Abstract
In eukaryotic cells, the nucleus is a complex and sophisticated organelle containing genomic DNA and supports essential cellular activities. Its surface contains many nuclear pore complexes (NPCs), channels for macromolecular transport between the cytoplasm and nucleus. It has been observed that the nuclear volume and the number of NPCs almost doubles during interphase in dividing cells, but the coordination of these events with the cell cycle was poorly understood, particularly in mammalian cells. Recently, we demonstrated that cyclin-dependent protein kinases (Cdks) control interphase NPC formation in dividing human cells. Cdks drive the very early step of NPC formation because Cdk inhibition suppressed the generation of “nascent pores,” which are considered to be immature NPCs, and disturbed expression and localization of some nucleoporins. Cdk inhibition did not affect nuclear volume, suggesting that these two processes have distinct regulatory mechanisms in the cell cycle. The details of our experimental systems and finding are discussed in more depth. With new findings recently reported, we also discuss possible molecular mechanisms of interphase NPC formation.
Key words: nuclear size, nuclear pore complex (NPC), cyclin-dependent protein kinases (Cdks), bio-imaging, cell-fusion
Introduction
Cell reproduction, which is fundamental to all life, occurs through an elaborate series of events known as the “cell cycle,” where genomic DNA and other cellular components are duplicated and then distributed into two daughter cells.1,2 In eukaryotic cells, cyclins and cyclin-dependent protein kinases (Cdks) are master regulators of the cell cycle.1,2
During the eukaryotic cell cycle, the volume of the nucleus almost doubles, but the mechanism for this growth during the cell cycle is poorly understood, particularly in mammalian cells.1 The number of nuclear pore complexes (NPCs), the channels for macromolecular transport between the cytoplasm and nucleus, also doubles as the cell cycle progresses.3–7 NPCs are supramolecular complexes, assembled from multiple copies of approximately 30 different proteins, termed nucleoporins (Nups). For the “economical” formation of NPCs during cell proliferation, their expression and formation process needs to be highly controlled. There must be a “global regulation” of interphase NPC formation coupled strictly with the cell cycle, to avoid a huge waste of energy.
Structural aspects of nuclei and NPCs have been characterized in detail. However, their formation and regulation, especially during interphase with a closed nuclear envelope, remained unclear.3–7 To address this question, we established novel techniques to study nuclear volume and NPC formation, and investigated how they were regulated during interphase in dividing human cells.8,9 Our results indicate that Cdks, especially Cdk1 and Cdk2, control NPC formation during interphase.9 Cdk inhibition suppressed the generation of the “nascent pores,” which are immature NPCs in the process of forming and interrupted expression and localization of some nucleoporins. Surprisingly, we also demonstrated that Cdk inhibition did not effect nuclear growth, revealing that nuclear growth and NPC formation have distinct regulation in the cell cycle.9
Novel Approaches for Investigating Nuclear Growth and NPC Formation during Interphase
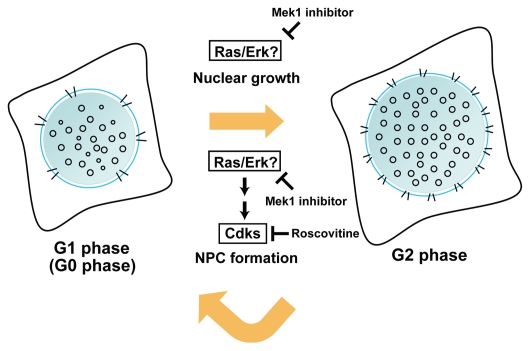
To measure nuclear volume and NPC density throughout the cell cycle, synchronized HeLa cells were viewed as three-dimensional image stacks under a fluorescence microscope after nuclear and NPC staining. Nuclear volume was measured using newly developed image processing software for the segmentation and extraction of nuclear area from the image stacks. NPC density was measured using manual counting. Both nuclear volume and NPC density gradually increased during cell cycle progression (Fig. 1), but the changes did not follow the same pattern. Inhibition of Cdk activity by roscovitine inhibited the increase in NPC density, but the nuclear volume changes were unaffected (Fig. 1). Nuclear growth was independent of cellular DNA content (Fig. 1F in ref. 9). Nuclear growth was suppressed with the Mek1 inhibitor, PD98059, suggesting that the Ras/Erk signaling pathway was involved in this process (Fig. 1).
