Abstract
Alcoholic fermentation (AF) conducted by Saccharomyces cerevisiae has been exploited for millennia in three important human food processes: beer and wine production and bread leavening. Most of the efforts to understand and improve AF have been made separately for each process, with strains that are supposedly well adapted. In this work, we propose a first comparison of yeast AFs in three synthetic media mimicking the dough/wort/grape must found in baking, brewing, and wine making. The fermentative behaviors of nine food-processing strains were evaluated in these media, at the cellular, populational, and biotechnological levels. A large variation in the measured traits was observed, with medium effects usually being greater than the strain effects. The results suggest that human selection targeted the ability to complete fermentation for wine strains and trehalose content for beer strains. Apart from these features, the food origin of the strains did not significantly affect AF, suggesting that an improvement program for a specific food processing industry could exploit the variability of strains used in other industries. Glucose utilization was analyzed, revealing plastic but also genetic variation in fermentation products and indicating that artificial selection could be used to modify the production of glycerol, acetate, etc. The major result was that the overall maximum CO2 production rate (Vmax) was not related to the maximum CO2 production rate per cell. Instead, a highly significant correlation between Vmax and the maximum population size was observed in all three media, indicating that human selection targeted the efficiency of cellular reproduction rather than metabolic efficiency. This result opens the way to new strategies for yeast improvement.
INTRODUCTION
Saccharomyces cerevisiae alcoholic fermentation (AF) has been exploited for several millennia throughout the world in a variety of food processes of crucial importance for humans. It has been used to produce alcoholic beverages, including beer and wine. These beverages seem to be connected to anthropic activity since the beginning of human civilization, as suggested by some archaeological records (11, 40, 47). Leavened bread, another human fundamental foodstuff, has made use of alcoholic fermentation for a long time: the study of baking practices revealed the use of yeast to leaven dough in ancient Egypt (57). At that time, alcoholic fermentation began due to the natural presence of indigenous yeast in wort/grape must/dough or, alternatively, via empirical leavening practice yet with total ignorance regarding the existence of microorganisms and their fermentative role. Indeed, beer, wine, and bread were produced with the help of yeast several thousand years before its role was formally proven by Pasteur in 1860 (45). Controlling alcoholic fermentation actually began with the development of yeast culture in the early 1880s, with the work of Emil Christian Hansen, head of the Carlsberg Laboratory in Copenhagen, Denmark. The use of pure yeast culture to initiate alcoholic fermentation progressively came to dominate worldwide practices, and the utilization of starter strains adapted to each food process is now the rule rather than the exception in brewing, wine making, and baking. Thus, several efforts have been undertaken to improve yeast strains used in the food and beverage industry, using various approaches, such as phenotypic selection of natural isolates (22), mutagenesis (13), clone and meiospore selection (13, 36, 50), breeding programs assisted by technological tests (13, 37, 38, 55) or quantitative trait locus (QTL) introgression (32, 37, 39), and genetic engineering (17, 68). Yeast improvement aimed at enhancing the efficiency of the fermentation process as well as the quality of the products, implying specific biotechnological targets regarding the nature of the final products: freezing tolerance, flocculation, aromatic contribution, and ethanol tolerance, as exhaustively described in the literature (18, 34, 47). Most of the efforts to understand or to improve alcoholic fermentation were specific to each food processing context, ignoring other genetic resources and information from other food processes. As a consequence, food processing strains are genetically clustered according to their industrial origin (21, 33), which reflects human activity. Although important differences exist between these food processes (e.g., the nature of assimilable sugar, temperature, process time, etc.), alcoholic fermentation is expected to be one of the main targets of human selection.
Alcoholic fermentation is a complex bioprocess that results from the interaction of numerous genetic, metabolic, and environmental parameters. This complexity may explain in part the failure of attempts to increase the fermentation rate through the overexpression of various glycolytic enzymes (16, 30, 31, 46, 58). For a relevant characterization of alcoholic fermentation in various food processes, it is thus necessary to consider different levels of cellular integration and to analyze their relationships. In particular, a high rate of fermentation can be reached in two ways: by enhancing the rate of fermentation per cell (i.e., high CO2 rate per cell) and/or by enhancing the number of cells per population. Such an issue, which is crucial to identifying the factors controlling fermentation rate, has never been investigated before and requires the analysis of the relationship between fermentation kinetics and population dynamics.
We propose the first comparison of yeast alcoholic fermentation and population growth dynamics in three different synthetic media mimicking the dough/wort/grape must found in baking, brewing, and wine making, respectively. We call these media bakery, brewery, and enology media, respectively. We compared the behaviors of nine different food processing S. cerevisiae strains, and we investigated different biological levels: we focused on the fermentation kinetics (AF kinetics traits), and the main products of alcoholic fermentation (metabolic traits) were quantified at the end of the process. Although some of these metabolites have a strong impact on the final quality of the beverages/foods produced, their variability within food processes and strains had never been investigated before. At the population level, we measured “life history traits,” which reflect population kinetics and have been found to be related to yeast evolution (23, 64).
The aims of this study were (i) to compare the behaviors of different yeast strains in the three main food processes (wine making, brewing, and baking); (ii) to examine the effect of human selection on food processing strains; (iii) to study the plasticity and genetic variability of the different fermentation products between food processes and strains; (iv) to analyze the relationships between AF kinetics parameters, metabolic traits, and life history traits during alcoholic fermentation; and (v) to propose a new rationale for strain selection.
MATERIALS AND METHODS
Biological material.
Twenty-six strains of Saccharomyces cerevisiae coming from different geographical origins and food processing industries (wine making, brewing, baking, and distilling) were initially selected to be representative of the main genetic clusters found in S. cerevisiae food processing strains (1). A previous work showed that strains from baking were mostly autotetraploids (1). To avoid possible effects of ploidy level, we excluded baking strains from the final panel. For each of the nine chosen food processing strains (Table 1 ), one meiospore was isolated with a micromanipulator (Singer MSM Manual; Singer Instrument, Somerset, United Kingdom). Six out of nine strains were homothallic (HO/HO) (294, 328, 382, F10, VL1, and BO213), so that the isolated meiospore gave rise to a fully homozygous diploid strain through mating type switch and further fusion of opposite-mating-type cells. For the three heterothallic strains (ho/ho) (963, A24, and 7327), the isolated haploid meiospore was diploidized via transient expression of the HO endonuclease: the strains were transformed with the pHS2 plasmid (kindly given by S. Himanshu) using the lithium acetate transformation protocol described by Gietz and Schiestl (26). After diploidization, the plasmid was eliminated from the strains through recurrent cultures on yeast extract-peptone-dextrose (YPD) medium. The resulting biological material was constituted by four wine-making strains (E1 to E4) (here called wine strains), two brewing strains (B1 and B2) (here called beer strains), and three distilling strains (D1 to D3) (here called distillery strains) (Table 1). All strains were currently grown at 30°C in YPD medium containing 1% yeast extract (Difco Laboratories, Detroit, MI), 1% Bacto peptone (Difco), and 2% glucose, supplemented or not with 2% agar.
Table 1.
Origins of Saccharomyces cerevisiae strains
| Food processing parental strain | Monosporic derivate | Collection/suppliera | Food origin | Area of origin | Reproductive mode | Ploidy level |
|---|---|---|---|---|---|---|
| CLIB-382 | B1 | CIRM-Levures | Brewery | Japan | Homothallic | Diploid |
| NRRL-Y-7327 | B2 | NRRL | Brewery | Tibet | Heterothallic | Diploid |
| CLIB-294 | D1 | CIRM-Levures | Distillery | France | Homothallic | Diploid |
| Alcotec 24 | D2 | Hambleton Bard | Distillery | UK | Heterothallic | Diploid |
| 963 | D3 | Confidential | Distillery | Confidential | Heterothallic | Not available |
| CLIB-328 | E1 | CIRM-Levures | Enology | UK | Homothallic | Diploid |
| BO213 | E2b | Laffort Oenologie | Enology | France | Homothallic | Diploid |
| VL1 | E3b | Laffort Oenologie | Enology | France | Homothallic | Diploid |
| F10 | E4b | Laffort Oenologie | Enology | France | Homothallic | Diploid |
CIRM-Levures, http://www.inra.fr/internet/Produits/cirmlevures; NRRL, http://nrrl.ncaur.usda.gov; Hambleton Bard, Chesterfield, United Kingdom; Laffort Oenologie, Bordeaux, France.
Synthetic fermentative media.
Three synthetic fermentative media were used; they differed in their levels of sugar and nitrogen, pH, osmotic pressure, and anaerobic growth factors in order to reflect the main changes of fermentation medium between brewing, baking, and wine-making contexts (see Table 2). Maltose is the main available sugar in wort and dough (14, 44), and yet most of the strains used in wine making cannot assimilate it (42). Moreover, previous work shows that the presence of maltose in the medium could affect glycolysis even for strains lacking the maltose permease (52). Therefore, to compare the basal fermentative behaviors of different food processing strains including those of wine-making origin, we decided to use glucose as the only carbon source in all three media. Although distillery strains were included, no distilling medium (here called distillery medium) was used. Indeed, the distilling process refers to distinct processes, including the production of distilled beverages such as cognac, whisky, tequila, or other spirits and the production of bioethanol fuel from various crops (sugarcane or beet molasses), etc. The compositions of these different distillery media vary greatly regarding sugar content, pH, osmotic pressure, nutrient limitation, or starvation, as illustrated for molasses composition (2), so that it was not possible to propose a single representative distillery medium.
