Abstract
The human cytomegalovirus UL111A gene is expressed during latent and productive infections, and it codes for homologs of interleukin-10 (IL-10). We examined whether viral IL-10 expressed during latency altered differentiation of latently infected myeloid progenitors. In comparison to infection with parental virus or mock infection, latent infection with a virus in which the gene encoding viral IL-10 has been deleted upregulated cytokines associated with dendritic cell (DC) formation and increased the proportion of myeloid DCs. These data demonstrate that viral IL-10 restricts the ability of latently infected myeloid progenitors to differentiate into DCs and identifies an immunomodulatory role for viral IL-10 which may limit the host's ability to clear latent virus.
TEXT
Human cytomegalovirus (HCMV) is a species-specific betaherpesvirus that infects a majority of the world's population (29). HCMV establishes and maintains a lifelong latent infection in primitive myeloid lineage cells (14, 22, 27, 48, 53, 58). Following terminal cell differentiation of these cells into myeloid dendritic cells (DCs) and macrophages, latent virus has the ability to reactivate, resulting in the production of new, infectious virions and often severe disease in immunocompromised individuals (11, 14, 28, 37, 49, 50, 59, 63). Only a subset of viral genes are transcriptionally active during latency (2, 8, 12, 13, 17, 23, 34, 47), including HCMV UL111A, a gene that encodes homologs of the potent immunomodulatory cytokine human interleukin-10 (hIL-10). UL111A is transcriptionally active during both productive and latent phases of infection and encodes several viral IL-10 proteins (17, 24–26, 46) which exert a diverse range of immunomodulatory functions, including inhibition of cytokine synthesis and major histocompatibility complex (MHC) expression by myeloid cells, stimulation of B cells, and suppression of DC maturation and cytotrophoblast function (5–7, 9, 16, 18, 36, 51, 52, 61). The vast majority of characterization of functions has come from studies using recombinant viral IL-10 proteins, although some have also assessed function during productive infection with viruses with deficient viral IL-10 proteins. Much less is known about the function of viral IL-10 in the context of latent infection, which, to date, has been limited to a single report that viral IL-10 expressed during latent infection of primary human myeloid progenitor cells modulates cell surface MHC class II levels and renders these cells refractory to recognition by both allogeneic and autologous CD4+ T cells (9). In this study, we examined (i) the production of proinflammatory cytokines linked to the control of cellular differentiation and (ii) the cellular differentiation pattern of primary human myeloid progenitor cells latently infected with parental virus or virus in which the gene encoding viral IL-10 has been deleted (viral IL-10 deletion viruses), providing evidence for a role of viral IL-10 in modulating the differentiation ability of latently infected cells.
Viral IL-10 inhibits mRNA and protein expression of proinflammatory cytokines by latently infected myeloid progenitor cells.
To determine whether HCMV UL111A-encoded viral IL-10 affected expression of proinflammatory cytokines involved in modulating myeloid cell differentiation, we first evaluated mRNA expression of interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α) in cells latently infected with RVAdIL10C, a recombinant virus that cannot express any viral IL-10 (33), or its parent strain AD169. Primary human CD34+ myeloid progenitors were latently infected as previously described (8, 22), and cells and culture supernatants were harvested at day 8 postinfection (p.i.). We have previously demonstrated that this viral IL-10 deletion virus infects and maintains a latent infection in CD34+ myeloid progenitors as efficiently as the parental virus does (9). Our routine analyses of viral genome load when new virus stocks were generated or when new infection experiments were undertaken confirmed equal rates of latent infection with these two viruses (see Fig. 3C; also data not shown). We also confirmed expression of viral IL-10 transcripts throughout the 8-day time course in cells infected with the parental virus, whereas these transcripts were not detected at any time in cells infected with the viral IL-10 deletion virus (see Fig. S1 in the supplemental material).
