Abstract
Filamentous fungi are the cause of serious human and plant diseases but are also exploited in biotechnology as production platforms. Comparative genomics has documented their genetic diversity, and functional genomics and systems biology approaches are under way to understand the functions and interaction of fungal genes and proteins. In these approaches, gene functions are usually inferred from deletion or overexpression mutants. However, studies at these extreme points give only limited information. Moreover, many overexpression studies use metabolism-dependent promoters, often causing pleiotropic effects and thus limitations in their significance. We therefore established and systematically evaluated a tunable expression system for Aspergillus niger that is independent of carbon and nitrogen metabolism and silent under noninduced conditions. The system consists of two expression modules jointly targeted to a defined genomic locus. One module ensures constitutive expression of the tetracycline-dependent transactivator rtTA2S-M2, and one module harbors the rtTA2S-M2-dependent promoter that controls expression of the gene of interest (the Tet-on system). We show here that the system is tight, responds within minutes after inducer addition, and allows fine-tuning based on the inducer concentration or gene copy number up to expression levels higher than the expression levels of the gpdA promoter. We also validate the Tet-on system for the generation of conditional overexpression mutants and demonstrate its power when combined with a gene deletion approach. Finally, we show that the system is especially suitable when the functions of essential genes must be examined.
INTRODUCTION
The metabolic versatility of filamentous fungi makes them outstanding cell factories in biotechnology. Important fungal metabolites that are commercially produced include pharmaceuticals, such as antibiotics and immunosuppressants; bulk commodities, such as organic acids; and enzymes exploited in various industrial sectors (25). Also, some filamentous fungi are phytopathogenic, causing huge agricultural losses, while others are causative agents of devastating human diseases (10). Within the last years, genome sequences for most of the important industrial, agricultural, and medical filamentous fungi have been published (for reviews, see references 5 and 12), thus facilitating multiple research activities, including genome-mining approaches to spot new metabolites and enzymes (9, 47), bioinformatics developments to reconstruct metabolic pathways (2), functional genomics attempts to study the functions of genes (11), and systems biology experiments to dissect the interactions of genes and proteins (1, 3).
A key resource for these approaches and the understanding of gene functions and networks is knockdown or knockout mutant collections, which provide a wealth of information. However, studies in Saccharomyces cerevisiae and higher eukaryotes, such as Drosophila and Caenorhabditis elegans, have shown that the majority of knockdown/knockout mutants have little, if any, discernible loss-of-function phenotype (13, 21). This observation is thought to result from the general robustness of biological systems and their buffering capacities against cellular variations, e.g., due to gene redundancies and compensatory pathways (19, 38). Moreover, mutants with essential genes deleted evade analysis, imposing further limitations on this approach. An alternative and complementary approach that overcomes these limitations is the generation of overexpression mutant collections. Attempts in yeast, plant, and insect systems have shown their power to assign gene functions where loss-of-function approaches have failed (38, 41, 48). However, there are limitations, as well. If metabolism-dependent promoters are used for overexpression studies, one is restricted in the choice of growth medium. In addition, the phenotypes observed might be ambiguous due to pleiotropic effects caused by metabolic changes. Moreover, if these systems mediate only an “on/off” situation, expression at a defined, user-specified level is not permitted. Therefore, tunable and metabolism-independent promoter systems have been developed for yeast, plant, insect, and mammalian systems, allowing more flexibility in experimental approaches (23, 24, 33, 46).
For filamentous fungi, an impressive set of promoters are available, allowing overexpression of a gene of interest (GOI). However, these promoters are either constitutively active and growth related (e.g., the Aspergillus niger glyceraldehyde-3-phosphate dehydrogenase promoter, PgpdA) or dependent on the carbon or nitrogen source (e.g., A. niger PglaA and PinuE, Aspergillus nidulans PalcA, Neurospora crassa Pqa-2, Ustilago maydis Pcrg1 and Pnar1, and Trichoderma reesei Pcbh1), and some are also leaky (e.g., PinuE and Pqa-2) (7, 22, 36, 37). In search of promoters that are tight, tunable, and metabolism independent, three systems have recently been tested for application in filamentous fungi: the thiamine promoter system (PthiA) in Aspergillus oryzae (40), the human estrogen receptor (hERα) system in A. nidulans and A. niger (32), and a system based on the Escherichia coli tetracycline resistance operon (Tet) in Aspergillus fumigatus (45). All three systems mediate gene expression in an inducer-dependent manner; however, the PthiA system is inactive under alkaline growth conditions, and hERα systems are either strongly inducible but leaky or tight but weakly inducible (32, 40). In comparison, the Tet system appeared to be the most promising for use in filamentous fungi (45).
Basically, the Tet system is a negative regulatory circuit of E. coli involving the repressor protein TetR, which binds to the operator sequence (tetO) of the Tn10 tetracycline resistance operon in the absence of tetracyclines. Transcription of the operon is thus shut down. However, the interaction of repressor and operator can be efficiently prevented by tetracycline, which forces TetR to dissociate from tetO. In consequence, transcription of the operon is initiated, thus conferring tetracycline resistance (4). Both TetR and tetO have been adapted for efficient use in eukaryotic systems (for detailed information, see references 15–18 and 42). In brief, TetR has been combined with the minimal transcriptional activation domain derived from the herpes simplex virus protein 16 (VP16). The generated hybrid transactivator, tTA, stimulates gene expression in the absence of tetracycline (the Tet-off system). This system has been modified so that tetracycline or its derivative doxycycline (Dox) induces but does not abolish binding to the operator sequence. For this purpose, the binding activities of tTA were modified and the reverse hybrid transactivator rtTA was generated (the Tet-on system). In order to increase the sensitivity of rtTA to Dox and to optimize rtTA expression in eukaryotic systems, additional mutations were introduced, eventually resulting in the reverse hybrid transactivator rtTA2S-M2. Finally, the tetracycline-responsive promoter was optimized for maximum expression levels by placing seven copies of the tetO sequence (tetO7) upstream of a minimal promoter (Pmin).
To judge, whether the Tet-on/Tet-off system is generally applicable to A. fumigatus, Vogt et al. constructed three plasmids, one containing the expression cassette ensuring constitutive expression of rtTA2S-M2 (PgpdA::rtTA2S-M2), one containing PgpdA::tTA, and one harboring the tetO7 sequence linked to a 175-bp minimal promoter sequence of PgpdA (Pmin) upstream of the E. coli hygromycin resistance gene hph (tetO7::Pmin::hph). When plasmid pairs were cotransfected to A. fumigatus, hygromycin-resistant clones were detected in either the presence or absence of Dox, demonstrating that the Tet system is in principle functional in A. fumigatus (45). However, the dynamics of the system and the effects of gene dosage on the efficacy and tightness of the system were not studied.