Table 2.
Main differences between the synthetic bakery, brewery, and enology media
| Main component | Bakery medium | Brewery medium | Enology medium |
|---|---|---|---|
| Glucose | 80 g liter−1 | 80 g liter−1 | 220 g liter−1 |
| Assimilable nitrogena | 0.4 g liter−1 | 0.4 g liter−1 | 0.2 g liter−1 |
| pH | 5.5 | 4.5 | 3.5 |
| Sorbitol (osmotic pressure) | 150 g liter−1 | No | No |
| Anaerobic growth factors | No | No | Yes |
The assimilable nitrogen was available from ammonium sulfate (one-third) and amino acids (two-thirds).
The enology medium was modified from the work of Marullo et al. (37) and Bely et al. (4, 5) and contained glucose (220 g liter−1), tartaric acid (3 g liter−1), citric acid (0.3 g liter−1), l-malic acid (0.3 g liter−1), and myoinositol (0.3 g liter−1). The available nitrogen was 200 mg liter−1, provided by 315 mg liter−1 (NH4)2SO4 (corresponding to 66.7 mg liter−1 nitrogen) and 0.67% (vol/vol) of a mixture of 18 amino acids containing l-tyrosine (9.4 mg liter−1), l-tryptophan (92.3 mg liter−1), l-isoleucine (16.9 mg liter−1), l-aspartic acid (22.9 mg liter−1), l-glutamic acid (62 mg liter−1), l-arginine (192.8 mg liter−1), l-leucine (24.9 mg liter−1), l-threonine (39.1 mg liter−1), l-glycine (9.4 mg liter−1), l-glutamine (260.2 mg liter−1), l-alanine (74.8 mg liter−1), l-valine (22.9 mg liter−1), l-methionine (16.2 mg liter−1), l-phenylalanine (19.5 mg liter−1), l-serine (40.4 mg liter−1), l-histidine (16.9 mg liter−1), l-lysine (8.8 mg liter−1), and l-cysteine (6.7 mg liter−1) in NaHCO3 (134.8 mg liter−1), corresponding to 133.3 mg liter−1 available nitrogen. The mineral salts were provided by KH2PO4 (2 g liter−1), MgSO4·7H2O (0.2 g liter−1), MnSO4 (4 mg liter−1), ZnSO4·7H2O (4 mg liter−1), CuSO4·5H2O (1 mg liter−1), KI (1 mg liter−1), CoCl2·6H2O (0.4 mg liter−1), (NH4)6Mo7O24·4H2O (1 mg liter−1), and H3BO3 (1 mg liter−1); the vitamins were as follows: biotin (0.04 mg liter−1), thiamine-HCl (1 mg liter−1), pyridoxine-HCl (1 mg liter−1), nicotinic acid (1 mg liter−1), calcium panthothenate (1 mg liter−1), and para-amino benzoic acid (1 mg liter−1). Finally, 0.02% (vol/vol) of a mixture of anaerobic growth factors was added, containing 1.5% (wt/vol) ergosterol and 0.5% (wt/vol) sodium oleate in Tween 80-ethanol (1:1, vol/vol). The pH was adjusted to 3.5 using KOH pellets.
The brewery medium was modified from the enology medium. The pH was adjusted to 4.5 using 40 mM citrate buffer, pH 4, replacing tartaric, citric, and malic acids. The glucose content was 80 g liter−1, and nitrogen content was modified as follows: 400 mg liter−1 available nitrogen, one-third coming from 630 mg liter−1 (NH4)2SO4 and two-thirds coming from 13.49% (vol/vol) of the mixture of amino acids described below. Brewery medium was not supplemented with anaerobic growth factors.
The bakery medium was modified from the enology medium. The pH was adjusted to 5.5 using 40 mM citrate buffer, pH 5.2, replacing tartaric, citric, and malic acids. The glucose content was 80 g liter−1, and nitrogen content was modified as follows: 400 mg liter−1 available nitrogen, one-third coming from 630 mg liter−1 (NH4)2SO4 and two-thirds coming from 13.49% (vol/vol) of the mixture of amino acids described below. Sorbitol (150 g liter−1, i.e., 0.82 mol liter−1) was also added in order to increase the osmotic pressure. Bakery medium was not supplemented with anaerobic growth factors.
Fermentation conditions.
The media were filtered through an 0.45-μm nitrate-cellulose membrane before inoculation. Precultures were run in diluted half-synthetic medium for 20 h at 24°C with orbital agitation (150 rpm), and population sizes were measured using a particle counter (Z2 Coulter counter; Beckman Coulter, Villepinte, France). The fermentative media were inoculated at 106 cells per ml, and fermentation triplicates were run in closed 1.2-liter glass reactors, locked to maintain anaerobiosis, with permanent stirring (≈150 rpm) at 22°C (5). CO2 was released through a sterile air outlet condenser. After yeast inoculation, the pH of the medium was not controlled, differing from a chemostat mode.
Fermentation kinetics.
The amount of CO2 released was determined by automatic measurement of glass reactor weight loss every 20 min (8, 19). The CO2 production rate (g liter−1 h−1) was calculated using a local polynomial regression fitting (loess function, R program [51]). Different kinetics parameters were calculated as previously described (37). The lag-phase time (h) was the time between inoculation and the beginning of CO2 release (CO2 production rate higher than 0.05 g liter−1 h−1). Similarly, the end of the fermentation was determined to be when the CO2 production rate dropped below 0.05 g liter−1 h−1. This point allowed us to estimate the AF time (alcoholic fermentation time; hours), which was the time necessary to ferment the sugars in the medium, excluding the lag phase. All strains were able to achieve the fermentation (i.e., to consume over 98.5% of initial sugar) except strains B1, B2, D1, D2, and D3 in enology medium. However, these five strains displayed sluggish fermentation under winery-type conditions, so that the corresponding AF time was higher than that for strains actually achieving AF. Thus, the AF time parameter was relevant to compare the global fermentation abilities of the strains, whatever the medium. Vmax (g liter−1 h−1) was the maximal CO2 production rate. Finally, CO2tot was the total amount of CO2 released at the end of the fermentation (g liter−1).
Population dynamics, cell size, and growth recovery.
The population growth and the cell size were monitored regularly using a particle counter (Z2 Coulter counter; Beckman Coulter, Villepinte, France); more than 20 samples per fermentation were taken from the inoculation time until the carrying capacity (maximum population size, K) was reached. During the exponential phase, samples were taken every 2 or 3 h in order to have a good estimate of the maximum rate of increase of the population (intrinsic growth rate, r). The experimental points were fitted with a logistic model that allowed estimation of K and r:
where Nt is the population size at time t, K is the carrying capacity (cells per ml), N0 is the initial population size, and r is the intrinsic growth rate (number of divisions per hour). At the end of the fermentation, the proportion of cells able to form colonies (CFU) after 2 days on YPD agar plates was determined and referred to as the growth recovery (%). This parameter is usually assimilated as an indirect measure of cell viability in microbiology (62). Finally, the cell size was measured at the end of alcoholic fermentation using a particle counter (Z2 Coulter counter; Beckman Coulter, Villepinte, France) and the mean cell size (diameter [μm]) was calculated and used for further analyses.
CO2-specific flux.
The CO2-specific flux (J, the CO2 production rate per cell, g h−1 cell−1) was calculated by dividing the CO2 production rate by the number of cells for a given volume. In particular, we calculated the Jmax, which is the maximum CO2 production rate per cell.
Dosage of alcoholic fermentation products.
At the end of the alcoholic fermentation, several dosages were made: ethanol concentration (percent volume) was determined by infrared reflectance (Infra-Analyzer 450; Technicon, Plaisir, France), and residual glucose (g liter−1) and acetic acid production (g liter−1) were measured by colorimetry (A460) in continuous flux (Sanimat, Montauban, France) in the supernatant. For the analyses of variance (ANOVAs), we considered a derived variable, ethanol/glucose ratio (in mol/mol), to compare ethanol yields. External glycerol (g liter−1) and residual nitrogen (g liter−1) were assayed by the enzymatic method (Boehringer kits 10 148 270 035 and 11 112 732 035; R-Biopharm, Darmstadt, Germany), and residual nitrogen was used to determine nitrogen consumption (%). Biomass dry weights were measured from 200 ml of final fermentation medium using a desiccator (SMO 01; Scaltec Instruments GmbH, Göttingen, Germany) and were expressed in g liter−1.