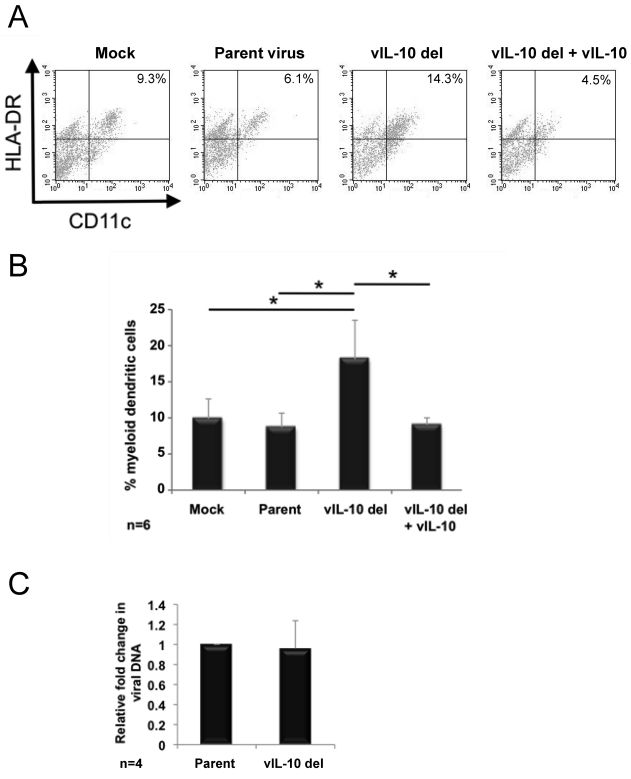
Fig. 3.
Increased generation of myeloid dendritic cells from myeloid progenitor cells latently infected with a viral IL-10 deletion virus. CD34+ myeloid progenitor cells were mock infected or latently infected with either parental virus (Parent) or a viral IL-10 deletion virus (vIL-10 del). Cultures of cells infected with vIL-10 del virus supplemented with recombinant viral IL-10 proteins were also generated (vIL-10 del + vIL-10). (A) Flow cytometry scatter plots of Lin− cells expressing HLA-DR and CD11c to identify myeloid DCs. (B) Graph depicting the percentage of myeloid DCs. (C) Graph depicting the fold change in viral genome load in cells infected with the viral IL-10 deletion virus relative to parental virus-infected counterparts. The number of independent biological replicate experiments (n) is shown. Error bars indicate the standard errors of the means. Significant differences (P value of <0.05) between the values for samples were determined by a one-tailed, paired Student's t test and are denoted by an asterisk.
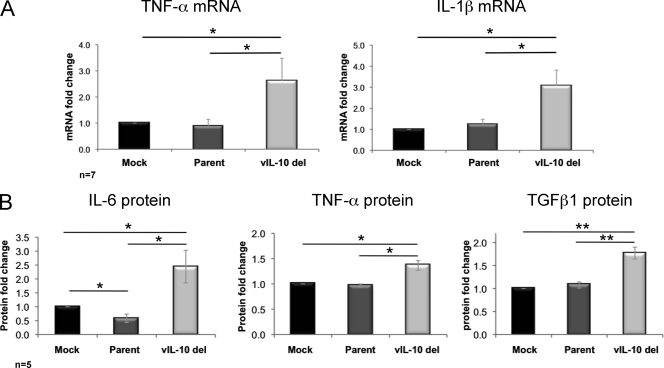
DNase-treated total RNA was reverse transcribed using SuperScript III (Invitrogen). mRNA expression was measured by quantitative reverse transcription-PCR (qRT-PCR) (Mx3000P qPCR system; Stratagene) at 50°C for 1 min, 95°C for 1 min, and then 50 cycles, with 1 cycle consisting of 95°C for 15 s and 60°C for 45 s. TNF-α and IL-1β mRNA were normalized to expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Data are presented as fold change of mRNA expression in infected samples relative to mock infection. Analysis of seven independent replicate experiments revealed a statistically significant increase in both TNF-α and IL-1β mRNA expression in cells latently infected with the viral IL-10 deletion virus compared to both mock-infected and parental virus-infected cells, which expressed comparable levels of these cytokine mRNAs (Fig. 1 A).
Fig. 1.
Upregulation of proinflammatory cytokines during latent infection with a viral IL-10 deletion virus. CD34+ myeloid progenitor cells were mock infected or latently infected with either parental virus (Parent) or a viral IL-10 deletion virus (vIL-10 del). (A) Fold mRNA change (relative to mock-infected cells) measured by qRT-PCR of TNF-α (primers TNF-α-F [F stands for forward] [5′-CCGTCTCCTACCAGACCAAG-3′] and TNF-α-R [R stands for reverse] [5′-CTGAGTCGGTCACCCTTCTC-3′]) and IL-1β (primers IL-1β-F [5′-GCTGAGGAAGATGCTGGTTC-3′] and IL-1β-R [5′-GTGATCGTACAGGTGCATCG-3′]) following normalization to the housekeeping gene GAPDH (primers GAPDH-F [5′-TCACCAGGGCTGCTTTTAAC-3′] and GAPDH-R [5′-GACAAGCTTCCCGTTCTCAG-3′]). (B) Fold change (relative to mock-infected cells) of secreted IL-6, TNF-α, and TGFβ1 proteins measured by ELISAs. The number of independent biological replicate experiments (n) is shown. Error bars indicate the standard errors of the means. Significant differences between values for the samples were determined by a one-tailed, paired Student's t test and are denoted by horizontal lines and asterisks as follows: *, P value of <0.05; **, P value of <0.01.
