Abstract
The membrane proteins IIC and IID of the mannose phosphotransferase system (Man-PTS) together form a membrane-located complex that serves as a receptor for several different bacteriocins, including the pediocin-like class IIa bacteriocins and the class IIc bacteriocin lactococcin A. Bacterial strains sensitive to class IIa bacteriocins readily give rise to resistant mutants upon bacteriocin exposure. In the present study, we have therefore investigated lactococcin A-resistant mutants of Lactococcus lactis as well as natural food isolates of Listeria monocytogenes with different susceptibilities to class IIa bacteriocins. We found two major mechanisms of resistance. The first involves downregulation of Man-PTS gene expression, which takes place both in spontaneous resistant mutants and in natural resistant isolates. The second involves normal expression of the Man-PTS system, but the underlying mechanism of resistance for these cells is unknown. In some cases, the resistant phenotype was linked to a shift in the metabolism; i.e., reduced growth on glucose due to reduction in Man-PTS expression was accompanied by enhanced growth on another sugar, such as galactose. The implications of these findings in terms of metabolic heterogeneity are discussed.
INTRODUCTION
Bacteriocins are peptides or proteins with antimicrobial activity against bacteria (9, 33). Many bacteriocins are produced by food-grade lactic acid bacteria that are naturally present in vegetables, meat, and dairy products, and since a number of these peptides can effectively kill food-spoiling and pathogenic bacteria, they are often considered promising agents for use in food preservation (9, 11). Most bacteriocins kill target cells by permeabilization of the cell membrane, and the activity is often very specific, since they employ specific receptors on the target cell surfaces. The target receptors of a few bacteriocins have been identified. For example, nisin and a number of other lantibiotic bacteriocins (peptides containing posttranslationally modified residues) use the cell wall precursor lipid II as a docking molecule on target cells (5, 6). Furthermore, it has been shown in recent years that a set of bacteriocins produced by both Gram-positive and Gram-negative species can employ the membrane components of the mannose phosphotransferase system (Man-PTS) on sensitive cells as receptor molecules. These bacteriocins include the pediocin-like bacteriocins (12, 15, 22, 40), the lactococcal bacteriocins lactococcin A and B (15), and microcin E492 from Klebsiella, which can target Man-PTS in the inner membrane of Escherichia coli (4).
The pediocin-like bacteriocins, also known as the class IIa bacteriocins, constitute a large group of peptides produced by lactic acid bacteria. Unlike lantibiotic bacteriocins, class IIa bacteriocins contain only nonmodified residues, except for one or two disulfide bridges; they are 36 to 49 amino acids (aa) long, are characterized by the presence of a conserved N-terminal motif (YGNGVxCxxxxCxVxWxxA, where x is any amino acid and underlining indicates invariant residues), and are known for their strong antilisterial activity (reviewed in reference 36). Lactococcin A, produced by Lactococcus lactis (25), is an unrelated bacteriocin of 54 nonmodified amino acids. This bacteriocin is a member of class IIc, which consists of linear, non-pediocin-like one-peptide bacteriocins (34).
The Man-PTS, which is a major sugar uptake system in Firmicutes and Gammaproteobacteria, consists of four domains: IIA, IIB, IIC, and IID (38). IIC and IID are membrane proteins that form reversible contacts with the cytosolic IIA and IIB domains (38). Only IIC and IID are involved as receptors for bacteriocins (15). It should be noted that lactococcin A and class IIa bacteriocins differ greatly in their inhibitory spectra: lactococcin A targets only the Man-PTS (ptn) from Lactococcus species, while the class IIa bacteriocins target the Man-PTSs from a wide range of genera, including Lactobacillus, Listeria, and Enterococcus, but somehow not the ptn system of Lactococcus (16, 25, 28). A recent study with reciprocal hybrid receptors of the lactococcal (ptn) and listerial (mpt) Man-PTSs has indeed revealed that lactococcin A differs from class IIa bacteriocins in the mode of receptor recognition: while lactococcin A appears to require several regions both on IIC (PtnC) and on IID (PtnD) for species-specific targeting, the specificity of the class IIa bacteriocins is dependent on a single extracellular loop in the IIC (MptC) protein (29).
It is frequently observed that sensitive strains give rise to resistant mutants upon exposure to class IIa bacteriocins (19, 44). The resistance frequency ranges from 10−4 to 10−9 depending on the species or genera tested, and in Listeria monocytogenes this phenotype has been linked to reduced expression of the Man-PTS genes (21, 41, 44). Interestingly, although class II bacteriocins are known to have strong antilisterial activity, natural isolates of L. monocytogenes have been observed to differ greatly in their sensitivities to these bacteriocins (27); however, the exact nature of these differences has not been investigated. Similarly, spontaneous mutants resistant to lactococcin A appear frequently but are poorly characterized. In the present study, we have assessed the status of the Man-PTS in a collection of Listeria isolates with different sensitivities to class IIa bacteriocins. A similar assessment was also performed on lactococcal mutants with different sensitivities to the class IIc bacteriocin lactococcin A in order to compare the mechanisms of resistance to these bacteriocins.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Unless otherwise stated, L. monocytogenes was grown in brain heart infusion (BHI) medium (Oxoid) at 37°C without shaking, and L. lactis was grown in M17 medium (Oxoid) supplemented with 0.4% (wt vol−1) glucose at 30°C without shaking. When appropriate, 10 μg ml−1 chloramphenicol was added to the growth medium. The bacterial strains used in this study are listed in Table 1.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Bacterial strains | ||