For internal glycogen and trehalose dosages, 10 ml of medium was sampled at the end of AF and washed twice with 4 ml of 5% (wt/vol) NaCl. After centrifugation (5 min, 2,750 × g), yeast pellets were chilled at −20°C. Trehalose was extracted by twice washing the pellets for 1 h at 4°C in 8 ml of 0.5 M trichloroacetic acid, with an additional wash with 4 ml of cold H2O. The pellets were kept at 4°C for further glycogen dosage (see below). After centrifugation (5 min, 2,750 × g), the washing supernatants were merged and adjusted to 25 ml with H2O. Trehalose was determined with anthrone as described by Roustan and Sablayrolles (51, 56). Briefly, 2.5 ml of cold anthrone solution (190 mg anthrone [Sigma-Aldrich, Lyon, France] in 83% [vol/vol] sulfuric acid) was added to 250 μl of the supernatant solution, boiled for 12 min, and immediately cooled on ice to stop the reaction. Optical density at 625 nm (OD625) was measured using a spectrophotometer (Lambda EZ201; Perkin-Elmer, Courtaboeuf, France). Gradual dilutions of trehalose dehydrate (Sigma) were used as standards. For glycogen dosage, the residual pellets were suspended in 0.5 ml of 8 M HCl, and glycogen was solubilized for 30 min at 60°C using 2 ml of dimethyl sulfoxide (DMSO; Sigma, Lyon, France). The suspension was cooled to room temperature, neutralized using 0.5 ml of 8 M NaOH, and buffered using 17 ml of 0.11 M citrate buffer (pH 4.5). Five hundred microliters of sample was used for an overnight hydrolysis at 37°C with 25 μl of amyloglucosidase (Roche). The released glucose was determined using a glucose oxidase kit (Sigma). Trehalose and glycogen concentrations were expressed in glucose equivalents per cell (g cell−1). As glycogen extraction is not considered to be exhaustive, minor variations may be meaningless.
Glucose allocation.
During alcoholic fermentation, the principal carbon flux goes from the assimilable carbon source (glucose in this work) to ethanol. Glucose catabolism is also used for biomass production and for the formation of AF by-products (acetic acid, glycerol, trehalose, and glycogen). A slight proportion of glucose is used for cellular maintenance functions during the stationary phase of fermentation but appears negligible compared to the other routes for glucose catabolism. Thus, to investigate glucose allocation to the main fermentation products, we expressed ethanol, acetic acid, and external glycerol in glucose equivalents (mol liter−1), taking into account that 1 mol of glucose produced 2 mol of ethanol, 2 mol of acetic acid, or 2 mol of glycerol. Since glycogen and trehalose accounted for 6% to 43% of the biomass depending on the medium and strain used, we subtracted glycogen and trehalose contents from biomass for glucose allocation studies. Biomass was also approximated in glucose equivalents: 1 mol of glucose produced 2 mol of ATP, which can itself be used for biomass production. Verduyn et al. (67) estimated that the maximum biomass yield from ATP was 28.3 g biomass per mol of ATP under fermentative conditions. Biomass (g liter−1) was thus divided by 56.6 (28.3 × 2) to obtain a biomass estimate expressed in glucose equivalents. Although glucose equivalents for biomass may vary depending on the environmental conditions, this calculation method allowed us to get an approximate value of the glucose allocation toward the different AF products.
Statistical analysis.
The variation of each trait was investigated using the lme4 package (R program), through the following mixed model of ANOVA: Z = μ + mediumi + strainj + blockk + positionl + medium × strainij + εijkl, where Z is the variable, medium is the medium effect (i = 1, 2, 3), strain is the strain effect (j = 1, …, 9), block is the random block effect (effect of each week of experimental repetition, k = 1, …, 11), position is the random position effect (glass reactor position, l = 1, …, 15), medium × strain is the interaction effect between medium and strain factors, and ε is the residual error. For further statistical analyses (principal component analysis [PCA], linear discriminant analysis [LDA], Spearman's correlations, etc.), the data were corrected for block and position effects (random effects). Since classical significance tests are controversial for fixed effects in mixed models, we used an alternative method based on Markov chain Monte Carlo (MCMC) sampling by means of R's languageR package version 1.0 (51; R. H. Baayen, 2009, “languageR: data sets and functions with Analyzing Linguistic Data: a Practical Introduction to Statistics” [http://cran.r-project.org/web/packages/languageR/index.html]). P values were then adjusted for multiple testing using Benjamini-Hochberg methods by means of R's multtest package, version 2.1.1 (7, 27, 51). For each variable, the homogeneity of the variance was assessed using a Levene test by means of R's car package, version 1.2-15 (51; J. Fox, 2009, “car: Companion to Applied Regression” [http://cran.r-project.org/web/packages/car/index.html]), as well as the normality of residual distribution using a Shapiro test (51). For AF time, r, acetic acid, trehalose, and nitrogen consumption variables, a log transformation was necessary to obtain normally distributed residues; for glycerol and ethanol/glucose variables, an inverse transformation was applied, while a square root transformation was used for glycogen and growth recovery.
To get a general overview of the data, a PCA was performed from the following variables: Vmax, lag-phase time, AF time, K, r, Jmax, CO2tot, ethanol, acetic acid, glycerol, glycogen, trehalose, biomass, cell size, growth recovery, and nitrogen consumption. PCA was run using the ade4 package from the R program on the ANOVA predicted mean for each trait and for each medium-strain combination. The data were corrected for the random effects and standardized, i.e., mean centered and scaled (12, 51).
For the food origin study, LDA was performed using R's mda package, version 0.3-4 (F. Leisch, K. Hornik, and B. D. Ripley, 2009, “mda: mixture and flexible discriminant analysis” [http://cran.r-project.org/web/packages/mda/index.html]) on the whole set of data corrected for random effects and using the same variables as those for the PCA.
Pairwise correlations between variables (mean of three replicates; data corrected for random effects) were studied using Spearman's rank correlations (ρ). Since 120 multiple correlations were computed, P values were corrected for multiple testing using Benjamini-Hochberg methods (7) by means of R's multtest package, version 2.1.1 (27, 51).
For Vmax study, multiple linear regressions were performed within each medium to estimate the contribution of K and Jmax to Vmax using the lm function in the R program (51). The entire data set and summarizing graphs for 15 kinetics and metabolic and life history traits are available as supplemental material (Table S2 and Fig. S1).
RESULTS
Multitrait genetic and plastic variation among strains grown in synthetic bakery, brewery, and enology media.
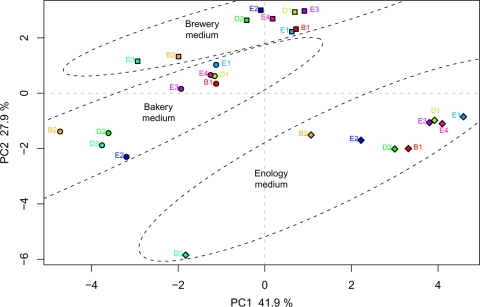
The fermentative abilities and population dynamics of nine fully homozygous strains from the wine-making, brewing, and distilling industries were evaluated in three synthetic media differing in their amount of glucose, nitrogen, pH, osmotic pressure, and anaerobic growth factors (Table 2). To obtain an initial overview of the variability between strains and media, a principal component analysis (PCA) was performed (Fig. 1), using a total of three parameters representative of the fermentation kinetics (lag-phase time, AF time, and Vmax); 10 metabolic traits, including flux data (Jmax); AF metabolites (CO2tot, ethanol, acetic acid, glycerol, glycogen, trehalose, biomass, and nitrogen consumption); and four life history traits (K, r, cell size, and growth recovery). The first two axes accounted for 69.8% of the total variation and allowed a clear clustering of both strains and culture media (Fig. 1). The first axis separated the enology medium from the bakery medium on the traits lag-phase time, K, cell size, trehalose, and nitrogen consumption. It also separated the strains within each medium, with B2 and D3 usually isolated from the group composed of E1, E3, E4, B1, and D1 strains. The two remaining strains, D2 and E2, displayed intermediate positions and clustered either with the B2-D3 group or with the second group, depending on the medium. The second axis separated the enology medium from the brewery medium on the basis of Vmax and glycerol traits essentially. This medium-based clustering revealed by the PCA mostly reflected the differences in fermentation kinetics (lag-phase time and Vmax) and population dynamics (K) between media as shown in Fig. 2 and 3 (see details below).
Fig. 1.
Principal component analysis (PCA) of nine food processing S. cerevisiae strains in three synthetic media (brewery, bakery, and enology). Fermentations run in bakery, brewery, and enology media are represented by circles, squares, and diamonds, respectively. B1 and B2, D1 to D3, and E1 to E4 stand for brewery, distillery, and enology strains, respectively. PCA was made from the following variables: Vmax, lag-phase time, AF time, K, r, Jmax, CO2tot, ethanol, acetic acid, glycerol, glycogen, trehalose, nitrogen consumption, biomass, cell size, and growth recovery. x axis, percentage of variation explained by principal component 1 (PC1) (41.9%); y axis, percentage of variation explained by PC2 (27.9%).
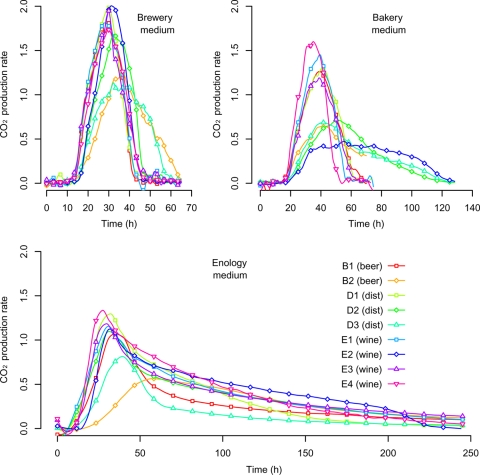
Fig. 2.