We next determined whether secreted protein levels of these cytokines were altered in culture supernatants from cells latently infected with parental or viral IL-10 deletion viruses or with their mock-infected counterparts. An enzyme-linked immunosorbent assay (ELISA) (SABiosciences or R&D Systems) was performed on culture supernatants (day 8 p.i.) from five independent biological replicates. This analysis was expanded to include protein levels of IL-6 and transforming growth factor β1 (TGFβ1), which also play important roles in myeloid cell differentiation (4, 10, 19, 30, 40, 55, 57) (Fig. 1B).
Protein levels of IL-6, TNF-α, and TGFβ1 were significantly higher in culture supernatants from cells latently infected with the viral IL-10 deletion virus (mean values of 18.2 ng/ml, 2.1 pg/ml, and 37.0 ng/ml, respectively) compared to both mock-infected cells (means of 8.3 ng/ml, 1.6 pg/ml, and 21.6 ng/ml, respectively) and parental virus-infected cells (means of 6.7 ng/ml, 1.5 pg/ml, and 24.1 ng/ml, respectively). There was also a modest, but statistically significant reduction of IL-6 protein in cultures infected with parental virus compared to mock-infected cultures. IL-1β protein levels from all infection settings remained below the limits of detection. These results demonstrate that the loss of the ability to express viral IL-10 during latent infection of primary myeloid progenitor cells increases expression of proinflammatory cytokines which play important roles in modulating cellular differentiation.
As an adjunct to analyses of these cytokines, we also measured both granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 expression by qRT-PCR (50 amplification cycles) from mock-infected cells or cells latently infected with parental or viral IL-10 deletion viruses. Little, if any, expression of either of these two cytokines was detected. Specifically, in analyses of four independent replicate experiments, only one showed any evidence of GM-CSF mRNA, and this was detected at a point close to the limits of detection. IL-4 mRNA was detected in only 2 out of 4 replicate experiments, and like GM-CSF, in the samples which did yield detection, this was close to the limit of detection of the assay. Furthermore, there was no quantitative difference in either GM-CSF or IL-4 mRNA between mock-infected cells and cells infected with parental virus and viral IL-10 deletion virus (data not shown). It was therefore concluded that GM-CSF and IL-4 do not play a significant role in the viral IL-10-mediated control of myeloid cell differentiation during latency.
Viral IL-10 expressed during latency inhibits differentiation of latently infected myeloid progenitor cells toward a DC phenotype.
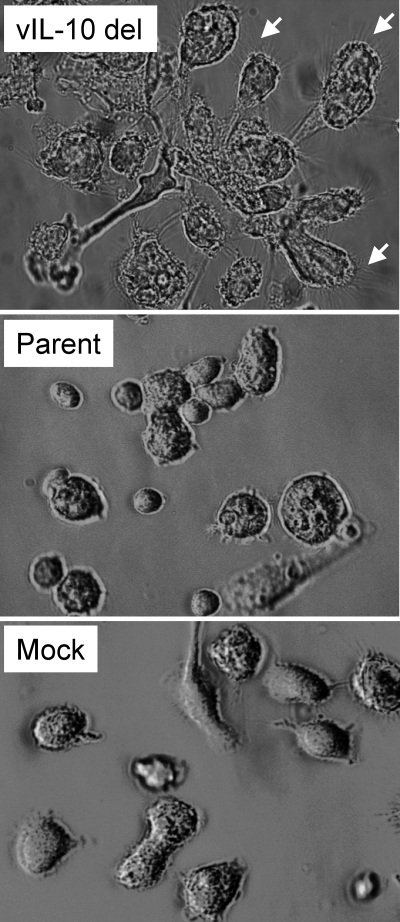
Myeloid DCs, together with monocytes, originate from CD34+ myeloid progenitor cells (20, 31, 38, 39, 45, 60). TNF-α, IL-6, IL-1β, and TGFβ1 have all been linked to initiation of DC differentiation. A cocktail of TNF-α, IL-1β, and IL-6 drives DC differentiation from CD14+ monocytes (10, 19), and studies have also demonstrated that TGFβ1 promotes DC differentiation from CD34+ myeloid progenitor cells (4, 30, 40, 55, 57). Our finding that a lack of ability to express viral IL-10 expressed during latency resulted in the upregulation of these proinflammatory cytokines (Fig. 1) raised the possibility that the latently infected myeloid cell differentiation state may be regulated by viral IL-10. In this respect, we observed notable differences in the morphology of cells of myeloid progenitor cultures mock infected or latently infected with parental or viral IL-10 deletion viruses (Fig. 2). Specifically, in comparison to mock-infected or parental virus-infected cells, loosely adherent cells in cultures latently infected with the viral IL-10 deletion virus were often more irregular in shape with more-pronounced, numerous spiky projections and were frequently grouped together in loose clumps (Fig. 2). These features were consistent with a DC morphology (1, 3, 32, 41, 43, 44, 62).
