system for targeted gene deletion in Clostridium thermocellum and creation of a pta mutant. Appl. Environ. Microbiol. doi: 10.1128/AEM.01484-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsai S. L., Oh J., Singh S., Chen R., Chen W. 2009. Functional assembly of minicellulosomes on the Saccharomyces cerevisiae cell surface for cellulose hydrolysis and ethanol production. Appl. Environ. Microbiol. 75:6087–6093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tyurin M. V., Desai S. G., Lynd L. R. 2004. Electrotransformation of Clostridium thermocellum. Appl. Environ. Microbiol. 70:883–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Zyl W. H., Lynd L. R., den Haan R., McBride J. E. 2007. Consolidated bioprocessing for bioethanol production using Saccharomyces cerevisiae. Adv. Biochem. Eng. Biotechnol. 108:205–235 [DOI] [PubMed] [Google Scholar]
- 30. Williams T. I., Combs J. C., Lynn B. C., Strobel H. J. 2007. Proteomic profile changes in membranes of ethanol-tolerant Clostridium thermocellum. Appl. Microbiol. Biotechnol. 74:422–432 [DOI] [PubMed] [Google Scholar]
- 31. Xu C., et al. 2010. Factors influencing cellulosome activity in consolidated bioprocessing of cellulosic ethanol. Bioresour. Technol. 101:9560–9569 [DOI] [PubMed] [Google Scholar]
- 32. Yang Y. T., Peredelchuk M., Bennett G. N., San K. Y. 2000. Effect of variation of Klebsiella pneumoniae acetolactate synthase expression on metabolic flux redistribution in Escherichia coli. Biotechnol. Bioeng. 69:150–159 [DOI] [PubMed] [Google Scholar]
- 33. Yoo J. S., Jung Y. J., Chung S. Y., Lee Y. C., Choi Y. L. 2004. Molecular cloning and characterization of CMCase gene (celC) from Salmonella typhimurium UR. J. Microbiol. 42:205–210 [PubMed] [Google Scholar]
- 34. Zhang K., Sawayab M. R., Eisenberg D. S., Liao J. C. 2008. Expanding metabolism for biosynthesis of nonnatural alcohols. Proc. Natl. Acad. Sci. U. S. A. 105:20653–20658 [DOI] [PMC free article] [PubMed] [Google Scholar]