Fermentation kinetics of nine food processing S. cerevisiae strains in three synthetic media (brewery, bakery, and enology). The food processing origin of the strain is indicated in parentheses; “dist” stands for distillery origin. CO2 production rate is expressed in g liter−1 h−1. In bakery and brewery media, all strains were able to complete fermentation (i.e., the residual sugar at the end of the fermentation was lower than 0.5 g liter−1). In enology medium, only the four wine strains displayed completed fermentation (mean residual sugar concentrations of 0.0, 0.0, 2.9, and 2.1 g liter−1 for E1, E2, E3, and E4, respectively), while beer and distillery strains displayed sluggish or stuck fermentation (residual sugar concentrations of 23.3, 37.2, 34.4, 7.3, and 102.8 g liter−1 for B1, B2, D1, D2, and D3, respectively). For figure visibility, only the first 250 h of fermentation in enology medium was represented.
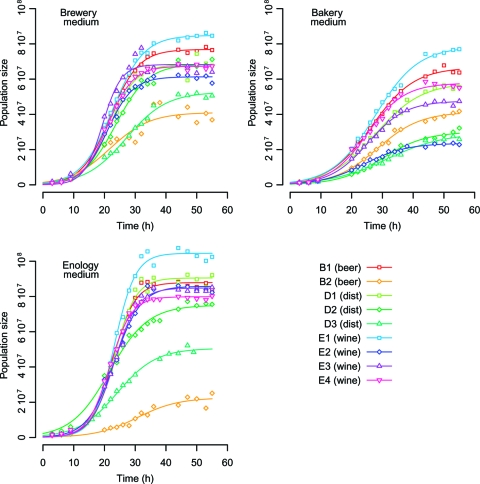
Fig. 3.
Population dynamics of nine food processing S. cerevisiae strains in three synthetic media (brewery, bakery, and enology). The food processing origin of the strain is indicated in parentheses; “dist” stands for distillery origin. Population size is expressed in number of cells per ml.
In the brewery medium, the CO2 production rate curve was bell shaped, with a short mean fermentation (AF) time of 37.85 ± 9.04 h and a high maximum CO2 production rate (Vmax, 1.72 ± 0.32 g liter−1 h−1), whereas in enology medium, all strains displayed a strongly asymmetrical curve with a long fermentation time (mean AF time of 273.58 ± 58.72 h) and a lower Vmax (1.09 ± 0.21 g liter−1 h−1) (Fig. 2; Table 3). The bakery medium displayed an intermediate profile with a mean AF time of 68.74 ± 29.04 h and a slightly lower Vmax of 1.03 ± 0.40 g liter−1 h−1. In the enology medium, the maximum population size K was higher ([7.15 ± 2.02] × 107 cells ml−1) than those in brewery medium ([6.24 ± 1.20] × 107 cells ml−1) and bakery medium ([4.94 ± 1.71] × 107 cells ml−1). Similarly, in the enology and brewery media, the intrinsic growth rate (r) was significantly higher (0.24 and 0.25 division per hour, respectively) than that in bakery medium (0.16 division per hour).
Table 3.
Results of the ANOVAs: mean values and sums of squares for alcoholic fermentation kinetics and metabolic and life history traits
| Parameter (unit)a | Mean value |
% of total sum of squaresb (df) |
|||||
|---|---|---|---|---|---|---|---|
| Bakery medium | Brewery medium | Enology medium | Medium (2) | Strain (8) | Medium by strain (16) | Residual (54) | |
| Vmax (g CO2 liter−1 h−1) | 1.03 | 1.72 | 1.09 | 47.62*** | 37.29*** | 13.84*** | 1.26 |
| AF time (h) | 68.74 | 37.85 | 273.58 | 88.76*** | 4.37*** | 6.16*** | 0.71 |
| Lag-phase time (h) | 17.72 | 14.10 | 12.09 | 45.44*** | 17.63*** | 21.86*** | 15.07 |
| K (cells ml−1) | 4.94 × 107 | 6.24 × 107 | 7.15 × 107 | 23.61*** | 56.36*** | 14.35*** | 5.68 |
| r (division h−1) | 0.16 | 0.25 | 0.24 | 40.58*** | 32.81*** | 8.38 | 18.23 |
| Ethanol/glucose (mol mol−1) | 0.41 | 0.48 | 0.45 | 66.81*** | 2.65 | 7.42 | 23.13 |
| Nitrogen consumption (%) | 71 | 85 | 95 | 64.21*** | 22.74*** | 6.79** | 6.26 |
| Jmax (g CO2 h−1 cell−1) | 2.75 × 10−11 | 3.34 × 10−11 | 2.54 × 10−11 | 29.83*** | 23.22*** | 26.03*** | 20.91 |
| Growth recovery (% cultivable cells) | 23 | 44 | 19 | 24.93*** | 38.42*** | 17.96** | 18.7 |
| Cell size (μm [diam]) | 5.61 | 5.91 | 5.19 | 36.63*** | 43.26*** | 12.38*** | 7.72 |
| Biomass (g liter−1) | 2.01 | 3.02 | 2.71 | 36.23*** | 22.25*** | 24.55*** | 16.97 |
| Glycerol (g liter−1) | 4.59 | 2.94 | 6.56 | 77.82*** | 14.55*** | 3.38** | 4.24 |
| Acetate (g liter−1) | 0.79 | 0.24 | 0.40 | 57.25*** | 28.28*** | 11.57*** | 2.89 |
| Trehalose (g cell−1) | 6.32 × 10−12 | 4.85 × 10−12 | 2.72 × 10−12 | 21.49*** | 49.37*** | 14.14* | 15.01 |
| Glycogen (g cell−1) | 1.23 × 10−12 | 1.76 × 10−12 | 4.02 × 10−12 | 51.73*** | 26.81*** | 12.97*** | 8.5 |
For some parameters, data transformation was necessary to obtain normally distributed residues: log transformation for AF time, r, acetic acid, and trehalose parameters; inverse transformation for glycerol and ethanol/glucose parameters; and, finally, square root transformation for glycogen and growth recovery.
With use of the Benjamini-Hochberg correction for multiple testing, significance is indicated as follows: *, significant at 5%; **, significant at 1%; ***, significant at 0.1%.
In each medium, strains E1, E3, E4, B1, and D1 tended to cluster together. Strains D3 and B2 were remote from the others due to markedly atypical behavior for most of the traits. They had lower fermentation and growth abilities than did the others in all media (Fig. 2 and 3). Strains E2 and D2 were close to the B2-D3 group in the bakery medium but joined the second group in brewery and enology media, with a clear-cut difference in population dynamics (Fig. 2) and fermentation kinetics (Fig. 3) due to poorer adaptation to the bakery medium. Regarding the food origin of the strains, enology, distillery, and brewery strains were mixed together within each medium, so that no clustering associated with food origin was observed.
Genetic and plastic variation for all AF kinetics and metabolic and life history traits.
Analysis of variance for each trait revealed that the medium effect was always significant (Table 3) and accounted for a large part of the total variation for most parameters (from 21.49% of variance explained by medium effect for trehalose to 88.76% explained for AF time). Strain effect was also highly significant and accounted for a large part of the variance for all parameters, yet to a lesser extent than medium effect. The only exception was for ethanol/glucose ratio, where no significant strain effect was evidenced. This result is congruent with current knowledge of ethanol yield, which is known to vary poorly according to the S. cerevisiae strain used. Finally, the medium-by-strain interaction effect was significant for all parameters but ethanol/glucose and r and accounted for 3.38% to 26.03% of the total phenotypic variation. This means that the ranking order of the strains was also medium dependent. For example, strain D2 had a shorter lag-phase time in enology medium but a higher value in brewery medium. Interestingly, although the global trend was a major medium effect followed by strain and medium-by-strain effects, four atypical variables were found: K, trehalose, cell size, and growth recovery, for which medium effect explained less variation than did strain effect. It is noteworthy that these four parameters are related to yeast growth ability (29). Strain effect accounted for 56.36% of total variation of K, while medium effect accounted for 23.61%. Therefore, even though significant plasticity was detected for these four traits, the genetic component of their variation was preeminent. From a biotechnological point of view, such traits are particularly interesting for traditional breeding programs (36). Altogether, these results revealed high plasticity among media for AF kinetics and metabolic traits and a strong genetic effect among the nine domesticated strains for life history traits.
The effect of the food process origins of the strains.