| Lactococcus lactis | ||
| IL1403 | Lactococcin A-sensitive strain | 7 |
| IL1403-Rlac-A | IL1403 clone resistant to lactococcin A (isolated from agar plate with 25 BU ml−1) | This study |
| IL1403-Rlac-B | IL1403 clone resistant to lactococcin A (isolated from agar plate with 25 BU ml−1) | This study |
| IL1403-Rlac-C | IL1403 clone resistant to lactococcin A (isolated from agar plate with 220 BU ml−1) | This study |
| IL1403-Rlac-D | IL1403 clone resistant to lactococcin A (isolated from agar plate with 220 BU ml−1) | This study |
| IL1403(p369) | IL1403 with p369 | This study |
| IL1403-Rlac-A(p369) | IL1403-Rlac-A with p369 | This study |
| IL1403-Rlac-B(p369) | IL1403-Rlac-B with p369 | This study |
| IL1403-Rlac-C(p369) | IL1403-Rlac-C with p369 | This study |
| IL1403-Rlac-D(p369) | IL1403-Rlac-D with p369 | This study |
| B100 | IL1403 with pMG36e | 15 |
| NZ9000 | L. lactis strain for nisin-controlled gene expression (nisRK integrated into the genome); lactococcin A sensitive | 30 |
| NZ9000-Rlac | NZ9000 clone resistant to lactococcin A (220 BU ml−1) | This study |
| NZ9000(pNZ8037) | NZ9000 carrying plasmid pNZ8037 | This study |
| NZ9000-Rlac(pNZ8037) | NZ9000-Rlac carrying plasmid pNZ8037 | This study |
| NZ9000(p423) | NZ9000 carrying p423 | This study |
| NZ9000-Rlac(p423) | NZ9000-Rlac carrying p423 | This study |
| Listeria monocytogenes | ||
| L31-H | Isolated from cheese; highly sensitive to class IIa bacteriocins | L. M. Rørvik |
| L196-H | Isolated from meat; highly sensitive to class IIa bacteriocins | L. M. Rørvik |
| L228-H | Isolated from meat; highly sensitive to class IIa bacteriocins | L. M. Rørvik |
| L361-I | Isolated from meat; intermediate sensitivity to class IIa bacteriocins | L. M. Rørvik |
| L852-I | Isolated from smoked salmon; intermediate sensitivity to class IIa bacteriocins | L. M. Rørvik |
| L1036-H | Isolated from seawater; highly sensitive to class IIa bacteriocins | L. M. Rørvik |
| L1040-L | Isolated at a fish-processing plant; low sensitivity to class IIa bacteriocins | L. M. Rørvik |
| L1207-H | Isolated at a fish processing plant; highly sensitive to class IIa bacteriocins | L. M. Rørvik |
| L1283-I | Isolated from smoked salmon; intermediate sensitivity to class IIa bacteriocins | L. M. Rørvik |
| L1310-I | Isolated at a fish-processing plant; intermediate sensitivity to class IIa bacteriocins | L. M. Rørvik |
| L1401-I | Isolated from chicken; intermediate sensitivity to class IIa bacteriocins | L. M. Rørvik |
| L1485-I | Isolated from chicken; intermediate sensitivity to class IIa bacteriocins | L. M. Rørvik |
| L2462-I | Isolated from chicken feces; intermediate sensitivity to class IIa bacteriocins | L. M. Rørvik |
| Plasmids | ||
| pMG36e | Lactococcal expression vector with strong P32 promoter; Eryr | 47 |
| pNZ8037 | Lactococcal expression vector containing nisin-responsive promoter; Camr | 13 |
| p369 | pMG36e with lcnA-flciA downstream of the P32 promoter; Eryr | 15 |
| p423 | pNZ8037 with ptnABCD downstream of the nisin-responsive-promoter; Camr | 15 |
Camr, chloramphenicol resistance; Eryr, erythromycin resistance.
Bacteriocins, bacteriocin assays, and growth analysis.
Bacteriocins were concentrated from culture supernatants by precipitation with 30% ammonium sulfate. The bacteriocin producers used were Pediococcus acidilactici LMGT2351 (35) for pediocin PA-1, Enterococcus faecium P13 (8) for enterocin P, Lactobacillus sakei Lb790(pSAK20, pSPP2) (3) for sakacin P, and L. lactis B190 (15) for lactococcin A.
Bacteriocin sensitivity was determined using microtiter plate assays where 100-fold dilutions of the test strains were exposed to 2-fold serial dilutions of bacteriocin (25). Alternatively, bacteriocin sensitivity was determined by a spot-on-lawn soft agar assay, where 2 μl of the concentrated supernatant was spotted directly onto a soft agar containing the test strain. Growth analysis was performed using a Bioscreen C system (Oy Growth Curves); overnight cultures were diluted 1,000-fold, and the optical density at 600 nm (OD600) was measured continuously.
DNA isolation and sequencing.
Total DNA was isolated from L. monocytogenes using a FastPrep FP120 bead-beater (Bio 101/Savant) and a QIAprep Miniprep kit (Qiagen) as described by Solheim et al. (42). The Man-PTS genes (mptACD) were amplified using primers mk64 (5′-ACGTGCATGCGCAATAAATATAGCGGGTAGC-3′) and mk65 (5′-ATCGCTCGAGTCGGTGAATATTGCACCAGC-3′), and the amplification product was sequenced using primers mk64, mk65, mk128 (5′-ATGTTTGCCCATCCAAGTGC-3′), and mk129 (5′-TTATCGGTTTCGTAGTAGCAG-3′). The mptA promoter region was amplified and sequenced using primers mk289 (5′-AAATGACTTTTTTAGAATTCCATCAA-3′) and mk291 (5′-GATTGCTTTAACGTTTTCTTGC-3′). rpoN was amplified and sequenced using primers mk306 (5′-ATGAAGACAATAAATGGAATTTAG-3′) and mk307 (5′-AAAAGACGTTTTTTGTCCCACA-3′). manR was amplified using primers mk292 (5′-TAGTCATGCTAAGATAAATACA-3′) and mk293 (5′-ATTATGAAAGTACTTCTGGTTGG-3′), and the amplification product was sequenced using primers mk292, mk293, mk294 (5′-GACTCTGGTACGTATAATAAACT-3′), mk295 (5′-TCAAGGTGTGGAAGATGATGA-3′), and mk296 (5′-TCATCATCTTCCACACCTTGA-3′). lmo0095 homologs were amplified and sequenced using primers mk299 (5′-AAATGACTTTTTTAGAATTCCATCAA-3′) and mk300 (5′-TCTATTTTAAGCACAAGATGCCT-3′), while resD was amplified and sequenced using primers mk301 (5′-TGAGTACTTATGAGTGAACAAGT-3′) and mk302 (5′-CTTAGTCTGTTTTATTAATCTTCTG-3′).
