Abstract
During HIV-1 assembly, Gag polypeptides multimerize to form an immature capsid and also package HIV-1 genomic RNA. Assembling Gag forms immature capsids by progressing through a stepwise pathway of assembly intermediates containing the cellular ATPase ABCE1, which facilitates capsid formation. The NC domain of Gag is required for ABCE1 binding, acting either directly or indirectly. NC is also critical for Gag multimerization and RNA binding. Previous studies of GagZip chimeric proteins in which NC was replaced with a heterologous leucine zipper that promotes protein dimerization but not RNA binding established that the RNA binding properties of NC are dispensable for capsid formation per se. Here we utilized GagZip proteins to address the question of whether the RNA binding properties of NC are required for ABCE1 binding and for the formation of ABCE1-containing capsid assembly intermediates. We found that assembly-competent HIV-1 GagZip proteins formed ABCE1-containing intermediates, while assembly-incompetent HIV-1 GagZip proteins harboring mutations in residues critical for leucine zipper dimerization did not. Thus, these data suggest that ABCE1 does not bind to NC directly or through an RNA bridge, and they support a model in which dimerization of Gag, mediated by NC or a zipper, results in exposure of an ABCE1-binding domain located elsewhere in Gag, outside NC. Additionally, we demonstrated that immature capsids formed by GagZip proteins are insensitive to RNase A, as expected. However, unexpectedly, immature HIV-1 capsids were almost as insensitive to RNase A as GagZip capsids, suggesting that RNA is not a structural element holding together immature wild-type HIV-1 capsids.
INTRODUCTION
The 55-kDa human immunodeficiency virus type 1 (HIV-1) Gag polyprotein is the only viral protein needed for the assembly and release of virus-like particles (VLPs). This process begins in the cytoplasm with the translation of Gag; continues with the targeting of Gag to the plasma membrane, where 3,000 to 5,000 Gag polyproteins multimerize to form a single immature HIV-1 capsid; and is completed when the fully assembled, spherical immature capsid undergoes budding, release, and maturation to form the cone-shaped mature viral capsid. The immature capsid is formed by way of an energy-dependent, multistep pathway in cells (11, 12, 23, 24, 27, 28, 35, 36). At a biochemical level, Gag progresses sequentially through a series of assembly intermediates, which have approximate sedimentation (S) coefficients of ∼10S, ∼80S, ∼150S, and ∼500S, before forming the completely assembled ∼750S immature capsid (11, 12, 27, 34). The high-molecular-weight assembly intermediates (∼80S, ∼150S, and ∼500S) contain Gag as well as cellular factors, including the ATP binding cassette protein E1 (ABCE1), a cellular protein that facilitates assembly (40). Successful progression of Gag through this assembly pathway requires specific motifs in Gag that are known to be critical for HIV-1 capsid assembly (11, 12, 27, 34). These motifs reside in the three domains of Gag that are required for assembly: matrix (MA), capsid (CA), and nucleocapsid (NC). In contrast, the fourth domain, p6, is not required for assembly and instead is critical for release (reviewed in references 13 and 14).
Because the function of the viral capsid is to protect the viral genome and properly deliver it to a target cell, the Gag polyprotein must also bind and encapsidate viral genomic RNA while undergoing assembly. This function is performed by the NC domain of Gag, which is required both for capsid assembly and for the binding and encapsidation of genomic and cellular RNA. The zinc fingers in NC govern specific encapsidation of retroviral genomic RNA, while the large number of basic residues in NC interact with nucleic acid nonspecifically (reviewed in reference 3). Additionally, the basic residues in NC are critical for capsid assembly (2, 4, 8, 10, 33). Consistent with this finding, NC basic residues are also required for the recruitment of Gag into the HIV-1 capsid assembly pathway and for binding to the assembly cofactor ABCE1 (11, 12, 26, 27, 40).
The observation that NC is critical both for assembly and for RNA binding has led to the proposal that NC-mediated RNA binding is critical for capsid formation, with RNA acting either by driving interprotein Gag-Gag interactions and/or by bridging Gag proteins together. To distinguish between these two models for the role of RNA in capsid formation, a number of groups have utilized GagZip chimeric proteins (1, 9, 19, 39), in which NC is replaced by a heterologous transcription factor leucine zipper (LZ) domain that facilitates protein dimerization or trimerization but does not bind RNA. Using the GagZip chimera, one can ask whether a domain that lacks the capacity to bind RNA and mediates dimerization entirely through strong protein-protein contacts is sufficient to promote Gag assembly. Such HIV-1 and Rous sarcoma virus (RSV) GagZip proteins have been found to assemble and release immature capsids that closely resemble those formed by wild-type (WT) Gag (1, 9, 19, 39), except that they contain very little genomic or cellular RNA (9, 38, 39). By demonstrating that an LZ domain lacking RNA binding ability can replace NC during capsid formation, these studies suggest that RNA acts indirectly to promote Gag-Gag interactions during assembly rather than acting directly to bridge Gag polypeptides. In contrast, proper encapsidation of RNA by the assembling virus requires RNA binding by NC, as evidenced by the dramatic reduction in RNA packaging observed upon replacement of NC with an LZ domain. Together these data support a model in which RNA binding by NC drives Gag-Gag interactions, which in turn promote capsid assembly.
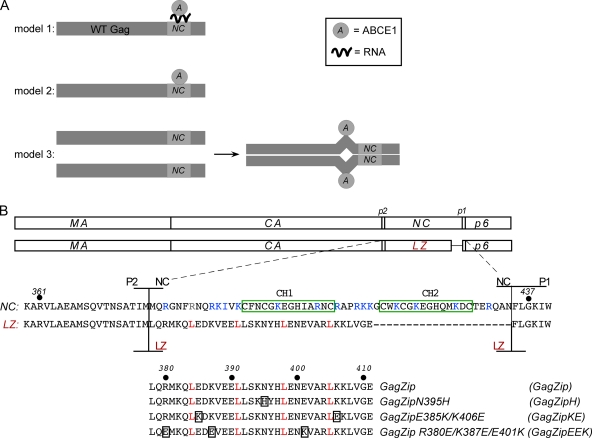
Because GagZip proteins allow the role of Gag oligomerization to be assessed independently of NC-mediated RNA binding, GagZip constructs can also be used to ask whether other functions of NC are dependent on Gag oligomerization rather than on NC-mediated RNA bridging. Specifically, it is known that the basic residues in NC are required for proper progression through the HIV-1 capsid assembly pathway (26) and for recruitment of the assembly cofactor ABCE1 present in the high-molecular-weight capsid assembly intermediates (11, 12, 40), but it is not clear whether RNA binding by NC plays a direct or an indirect role in these events. With respect to ABCE1 binding, at least three models can be envisioned (Fig. 1 A): (i) binding of ABCE1 to Gag could be initiated or maintained via an NC-dependent RNA bridge; (ii) ABCE1 could bind directly to NC through RNA-independent protein-protein contacts; or (iii) RNA-mediated oligomerization of NC could result in the exposure of a cryptic ABCE1 binding site present in a different region of Gag.
Fig. 1.
Model for ABCE1 binding and amino acid alignments of GagZip constructs. (A) Three models for the binding of ABCE1 to Gag. (Model 1) The binding of ABCE1 to Gag could be mediated through RNA recruited by NC. (Model 2) ABCE1 could bind directly to NC through protein-protein interactions. (Model 3) NC-mediated dimerization of Gag could expose a cryptic ABCE1 binding site in another region of Gag. (B) Amino acid alignments of Gag, GagZip, and GagZip mutants. Line diagrams show the domains of WT Gag (matrix [MA], capsid [CA], nucleocapsid [NC], and p6, along with two spacers, p2 and p1) and the GagZip chimeric protein used in this study. The sequences below show the amino acids in and around NC and the leucine zipper (LZ). The two zinc fingers in NC are boxed in green; basic residues in NC are indicated in blue; and amino acids in the d position of the LZ heptad repeat are indicated in red (see Fig. S1 in the supplemental material for more details). The alignment at the bottom shows the leucine zipper sequences of GagZip and the GagZip mutants, with mutated residues boxed.
In this study, we utilized GagZip proteins to distinguish between these models for the role of NC in ABCE1 binding and the formation of assembly intermediates. Here we demonstrate that dimerization-competent GagZip proteins bind to ABCE1 just as well as WT Gag and also form ABCE1-containing complexes that closely resemble previously described HIV-1 capsid assembly intermediates. Thus, it appears that NC- or LZ-mediated oligomerization is required for Gag-ABCE1 binding but NC-mediated RNA binding is dispensable. Additionally, our data suggest that ABCE1 does not bind directly to NC. Together, these findings argue against the first two models for ABCE1 binding described above and support the third model, in which dimerization of Gag exposes a site for ABCE1 binding elsewhere in Gag. Finally, we also analyzed the role of RNA in the structural integrity of immature HIV-1 WT and GagZip capsids. As expected, fully assembled immature HIV-1 GagZip capsids, which contain very little RNA, are insensitive to RNase A. However, unexpectedly, fully assembled WT immature HIV-1 capsids retained their structural integrity upon digestion of >99% of VLP RNA with RNase A. These data raise the possibility that, at least for HIV-1, RNA does not act as a structural element that is required for the integrity of immature capsids.
MATERIALS AND METHODS
Plasmids.
