Abstract
Fe(III)-reducing soil enrichment cultures can tolerate 100 μM Cu and Cd, 150 μM Co, 600 μM Ni, and 2,500 μM Zn. Metal-tolerant cultures were dominated by Geobacter-related Deltaproteobacteria and Gram-positive Firmicutes spp. (Clostridia and Sedimentibacter). A Cd- and Cu-tolerant Fe(III)-reducing coculture of Desulfosporosinus and Desulfitobacterium indicated the importance of the Firmicutes for Fe(III) reduction in the presence of metals.
INTRODUCTION
Toxic metals can be immobilized on surface sorption sites of soil Fe(III) minerals or can be included in the mineral structure (4, 29). Fe(III)-reducing bacteria (FeRB) can facilitate the release of these metals by reductive dissolution of Fe(III) oxides (9, 17) and bioreduction of Fe(III) oxide-bound trace metals (42). This release might enhance metal stress, suggesting that metal tolerance should be an important attribute for FeRB. Acidophilic FeRB can tolerate millimolar concentrations of Cd, Cu, Ni, and Zn (12), which might be a prerequisite to survival in habitats where low pH facilitates high metal solubility. In contrast, neutrophilic FeRB like Shewanella spp. tolerate only μM concentrations (34, 36). Geobacter spp. have not been tested to the best of our knowledge, but metal tolerance proteins are expressed during growth in uranium-contaminated sediments, which might be connected to metal resistance (19).
Near Ronneburg (Thuringia, Germany), uranium mining caused severe environmental contamination with metals and radionuclides (20). In creek bank alluvial soils of the Gessenbach, a main drainage system of upstream mining sites (41), high heavy metal concentrations occur both in solid phase and in the pore water of a ground- and surface water-influenced, oxidized, iron-rich Btlc horizon of a Luvic Gleysol. We demonstrated the solubilization of Co, Ni, Zn, As, and U in Btlc soil microcosms during biostimulated microbial Fe(III) reduction that was associated with the activity of microorganisms related to Delta- and Betaproteobacteria, Acidobacteria, and Firmicutes (7). The aims of this study were to (i) determine the heavy metal fraction of the solid phase, which could be released during reductive dissolution of Fe(III) oxides, (ii) estimate the effect of heavy metals on the activity of FeRB in the Gessenbach creek bank soil, and (iii) identify metal-tolerant FeRB, because the permanent exposure to contaminants during the last 50 years should have promoted metal tolerance.
Soil geochemistry.
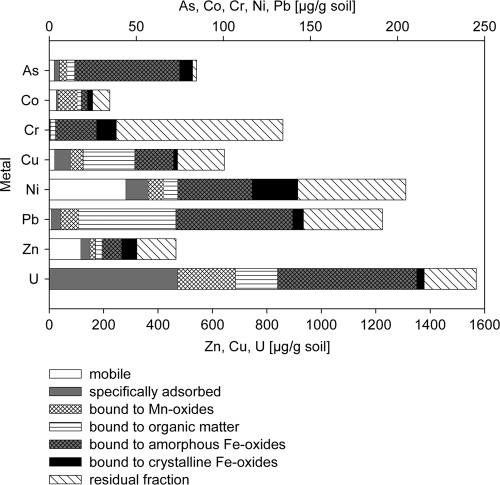
Putative binding forms of heavy metals in the Btlc soil solid phase were determined by sequential extraction (8, 43) in samples collected in August 2006. Metal concentrations were analyzed with either ICP-MS (inductively coupled plasma-mass spectrometry) or ICP-OES (optical emission spectrometry) (8). Most metals (20 to 40%) and even 80% of As in Btlc soil were detected in fraction 5, which is representative for amorphous Fe(III) oxides (Fig. 1). A considerable amount of uranium (30%) was recovered in the specifically adsorbed fraction, whereas only Zn and Ni were primarily recovered in the mobile fraction. Zn and Ni also dominated the heavy metal pore water concentration of the creek bank soil, which was sampled monthly from June to November 2007 (7). Pore water heavy metal and As concentrations always peaked in the Btlc horizon (see Fig. S1 in the supplemental material) and reached maximum concentrations of 38.6, 16.4, 3.9, 1.5, 0.6, and 0.3 μM for Zn, Ni, Al, Cu, Co, and Cd, respectively. Pore water Fe(II) concentrations, measured as previously described (7), increased with depth, starting at the transition between the horizons Btlc and Br1 (see Fig. S1). In the deeper horizons Br1 and Br2, pore water concentrations of heavy metals and As were low, although the solid phase was enriched with heavy metals (data not shown) caused by the permanent contact of contaminated groundwater and creek water.