The PCA (Fig. 1) showed no particular clustering according to the food process origins of the strains, indicating that the food origin effect was lower than the medium and strain effects previously identified. However, to investigate food origin effect in depth, a linear discriminant analysis (LDA) was made and allowed reliable discrimination of the different food origins (beer, distillery, and wine strains): the a posteriori probability of correctly inferring the food origin of the strains was 0.74. The main variables accounting for food origin effect were two fermentation kinetics parameters, CO2tot and AF time (explaining 17.7% and 11.6%, respectively, of the variance of the two LDA axes), and a storage carbohydrate, trehalose (explaining 8.1% of the variance). This reflects the fact that the wine strains displayed reduced AF time regardless of the medium (33.0, 57.0, and 262.5 h mean AF times in brewery, bakery, and enology media, respectively) compared to beer strains (44.4, 78.9, and 277.8 h in brewery, bakery, and enology media, respectively) and distillery strains (39.8, 77.7, and 288.8 h in brewery, bakery, and enology media, respectively). Wine strains were also able to produce on average more CO2 than did beer and distillery strains in enology medium (98.4, 67.5, and 88.1 g liter−1 for wine, beer, and distillery strains, respectively, in enology medium). Indeed, only the four wine strains (E1, E2, E3, and E4) were able to achieve the fermentation under winery-type conditions (i.e., to consume over 98.5% of initial sugar) while the five remaining strains displayed incomplete (sluggish) fermentation, with final sugar consumption ranging from 53.2% (D3) to 96.7% (D2). Regarding carbohydrate storage content, beer strains displayed higher trehalose levels in bakery and brewery media (11.0 × 10−12 g cell−1 and 8.5 × 10−12 g cell−1, respectively) than did distillery strains (5.2 × 10−12 g cell−1 and 4.6 × 10−12 g cell−1 in bakery and brewery media, respectively) and wine ones (4.8 × 10−12 g cell−1 and 2.7 × 10−12 g cell−1 in bakery and brewery media, respectively).
Allocation of catabolized glucose in the cells.
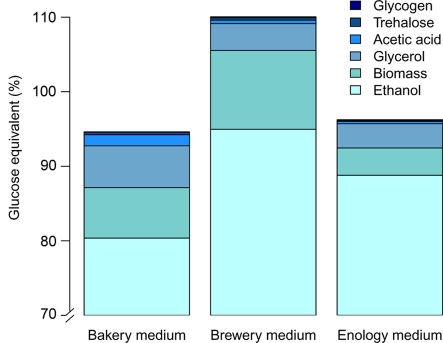
To investigate the variation of glucose allocation to the different AF products by the cells, we measured the storage carbohydrates (trehalose and glycogen) and the biomass (without storage carbohydrates), as well as the acetic acid, glycerol, and ethanol produced at the end of the alcoholic fermentation, and we expressed them in glucose equivalents (mol liter−1; see Table S1 in the supplemental material). The total sum of fermentation products was close to 100% of catabolized glucose (95.1%, 110.5%, and 96.4% in bakery, brewery, and enology media, respectively) (Fig. 4), indicating that our glucose balance explained in a satisfactory way the sugar utilization. Accumulation of small errors in dosages of the different fermentation by-products and approximation in conversion of biomass to glucose equivalents may explain the slight under-/overestimation of glucose utilization.
Fig. 4.
Percentages of glucose allocated to ethanol, biomass, glycerol, acetic acid, trehalose, and glycogen production during alcoholic fermentation of nine food processing S. cerevisiae strains in three synthetic media (brewery, bakery, and enology). Biomass was taken into account without glycogen and trehalose contents in glucose balance analysis and was approximated in glucose equivalents using the equation of Verduyn et al. (67). Variance analysis applied to glucose allocation data showed that all six fermentation products displayed significant medium effect. Strain and medium-by-strain effects were also found to be significant for all parameters but the proportion of glucose allocated to ethanol.
We observed clear and significant differences of glucose allocation depending on the medium (Fig. 4; see also Table S1 in the supplemental material). In bakery medium, 80.9% of the consumed glucose was used for ethanol production, while higher yields were observed in the enology and brewery media, with 89.0% and 95.3% of sugar entering the glucose-ethanol flux, respectively. The distribution of the other minor fermentation products was also strongly medium dependent, as illustrated in Fig. 4. While a large fraction of glucose was devoted to biomass production in brewery medium (10.7%), only 6.8% and 3.6% were used for biomass in bakery and enology media, respectively. The proportion of glucose going to glycerol also varied greatly, from 3.3% and 3.6% in enology and brewery media, respectively, and as much as 5.6% in bakery medium. Glycerol is known to be produced to counterbalance hyperosmolarity (9, 35). It is thus consistent that the higher glycerol content was found in the medium displaying the higher osmotic pressure (bakery). A higher proportion of acetic acid was also made in bakery medium (1.53%) than in brewery and enology media (0.46% and 0.31%, respectively). Finally, less than 0.5% initial glucose was used for glycogen and trehalose storage in all three media. However, allocation to the two storage molecules differed. While in enology medium, carbohydrate storage was principally made from glycogen (0.14% compared to 0.09% from trehalose); in brewery and bakery media, it was trehalose (0.33% and 0.31% trehalose, respectively, compared to 0.13% and 0.06% glycogen). In addition, although very little initial sugar content is dedicated to trehalose production, we found a significant correlation between trehalose and growth recovery (ρ = 0.57, P < 0.01) in accordance with previous data suggesting that trehalose was related to cell growth ability after quiescence (24, 59, 63, 69).
Analysis of glucose allocation within each medium also revealed significant differences between strains. In bakery media, subcluster 1 (B2, D3, D2, and E2) was formed of strains making, from 1 gram of glucose, more glycerol and more acetic acid but less biomass than the subcluster 2 strains made. Despite overall nonsignificant strain and medium-by-strain effects on the ethanol yield, strain B2 in the enology medium differed from other strains by producing more ethanol with the same amount of glucose but was unable to complete fermentation (residual sugar of 37.2 g liter−1). In addition, B2 differed by producing more trehalose than did the other strains.
Relationships between AF kinetics and metabolic and life history traits.
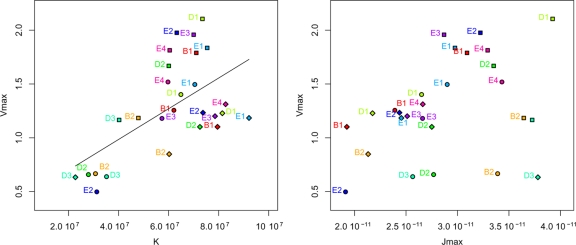
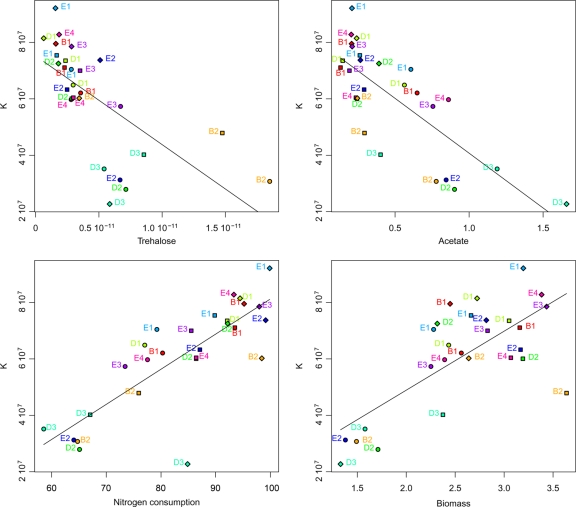
We examined the phenotypic relationships between kinetics and metabolic and life history traits on all media. First, we tested whether a high fermentation speed (using Vmax, a relevant indicator of fermentation rate) was correlated with a higher rate of CO2 production per cell (Jmax) and/or with an increased number of cells (K) (Fig. 5). Interestingly, we found that Vmax was significantly correlated with K (ρ = 0.48, P < 0.05), while no correlation was found between Vmax and Jmax (P > 0.1). We then examined the relationship between K and other fermentation traits. Considering by-products of technological interest, we found significant trade-offs between K and acetic acid (ρ = −0.76, P < 0.001) (Fig. 6), as well as between K and trehalose (ρ = −0.84, P < 0.001) (Fig. 6). As expected, K was also positively correlated with relative nitrogen consumption (ρ = 0.82, P < 0.001) (Fig. 6).
Fig. 5.
Relationships between Vmax, Jmax, and K. Fermentations run in bakery, brewery, and enology media are represented by circles, squares, and diamonds, respectively. B1 and B2, D1 to D3, and E1 to E4 stand for brewery, distillery, and enology strains, respectively. Significant correlation was found between Vmax and K (ρ = 0.48 and P < 0.05), while there was no relation between Vmax and Jmax (P > 0.1). Vmax was expressed in g liter−1 h−1, Jmax was expressed in g h−1 cell−1, and K was expressed in cells ml−1.
Fig. 6.
Relationships between K and trehalose, acetic acid, nitrogen consumption, and biomass. Symbols are the same as those in Fig. 5. Significant correlation was found between K and trehalose (ρ = −0.84, P < 0.001), K and acetic acid (ρ = −0.76, P < 0.001), K and nitrogen consumption (ρ = 0.82, P < 0.001), and K and biomass (ρ = 0.60, P < 0.01). K was expressed in cells ml−1, trehalose was expressed in g cell−1, acetic acid was expressed in g liter−1, nitrogen consumption was expressed as the percentage of initial nitrogen content consumed, and biomass was expressed in g liter−1.
The relationship between the population size (K) and biomass was also studied. While biomass was intuitively thought to depend on both K and cell size, biomass and K were found to be significantly correlated (ρ = 0.60, P < 0.01) (Fig. 6), while no correlation was detected for biomass and cell size.