Figure 1.
Nuclear volume and the number of nuclear pore complexes (NPCs) almost double during interphase in dividing cells. NPC formation, but not nuclear growth, is governed by cyclin-dependent kinases (Cdks) from G1 (left) to G2 (right) phase. The nuclear size and NPC density of quiescent G0 nuclei in normal human fibroblasts was similar to those of G1 nuclei.9,28 The Mek1 inhibitor, PD98059, blocked NPC formation during interphase. Ras/Erk signaling occurs upstream of Cdks in the regulation of NPC formation.
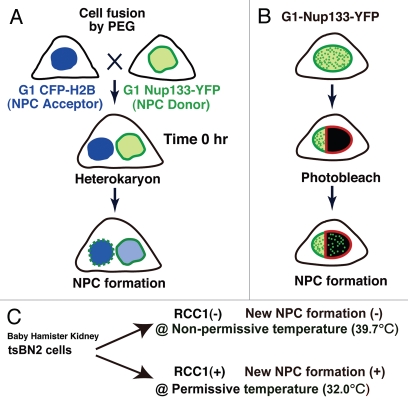
The manual counting of NPCs to examine density proved laborious and prone to error, and the low resolution of light microscopy was insufficient to distinguish between adjacent NPCs. Increases in the nuclear surface area with cell cycle progression also affected the NPC density. To overcome these problems, we used two novel approaches to directly visualize newly formed NPCs on nuclear surfaces during interphase (Fig. 2A and B). In the heterokaryon method, cells expressing Nup133-YFP or YFP-Nup107 were used as NPC donor cells and CFP-histone 2B (H2B)-expressing cells were used as NPC acceptor cells (Fig. 2A). The two cell types were mixed at an appropriate density and polyethylene glycol (PEG) treatment resulted in the formation of heterokaryons (Fig. 2A). Newly formed NPCs were visualized on the NPC acceptor nuclei in the heterokaryons (Fig. 2A). In the photobleaching method, selected nuclear surface regions in G1-phase cells expressing Nup133-YFP or YFP-Nup107 were photobleached using a 488 nm laser. After bleaching, newly formed NPCs appeared as bright dots in the bleached areas (Fig. 2B; also reviewed in ref. 8). These two procedures are complementary. The heterokaryon method reveals the formation of new NPCs on whole acceptor nuclei; it has a low background signal, and does not risk laser damage to the cells. Photobleaching offers the advantage of selective monitoring of the particular cells of interest, as in the case of Cdk siRNA experiments. Using both procedures, we were able to demonstrate that interphase NPC formation depended on Cdks activity.
Figure 2.
(A) Schematic representation of the heterokaryon procedure. HeLa cells expressing Nup133-YFP or YFP-Nup107 are used as NPC donor cells and HeLa cells expressing CFP-histone 2B (H2B) were used as NPC acceptor cells (top). G1-synchronized donor and acceptor cells were treated with 50% (w/v) polyethylene glycol (PEG) to make heterokaryons (middle). Many bright fluorescent dots appear on the NPC acceptor nuclear surface, which has the brighter CFP-H2B signal, in the heterokaryon cells. The bright foci represent newly formed NPCs (bottom). (B) The photobleaching method. Nuclear surface regions in G1-phase cells expressing Nup133-YFP or YFP-Nup107 were photobleached by a 488 nm laser (middle). At 16 h after bleaching, many bright YFP dots appear in the bleached areas, representing newly synthesized NPCs (bottom). (C) In the tsBN2 cell, which is a temperature-sensitive mutant of RCC1 that is a guanine nucleotide exchange factor for Ran, a loss of RCC1 inhibits NPC formation during interphase. This allows the inhibition or initiation of NPC formation by changes in temperature. In this system, interphase NPC formation actively occurs at permissive temperature (32°C), but not at non-permissive temperature (39.7°C).