As detailed understanding of the performance of the Tet system is indispensable for future applications in filamentous fungi, we decided to redesign the Tet-on system and to systematically evaluate it using the industrial model fungus A. niger as the host and the codon-optimized luciferase gene mluc (14) as a reporter. Different A. niger transformants containing one or multiple copies of the newly constructed Tet-on system were generated. The strains were cultivated at microtiter plate (MTP) and bioreactor scales in the presence or absence of Dox, and luciferase activities were determined at minute intervals. The data obtained demonstrate that the Tet-on system is tight under noninduced conditions and can respond within minutes after Dox addition. It allows tunable gene control, which can be user specified by varying the Dox concentration or gene copy numbers. Most importantly, the system reaches high expression levels comparable to those of the strong gpdA promoter. To demonstrate the utility of this tool, we finally used our Tet-on version to establish overexpression mutants and report on a Tet-on-based strategy to establish mutants that have essential genes deleted but are viable under selected conditions. The data from this work suggest that the Tet-on system can serve as a general and excellent tool for functional genomics and systems biology approaches in filamentous fungi.
MATERIALS AND METHODS
Strains, culture conditions, and molecular techniques.
The A. niger strains used in this study are given in Table 1 and were cultivated in minimal medium (MM) (6) containing 1% glucose as a carbon source or in complete medium (CM), consisting of MM supplemented with 1% yeast extract and 0.5% Casamino Acids. Uridine (10 mM) was added when required. Fermentation medium (FM) is composed of 0.75% glucose, 0.45% NH4Cl, 0.15% KH2PO4, 0.05% KCl, 0.05% MgSO4, 0.1% salt solution (6), and 0.003% yeast extract. The pH of the FM was adjusted to pH 3. General cloning procedures in E. coli were done according to the methods described by Sambrook and Russell (39). Transformation of A. niger, selection procedures, genomic-DNA extraction, and Southern analyses were performed using recently described protocols (28). Quantitative real-time PCR was carried out as described previously (31). The primer pairs for mluc, gpdA, and H2B (used as a reference gene) are summarized in Table S1 in the supplemental material.
Table 1.
A. niger strains used in this study
| Strain | Code | Relevant genotype (comment) | Source |
|---|---|---|---|
| N402 | Wild type | Laboratory collection | |
| AB4.1 | pyrG− | 44 | |
| MA70.15 | pyrG− ΔkusA | 26 | |
| MA77.1 | pyrG+ ΔkusA (MA70.15 derivative) | This work | |
| MA78.6 | ΔkusA (N402 derivative) | 8 | |
| MA169.4 | pyrG−kusA− | 8 | |
| VG5.1 | L0 | pyrG+ ΔkusA (transformed with pVG2.2; single copy) | This work |
| VG6.1 | L1 | pyrG+ ΔkusA (transformed with pVG4.1; single copy) | This work |
| VG6.3 | L1 | pyrG+ ΔkusA (transformed with pVG4.1; single copy) | This work |
| VG7.2 | L0 | pyrG+ (transformed with pVG2.2; single copy) | This work |
| VG8.27 | L1 | pyrG+ (transformed with pVG4.1; single copy) | This work |
| VG8.1 | L2 | pyrG+ (transformed with pVG4.1; double copy) | This work |
| VG8.2 | L3 | pyrG+ (transformed with pVG4.1; triple copy) | This work |
| MA147.8 | ΔcfrA | ΔcfrA pyrG+ ΔkusA (MA70.15 derivative) | This work |
| MA148.7 | ΔcfrB | ΔcfrB pyrG+ ΔkusA (MA70.15 derivative) | This work |
| MA149.2 | ΔcfrC | ΔcfrC pyrG+ ΔkusA (MA70.15 derivative) | This work |
| MA151.1 | ΔcfrE | ΔcfrE pyrG+ ΔkusA (MA70.15 derivative) | This work |
| MA152.5 | ΔcfrF | ΔcfrF pyrG+ ΔkusA (MA70.15 derivative) | This work |
| MA153.1 | ΔcfrG | ΔcfrG pyrG+ ΔkusA (MA70.15 derivative) | This work |
| MA173.18 | OEcfrA | TetO7::Pmin::cfrA pyrG+ (AB4.1 derivative) | This work |
| MA174.5 | OEcfrB | TetO7::Pmin::cfrB pyrG+ (AB4.1 derivative) | This work |
| MA175.3 | OEcfrC | TetO7::Pmin::cfrC pyrG+ (AB4.1 derivative) | This work |
| MA177.6 | OEcfrE | TetO7::Pmin::cfrE pyrG+ (AB4.1 derivative) | This work |
| MA178.2 | OEcfrF | TetO7::Pmin::cfrF pyrG+ (AB4.1 derivative) | This work |
| MA179.6 | OEcfrG | TetO7::Pmin::cfrG pyrG+ (AB4.1 derivative) | This work |
| MA216.9 | OEactA | TetO7::Pmin::actA pyrG+ (MA169.4 derivative) | This work |
| MA224.1 | Δ-OEactA | TetO7::Pmin::actA pyrG+ ΔactA hygr (MA216.9 derivative) | This work |
Cultivation in 96-well MTPs.
In each well of 96-well MTPs, 104 freshly harvested spores of A. niger transformants were inoculated in 100 μl CM or MM and cultivated at 30°C for a maximum of 24 h. At 0, 6, or 16 h after inoculation, 30 μl of Dox was added to induce the expression of mluc (diluted in MM or CM; final concentrations, 0 to 500 μg/ml) and 70 μl of the substrate luciferin (diluted in MM or CM; final concentration, 1.4 mM) to detect mLuc activity. Luminescence (luminescent counts per second [LCPS]) and biomass formation were measured in situ every 5 min using Victor3 (Perkin Elmer) at 537 and 620 nm, respectively. Three independently growing cultures were analyzed for every experimental condition.
Bioreactor cultivation.