Fig. 1.
Concentrations of selected metals obtained by sequential extraction from Btlc soil in different fractions, which can be correlated to the following putative binding forms: mobile (fraction 1), specifically adsorbed (fraction 2), bound to manganese oxides (fraction 3), bound to organic material (fraction 4), bound to amorphous Fe(III) oxides (fraction 5), bound to crystalline Fe(III) oxides (fraction 6), and bound to the residual fraction, presumably mainly silicates (fraction 7).
Metal tolerance of Fe(III)-reducing enrichment cultures.
Based on in situ concentrations, four concentrations of Cd, Cu, Ni, or Zn were added to Btlc soil suspensions cultured in media selective for Fe(III) reducers (Table 1). In addition, mixes of metals representing Btlc pore water concentrations (mix 1) or Btlc bioavailable concentrations (mix 2), which are assumed to be represented by fractions 1 and 2, were added. For the enrichment cultures, 1 ml of soil suspension (9 ml of 0.7% NaCl solution added to 1 g of soil) derived from Btlc soil obtained in April 2008 was added to 9 ml of a basal medium (23), with 1 mM each of ethanol and lactate as carbon sources, 14 mM amorphous Fe(OH)3 (24) as the electron acceptor, and 0.5 mM FeCl2 as the reductant. Uninoculated controls received only 1 ml of NaCl solution. The final pH was 6.5 to 6.8. Fe(II) formation was measured at selected time intervals (7, 22). Dissolved metal concentrations were measured to check for precipitation or adsorption in selected treatments by ICP-MS (7) or ICP-OES (8). In a subsequent experiment, more concentrations were tested to specify the metal tolerance, if necessary.
Table 1.
Rates of Fe(II) formation in metal-amended Fe(III)-reducing Btlc soil enrichment cultures in the presence and absence of metals
| Treatment | Concn added (μM) | Fe(II) formation rate ± SD [nmol Fe(II) day−1 ml−1]c |
|---|---|---|
| Control | No metal added | 509.17 ± 1.66 A/AB |
| Mix 1a | Low | 416.20 ± 51.92 B |
| Mix 2b | High | −3.27 ± 2.56 C |
| CdCl2 | 0.15 | 358.33 ± 33.38 B |
| 0.3 | 392.13 ± 9.06 A,B | |
| 15 | 337.83 ± 11.76 B | |
| 30 | 279.30 ± 104.08 B | |
| CoCl2 | 0.15 | 497.10 ± 47.72 AB |
| 0.35 | 551.57 ± 39.37 A | |
| 50 | 428.70 ± 39.02 AB | |
| 150 | 382.47 ± 89.73 B | |
| CuCl2 | 0.5 | 389.37 ± 52.05 B |
| 1.1 (0.8/0.02)d | 481.47 ± 43.16 A | |
| 150 | 19.90 ± 3.56 C | |
| 1,500 | 17.97 ± 8.56 C | |
| NiCl2 | 10 | 434.63 ± 18.07 B |
| 20 | 527.47 ± 33.33 A | |
| 150 (73/35)d | 290.70 ± 31.19 C | |
| 1,000 | 7.50 ± 0.17 D | |
| CrCl3 | 0.06 | 404.07 ± 29.98 A |
| 1 | 454.63 ± 43.92 A | |
| 10 | 334.57 ± 144.03 A | |
| 100 | 420.50 ± 24.18 A | |
| ZnCl2 | 20 | 463.00 ± 57.77 A |
| 40 | 479.03 ± 34.46 A | |
| 500 | 464.77 ± 42.05 A | |
| 2,500 (114/35)d | 245.67 ± 95.64 B | |
| AlCl3 | 2 | 531.43 ± 23.13 A |
| 4 | 403.87 ± 16.97 BC | |
| 100 | 378.97 ± 67.02 C | |
| 1,500 | 386.67 ± 42.67 C | |
| PbCl2 | 3 | 384.80 ± 19.70 A |
| 6 | 444.23 ± 68.35 A | |
| 20 | 399.17 ± 57.64 A | |
| 100 | 411.03 ± 87.65 A |
Components of mix 1: 0.3 μM CdCl2, 0.35 μM CoCl2, 1.1 μM CuCl2, 20 μM NiCl2, 0.06 μM CrCl3, 40 μM ZnCl2, 4 μM AlCl3, and 6 μM PbCl2.
