Abstract
The synthesis of reactive oxygen species (ROS) is one of the first events following pathogenic interactions in eukaryotic cells, and NADPH oxidases are involved in the formation of such ROS. The nox1 gene of Trichoderma harzianum was cloned, and its role in antagonism against phytopathogens was analyzed in nox1-overexpressed transformants. The increased levels of nox1 expression in these transformants were accompanied by an increase in ROS production during their direct confrontation with Pythium ultimum. The transformants displayed an increased hydrolytic pattern, as determined by comparing protease, cellulase, and chitinase activities with those for the wild type. In confrontation assays against P. ultimum the nox1-overexpressed transformants were more effective than the wild type, but not in assays against Botrytis cinerea or Rhizoctonia solani. A transcriptomic analysis using a Trichoderma high-density oligonucleotide (HDO) microarray also showed that, compared to gene expression for the interaction of wild-type T. harzianum and P. ultimum, genes related to protease, cellulase, and chitinase activities were differentially upregulated in the interaction of a nox1-overexpressed transformant with this pathogen. Our results show that nox1 is involved in T. harzianum ROS production and antagonism against P. ultimum.
INTRODUCTION
Trichoderma is a fungal genus that includes species in current use as biological control agents due to their ability to antagonize other fungi (28). The ability of Trichoderma species to suppress plant diseases caused by phytopathogenic fungi has long been known (19). The antagonistic properties of Trichoderma have been related to mechanisms of action such as the production of antibiotics (48, 52) and/or hydrolytic enzymes (6) and competition for nutrients (11). It is also known that these fungi have the ability to interact with plants, inducing resistance to biotic and abiotic stresses and promoting plant growth (17, 21). These characteristics have encouraged extensive research into the use of Trichoderma strains as biocontrol agents (BCAs) to combat fungal and oomycetous diseases (28, 35), Trichoderma harzianum being the most cited species as an active agent in a variety of commercial biopesticides and biofertilizers (62).
One of the earliest manifestations of defense responses in animals and plants is the production of reactive oxygen species (ROS) by specific NADPH oxidases (Nox) (22, 59). Three different Nox subfamilies have been found in the kingdom Fungi (2): NoxA, which possesses domains for the catalytic core but no additional motifs and which is very similar in structure to human gp91phox (23, 55); NoxB, which has an additional N-terminal extension with no evident functional motifs (30, 56); and NoxC, which has an even longer N-terminal region that contains a putative calcium-binding EF-hand motif, similar to the respiratory burst oxidase homolog (Rboh) enzymes from plants (24). The most widely studied member of this group of enzymes is the mammalian gp91phox, which is responsible for the phagocytic oxidative burst in response to microbial pathogens. In activated macrophages, Nox enzymes produce superoxide, and its dismutation product, hydrogen peroxide, can kill pathogens directly or through the activation of proteases (42). Plant cells are also capable of an oxidative burst in response to pathogen recognition (14). Arabidopsis thaliana possesses 10 Rboh genes, and their enzymes are involved in plant defense signaling, programmed cell death, and root hair growth (12, 58).
Functional analyses of gp91phox homologs from filamentous fungi have shown that these enzymes play a key role in fungal development and defense. A noxA disruption in Aspergillus nidulans blocks fungal sexual development since fruiting bodies cannot be formed (23). Similar phenotypes have been observed in Podospora anserina and Neurospora crassa after nox gene deletions, demonstrating that Nox enzymes are critical for the development of sexual structures in filamentous fungi (2, 30). Additional studies have reported that the Magnaporthe grisea nox1 gene is also involved in the development of appressoria, which are important structures during plant infection processes (10). Nox-mediated fungal ROS production has been also related to mechanisms for defense against other fungi (15, 51).
This paper reports the isolation and characterization of the nox1 gene in T. harzianum T34 and its functional analysis through a gene overexpression strategy. Transformants with higher nox1 expression levels showed more antagonistic activity than the wild-type strain against the phytopathogen Pythium ultimum, but not against Rhizoctonia solani or Botrytis cinerea. Increased ROS production correlated with increased induction of protease, cellulase, and chitinase activities, which were the main overproduced enzymes in the nox1 transformants in P. ultimum interaction conditions.
MATERIALS AND METHODS
Microorganisms.
Trichoderma harzianum T34 (CECT 2413; Spanish Type Culture Collection, Burjassot, Spain) was used in this study. The fungal phytopathogens Rhizoctonia solani CECT 2815 and Botrytis cinerea 98, isolated by us from diseased strawberry plants, and the oomycete Pythium ultimum 8, obtained from the ArBoPaVe collection of the University of Naples (Italy), were used as targets in dual confrontation assays. Strains were cultured on potato dextrose agar (PDA; Difco-Becton Dickinson, Sparks, MD).
Source of the nox1 gene: EST database and cDNA library.
The L03T34P075R07015 clone with accession number AJ897753 (hereafter referred to as EST 7015) was generated from the T. harzianum T34 cDNA library L03, one of the 26 cDNA libraries that were constructed for the TrichoEST project, using different mRNA populations from 10 strains belonging to eight Trichoderma species (including T. harzianum T34) expressed under conditions that simulate biocontrol processes (45). The growth conditions for the T34 strain used for preparing the L03 cDNA library have been described previously (66). Expressed sequence tags (ESTs) were generated by sequencing cDNA clones from the 5′ end, and an EST database was compiled with 13,814 unique ESTs (66, 67).
Sequence analyses.
Sequences were analyzed using the DNAstar package (Lasergene, Madison, WI). Transmembrane domains were identified using the TopPred II program (9). The NCBI database was used for protein domain identification (31).
PCR procedures.
nox1 cDNA was amplified by PCR with the primers nadph8 (5′-ATGGCTGGCCAACTCACATAT-3′) and nadph9 (5′-TTAAAAGTGCTCTTTCCAGAAGC-3′) and with phagemid DNA from the cDNA library L03 as the template using the Taq polymerase system (Biotools, Edmonton, Canada), according to the manufacturer's instructions. PCR was carried out for 35 cycles of 1 min at 94°C, 1 min 30 s at 59°C, and 1 min 30 s at 72°C. To construct the pJL7015 plasmid, the nox1 cDNA was amplified by PCR using the oligonucleotides X5 (5′-TCTAGAATGGCTGGCCAACTC-3′) and X3 (5′-TCTAGATTAAAAGTGCTCTTTCC-3′), which contain XbaI recognition sites, and the conditions described above. Screening of T. harzianum T34 nox1-overexpressed transformants was performed by PCR with the primers Cyc-F (5′-ACAACCTGAAGTCTAGGTCCC-3′) and nadph11 (5′-ATCCTCTCACTGAGCTCAAG-3′) to amplify a 1,431-bp fragment from the pJL7015 plasmid. The oligonucleotide pair ble (5′-CCTTTCAGTTCGAGCTTTCCC-3′) and ble-r (5′-GGGACCTAGACTTCAGGTTGT-3′) served to amplify the phleomycin resistance gene (ble), which was used as a probe in a Southern analysis under the reaction conditions described above.