In both procedures the use of cells expressing fluorescently tagged scaffold Nups, like Nup133-YFP or YFP-Nup107, was essential. The scaffold Nups are the most immobile components of NPCs. D'Angelo et al. recently reported that Nup107 is not exchanged after incorporation into NPCs in a myoblast fusion system.10 When we used Nup62-YFP-expressing cells, the NPC acceptor nuclei acquired Nup62-YFP signals without interphase NPC formation, likely due to the rapid turnover of Nup62-YFP within NPCs (Maeshima K, et al. unpublished). Additionally, in both systems, the fraction of Nup107 or Nup133 that was fluorescently labeled and how much excess YFP-Nup107 or Nup133-YFP was expressed are also critical issues, because they have important implications in the interpretation of the data. When the expression levels of exogenous and endogenous Nups were examined, we observed that endogenous proteins were dramatically downregulated, possibly to maintain a constant level of Nup107 or Nup133 protein (Sup. Fig. 4 in ref. 9). Using Nup62 as a normalization control, the total signal intensity of YFP-Nup107 and endogenous Nup107 in the stable cell line was similar to the signal intensity of endogenous Nup107 in the control cells (0.9823). The corresponding ratio for Nup133 was 1.853, indicating slight overproduction. In the stable cell lines, 62.9% of Nup107 proteins and 84.6% of Nup133 proteins were labeled fluorescently. The amount of endogenous Nup62 was also markedly reduced in cells stably expressing Nup62-YFP (Maeshima K, et al. unpublished). These results suggest that ectopic Nup expression is unlikely to be problematic in our systems, and that the cellular system maintains Nups at constant levels. The existence of such a Nup monitoring system is an intriguing topic for further investigation.
Possible Mechanisms for Cdk-Mediated Control of NPC Formation during Interphase
We found that Cdk inhibition almost entirely blocked interphase NPC formation (Fig. 1), but the fluorescence intensities of several YFP-tagged nucleoporins from existing NPCs remained unchanged (Maeshima K, et al. unpublished). Furthermore, the observed size and global structure of NPCs on freeze-fractured surfaces were comparable between roscovitine-treated and control cells (Maeshima K, et al. unpublished). These results suggest that Cdk activity is required for the early stages of NPC formation, but not for structural maintenance of NPCs. How can Cdk control the formation of new NPCs during interphase? To obtain clues to answer this question, we examined the expression level and cellular localization of various nucleoporins in Cdk-inhibited cells.9 Nups 62, 88, 93, 98, 107 and 133 were expressed at similar levels in roscovitine-treated and control cells. Nups 62, 107 and 133 were co-localized in the cytoplasm, forming large dots or foci on Cdk inhibition. Elys/Mel28, Pom121, Nup153 and RanBP2 expression was reduced by 30–40% in roscovitine-treated cells compared with control cells.9 Several groups recently reported the importance of Pom121 for the recruitment of the Nup107-160 complex into new assembly sites of NPC on closed nuclear envelopes.11–13 These observations agree closely with live-cell imaging of NPC formation.14 Downregulation of Pom121 by Cdk-inhibition could be a reason to suppress new NPC formation during interphase (Fig. 3) since Pom121 may be the seed for interphase NPC formation (Fig. 3).
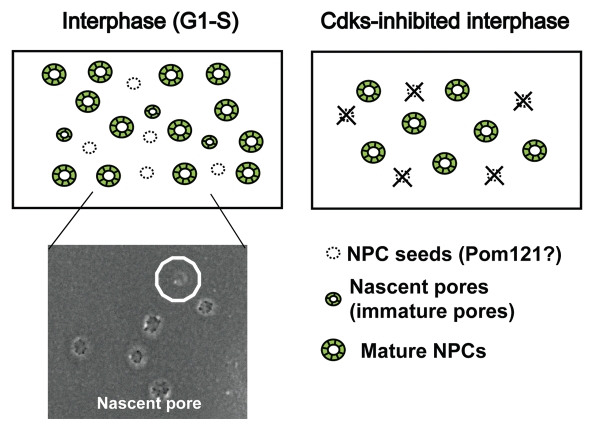
Figure 3.
Model of interphase NPC formation. Based on the location of the NPC seeds, it appears that in G1/S phase, construction proceeds from the nascent pores to mature NPCs (left). SEM image shows a nascent pore (marked with circle) (left bottom). In Cdk-inhibited cells, there are no NPC seeds. Nascent pores and subsequently mature NPCs cannot form (right).