Components of mix 2: 30 μM CdCl2, 150 μM CoCl2, 1,500 μM CuCl2, 1,000 μM NiCl2, 10 μM CrCl3, 2,500 μM ZnCl2, 1,500 μM AlCl3, and 20 μM PbCl2.
A, B, C, and D indicate groups with significant (P < 0.05) differences in Fe(II) formation rates within the concentration range tested. The control is included in all treatments as a metal concentration of 0. Each metal and mix were tested separately. The control belongs to group A for the Cd, Cr, Zn, Pb, Cu, Ni, and mixed metal treatments and to groups A and B for the Co and Al treatments.
Values shown in parentheses are the concentrations measured by ICP-MS at the end of the incubation in noninoculated controls/concentrations measured by ICP-MS at the end of the incubation in inoculated cultures.
Metal amendment affected Fe(II) formation rates (Table 1 and data not shown). Maximum pore water metal concentrations (mix 1) had no effect, but bioavailable solid-phase concentrations (mix 2) completely inhibited Fe(III) reduction. Also, addition of 150 μM Cu or 1,000 μM Ni caused complete inhibition of Fe(II) formation. In the presence of 100 μM Cu, 150 or 600 μM Ni, or 2,500 μM Zn, Fe(II) formation rates were reduced. Additionally, the lag phase was extended by about 2 weeks in cultures with 2,500 μM Zn. The addition of Co did not decrease Fe(II) formation rates, but the time before Fe(II) formation started was delayed by 1 week in the presence of 150 μM Co compared to that in the presence of controls with no metals added. Cd was tolerated at 100 μM, but at 500 μM, no Fe(II) formation was observed. For Al, Cr, and Pb, no toxic effects with up to 10 mM Al, 100 μM Cr, and 1,000 μM Pb were observed. The presence of As at up to 800 μM did not inhibit Fe(II) formation, but rates decreased at 1,600 μM As. These data indicated that Cu and Ni should be the main inhibitive contaminants in Btlc soil in situ. However, multiple effects of metal combinations cannot be ruled out.
Metal tolerances were comparable to those reported for Shewanella strains that cannot grow in the presence of 150 μM Co, 150 to 400 μM Zn, 75 to 150 μM Cd, or 150 to 400 μM Cu when cultivated aerobically in 10% LB broth (36). Anoxic hematite bioreduction by Shewanella putrefaciens is 50% inhibited by 210 μM Zn but not by 1.63 mM Ni (27). Clostridia, which can use Fe(III) as a sink for excess electrons from fermentation (16), can grow in the presence of 24 μM Cd but not 24 μM Cu (14). Cd solution concentrations of 2.8 to 1,503 μM can inhibit 50% of the Fe(III) reduction rate in different soils, depending on soil type (38). However, metal tolerances of those FeRB might be lower than those reported due to interactions of the amended metals with Fe(III) oxides or other medium components and due to metal sorption on cell surfaces (36, 37). When metal concentrations were checked at the end of our experiment, only 49, 5, or 73% of amended Ni, Zn, or Cu, respectively, were still dissolved in the medium of noninoculated controls, and even 23, 1, or 2% of those were dissolved in the inoculated cultures. Therefore, FeRB supposedly did not face the total concentration of amended metals, and their tolerance to dissolved metals should be much lower. Similarly, a previous study reported that the Cu tolerance of Shewanella varies between 75 and 750 mM depending on the nutrient load of the medium (36).