Freshly harvested conidia (2.5 × 108) from the different A. niger strains were used to inoculate 1 liter FM. Cultivations were performed in a BioFlo/CelliGen 115 bioreactor (New Brunswick Scientific), where the temperature (set to 30°C), pH (set to 3), and agitation speed were controlled on line. The dissolved oxygen tension was at all times above 40% to ensure sufficient oxygen supply. The cultivation program followed two phases. (i) During the first 5 h of cultivation, the agitation speed was set to 250 rpm, and aeration was performed via the headspace (1 liter min−1). These precautions help to reduce loss of hydrophobic conidia via the exhaust gas. (ii) After this period, more than 90% of the spores had started to germinate. Therefore, the agitation speed was set to 750 rpm and air was supplied through a ring sparger system to allow optimal mixing and oxygen transfer (1 liter min−1). To avoid foam formation, 0.01% (vol/vol) polypropylene glycol was added as an antifoam agent to the culture broth. Dox was added to the culture (final concentration, 5 or 20 μg/ml) during the early exponential growth phase, i.e., when the culture reached a density of 1 g (dry weight) biomass/kg culture broth (run time, about 17.5 h). Mycelial samples were taken after certain points in time (before and after Dox addition) to determine the biomass concentration and luciferase activity using a microtiter plate-based reading assay. In doing so, 130 μl freshly harvested biomass samples were mixed with 70 μl luciferin (diluted in FM; final concentration, 1.4 mM), and the luminescence and biomass were measured at 537 and 620 nm, respectively, using Victor3 (Perkin Elmer). All measurements were performed at least in triplicate.
Cloning of a Tet-on reporter-control construct.
The expression vector pVG4.1 (PgpdA::rtTA::TcgrA-tetO7::Pmin::mluc::TtrpC-pyrG*) and the negative-control vector pVG2.2, which lacks the mluc open reading frame (ORF) (PgpdA::rtTA::TcgrA-tetO7::Pmin::TtrpC-pyrG*), were constructed as described in detail in Fig. S1 in the supplemental material. Both plasmids and information on the respective DNA sequences have been deposited at the Fungal Genetics Stock Center (FGSC) (http://www.fgsc.net). The PgpdA::rtTA::TcgrA fragment was taken from plasmid p474 (obtained from FGSC [45]) and tetO7::Pmin, as well as TtrpC fragments from plasmid p500 (obtained from FGSC [45]). The pyrG* allele was taken from plasmid pAB94 (43) in order to facilitate integration of the constructs at the endogenous pyrG locus of A. niger. The pyrG− strains AB4.1 and MA70.15 were used as recipient strains for transformation. Strain MA70.15 is deleted in the ku70-homologous kusA gene, thus allowing targeted integration at high frequencies (26). Uracil prototroph transformants were selected, purified, and subjected to Southern analyses according to the method of Meyer et al. (28) to confirm homologous integrations of pVG2.2 and pVG4.1 at the pyrG locus. Isolated strains carrying one or more copies of both plasmids are given in Table 1.
Construction of cfr deletion and overexpression strains.
Constructs to delete the cfrA (An08g08280), cfrB (An07g07170), cfrC (An16g05020), cfrE (An11g05330), cfrF (An18g03740), or cfrG (An17g02350) gene were made as follows: cfrX 5′ flanking sequences (length, ∼700 bp) were obtained as KpnI-XhoI fragments by PCR using genomic DNA from strain N402 as a template and the primer pairs cfrXP1KpnI and cfrXP2XhoI. cfrX 3′ flanking sequences (length, ∼700 bp) were obtained as HindIII-NotI fragments from N402 genomic DNA by PCR using the primer pairs cfrXP3HindIII and cfrXP4NotI (the only exception was cfrG; there, the 5′ flanking sequence was NotI-HindIII and the 3′ flanking sequence was XhoI-KpnI). The respective 5′ PCR products were digested with KpnI and XhoI and ligated into KpnI-XhoI-opened pBluescript II SK(+) (Fermentas). A 1.7-kb XhoI-HindIII fragment of the A. oryzae pyrG gene was isolated from pMA172 (8), and the cfrX 3′ flanking sequences were digested with HindIII and NotI. Both fragments were ligated into XhoI-NotI-opened pBluescript II SK(+) vector containing the respective cfrX 5′ flanking sequence. The pyrG− strain MA70.15 was used as a recipient strain for transformation. Successful deletions were identified via Southern analyses.
cfr overexpression (OEcfr) constructs were made as follows. The ORF was obtained by PCR using N402 genomic DNA as a template and the primer pairs cfrXP5PmeI and cfrXP6PmeI, which introduced PmeI overhangs. The PCR products were ligated into pJET1.2 (Fermentas), sequenced, and cloned in the right orientation via PmeI restriction into plasmid pVG2.2. The plasmids obtained (pVG2.2-cfrX) were transformed into the pyrG− strain AB4.1 using conferred uracil prototrophy for selection. Uracil prototroph transformants were purified and subjected to Southern analyses to confirm homologous integrations of pVG2.2-cfrX at the pyrG locus. The strains are summarized in Table 1.
Construction of conditional actin overexpression and loss-of-function strains.
In order to establish a conditional actA overexpression strain, the actA ORF was cloned into pVG2.2, and the plasmid obtained, pVG2.2-actA, was used to transform the pyrG− strain MA169.4, which contains a transiently disrupted kusA allele for improved gene targeting (8). A pyrG+ transformant carrying a single copy of PgpdA::rtTA::TcgrA-tetO7::Pmin::actA::TtrpC at the pyrG locus was isolated by Southern analysis and used for further analysis. This strain, named MA216.9, was used as a recipient strain to delete the actin gene. In doing so, an actin disruption cassette was made, consisting of the hygromycin resistance cassette (a 3.1-kb fragment obtained from pAN7-1 [35] via HindIII/XhoI restriction) flanked by ∼700-bp-long sequence stretches of actA. Note that the 5′ flanking sequence consisted of about 400 bp of the 5′ untranslated region and the first 300 bp of the actA ORF. This construct was transformed into MA216.9, and putative actA loss-of-function strains were isolated and purified on plates containing 100 μg/ml hygromycin and 4 μg/ml Dox. Successful deletion of actA was verified by Southern analysis.
RESULTS
Establishing a Tet-on system for A. niger.