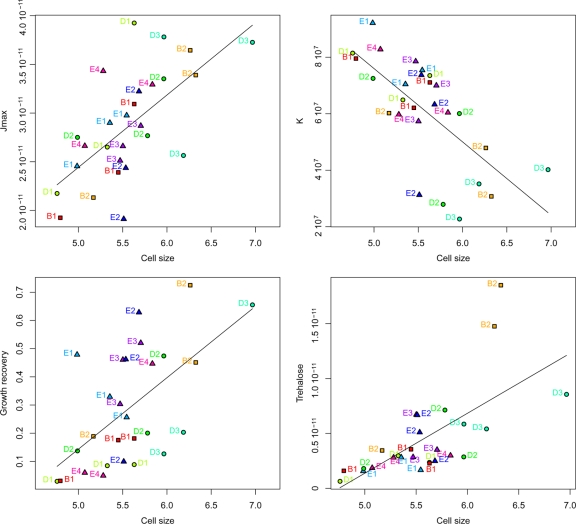
Regarding cell size, a positive significant phenotypic correlation was found between mean cell size and the maximum CO2 production rate per cell (Jmax) (ρ = 0.65, P < 0.01) (Fig. 7). As previously found (61), K was found to be negatively correlated with cell size (ρ = −0.69, P < 0.001) (Fig. 7). Interestingly, cell size and growth recovery were positively correlated (ρ = 0.58, P < 0.01) (Fig. 7), as were cell size and trehalose (ρ = 0.70, P < 0.001) (Fig. 7).
Fig. 7.
Relationships between cell size, Jmax, K, growth recovery, and trehalose. Symbols are the same as those in Fig. 5. Significant correlations were found between cell size and Jmax (ρ = 0.65, P < 0.01), cell size and K (ρ = −0.69, P < 0.001), cell size and growth recovery (ρ = 0.58, P < 0.01), and cell size and trehalose (ρ = 0.70, P < 0.001). Jmax was expressed in g liter−1 h−1 cell−1, cell size was expressed in μm (mean diameter), K was expressed in cells ml−1, growth recovery was expressed as the frequency of cultivable cells, and trehalose was expressed in g cell−1.
To explore the genetic relationships between Vmax, Jmax, and K, we also analyzed their relationships within each medium individually, using multiple linear regressions (Table 4). We confirmed that K was the main component of Vmax variation and accounted for 78.7%, 86.0%, and 94.5% of total Vmax variation in brewery, enology, and bakery media, respectively (Table 4). In contrast, Jmax was found to be significantly related to Vmax only in bakery medium and accounted for very little Vmax variation (3.24%) (Table 4).
Table 4.
Sums of squares of the multiple linear regressions for Vmax in bakery, brewery, and enology media
| Factor | df | % of total sum of squaresa |
||
|---|---|---|---|---|
| Bakery medium | Brewery medium | Enology medium | ||
| K | 1 | 92.52*** | 79.30** | 82.97*** |
| Jmax | 1 | 4.97* | 0.63 | 3.42 |
| Residual | 6 | 2.51 | 20.08 | 13.61 |
Significance is indicated as follows: *, significant at 5%; **, significant at 1%; ***, significant at 0.1%.
DISCUSSION
Enology, brewery, and bakery media exhibit relevant fermentation differences.
The yeast S. cerevisiae has been used for millennia in three essential human food processes: baking, brewing, and wine making, while the very beginning of the 21st century saw the world ethanol production for transport fuel tripling. In the last century, the constant need for process optimization led to the development of yeast improvement programs for each food process individually. Selection of yeast strains of interest requires knowledge of yeast genetic and plastic variation. We developed three synthetic media reflecting the main differences between baking, brewing, and wine-making processes, and we studied the fermentative behavior of nine S. cerevisiae strains of various industrial origins. This experimental design allowed relevant exploration of the alcoholic fermentation process in various medium-strain combinations. As expected, we found that the environment was the main factor shaping alcoholic fermentation, followed by the genetic factor and finally by the interaction between environment and genotype.
Strain behavior reflects weak human selection for food processing.
Interestingly, we found that the effect of the food processing origin of the strains (wine, beer, and distillery) was lower than the strain and medium effects, indicating generally weak human selection for food processing. The main impact of the origin was on the fermentation achievement: wine strains produced more CO2 in less time, especially in enology medium, where they were able to consume all the sugar, while the beer and distillery strains displayed slow or incomplete fermentations. As expected, it seems that wine strains were selected for their ability to complete fermentation under high sugar concentrations (as found in grape must), which are a key feature in wine making. In addition to fermentation completion, trehalose was shown to discriminate beer strains from distillery and wine ones in brewery and bakery media. Previous work showed that trehalose content was a key parameter for the brewery process: beer production generally includes a pitching step to produce biomass before wort inoculation. The viability of pitching yeast is crucial for the subsequent alcoholic fermentation stage and was shown elsewhere to be directly related to trehalose content (29). By selecting for high growth recovery of pitching yeast in beer production, humans may have indirectly selected for increased trehalose content. Apart from fermentation completion and trehalose, no signature of selection by a specific food process was found to be prominent. This revealed that yeast selection programs can easily exploit genetic variation of strains of various food process origins.
Glucose allocation displays plastic and genetic variability.
The allocation of catabolized glucose to fermentation products at the end of fermentation varies among the three media and nine strains. The proportion of sugar used for the formation of the different AF by-products varies to a large extent, depending essentially on the medium. A large variability was also found between strains, except for the ethanol/glucose ratio, which displays intermedium variation but varies weakly between genotypes, in accordance with current knowledge (48). Ethanol yield is a crucial issue in wine making: the most recent improvements in viticulture practices led to grape musts with increased initial sugar content and subsequently wine with higher alcoholic content, while the inverse tendency is required for public health considerations. Our results confirm that the natural yeast variability for ethanol/glucose ratio is too low to be exploited through traditional breeding programs in bakery, brewery, and enology media. Indeed, approaches aiming at lowering ethanol yield (for a wine-making or brewing purpose) focus on rerouting the carbon fluxes toward minor AF by-products such as glycerol through enzymatic engineering, with some success (17, 41, 43, 53).
We observed genetic and plastic variation related to all fermentation by-products but not the ethanol/glucose ratio, and this allowed yeast improvement through standard breeding programs. For example, the genetic component explained about 35% of acetic acid variation, in accordance with previous work showing that acetate production was easily optimized through breeding (28, 37). Similary, glycerol production can be improved using selection programs as previously shown (54).
Alcoholic fermentation is driven by population size rather than specific flux.
In microorganisms, the AF ability is usually confounded with the glycolytic specific flux, which, per definition, is considered at the cellular level. This is usually thought to be the main parameter that controls the fermentation kinetics of the whole population. In this context, several works searched for the factors controlling the glycolytic flux: in particular, hexose transport and some glycolytic enzymes such as phosphofructokinase or pyruvate kinase have been suggested to be rate-limiting steps of glycolysis, through in vivo and/or in silico analyses (10, 15, 20, 25, 49). Increasing these factors was intuitively supposed to increase the glycolytic flux and, therefore, the global fermentative ability that was exploited for industrial purposes (46). However, this hypothesis did not take into account that the fermentative ability, at the population level, is the result of both specific flux (i.e., flux per cell) and cell number. While no correlation was found between Jmax and Vmax in our experimental design, the variation of the maximum population size K explained 79% to 95% of Vmax variation, confirming that the major factors controlling fermentative ability were population parameters.
Considering K for a new selection approach.
The correlation found between K and Vmax suggests that increasing K would also increase fermentative ability. Accordingly, previous data showed that a higher carrying capacity was associated with a higher CO2 production rate in nearly isogenic S. cerevisiae strains (38), and Varela et al. (66) showed that adding biomass reduced the time to achieve sugar consumption in slow fermentations. Using K as a selection criterion for AF would therefore increase the rate of fermentation. Since no genetic variation was detected for the ethanol/glucose yield, increasing K does not seem to be associated with a reduction of the ethanol yield but rather seems to be related to a reduction of the yield of other by-products. Indeed, we showed that K was negatively correlated with acetate formation. This suggests that increasing K by selection may also allow decreasing acetic acid. Indeed, excess acetate is responsible for a well-known off-flavor (vinegary taste) in wine making and brewing and is the object of attempts at underproduction through mixed culture, including S. cerevisiae and other yeast species, for example (6). The trade-off between K and acetic acid was previously shown under hyperosmotic conditions (3) and could be related to the redox-equilibrating process: cell growth is associated with NADH excess that must be regenerated quickly to maintain the cellular redox balance. The glycerol pathway is usually thought to be the main way to reoxidize the NADH excess due to biomass formation (65). However, in our experiment, there was no significant correlation between glycerol content and biomass or K, suggesting that other reoxidization pathways might be activated. Another NADH-consuming pathway is through acetoin formation; it would be interesting to measure and correlate this pathway with cell growth under our conditions. Finally, the third way to reoxidize NADH is through the last step of ethanol production mediated by aldehyde dehydrogenase isozymes. It can be hypothesized that, under excess-NADH conditions, acetaldehyde may be preferentially routed toward ethanol formation rather than toward acetate production, leading to an indirect negative correlation between acetate and K. This would have an undetectable effect on ethanol yield, the proportion of glucose entering the acetate route being small (≈2%) compared to the glucose-ethanol highway (>76%) and below the experimental variation (≈4%). Although undetectable at the ethanol level, the modulation of the ethanol/acetate ratio can be hypothesized as an additional alternative for the redox-equilibrating purpose. Besides, whatever the origin of this trade-off, such a negative correlation could be exploited for a biotechnological purpose, either to improve fermentative ability or/and to lower acetic acid production.