All plasmids utilized in this study were generated in the HIV-1 provirus strain LAI with a deletion in env (HIV-1ΔEnv) (21). To precisely replace NC in this provirus backbone with a GCN4 zipper in the backbone of HIVΔEnv, we amplified an insert by two rounds of PCR using either HIVΔEnv or a construct containing a GCN4 zipper obtained from H. Göttlinger (1) as a template (primers are described in Table S1 in the supplemental material). In the first round, three reactions were performed to yield three fragments with overlapping complementary ends: (i) an HIV-1ΔEnv template produced with the 5′ Gag and 3′ P2/Zip primers, (ii) a GCN4 template produced with the 5′ P2/Zip and 3′ Zip/p1 primers, and (iii) an HIV-1ΔEnv template produced with the 5′ Zip/p1 and 3′ Pol primers. The three fragments were gel purified, and each was used in a second PCR, along with primers 5′ Gag and 3′ Pol, to generate a fragment containing CA-P2-zip-P1-P6-Pol with the exact junctions in the primers. A unique AgeI site (in CA) and a unique NheI site (in Pol) were used to ligate the insert back into the provirus to generate HIV-1 GagZip. Site-directed mutagenesis using HIV-1 GagZip as a template was used to generate GagZip N395H, GagZip E385K/K406E, GagZip R380E/K387E/E401K, GagZip G2A, and GagZip WM184/185AA. Site-directed mutagenesis using the original HIV-1ΔEnv plasmid encoding WT Gag was used to generate Gag G2A, GagKR9A, and Gag WM184/185AA. All plasmid constructions were confirmed by sequencing. GagΔNCΔp6 (previously termed HIV-1 Gag Tr) has been described previously (11, 12). The Moloney murine leukemia virus (MLV) Pro− construct was made by engineering a D31L mutation into the protease of an MLV proviral construct (obtained from A. Dusty Miller) using standard cloning techniques.
Transfection, cell harvest, immunoprecipitation, and Western blotting.
COS-1 cells were transfected with the plasmids indicated on the figures, using polyethylenimine (Polysciences). Cells were harvested 38 h posttransfection in NP-40 buffer (which contains 0.625% NP-40, 10 mM Tris acetate [pH 7.4], 50 mM potassium acetate, 100 mM NaCl, and 10 mM EDTA), as described previously (26). Cell lysates were subjected to immunoprecipitation with an antibody against ABCE1 (αABCE1) as described previously (26). Lysates and immunoprecipitation eluates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting with either αABCE1 (40), a murine monoclonal antibody directed against HIV-1 Gag p24 (an HIV-1 p24 hybridoma [183-H12-5C] obtained from Bruce Chesebro through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH), or a goat polyclonal anti-MLV CA antiserum generated by NCI and obtained from Stephen Goff and Michael Emerman.
Gradient analysis.
Cell lysates or VLP preparations harvested in NP-40 buffer were subjected to velocity sedimentation for 45 min at 4°C in a Beckman MLS-50 rotor at 45,000 rpm (217,000 × g). The following sucrose solutions, made in the NP-40 buffer described above, were used for step gradients: (i) 675 μl each of 10%, 15%, 20%, 40%, 50%, 66%, and 80% sucrose for Fig. 2B and 9C and (ii) 675 μl each of 10%, 15%, 40%, 50%, 60%, 70%, and 80% sucrose for Fig. 4, 5, and 7. The first of these two gradients is better for separating the ∼500S and 750S peaks, while the second gradient is better for separating the ∼80S/150S peak from the ∼500S peak. Note that neither of these gradients separates Gag or GagZip monomers from dimers. Gradients were fractionated from top to bottom, and aliquots of fractions were analyzed by SDS-PAGE, followed by Western blotting or immunoprecipitation, as indicated in the figure legends. The method for estimating the migration of particles with different S values in gradients has been described previously (27).
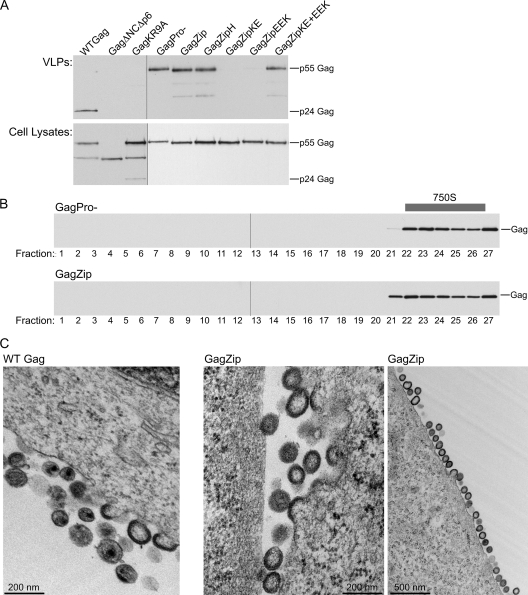
Fig. 2.
VLP production by GagZip proteins is inhibited by mutations that disrupt zipper dimerization. (A) COS-1 cells were transfected with WT Gag, GagZip, or the indicated mutants. Shown are results for equivalent aliquots of VLPs harvested by pelleting through sucrose (top) or cell lysates (bottom), analyzed by Western blotting for Gag. The migrations of uncleaved p55 Gag and cleaved p24 Gag are indicated to the right of the blots. (B) 293T cells were transfected with GagPro− or GagZip, and VLPs isolated from the medium were analyzed by velocity sedimentation on sucrose step gradients. Fractions were analyzed by Western blotting for Gag and are shown with the S value increasing from left to right. The position of ∼750S capsids in the gradients is indicated. The experiments for panels A and B were performed 3 times, and results of one representative experiment are shown in each panel. (C) Thin-section TEM of infected COS-1 cells shows WT Gag (left) and GagZip (center and right) capsids assembling at the plasma membrane.
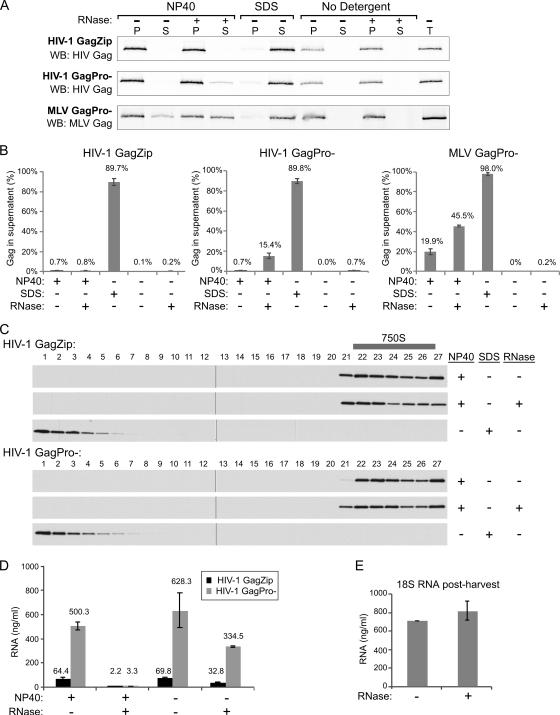
Fig. 9.
De-enveloped immature HIV-1 capsids are largely resistant to RNase A treatment. (A and B) 293T cells were transfected to express HIV-1 GagZip, HIV-1 GagPro−, or MLV GagPro−. VLPs harvested from the medium were treated with NP-40 or with SDS or were left untreated (No Detergent). Detergent-treated and untreated VLPs were subsequently treated with RNase A or buffer. After treatment, capsids were repelleted, and equivalent aliquots of the supernatant (S) and pellet (P) were compared to the VLP total before treatment (T) by Western blotting for HIV-1 or MLV Gag. The experiment was performed, and the results quantified, 3 times. (A) Western blots from one representative experiment. (B) Averages of results of 3 independent experiments, with the amount of Gag in the supernatant upon repelleting shown as percent total Gag. Error bars indicate standard errors of the mean. (C) HIV-1 VLPs were de-enveloped with NP-40, treated with RNase A or buffer, and analyzed by velocity sedimentation on sucrose step gradients as for Fig. 2B. Fractions were analyzed by Western blotting for HIV- 1 Gag and are shown with S values increasing from left to right. The position of 750S capsids in the gradients is indicated. (D) HIV- 1 GagZip and HIV-1 GagPro− VLPs that had been de-enveloped with NP-40 and treated with RNase A where indicated were also analyzed for total RNA. Error bars indicate standard deviations. (E) As a control to determine whether RNase A continued to be active after samples were harvested in Trizol, 18 S rRNA (final concentration, 1,000 ng/ml) was added to RNase A- or buffer-treated mock samples immediately following Trizol treatment, and total RNA was quantified.
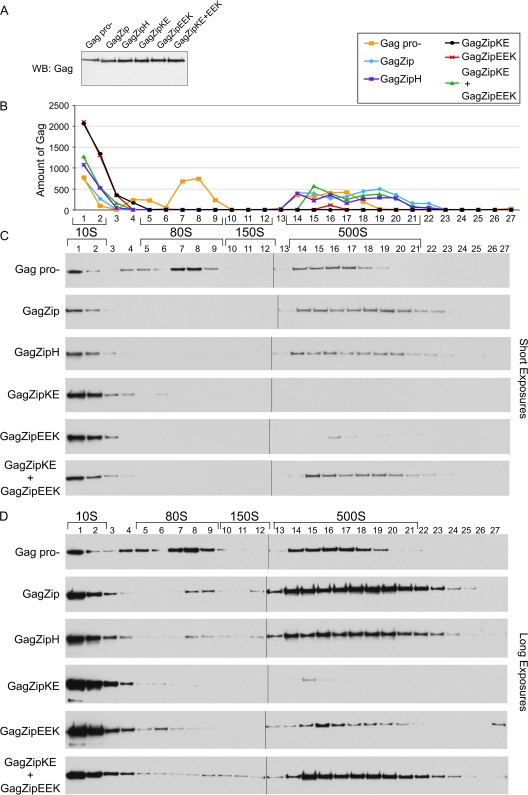
Fig. 4.
Assembling GagZip proteins form complexes corresponding to the 80S and 500S assembly intermediates formed by WT Gag. (A) COS-1 cells were transfected with the indicated constructs, and cell lysates were analyzed by Western blotting for Gag. (B) Quantification of Western blots from panel C, showing amounts of Gag (in arbitrary units). (C) Cell lysates from the experiment for panel A were subjected to velocity sedimentation on sucrose step gradients to separate out individual assembly intermediates, which were analyzed by Western blotting to show the amount of Gag in each fraction. The positions of the ∼10S, ∼80S, ∼150S, and ∼500S assembly intermediates are indicated by brackets. (D) Long exposures of blots shown in panel C. The experiment was performed 3 times, and results of one representative example are shown.
Fig. 5.