RNA isolation, cDNA synthesis, and RT-PCR.
L. monocytogenes cells were harvested by centrifugation of cultures in the exponential-growth phase (OD600 of 0.6), and RNA isolation, DNase treatment, and cDNA synthesis were performed as described previously (28). Reverse transcription-PCR (RT-PCR) was carried out using primers mk199 (5′-CAGCCATTAATCGCATGTACA-3′) and mk200 (5′-CGAAGAACGGCCATACTTCT-3′) targeting mptC, mk201 (5′-GTAGCATGGCGCTCTACGT-3′) and mk202 (5′-ACGAACATCCCGAGTATCGA-3′) targeting mptD, and 1F (5′-GAGTTTGATCCTGGCTCAG-3′) and mk203 (5′-TTAGCCGTGGCTTTCTGGT-3′) targeting the 16S rRNA housekeeping gene. Primers were designed based on the genome sequence of L. monocytogenes EGD-e (18).
Isolation of lactococcin A-resistant mutants.
One bacteriocin unit (BU) was defined as the amount of lactococcin A required to produce 50% growth inhibition in a 200-μl L. lactis IL1403 culture. In order to generate lactococcin A-resistant mutants, L. lactis IL1403 and NZ9000 cultures were plated onto GM17 agar with a layer of soft agar containing 25 BU ml−1, 70 BU ml−1, or 220 BU ml−1. Bacteriocin-resistant colonies were cultivated in bacteriocin-free medium for at least 100 generations before the bacteriocin sensitivity was assessed by microtiter plate assays.
Protein purification and SDS-PAGE.
Plasmid p369 was transformed into wild-type L. lactis IL1403 and into four resistant clones for constitutive expression of the Flag-tagged lactococcin A immunity gene flciA. Cells were grown to an OD600 of 0.5, harvested by centrifugation at 7,000 × g, and washed with ice-cold Tris-buffered saline (TBS). Cells were lysed mechanically using a FastPrep FP120 instrument (Savant Instruments Inc., Holbrook, NY). The Flag-tagged protein fLciA was then immunoprecipitated using M2 anti-Flag agarose according to the manufacturer's protocol (Sigma-Aldrich, St. Louis, MO). The eluted proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 4% stacking gel and a 15% separation gel and were visualized by silver staining.
Transformation.
L. lactis was transformed by electroporation as described by Holo and Nes (24).
RESULTS
Natural L. monocytogenes isolates resistant to class IIa bacteriocins display reduced Man-PTS gene expression.
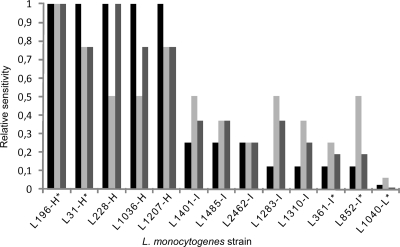
A distinctive feature of class IIa bacteriocins is their strong antilisterial activity (16). However, it has been reported previously that a large collection of 200 food and food industry Listeria isolates that had not been exposed to class IIa bacteriocins prior to collection displayed great variation in sensitivity when challenged with class IIa bacteriocins (27). Thirteen L. monocytogenes isolates from this collection were selected in order to examine the molecular nature of these variations. Based on differences in the MIC values of the class IIa bacteriocins pediocin PA-1 (a 44-aa peptide belonging to subgroup 1 of the class IIa bacteriocins), enterocin P (44 aa; belonging to subgroup 3), and sakacin P (43 aa; belonging to subgroup 1) (Fig. 1), the isolates were divided into three groups: (i) a highly sensitive group, containing isolates L31-H, L196-H, L228-H, L1036-H, and L1207-H, (ii) an intermediately sensitive group, containing isolates L361-I, L852-I, L1283-I, L1310-I, L1401-I, L1485-I, and L2462-I, and (iii) a low-sensitivity group with only one member, L1040-L. The differences in MIC values between the most sensitive and the least sensitive strain were 43-, 16-, and 85-fold for pediocin PA-1, enterocin P, and sakacin P, respectively. In general, strains displayed less variation in sensitivity to enterocin P than to the other two bacteriocins.
Fig. 1.
Relative sensitivities of L. monocytogenes isolates to the bacteriocins pediocin PA-1 (filled bars), enterocin P (light shaded bars), and sakacin P (dark shaded bars). The MIC was defined as the amount of bacteriocin required to produce 50% growth inhibition in a 200-μl culture. The MIC of the most sensitive strain (L196-H) was taken to be 1, and the MICs of the other strains were determined relative to this (relative sensitivity = 1/MIC). Asterisks mark strains chosen for further analysis.
In L. monocytogenes, the Man-PTS system is encoded by the mptACD genes; MptC and MptD constitute the membrane-located receptor complex (IIC and IID). The mptACD genes were sequenced in five isolates with different bacteriocin susceptibilities (L31-H, L196-H, L361-I, L852-I, and L1040-L) in order to investigate whether the observed differences in susceptibility to bacteriocins between the isolates could result from sequence variations in the receptor. Some nucleotide variations were observed, but the resulting amino acid sequences of MptA, MptC, and MptD were identical in all isolates except for a single polymorphism found in MptC (Ile-150 in L31-H, L196-H, and L1040-L as opposed to Val-150 in L361-I and L852-I). However, this polymorphism is unlikely to have any significant effect on receptor potency, since the same amino acid (Ile-150) was found in both the most sensitive (L196-H) and the least sensitive (L1040-L) isolate.
It is known that some resistant mutants of L. monocytogenes and Enterococcus faecalis arising from exposure to class IIa bacteriocins show lower Man-PTS gene expression than wild-type sensitive cells (21, 37, 41, 44). Therefore, semiquantitative RT-PCR with primers targeting mptC and mptD was performed to investigate their expression levels in the five isolates (Fig. 2). The results demonstrate that expression of the receptor genes mptC and mptD was much lower in the isolate with low bacteriocin susceptibility (L1040-L) than in the isolates with high and intermediate susceptibilities (L31-H, L196-H, L361-I, and L852-I). This result corresponds well with a previous study on Lactobacillus sakei strains that showed a correlation between the Man-PTS gene expression level and the degree of sensitivity to class IIa bacteriocins (28).