Construction of the pJL7015 vector and Trichoderma transformation.
nox1 cDNA was amplified by PCR with the primer pair X5 and X3 and cloned to the pGEM-T Easy vector. After digestion with XbaI, the 1.8-kb fragment corresponding to the nox1 cDNA was ligated to the pLMG vector (36), previously digested with XbaI. The nox1 expression cassette of 3.6 kb, which contains the gpdA (glyceraldehyde 3-phosphate dehydrogenase) gene promoter from A. nidulans and the cbh2 (cellobiohydrolase II) termination region from Trichoderma reesei, was isolated by digestion with HindIII and cloned into plasmid pJL43b1. The resulting 8.11-kb vector, pJL7015, contained the ble gene from Streptoalloteichus hindustanus under the control of the gpdA gene promoter. Plasmid pJL7015 was used to transform protoplasts of T. harzianum T34 as previously described (8), and the transformants were selected for phleomycin resistance.
DNA manipulations.
Fungal DNA was extracted by following the method of Raeder and Broda (41). Mycelia were recovered by filtration from potato dextrose broth (PDB) cultures, washed twice with water, frozen in liquid nitrogen, lyophilized, and ground. Bacterial DNAs were obtained using a routine miniprep procedure. For Southern analysis, 10 μg of genomic DNA was digested with EcoRI and restriction fragments were separated in 0.7% (wt/vol) agarose gels and transferred to a Hybond-N+ membrane (Amersham, Piscataway, NJ). The ble gene was labeled with the PCR DIG labeling mix kit (Roche, Penzberg, Germany) by following the supplier's protocols and used as a probe. Hybridizations were carried out for 16 h at 65°C. Membranes were washed under high-stringency conditions. Immunological detection was performed using the DIG nucleic acid detection kit (Roche), according to the manufacturer's instructions.
Confrontation assays in vitro.
Confrontations between the Trichoderma strains and the phytopathogens B. cinerea, R. solani, and P. ultimum were carried out. Agar plugs cut from the growing edge of a 4-day colony of each phytopathogen were placed 2 cm from the borders of petri dishes containing PDA covered with sterile cellophane sheets. B. cinerea was allowed to grow at 25°C for 1 day before the sowing of Trichoderma strains at 2 cm from the borders on the opposite sides of the same petri dishes where this target pathogen was grown. In the same way, R. solani, P. ultimum, and T. harzianum strains were sown at the same time. Trichoderma-Trichoderma confrontations were used as a control condition. Mycelia were collected from the 5-mm interaction zone between the microorganisms confronted in dual cultures and used for both RNA and protein extractions, which were included in analyses of gene expression and hydrolytic activity, respectively. In vitro confrontations using PDA plates that were not covered with cellophane sheets were also conducted in triplicate as described above in order to analyze the antagonism of Trichoderma against these three phytopathogens. Dual cultures were photographed after 5 days.
Superoxide production in situ assay.
Plates containing Trichoderma-pathogen confrontations, conducted as described above, were incubated for 5 days, after which 1 ml of an NBT solution (0.05% Nitro Blue Tetrazolium in 50 mM phosphate [pH 7.5 buffer]) (Sigma-Aldrich Química S.A., Madrid, Spain) was added to the interaction zone and then plates were photographed.
Activity assays.
Mycelia collected from interaction zones between microorganisms were homogenized in 100 mM Tris (pH 7.5 buffer) at 4°C for 1 h using a Thermomixer (Eppendorf, Hamburg, Germany), and then the supernatants were recovered by centrifugation at 12,000 × g at 4°C for 20 min. Quantitative protein determination was performed with the Bradford assay (7), with bovine serum albumin as a protein standard. Ten micrograms of total proteins was included in each enzymatic assay. Tests were performed in triplicate, and the data represent mean values with standard deviations.
NADPH oxidase activity was determined in a colorimetric assay by measuring the reduction of the tetrazolium dye 3′-(1-[phenylamino-carbonyl]-3,4-tetrazolium)-bis(4-methoxy-6-nitro) benzenesulfonic acid hydrate (XTT) by O2− radicals at 470 nm (1). In this assay, XTT is reduced by HO2−/O2− to a soluble formazan that can be readily quantified in solution. The reaction mixture (0.25 ml) contained 0.3 mM XTT (Sigma-Aldrich Química), 100 μM NADPH in 100 mM Tris (pH 7.5 buffer), and 10 μg of proteins from protein extract. The reaction was initiated by the addition of NADPH and XTT, and the subsequent reduction was determined after incubation at room temperature for 10 min. Total activity corresponds to nmol of O2− produced in 1 min, and specific activity corresponds to nmol of O2− produced in 1 min per mg of protein.
Protease activity was determined in a colorimetric assay by measuring the hydrolysis of azocasein at 366 nm, as described previously (18). Azocasein is a nonspecific protease substrate that, when it is hydrolyzed, releases the azo dye into the media, where it is detected by measuring absorbance. The reaction mixture (0.325 ml), containing 1% (wt/vol) azocasein (Sigma-Aldrich Química) in 50 mM sodium acetate (pH 5.5 buffer) and 10 μg of proteins from protein extracts, was incubated at 30°C for 1 h. Total activity corresponds to nmol of azocasein hydrolyzed in 1 min, and specific activity corresponds to nmol of azocasein hydrolyzed in 1 min per mg of protein.
Cellulase activity was determined in a colorimetric assay by measuring the release of reducing groups during the hydrolysis of carboxymethylcellulose (CMC) at 520 nm (37, 53). The reaction mixture (0.25 ml), containing 1% (wt/vol) CMC (Sigma-Aldrich Química) in 50 mM sodium acetate (pH 5.5 buffer) and 10 μg of proteins from protein extracts, was incubated at 37°C for 1 h. Total activity corresponds to nmol of glucose released in 1 min, and specific activity corresponds to nmol of glucose released in 1 min per mg of protein.
Chitinase activity was determined in a colorimetric assay by measuring the release of N-acetylglucosamine during the hydrolysis of chitin at 585 nm. The reaction mixture (0.25 ml), containing 0.1% (wt/vol) colloidal chitin (Sigma-Aldrich Química) in 50 mM sodium acetate (pH 5.0 buffer) and 10 μg of protein from protein extracts, was incubated at 37°C in a water bath with constant shaking for 2 h. The N-acetylglucosamine released into the reaction mixture was estimated as previously described (44). Total activity corresponds to nmol of N-acetylglucosamine released in 1 min, and specific activity corresponds to nmol of N-acetylglucosamine released in 1 min per mg of protein.
Protease and cellulase activities were also measured, in triplicate, in mycelia from T34, Tnox2, and Tnox5 strains after 48 h growth in PDB medium supplemented with 1% CMC.
Quantitative real-time PCR analyses.