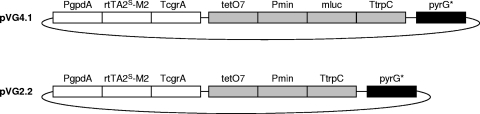
Aiming at the highest control of the genomic integration of the system, thus allowing direct comparison of different transformants, we decided to redesign the Tet-on system and to integrate all of its components into a single plasmid. In doing so, plasmid pVG4.1 was constructed, comprising both the PgpdA::rtTA2S-M2 and the tetO7::Pmin::mluc expression cassette, as well as a third cassette, pyrG*, necessary for selection and gene targeting (Fig. 1; for cloning details, see Fig. S1 in the supplemental material). As a control plasmid, pVG2.2, which is identical to pVG4.1 but lacks the mluc gene, was generated. Strain MA70.15 (pyrG− ΔkusA) and strain AB4.1 (pyrG−) were used as recipient strains, in order to obtain single-copy or multicopy transformants, respectively (26). Transformants selected for growth in the absence of uridine were isolated and purified. Twenty transformants were screened via Southern hybridizations for the presence of single or multiple plasmid copies at the pyrG locus. Strains harboring one homologously integrated copy of the control construct pVG2.2 were named L0 (for no mluc copy), and strains harboring one or two copies of pVG4.1 at the pyrG locus were named L1 and L2 (for one or two mluc copies). We also identified one strain that contained three copies of pVG4.1 (L3), two of which integrated in tandem at the pyrG locus while one copy integrated heterologously in the genome of A. niger (Table 1; see Fig. S2 in the supplemental material).
Fig. 1.
The Tet-on system for A. niger. Plasmid pVG4.1 comprises one module ensuring constitutive expression of the tetracycline-dependent transactivator (PgpdA::rtTA2S-M2::TcrgA, with the A. nidulans PgpdA promoter and the A. fumigatus crgA terminator), a second module enabling mluc reporter gene expression in an rtTA2-M2-dependent manner (tetO7::Pmin::mluc::TtrpC, with tetO7::Pmin as an rtTA2S-M2-dependent promoter and the A. nidulans trpC terminator), and a third module, pyrG*, necessary for homologous integration of the plasmid at the pyrG locus of A. niger. The control plasmid pVG2.2 is identical to pVG4.1 but lacks the mluc gene. For cloning details and plasmid and fragment sizes, as well as restriction endonuclease sites, see Fig. S1 in the supplemental material.
To assess any possible influence of Dox on the growth of A. niger, 104 spores of wild-type strains (N402 and MA78.6) and selected transformants (L0 to L3) were spotted on MM and CM agar plates containing different Dox concentrations. The plates were incubated at 30°C for up to 7 days, and the colony diameter was measured daily. When Dox concentrations up to 125 μg/ml were used, no effect on the growth rate and morphology was detectable. However, concentrations above this level inhibited growth of all A. niger strains (∼20% growth inhibition in the presence of 200 μg/ml Dox) (data not shown).
System evaluation on an MTP scale.
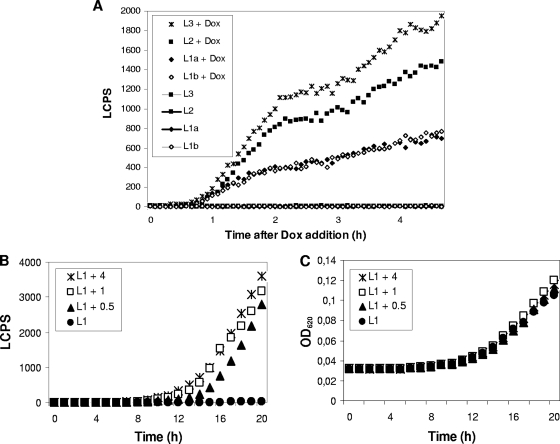
In order to study the effect of mluc copy numbers on luciferase activity, L1 to L3 strains were cultivated in MM for 16 h, after which 0 or 50 μg/ml Dox was added and luminescence values were recorded at minute intervals. In the absence of Dox, no luminescence values were detectable for strains L1, L2, and L3, suggesting that the Tet-on system is tight (Fig. 2A). When 50 μg/ml Dox was present, mluc expression was induced in a gene dosage-dependent manner: luciferase activity was much more highly stimulated in L2 than in L1 but was highest in L3. Most importantly, two independent L1 clones (VG6.1 and VG6.3) showed exactly the same kinetics of luciferase expression, reflecting very good reproducibility of the Tet-on system (Fig. 2A).
Fig. 2.
Gene dosage- and concentration-dependent luciferase activities on an MTP scale. (A) One thousand spores of different strains were inoculated in MM for 16 h (30°C), after which 0 or 50 μg/ml Dox was added and luciferase activities were measured (in LCPS). The strains used were L1a (i.e., strain VG6.1), L1b (i.e., strain VG6.3), L2 (i.e., strain VG8.1), and L3 (i.e., strain VG8.2). (B and C) Different amounts of Dox were added directly to 104 spores of an L1 strain (VG6.1) in MM, and the LCPS and optical density at 620 nm (OD620) were recorded over time during cultivation. Note that luciferase activities became detectable only after germination had been completed (after ∼7 to 8 h).
To follow inducer-dependent responses, spores of a selected L1 strain were cultivated in MM in the presence of various Dox concentrations, and luminescence and cell density were recorded up to 20 h of cultivation. As depicted in Fig. 2B and C, 0.5 μg/ml Dox was sufficient to induce mluc expression. When higher Dox concentrations were applied, the responses were faster and reached higher levels. To calculate the response time of the system, i.e., how long it takes for Dox to provoke measurable luminescence activities, we cultivated spores from an L0 strain (negative control) and an L1 and L2 strain in MM or CM and added different Dox concentrations after 6 h of growth. The response time was defined as the time when luminescence values of L1 and L2 exceeded the threshold level defined by L0. (Note that in L0, i.e., in the mluc-negative control strains VG5.1 and VG7.2, background luminescence values scatter around 50. This value could be a result of some nonspecific autoluminescence of A. niger and was set as the threshold value.) As shown in Table 2, the system responded within either a few minutes or a few hours, demonstrating that rapid or delayed induction and a strong or a weak response can be adjusted by varying gene copy numbers and/or inducer concentrations.
Table 2.
Reaction times of the Tet-on system in MM and CM
| Concn of Dox (μg/ml) | Reaction time (h)a |
|||
|---|---|---|---|---|
| L1 |
L2 |
|||
| MM | CM | MM | CM | |
| 0.5 | 6 | 6 | 2 | 1 |
| 2 | 3 | 3 | 1 | 1 |
| 4 | 2 | 2 | 1 | 0.1 |
| 20 | 1 | ND | 1 | ND |
| 40 | 1 | ND | 0.2 | ND |
Reaction times were determined as the time points when the luminescence counts of the cultures exceeded the threshold value defined by L0. L1, strain VG6.1; L2, strain VG8.1; ND, not determined.