Fig. 2.
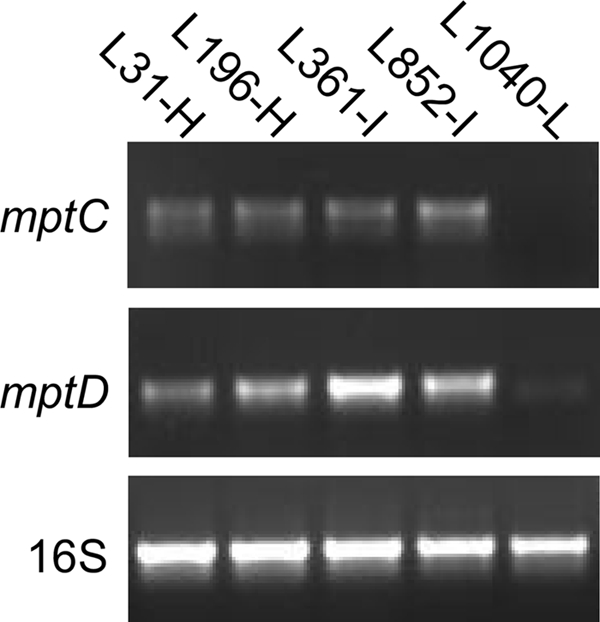
RT-PCR with primers targeting mptC, mptD, and the housekeeping gene 16S rRNA (control) in five different L. monocytogenes strains (L31-H, L196-H, L361-I, L852-I, and L1040-L).
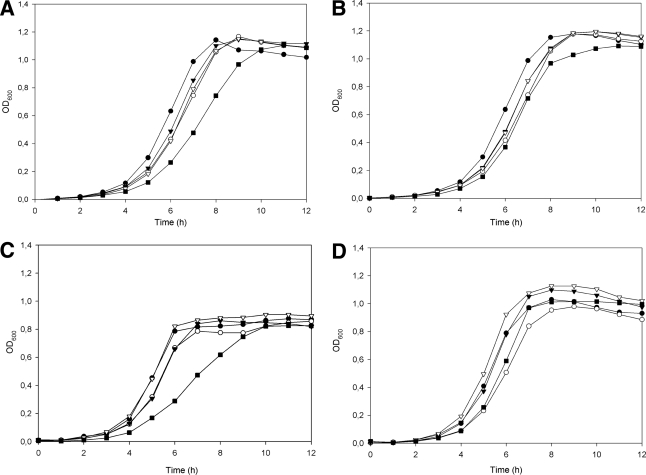
MptACD is a major glucose uptake system in L. monocytogenes, although glucose can also be transported by alternative PTSs (43). Growth analysis demonstrated that L1040-L grew considerably more slowly than L31-H, L196-H, L361-I, and L852-I in both M17 medium supplemented with 0.4% glucose and BHI medium (containing glucose) (Fig. 3 A and C). On the other hand, when the carbon source was changed to cellobiose, which is transported by other PTSs (43), the growth rates were similar for all five strains (Fig. 3B and D). Thus, the low susceptibility of L. monocytogenes strain L1040-L to class IIa bacteriocins is caused by reduced expression of Man-PTS genes, resulting in reduced growth on glucose. However, the smaller variation in sensitivity between the highly and intermediately susceptible isolates remains enigmatic, since this sensitivity variation could not be correlated with differences in mpt expression levels (Fig. 2).
Fig. 3.
Growth of L. monocytogenes strains L31-H (•), L196-H (○), L361-I (▾), L852-I (▿), and L1040-L (▪) in M17 medium supplemented with 0.4% glucose (A), M17 medium supplemented with 0.4% glucose and 0.4% cellobiose (B), BHI (C), and BHI with 0.4% cellobiose (D).
The regulation of mpt gene expression in Listeria has been studied extensively, and several regulatory factors have been identified, including the σ54 factor RpoN (2, 12), the σ54-associated activator ManR (12, 50), the response regulator ResD (31), and Lmo0095 (48, 49), whose function is unknown. Interestingly, a transversion mutation (Ala356Gly) in the E. faecalis ManR homolog MptR has been identified in several spontaneous mutants resistant to class IIa bacteriocins, and downregulation of mpt gene expression has been attributed to this mutation (37). In order to find out whether similar polymorphisms in the regulatory genes could account for the low mpt expression in strain L1040-L, four known regulatory genes (rpoN, manR, resD, and lmo0095), as well as the mpt promoter region, were sequenced in isolates L31-H, L196-H, L361-I, L852-I, and L1040-L. Some differences in amino acid sequence between the strains were found (Table 2); however, most of these polymorphisms are unlikely to have any effect, since similar amino acids were found in strains with high (L31-H, L196-H, L361-I, L852-I) and low (L1040-L) mpt expression. The exceptions are two polymorphic amino acid positions in the manR gene that were unique to L1040-L; Glu was replaced with Lys and Tyr with Cys at positions 321 and 690, respectively. The important role of manR in the control of Man-PTS gene expression has been studied in the related bacterium Listeria innocua. Xue and Miller (50) showed that deletion of manR reduced the level of mpt gene expression 100-fold from that in control cells. In another study (12), a manR interruption mutant generated in L. monocytogenes was found not only to be severely depleted in Man-PTS gene expression but also to have acquired at least 500-fold resistance to the class IIa bacteriocin mesentericin Y105. However, whether the polymorphisms in the manR gene of strain L1040-L are responsible for the reduced expression of Man-PTS observed for this isolate awaits further investigation.
Table 2.