Identification of metal-tolerant microorganisms.
To identify metal-tolerant FeRB, samples of three cultures, containing the highest concentrations tested of each metal that still allowed Fe(II) formation (1.1 μM Cu, 150 μM Ni, and 2,500 μM Zn), were pooled for clone library construction (see the supplemental material). The 16S rRNA gene-based clone library almost exclusively contained sequences related to Deltaproteobacteria (69%) and the Firmicutes (29%) (see Table S1 in the supplemental material). The importance of these groups is similar to that found in biostimulated soil microcosm studies (7). All Deltaproteobacteria clones were related to Geobacter, a genus that dominates during uranium reduction in bioremediation experiments (3, 18). Within the Firmicutes, several genera which are known to reduce Fe(III) by shunting electrons from fermentation to Fe(III) were detected (16). Microbial community fingerprints of these cultures generated by denaturing gradient gel electrophoresis (DGGE) (see the supplemental material) demonstrated that some prominent bands were present in all cultures but that more bands were present in Cu-amended cultures than in Zn- and Ni-amended cultures. By sequencing the three main DGGE bands of the Zn-amended culture, all were identified as Firmicutes spp. related to Gram-positive fermenters of Sedimentibacter and Clostridiales strains that have not been reported for Fe(III) reduction (Fig. 2). One sequence was closely related to Sedimentibacter strains found in dechlorinating enrichments (5, 10). The Clostridiales-related sequence shared the highest identity with a clone obtained from a tar oil-impacted aquifer (40); the closest cultured relative was Clostridium aminobutyricum (95% identity). The same bands were also observed in the 150 μM Ni and 1.1 μM Cu treatment, indicating that the Firmicutes might also be important in those cultures. Studies of bacterial metal sorption indicated that isolated cell walls from Gram-positive organisms bind more metal than isolated envelopes of Gram-negative bacteria (37). Due to cellular metal sequestration, fermentative Firmicutes spp. may be important for ameliorating metal toxicity to FeRB within a community, as was seen in a bacterial consortium from metal-contaminated sediments (25). However, it cannot be ruled out that Firmicutes spp. might have survived as spores in the soil (5, 39) and were activated after nutrient addition in the enrichment cultures.
Fig. 2.
Microbial community DGGE patterns generated from metal-amended cultures. The main bands that were identified by sequencing are labeled, and the top BLAST hit is given with its GenBank accession number and percent identity. Band 1, Sedimentibacter clone VE117 (GenBank accession no. EF681724.1) with 99% identity; band 2, Clostridiales bacterium clone D10_24 (GenBank accession no. EU266794.1) with 99% identity.
Establishment of a metal-tolerant FeRB coculture.
The metal-amended Fe(III)-reducing enrichment cultures were used for isolation attempts, with a modified agar shake technique (see the supplemental material). An isolated colony composed of uniform rod-shaped cells under a light microscope was obtained after the fifth transfer. When transferred to liquid medium, 25% of the supplemented Fe(III) was reduced, with lactate and ethanol as the electron donors. Consumption of lactate and ethanol resulted in the accumulation of propionate and acetate (data not shown). Although the isolate was thought to be a pure culture, 16S rRNA sequence analysis revealed that it was actually a coculture of two strains. The two strains, DA-1 and DA-2, were affiliated with the family Peptococcaceae within the Firmicutes, with strain DA-1 being most closely related to the Desulfosporosinus lacus strain STP12T (GenBank accession no. AJ582757) and an uncultured bacterium clone, GIF4 (GenBank accession no. AF407196), derived from monochlorobenzene-contaminated groundwater (1) (98% sequence similarity to both). Strain DA-2 was most closely related to Desulfitobacterium dichloroeliminans strain LMG (GenBank accession no. AJ565938) (96% sequence similarity).