Interestingly, K is one of the few parameters for which the strain effect explained more variation than the medium effect. Since the genetic component has proven to be preeminent, the exploitation of yeast genetic variability should allow the identification of strains with high carrying capacity associated with enhanced fermentative potential. It is noteworthy that the great variability of S. cerevisiae has been poorly exploited in improvement programs, essentially because estimating the fermentative ability of a strain requires expensive and time-consuming experiments. Here, we proposed a new strategy to preselect S. cerevisiae strains with good fermentative potential, based on their carrying capacity: measuring K can be achieved using a particle counter or a flow cytometer for numerous strains in a short time, allowing the evaluation of hundreds of poorly studied yeast strains available in collection centers. Strains displaying high carrying capacities could then enter traditional breeding programs involving crosses with well-characterized industrial strains, for example (38).
“Ant” strategy versus “grasshopper” strategy and their use for selection.
In a previous work, trade-offs between some life history traits had been found, leading to a continuum of life history strategies distributed between two extremes. “Grasshoppers” have a large cell size and a high rate of glucose consumption but have a low carrying capacity; they illustrate the “selfish” strategy in which individual benefits prevail over population considerations (60, 61, 69). In contrast, “ants” have a small cell size and consume glucose at a low rate, which allows a high carrying capacity to be reached; this evokes a “cooperative” strategy. Ant and grasshopper strategies were also identified in this work. In addition, we found that grasshoppers appeared to be more able than ants to resume growth at the end of the fermentation process. Although this result needs to be confirmed with additional strains from various ecological niches, it could explain why the grasshopper strategy was maintained through evolution. It is notable that almost all these parameters (K, cell size, and growth recovery) were found to display less plasticity than were other fermentation parameters (strain effect was larger than medium effect). This suggests that the ant or grasshopper status is above all genetically determined, although some plasticity exists. Indeed, only one strain changed its strategy depending on the medium: E2 displayed a grasshopper strategy in bakery medium and an ant strategy in enology and brewery media. The eight remaining strains displayed stable strategies in all three media.
From a technological viewpoint, ant strains are of peculiar interest since they favor increased maximum population size, a trait closely related to fermentation rate. Spor et al. (60) showed that, in a broad collection of yeast strains from various ecological niches, industrial strains were paradoxically among the grasshoppers, while extreme ants can be found in forest and laboratory environments. Thus, it could be interesting to measure the carrying capacities of such strains under different food processing conditions, in order to include them in breeding programs to improve fermentative ability.
Supplementary Material
ACKNOWLEDGMENTS
We thank the anonymous reviewers for their comments, which helped improve the manuscript.
This work was supported by the ANR program “blanc” Adaptalevure NT05-4_45721 and the ANR program “ALIA” HeterosYeast ANR-08-ALIA-9.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 25 February 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Albertin W., et al. 2009. Evidence for autotetraploidy associated with reproductive isolation in Saccharomyces cerevisiae: towards a new domesticated species. J. Evol. Biol. 22:2157–2170 [DOI] [PubMed] [Google Scholar]
- 2. Attfield P. V. 1997. Stress tolerance: the key to effective strains of industrial baker's yeast. Nat. Biotechnol. 15:1351–1357 [DOI] [PubMed] [Google Scholar]
- 3. Bely M., Rinaldi A., Dubourdieu D. 2003. Influence of assimilable nitrogen on volatile acidity production by Saccharomyces cerevisiae during high sugar fermentation. J. Biosci. Bioeng. 96:507–512 [DOI] [PubMed] [Google Scholar]
- 4. Bely M., Sablayrolles J. M., Barre P. 1990. Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in enological conditions. J. Ferment. Bioeng. 70:246–252 [Google Scholar]
- 5. Bely M., Sablayrolles J. M., Barre P. 1990. Description of alcoholic fermentation kinetics—its variability and significance. Am. J. Enol. Vitic. 41:319–324 [Google Scholar]
- 6. Bely M., Stoeckle P., Masneuf-Pomarede I., Dubourdieu D. 2008. Impact of mixed Torulaspora delbrueckii-Saccharomyces cerevisiae culture on high-sugar fermentation. Int. J. Food Microbiol. 122:312–320 [DOI] [PubMed] [Google Scholar]
- 7. Benjamini Y., Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57:289–300 [Google Scholar]
- 8. Bezenger M. C., Navarro J. M., Abbal P., Sablayrolles J. M. 1985. Suivi de fermentation a I'aide d'un microordinateur personnel, application a la fermentation alcoolique en oenologie. Ind. Agric. Aliment. 102:1283–1291 [Google Scholar]
- 9. Blomberg A., Adler L. 1989. Roles of glycerol and glycerol-3-phosphate dehydrogenase (NAD+) in acquired osmotolerance of Saccharomyces cerevisiae. J. Bacteriol. 171:1087–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boiteux A., Hess B. 1981. Design of glycolysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 293:5–22 [DOI] [PubMed] [Google Scholar]
- 11. Cavalieri D., McGovern P. E., Hartl D. L., Mortimer R., Polsinelli M. 2003. Evidence for Saccharomyces cerevisiae fermentation in ancient wine. J. Mol. Evol. 57(Suppl. 1):S226–S232 [DOI] [PubMed] [Google Scholar]
- 12. Chessel D., Dufour A. B., Thioulouse J. 2004. The ade4 package-I-one-table methods. R News 4:5–10 [Google Scholar]
- 13. Codon A. C., et al. 2003. New Saccharomyces cerevisiae baker's yeast displaying enhanced resistance to freezing. J. Agric. Food Chem. 51:483–491 [DOI] [PubMed] [Google Scholar]
- 14. Coghe S., D'Hollander H., Verachtert H., Delvaux F. R. 2005. Impact of dark specialty malts on extract composition and wort fermentation. J. Inst. Brew. 111:51–60 [Google Scholar]
- 15. Cortassa S., Aon M. A. 1994. Metabolic control analysis of glycolysis and branching to ethanol production in chemostat cultures of Saccharomyces cerevisiae under carbon, nitrogen, or phosphate limitations. Enzyme Microb. Technol. 16:761–770 [Google Scholar]
- 16. Davies S. E., Brindle K. M. 1992. Effects of overexpression of phosphofructokinase on glycolysis in the yeast Saccharomyces cerevisiae. Biochemistry 31:4729–4735 [DOI] [PubMed] [Google Scholar]
- 17. Dequin S. 2001. The potential of genetic engineering for improving brewing, wine-making and baking yeasts. Appl. Microbiol. Biotechnol. 56:577–588 [DOI] [PubMed] [Google Scholar]
- 18. Donalies U. E., Nguyen H. T., Stahl U., Nevoigt E. 2008. Improvement of Saccharomyces yeast strains used in brewing, wine making and baking. Adv. Biochem. Eng. Biotechnol. 111:67–98 [DOI] [PubMed] [Google Scholar]
- 19. El Haloui N., Picque D., Corrieu G. 1988. Alcoholic fermentation in winemaking: on-line measurement of density and carbon dioxide evolution. J. Food Eng. 8:17–30 [Google Scholar]
- 20. Evans P. R., Farrants G. W., Hudson P. J. 1981. Phosphofructokinase: structure and control. Philos. Trans. R. Soc. Lond. B Biol. Sci. 293:53–62 [DOI] [PubMed] [Google Scholar]
- 21. Fay J. C., Benavides J. A. 2005. Evidence for domesticated and wild populations of Saccharomyces cerevisiae. PLoS Genet. 1:66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fleet G. H. 2008. Wine yeasts for the future. FEMS Yeast Res. 8:979–995 [DOI] [PubMed] [Google Scholar]
- 23. Flores-Moya A., Costas E., Lopez-Rodas V. 2008. Roles of adaptation, chance and history in the evolution of the dinoflagellate Prorocentrum triestinum. Naturwissenschaften 95:697–703 [DOI] [PubMed] [Google Scholar]
- 24. Francois J., Parrou J. L. 2001. Reserve carbohydrates metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 25:125–145 [DOI] [PubMed] [Google Scholar]
- 25. Galazzo J. L., Bailey J. E. 1990. Fermentation pathway kinetics and metabolic flux control in suspended and immobilized Saccharomyces cerevisiae. Enzyme Microb. Technol. 12:162–172 [Google Scholar]
- 26. Gietz R. D., Schiestl R. H. 1991. Applications of high efficiency lithium acetate transformation of intact yeast cells using single-stranded nucleic acids as carrier. Yeast 7:253–263 [DOI] [PubMed] [Google Scholar]