Polymorphisms identified in the L. monocytogenes genes lmo0095, manR, resD, and rpoN
| Gene | Amino acid position | Amino acid in the following L. monocytogenes straina: |
||||
|---|---|---|---|---|---|---|
| L31-H | L196-H | L361-I | L852-I | L1040-L | ||
| lmo0095 | 95 | T | T | A | A | T |
| 96 | D | D | E | E | D | |
| 104 | G | D | D | D | G | |
| 114 | E | E | Q | Q | E | |
| 150 | Q | Q | E | E | Q | |
| manR | 86 | N | N | S | S | N |
| 91 | D | D | E | E | D | |
| 204 | D | D | E | E | D | |
| 321b | E | E | E | E | K | |
| 690b | Y | Y | Y | Y | C | |
| resD | 174 | R | R | K | K | R |
| rpoN | 183 | S | A | S | S | T |
| 284 | N | N | S | S | N | |
| 295 | N | N | S | S | N | |
| 363 | T | T | I | I | T | |
| 373 | K | K | M | M | K | |
L31-H and L196-H have high sensitivity to class IIa bacteriocin; L361-I and L852-I have intermediate sensitivity; and L1040-L has low sensitivity.
Position with unique polymorphisms in the low-sensitivity strain L1040-L.
Reduced Man-PTS expression is found in spontaneous mutants resistant to lactococcin A.
In order to compare resistance to class IIa bacteriocins with the mechanism of resistance to another Man-PTS-targeting bacteriocin, lactococcin A-resistant mutants were generated by exposing the sensitive strain L. lactis IL1403 to three different concentrations of lactococcin A on agar plates. The frequency of resistance was approximately 1.5 × 10−5 for the lowest lactococcin A concentration (25 BU ml−1) and about 5 × 10−7 for the two higher concentrations (70 BU ml−1 and 220 BU ml−1). The MICs for resistant mutants of IL1403 (35 independent mutants tested) increased 16 to 67 times over that for the wild type, and the lactococcin A-resistant phenotype was stably maintained after growth in bacteriocin-free medium for at least 100 generations.
The Man-PTS receptor for lactococcin A in L. lactis is encoded by the ptnABCD genes, which are homologs of mptACD in L. monocytogenes. The ptnABCD genes were sequenced in the wild-type strain IL1403 as well as in four resistant mutants (Rlac-A and Rlac-B, isolated from the agar plate with 25 BU ml−1 lactococcin A, and Rlac-C and Rlac-D, isolated from the plate with 220 BU ml−1 lactococcin A), but no differences were found, demonstrating that lactococcin A resistance did not result from mutations in the receptor genes.
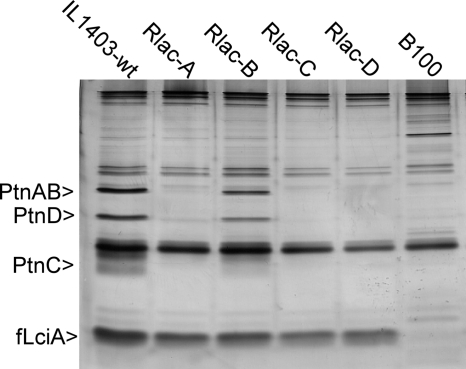
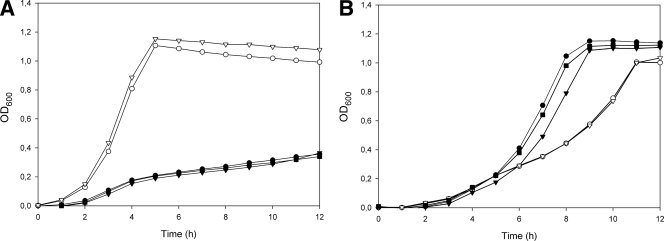
In a previous study, we have shown that in immune L. lactis organisms that are exposed to lactococcin A, the immunity protein (LciA) specifically binds to the PtnABCD proteins to form a complex that prevents pore formation (15). By immunoprecipitation (using antibodies targeting a Flag-tagged version of the immunity protein, fLciA), the Ptn proteins are thus readily copurified with fLciA (15), and this method was used to assess the amounts of PtnABCD proteins in the four resistant mutants of L. lactis IL1403. As expected, high levels of the Man-PTS proteins PtnAB, PtnC, and PtnD copurified with fLciA in the wild-type strain (Fig. 4). In three of the four resistant mutants tested (Rlac-A, Rlac-C, and Rlac-D), the PtnAB, PtnC, and PtnD protein bands were absent or very weak, clearly demonstrating that the level of PtnABCD was downregulated in these cells. In the last resistant mutant (Rlac-B), the amounts of precipitated Man-PTS proteins were similar to those found in wild-type cells, indicating that the Man-PTS expression level was not significantly reduced in this mutant. These results corresponded well with the findings of the subsequent growth analysis (Fig. 5): mutants Rlac-A, Rlac-C, and Rlac-D, with markedly reduced expression of Man-PTS genes, grew significantly more slowly than the wild type and mutant Rlac-B in GM17 medium containing glucose as the major carbon source. On the other hand, when galactose, which is transported independently of Man-PTS, was used as the carbon source, the resistant clones with downregulated Man-PTSs displayed notably higher growth rates than both the wild-type strain and the Rlac-B mutant, suggesting that the resistant mutants Rlac-A, Rlac-C, and Rlac-D have compensated for the reduced glucose uptake by activating galactose metabolism.
Fig. 4.
Differential expression of PtnABCD. The silver-stained SDS-PAGE gel shows fLciA and its copurified proteins in wild-type (wt) L. lactis IL1403 and lactococcin A-resistant mutants Rlac-A, Rlac-B, Rlac-C, and Rlac-D. All clones contain plasmid p369 for expression of fLciA, except for the negative control B100 (IL1403 with an empty plasmid). The identities of the protein bands have been determined previously by mass spectrometry (15).
Fig. 5.
Growth of wild-type L. lactis IL1403 (○) compared to that of the four lactococcin A-resistant mutants Rlac-A (•), Rlac-B (▿), Rlac-C (▾), and Rlac-D (▪) in M17 medium supplemented with glucose (A) or galactose (B).