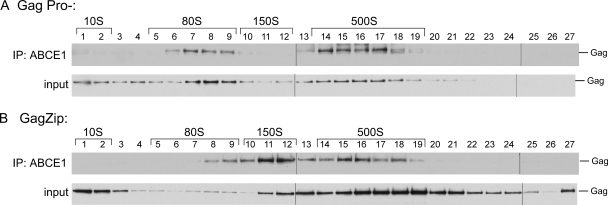
The 80S/150S and 500S complexes formed by GagZip proteins are associated with ABCE1. Lysates of COS-1 cells expressing GagPro− (A) or GagZip (B) were subjected to velocity sedimentation and fractionation in order to separate each assembly intermediate, as for Fig. 4. Each fraction was then subjected to immunoprecipitation (IP) using αABCE1. The top blot in each panel shows immunoprecipitation eluates analyzed by Western blotting for Gag. The bottom blot in each panel shows equivalent amounts of immunoprecipitation inputs, analyzed by Western blotting for Gag. The positions of the ∼10S, ∼80S, ∼150S, and ∼500S assembly intermediates are indicated by brackets. The experiment was performed 3 times, and one representative example is shown.
Fig. 7.
The progression of GagZip through the assembly pathway requires the WM184/185 motif in CA but not the myristoylation motif in MA. (A) COS-1 cells were transfected with the indicated constructs. Cell lysates and VLPs were harvested and analyzed by Western blotting as for Fig. 2A. (B) Lysates from cells transfected as for panel A were subjected to velocity sedimentation on sucrose step gradients in order to separate out individual assembly intermediates. Each fraction was analyzed by Western blotting for Gag. The positions of the 10S, 80S, 150S, and 500S assembly intermediates are indicated by brackets. The experiment was performed 4 times, and one representative example is shown.
Infections.
Pseudotyped virus particles were generated by transfecting 293T cells in 6-well plates with a plasmid encoding a WT or mutant viral genome (1.5 μg), a 2nd-generation packaging vector (psPAX2, obtained from Didier Trono; 1 μg), and a vesicular stomatitis virus glycoprotein (VSV-G) plasmid (pMD2.G, obtained from Didier Trono; 0.2 μg). Virus titers were determined on TZM-bl cells (obtained from John C. Kappes, Xiaoyun Wu, and Tranzyme Inc. through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) using 20 μg/ml DEAE dextran and spinoculation (2 h at 2,500 rpm in a Beckman Allegra 6 centrifuge using a GH-3.8 rotor). COS-1 cells were plated in 6-well plates (300,000 cells per well), and were infected with virus at a multiplicity of infection (MOI) of 10 to 50 using 20 μg/ml DEAE dextran and spinoculation as described above. Following incubation with the virus for 12 to 15 h, cells were washed 3 times with complete medium to remove the virus inoculum. Cells were harvested for transmission electron microscopy (TEM) experiments ∼36 h postinfection, as described below.
VLP preparation and RNase A treatment.
COS-1 or 293T cells were transfected with the indicated constructs; the medium was changed at 6 h posttransfection; and the medium was collected 38 h posttransfection. For Fig. 9 and 2B, indinavir was added to the GagZip plates at a final concentration of 5 μM during medium collection to ensure the production of immature particles. The medium was centrifuged (for 10 min at 2,000 rpm in a Beckman Allegra 6R centrifuge using a GH-3.8 rotor), passed through a syringe filter (pore size, 0.45 μm) to remove cells, and then pelleted through a 30% sucrose cushion for 30 min at 4°C in a Beckman L-90 centrifuge at 60,000 rpm using an SW60 Ti rotor (485,000 × g). The medium was aspirated; pellets were harvested in NP-40 buffer; and aliquots were analyzed by SDS-PAGE and Western blotting with an antibody to Gag as described above.
In RNase A treatment experiments, the protocol of Muriaux et al. (29) was followed. Briefly, 293T cells were transfected with the indicated constructs, and the medium was harvested at 44 h posttransfection, as described above. The medium was pelleted through 20% sucrose–TNE (100 mM NaCl, 10 mM Tris-HCl [pH 7.4], 1 mM EDTA) for 45 min at 4°C in a Beckman L90 centrifuge at 27,000 rpm using an SW40 Ti rotor (115,000 × g). The supernatant was aspirated, and sucrose was decanted. VLPs were resuspended in 1× TN (100 mM NaCl, 50 mM Tris-HCl [pH 7.4]) for 1 h on ice, further resuspended by gentle pipetting, transferred to 1.5-ml microcentrifuge tubes, and then either de-enveloped with NP-40 (final concentration, 1%; Roche), denatured with SDS (final concentration, 1%), or left untreated for 30 min at room temperature. All samples, except those treated with SDS, were then treated with either RNase A (final concentration, 1.3 mg/ml; Qiagen) or an equal volume of 1× TN buffer for 2 h at 26°C. VLPs were then repelleted through a 10-μl 20% sucrose cushion (containing the same salts and detergents as the treated samples) at 14,000 rpm in a Beckman microcentrifuge (18,000 × g) using 0.2-ml tubes. Equivalent aliquots of supernatants and pellets were analyzed by SDS-PAGE and Western blotting as described above. Ponceau staining was performed to ensure that inputs of MLV, HIV, and GagZip capsids in detergent and RNase A treatment experiments were approximately equivalent (data not shown). RiboGreen analysis of VLPs was used to demonstrate that RNase A was enzymatically active (data not shown).
RiboGreen analysis of VLP RNA.
In two of the RNase A experiments described above, aliquots of the HIV-1 GagZip and HIV-1 GagPro− VLP preparations described above were used for total-RNA measurement without normalization to the MLV VLP preparation. These VLP samples were de-enveloped with NP-40 where indicated and were treated with RNase A or buffer in parallel with the samples described above. Following the treatments, VLP preparations were immediately harvested in Trizol (Invitrogen), and the RNA was precipitated according to the manufacturer's instructions. Pellets were resuspended in 1× DNase I buffer (Ambion) and were incubated at 37°C for 20 min with 1 μl of DNase I (Ambion), and the RNA concentration was determined using RiboGreen (Invitrogen). To determine whether RNase A was completely inactivated by denaturation in Trizol, mock samples were prepared with 1× TN, treated with NP-40 as described above, treated with 1× TN buffer or RNase A as described above, and lysed with Trizol. Following lysis, 18S RNA was added to a final concentration of 1 μg/ml to each sample, and total RNA was extracted and measured using RiboGreen.
Quantitative analysis of Western blots.
For Fig. 4, semiquantitative Western blotting was performed using enhanced chemiluminescence (ECL) and film. A standard curve was included in each blot to allow the selection of films with signals that were equivalent and within the linear range of the standard curve. Densitometry was performed on scanned films using ImageJ. For Fig. 9, quantitative Western blotting was performed using the LiCor Odyssey system.
Standard TEM.
For standard TEM (Fig. 2C and 8A), infected cells were harvested in Karnovsky's fixative (2% paraformaldehyde and 2.5% glutaraldehyde, buffered with 0.2 M cacodylate), osmicated with 2% OsO4–0.2 M cacodylate buffer for 2 h, dehydrated with increasing percentages of ethanol, infiltrated overnight in propylene oxide–Epon 812 resin, and cured overnight at 60°C. Sections (thickness, 70 to 90 nm) were poststained with uranyl acetate and lead citrate. Samples were processed by the Fred Hutchinson Electron Cancer Research Center Electron Microscopy Resource (Seattle, WA). Sections were examined using a JEOL 1230 transmission electron microscope with Gatan digital micrograph software.
Fig. 8.
GagZip G2A forms cytoplasmic capsid-like structures and can be found colocalized with cytoplasmic ABCE1. (A) Infected COS-1 cells expressing GagZip G2A were analyzed by TEM. (Left and center panels) Regions of plasma membranes (indicated by black arrowheads) from two adjacent cells. Capsid-like structures were not found at the plasma membrane but were found in the cytoplasm (indicated by white arrowheads). (Right panel) Additional regions of the cytoplasm containing capsid-like structures. Bars, 200 nm. (B) Infected COS-1 cells expressing GagZip G2A were also subjected to immunogold labeling, as for Fig. 6A to C, with Gag labeled by 6-nm-diameter gold particles and ABCE1 labeled by 15-nm-diameter gold particles. Panel 1 shows a representative region of the plasma membrane. Panels 2 to 5 show areas of the cytoplasm. White arrowheads indicate clusters of Gag colocalized with ABCE1 in the cytoplasm. Black arrowheads point to the plasma membrane. Boxes indicate large clusters of Gag colocalized with ABCE1, with underlying areas of electron density that are ≥100 nm in diameter. Bars, 200 nm.
Immunogold double labeling and quantitation.
For immunogold labeling, infected cells were harvested in 3% paraformaldehyde–0.025% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4), pelleted, and subjected to high-pressure freezing using the Leica EMPACT2 high-pressure freezer, followed by freeze substitution. Immunogold samples were infiltrated overnight with LR White embedding resin (London Resin Company Ltd., Reading, Berkshire, England) in ethanol, placed in straight LR White, embedded in gelatin capsules (Electron Microscopy Sciences, Hatfield, PA), and cured overnight in a UV light cryochamber at 4°C. Sections (thickness, ∼50 nm) were placed on grids, treated with 0.05 M glycine for 20 min at room temperature (RT), rinsed in phosphate-buffered saline (PBS), blocked for 45 min with 1% bovine serum albumin, and washed in PBS and 0.1% bovine serum albumin-C (BSA-C; Electron Microscopy Sciences, Hatfield, PA). The monoclonal antibody directed against HIV-1 Gag, described above, was used at a final concentration of 0.25 mg/ml and was detected with goat anti-mouse IgG conjugated to 6-nm-diameter gold particles diluted 1:1 in 0.1% BSA-C in PBS (EM Services, Singapore). Affinity-purified, peptide-specific rabbit αABCE1 was prepared as described in reference 40, desalted using a Zeba desalting column (Pierce), concentrated to 1.0 mg/ml, diluted 1:1 with 0.1% BSA-C and 0.01% Tween 20 in PBS, and detected with goat anti-rabbit IgG conjugated to 15-nm-diameter gold particles diluted 1:1 with 0.1% BSA-C and 0.01% Tween 20 in PBS (EM Services, Singapore). For double immunolabeling, grids were subjected to the following series of 1-h incubations: αABCE1 as a primary antibody, gold-conjugated goat anti-rabbit IgG as a secondary antibody, αGag as a primary antibody, and gold-conjugated goat anti-mouse IgG as a secondary antibody. Between incubations, grids were rinsed 3 times with PBS and 3 times with 0.1% BSA-C in PBS. Sections were then fixed with 2% glutaraldehyde in PBS for 10 min at RT and were rinsed in deionized water. Samples were poststained with uranyl acetate for 15 min and with lead citrate for 5 min and were examined as described for standard TEM above. WT Gag, GagΔNCΔp6, GagZip, and GagZip G2A sections were processed in parallel on the same day in each experiment, and the double-labeling experiment was repeated twice. Single-label controls demonstrating the specificity of the secondary antibodies and the lack of labeling in the absence of the primary antibody have been performed previously (12). Note that the procedures involved in preparing samples for immunogold labeling maximize epitope preservation at the expense of morphology; thus, ultrastructural details are better resolved by standard TEM.