Fig. 2.
Cell morphology changes during latent infection with a viral IL-10 deletion virus. Light microscopy views of myeloid progenitor cells on day 8 after latent infection with a viral IL-10 deletion virus (vIL-10 del) or parental virus (Parent) or mock infection (Mock). The small white arrows highlight fine spiky projections on clumping cells in cultures infected with vIL-10 del virus.
We therefore performed flow cytometry to quantify myeloid DC formation from myeloid progenitors after mock infection or infection with parental virus or viral IL-10 deletion virus. On day 8 p.i., cells were stained with anti-CD11c antibody conjugated to allophycocyanin (anti-CD11c–APC) (BD Biosciences), anti-HLA-DR–peridinin chlorophyll protein complex (PerCP; BD Biosciences), anti-lineage 1 cocktail–fluorescein isothiocyanate (FITC) (containing antibodies against CD3, CD14, CD16, CD19, CD20, and CD56; BD Biosciences), or their respective isotype control antibodies. Data were acquired using FACSCalibur or FACSCanto flow cytometers and analyzed by CellQuest or FlowJo software, respectively (BD Biosciences).
The gated lineage-negative (Lin−) cell population was analyzed for expression of CD11c and HLA-DR, with Lin− CD11c+ HLA-DR+ cells defined as myeloid DCs. Six independent replicate experiments were analyzed. In contrast to mock- and parental virus-infected cultures, which yielded a comparable proportion of myeloid DCs, latent infection with the viral IL-10 deletion virus resulted in the formation of a significantly higher proportion of myeloid DCs (Fig. 3 A and B). Addition of 100 ng/ml of recombinant viral IL-10 proteins (18) to viral IL-10 deletion virus infected-cultures on day 4 and day 6 p.i. completely abrogated this increased myeloid DC formation (Fig. 3A and B), indicating that the increased proportion of myeloid DC formed in cultures latently infected with the viral IL-10 deletion virus was due to a lack of ability to express viral IL-10.
We also measured viral DNA by qPCR to determine whether the increase in the proportion of DCs resulted in any change to viral genome load. In 4 independent replicate infection experiments, viral DNA levels remained highly comparable in cells infected with the viral IL-10 deletion virus and parental virus-infected counterparts (Fig. 3C). In addition, viral transcription from the major immediate-early region was detected only at very low levels, and there was no difference between parental and viral IL-10 deletion virus. These data indicated that there is no increased viral activity, for example due to initiation of spontaneous reactivation, in cells infected with the viral IL-10 deletion virus.
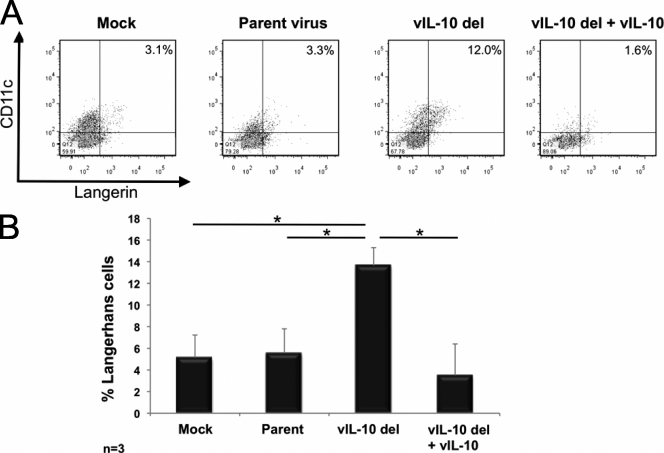
In an extension of the analyses of cell phenotype, we included staining with anti-CD207–phycoerythrin (PE; ImmunoTech) to detect langerin, a marker expressed by Langerhans cells (a subset of myeloid DCs). Analysis of three independent replicates revealed that in comparison to mock infection or latent infection with parental virus, latent infection of myeloid progenitors with the viral IL-10 deletion virus resulted in the formation of more CD11c+ langerin+ Langerhans cells (Fig. 4). As before, this increase was blocked by addition of recombinant viral IL-10 proteins to viral IL-10 deletion virus-infected cultures (Fig. 4).