The results from protein and growth analyses suggest that exposure of L. lactis to lactococcin A generates two different types of resistant cells: type 1 mutants (such as Rlac-A, Rlac-C, and Rlac-D), with downregulation of Man-PTS expression, reduced growth on glucose, and enhanced growth on galactose, and type 2 mutants (such as Rlac-B), with normal Man-PTS expression and wild-type-like growth profiles on glucose and galactose. To determine the relative frequencies of these two types of mutants, the glucose and galactose growth profiles of 35 lactococcin A-resistant L. lactis IL1403 mutants were monitored. Interestingly, all the mutants (12 out of 12 tested) obtained from the agar plates containing the higher concentrations of lactococcin A (70 and 220 BU ml−1) belonged to type 1, while among the mutants obtained from the agar plate with a low lactococcin A concentration (25 BU ml−1), 39% (9 of 23) belonged to type 1 and 61% (14 of 23) to type 2. These findings indicate that downregulation of Man-PTS expression is the main resistance mechanism arising from exposure to high bacteriocin concentrations, while a second resistance mechanism (associated with normal Man-PTS expression) can play an important role at lower bacteriocin concentrations.
Expression of cloned receptor genes in a spontaneous resistant mutant restores the sensitive phenotype.
L. lactis NZ9000 is a strain that has been constructed to allow heterologous gene expression based on the nisin regulatory system (30). In order to examine whether expression of cloned receptor genes in lactococcin A-resistant mutants can render the cells sensitive to lactococcin A, we took advantage of NZ9000 as an expression host. In a manner similar to that for IL1403, NZ9000 was exposed to lactococcin A (220 BU ml−1 in soft agar) to generate resistant mutants. The resistance frequency for this strain was 1,000 times higher than that for IL1403 (5 × 10−4 versus 5 × 10−7), and MIC values for five randomly selected mutants showed that they were 3 to 10 times less sensitive to lactococcin A than was wild-type NZ9000. All five mutants displayed a type 1 resistant phenotype with a reduced growth rate on glucose, suggesting that the expression of Man-PTS was downregulated, and when ptnABCD were expressed from a plasmid in one of the resistant mutants (NZ9000-Rlac), bacteriocin sensitivity was indeed restored (Fig. 6).
Fig. 6.
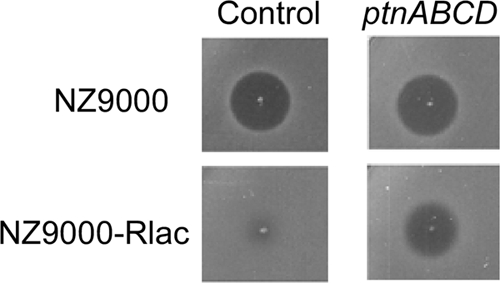
Lactococcin A sensitivities of wild-type L. lactis NZ9000 and the resistant clone NZ9000-Rlac with an empty plasmid (control) and with plasmid p432 expressing ptnABCD. Expression of ptnABCD was induced by the addition of 1 ng ml−1 nisin to the soft agar. Lactococcin A sensitivity is seen as clear zones. Expression of ptnABCD rendered the resistant clone sensitive; however, expression of ptnABCD in the wild-type control NZ9000 did not affect the bacteriocin sensitivity of this strain.
DISCUSSION
The results presented in this study suggest that two different mechanisms confer resistance to Man-PTS-targeting bacteriocins in Listeria and Lactococcus. The first and main mechanism involves the downregulation of Man-PTS gene expression, leading to resistance to bacteriocins due to limited amounts of, or the absence of, receptor proteins, and we demonstrate that downregulation of Man-PTS expression is found both among naturally resistant isolates and among laboratory-induced resistant mutants. This resistance mechanism is often associated with highly resistant cells and has indeed been reported in previous studies dealing with class IIa bacteriocin resistance (21, 41, 44). The Man-PTS expression level is, however, not the only factor determining sensitivity to these bacteriocins (Fig. 2, 4, and 6), because in the second mechanism, which normally occurs in cells with intermediate resistance, we found relatively high Man-PTS gene expression, at levels comparable to those found in wild-type and sensitive cells. Although the exact nature of the second resistance mechanism is still unknown, some circumstances suggest that cell surface changes affecting the interaction between the bacteriocin and its membrane-located receptor might be involved. For instance, previous work has shown that bacteriocin-resistant L. monocytogenes mutants display a variety of altered phenotypes compared to the sensitive wild-type cells, e.g., differences in membrane composition and cell surface charge (44–46). In preliminary work, we observed that lactococcin A-resistant cells of L. lactis somehow attached better to glass slides submerged in a bacterial culture than did wild-type cells (data not shown), indicating a change on the membrane surface that affected their affinity for the glass surface. It should also be noted that a number of genetic loci in L. monocytogenes that are involved in resistance to the lantibiotic bacteriocin nisin, such as the cell wall synthesis gene dltA (1), the penicillin-binding protein gene lmo2229 (20), and the transporter gene anrB (10), appear to play a direct role in cell envelope composition, and these genes might confer general bacteriocin resistance. Future studies to decipher the molecular nature underlying such bacteriocin resistance will therefore focus primarily on unraveling differences in the cell envelope between wild-type and resistant cells.
During normal growth with glucose as the primary carbon source, the expression of Man-PTS is high, while the metabolic pathways for alternative sugars are commonly repressed, and only when glucose is no longer available are these alternative pathways turned on. This regulatory phenomenon is generally referred to as carbon catabolite repression (14). In this context, it was interesting that lactococcin A-resistant mutants displayed a reduced ability to grow on glucose, but enhanced growth on the alternative sugar galactose, relative to the growth of wild-type cells. Exposure to bacteriocins has thus generated resistant cells in which the alternative galactose pathway has been derepressed as a result of downregulated Man-PTS expression.