To quantify colocalization, randomly selected regions of membrane depicting Gag labeling at the plasma membrane were obtained from cells expressing WT HIV-1 Gag, HIV-1 GagZip, or GagΔNCΔp6. To obtain randomly selected images, each grid was divided into nine nearly equivalent regions. Within each region, photographs were taken of Gag labeling at the membrane of a randomly selected cell at low power. Once all the images were obtained, sites of assembly, referred to as regions of clustered Gag (RCG), were marked. An RCG was defined as a 100-nm-diameter zone (approximately equivalent to the diameter of an immature VLP) plus an additional 35-nm zone. The additional 35 nm was included because this is the maximum distance of a gold particle from the site of the antigen (the 10-nm length of the primary antibody plus the 10-nm length of the secondary antibody plus the 15-nm diameter of the large gold particle). To be counted, RCG had to contain at least three 6-nm-diameter gold particles (representing Gag). Sites where membrane deformation, dark staining, and Gag labeling were evident were marked first. Next, the remaining sites that met the criteria described above were marked, starting from one edge of the photograph and moving sequentially to the other. Clusters of Gag label that overlapped with an existing marked site, were less than 100 nm from the edge of the image field, or were further than 100 nm from the membrane in any direction were excluded from the analysis. For each RCG marked, the 100-nm inner diameter of the site was first centered on the Gag cluster and then aligned tangentially with the membrane such that the 100-nm zone was entirely within the cell. Colocalization was determined by examining each RCG for ABCE1 label. If ABCE1 labeling occurred within the 135-nm-diameter RCG zone, it was scored as positive. The percentage of colocalization was defined as the number of positive RCG as a percentage of total RCG. Error bars in Fig. 6D represent standard errors of the means (SEM) from the two separate labeling experiments, from which the total numbers of RCG were 386 for WT Gag, 380 for GagZip, and 795 for GagΔNCΔp6, obtained from 84, 109, and 120 fields, respectively.
Fig. 6.
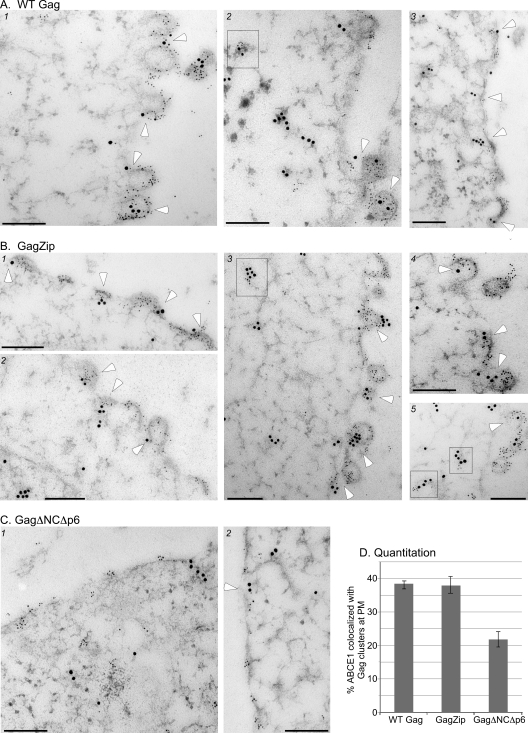
Quantitative immunogold double labeling demonstrates the recruitment of ABCE1 to sites of GagZip assembly. Infected COS-1 cells were fixed, subjected to high-pressure freezing, sectioned, and immunogold labeled. The anti-Gag antibody was detected by goat anti-mouse IgG coupled to 6-nm-diameter gold particles, while the antibody to ABCE1 was detected by goat anti-rabbit IgG coupled to 15-nm-diameter gold particles. White arrowheads indicate events that were scored as ABCE1 colocalized with Gag at the plasma membrane (PM) during quantitation. Boxes indicate clusters of Gag in the cytoplasm that are colocalized with ABCE1. Bars, 200 nm. (A) Three representative images of cells infected to express the WT HIV-1 provirus, showing early (e.g., panel 3) and late assembly events at the plasma membrane. (B) Five representative images of cells infected to express a provirus encoding HIV-1 GagZip, showing early (e.g., panel 1) and late assembly events at the plasma membrane. (C) Two representative images of cells infected to express a provirus encoding assembly-defective HIV-1 GagΔNCΔp6. (D) The number of independent Gag clusters at the plasma membrane that were colocalized with ABCE1 was quantified and is expressed in the bar graph as the percentage of total Gag clusters at the plasma membrane.
RESULTS
To analyze whether HIV-1 GagZip mutants form intracellular assembly intermediates, we generated a series of dimerization-competent and dimerization-defective GagZip mutants, outlined in Fig. 1B. To make the HIV-1 GagZip construct, the NC region of Gag in the nearly complete HIV-1ΔEnv genome was replaced with a GCN4 LZ domain (Fig. 1B), creating a GagZip chimera similar to that used by Accola et al. (1). In this construct, the GagPol frameshift region was altered, thereby reducing GagPol production and Gag cleavage, but the p6 region required for budding was left intact. We also generated 3 mutants in the leucine zipper region of the GagZip constructs (Fig. 1B). The first of these (the N395H mutant, referred to as GagZipH) replaces a polar residue in the dimer interface with a bulky charged residue. The N395H mutation would not be expected to disrupt homodimerization, because N395 is a destabilizing residue that allows dimerization to be reversible and is especially tolerant of substitutions (31). In contrast, the second and third mutations (GagZip E385K/K406E, referred to as GagZipKE, and GagZip R380E/K387E/E401K, referred to as GagZipEEK) would be expected to significantly reduce homodimerization when expressed independently, since charged residues in these two constructs disrupt important interhelical pairs that form electrostatic interactions (32) (see Fig. S1 in the supplemental material). However, because they contain complementary mutations, the GagZipKE and GagZipEEK mutants would be expected to heterodimerize when expressed together (see Fig. S1). Additionally, as positive controls, we utilized HIV-1ΔEnv genomes containing assembly-competent wild-type Gag (WT Gag) and WT Gag with an inactivated protease (GagPro−). As negative controls, we included HIV-1ΔEnv genomes containing assembly-incompetent GagΔNCΔp6 (in which NC, p1, p2, and p6 are deleted) and GagKR9A (in which 9 basic residues in NC are converted to alanines).
Biochemical and morphological analyses demonstrate that HIV-1 GagZip capsids resemble WT immature capsids.
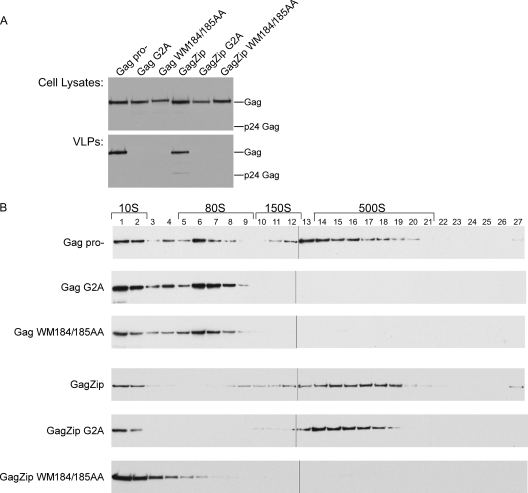
To assess the production and release of virus-like particles (VLPs) by these constructs, the medium from transfected COS-1 cells was first pelleted through sucrose cushions and then analyzed by Western blotting (Fig. 2 A). All constructs behaved as expected on the basis of previous reports (1, 8, 9, 11, 12, 17, 26, 39, 40). Specifically, GagZip formed VLPs at levels comparable to those of the positive controls, HIV-1 WT Gag and GagPro−. Additionally, the assembly-competent GagZipH mutant formed VLPs, as did the complementing mutants GagZipKE and GagZipEEK when coexpressed. However, when expressed independently, the GagZipKE and GagZipEEK mutants, which contain disrupted dimerization domains, failed to release particles, and thus resembled the negative controls, GagΔNCΔp6 and GagKR9A. Analysis of cell lysates (Fig. 2A) demonstrated that all constructs were expressed at comparable levels. WT Gag formed VLPs containing cleaved Gag (p24), while GagPro− constructs produced VLPs that contained uncleaved Gag (p55). GagZip VLPs contained largely uncleaved Gag, due to reduced expression of the WT viral protease. Since all these constructs contain a wild-type version of the p6 domain that governs budding, differences in VLP production likely reflect differences at the level of capsid assembly. Thus, we concluded that, as expected from the results of a previous study (39), the dimerization-competent GagZip and GagZipH proteins are assembly competent, while the dimerization-incompetent mutants GagZipKE and GagZipEEK are assembly defective unless they are coexpressed to allow heterodimerization.
Completely assembled wild-type immature capsids found in released virus can be distinguished from intracellular assembly intermediates by their larger S values. To determine the S value of completely assembled GagZip capsids found in released virus, the medium from 293T cells transfected with GagPro− or GagZip was harvested, treated with a nonionic detergent (to remove envelopes but leave immature capsids intact), and subjected to velocity sedimentation on sucrose gradients (Fig. 2B). Immature capsids produced by cells transfected with GagZip formed a broad peak at the same position on velocity sedimentation gradients as observed for capsids found in the medium of cells expressing GagPro−, which have been estimated to have an S value of ∼750 (27). Finally, cells expressing WT Gag, Gag mutants, or GagZip constructs were also examined by thin-section transmission electron microscopy (TEM). As expected given previously published EM images of GagZip capsids (9, 19), we found that capsids formed by HIV-1 GagZip were nearly indistinguishable from immature capsids formed by WT HIV-1 Gag (Fig. 2C). Thus, as determined by velocity sedimentation and TEM analyses, the size and morphology of immature capsids formed by GagZip are similar to those of immature capsids formed by WT Gag.