Fig. 4.
Increased generation of Langerhans cells from myeloid progenitor cells latently infected with a viral IL-10 deletion virus. CD34+ myeloid progenitor cells were mock infected or latently infected with either parental virus (Parent) or a viral IL-10 deletion virus (vIL-10 del). Cultures of vIL-10 del virus-infected cells supplemented with recombinant viral IL-10 proteins were also generated (vIL-10 del + vIL-10). (A) Flow cytometry scatter plots of cells expressing CD11c and langerin to identify Langerhans cells. (B) Graph depicting the percentage of Langerhans cells. The number of independent biological replicate experiments (n) is shown. Error bars indicate the standard errors of the means. Significant differences (P value of <0.05) between the values for samples were determined by a one-tailed, paired Student's t test and are denoted by an asterisk.
It is not certain why latent HCMV would encode a function via viral IL-10 to inhibit formation of lymphoid cells (LCs). However, the known roles of LCs may provide some insights. LC differentiation from CD34+ myeloid progenitors latently infected with viral IL-10 deletion virus could be explained by increased TGFβ1 expression by these cells. TGFβ1 stimulation is required for LC differentiation from CD34+ myeloid progenitors both in vivo and in vitro (4, 15, 56). Immature LCs are particularly effective in processing antigens into MHC class II complexes for presentation to antigen-specific T cells (42). Upon a maturation stimulus, surface MHC class II expression increases (35), resulting in efficient antigen cross-presentation and CD8+ cytotoxic T cell activation (21, 54). Therefore, blocking of LC differentiation by viral IL-10 may result in the reduced ability of latently infected cells to present viral antigens.
We did not identify a significant difference in the timing or frequency of reactivation of viral IL-10 deletion virus in comparison to parental virus using our previously described reactivation assay, whereby latently infected cells were cocultured with monolayers of primary human foreskin fibroblasts to stimulate reactivation, and monolayers were monitored daily for the appearance of cytopathic effect (CPE) as an indicator of virus reactivation (8, 9, 22). Specifically, in four independent experiments, both parental virus and viral IL-10 deletion virus reactivated at the same time (mean time of 12 days after reactivation stimulus), and the frequencies of reactivation were very similar for the parental virus (1.2 × 10−4) and viral IL-10 deletion virus (1.3 × 10−4). These results were comparable to the rates of reactivation that we have previously reported for these viruses (9).
Reeves et al. demonstrated that HCMV could be reactivated from naturally infected CD34+ progenitors after their ex vivo differentiation to become mature DCs (37). On this basis, if the presence of viral IL-10 suppressed DC differentiation and reactivation by maintaining the cells in the DC progenitor stage, it could be predicted that cells infected with the viral IL-10 deletion virus might reactivate at a higher rate than the parental strain. However, it is possible that the presence of viral IL-10 promotes differentiation to a different mature myeloid cell type, such as a macrophage. In this respect, ex vivo differentiation of naturally infected myeloid cells to a macrophage has been shown to result in reactivation from latency (49, 50). Thus, both macrophages and mature DCs support virus reactivation, and so any skewing of differentiation by viral IL-10 between these two terminally differentiated cell types may not alter the frequency of reactivation. If viral IL-10 did indeed suppress reactivation by maintaining DCs in the progenitor stage and did not skew differentiation to a different cell type such as a macrophage, the magnitude of the increase in the proportion of DCs may not have been sufficient to result in a detectable difference in reactivation frequency as measured by this assay. Defining whether the presence of viral IL-10 expression during latency inhibits differentiation/maturation of DC-committed progenitors or whether it acts at an earlier stage of differentiation by reprogramming more-primitive myeloid progenitors to differentiate toward a different cell type such as a macrophage, will be important components of future studies to fully delineate the role of viral IL-10 during latency and reactivation. Examination of the role of viral IL-10 in modulating myeloid cell differentiation during latent infection with viruses based upon a low-passage clinical strain (in addition to the laboratory strain AD169 used in the current study) will also be an important consideration for subsequent analyses of viral IL-10 function.
In summary, this study identifies a role for viral IL-10 in modulating the differentiation of latently infected myeloid progenitor cells. Latent infection of these progenitors with a virus unable to express viral IL-10 resulted in upregulation of cellular cytokines which were likely to create a favorable environment for DC formation, together with skewing of differentiation toward a myeloid DC type, at least some of which were Langerhans cells. Inhibition of the DC differentiation pathway by viral IL-10 may provide an advantage to latent HCMV in the context of evasion of the host's immune system. As DCs are the most potent antigen-presenting cell type, suppression of differentiation of latently infected myeloid progenitors toward a DC is likely to enhance the ability of latent virus to limit presentation of latency-associated viral peptides to HCMV-specific T cells. In this respect, we reported that latently infected cells were unable to evade detection by CD4+ T cells in the absence of the ability to express viral IL-10 and that this was concomitant with increased MHC class II by these latently infected cells (9). Thus, modulation of DC differentiation by viral IL-10 may act to render latently infected cells less immunogenic and so limit/evade immune detection to enhance the ability of HCMV to persist in a latent state in the human host.