Trichoderma mycelia collected from confrontation assay interaction zones were frozen, lyophilized, ground, and used for RNA extraction with Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. cDNAs were synthesized from 1 μg of total RNA using the AffinityScript quantitative PCR (QPCR) cDNA synthesis kit (Stratagene, La Jolla, CA) with an oligo(dT) primer. Then, 0.5 μl of the cDNA was used in the subsequent PCR. Quantitative real-time PCR was performed using an ABI Prism 7000 sequence detection system with Brilliant SYBR green QPCR master mix (Stratagene). All PCRs were performed in triplicate in a total volume of 12 μl for 40 cycles under the following conditions: denaturation, 95°C, 30 s; annealing, 60°C, 1 min; extension, 72°C, 1 min. Threshold cycles (CT) were determined using the 7000 SDS system software, and CT values were calculated using the β-tubulin gene as an endogenous control. Data are expressed as log10 of 2−ΔΔCT (26), and three biological replicates were used for statistical analysis. The following specific primer pairs were used (GenBank accession numbers of encoded proteins are in parentheses): Nox1 (HM565864), 5′-CACCACCTGTTCATCCC-3′ and 5′-GTCAAATGGCGAGAATCC-3′; catalase (XP_002480230), 5′-ACTGCATTGTCCGTTTCT-3′ and 5′-AGTTGCCCTCCTCTGTG-3′; cellulase signaling protein OOC1 (AAY25948), 5′-CAATGCTCCGACAATTACAG-3′ and 5′-CACCGATCACAGCACAGCA-3′; trypsin-like protease (CAC80694), 5′-CTGCCATCACTCCTCGT-3′ and 5′-AGAAGTGCGAACCACCA-3′; and β-tubulin, 5′-TTCTTGCATTGGTACACTAGCG-3′ and 5′-ATCGTTCATGTTGGACTCAGCC-3′.
Northern blot analysis.
Four-month-old olive plants (Olea europaea L., var. Picual) were cultured in a 250-ml Erlenmeyer flask containing 100 ml of liquid Murashige and Skoog (MS) medium (Duchefa Biochemie B.V., Haarlem, Netherlands) inoculated with 105 conidial germlings ml−1 of T. harzianum T34, which was incubated in an orbital shaker at 80 rpm and 25°C. After 4, 8, or 24 h of plant-Trichoderma interaction, mycelia were collected, washed with distilled water, frozen, lyophilized, and kept at −80°C until total RNA extraction. In parallel, fungal mycelia from cultures without olive plants were also obtained (control). Conidial germlings were obtained from 15-h-old cultures of strain T34 in 200 ml of minimal medium (MM) (40) shaken at 200 rpm and incubated at 25°C. nox1 gene expression was analyzed by Northern blot hybridization, and 20 μg of total RNA was separated on a 1.2% formaldehyde-agarose gel and transferred to a Hybond-N+ membrane. Blots were hybridized with 32P-labeled nox1 or 18S ribosomal DNA (rDNA) probes. Hybridization conditions and membrane washes were as described above for Southern analysis.
Microarray assay.
Mycelia collected from the interaction zone in confrontations between P. ultimum and the T. harzianum T34 or Tnox5 strain were used for RNA extraction as described above and then purified using the RNeasy MinElute cleanup kit (Qiagen, Hilden, Germany). The quality and quantity of the RNA were determined using a Nanodrop spectrophotometer. High-quality purified RNAs, 40 μg per set of three microarrays, were submitted to Roche-NimbleGen (Madison, WI), where cDNAs were synthesized, amplified, labeled, and then used for subsequent hybridizations.
A self-designed Trichoderma high-density oligonucleotide (HDO) microarray (Roche-NimbleGen) was constructed by a method similar to that for a previous Trichoderma HDO microarray (49). The microarray was composed of 392,779 60-mer probes designed against 14,081 EST-derived transcripts and 11,100 genes of Trichoderma atroviride and 11,643 of Trichoderma virens.
Digitalization of the fluorescent signals emitted after the hybridization was performed with the Gene Spring GX program (Agilent Technologies, Santa Clara, CA). This program allows the statistical analysis and identification of induced and repressed genes in each experimental condition. Background correction, normalization, and expression analysis from the data were performed using the RMA algorithm (20). The significance of the differential expression under the two conditions compared was determined by statistical analysis performed with the t test algorithm (5).
Statistical analyses.
The mean values of the assays carried out in triplicate were recorded in the StatView 4.01 program and analyzed using a Fisher exact test.
Nucleotide sequence accession number.
The DNA sequence of nox1 was determined and deposited as GenBank accession number HM565864.
RESULTS
The T. harzianum T34 nox1 gene.
A 690-bp fragment derived from the T. harzianum T34 EST 7015 was used as the probe to screen a lambda genomic DNA library (27). A total of 2,613 bp containing 547 bp of the promoter region and 267 bp corresponding to the terminator region was sequenced from one positive phage. The nox1 cDNA was amplified from L03 library phagemids and cloned into pGEM-T Easy. The nox1 coding region contains 1,799 bp with two introns. The open reading frame (ORF) contains 1,674 bp and encodes a protein of 557 amino acids with a theoretical molecular mass of 64.1 kDa and an isoelectric point of 8.8. The highest degree of similarity (87% amino acid sequence identity) was found with the NoxA protein from Epichloe festucae (GenBank accession number BAE72680). Additionally, one homologous gene was detected in the publicly available genomes of T. reesei (http://genome.jgi-psf.org/Trire2/Trire2.home.html) (95% identity of encoded protein with ID_79489 protein), T. atroviride (http://genome.jgi-psf.org/Triat2/Triat2.info.html) (94% identity of encoded protein with ID_302802 protein), and T. virens (http://genome.jgi-psf.org/TriviGv29_8_2/TriviGv29_8_2.info.html) (99% identity of encoded protein with ID_32702 protein). Bioinformatic analysis of the promoter region showed protein-binding motifs that may give hints as to its regulation, such as HAP1 (central regulator of oxygen metabolism genes), STUAP (involved in sexual development in fungi), and a region binding to the WRKY transcription factor, which regulates defense pathogenesis-related (PR) genes in plants.
Analysis of the 557 amino acids of the predicted T. harzianum T34 Nox1 protein revealed the presence of six transmembrane domains and one flavin adenine dinucleotide (FAD)/NADPH binding cytosolic domain as described previously for gp91phox. The Nox1 structure was similar to that described for fungal NoxA enzymes.
nox1 expression analysis.
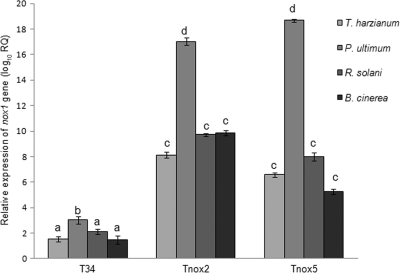
Mycelia from the area of interaction between T. harzianum T34 and phytopathogen B. cinerea, R. solani, or P. ultimum were recovered and used to analyze nox1 expression by quantitative reverse transcription-PCR (RT-PCR). As shown in Fig. 1, the highest transcript levels were detected in mycelia from T34-P. ultimum interactions, followed by those detected in T34-R. solani interactions. Among the four interactions considered, statistically significant higher nox1 transcript levels were observed only in mycelia from T34-P. ultimum interactions compared to those observed in mycelia from T34-T34 interactions. Moreover, high transcript levels were also detected when the fungus was cultured for 4 h in the presence of olive plants (Fig. 2).
Fig. 1.