System evaluation on a bioreactor scale.
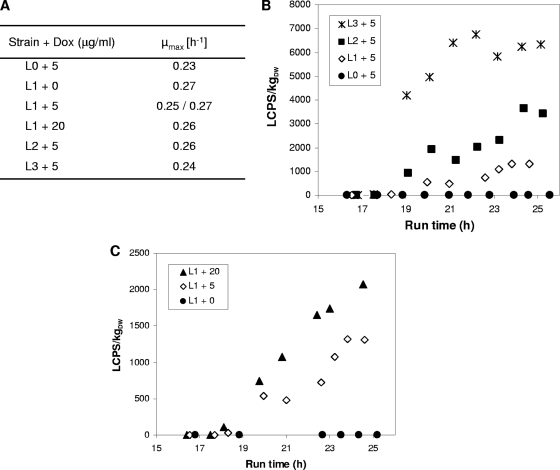
The Tet-on system showed excellent dose-response characteristics in MTP cultivations, and we therefore wished to assess its performance under controlled growth conditions in bioreactors, i.e., under cultivation conditions required for systems biology research. For this purpose, we conducted single or duplicate batch runs of strains L0 to L3 (see Materials and Methods) and added different Dox concentrations when the cultures entered the early exponential growth phase (run time, about 17.5 h). Mycelial samples were taken till the end of the exponential growth phase (run time, about 26 h), and luciferase activities were determined. As shown in Fig. 3A, strains L0, L1, L2, and L3 reached similar maximum growth rates (μmax). This suggests that, as already observed during the MTP-based experiments, expression of the system components rtTA2S-M2 and mLuc, as well as addition of Dox, did not impair the growth of A. niger. Figure 3B and Table 3 reflect the compilation of mLuc data obtained from runs in which expression of luciferase was induced in L0, L1, L2, and L3 cultures using 0 or 5 μg/ml Dox. In agreement with the MTP data, increased copy numbers of mluc resulted in enhanced reporter activities. Most importantly, in the absence of Dox, virtually no reporter activities were measurable in L1, L2, and L3 strains, and the tightness of the system was determined to be higher than 99.5%. Once Dox was added, however, mluc expression rapidly switched on and was induced several thousandfold.
Fig. 3.
Gene dosage- and concentration-dependent luciferase activities on a bioreactor scale. (A) Growth rates determined from different bioreactor runs in the presence or absence of Dox. (B) Luciferase profiles of different bioreactor runs. Reporter gene expression was induced after a run time of ∼17.5 h with 5 μg/ml Dox. (C) Luciferase profiles of the Tet-on system dependent on different Dox concentrations (0, 5, or 20 μg/ml Dox). L0, strain VG7.2; L1, strain VG8.27; L2, strain VG8.1; L3, strain VG8.2. DW, dry weight.
Table 3.
Gene dosage-dependent tightness and fold induction of the Tet-on system
| Run code | Strain used | Concn of Dox (μg/ml)a | LCPS/kgDWb before Dox addition (at 17 h) | Tightnessc (%) | LCPS/kgDW (at 26 h) | Fold inductiond |
|---|---|---|---|---|---|---|
| L0 + 5 | VG7.2 | 5 | 0.82 | 1.41 | ||
| L0 + 5 | VG7.2 | 5 | 0.80 | 1.35 | ||
| L1 + 0 | VG8.27 | 0 | 1.39 | 99.96 | 1.38 | 0 |
| L1 + 5 | VG8.27 | 5 | 1.52 | 99.96 | 1,316 | 950 |
| L1 + 5 | VG8.27 | 5 | 0.85 | 99.96 | 1,312 | 950 |
| L2 + 5 | VG8.1 | 5 | 9.21 | 99.82 | 4,466 | 3,200 |
| L3 + 5 | VG8.2 | 5 | 32.1 | 99.51 | 6,403 | 4,600 |
Dox was added after about 17.5 h run time.
DW, dry weight.
The tightness (%) of the system was calculated from the LCPS/kgDW values at 26 h (t2) and 17 h (t1) using the following formula: 100 − 100 × (LNt1 − L0t1)/(LNt2 − L0t2), where LN is L0, L1, L2, or L3.
Fold induction was calculated as follows: LNt2/(L1 + 0)t2.
To determine the response time under bioreactor conditions, we induced mluc expression in the L1 strain using 0, 5, or 20 μg/ml Dox and ascertained the time point when the L0 threshold level was crossed. Dox at a concentration of 5 μg/ml elicited mLuc activity after 9 min and 20 μg/ml Dox elicited mLuc activity after 3 min. In addition, the higher Dox amount not only caused faster response of the system, it also facilitated higher mLuc levels (Fig. 3C), demonstrating that the system allows not only a qualitative and rapid on-off shift, but also quantitative fine-tuning.
Finally, we wished to know whether expression levels achieved with the Tet-on system can bear comparison with expression levels of the strong gpdA promoter, one of the most commonly used promoters for protein overproduction in filamentous fungi. We therefore used real-time PCR to measure mluc and gpdA mRNA amounts from selected samples, which were harvested from L1 and L2 cultures induced with 5 μg/ml Dox. As summarized in Table 4, the Tet-on system was able to induce mluc transcription up to levels comparable with gpdA transcript levels. As the transcript stabilities of both mluc and gpdA mRNAs might differ, a direct quantitative comparison is of course not legitimate. However, the fact that the values obtained for mluc and gpdA transcript amounts are on the same order of magnitude strongly suggests that the Tet-on system can also be used as a production system for controlled overexpression for proteins of interest.
Table 4.
Strength of the Tet-on system compared to the gpdA promoter
| Run code | Strain used | Run time (h) | Time after Dox addition (h) | mluc expression compared to gpdA expression (%)a |
|---|---|---|---|---|
| L1 + 5 | VG8.27 | 20.9 | 3.5 | 18 ± 2 |
| 24.4 | 6.9 | 72 ± 1 | ||
| L2 + 5 | VG8.1 | 21.2 | 3.7 | 84 ± 16 |
| 25.1 | 7.6 | 810 ± 110 |
Determined by quantitative real-time reverse transcription (RT)-PCR using H2B as a reference gene. Mean values from technical triplicates are given.