The molecular switch that turns off or downregulates Man-PTS expression in resistant cells is a central but still poorly understood aspect of bacteriocin resistance. Most probably, the resistance phenotype is manifested in stable genetic changes, since we and others have observed that the resistance phenotype is not lost after hundreds of generations in nonselective medium. Indeed, some mutations have been found in important regulatory genes involved in Man-PTS expression. For instance, the gene activator MptR/ManR could represent such a genetically variable hot spot, since polymorphisms in this gene have been detected in resistant isolates of both E. faecalis (37) and L. monocytogenes (the present study). Nevertheless, given the high frequencies of bacteriocin resistance resulting from reduced Man-PTS expression, as seen for several different bacteria (e.g., L. lactis, L. monocytogenes, and E. faecalis), it is tempting to speculate that downregulation of Man-PTS expression is not due primarily to regular spontaneous mutations but rather to a process that causes metabolic variability in a bacterial culture. In recent years, it has been established that bacterial monocultures exhibit stochastic switching of gene expression in order to generate phenotypically heterogeneous populations, and bacteria can use this heterogeneity as a survival strategy to cope with stressful and fluctuating environments (26, 32, 39). Since the Man-PTS is involved in global carbon catabolite control (2, 14, 37, 48), instability in Man-PTS gene expression could be used as a mechanism to generate phenotypic heterogeneity with respect to carbon source utilization. Moreover, the Man-PTS is a known vulnerable spot for biological attack, since it is used as a target for several antimicrobial agents, including different classes of bacteriocins as well as bacteriophages (4, 15, 17, 23). Stochastic Man-PTS gene expression could thus be seen as a defense mechanism to ensure that at least a small subpopulation of cells in a bacterial culture could escape from such extracellular attacks. Further investigation may reveal whether population heterogeneity indeed contributes to the high resistance frequency observed for Man-PTS-targeting bacteriocins.
ACKNOWLEDGMENTS
This work was supported by a grant from The Research Council of Norway.
We thank Liv-Marit Rørvik of the Norwegian School of Veterinary Science for providing the L. monocytogenes strains and Zhian Salehian and Mari Christine Brekke for technical assistance.
Footnotes
Published ahead of print on 18 March 2011.
REFERENCES
- 1. Abachin E., et al. 2002. Formation of d-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 43:1–14 [DOI] [PubMed] [Google Scholar]
- 2. Arous S., et al. 2004. Global analysis of gene expression in an rpoN mutant of Listeria monocytogenes. Microbiology 150:1581–1590 [DOI] [PubMed] [Google Scholar]
- 3. Axelsson L., Katla T., Bjornslett M., Eijsink V. G., Holck A. 1998. A system for heterologous expression of bacteriocins in Lactobacillus sake. FEMS Microbiol. Lett. 168:137–143 [DOI] [PubMed] [Google Scholar]
- 4. Bieler S., Silva F., Soto C., Belin D. 2006. Bactericidal activity of both secreted and nonsecreted microcin E492 requires the mannose permease. J. Bacteriol. 188:7049–7061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breukink E., et al. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361–2364 [DOI] [PubMed] [Google Scholar]
- 6. Brötz H., et al. 1998. Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin, epidermin and other lantibiotics. Mol. Microbiol. 30:317–327 [DOI] [PubMed] [Google Scholar]
- 7. Chopin A., Chopin M. C., Moillo-Batt A., Langella P. 1984. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid 11:260–263 [DOI] [PubMed] [Google Scholar]
- 8. Cintas L. M., Casaus P., Havarstein L. S., Hernandez P. E., Nes I. F. 1997. Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl. Environ. Microbiol. 63:4321–4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cleveland J., Montville T. J., Nes I. F., Chikindas M. L. 2001. Bacteriocins: safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 71:1–20 [DOI] [PubMed] [Google Scholar]
- 10. Collins B., Curtis N., Cotter P. D., Hill C., Ross R. P. 2010. The ABC transporter AnrAB contributes to the innate resistance of Listeria monocytogenes to nisin, bacitracin, and various beta-lactam antibiotics. Antimicrob. Agents Chemother. 54:4416–4423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cotter P. D., Hill C., Ross R. P. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777–788 [DOI] [PubMed] [Google Scholar]
- 12. Dalet K., Cenatiempo Y., Cossart P., Hechard Y. 2001. A σ54-dependent PTS permease of the mannose family is responsible for sensitivity of Listeria monocytogenes to mesentericin Y105. Microbiology 147:3263–3269 [DOI] [PubMed] [Google Scholar]
- 13. de Ruyter P. G., Kuipers O. P., de Vos W. M. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deutscher J., Francke C., Postma P. W. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70:939–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diep D. B., Skaugen M., Salehian Z., Holo H., Nes I. F. 2007. Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc. Natl. Acad. Sci. U. S. A. 104:2384–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eijsink V. G., Skeie M., Middelhoven P. H., Brurberg M. B., Nes I. F. 1998. Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl. Environ. Microbiol. 64:3275–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Esquinas-Rychen M., Erni B. 2001. Facilitation of bacteriophage lambda DNA injection by inner membrane proteins of the bacterial phosphoenol-pyruvate: carbohydrate phosphotransferase system (PTS). J. Mol. Microbiol. Biotechnol. 3:361–370 [PubMed] [Google Scholar]
- 18. Glaser P., et al. 2001. Comparative genomics of Listeria species. Science 294:849–852 [DOI] [PubMed] [Google Scholar]
- 19. Gravesen A., Jydegaard Axelsen A. M., Mendes da Silva J., Hansen T. B., Knochel S. 2002. Frequency of bacteriocin resistance development and associated fitness costs in Listeria monocytogenes. Appl. Environ. Microbiol. 68:756–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gravesen A., et al. 2004. pbp2229-mediated nisin resistance mechanism in Listeria monocytogenes confers cross-protection to class IIa bacteriocins and affects virulence gene expression. Appl. Environ. Microbiol. 70:1669–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gravesen A., et al. 2002. High-level resistance to class IIa bacteriocins is associated with one general mechanism in Listeria monocytogenes. Microbiology 148:2361–2369 [DOI] [PubMed] [Google Scholar]