Scanning electron microscopy (SEM) of the freeze-fractured nuclear surfaces identified small NPCs, “the nascent pores” (Fig. 3) in the G1/S phase of HeLa cells, but not in roscovitine-treated cells.9 Recently, using baby hamster kidney tsBN2 cells, a temperature-sensitive mutant of RCC1, we established a system allowing the inhibition or initiation of interphase NPC formation by changing the temperature.8 In this system, interphase NPC formation actively occurs at permissive temperature (32°C), but not at non-permissive temperature (39.7°C) (Fig. 2C) although detailed mechanism of the inhibition remains unclear. Again, we only found nascent pores in tsBN2 cells when new NPC formation was active during interphase (Sup. Fig. 7 in ref. 9). These findings indicate that the nascent pores are not species- or cell-type-specific and suggest they could be immature pores under construction. The nascent pores we observed were evenly distributed and not paired or clustered (Maeshima K, et al. unpublished), indicating that they were not generated by splitting of preexisting NPCs. Because we assume that the nascent pores are a rate-limiting step of immature pores under construction, a structural analysis of the nascent pores may provide new clues to NPC formation.
How Can Cdk Inhibitors Work in Cells?
To examine the global regulation of interphase NPC formation, we used small-molecule inhibitors, which are widely used to examine protein function (reviewed in ref. 15). An advantage of such inhibitors is they act rapidly to inactivate function, whereas small-interfering RNA (siRNA) do not affect function for 48–72 h or longer after transfection. Here, we discuss the possible in vivo target(s) of roscovitine and purvalanol A, both of which inhibit the kinase activity by competing with ATP at the ATP binding site of the kinase.16,17
Reported in vitro targets of roscovitine are Cdks 1, 2, 5, 7 and 9 (IC50 < 1 µM).16 A further Cdk inhibitor, purvalanol A, also used in our study, is similar to roscovitine in its selectivity.17 Cdk1 (and also Cdk2) are major cell cycle drivers in mammalian cells, whereas Cdk5 has neuron-specific expression and function.1,18 Cdk7 functions as a Cdk-activating kinase and is essential for activity of the transcription factor TFIIH.1,18 Cdk9 is an elongation factor for RNA polymerase II-directed transcription and functions by phosphorylating the C-terminal domain of the largest subunit of RNA polymerase II.1,18
In addition to the involvement of Cdk1 and Cdk2 in interphase NPC formation,9 does Cdk7 or Cdk9 have an effect on interphase NPC formation? We cannot exclude this possibility, but we think it is unlikely that the suppression of general transcription through Cdk7 and Cdk9 inhibition would have major effects on NPC formation, for the following reasons. First, neither roscovitine nor purvalanol A affects nuclear growth during interphase.9 Expression levels of all inner nuclear membrane proteins examined were similar between roscovitine-treated and control cells.9 Second, inhibition of Cdk9 activity by flavopridol, which is currently thought to mainly target Cdk9,19 leads to dramatic suppression of global mRNA production;20 this is not observed with roscovitine.21 Thus, from our study, we conclude that Cdk1 and Cdk2 are the most likely major targets of roscovitine and purvalanol A.
Distinct Assembly Mechanisms between Post-Mitotic NPC Assembly and Interphase NPC Formation
In the metazoan cell cycle, there are two periods of NPC formation: post-mitotic NPC assembly and interphase NPC formation.3–7 Post-mitotic NPC assembly occurs concomitantly with the formation of NEs around chromatin.22 It begins in early anaphase,14,23 with the recruitment of the Nup107-160 scaffold complex to the chromatin. The recruitment is mediated by Elys/Mel28.24–26 During interphase, the number of NPCs on the nuclear surface doubles in preparation for re-entry into mitosis.27 Much less was known about the mechanism of interphase NPC formation than about post-mitotic processes.4–7
Although we showed that Cdk activity was essential for interphase NPC formation, Cdk activity was not required for post-mitotic NPC assembly, demonstrating that post-mitotic NPC assembly and interphase NPC formation are differentially regulated.9 Consistent with this finding, other groups recently determined distinct aspects of post-mitotic NPC assembly and interphase NPC formation. Hetzer's group demonstrated that Elys/Mel28 is required for post-mitotic NPC assembly, but not during interphase.11 Ellenberg's group also showed that interphase NPC formation is initiated by slow accumulation of Pom121 followed by more rapid association of the soluble NPC subcomplex Nup107-160.14 In the case of post-mitotic NPC assembly, an inverse order of recruitment from Nup107-160 to Pom121 occurs.14 These findings indicate distinct molecular mechanisms between post-mitotic NPC assembly and interphase NPC formation.