Application of the Tet-on system for overexpression studies.
To examine the utility of the Tet-on system for functional gene studies, we constructed overexpression mutants for six predicted ORFs selected from the genome sequence of A. niger (34). The functions of these ORFs, temporarily named cfrA, cfrB, cfrC, cfrE, cfrF, and cfrG, are completely unknown. However, our previous transcriptomic analyses suggested that these genes might play important roles in the survival of A. niger under stress conditions (27). We have thus rationally selected these six candidate genes for our analyses. The sequences of the GOIs were integrated into pVG2.2, downstream of the tetO7::Pmin sequence, and the six plasmids obtained were used to transform strain AB4.1 (pyrG−). Transformants carrying integrations of tetO7::Pmin::GOI at the pyrG locus were identified using Southern blot analyses (data not shown). For comparison, deletion strains of the GOIs using MA70.15 (pyrG− ΔkusA) as a recipient strain were constructed, as well (Table 1 and data not shown).
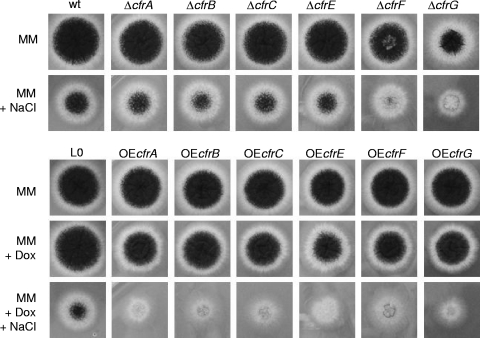
The OEcfr and cfr deletion (Δcfr) strains were cultivated on MM agar plates in the absence or presence of 1 M NaCl (Fig. 4). Phenotypic analysis of the Δcfr strains revealed that only two out of six Δcfr strains (ΔcfrF and ΔcfrG) showed a growth- and sporulation-inhibited phenotype, implying that only cfrF and cfrG, but not the other four cfr genes, are important for the survival of A. niger under salt stress. In contrast, all six OEcfr strains showed a clear stress phenotype under induced conditions (MM agar plates supplemented with Dox and NaCl), strongly indicating that each of the six cfr genes is important for the A. niger salt stress response and not only cfrF and cfrG, as inferred from the deletion analysis. Hence, the conditional Tet-on system can uncover functional information for A. niger genes that lack a loss-of-function phenotype.
Fig. 4.
Phenotypes of Δcfr and OEcfr strains under different growth conditions. One thousand spores of the different strains were inoculated on MM supplemented with 1 M NaCl and/or 20 μg/ml Dox. The plates were cultivated for 72 h at 30°C. MA77.1 (wild type [wt]) and VG5.1 (L0) were used as corresponding control strains. Note that none of the six OEcfr strains displayed a mutant phenotype when cultivated under noninduced conditions (MM agar plates lacking Dox), demonstrating that the Tet-on system is indeed tight.
Application of the Tet-On system for loss-of-function studies.
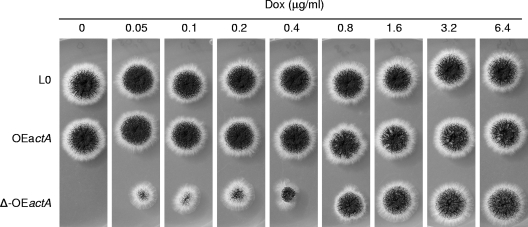
We finally tested the applicability of the system for the establishment of conditional knockout mutants. Our interest was especially focused on the question of whether it is possible to construct mutants that are viable under certain conditions although with essential genes deleted. The approach pursued the idea that deletion of an essential gene of interest in a tetO7::Pmin::GOI background strain could result in a viable strain when cultivated in the presence of Dox but in a lethal phenotype when cultivated in medium lacking Dox. To test this approach, we selected the γ-actin gene (actA), which is essential for filamentous fungi, as a candidate gene. Following the approach described above for the OEcfr strains, we constructed an OEactA strain, which was indistinguishable from the control strain, L0, in the absence and presence of Dox (Fig. 5). This OEactA strain was transformed with an actA disruption cassette gene containing hygromycin as a selection marker (see Materials and Methods). Three primary transformants (′Δ-OEactA′) were isolated and purified on plates containing 100 μg/ml hygromycin and 4 μg/ml Dox and were verified by Southern analysis to lack actA (data not shown). As none of the three Δ-OEactA strains was able to grow on medium lacking Dox (Fig. 5 and data not shown), we concluded that this condition indeed led to a complete knockout situation. However, growth of the Δ-OEactA strains was rescued in medium containing increasing amounts of Dox (Fig. 5), most likely because expression of actA was induced by the Tet-on system. Hence, the approach followed here can be used to generate and study three different phenotypes in a single strain: the knockout phenotype, the wild-type phenotype, and the overexpression phenotype.
Fig. 5.
Phenotypes of OEactA and Δ-OEactA strains in the presence or absence of Dox. One thousand spores of both strains were inoculated on MM supplemented with the indicated Dox concentrations. The plates were cultivated for 65 h at 30°C. VG5.1 (L0) was used as a control strain.
DISCUSSION
The postgenomic era calls for new approaches to substantially understand the growth, metabolism, and behavior of filamentous fungi. To better exploit these organisms in biotechnology and to understand the emergence and progression of fungal diseases, functional genomics and systems biology attempts are under way to uncover the relationship between fungal genomes and phenotypes. Both approaches require that expression of the genes of interest can be controlled quantitatively and temporally. However, the ability to control gene expression in filamentous fungi is so far restricted to metabolism-dependent promoters and/or to promoters that do not allow fine-tuning.