Members of the Peptococcaceae were detected previously in biostimulated Fe(III)-reducing Btlc microcosms at the end of incubation when sulfate reduction also occurred (7). D. lacus can reduce sulfate and, unlike other strains, also Fe(III) in the presence of lactate and can grow fermentatively with pyruvate and lactate (28, 33). Since sulfate was not present in the enrichment cultures, DA-1 had to grow either fermentatively on lactate or by coupling growth to Fe(III) reduction. Although D. lacus was isolated from a pristine environment, members of this genus have regularly been detected in various contaminated sites, including radionuclide-contaminated sediment (26, 30, 35). D. lacus tolerates 2 mM As but is inhibited by 10 mM As, 10 μM Cd, 0.4 mM Cr, and 10 μM Zn (28). These concentrations are similar to the dissolved metal concentrations shown in our enrichments. DA-2 was distantly related to Desulfitobacterium spp., which are typically isolated from contaminated environments where they dechlorinate halogenated compounds or respire metals, such as As(V), Fe(III), Mn(IV), and Cr(VI) (14, 33).
Potential Fe(III) reduction rates of creek bank soil.
Since the presence of Fe(II) in the pore water suggested ongoing in situ Fe(III) reduction, we incubated soil obtained from the Btlc and the deeper permanently reduced Br1 and Br2 horizons as slurries under anoxic conditions (7, 32) and calculated Fe(III) reduction rates from the regression slope during the linear increase of Fe(II). Initial Fe(II) formation rates in Btlc microcosms were negligible or did not exceed 2 μmol day−1 g (wet weight) soil−1 despite the high Fe(III) and carbon contents of this horizon (Fig. 3). Biostimulation with lactate or ethanol increased initial Fe(II) formation rates by up to four times, suggesting that an additional energy source is necessary to drive energy-consuming detoxification processes, like ATP-driven efflux pumps or the production of metal-binding compounds (2, 6, 11, 31). In contrast, the Br1 and Br2 horizons showed higher Fe(II) formation rates of up to 6 μmol day−1 g (wet weight) soil−1 independent of carbon amendment (Fig. 3), which corresponded with the higher Fe(II) concentrations detected in the pore water of the Br1 and Br2 horizons. The low heavy metal pore water concentrations in these horizons suggest effective metal attenuation processes. 35S tracer studies recently demonstrated that both of the reduced horizons have ongoing sulfate-reducing activity (32) and that the release of sulfide may have lead to the retention of metals as metal sulfides (13, 15, 21). Therefore, metabolically diverse sulfate reducers that can switch to Fe(III) reduction could fill an important niche in stratified contaminated soils. In addition, FeRB, like Geobacter spp. and fermentative FeRB, might profit from metal sulfide precipitation as a by-product of sulfate reduction. All together, in the presence of metal contaminants, microbial Fe(III) reduction may be dependent on (i) the presence of a sufficient carbon source to fuel detoxification, (ii) metal scavenging by cellular metal sequestration, and (iii) the metabolic diversity of nonclassical FeRB.
Fig. 3.