Supplementary Material
Acknowledgments
We thank Bodo Plachter and Sandra Pepperl-Klindworth (Institute for Virology, University Medical Center of the University of Mainz) for kindly providing the viral IL-10 deletion and parental viruses and Eve Diefenbach and Winnie Garcia (Westmead Millennium Institute) for assistance in generating recombinant viral IL-10 proteins.
Flow cytometry was performed in the Flow Cytometry Core Facility that is supported by Westmead Millennium Institute, Australian National Health and Medical Research Council (NHMRC), and Cancer Institute New South Wales. S.A. and J.Z.C. were recipients of an Australian Postgraduate Award and a Westmead Medical Research Foundation Stipend Enhancement Award. This work was supported by NHMRC grant funding awarded to B.S. and A.A.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 18 May 2011.
REFERENCES
- 1. Anton D., Dabadghao S., Palucka K., Holm G., Yi Q. 1998. Generation of dendritic cells from peripheral blood adherent cells in medium with human serum. Scand. J. Immunol. 47:116–121 [DOI] [PubMed] [Google Scholar]
- 2. Bego M., Maciejewski J., Khaiboullina S., Pari G., St. Jeor S. 2005. Characterization of an antisense transcript spanning the UL81-82 locus of human cytomegalovirus. J. Virol. 79:11022–11034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burns S., et al. 2004. Maturation of DC is associated with changes in motile characteristics and adherence. Cell Motil. Cytoskeleton 57:118–132 [DOI] [PubMed] [Google Scholar]
- 4. Caux C., et al. 1999. Respective involvement of TGF-beta and IL-4 in the development of Langerhans cells and non-Langerhans dendritic cells from CD34+ progenitors. J. Leukoc. Biol. 66:781–791 [DOI] [PubMed] [Google Scholar]
- 5. Chang W. L., Barry P. A., Szubin R., Wang D., Baumgarth N. 2009. Human cytomegalovirus suppresses type I interferon secretion by plasmacytoid dendritic cells through its interleukin 10 homolog. Virology 390:330–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang W. L., Baumgarth N., Yu D., Barry P. A. 2004. Human cytomegalovirus-encoded interleukin-10 homolog inhibits maturation of dendritic cells and alters their functionality. J. Virol. 78:8720–8731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheeran M. C., Hu S., Sheng W. S., Peterson P. K., Lokensgard J. R. 2003. CXCL10 production from cytomegalovirus-stimulated microglia is regulated by both human and viral interleukin-10. J. Virol. 77:4502–4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheung A. K., Abendroth A., Cunningham A. L., Slobedman B. 2006. Viral gene expression during the establishment of human cytomegalovirus latent infection in myeloid progenitor cells. Blood 108:3691–3699 [DOI] [PubMed] [Google Scholar]
- 9. Cheung A. K., et al. 2009. The role of the human cytomegalovirus UL111A gene in downregulating CD4+ T cell recognition of latently infected cells: implications for virus elimination during latency. Blood 114:4128–4137 [DOI] [PubMed] [Google Scholar]
- 10. Choi G. S., Kang J. M., Lee M. G. 2000. Analysis of methods for the generation of dendritic cells from human peripheral blood monocytes. Yonsei Med. J. 41:642–650 [DOI] [PubMed] [Google Scholar]
- 11. De La Melena V. T., et al. 2001. Kinetics and development of CMV-accelerated transplant vascular sclerosis in rat cardiac allografts is linked to early increase in chemokine expression and presence of virus. Transplant. Proc. 33:1822–1823 [DOI] [PubMed] [Google Scholar]
- 12. Goodrum F., Jordan C. T., Terhune S. S., High K., Shenk T. 2004. Differential outcomes of human cytomegalovirus infection in primitive hematopoietic subpopulations. Blood 104:687–695 [DOI] [PubMed] [Google Scholar]
- 13. Goodrum F., Reeves M., Sinclair J., High K., Shenk T. 2007. Human cytomegalovirus sequences expressed in latently infected individuals promote a latent infection in vitro. Blood 110:937–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hahn G., Jores R., Mocarski E. S. 1998. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. U. S. A. 95:3937–3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jaksits S., et al. 1999. CD34+ cell-derived CD14+ precursor cells develop into Langerhans cells in a TGF-beta 1-dependent manner. J. Immunol. 163:4869–4877 [PubMed] [Google Scholar]
- 16. Jaworowski A., et al. 2009. Enhanced monocyte Fc phagocytosis by a homologue of interleukin-10 encoded by human cytomegalovirus. Virology 391:20–24 [DOI] [PubMed] [Google Scholar]