- 27. Gilbert H. N., Pollard K. S., van der Laan M. J., Dudoit S. 2009. Resampling-based multiple hypothesis testing with applications to genomics: new developments in the R/Bioconductor package multtest. Working paper 249. University of California at Berkeley Division of Biostatistics Working Paper Series. University of California, Berkeley, CA [Google Scholar]
- 28. Giudici P., Zambonelli C. 1992. Biometric and genetic study on acetic production for breeding of wine yeast. Am. J. Enol. Vitic. 43:370–374 [Google Scholar]
- 29. Guldfeldt L. U., Arneborg N. 1998. The effect of yeast trehalose content at pitching on fermentation performance during brewing fermentations. J. Inst. Brew. 104:37–39 [Google Scholar]
- 30. Hauf J., Zimmermann F. K., Muller S. 2000. Simultaneous genomic overexpression of seven glycolytic enzymes in the yeast Saccharomyces cerevisiae. Enzyme Microb. Technol. 26:688–698 [DOI] [PubMed] [Google Scholar]
- 31. Heinisch J. 1986. Isolation and characterization of the two structural genes coding for phosphofructokinase in yeast. Mol. Gen. Genet. 202:75–82 [DOI] [PubMed] [Google Scholar]
- 32. Javelot C., Girard P., Colonna-Ceccaldi B., Vladescu B. 1991. Introduction of terpene-producing ability in a wine strain of Saccharomyces cerevisiae. J. Biotechnol. 21:239–252 [Google Scholar]
- 33. Legras J. L., Merdinoglu D., Cornuet J. M., Karst F. 2007. Bread, beer and wine: Saccharomyces cerevisiae diversity reflects human history. Mol. Ecol. 16:2091–2102 [DOI] [PubMed] [Google Scholar]
- 34. Lodolo E. J., Kock J. L., Axcell B. C., Brooks M. 2008. The yeast Saccharomyces cerevisiae—the main character in beer brewing. FEMS Yeast Res. 8:1018–1036 [DOI] [PubMed] [Google Scholar]
- 35. Mager W. H., Varela J. C. 1993. Osmostress response of the yeast Saccharomyces. Mol. Microbiol. 10:253–258 [PubMed] [Google Scholar]
- 36. Marullo P., Bely M., Masneuf-Pomarède I., Aigle M., Dubourdieu D. 2004. Inheritable nature of enological quantitative traits is demonstrated by meiotic segregation of industrial wine yeast strains. FEMS Yeast Res. 4:711–719 [DOI] [PubMed] [Google Scholar]
- 37. Marullo P., et al. 2006. Breeding strategies for combining fermentative qualities and reducing off-flavor production in a wine yeast model. FEMS Yeast Res. 6:268–279 [DOI] [PubMed] [Google Scholar]
- 38. Marullo P., et al. 2009. Genetic improvement of thermo-tolerance in wine Saccharomyces cerevisiae strains by a backcross approach. FEMS Yeast Res. 9:1148–1160 [DOI] [PubMed] [Google Scholar]
- 39. Marullo P., Yvert G., Bely M., Aigle M., Dubourdieu D. 2007. Efficient use of DNA molecular markers to construct industrial yeast strains. FEMS Yeast Res. 7:1295–1306 [DOI] [PubMed] [Google Scholar]
- 40. Meussdoerffer F. G. 2009. A comprehensive history of beer brewing, p. 1–42In Esslinger H. M. (ed.), Handbook of brewing: processes, technology, markets. Wiley-VCH, Weinheim, Germany [Google Scholar]
- 41. Michnick S., Roustan J. L., Remize F., Barre P., Dequin S. 1997. Modulation of glycerol and ethanol yields during alcoholic fermentation in Saccharomyces cerevisiae strains overexpressed or disrupted for GPD1 encoding glycerol 3-phosphate dehydrogenase. Yeast 13:783–793 [DOI] [PubMed] [Google Scholar]
- 42. Naumov G. I., Naumova E. S., Michels C. A. 1994. Genetic variation of the repeated MAL loci in natural populations of Saccharomyces cerevisiae and Saccharomyces paradoxus. Genetics 136:803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nevoigt E., Stahl U. 1996. Reduced pyruvate decarboxylase and increased glycerol-3-phosphate dehydrogenase [NAD+] levels enhance glycerol production in Saccharomyces cerevisiae. Yeast 12:1331–1337 [DOI] [PubMed] [Google Scholar]
- 44. Panadero J., Randez-Gil F., Prieto J. A. 2005. Validation of a flour-free model dough system for throughput studies of baker's yeast. Appl. Environ. Microbiol. 71:1142–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pasteur L. 1860. Mémoire sur la fermentation alcoolique. Ann. Chim. Phys. 58:323–426 [Google Scholar]
- 46. Peter Smits H., et al. 2000. Simultaneous overexpression of enzymes of the lower part of glycolysis can enhance the fermentative capacity of Saccharomyces cerevisiae. Yeast 16:1325–1334 [DOI] [PubMed] [Google Scholar]
- 47. Pretorius I. S. 2000. Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast 16:675–729 [DOI] [PubMed] [Google Scholar]
- 48. Pretorius I. S., Bauer F. F. 2002. Meeting the consumer challenge through genetically customized wine-yeast strains. Trends Biotechnol. 20:426–432 [DOI] [PubMed] [Google Scholar]
- 49. Pritchard L., Kell D. B. 2002. Schemes of flux control in a model of Saccharomyces cerevisiae glycolysis. Eur. J. Biochem. 269:3894–3904 [DOI] [PubMed] [Google Scholar]
- 50. Ramirez M., Regodon J. A., Perez F., Rebollo J. E. 1999. Wine yeast fermentation vigor may be improved by elimination of recessive growth-retarding alleles. Biotechnol. Bioeng. 65:212–218 [DOI] [PubMed] [Google Scholar]
- 51. R. Development Core Team 2010. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 52. Reijenga K. A., et al. 2001. Control of glycolytic dynamics by hexose transport in Saccharomyces cerevisiae. Biophys. J. 80:626–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Remize F., Roustan J. L., Sablayrolles J. M., Barre P., Dequin S. 1999. Glycerol overproduction by engineered Saccharomyces cerevisiae wine yeast strains leads to substantial changes in by-product formation and to a stimulation of fermentation rate in stationary phase. Appl. Environ. Microbiol. 65:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Remize F., Sablayrolles J. M., Dequin S. 2000. Re-assessment of the influence of yeast strain and environmental factors on glycerol production in wine. J. Appl. Microbiol. 88:371–378 [DOI] [PubMed] [Google Scholar]
- 55. Romano P., Soli G., Suzzi G., Grazia L., Zambonelli C. 1985. Improvement of a wine Saccharomyces cerevisiae strain by a breeding program. Appl. Environ. Microbiol. 50:1064–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roustan J. L., Sablayrolles J. M. 2002. Trehalose and glycogen in wine-making yeasts: methodological aspects and variability. Biotechnol. Lett. 24:1059–1064 [Google Scholar]
- 57. Samuel D. 1996. Investigation of ancient Egyptian baking and brewing methods by correlative microscopy. Science 273:488–490 [DOI] [PubMed] [Google Scholar]
- 58. Schaaff I., Heinisch J., Zimmermann F. K. 1989. Overproduction of glycolytic enzymes in yeast. Yeast 5:285–290 [DOI] [PubMed] [Google Scholar]
- 59. Shi L., Sutter B. M., Ye X., Tu B. P. 2010. Trehalose is a key determinant of the quiescent metabolic state that fuels cell cycle progression upon return to growth. Mol. Biol. Cell 21:1982–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Spor A., et al. 2009. Niche-driven evolution of metabolic and life-history strategies in natural and domesticated populations of Saccharomyces cerevisiae. BMC Evol. Biol. 9:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Spor A., Wang S., Dillmann C., de Vienne D., Sicard D. 2008. “Ant” and “grasshopper” life-history strategies in Saccharomyces cerevisiae. PLoS One 3:e1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Takahashi S., Ando A., Takagi H., Shima J. 2009. Insufficiency of copper ion homeostasis causes freeze-thaw injury of yeast cells as revealed by indirect gene expression analysis. Appl. Environ. Microbiol. 75:6706–6711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thevelein J. M. 1984. Regulation of trehalose mobilization in fungi. Microbiol. Rev. 48:42–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Travisano M., Mongold J. A., Bennett A. F., Lenski R. E. 1995. Experimental tests of the roles of adaptation, chance, and history in evolution. Science 267:87–90 [DOI] [PubMed] [Google Scholar]
- 65. Van Dijken J. P., Scheffers W. A. 1986. Redox balances in the metabolism of sugars by yeasts. FEMS Microbiol. Lett. 32:199–224 [Google Scholar]
- 66. Varela C., Pizarro F., Agosin E. 2004. Biomass content governs fermentation rate in nitrogen-deficient wine musts. Appl. Environ. Microbiol. 70:3392–3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Verduyn C., Postma E., Scheffers W. A., van Dijken J. P. 1990. Energetics of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J. Gen. Microbiol. 136:405–412 [DOI] [PubMed] [Google Scholar]
- 68. Volschenk H., Viljoen-Bloom M., Subden R. E., van Vuuren H. J. 2001. Malo-ethanolic fermentation in grape must by recombinant strains of Saccharomyces cerevisiae. Yeast 18:963–970 [DOI] [PubMed] [Google Scholar]
- 69. Wang S., et al. 2011. Switch between life history strategies due to changes in glycolytic enzyme gene dosage in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 77:452–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wiemken A. 1990. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie Van Leeuwenhoek 58:209–217 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.