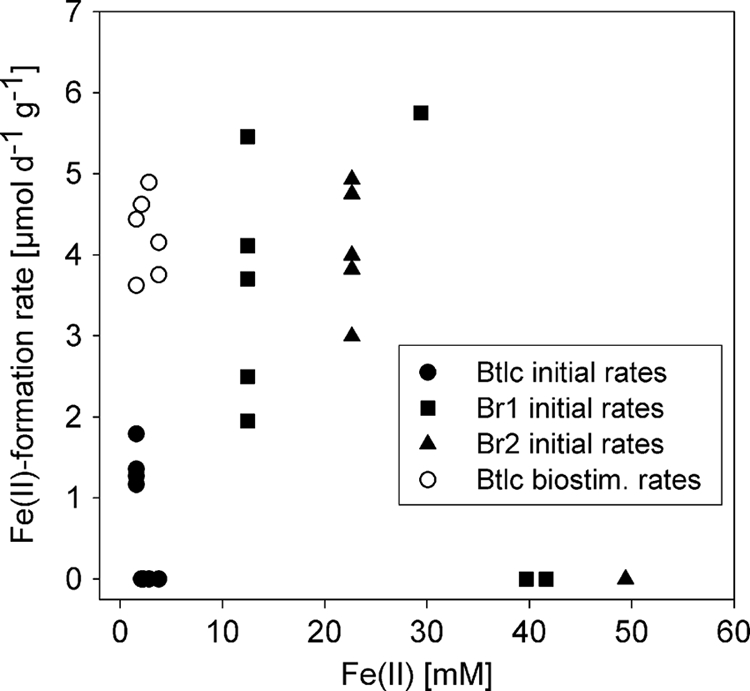
Fe(II) formation rates obtained in anoxic soil microcosms in relation to the initial Fe(II) concentrations. Initial Fe(II) formation rates were determined in carbon-unamended soil microcosms of horizons Btlc, Br1, and Br2, whereas biostimulated Fe(II) formation rates were determined in carbon-amended Btlc microcosms.
Supplementary Material
Acknowledgments
This work was supported by the German Science Foundation (DFG) within the Research Training Group 1257, “Alteration and Element Mobility at the Microbe Mineral Interface,” which is part of the Jena School for Microbial Communication (JSMC).
We thank Dirk Merten for helpful discussions and Sylvia Löffler, Jana Sitte, Christian Kaufmann, and Claudia Lüdecke for technical assistance.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 4 March 2011.
REFERENCES
- 1. Alfreider A., Vogt C., Babel W. 2002. Microbial diversity in an in situ reactor system treating monochlorobenzene contaminated groundwater as revealed by 16S ribosomal DNA analysis. Syst. Appl. Microbiol. 25:232–240 [DOI] [PubMed] [Google Scholar]
- 2. Amoroso M. J., Schubert D., Mitscherlich P., Schumann P., Kothe E. 2000. Evidence for high affinity nickel transporter genes in heavy metal resistant Streptomyces spec. J. Basic Microbiol. 40:295–301 [DOI] [PubMed] [Google Scholar]
- 3. Anderson R. T., et al. 2003. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 69:5884–5891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bradl H. B. 2004. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 277:1–18 [DOI] [PubMed] [Google Scholar]
- 5. Breitenstein A., et al. 2002. Reclassification of Clostridium hydroxybenzoicum as Sedimentibacter hydroxybenzoicus gen. nov., comb. nov., and description of Sedimentibacter saalensis sp. nov. Int. J. Syst. Evol. Microbiol. 52:801–807 [DOI] [PubMed] [Google Scholar]
- 6. Bruins M. R., Kapil S., Oehme F. W. 2000. Microbial resistance to metals in the environment. Ecotoxicol. Environ. Saf. 45:198–207 [DOI] [PubMed] [Google Scholar]
- 7. Burkhardt E.-M., et al. 2010. Impact of biostimulated redox processes on metal dynamics in an iron-rich creek soil of a former uranium mining area. Environ. Sci. Technol. 44:177–183 [DOI] [PubMed] [Google Scholar]
- 8. Burkhardt E.-M., Meißner S., Merten D., Büchel G., Küsel K. 2009. Heavy metal retention and microbial activities in geochemical barriers formed in glacial sediments subjacent to a former uranium mining leaching heap. Chem. Erde 69(Suppl. 2):21–34 [Google Scholar]
- 9. Cambier P., Charlatchka R. 1999. Influence of reducing conditions on the mobility of divalent trace metals in soils, p. 159–175 In Selim H. M., Iskandar I. K. (ed.), Fate and transport of heavy metals in the vadose zone. Lewis Publishers, Boca Raton, FL [Google Scholar]
- 10. Cheng D., Chow W. L., He J. 2010. A Dehalococcoides-containing co-culture that dechlorinates tetrachloroethene to trans-1,2-dichloroethene. ISME J. 4:88–97 [DOI] [PubMed] [Google Scholar]
- 11. Dimkpa C. O., et al. 2008. Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere 74:19–25 [DOI] [PubMed] [Google Scholar]
- 12. Dopson M., Baker-Austin C., Koppineedi P. R., Bond P. L. 2003. Growth in sulfidic mineral environments: metal resistance mechanisms in acidophilic micro-organisms. Microbiology 149:1959–1970 [DOI] [PubMed] [Google Scholar]