In this study, we developed an artificial gene expression system based on the Tet-on system for the industrial model fungus A. niger and systematically evaluated its performance using different settings. As a reporter gene, we used the luciferase gene mluc, encoding a reporter that is sensitive and nondestructive and has a short half-life (14, 29), thus being especially suited to monitor and measure temporal changes in transcription on line. We demonstrated that on both MTP and bioreactor scales, the Tet-on system can be kept silent in the absence of Dox but becomes rapidly and strongly induced after Dox addition in a gene dosage- and inducer concentration-dependent manner. Hence, the system enables precise gene expression control, which is an essential prerequisite for systems biology approaches. In addition, the Tet-on system provides a wide range of promoter strengths, from very low to very high levels, which are competitive even with the strong gpdA promoter. Most importantly, the newly designed Tet-on system for A. niger has significant advantages over the previously described Tet-on system for A. fumigatus (45). On one hand, it allows site-specific integration of the system at the pyrG locus, which ensures stringent control over gene expression. On the other hand, the presence of both the regulator and response modules on a single plasmid ensures that the transactivator rtTA2S-M2 cannot be titrated away if multicopy integrations are aimed for (e.g., when very high expression levels of the GOI are intended and some background expression is acceptable). Irrespective of which of the two Aspergillus Tet-on systems is applied, the overall performance and efficiency of the system depends on the activity of the gpdA promoter, which has to ensure high-level expression of rtTA2S-M2. To the best of our knowledge, no condition under which the gpdA promoter is silent is known.
We foresee many implementations of the Tet-on system in filamentous fungi, and we demonstrated in this work the proof-of-principle for two possible applications in A. niger. First, we showed that the Tet-on system can be used for the establishment of conditional overexpression mutants. As discussed above, loss-of-function screens in yeast, flies, and worms in the majority of genes did not uncover gene functions, in contrast to gain-of-function screens (38, 41). We thus rationally selected six cfr genes from the genome of A. niger and constructed the respective deletion and Tet-on-based overexpression strains. Based on our previous transcriptomic analyses (27), we suspected that these cfr genes might be important for the survival of A. niger under stress conditions. Whereas the analysis of Δcfr strains illuminated a potential role for two out of six cfr genes in stress resistance, all six OEcfr strains displayed a salt stress phenotype, suggesting that Tet-on-based overexpression can indeed be applied as an effective functional genomics tool for A. niger. Second, we demonstrated the power of combining the Tet-on system with a deletion approach. Using the gene actA as an example, we showed that it is possible to turn actA expression on or off in a Δ-ΟΕactA strain and that it is thus possible to generate a conditional viable strain, although with an essential gene deleted. This approach permits unprecedented flexibility in dissecting gene functions; within a single strain, a loss-of-function, an overexpression, and a wild-type situation can be adjusted in a Dox-dependent manner.
Many more applications of the Tet-on system can be envisioned for A. niger and other filamentous fungi. For example, conditional knockdown mutants can be generated by expressing the gene of interest in an antisense direction from the tetO7::Pmin promoter. For systems biology approaches, the Tet-on system offers excellent options as well, e.g., for studying the interaction and cross talk of signaling cascades and metabolic pathways. For example, the expression of a gene of interest (e.g., encoding a signaling protein) can be rapidly modulated at a specific time, and the temporal response of the respective signaling cascade(s) and the performance of gene/protein networks can be studied. Finally, the Tet-on system appears to be perfectly suited for the production of recombinant proteins in A. niger. Controlled gene expression in microbial production strains is often needed for maximum protein yield and quality (20, 30) and has to be monitored, especially when expressing potentially toxic products. The tightness and strong inducibility of the Tet-on system suggests that it can become an efficient tool for protein overproduction in filamentous fungi.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Sara Altuntas and Frank Sonntag for help in cloning experiments and phenotypic analyses. We thank Erasmus for supporting the research stay of F.W. and F.S. in our laboratory.
This project was carried out within the research program of the Kluyver Centre for Genomics of Industrial Fermentation, which is part of the Netherlands Genomics Initiative/Netherlands Organization for Scientific Research.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 4 March 2011.
REFERENCES
- 1. Andersen M. R., Nielsen J. 2009. Current status of systems biology in Aspergilli. Fungal Genet. Biol. 46(Suppl. 1):S180–S190 [DOI] [PubMed] [Google Scholar]
- 2. Andersen M. R., Nielsen M. L., Nielsen J. 2008. Metabolic model integration of the bibliome, genome, metabolome and reactome of Aspergillus niger. Mol. Syst. Biol. 4:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker C. L., Kettenbach A. N., Loros J. J., Gerber S. A., Dunlap J. C. 2009. Quantitative proteomics reveals a dynamic interactome and phase-specific phosphorylation in the Neurospora circadian clock. Mol. Cell 34:354–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beck C. F., Mutzel R., Barbe J., Muller W. 1982. A multifunctional gene (tetR) controls Tn10-encoded tetracycline resistance. J. Bacteriol. 150:633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bennett J. W. 2009. Aspergillus: a primer for the novice. Med. Mycol. 47(Suppl. 1):S5–S12 [DOI] [PubMed] [Google Scholar]
- 6. Bennett J. W., Lasure L. 1991. More gene manipulations in fungi. Academic Press, San Diego, CA [Google Scholar]
- 7. Brachmann A., Weinzierl G., Kamper J., Kahmann R. 2001. Identification of genes in the bW/bE regulatory cascade in Ustilago maydis. Mol. Microbiol. 42:1047–1063 [DOI] [PubMed] [Google Scholar]
- 8. Carvalho N. D., Arentshorst M., Jin Kwon M., Meyer V., Ram A. F. 2010. Expanding the ku70 toolbox for filamentous fungi: establishment of complementation vectors and recipient strains for advanced gene analyses. Appl. Microbiol. Biotechnol. 87:1463–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiang Y. M., Lee K. H., Sanchez J. F., Keller N. P., Wang C. C. 2009. Unlocking fungal cryptic natural products. Nat. Prod Commun. 4:1505–1510 [PMC free article] [PubMed] [Google Scholar]