Assembly-competent GagZip proteins associate with endogenous ABCE1.
Previously, assembly-competent Gag has been shown to progress sequentially through ∼10S, ∼80S, ∼150S, and ∼500S complexes before culminating in the formation of the completely assembled ∼750S immature capsid (12, 27), thereby demonstrating that these intracellular complexes represent bona fide assembly intermediates. Additionally, it has been shown that ABCE1, a cofactor for immature capsid assembly (40), is associated with assembly intermediates (11, 12, 40). Notably, the association between Gag and ABCE1 is disrupted if multiple basic residues in the NC domain of Gag are replaced with alanines (26).
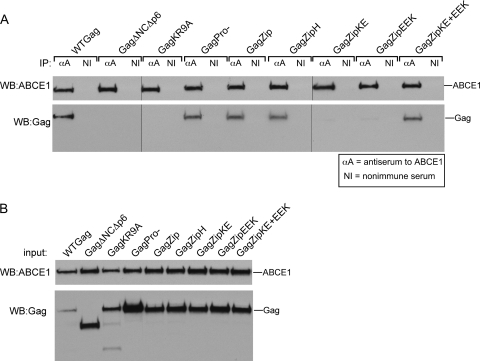
The finding that GagZip chimeric proteins can assemble even though they lack the NC domain raised the possibility that GagZip proteins might assemble through an alternate, ABCE1-independent assembly pathway. If this were the case, αABCE1 should immunoprecipitate endogenous ABCE1 but should not coimmunoprecipitate GagZip proteins. To test this possibility, we performed immunoprecipitations using αABCE1 on lysates of COS-1 cells that were transfected to express proviruses encoding WT Gag, Gag mutants, or the GagZip proteins (Fig. 3 A). Western blotting for ABCE1 demonstrated that endogenous ABCE1 was immunoprecipitated to equivalent extents from all the cell lysate samples (Fig. 3A). Western blotting for Gag revealed that αABCE1 coimmunoprecipitated WT Gag and GagPro− but not the assembly-incompetent Gag mutants GagΔNCΔp6 and GagKR9A, as expected from previous studies (11, 12, 26, 40). However, surprisingly, αABCE1 coimmunoprecipitated the assembly-competent GagZip and GagZipH proteins to the same extent as WT Gag and GagPro−. Additionally, while the assembly-defective GagZipKE and GagZipEEK mutants were not coimmunoprecipitated by αABCE1 when expressed separately, they were coimmunoprecipitated by αABCE1 when they were rendered assembly competent through coexpression. Analysis of equivalent aliquots of total-cell lysates confirmed that the expression of all constructs was comparable (Fig. 3B). Thus, unexpectedly, the assembly-competent GagZip proteins associate with endogenous ABCE1 in cells, as observed for WT Gag, while the assembly-defective GagZip constructs do not, unless they are coexpressed to allow heterodimerization. Together, these data suggest that GagZip proteins form ABCE1-containing intermediates during their assembly and that the binding site for ABCE1 likely resides in some domain in Gag other than NC, as shown in Fig. 1A, model 3.
Fig. 3.
ABCE1 is associated with WT GagZip and assembly-competent GagZip mutants but not with assembly-incompetent GagZip mutants. (A) COS-1 cells transfected with WT and mutant Gag and GagZip constructs were subjected to immunoprecipitation with an antibody to ABCE1 (αA) or a nonimmune antibody (NI) and were Western blotted (WB) for ABCE1 or HIV-1 Gag. (B) Input cell lysates used for the immunoprecipitations for panel A were Western blotted as described for panel A. The experiment was performed 3 times, and results of one representative experiment are shown.
Assembling GagZip proteins form high-molecular-weight complexes that resemble HIV-1 capsid assembly intermediates.
To directly test the hypothesis that assembly-competent GagZip proteins form complexes that resemble the assembly intermediates formed by WT Gag, cells expressing equivalent amounts of each construct (Fig. 4 A) were harvested in a nonionic-detergent-containing buffer to solubilize membranes and were analyzed by velocity sedimentation to separate Gag-containing complexes of various sizes. Quantitative analysis (Fig. 4B) of the amount of Gag in each complex was performed on Western blots, using short exposures (Fig. 4C). Titration controls demonstrated that in these short exposures, Gag expression for almost every lane was within the linear range for quantitation of the Western blot, and the total amount of Gag present in the gradient Western blot was approximately equivalent for each group (data not shown). These short exposures revealed that GagPro− formed the intracellular ∼10S, ∼80S/150S, and ∼500S assembly intermediates, as described previously (11, 12, 27, 40). In these gradients, the ∼80S and 150S complexes are not fully resolved and are therefore referred to as the ∼80S/150S complex. Additionally, these short exposures demonstrated that the assembly-competent GagZip and GagZipH proteins formed ∼10S and ∼500S complexes. Upon analysis of the assembly-defective GagZipKE construct, only the ∼10S complex could be seen, while the assembly-defective GagZipEEK construct formed the ∼10S complex and a very small amount of the ∼500S complex. When GagZipKE and GagZipEEK were coexpressed, the gradient pattern resembled that of the assembly-competent GagZip and GagZipH constructs, with production of the ∼10S complex and a significant amount of the ∼500S Gag complex.
To investigate whether assembly-competent GagZip constructs also form the ∼80S/150S complex but in smaller amounts than WT Gag, we examined longer exposures of the gradients (Fig. 4D). The signals present in these long exposures were not in the linear range of the Western blot and therefore were not amenable to quantitation. However, these longer exposures clearly demonstrated that the assembly-competent GagZip, GagZipH, and coexpressed GagZipKE+EEK constructs formed discrete peaks in the ∼80S/150S region. On these long exposures, assembly-defective constructs also contained some Gag in the ∼80S/150S region of the gradient and a small amount in the ∼500S region. Thus, we concluded that assembly-competent GagZip proteins form complexes that closely resemble the intracellular ∼80S/150S and ∼500S assembly intermediates formed by WT Gag and GagPro−, although the amounts of each complex at steady state differ, with assembly-competent GagZip proteins forming smaller ∼80S/150S peaks and larger ∼500S peaks than GagPro−. Additionally, we found that dimerization-defective GagZip proteins form predominantly the ∼10S complex. In this respect, the assembly-defective GagZip constructs resemble the assembly-incompetent GagΔNCΔp6 and GagKR10A constructs, which were previously shown to be arrested in the form of the ∼10S complex (11, 12, 26, 27).
High-molecular-weight GagZip complexes contain ABCE1.
We have demonstrated previously that the high-molecular-weight assembly intermediates contain Gag in association with ABCE1, while the ∼10S assembly intermediate and the released 750S completed immature capsid do not (11, 12, 27). The finding that GagZip is immunoprecipitated from total-cell lysates by αABCE1 suggested that the intracellular ∼80S/150S and ∼500S complexes formed by GagZip might contain Gag in association with ABCE1. To determine if this is the case, we examined each fraction of GagZip and GagPro− gradients by immunoprecipitation with αABCE1 (Fig. 5). Gag was coimmunoprecipitated by αABCE1 from the ∼80S/150S and ∼500S regions of the GagPro− gradient, as shown previously (40). Additionally, GagZip was coimmunoprecipitated by αABCE1 from the ∼80S/150S and ∼500S regions of the gradient, confirming that the high-molecular-weight intracellular complexes formed by GagZip closely resemble the assembly intermediates previously described for WT Gag and GagPro−. ABCE1 was not found in GagZip VLPs (data not shown), consistent with our previous finding that ABCE1 is released from assembling WT Gag and GagPro− after assembly is completed and upon virus release (11, 12, 40).
Immunogold labeling and TEM demonstrate that complexes containing GagZip and ABCE1 are present at plasma membrane sites of assembly and in the cytoplasm.
To corroborate the finding that GagZip, like WT Gag, forms ABCE1-containing assembly intermediates, we analyzed thin sections of infected cells using double immunogold labeling. For these experiments, we generated pseudotyped virus stocks containing replication-competent or replication-defective viral genomes by cotransfecting WT Gag, GagZip, or GagΔNCΔp6 genomes along with GagPol helper constructs and VSV-G. Sections of cells infected to express WT Gag, GagZip, or GagΔNCΔp6 genomes were immunolabeled with antibodies specific for the CA domain of Gag (indicated by small gold particles) and for endogenous ABCE1 (indicated by large gold particles) and were analyzed by TEM (Fig. 6). In micrographs of infected cells expressing WT Gag, we frequently observed Gag and endogenous ABCE1 colocalized in capsid-like structures assembling at the plasma membrane, as described previously (12). A similar pattern and degree of Gag and ABCE1 colocalization was also observed in infected cells expressing GagZip. Indeed, colocalization in cells expressing GagZip was essentially indistinguishable from that in cells expressing WT Gag. In both groups, double labeling occurred on structures representing early stages of assembly (in which only a small patch of Gag was present at the plasma membrane, with no membrane deformation), intermediate stages of assembly (in which larger amounts of Gag protein were present, with some membrane deformation), and late stages (in which the capsid structure was almost completely spherical and had formed a large bud protruding from the membrane), as shown in Fig. 6. These findings are consistent with the results of coimmunoprecipitations demonstrating that ABCE1 is associated with Gag in ∼80S/150S and ∼500S assembly intermediates (Fig. 5).