- 13. Ehrlich H. L. 1999. Microbes as geologic agents: their role in mineral formation. Geomicrobiol. J. 16:135–153 [Google Scholar]
- 14. Finneran K. T., Forbush H. M., Gaw VanPraagh C. V., Lovley D. R. 2002. Desulfitobacterium metallireducens sp. nov., an anaerobic bacterium that couples growth to the reduction of metals and humic acids as well as chlorinated compounds. Int. J. Syst. Evol. Microbiol. 52:1929–1935 [DOI] [PubMed] [Google Scholar]
- 15. Fortin D., Southam G., Beveridge T. J. 1994. Nickel sulfide, iron-nickel sulfide and iron sulfide precipitation by a newly isolated Desulfotomaculum species and its relation to nickel resistance. FEMS Microbiol. Ecol. 14:121–132 [Google Scholar]
- 16. Francis A. J., Dodge C. J. 1988. Anaerobic microbial dissolution of transition and heavy metal oxides. Appl. Environ. Microbiol. 54:1009–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Francis A. J., Dodge C. J. 1990. Anaerobic microbial remobilization of toxic metals coprecipitated with iron oxide. Environ. Sci. Technol. 24:373–378 [Google Scholar]
- 18. Holmes D. E., Finneran K. T., O'Neil R. A., Lovley D. R. 2002. Enrichment of members of the family Geobacteraceae associated with stimulation of dissimilatory metal reduction in uranium-contaminated aquifer sediments. Appl. Environ. Microbiol. 68:2300–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holmes D. E., et al. 2009. Transcriptome of Geobacter uraniireducens growing in uranium-contaminated subsurface sediments. ISME J. 3:216–230 [DOI] [PubMed] [Google Scholar]
- 20. Jakubick A. T., Gatzweiler R., Mager D., Robertson A. M. 1997. The Wismut waste rock pile remediation program of the Ronneburg mining district, Germany, p. 1285–1301 Abstr. 4th Int. Conf. Acid Rock Drainage, Vancouver, BC, Canada [Google Scholar]
- 21. Krumholz L. R., Elias D. A., Suflita J. M. 2003. Immobilization of cobalt by sulfate-reducing bacteria in subsurface sediments. Geomicrobiol. J. 20:61–72 [Google Scholar]
- 22. Küsel K., Roth U., Drake H. L. 2002. Microbial reduction of Fe(III) in the presence of oxygen under low pH conditions. Environ. Microbiol. 4:414–421 [DOI] [PubMed] [Google Scholar]
- 23. Lin B., et al. 2007. Phylogenetic and physiological diversity of dissimilatory ferric iron reducers in sediments of the polluted Scheldt estuary, Northwest Europe. Environ. Microbiol. 9:1956–1968 [DOI] [PubMed] [Google Scholar]
- 24. Lovley D. R., Phillips E. J. P. 1986. Organic matter mineralisation with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 51:683–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Markwiese J. T., Colberg P. J. S. 2000. Bacterial reduction of copper-contaminated ferric oxide: copper toxicity and the interaction between fermentative and iron-reducing bacteria. Arch. Environ. Contam. Toxicol. 38:139–146 [DOI] [PubMed] [Google Scholar]
- 26. Nevin K. P., Finneran K. T., Lovley D. R. 2003. Microorganisms associated with uranium bioremediation in a high-salinity subsurface sediment. Appl. Environ. Microbiol. 69:3672–3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paul C. C., Stone J. J. 2009. Effects of nickel and soil humic acid during biological hematite reduction by Shewanella putrefaciens CN32. Environ. Eng. Sci. 26:841–848 [Google Scholar]