- 22. Héchard Y., Pelletier C., Cenatiempo Y., Frere J. 2001. Analysis of σ54-dependent genes in Enterococcus faecalis: a mannose PTS permease (EIIMan) is involved in sensitivity to a bacteriocin, mesentericin Y105. Microbiology 147:1575–1580 [DOI] [PubMed] [Google Scholar]
- 23. Héchard Y., Sahl H.-G. 2002. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 84:545–557 [DOI] [PubMed] [Google Scholar]
- 24. Holo H., Nes I. F. 1989. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl. Environ. Microbiol. 55:3119–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holo H., Nilssen O., Nes I. F. 1991. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J. Bacteriol. 173:3879–3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaern M., Elston T. C., Blake W. J., Collins J. J. 2005. Stochasticity in gene expression: from theories to phenotypes. Nat. Rev. Genet. 6:451–464 [DOI] [PubMed] [Google Scholar]
- 27. Katla T., Naterstad K., Vancanneyt M., Swings J., Axelsson L. 2003. Differences in susceptibility of Listeria monocytogenes strains to sakacin P, sakacin A, pediocin PA-1, and nisin. Appl. Environ. Microbiol. 69:4431–4437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kjos M., Nes I. F., Diep D. B. 2009. Class II one-peptide bacteriocins target a phylogenetically defined subgroup of mannose phosphotransferase systems on sensitive cells. Microbiology 155:2949–2961 [DOI] [PubMed] [Google Scholar]
- 29. Kjos M., Salehian Z., Nes I. F., Diep D. B. 2010. An extracellular loop of the mannose phosphotransferase system component IIC is responsible for specific targeting by class IIa bacteriocins. J. Bacteriol. 192:5906–5913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuipers O. P., de Ruyter P. G. G. A., Kleerebezem M., de Vos W. M. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15–21 [Google Scholar]
- 31. Larsen M. H., Kallipolitis B. H., Christiansen J. K., Olsen J. E., Ingmer H. 2006. The response regulator ResD modulates virulence gene expression in response to carbohydrates in Listeria monocytogenes. Mol. Microbiol. 61:1622–1635 [DOI] [PubMed] [Google Scholar]
- 32. Losick R., Desplan C. 2008. Stochasticity and cell fate. Science 320:65–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nes I. F., et al. 1996. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Van Leeuwenhoek 70:113–128 [DOI] [PubMed] [Google Scholar]
- 34. Nes I. F., Yoon S.-S., Diep D. B. 2007. Ribosomally synthesized antimicrobial peptides (bacteriocins) in lactic acid bacteria: a review. Food Sci. Biotechnol. 16:675–690 [Google Scholar]
- 35. Nieto Lozano J. C., Meyer J. N., Sletten K., Pelaz C., Nes I. F. 1992. Purification and amino acid sequence of a bacteriocin produced by Pediococcus acidilactici. J. Gen. Microbiol. 138:1985–1990 [DOI] [PubMed] [Google Scholar]
- 36. Nissen-Meyer J., Rogne P., Oppegard C., Haugen H. S., Kristiansen P. E. 2009. Structure-function relationships of the non-lanthionine-containing peptide (class II) bacteriocins produced by Gram-positive bacteria. Curr. Pharm. Biotechnol. 10:19–37 [DOI] [PubMed] [Google Scholar]
- 37. Opsata M., Nes I. F., Holo H. 2010. Class IIa bacteriocin resistance in Enterococcus faecalis V583: the mannose PTS operon mediates global transcriptional responses. BMC Microbiol. 10:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Postma P. W., Lengeler J. W., Jacobson G. R. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raj A., van Oudenaarden A. 2008. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135:216–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramnath M., Arous S., Gravesen A., Hastings J. W., Héchard Y. 2004. Expression of mptC of Listeria monocytogenes induces sensitivity to class IIa bacteriocins in Lactococcus lactis. Microbiology 150:2663–2668 [DOI] [PubMed] [Google Scholar]
- 41. Ramnath M., Beukes M., Tamura K., Hastings J. W. 2000. Absence of a putative mannose-specific phosphotransferase system enzyme IIAB component in a leucocin A-resistant strain of Listeria monocytogenes, as shown by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Appl. Environ. Microbiol. 66:3098–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Solheim M., Aakra A., Snipen L. G., Brede D. A., Nes I. F. 2009. Comparative genomics of Enterococcus faecalis from healthy Norwegian infants. BMC Genomics 10:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stoll R., Goebel W. 2010. Identification of the major PEP-phosphotransferase systems (PTS) for glucose, mannose and cellobiose of Listeria monocytogenes and their significance for extra- and intracellular growth. Microbiology 156:1069–1083 [DOI] [PubMed] [Google Scholar]
- 44. Tessema G. T., Moretro T., Kohler A., Axelsson L., Naterstad K. 2009. Complex phenotypic and genotypic responses of Listeria monocytogenes strains exposed to the class IIa bacteriocin sakacin P. Appl. Environ. Microbiol. 75:6973–6980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vadyvaloo V., et al. 2004. Cell-surface alterations in class IIa bacteriocin-resistant Listeria monocytogenes strains. Microbiology 150:3025–3033 [DOI] [PubMed] [Google Scholar]
- 46. Vadyvaloo V., Hastings J. W., van der Merwe M., Rautenbach M. J. 2002. Membranes of class IIa bacteriocin-resistant Listeria monocytogenes cells contain increased levels of desaturated and short-acyl-chain phosphatidylglycerols. Appl. Environ. Microbiol. 68:5223–5230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van de Guchte M., van der Vossen J. M., Kok J., Venema G. 1989. Construction of a lactococcal expression vector: expression of hen egg white lysozyme in Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 55:224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vu-Khac H., Miller K. W. 2009. Regulation of mannose phosphotransferase system permease and virulence gene expression in Listeria monocytogenes by the EIItMan transporter. Appl. Environ. Microbiol. 75:6671–6678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xue J., et al. 2005. Novel activator of mannose-specific phosphotransferase system permease expression in Listeria innocua, identified by screening for pediocin AcH resistance. Appl. Environ. Microbiol. 71:1283–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xue J., Miller K. W. 2007. Regulation of the mpt operon in Listeria innocua by the ManR protein. Appl. Environ. Microbiol. 73:5648–5652 [DOI] [PMC free article] [PubMed] [Google Scholar]