- 10. De Lucca A. J. 2007. Harmful fungi in both agriculture and medicine. Rev. Iberoam. Micol. 24:3–13 [PubMed] [Google Scholar]
- 11. Dunlap J. C., et al. 2007. Enabling a community to dissect an organism: overview of the Neurospora functional genomics project. Adv. Genet. 57:49–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galagan J. E., Henn M. R., Ma L. J., Cuomo C. A., Birren B. 2005. Genomics of the fungal kingdom: insights into eukaryotic biology. Genome Res. 15:1620–1631 [DOI] [PubMed] [Google Scholar]
- 13. Giaever G., et al. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418:387–391 [DOI] [PubMed] [Google Scholar]
- 14. Gooch V. D., et al. 2008. Fully codon-optimized luciferase uncovers novel temperature characteristics of the Neurospora clock. Eukaryot. Cell 7:28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gossen M., Bonin A. L., Bujard H. 1993. Control of gene activity in higher eukaryotic cells by prokaryotic regulatory elements. Trends Biochem. Sci. 18:471–475 [DOI] [PubMed] [Google Scholar]
- 16. Gossen M., Bonin A. L., Freundlieb S., Bujard H. 1994. Inducible gene expression systems for higher eukaryotic cells. Curr. Opin. Biotechnol. 5:516–520 [DOI] [PubMed] [Google Scholar]
- 17. Gossen M., Bujard H. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. U. S. A. 89:5547–5551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gossen M., et al. 1995. Transcriptional activation by tetracyclines in mammalian cells. Science 268:1766–1769 [DOI] [PubMed] [Google Scholar]
- 19. Hartman J. L. T., Garvik B., Hartwell L. 2001. Principles for the buffering of genetic variation. Science 291:1001–1004 [DOI] [PubMed] [Google Scholar]
- 20. Hartner F. S., et al. 2008. Promoter library designed for fine-tuned gene expression in Pichia pastoris. Nucleic Acids Res. 36:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamath R. S., et al. 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421:231–237 [DOI] [PubMed] [Google Scholar]
- 22. Larrondo L. F., Colot H. V., Baker C. L., Loros J. J., Dunlap J. C. 2009. Fungal functional genomics: tunable knockout-knock-in expression and tagging strategies. Eukaryot. Cell 8:800–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maya D., Quintero M. J., de la Cruz Munoz-Centeno M., Chavez S. 2008. Systems for applied gene control in Saccharomyces cerevisiae. Biotechnol. Lett. 30:979–987 [DOI] [PubMed] [Google Scholar]
- 24. McGuire S. E., Roman G., Davis R. L. 2004. Gene expression systems in Drosophila: a synthesis of time and space. Trends Genet. 20:384–391 [DOI] [PubMed] [Google Scholar]
- 25. Meyer V. 2008. Genetic engineering of filamentous fungi—progress, obstacles and future trends. Biotechnol. Adv. 26:177–185 [DOI] [PubMed] [Google Scholar]
- 26. Meyer V., et al. 2007. Highly efficient gene targeting in the Aspergillus niger kusA mutant. J. Biotechnol. 128:770–775 [DOI] [PubMed] [Google Scholar]
- 27. Meyer V., et al. 2007. Survival in the presence of antifungals: genome-wide expression profiling of Aspergillus niger in response to sublethal concentrations of caspofungin and fenpropimorph. J. Biol. Chem. 282:32935–32948 [DOI] [PubMed] [Google Scholar]
- 28. Meyer V., Ram A. F., Punt P. J. 2010. Genetics, genetic manipulation, and approaches to strain improvement of filamentous fungi, p. 318–329 In Demain A. L., Davis J. (ed.), Manual of industrial microbiology and biotechnology, 3rd ed. Wiley, New York, NY [Google Scholar]
- 29. Millar A. J., Short S. R., Chua N. H., Kay S. A. 1992. A novel circadian phenotype based on firefly luciferase expression in transgenic plants. Plant Cell 4:1075–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nevoigt E. 2008. Progress in metabolic engineering of Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 72:379–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nowrousian M., Ringelberg C., Dunlap J. C., Loros J. J., Kuck U. 2005. Cross-species microarray hybridization to identify developmentally regulated genes in the filamentous fungus Sordaria macrospora. Mol. Genet. Genomics 273:137–149 [DOI] [PubMed] [Google Scholar]
- 32. Pachlinger R., Mitterbauer R., Adam G., Strauss J. 2005. Metabolically independent and accurately adjustable Aspergillus sp. expression system. Appl. Environ. Microbiol. 71:672–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Padidam M. 2003. Chemically regulated gene expression in plants. Curr. Opin. Plant Biol. 6:169–177 [DOI] [PubMed] [Google Scholar]
- 34. Pel H. J., et al. 2007. Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat. Biotechnol. 25:221–231 [DOI] [PubMed] [Google Scholar]
- 35. Punt P. J., Oliver R. P., Dingemanse M. A., Pouwels P. H., van den Hondel C. A. 1987. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 56:117–124 [DOI] [PubMed] [Google Scholar]
- 36. Punt P. J., et al. 2002. Filamentous fungi as cell factories for heterologous protein production. Trends Biotechnol. 20:200–206 [DOI] [PubMed] [Google Scholar]
- 37. Rahman Z., et al. 2009. Evaluation and characterization of Trichoderma reesei cellulase and xylanase promoters. Appl. Microbiol. Biotechnol. 82:899–908 [DOI] [PubMed] [Google Scholar]
- 38. Rørth P., et al. 1998. Systematic gain-of-function genetics in Drosophila. Development 125:1049–1057 [DOI] [PubMed] [Google Scholar]
- 39. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 40. Shoji J. Y., Maruyama J., Arioka M., Kitamoto K. 2005. Development of Aspergillus oryzae thiA promoter as a tool for molecular biological studies. FEMS Microbiol. Lett. 244:41–46 [DOI] [PubMed] [Google Scholar]
- 41. Sopko R., et al. 2006. Mapping pathways and phenotypes by systematic gene overexpression. Mol. Cell 21:319–330 [DOI] [PubMed] [Google Scholar]
- 42. Urlinger S., et al. 2000. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc. Natl. Acad. Sci. U. S. A. 97:7963–7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Gorcom R. F., van den Hondel C. A. 1988. Expression analysis vectors for Aspergillus niger. Nucleic Acids Res. 16:9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Hartingsveldt W., Mattern I. E., van Zeijl C. M., Pouwels P. H., van den Hondel C. A. 1987. Development of a homologous transformation system for Aspergillus niger based on the pyrG gene. Mol. Gen. Genet. 206:71–75 [DOI] [PubMed] [Google Scholar]
- 45. Vogt K., Bhabhra R., Rhodes J. C., Askew D. S. 2005. Doxycycline-regulated gene expression in the opportunistic fungal pathogen Aspergillus fumigatus. BMC Microbiol. 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weber W., Lienhart C., Baba M. D., Fussenegger M. 2009. A biotin-triggered genetic switch in mammalian cells and mice. Metab. Eng. 11:117–124 [DOI] [PubMed] [Google Scholar]
- 47. Yuan X. L., et al. 2006. Database mining and transcriptional analysis of genes encoding inulin-modifying enzymes of Aspergillus niger. Microbiology 152:3061–3073 [DOI] [PubMed] [Google Scholar]
- 48. Zhang J. Z. 2003. Overexpression analysis of plant transcription factors. Curr. Opin. Plant Biol. 6:430–440 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.