Quantitative RT-PCR analysis of the nox1 transcript of T. harzianum T34 (wild type) and the homologous nox1-overexpressed transformants Tnox2 and Tnox5. The experiments were carried out with mycelia obtained from the interaction zone of Trichoderma confrontations with T. harzianum itself, P. ultimum, R. solani, or B. cinerea. T. harzianum T34 β-tubulin was used as an internal reference gene. Data are expressed as log10 relative quantification (RQ) (2−ΔΔCT). Bars represent the standard deviations of the mean values of two biological replicates. Histograms with different letters are significantly different (P < 0.0001).
Fig. 2.
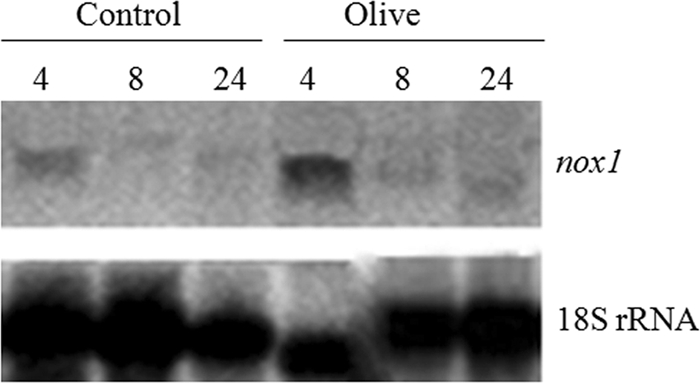
Northern analysis of the nox1 gene of T. harzianum T34 in the presence of olive plants. The experiments were carried out with mycelia obtained after growing the fungus in MS medium for 4, 8, or 24 h without plants (control) or in the presence of olive plants. The nox1 ORF and 18S rRNA gene were used as probes.
Overexpression of nox1 in T. harzianum T34.
In order to characterize the nox1 gene functionally, the pJL7015 plasmid was constructed, linearized with ApaI to facilitate its integration, and used to transform T. harzianum T34. Ten transformants showing phleomycin resistance were checked by PCR. A 1.4-kb PCR product was amplified in all transformants analyzed using the primer pair Cyc-F and nadph11. Four PCR-positive nox1-overexpressed transformants were checked by Southern blotting to determine the additional nox1 copies due to the insertion of the transformation cassette in the T. harzianum genome using EcoRI-digested genomic DNA and a fragment of the ble gene (with one EcoRI cut inside) as a probe (Fig. 3). DNA from the wild type was included as a control. Several blotted bands corresponding to the ble gene were observed only in DNAs from all transformant strains, indicating that the transformation cassette had been inserted several times into the four overexpressed transformant genomes. At this stage, a Nox activity assay of three transformants, Tnox1, Tnox2, and Tnox5, which showed different patterns of integration of the transformation cassette, was performed. Protein extracts obtained from dual confrontation experiments of wild-type or transformant strains against Pythium, Rhizoctonia, or Trichoderma (control condition) were assayed for superoxide production. As shown in Table 1, higher activity levels were detected in nox1-overexpressed transformant strains than in the wild type in Pythium confrontations. Except for the Tnox1 transformant, all Trichoderma strains showed lower activity levels in Rhizoctonia confrontations than those observed in control confrontations. Tnox2 and Tnox5 strains that produced the highest quantities of superoxide were selected and used in further characterization studies. In addition, a higher superoxide production in Tnox2- or Tnox5-P. ultimum interactions than in wild-type or R. solani interactions was observed in a staining assay with NBT (see Fig. S1 in the supplementary material).
Fig. 3.
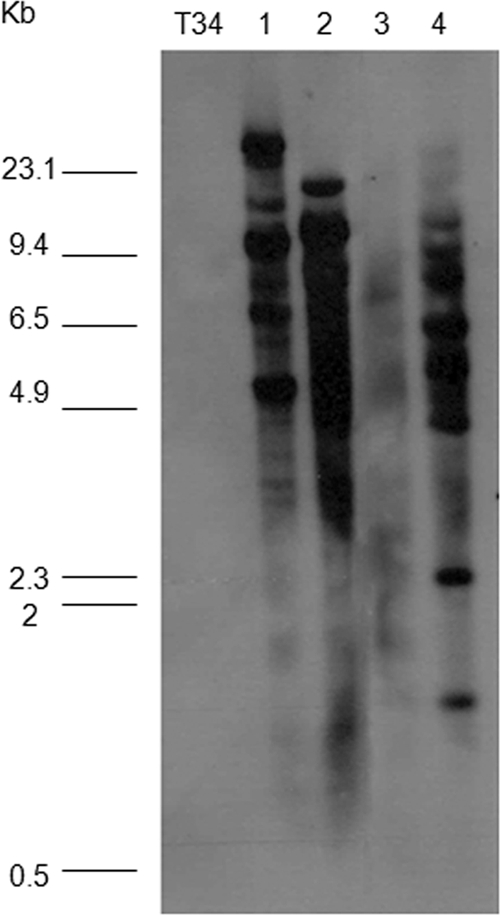
Southern blots of EcoRI-digested genomic DNA of Trichoderma strains hybridized with the ble gene as a probe. DNA was obtained from wild-type T. harzianum (T34) and the nox1-overexpressed transformant strains Tnox1 (lane 1), Tnox2 (lane 2), Tnox4 (lane 3), and Tnox5 (lane 4). Molecular size standards of HindIII-digested λ DNA are at the left.
Table 1.
NADPH oxidase activity measured in intracellular protein extracts from interaction zones in dual cultures of T. harzianum T34 or nox1-overexpressed transformants and itself, P. ultimum, or R. solani
| Strain | Mean NADPH activitya (nmol min−1/mg protein) ± SD for: |
||
|---|---|---|---|
| T-Tb | T. harzianum-P. ultimum | T. harzianum-R. solani | |
| T34 | 38.44 ± 1.85 | 33.07 ± 1.57 | 27.67 ± 0.97 |
| Tnox1 | 27.30 ± 1.08* | 35.76 ± 0.94 | 32.07 ± 3.46* |
| Tnox2 | 65.19 ± 4.17* | 68.08 ± 2.97* | 32.28 ± 0.56* |
| Tnox5 | 54.57 ± 0.32* | 60.08 ± 5.71* | 44.23 ± 4.37* |
*, significant difference with the T34 wild type (P < 0.0001).
T-T, interaction of T. harzianum with itself.
We analyzed the expression of the nox1 gene in the wild type and the two previously selected transformant strains in the following interaction zones (Fig. 1): Trichoderma-Pythium, Trichoderma-Rhizoctonia, Trichoderma-Botrytis, and Trichoderma-Trichoderma. Under identical growth conditions, the Tnox2 and Tnox5 transformants showed nox1 transcript levels higher than that of the endogenous nox1 gene in the wild-type strain. Figure 1 shows that the greatest differences in nox1 expression between the transformant strains and the wild type were observed in mycelia obtained from P. ultimum confrontations.
Confrontation assays.