- 17. Jenkins C., Abendroth A., Slobedman B. 2004. A novel viral transcript with homology to human interleukin-10 is expressed during latent human cytomegalovirus infection. J. Virol. 78:1440–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jenkins C., et al. 2008. Immunomodulatory properties of a viral homolog of human interleukin-10 expressed by human cytomegalovirus during the latent phase of infection. J. Virol. 82:3736–3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jonuleit H., et al. 1997. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur. J. Immunol. 27:3135–3142 [DOI] [PubMed] [Google Scholar]
- 20. Katz S. I., Tamaki K., Sachs D. H. 1979. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature 282:324–326 [DOI] [PubMed] [Google Scholar]
- 21. Klechevsky E., et al. 2008. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity 29:497–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kondo K., Kaneshima H., Mocarski E. 1994. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc. Natl. Acad. Sci. U. S. A. 91:11879–11883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kondo K., Xu J., Mocarski E. S. 1996. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc. Natl. Acad. Sci. U. S. A. 93:11137–11142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kotenko S. V., Saccani S., Izotova L. S., Mirochnitchenko O. V., Pestka S. 2000. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl. Acad. Sci. U. S. A. 97:1695–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin Y. L., Chang P. C., Wang Y., Li M. 2008. Identification of novel viral interleukin-10 isoforms of human cytomegalovirus AD169. Virus Res. 131:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lockridge K. M., et al. 2000. Primate cytomegaloviruses encode and express an IL-10-like protein. Virology 268:272–280 [DOI] [PubMed] [Google Scholar]
- 27. Mendelson M., Monard S., Sissons P., Sinclair J. 1996. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J. Gen. Virol. 77:3099–3102 [DOI] [PubMed] [Google Scholar]
- 28. Minton E. J., Tysoe C., Sinclair J. H., Sissons J. G. 1994. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J. Virol. 68:4017–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mocarski E. S., Shenk T., Pass R. F. 2007. Cytomegaloviruses, p. 2701–2772 In Knipe D. M., Howley P. M. (ed.), Fields virology, 5th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 30. Mollah Z. U., et al. 2003. Interleukin-3 in cooperation with transforming growth factor beta induces granulocyte macrophage colony stimulating factor independent differentiation of human CD34+ hematopoietic progenitor cells into dendritic cells with features of Langerhans cells. J. Investig. Dermatol. 121:1397–1401 [DOI] [PubMed] [Google Scholar]
- 31. O'Doherty U., et al. 1994. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology 82:487–493 [PMC free article] [PubMed] [Google Scholar]
- 32. Olweus J., et al. 1997. Dendritic cell ontogeny: a human dendritic cell lineage of myeloid origin. Proc. Natl. Acad. Sci. U. S. A. 94:12551–12556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pepperl-Klindworth S., et al. 2006. Cytomegalovirus interleukin-10 expression in infected cells does not impair MHC class I restricted peptide presentation on bystanding antigen-presenting cells. Viral Immunol. 19:92–101 [DOI] [PubMed] [Google Scholar]
- 34. Petrucelli A., Rak M., Grainger L., Goodrum F. 2009. Characterization of a novel Golgi apparatus-localized latency determinant encoded by human cytomegalovirus. J. Virol. 83:5615–5629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pierre P., et al. 1997. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature 388:787–792 [DOI] [PubMed] [Google Scholar]
- 36. Raftery M. J., et al. 2004. Shaping phenotype, function, and survival of dendritic cells by cytomegalovirus-encoded IL-10. J. Immunol. 173:3383–3391 [DOI] [PubMed] [Google Scholar]
- 37. Reeves M. B., MacAry P. A., Lehner P. J., Sissons J. G., Sinclair J. H. 2005. Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers. Proc. Natl. Acad. Sci. U. S. A. 102:4140–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reid C. D., et al. 1990. Identification of hematopoietic progenitors of macrophages and dendritic Langerhans cells (DL-CFU) in human bone marrow and peripheral blood. Blood 76:1139–1149 [PubMed] [Google Scholar]
- 39. Reid C. D., Stackpoole A., Meager A., Tikerpae J. 1992. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow. J. Immunol. 149:2681–2688 [PubMed] [Google Scholar]