Previously, we identified WT Gag colocalized with ABCE1 at endogenous plasma membrane sites of assembly (12) but were not able to comment on whether complexes containing WT Gag and ABCE1 are also present in the cytoplasm, due to high levels of background staining in the cytoplasm. In the current study, the use of high-pressure freezing rather than prolonged chemical fixation of samples resulted in reduced background staining and allowed gold labeling in the cytoplasm to be assessed. We observed that cells expressing WT Gag frequently contained small clusters of Gag in the cytoplasm and that in some cases, these colocalized with endogenous ABCE1 (example in Fig. 6A, panel 2), suggesting that Gag is recruited into smaller ABCE1-containing intermediates before membrane targeting occurs. Similar clusters of Gag colocalized with ABCE1 could be found in infected cells expressing GagZip (examples in Fig. 6B, panels 3 and 5).
Cells expressing assembly-defective GagΔNCΔp6 differed dramatically from cells expressing WT Gag or GagZip (Fig. 6C). In these cells, partially or completely formed capsid-like structures were never observed, but label representing GagΔNCΔp6 was frequently found at the plasma membrane. GagΔNCΔp6 at the plasma membrane displayed less colocalization with ABCE1 than did WT Gag, as observed previously (12). However, because endogenous ABCE1 is present throughout the cytoplasm of normal cells, it is found near the plasma membrane even in uninfected cells (12) (data not shown). To distinguish between this random background of ABCE1 at the plasma membrane and specific recruitment of ABCE1 into assembling capsids, we quantified the number of times that ABCE1 was found within 135 nm of a Gag cluster at the plasma membrane in each group (Fig. 6D). Note that for a variety of reasons, presented in the discussion, immunogold labeling is expected to detect only a fraction of the ABCE1 present in the region of an assembling capsid. Nevertheless, despite the underrepresentation of ABCE1 colocalization at sites of capsid assembly, 2-fold enrichment of ABCE1 labeling at the plasma membrane was observed for the WT Gag positive control relative to assembly-defective GagΔNCΔp6, indicating specific recruitment of ABCE1 to sites of assembly. These results were similar to what we have observed previously for WT Gag (12). Importantly, the same level of ABCE1 enrichment at plasma membrane sites of assembly relative to GagΔNCΔp6 was observed for GagZip, indicating that assembling GagZip recruits endogenous ABCE1 to a similar extent as does WT Gag. Thus, this quantitative morphological analysis, along with the coimmunoprecipitation data, demonstrates that GagZip forms ABCE1-containing assembly intermediates that closely resemble those formed by WT Gag.
Formation of ABCE1-containing GagZip assembly intermediates is dependent on the WM184/185 motif in CA but not on the myristoylation motif in MA.
A key feature of the HIV-1 capsid assembly pathway is that progression past specific assembly intermediates is dependent on one or more motifs in Gag that are known to be required for assembly (11, 12, 27). To examine whether the ABCE1-containing complexes formed by GagZip display the same dependence on well-defined assembly motifs as WT Gag, we introduced the WM184/185AA mutation into proviruses encoding Gag or GagZip. Mutations of these residues, which are located at the CA dimer interface (15, 37), cause a significant reduction in particle formation in cells (20, 30, 37) and inhibit Gag-Gag interactions in vitro (6). Thus, these residues are thought to mediate important Gag-Gag interactions during HIV-1 capsid assembly.
COS-1 cells were transfected to express WT and mutant proviruses, and the media were harvested for analysis of VLP release (Fig. 7 A), while cell lysates were analyzed for the pattern of assembly intermediates present at steady state (Fig. 7B). As expected, cells expressing GagPro− released VLPs and formed the ∼10S, ∼80S/150S, and ∼500S assembly intermediates. In contrast, cells expressing Gag WM184/185AA failed to form VLPs and displayed only the ∼10S and ∼80S intracellular assembly intermediates, indicating arrest of Gag WM184/185AA at the ∼80S assembly intermediate. When the WM184/185AA mutation was introduced into the provirus encoding GagZip, VLPs were not formed, and intracellular Gag was arrested in the form of the ∼10S assembly intermediate. Thus, the progression of both WT Gag and GagZip through the assembly pathway is dependent on the WM184/185 motif in the CA domain of Gag, although these residues act at different steps in the pathway when encoded by WT Gag versus GagZip.
We also examined the effect of the G2A mutation in MA on GagZip VLP formation (Fig. 7A) and progression through the assembly pathway (Fig. 7B). The well-studied G2A mutation inactivates the myristoylation signal at the N terminus of Gag, thereby preventing myristoylation, membrane targeting, completion of immature-capsid assembly, and VLP release (5, 18). Because it is known that nonmyristoylated Gag can assemble when expressed to high levels using baculovirus systems (7, 16), we reduced the amount of GagZip plasmid used to ∼25% of the levels used for Fig. 2 through 4, and we also equalized the expression of all constructs examined (Fig. 7A). Cells expressing Gag G2A or GagZip G2A failed to release VLPs, as expected given that myristoylation of Gag is required for plasma membrane targeting (5, 18). Additionally, Gag G2A failed to progress beyond the ∼80S step in the assembly pathway, as reported previously (27). In contrast, GagZip G2A progressed to the late events in the assembly pathway, forming the ∼10S, ∼80S/150S, and ∼500S assembly intermediates. No 750S completed capsid was observed on gradients that were optimized to separate the ∼500S and 750S peaks (data not shown). Thus, unlike Gag G2A, GagZip G2A failed to be arrested at the ∼80S assembly intermediate and instead progressed to the ∼500S step, despite its inability to be targeted to the plasma membrane. Finally, we also observed that, as expected, all the Gag constructs that formed the ∼500S and/or ∼80S/150S complex in Fig. 7B were also coimmunoprecipitated by αABCE1 (data not shown).
Together, the data in Fig. 7 demonstrate that the progression of GagZip through ABCE1-containing assembly intermediates displays both similarities and differences from that of WT Gag. Specifically, even at low levels of expression, GagZip does not appear to require myristoylation to form the late ∼500S assembly intermediate, unlike WT Gag, which is arrested at the ∼80S step when the myristoylation signal is mutated. In contrast, the WM184/185 motif is critical for the progression of both WT Gag and GagZip past early steps in the assembly pathway.
TEM analysis and immunogold labeling reveal that GagZip G2A forms capsid-like structures in the cytoplasm of infected cells and associates with ABCE1.
The finding that GagZip G2A assembles (Fig. 7B) but fails to release VLPs (Fig. 7A) suggested that GagZip G2A may form intracytoplasmic capsid-like structures. To better assess the morphology and subcellular localization of the capsids formed by GagZip G2A, we analyzed infected COS-1 cells expressing GagZip G2A by standard TEM (Fig. 8 A). While no assembling Gag was observed at the plasma membrane in cells expressing GagZip G2A, structures with electron-dense rims and diameters as large as ∼100 nm were observed in the cytoplasm of cells expressing GagZip G2A (Fig. 8), but not in cells expressing Gag G2A (data not shown). As expected, these structures did not appear to be membrane bound. Thus, both the velocity sedimentation data and the TEM analyses indicate that GagZip G2A assembles into capsid-like structures in the cytoplasm and support the notion that GagZip proteins can assemble capsid-like structures in a myristoylation-independent manner.
To assess the ABCE1 association of assembling GagZip G2A proteins, we examined immunogold double-labeled sections from infected cells expressing GagZip G2A (Fig. 8B). In these cells, Gag was not observed at the plasma membrane, in contrast to cells expressing GagPro−, GagZip, or GagΔNCΔp6, which were analyzed in parallel. However, Gag labeling was abundant in the cytoplasm, and some of this cytoplasmic Gag labeling was closely associated with ABCE1 labeling. Notably, ABCE1 was associated with both small and large clusters of Gag. Indeed, in some cases, ABCE1 was associated with clusters of Gag that were superimposed on electron-dense cytoplasmic structures ∼100 nm in diameter (examples in Fig. 8B, panels 3 and 5). Thus, these data, along with coimmunoprecipitation data (data not shown), indicate that ABCE1 is recruited to cytoplasmic sites of GagZip G2A assembly.
Immature GagZip capsids are highly RNase A resistant, and WT immature HIV-1 capsids display an unexpectedly high degree of RNase A resistance.
The finding that HIV-1 GagZip proteins assemble into structures that closely resemble immature capsids (1, 9, 19, 39) (Fig. 2B and C), even though they contain levels of RNA that are <10% those of wild-type HIV-1 capsids (9, 38, 39), suggests that RNA is not required for the integrity of GagZip capsids. While this property could be unique to GagZip capsids, alternatively, it is also possible that the integrity of WT immature HIV-1 capsids is not dependent on RNA. Arguing against the latter possibility, a previous study (29) demonstrated that RNase A disrupts the integrity of Moloney murine leukemia virus (MLV) capsids and proposed that RNA is a structural element critical for the integrity of immature retroviral capsids. Because that study did not examine the role of RNA in maintaining the integrity of immature HIV-1 capsids, we addressed that question here by comparing the RNase A sensitivity of de-enveloped immature HIV-1 and MLV capsids to that of immature HIV-1 GagZip capsids (Fig. 9 A and B). Immature HIV-1, MLV, or HIV-1 GagZip VLPs were harvested by pelleting through sucrose cushions, de-enveloped with nonionic detergent (1% NP-40), treated with RNase A (1.3 mg/ml for 2 h at 26°C) or buffer, and repelleted, using a previously described protocol (29). When capsid integrity was disrupted by denaturing VLPs with ionic detergent (1% SDS), Gag in all samples was found in the supernatant upon repelleting, as expected. De-envelopment alone, without RNase A treatment, resulted in the release of <1% of HIV-1 GagZip or HIV-1 Gag and 20% of MLV Gag into the supernatant upon repelleting. De-envelopment followed by RNase A treatment resulted in the release of <1% of GagZip, 15% of HIV-1 Gag, and 46% of MLV Gag into the supernatant upon repelleting. The failure of RNase A treatment to disrupt GagZip capsid integrity was not surprising given that GagZip capsids are largely devoid of RNA (9, 38, 39). However, unexpectedly, de-enveloped immature HIV-1 capsids were also relatively RNase A insensitive, with 85% of HIV-1 Gag remaining in intact capsids and repelleting after treatment with RNase A. Velocity sedimentation analysis confirmed that RNase A treatment did not disrupt capsid integrity, unlike SDS treatment (Fig. 9C). On longer exposures, a small amount of Gag could be seen at the top of the gradients in the RNase A-treated HIV-1 GagPro− samples (data not shown), consistent with the small amount of Gag released into the supernatant in Fig. 9B.