- 28. Ramamoorthy S., et al. 2006. Desulfosporosinus lacus sp. nov., a sulfate-reducing bacterium isolated from pristine freshwater lake sediments. Int. J. Syst. Evol. Microbiol. 56:2729–2736 [DOI] [PubMed] [Google Scholar]
- 29. Schwertmann U., Taylor R. M. 1989. Iron oxides, p. 379–438 In Dixon J. B., Weed S. B. (ed.), Minerals in soil environments. SSSA book series no. 1. Soil Science Society of America, Madison, WI [Google Scholar]
- 30. Shelobolina E. S., O'Neill K., Finneran K. T., Hayes L. A., Lovley D. R. 2003. Potential for in situ bioremediation of a low-pH, high-nitrate uranium-contaminated groundwater. Soil Sediment Contam. 12:865–884 [Google Scholar]
- 31. Silver S. 1996. Bacterial resistances to toxic metal ions—a review. Gene 179:9–19 [DOI] [PubMed] [Google Scholar]
- 32. Sitte J., et al. 2010. Sulfate-reducing activity linked to metal retention in contaminated soil located at a former uranium-mining district (Ronneburg, Germany). Appl. Environ. Microbiol. 76:3143–3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spring S., Rosenzweig F. 2006. The genera Desulfitobacterium and Desulfosporosinus: taxonomy, p. 771–786 In Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E. (ed.), The prokaryotes. Springer, New York, NY [Google Scholar]
- 34. Stone J. J., Burgos W. D., Royer R. A., Dempsey B. A. 2006. Impact of zinc on biological Fe(III) and nitrate reduction by Shewanella putrefaciens CN32. Environ. Eng. Sci. 23:691–704 [Google Scholar]
- 35. Suzuki Y., Kelly S. D., Kemner K. M., Banfield J. F. 2002. Nanometre-size products of uranium bioreduction. Nature 419:134. [DOI] [PubMed] [Google Scholar]
- 36. Toes A.-C. M., Geelhoed J. S., Kuenen J. G., Muyzer G. 2008. Characterization of heavy metal resistance of metal-reducing Shewanella isolates from marine sediments. Geomicrobiol. J. 25:304–314 [Google Scholar]
- 37. Urrutia M. M. 1997. General bacterial sorption processes, p. 39–66 In Wase J. D. A., Forster C. (ed.), Biosorbents for metal ions. Taylor and Francis, Bristol, PA [Google Scholar]
- 38. Welp G., Brümmer G. W. 1997. Microbial toxicity of Cd and Hg in different soils related to total and water-soluble contents. Ecotoxicol. Environ. Saf. 38:200–204 [DOI] [PubMed] [Google Scholar]
- 39. Wiegel J., Tanner R., Rainey F. A. 2006. An introduction to the family Clostridiaceae, p. 654–678 In Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E. (ed.), The prokaryotes. Springer, New York, NY [Google Scholar]
- 40. Winderl C., Anneser B., Griebler C., Meckenstock R. U., Lueders T. 2008. Depth-resolved quantification of anaerobic toluene degraders and aquifer microbial community patterns in distinct redox zones of a tar oil contaminant plume. Appl. Environ. Microbiol. 74:792–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wismut GmbH 2006. Umweltbericht Wismut 2005. Wismut GmbH, Chemnitz, Germany [Google Scholar]
- 42. Zachara J. M., Fredrickson J. K., Smith S. C., Gassman P. L. 2001. Solubilization of Fe(III) oxide-bound trace metals by a dissimilatory Fe(III) reducing bacterium. Geochim. Cosmochim. Acta 65:75–93 [Google Scholar]
- 43. Zeien H., Brümmer G. W. 1989. Chemische Extraktionen zur Bestimmung von Schwermetallbindungsformen in Böden. Mitt. Dtsch. Bodenkundl. Ges. 59:505–510 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.