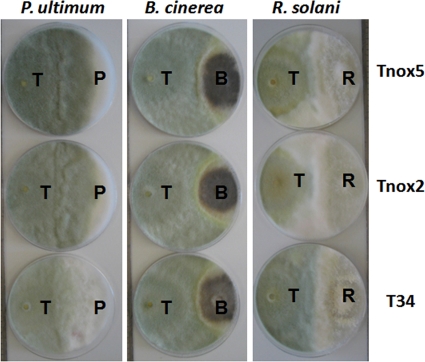
Plate confrontation experiments between T34 or nox1-overexpressed transformants Tnox2 and Tnox5 and the pathogens B. cinerea, R. solani, and P. ultimum were also carried out for 5 days at 25°C to investigate the effect of nox1 overexpression on T. harzianum T34 antifungal activity. Plates were photographed at the end of the confrontation assay. In all cases, after this incubation time the three pathogens completely covered the surfaces of 90-mm-diameter PDA plates used as controls (data not shown). As shown in Fig. 4, Trichoderma strains overgrew the colonies of R. solani and P. ultimum and surrounded the colonies of B. cinerea in the dual cultures. No differences between the wild type and nox1-overexpressed transformant strains in inhibition of the pathogens B. cinerea and R. solani were observed. However, differences in growth inhibition and sporulation between T34 and the nox1-overexpressed transformants were observed in the Pythium confrontation plates. Smaller P. ultimum colony diameters were observed in nox1 transformant confrontations than in those corresponding to the wild type. Sporulation in the pathogen overgrowing area was also more abundant in the nox1 transformant confrontations.
Fig. 4.
Dual cultures of strains T34 and the nox1-overexpressed transformants Tnox2 and Tnox5 of T. harzianum (T) and the pathogens P. ultimum (P), B. cinerea (B), and R. solani (R) on PDA medium. T. harzianum, P. ultimum, and R. solani were sown at the same time. B. cinerea was allowed to grow for 1 day before Trichoderma sowing. Plates were incubated at 25°C for 5 days.
Transcriptomic analysis of nox1 during the T. harzianum-P. ultimum interaction.
The role of nox1 in the Trichoderma-Pythium interaction was analyzed using a Trichoderma HDO microarray (GEO submission: GSE24630). Full microarray data are shown in Table S1 in the supplemental material. After statistical analysis of normalized hybridization data, comparison of the T34-P. ultimum and Tnox5-P. ultimum transcriptomes showed that 108 Trichoderma genes, with fold changes (FC) ≥4 and P values ≤0.05, were differentially expressed in the Tnox5 nox1-overexpressed transformant. One hundred of them were upregulated, and eight were downregulated. The differentially regulated genes were classified into several biological processes of the Gene Ontology Consortium (GO). Proteolysis and binding and cellular transport processes were highly represented in Tnox5 during the interaction with P. ultimum.
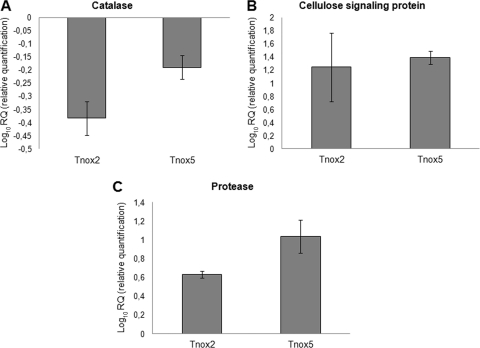
To check the level of reliability of the array-based data, we selected two upregulated genes and one downregulated gene in the Tnox5-Pythium interaction and analyzed their expression levels by quantitative real-time PCR. In addition, the expression of these three genes in the Tnox2-Pythium interaction was analyzed. As expected, the catalase gene downregulated in the microarray data was also downregulated in the two transformant strains (Fig. 5A). Also, higher transcript levels of genes encoding the cellulose signaling protein and the trypsin-like protease (Fig. 5B and C, respectively) were detected in Tnox2 and Tnox5 transformant strains than in the wild-type T34 during the interaction with Pythium.
Fig. 5.
Quantitative RT-PCR analysis of catalase (A), cellulose signaling (B), and trypsin-like protease (C) genes in the nox1-overexpressed transformants Tnox2 and Tnox5. Values (2−ΔΔCT) correspond to relative measurements against the nox1 transcript in the wild-type T. harzianum T34 (2−ΔΔCT = 1). Mycelia were obtained from the interaction area of T. harzianum-P. ultimum dual cultures. T. harzianum T34 β-tubulin was used as an internal reference gene. Bars represent standard deviations of the mean values of three biological replicates.
Most upregulated genes represented, with FC values between 4.1 and 19.3, were related to genes encoding (i) proteases (19%), including aspartic, subtilisin serine, and trypsin-like proteases; (ii) hydrophobins (7%); (iii) hypothetical proteins with domains related to fungal pathogenesis (5%); (iv) oligopeptide transporters (4%); (v) proteins involved in cell wall (CW) integrity (4%); (vi) cellulose signaling proteins (2%); and (vii) chitinases (2%). Most downregulated genes represented were those related to catalases (2%).
Protease, cellulose, and chitinase activities.
Taking into account that an enhanced antagonistic activity of nox1 transformants against P. ultimum, an oomycete with cellulose and minor amounts of chitin in its CW, was observed and that the highest number of upregulated genes were protease genes in the microarray assay, we compared the protease, cellulase, and chitinase activities in the wild-type and nox1-overexpressed transformant strains during their interaction with T. harzianum itself, P. ultimum, or R. solani. As shown in Table 2, higher levels of cellulase activity were detected in the two transformants than in the wild-type strain; these differences were statistically significant for both Trichoderma-Trichoderma and Trichoderma-Pythium confrontations but not for Trichoderma-Rhizoctonia confrontations. A similar relationship between the transformants and the wild-type strain was observed when protease and chitinase activities were analyzed under the different interaction conditions considered. Protease and cellulase activity levels detected in wild-type or transformant strains after 48 h of growth in liquid medium with 1% CMC were similar to those measured in Trichoderma-Pythium interactions.
Table 2.
Protease, cellulase, and chitinase activitiesa
| Enzyme and strain | Mean activity (nmol min−1/mg protein) ± SD for: |
|||
|---|---|---|---|---|
| T-T | T-P | T-R | CMC | |
| Protease | ||||
| T34 | 0.1 ± 0.2A | 2.7 ± 0.8C | 3.0 ± 1.1C | 3.1 ± 0.35C |
| Tnox2 | 1.6 ± 0.1B | 6.8 ± 0.8D | 4.2 ± 0.8C | 6.8 ± 0.34D |
| Tnox5 | 1.1 ± 0.6B | 5.5 ± 0.6D | 4.2 ± 0.1C | 6.6 ± 0.33D |
| Cellulase | ||||
| T34 | 77.7 ± 11.1A | 83.3 ± 5.5A | 94.4 ± 27.7A | 91.89 ± 1.6A |
| Tnox2 | 216.6 ± 2.7B | 244.4 ± 5.5C | 127.7 ± 11.1A | 222.7 ± 1.54C |
| Tnox5 | 172.2 ± 5.5C | 227.7 ± 5.5C | 116.6 ± 11.1A | 232.5 ± 1.57C |
| Chitinase | ||||
| T34 | 18.1 ± 1.6A | 21.8 ± 1.4A | 16.6 ± 0.2A | NDb |
| Tnox2 | 23.7 ± 1.8B | 38.0 ± 1.5C | 13.3 ± 1.6A | ND |
| Tnox5 | 23.2 ± 1.4B | 32.2 ± 1.8C | 14.7 ± 1.9A | ND |
Activities were measured in intracellular protein extracts from interaction zones in dual cultures of T. harzianum T34 or each nox1-overexpressed transformant and itself (T-T), P. ultimum (T-P), or R. solani (T-R), and protease and cellulase activities in T34 and transformant intracellular protein extracts obtained from 48-h liquid cultures in PDB supplemented with 1% CMC were also measured. For each activity, values followed by different superscript letters are significantly different (P < 0.001).