Analysis of de-enveloped HIV-1 or GagZip capsids after RNase A treatment revealed that >95% of the RNA in GagZip capsids and >99% of the RNA in HIV-1 capsids was degraded by the RNase A treatments we used (Fig. 9D). We also assessed whether RNase A was fully inactivated when samples were denatured with Trizol during sample preparation. To test this, we performed parallel controls, in which purified 18S rRNA was added to RNase A- or buffer-treated mock samples immediately after the samples were denatured for analysis. The finding that 18S rRNA remained largely intact when added after sample denaturation argues that RNase A was active only during the RNase A treatment period (Fig. 9E). Taken together, our data indicate that immature HIV-1 capsids are almost as insensitive to RNase A treatment as immature HIV-1 GagZip capsids, which lack RNA. Thus, these data suggest that loss of capsid integrity upon RNase A treatment is not a general property of immature retroviral capsids.
DISCUSSION
In this study we provide strong evidence that assembling HIV-1 GagZip chimeric proteins form ∼80S/150S and ∼500S complexes that correspond to previously defined HIV-1 capsid assembly intermediates. In addition to having the same S value as capsid assembly intermediates, the complexes formed by GagZip contain the cellular assembly cofactor ABCE1, closely resemble WT assembly intermediates upon analysis by EM immunogold double labeling, and are dependent on at least one specific assembly motif in Gag for their formation. The failure of GagZip mutants that are defective in dimerization and assembly to form ABCE1-containing complexes further supports the notion that GagZip proteins progress through the capsid assembly pathway. Finally, our study also demonstrates that, as expected, immature capsids formed by GagZip proteins are insensitive to RNase A treatment. However, we also found that immature HIV-1 capsids are nearly as resistant to RNase A treatment as GagZip proteins, suggesting that the structural integrity of WT immature HIV-1 capsids, like that of GagZip capsids, is not highly dependent on RNA.
The finding that HIV-1 GagZip proteins appear to form ABCE1-containing capsid assembly intermediates has three important implications for our understanding of how HIV-1 capsid assembly intermediates are formed. First, these results indicate that RNA recruited by NC does not play a direct role in binding ABCE1 to Gag. While we had previously demonstrated that basic residues in NC are critical for the binding of ABCE1 to WT Gag proteins (26), it remained unclear whether this is due to the ability of basic residues in NC to promote Gag-Gag interactions or to the ability of NC to bind RNA. Our finding that the Gag-ABCE1 interaction was not disrupted by treatment of assembly intermediates with RNase A (26) suggested that ABCE1-Gag binding is not maintained by RNA but left open the possibility that RNA could be required to initiate ABCE1-Gag binding. In contrast, the approach we took here, in which we replaced the dual-function NC domain of Gag with an LZ domain that strongly promotes protein dimerization but lacks any RNA binding properties, demonstrates definitively that the RNA binding properties of NC are entirely dispensable for ABCE1-Gag binding and progression through the assembly pathway.
Second, these data demonstrate that the ABCE1-binding domain does not reside within NC alone, since replacement of NC with the heterologous LZ domain does not alter ABCE1 binding. These observations suggest a model in which oligomerization, the one feature that NC and LZ have in common, is a prerequisite for ABCE1-Gag binding. In such a model, NC (or LZ) enables ABCE1-Gag binding by promoting an oligomerization-induced conformational change in Gag that, in turn, exposes a cryptic ABCE1-binding domain elsewhere in Gag (Fig. 1A, model 3). Consistent with such a model, mutations that specifically disrupt residues critical for oligomerization by NC or LZ completely abrogate ABCE1 binding (Fig. 3). While further studies will be required to fully test this model and to define the ABCE1-binding domain within Gag, mutational analyses point to candidate regions of Gag that could contain a binding site for ABCE1 that becomes exposed following Gag oligomerization. Specifically, our previous analysis of Gag deletions demonstrated that an ABCE1-binding domain is unlikely to reside in most of MA or the N-terminal two-thirds of CA (26). However, an ABCE1-binding domain could be present in one of the three regions of Gag that were not directly examined in that study, namely, the first 12 residues of MA, the C terminus of CA distal to the major homology region (amino acids 304 to 363), or the p2 domain (amino acids 364 to 374).
Third, our findings underscore the universality of the ABCE1 capsid assembly pathway. We have previously demonstrated that Gag proteins of other primate lentiviruses, including HIV-2 and simian immunodeficiency viruses SIVmac and SIVagm, form ABCE1-containing assembly intermediates (11). However, it remains possible that not all Gag proteins assemble through this particular pathway, although to date we have found no evidence for assembly of HIV-1 or primate lentiviruses through any other pathways. If an alternate assembly pathway exists, one might expect that GagZip proteins, which lack a domain present in all retroviral Gag proteins, might utilize such a pathway. Our finding that assembly-competent, but not assembly-incompetent, GagZip proteins form ABCE1-containing intermediates lends support to the notion that progression through ABCE1-containing intermediates is a universal feature of primate lentiviral capsid assembly.
The association between GagZip and ABCE1 observed by coimmunoprecipitation was corroborated by our quantitative immunogold double-labeling experiments. Since colocalization scores instances in which two proteins are in close proximity while coimmunoprecipitation detects only proteins that are bound to each other, it is not surprising that our colocalization experiments displayed a higher background. Specifically, the colocalization analysis demonstrated that in 21% of cases, the GagΔNCΔp6 negative control was in close proximity to ABCE1 (Fig. 6D), while the coimmunoprecipitation data showed essentially no association between GagΔNCΔp6 and ABCE1 (Fig. 3A). Taken together, these data suggest that most of the GagΔNCΔp6 found near ABCE1 is not bound to ABCE1, while much of the WT Gag or GagZip found near ABCE1 is physically bound to ABCE1. Conversely, ABCE1 in association with late assembly intermediates is likely underrepresented in our colocalization experiments for a number of reasons. These include the fact that our thin sections (thickness, 50 nm) typically capture only a fraction of the 100-nm-diameter assembling virus particle, thereby leaving uncounted any ABCE1 that is associated with that particle outside the plane of section. Moreover, penetration of antibodies through the full 50-nm-thick section is unlikely. Additionally, the ratio of ABCE1 to Gag may be relatively low in Gag-rich late assembly intermediates. These inherent differences of higher background and the underrepresentation of positive colocalization events in immunogold double-labeling experiments are reflected in the data presented, as expected. Regardless, the EM colocalization data presented here advance our understanding of assembly intermediates by providing a morphological and ultrastructural view of ABCE1 in assembling GagZip capsids. Additionally, for the first time, we were able to generate ultrastructural evidence of cytoplasmic complexes containing ABCE1 colocalized with WT Gag, GagZip, and GagZip G2A.
To examine whether GagZip proteins utilize the same capsid assembly pathway used by WT Gag, we took advantage of a key feature of HIV-1 capsid assembly intermediates—the fact that the progression of Gag through specific steps in the assembly pathway is dependent on well-defined motifs in Gag known to be required for capsid assembly (11, 12, 27, 40). Here we demonstrated that the WM184/185 motif located at the CA dimer interface, which was initially found by von Schwedler et al. to be critical for HIV-1 particle formation (37) and was subsequently shown to play a role in membrane targeting as well (20, 30), is critical for the progression of WT Gag and GagZip to the ∼500S assembly intermediate. Thus, not only do assembling WT Gag and GagZip form ABCE1-containing complexes, but additionally, both require the WM184/185 motif in CA to progress through the pathway, indicating important similarities in the ways in which they assemble.
Our mutational studies also demonstrated differences in how WT Gag and GagZip progress through the assembly pathway. WT Gag requires myristoylation in order to be targeted to membranes and assemble into capsids (5, 17); consistent with this, when myristoylation is blocked by the G2A mutation, Gag fails to progress through the assembly pathway, is arrested at the ∼80S assembly intermediate, and does not form 500S intermediates or completed capsids (27). In contrast, we demonstrated here with biochemical and EM data that GagZip G2A progresses to the ∼500S step in the assembly pathway, forming capsid-like structures in the cytoplasm rather than at the plasma membrane. Thus, GagZip requires myristoylation for membrane targeting but not for progression to the late stages of capsid formation. It should be noted that our data are consistent with those of Crist et al., who mentioned that nonmyristoylated GagZip can form cytoplasmic capsid-like structures (9), and with the finding by Li et al. that nonmyristoylated GagZip undergoes extensive multimerization, unlike nonmyristoylated Gag (25). Here we also ruled out the possibility that assembly of nonmyristoylated GagZip was due to overexpression, and we matched its expression to that of the assembly-defective Gag G2A control. Indeed, we obtained the same results when we reduced the amount of transfected GagZip G2A plasmid to 10-fold less than what was used in Fig. 7 (data not shown). These data suggest that the assembly of capsids in the cytoplasm by GagZip G2A is not concentration dependent, in contrast to what has been observed for Gag G2A (7, 16). Our data also suggest that although nonmyristoylated GagZip progresses to the late stages of the capsid assembly pathway, it fails to form a 750S immature capsid and to complete assembly, unlike GagZip and WT Gag. Instead, GagZip G2A appears to remain arrested in the form of an ABCE1-containing ∼500S capsid-like late assembly intermediate.
The difference in assembly competence between Gag G2A and GagZip G2A most likely results from the fact that LZ is a much more effective dimerization domain than NC. It is known that WT Gag oligomerizes to a limited extent in the cytoplasm, with extensive multimerization occurring only after Gag is targeted to the plasma membrane (22, 25, 32). These data suggest that NC is a relatively weak multimerization domain and that other events, such as membrane targeting, are required to facilitate higher-order multimerization of Gag. In contrast, because LZ is highly effective at promoting protein-protein interactions, GagZip proteins are able to progress to late stages of the assembly pathway in the absence of facilitating events such as membrane targeting. Because LZ is a strong dimerization domain, unlike NC, it is not surprising that mutations in Gag affect WT Gag and GagZip differently. Notably, while our goal was to compare WT and GagZip capsids in order to gain insights into the assembly process, Gag and GagZip should not be fully equated, since they are fundamentally different.