- 40. Riedl E., Strobl H., Majdic O., Knapp W. 1997. TGF-beta 1 promotes in vitro generation of dendritic cells by protecting progenitor cells from apoptosis. J. Immunol. 158:1591–1597 [PubMed] [Google Scholar]
- 41. Romani N., et al. 1994. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 180:83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Romani N., et al. 1989. Presentation of exogenous protein antigens by dendritic cells to T cell clones. Intact protein is presented best by immature, epidermal Langerhans cells. J. Exp. Med. 169:1169–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Romani N., et al. 1996. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J. Immunol. Methods 196:137–151 [DOI] [PubMed] [Google Scholar]
- 44. Sallusto F., Lanzavecchia A. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Santiago-Schwarz F., Belilos E., Diamond B., Carsons S. E. 1992. TNF in combination with GM-CSF enhances the differentiation of neonatal cord blood stem cells into dendritic cells and macrophages. J. Leukoc. Biol. 52:274–281 [PubMed] [Google Scholar]
- 46. Slobedman B., Barry P. A., Spencer J. V., Avdic S., Abendroth A. 2009. Virus-encoded homologs of cellular interleukin-10 and their control of host immune function. J. Virol. 83:9618–9629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Slobedman B., et al. 2010. Human cytomegalovirus latent infection and associated viral gene expression. Future Microbiol. 5:883–900 [DOI] [PubMed] [Google Scholar]
- 48. Slobedman B., Mocarski E. S. 1999. Quantitative analysis of latent human cytomegalovirus. J. Virol. 73:4806–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Soderberg-Naucler C., Fish K. N., Nelson J. A. 1997. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91:119–126 [DOI] [PubMed] [Google Scholar]
- 50. Soderberg-Naucler C., et al. 2001. Reactivation of latent human cytomegalovirus in CD14+ monocytes is differentiation dependent. J. Virol. 75:7543–7554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Spencer J. V. 2007. The cytomegalovirus homolog of interleukin-10 requires phosphatidylinositol 3-kinase activity for inhibition of cytokine synthesis in monocytes. J. Virol. 81:2083–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Spencer J. V., Cadaoas J., Castillo P. R., Saini V., Slobedman B. 2008. Stimulation of B lymphocytes by cmvIL-10 but not LAcmvIL-10. Virology 374:164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stanier P., et al. 1989. Persistence of cytomegalovirus in mononuclear cells in peripheral blood from blood donors. BMJ 299:897–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stoitzner P., et al. 2006. Langerhans cells cross-present antigen derived from skin. Proc. Natl. Acad. Sci. U. S. A. 103:7783–7788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Strobl H., et al. 1997. flt3 ligand in cooperation with transforming growth factor-beta1 potentiates in vitro development of Langerhans-type dendritic cells and allows single-cell dendritic cell cluster formation under serum-free conditions. Blood 90:1425–1434 [PubMed] [Google Scholar]
- 56. Strobl H., Knapp W. 1999. TGF-beta1 regulation of dendritic cells. Microbes Infect. 1:1283–1290 [DOI] [PubMed] [Google Scholar]
- 57. Strobl H., et al. 1996. TGF-beta 1 promotes in vitro development of dendritic cells from CD34+ hemopoietic progenitors. J. Immunol. 157:1499–1507 [PubMed] [Google Scholar]
- 58. Taylor-Wiedeman J., Sissons J. G., Borysiewicz L. K., Sinclair J. H. 1991. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J. Gen. Virol. 72:2059–2064 [DOI] [PubMed] [Google Scholar]
- 59. Taylor-Wiedeman J., Sissons P., Sinclair J. 1994. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J. Virol. 68:1597–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thomas R., Davis L. S., Lipsky P. E. 1993. Isolation and characterization of human peripheral blood dendritic cells. J. Immunol. 150:821–834 [PubMed] [Google Scholar]
- 61. Yamamoto-Tabata T., McDonagh S., Chang H. T., Fisher S., Pereira L. 2004. Human cytomegalovirus interleukin-10 downregulates metalloproteinase activity and impairs endothelial cell migration and placental cytotrophoblast invasiveness in vitro. J. Virol. 78:2831–2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhou T., et al. 2005. Effect of interleukin-10 on the phenotype and function of cultured human dendritic cells. Chin. Med. J. 118:1299–1302 [PubMed] [Google Scholar]
- 63. Zhuravskaya T., et al. 1997. Spread of human cytomegalovirus (HCMV) after infection of human hematopoietic progenitor cells: model of HCMV latency. Blood 90:2482–2491 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.