ND, no data.
DISCUSSION
The present study provides a contribution to understanding the role of a T. harzianum protein related to ROS production in the interaction between a biocontrol agent and three phytopathogenic preys. The isolation and characterization of a novel Trichoderma gene coding for a Nox protein are reported.
ROS production by specific Nox proteins has been related to defense responses in animals and plants (22, 59), and three Nox subfamilies have been described in the kingdom Fungi (2). EST 7015, showing high identity with ESTs for Nox proteins in a BlastX analysis, was identified in a T. harzianum T34 cDNA library constructed under antagonistic conditions (66). Southern blot analysis showed that nox1 was present as a single copy in the genome of T. harzianum T34 (data not shown), and this result is in agreement with the presence of a single homologous gene in the three Trichoderma genomes available on line.
It has previously been demonstrated that nox genes are induced by the presence of pathogens in mammals (46) and plants (57, 60). We observed motifs for binding the regulators of oxygen metabolism, sexual development, and defense PR proteins in the nox1 promoter region. These data are in agreement with the role of Nox proteins as critical enzymes for sexual development (2, 24, 30) and sclerotial differentiation (39) in filamentous fungi, plant infection processes (10), and antimicrobial activity due to their involvement in ROS production (15, 51).
Bearing in mind that some fungi are endowed with the ability to recognize potential contestants and have built up a response that involves cell death as a result of the generation of ROS through NADPH oxidase activity at the contact zone between fungi (51), that this gene was also induced by plant material (Fig. 2), and that cellulose is a major component of Pythium and plant CWs, it seems clear that this polysaccharide must be related to nox1 induction. In contrast, B. cinerea and R. solani are ascomycete and basidiomycete fungi, respectively, without cellulose in their CWs, and no increase in nox1 expression was detected in the zones of interaction between T. harzianum T34 and these two phytopathogens.
Since the frequency of homologous recombination in T. harzianum is very low (29) and generation of null mutants of this species has been achieved only twice (47, 48), the function of the nox1 gene was studied following a homologous overexpression approach.
Because Southern analysis revealed that several copies of the transformation cassette were inserted into the genomes of transformant strains, superoxide production served to select two nox1-overexpressed transformants. The Tnox2 and Tnox5 transformants produced higher quantities of superoxide than the wild-type strain under both control and Pythium confrontation conditions. Although an increased nox1 expression level had been detected only in Pythium interactions, similar Nox1 activity values were measured in transformant-Pythium confrontations and their controls (Table 1). According to nox1 expression results (Fig. 1), we have observed higher superoxide in situ production in the zones of interaction between the transformants and Pythium than those from Rhizoctonia or Trichoderma confrontations, where stain differences were not observed (see Fig. S1 in the supplementary material). Dual-culture experiments showed that the wild-type and transformant strains displayed the same behavior against R. solani or B. cinerea. However, against P. ultimum, nox1-overexpressed transformants displayed more biocontrol efficiency than the wild type. This suggests that nox1 is involved in T. harzianum antagonistic activity against the oomycete P. ultimum, but not against R. solani or B. cinerea. It is well documented that ROS can induce sclerotial metamorphosis in R. solani (39), but this pathogen can also repress ROS formation through the production of the scavenger ascorbic acid in certain concentration gradients and proportions in response to oxidative stress caused by ROS during mycelial and sclerotial differentiation (13). On the other hand, B. cinerea seems to be better adapted to oxidative stress than other phytopathogens since it has been observed that ROS function generated by Nicotiana benthamiana has a positive role in expansion of disease lesions during B. cinerea-plant interaction (3).
The transcriptomic response of T. harzianum T34 to nox1 overexpression was analyzed in interactions with P. ultimum using HDO microarrays. Compared to results for T34, the largest number of upregulated genes (19%) of the Tnox5 transformant in contact with P. ultimum were genes encoding proteases, including aspartic, subtilisin serine, and trypsin-like proteases, all of which were related to a biocontrol function and previously described in Trichoderma spp. (25, 54, 63, 68). Other highly represented differential genes were genes encoding hydrophobins (7%), also identified in proteomic studies as overexpressed T. atroviride proteins in interactions with bean roots and R. solani (32) or related to plant root colonization in Trichoderma asperellum (64). Hydrophobins have also been found to be a part of the antioxidant protective system of Neurospora crassa (16). Other differentially upregulated genes identified in Tnox5-P. ultimum interaction were those encoding hypothetical proteins with PR domains (5%), oligopeptide transporters (4%) induced by chitin in Trichoderma spp. (65), proteins involved in the maintenance of CW integrity (4%) (61), and cellulose signaling proteins (2%), induced only by cellulose in T. reesei (50).
That ROS promote the modification of cellular proteins and that intracellular proteolytic enzymes selectively degrade oxidized proteins is well documented (38). It has also been proposed that the proteasome is the major player involved in the removal of these oxidized proteins (43). However, proteolytic genes related to the proteasome, the ubiquitin pathway, and ROS detoxification were not differentially expressed in the T. harzianum-P. ultimum microarray assay. The induction of several proteases, an amine oxidase, and proteins related to cellulose signaling and pathogenesis, as well as the repression of catalase genes, suggests that Nox1 would be involved in a mechanism of defense against pathogens in a way similar to plant defense responses. In plants, the suppression of catalase or ascorbate peroxidase activities during attack by pathogens results in an overaccumulation of ROS and the activation of defense responses (34).
ROS production by NADPH oxidases is a universal signaling system among multicellular organisms, and the battery of ROS scavenging systems present in cells, including ascorbate peroxidases, glutathione, superoxide dismutases, and catalases, ensures rapid turnover of the ROS to maintain ROS homeostasis (55, 59). Then, it is generally accepted that a high catalase activity is related to high ROS levels as a mechanism for protection against oxidative damage. However, the repression of catalase genes observed in overexpressed Trichoderma transformants during the contact with P. ultimum indicates that high ROS levels downregulate catalase genes. This is in agreement with a recent study that describes how high ROS levels produce a hypermethylation of catalase promoters that reduce the expression of catalase genes in human tumoral cell lines (33).
A direct correlation between hydrolase production and biocontrol activity has been reported in transcriptomic studies of Trichoderma spp. (28, 66, 67). The detection of higher protease, cellulose, and chitinase activities in nox1-overexpressed transformants than in T34 in P. ultimum interactions is in agreement with the biocontrol efficiency observed in dual cultures and the microarray transcriptomic results. The fact that protease and cellulase activity levels detected in Trichoderma strains in the presence of CMC were similar to those observed in Trichoderma-Pythium interactions indicates that cellulose alone is the triggering factor of these Trichoderma hydrolytic activities. These results are in agreement with the nox1 expression studies indicating that cellulose from Pythium CWs is the inducing factor for the nox1 gene. The nox1-overexpressed transformants also displayed more chitinase activity in the interaction with P. ultimum than in that with R. solani. In this case, the higher chitinase activity could be due to the residual chitin present in Pythium CWs (4). The increased ROS levels measured in nox1 transformants in interactions with P. ultimum seem be involved in their increased hydrolytic activities and therefore in the biocontrol efficiency of nox1 transformants against this pathogen.