We identified another situation in which the effect of a mutation was context dependent. In the GagZip context, the WM184/185AA mutation arrests GagZip at the 10S assembly intermediate. However, in the context of WT Gag, the same mutation arrests Gag at the 80S assembly intermediate (Fig. 7). While this study was focused mainly on examining GagZip, our analysis of the WM184/185AA mutation also sheds new light on how WT Gag progresses through the assembly pathway. Specifically, our data demonstrate that in the context of full-length WT Gag assembling in cells, the WM184/185 residues in CA are required for progression from the 80S to the 500S intermediate and therefore act downstream from the basic residues in NC, which are required for Gag to progress into the 80S assembly intermediate (11, 26).
Finally, our studies also demonstrate that immature HIV-1 capsids, which contain RNA, are only slightly more sensitive to RNase A than HIV-1 GagZip capsids, which lack RNA. Our controls demonstrated that >99% of the RNA in the immature HIV-1 particles was degraded by RNase A and that this degradation occurred during the RNase A treatment period and not during subsequent sample preparation. Importantly, we cannot rule out the possibility that the RNA remaining in immature HIV-1 capsids after RNase treatment, which amounted to <1% of the total VLP RNA present initially, is critical for capsid integrity. Nevertheless, both the ability of HIV-1 GagZip to assemble into immature capsids and the finding that immature HIV-1 capsids retain their structure after the digestion of >99% of VLP RNA suggest that the integrity of fully assembled immature HIV-1 capsids may be largely dependent on protein-protein interactions rather than on RNA bridging.
Supplementary Material
ACKNOWLEDGMENTS
We thank Heinrich Göttlinger for providing a GCN4-containing plasmid that we used in our GagZip plasmid constructions; A. Dusty Miller for providing the MLV genome; Stephen Goff and Michael Emerman for providing MLV antisera; Didier Trono for providing the psPAX2 and pMD2.G plasmids (via Addgene); Justine Brown for assistance with plasmid construction; Bobbie Schneider, Steve MacFarlane, and the Fred Hutchinson Cancer Research Institute for assistance with TEM; and Christopher Peterson for comments on the manuscript. Additionally, the following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: the HIV-1 p24 hybridoma (183-H12-5C) from Bruce Chesebro and TZM-bl cells from John C. Kappes, Xiaoyun Wu, and Tranzyme Inc.
This study was supported by NIH R01AI48389 (to J.R.L.), an ARRA supplement to NIH R01AI48389, and NSF graduate fellowship DGE0203031 to J.C.R.
J.R.L. is a cofounder of Prosetta Antiviral, Inc.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 4 May 2011.
REFERENCES
- 1. Accola M. A., Strack B., Göttlinger H. G. 2000. Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain. J. Virol. 74:5395–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bennett R. P., Nelle T. D., Wills J. W. 1993. Functional chimeras of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J. Virol. 67:6487–6498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berkowitz R., Fisher J., Goff S. P. 1996. RNA packaging. Curr. Top. Microbiol. Immunol. 214:177–218 [DOI] [PubMed] [Google Scholar]
- 4. Bowzard J. B., et al. 1998. Importance of basic residues in the nucleocapsid sequence for retrovirus Gag assembly and complementation rescue. J. Virol. 72:9034–9044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bryant M., Ratner L. 1990. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc. Natl. Acad. Sci. U. S. A. 87:523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burniston M. T., Cimarelli A., Colgan J., Curtis S. P., Luban J. 1999. Human immunodeficiency virus type 1 Gag polyprotein multimerization requires the nucleocapsid domain and RNA and is promoted by the capsid-dimer interface and the basic region of matrix protein. J. Virol. 73:8527–8540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chazal N., Carriere C., Gay B., Boulanger P. 1994. Phenotypic characterization of insertion mutants of the human immunodeficiency virus type 1 Gag precursor expressed in recombinant baculovirus-infected cells. J. Virol. 68:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cimarelli A., Sandin S., Hoglund S., Luban J. 2000. Basic residues in human immunodeficiency virus type 1 nucleocapsid promote virion assembly via interaction with RNA. J. Virol. 74:3046–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crist R. M., et al. 2009. Assembly properties of human immunodeficiency virus type 1 Gag-leucine zipper chimeras: implications for retrovirus assembly. J. Virol. 83:2216–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dawson L., Yu X. F. 1998. The role of nucleocapsid of HIV-1 in virus assembly. Virology 251:141–157 [DOI] [PubMed] [Google Scholar]
- 11. Dooher J. E., Lingappa J. R. 2004. Conservation of a step-wise, energy-sensitive pathway involving HP68 for assembly of primate lentiviral capsids in cells. J. Virol. 78:1645–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dooher J. E., Schneider B. L., Reed J. C., Lingappa J. R. 2007. Host ABCE1 is at plasma membrane HIV assembly sites and its dissociation from Gag is linked to subsequent events of virus production. Traffic 8:195–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freed E. O. 1998. HIV-1 Gag proteins: diverse functions in the virus life cycle. Virology 251:1–15 [DOI] [PubMed] [Google Scholar]
- 14. Freed E. O. 2002. Viral late domains. J. Virol. 76:4679–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gamble T. R., et al. 1997. Structure of the carboxyl-terminal dimerization domain of the HIV-1 capsid protein. Science 278:849–853 [DOI] [PubMed] [Google Scholar]
- 16. Gheysen D., et al. 1989. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell 59:103–112 [DOI] [PubMed] [Google Scholar]
- 17. Göttlinger H. G., Dorfman T., Sodroski J. G., Haseltine W. A. 1991. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. U. S. A. 88:3195–3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Göttlinger H. G., Sodroski J. G., Haseltine W. A. 1989. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. U. S. A. 86:5781–5785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson M. C., Scobie H. M., Ma Y. M., Vogt V. M. 2002. Nucleic acid-independent retrovirus assembly can be driven by dimerization. J. Virol. 76:11177–11185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joshi A., Nagashima K., Freed E. O. 2006. Mutation of dileucine-like motifs in the human immunodeficiency virus type 1 capsid disrupts virus assembly, Gag-Gag interactions, Gag-membrane binding, and virion maturation. J. Virol. 80:7939–7951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kimpton J., Emerman M. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J. Virol. 66:2232–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kutluay S. B., Bieniasz P. D. 2010. Analysis of the initiating events in HIV-1 particle assembly and genome packaging. PLoS Pathog. 6:e1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee Y. M., Liu B., Yu X. F. 1999. Formation of virus assembly intermediate complexes in the cytoplasm by wild-type and assembly-defective mutant human immunodeficiency virus type 1 and their association with membranes. J. Virol. 73:5654–5662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee Y. M., Yu X. F. 1998. Identification and characterization of virus assembly intermediate complexes in HIV-1-infected CD4+ T cells. Virology 243:78–93 [DOI] [PubMed] [Google Scholar]
- 25. Li H., Dou J., Ding L., Spearman P. 2007. Myristoylation is required for human immunodeficiency virus type 1 Gag-Gag multimerization in mammalian cells. J. Virol. 81:12899–12910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lingappa J. R., Dooher J. E., Newman M. A., Kiser P. K., Klein K. C. 2006. Basic residues in the nucleocapsid domain of Gag are required for interaction of HIV-1 Gag with ABCE1 (HP68), a cellular protein important for HIV-1 capsid assembly. J. Biol. Chem. 281:3773–3784 [DOI] [PubMed] [Google Scholar]
- 27. Lingappa J. R., Hill R. L., Wong M. L., Hegde R. S. 1997. A multistep, ATP-dependent pathway for assembly of human immunodeficiency virus capsids in a cell-free system. J. Cell Biol. 136:567–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morikawa Y., Goto T., Momose F. 2004. Human immunodeficiency virus type 1 Gag assembly through assembly intermediates. J. Biol. Chem. 279:31964–31972 [DOI] [PubMed] [Google Scholar]
- 29. Muriaux D., Mirro J., Harvin D., Rein A. 2001. RNA is a structural element in retrovirus particles. Proc. Natl. Acad. Sci. U. S. A. 98:5246–5251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ono A., Waheed A. A., Joshi A., Freed E. O. 2005. Association of human immunodeficiency virus type 1 Gag with membrane does not require highly basic sequences in the nucleocapsid: use of a novel Gag multimerization assay. J. Virol. 79:14131–14140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Shea E. K., Klemm J. D., Kim P. S., Alber T. 1991. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science 254:539–544 [DOI] [PubMed] [Google Scholar]
- 32. O'Shea E. K., Rutkowski R., Kim P. S. 1992. Mechanism of specificity in the Fos-Jun oncoprotein heterodimer. Cell 68:699–708 [DOI] [PubMed] [Google Scholar]
- 33. Sandefur S., Varthakavi V., Spearman P. 1998. The I domain is required for efficient plasma membrane binding of human immunodeficiency virus type 1 Pr55Gag. J. Virol. 72:2723–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singh A. R., Hill R. L., Lingappa J. R. 2001. Effect of mutations in Gag on assembly of immature human immunodeficiency virus type 1 capsids in a cell-free system. Virology 279:257–270 [DOI] [PubMed] [Google Scholar]
- 35. Tritel M., Resh M. D. 2000. Kinetic analysis of human immunodeficiency virus type 1 assembly reveals the presence of sequential intermediates. J. Virol. 74:5845–5855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tritel M., Resh M. D. 2001. The late stage of human immunodeficiency virus type 1 assembly is an energy-dependent process. J. Virol. 75:5473–5481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. von Schwedler U. K., Stray K. M., Garrus J. E., Sundquist W. I. 2003. Functional surfaces of the human immunodeficiency virus type 1 capsid protein. J. Virol. 77:5439–5450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zennou V., Perez-Caballero D., Gottlinger H., Bieniasz P. D. 2004. APOBEC3G incorporation into human immunodeficiency virus type 1 particles. J. Virol. 78:12058–12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Y., Qian H., Love Z., Barklis E. 1998. Analysis of the assembly function of the human immunodeficiency virus type 1 Gag protein nucleocapsid domain. J. Virol. 72:1782–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zimmerman C., et al. 2002. Identification of a host protein essential for assembly of immature HIV-1 capsids. Nature 415:88–92 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.