Taking our results as a whole, they indicate that the T. harzianum nox1 gene is involved in control of the oomycete P. ultimum by maintaining high ROS levels, which are accompanied by the upregulation of protease-, cellulase-, and chitinase-encoding genes, leading to an increase in the corresponding enzymatic activities.
Supplementary Material
ACKNOWLEDGMENTS
Research project funding was from the Junta de Castilla y León (GR67) and the Spanish Ministry of Science and Innovation (AGL2008-0512/AGR and AGL2009-13431).
Thanks are due to Ricardo López Pérez for microarray data analysis.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 18 March 2011.
REFERENCES
- 1. Able A. J., Guest D. I., Sutherland M. W. 1998. Use of a new tetrazolium-based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores of Phytophthora parasitica var. nicotianae. Plant Physiol. 117:491–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aguirre J., Rios-Momberg M., Hewitt D., Hansberg W. 2005. Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 13:111–118 [DOI] [PubMed] [Google Scholar]
- 3. Asai S., Yoshioka H. 2009. Nitric oxide as a partner of reactive oxygen species participates in disease resistance to nectrotophic pathogen Botryis cinerea in Nicotiana benthamiana. Mol. Plant Microbe Interact. 22:619–629 [DOI] [PubMed] [Google Scholar]
- 4. Asiegbu F. O., Lönneborg A. M., Johansson M. 1996. Chitin and glucans detected in the cell walls of Pythium dimorphum—an oomycetous fungus. Eur. J. Forest Pathol. 26:315–321 [Google Scholar]
- 5. Baldi P., Long A. D. 2001. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics 17:509–519 [DOI] [PubMed] [Google Scholar]
- 6. Benítez T., Rincón A. M., Limón M. C., Codón A. C. 2004. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 7:249–260 [PubMed] [Google Scholar]
- 7. Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 8. Cardoza R. E., Vizcaíno J. A., Hermosa M. R., Monte E., Gutiérrez S. 2006. A comparison of the phenotypic and genetic stability of recombinant Trichoderma spp. generated by protoplast- and Agrobacterium-mediated transformation. J. Microbiol. 44:383–395 [PubMed] [Google Scholar]
- 9. Claros M. G., von Heijne G. 1994. TopPred II: an improved software for membrane protein structure predictions. Comput. Appl. Biosci. 10:685–686 [DOI] [PubMed] [Google Scholar]
- 10. Egan M. J., Wang Z., Jones M. A., Smirnoff N., Talbot N. J. 2007. Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc. Natl. Acad. Sci. U. S. A. 104:11772–11777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elad Y. 2000. Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop Prot. 19:709–714 [Google Scholar]
- 12. Foreman J., et al. 2003. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446 [DOI] [PubMed] [Google Scholar]
- 13. Georgiou C. D., Petropoulou K. P. 2001. Effect of the antioxidant ascorbic acid on sclerotial differentiation in Rhizoctonia solani. Plant Pathol. 50:594–600 [Google Scholar]
- 14. Grant M., et al. 2000. The RPMI plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J. 23:441–450 [DOI] [PubMed] [Google Scholar]
- 15. Haedens V., Malagnac F., Silar P. 2005. Genetic control of an epigenetic cell degeneration syndrome in Podospora anserine. Fungal Genet. Biol. 42:564–577 [DOI] [PubMed] [Google Scholar]
- 16. Hansberg W., Aguirre J. 1990. Hyperoxidant states cause microbial cell differentiation by cell isolation from dioxygen. J. Theor. Biol. 142:201–221 [DOI] [PubMed] [Google Scholar]
- 17. Harman G. E., Howell C. R., Viterbo A., Chet I., Lorito M. 2004. Trichoderma species—opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2:43–56 [DOI] [PubMed] [Google Scholar]
- 18. Holwerda B. C., Rogers J. C. 1992. Purification and characterization of aleurain: a plant thiol protease functionally homologous to mammalian cathepsin H. Plant Physiol. 99:848–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Howell C. R. 2003. Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant Dis. 87:4–10 [DOI] [PubMed] [Google Scholar]
- 20. Irizarry R. A., et al. 2003. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264 [DOI] [PubMed] [Google Scholar]
- 21. Kuc J. 2001. Concepts and direction of induced systemic resistance in plants and its application. Eur. J. Plant Pathol. 107:7–12 [Google Scholar]
- 22. Lambeth J. D. 2004. Nox enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4:181–189 [DOI] [PubMed] [Google Scholar]
- 23. Lara-Ortiz T., Riveros-Rosas H., Aguirre J. 2003. Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol. Microbiol. 50:1241–1255 [DOI] [PubMed] [Google Scholar]
- 24. Lewit-Bentley A., Rety S. 2000. EF-hand calcium-binding proteins. Curr. Opin. Struct. Biol. 10:637–643 [DOI] [PubMed] [Google Scholar]
- 25. Liu Y., Yang Q. 2007. Cloning and heterologous expression of aspartic protease SA76 related to biocontrol in Trichoderma harzianum. FEMS Microbiol. Lett. 227:173–181 [DOI] [PubMed] [Google Scholar]
- 26. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 27. Lora J. M., De la Cruz J., Llobell A., Benítez T., Pintor-Toro J. A. 1995. Molecular characterization and heterologous expression of an endo-beta-1,6-glucanase gene from the mycoparasitic fungus Trichoderma harzianum. Mol. Gen. Genet. 247:639–645 [DOI] [PubMed] [Google Scholar]
- 28. Lorito M., Woo S. L., Harman G. E., Monte E. 2010. Translational research on Trichoderma: from 'omics to the field. Annu. Rev. Phytopathol. 48:395–417 [DOI] [PubMed] [Google Scholar]
- 29. Mach R. L., Zeilinger S. 1998. Genetic transformation of Trichoderma and Gliocladium, p. 225–242 In Kubicek C. P., Harman G. E. (ed.), Trichoderma and Gliocladium. Taylor & Francis, Ltd., London, United Kingdom [Google Scholar]
- 30. Malagnac F., Lalucque H., Lepere G., Silar P. 2004. Two NADPH oxidase isoforms are required for sexual reproduction and ascospore germination in the filamentous fungus Podospora anserine. Fungal Genet. Biol. 41:982–997 [DOI] [PubMed] [Google Scholar]
- 31. Marchler-Bauer A., et al. 2003. CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 31:383–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marra R., et al. 2006. Study of the three-way interaction between Trichoderma atroviride, plant and fungal pathogens by using a proteomic approach. Curr. Genet. 50:307–321 [DOI] [PubMed] [Google Scholar]
- 33. Min J. Y., Lin S.-O., Jung G. 2010. Downregulation of catalase by reactive oxygen species via hypermethylation of CpG island II on the catalase promoter. FEBS Lett. 584:2427–2432 [DOI] [PubMed] [Google Scholar]
- 34. Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7:405–410 [DOI] [PubMed] [Google Scholar]
- 35. Monte E. 2001. Understanding Trichoderma: between biotechnology and microbial ecology. Int. Microbiol. 4:1–4 [DOI] [PubMed] [Google Scholar]
- 36. Montero-Barrientos M., et al. 2008. Overexpression of a Trichoderma HSP70 gene increases fungal resistance to heat and other abiotic stresses. Fungal Genet. Biol. 45:1506–1513 [DOI] [PubMed] [Google Scholar]
- 37. Nelson N. J. 1944. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 153:375–380 [Google Scholar]
- 38. Okada K., et al. 1999. 4-Hydroxy-2-nonenal-mediated impairment of intracellular proteolysis during oxidative stress. J. Biol. Chem. 274:23787–23793 [DOI] [PubMed] [Google Scholar]
- 39. Papapostolou I., Georgiou C. D. 2010. Superoxide radical induces sclerotial differentiation in filamentous phytopathogenic fungi: a superoxide dismutase mimetics study. Microbiology 156:960–966 [DOI] [PubMed] [Google Scholar]
- 40. Penttilä M., Nevalainen H., Ratto M., Salminen E., Knowles J. 1987. A versatile transformation system for the cellulolytic filamentous fungus Trichoderma reesei. Gene 61:155–164 [DOI] [PubMed] [Google Scholar]
- 41. Raeder U., Broda P. 1985. Rapid preparation of DNA from filamentous fungi. Lett. Appl. Microbiol. 1:17–20 [Google Scholar]
- 42. Reeves E. P., et al. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416:291–297 [DOI] [PubMed] [Google Scholar]
- 43. Reinheckel T., et al. 1998. Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem. J. 335:637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reissig J. L., Strominger J. L., Leloir L. F. 1955. A modified colorimetric method for the stomation of N-acetylamino sugars. J. Biol. Chem. 217:959–966 [PubMed] [Google Scholar]
- 45. Rey M., Llobell A., Monte E., Scala F., Lorito M. 2004. Genomics of Trichoderma, p. 225–248 In Khachatourians G. G. (ed.), Fungal genomics, vol. 4 Elsevier Science, Amsterdam, Netherlands [Google Scholar]
- 46. Roos D., et al. 1996. Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood 87:1663–1681 [PubMed] [Google Scholar]
- 47. Rosado I. V., et al. 2007. QID74 cell wall protein of Trichoderma harzianum is involved in cell protection and adherence to hydrophobic surfaces. Fungal Genet. Biol. 44:950–964 [DOI] [PubMed] [Google Scholar]
- 48. Rubio M. B., Hermosa R., Reino J. L., Collado I. G., Monte E. 2009. Thctf1 transcription factor of Trichoderma harzianum is involved in 6-pentyl-2H-pyran-2-one production and antifungal activity. Fungal Genet. Biol. 46:17–27 [DOI] [PubMed] [Google Scholar]
- 49. Samolski I., de Luis A., Vizcaíno J. A., Monte E., Suárez M. B. 2009. Gene expression analysis of the biocontrol fungus Trichoderma harzianum in the presence of tomato plants, chitin, or glucose using a high-density oligonucleotide microarray. BMC Microbiol. 9:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schmoll M., Kubicek C. P. 2005. ooc1, a unique gene expressed only during growth of Hypocrea jecorina (anamorph: Trichoderma reesei) on cellulose. Curr. Genet. 48:126–133 [DOI] [PubMed] [Google Scholar]
- 51. Silar P. 2005. Peroxide accumulation and cell death in filamentous fungi induced by contact with a contestant. Mycol. Res. 109:137–149 [DOI] [PubMed] [Google Scholar]
- 52. Sivasithamparam K., Ghisalberti E. L. 1998. Secondary metabolism in Trichoderma and Gliocladium, p. 139–191 In Kubicek C. P., Harman G. E. (ed.), Trichoderma and Gliocladium, Taylor & Francis, London, United Kingdom [Google Scholar]
- 53. Somogyi M. 1952. Notes on sugar determination. J. Biol. Chem. 195:19–23 [PubMed] [Google Scholar]
- 54. Suárez M. B., et al. 2005. Proteomic analysis of secreted proteins from Trichoderma harzianum. Identification of a fungal cell wall-induced aspartic protease. Fungal Genet. Biol. 42:924–934 [DOI] [PubMed] [Google Scholar]
- 55. Takemoto D., Tanaka A., Scott B. 2007. NADPH oxidases in fungi: diverse roles of reactive oxygen species in fungal cellular differentiation. Fungal Genet. Biol. 44:1065–1076 [DOI] [PubMed] [Google Scholar]
- 56. Tanaka A., Christensen M. J., Takemoto D., Park P., Scott B. 2006. Reactive oxygen species play a role in regulating a fungus-perennial ryegrass mutualistic association. Plant Cell 18:1052–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Torres M. A., Dangl J. L., Jones J. D. G. 2002. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. U. S. A. 99:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Torres M. A., Jones J. D. G., Dangl J. L. 2005. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat. Genet. 37:1130–1134 [DOI] [PubMed] [Google Scholar]
- 59. Torres M. A., Dangl J. L. 2005. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 8:397–403 [DOI] [PubMed] [Google Scholar]
- 60. Torres M. A., et al. 1998. Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J. 14:365–370 [DOI] [PubMed] [Google Scholar]
- 61. Verna J., Lodder A., Lee K., Vagts A., Ballester R. 1997. A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 94:13804–13809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vinale F., et al. 2006. Major secondary metabolites produced by two commercial Trichoderma strains active against different phytopathogens. Lett. Appl. Microbiol. 43:143–148 [DOI] [PubMed] [Google Scholar]
- 63. Viterbo A., Chet I. 2006. TasHyd1, a new hydrophobin gene from the biocontrol agent Trichoderma asperellum, is involved in plant root colonization. Mol. Plant Pathol. 7:249–258 [DOI] [PubMed] [Google Scholar]
- 64. Viterbo A., Harel M., Chet I. 2004. Isolation of two aspartyl proteases from Trichoderma asperellum expressed during colonization of cucumber roots. FEMS Microbiol. Lett. 238:151–158 [DOI] [PubMed] [Google Scholar]
- 65. Vizcaíno J. A., et al. 2006. ThPTR2, a di/tri-peptide transporter gene from Trichoderma harzianum. Fungal Genet. Biol. 43:234–246 [DOI] [PubMed] [Google Scholar]
- 66. Vizcaíno J. A., et al. 2006. Generation, annotation and analysis of ESTs from Trichoderma harzianum CECT 2413. BMC Genomics 7:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vizcaíno J. A., et al. 2007. Generation, annotation, and analysis of ESTs from four different Trichoderma strains grown under conditions related to biocontrol. Appl. Microbiol. Biotechnol. 75:853–862 [DOI] [PubMed] [Google Scholar]
- 68. Yang J. K., et al. 2005. Isolation and characterization of a serine protease from the nematophagous fungus, Lecanicillium psalliotae, displaying nematicidal activity. Biotechnol. Lett. 27:1123–1128 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.