NPC Formation, Nuclear Growth and Tumor Cells
In our previous study, we showed that the NPC density of quiescent G0 nuclei in normal human fibroblasts was similar to that of G1 nuclei.28 However, the NPC density in replicative senescent cells was much higher, even though both the quiescent and senescent cells were in a non-dividing state.28 This is not so surprising because the proliferation signaling (Ras/Erk) activates Cdks.1 Indeed, the Mek1 inhibitor, PD98059, blocked NPC formation during interphase (Fig. 1) (Maeshima K, et al. unpublished result). In G0 cells, Ras/Erk signaling is inactivated, and the NPC density may remain low, as in G1 nuclei. In contrast, the activation of Ras/Erk signaling by oncogenic Ras was reported to induce senescence of human fibroblasts,29 suggesting that activation of Ras/Erk signaling increased NPC density in the senescent cells.
Similarly, in normal human fibroblasts, G0 cells induced by serum depletion have smaller nuclei9 than senescent cells.30 The large nuclei in senescent cells may be due to the activation of Ras/Erk signaling by oncogenic Ras. This theory is in good agreement with the finding that inhibition of Mek1, but not of Cdks, blocked nuclear growth during interphase.9 These results suggest a general relationship between nuclear size and Ras/Erk signaling. Interestingly, when Cdk activity was suppressed in our study, the nuclei were larger than in the control cells. However, this can be explained by roscovitine transiently stimulating the Ras/Erk pathway,21 again showing a close link of nuclear size and Ras/Erk signaling.
Two groups using budding and fission yeasts reported that yeast nuclei undergo a continuous increase in volume, independent of the cell cycle.31,32 Thus, our finding that nuclear growth does not depend on Cdk activity and is independent of the cellular DNA content may be universal in eukaryotes. The Ras/Erk pathway is not well conserved in the budding yeast; in fission yeast, there are three pathways (Ras1-Spk1/Pmk1-MAPK/Sty1/Spc1/Phh1-SAPK), which are equivalent or related to Ras/Erk signaling in mammalian cells.33–35 It is intriguing to investigate involvement of these pathways in the regulation of nuclear size in fission yeast. It is also well known that an increased ratio of nuclear volume to cell volume is a prominent diagnostic marker of many tumor cells.36,37 We suggest that the increased nuclear volume ratio in some tumor cells might be a result of activated Ras/Erk signaling (e.g., oncogenic ras mutations) because Ras/Erk signaling is activated in many tumor cells. Activation of Ras/Erk signaling might also explain why many tumors have a high density of NPCs, while terminally differentiated cells such as erythrocytes have a low density (Fig. 1).38 Further research to assess the relationship between nuclear structure and the activation of Ras/Erk signaling would be useful in clinical oncology research.
In conclusion, our findings reveal that Cdks govern NPC formation, but not nuclear growth, during interphase in human dividing cells, suggesting that NPC formation and nuclear growth have distinct regulatory mechanisms (Fig. 1). Additionally, the imaging and quantification procedures developed in our study will provide useful tools for furthering our understanding of the mechanism of NPC formation and nuclear growth during interphase (Fig. 2A and B).
Acknowledgements
We are grateful to Dr. Funakoshi, Ms. Watanabe, Ms. Nakatomi, Mr. Nishimura, Dr. Yahata, Dr. Imamoto, Dr. Hashikawa and Dr. Yokota, who contributed to the work published in Nat Struct Mol Biol. We also thank Drs. T. Toda, M. Narita, J. Ellenberg and H. Araki for helpful discussions and useful suggestions for this manuscript.
Extra View to: Maeshima K, Lino H, Hihara S, Funakoshi T, Watanabe A, Nishimura M, et al. Nuclear pore formation but not nuclear growth is governed by cyclin-dependent kinases (Cdks) during interphase. Nat Struct Mol Biol. 2010;17:1065–1071. doi: 10.1038/nsmb.1878.
References
- 1.Morgan DO. The Cell Cycle—Principles of Control. 2007 [Google Scholar]
- 2.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. (Fifth Edition) 2007 [Google Scholar]
- 3.Tran EJ, Wente SR. Dynamic nuclear pore complexes: life on the edge. Cell. 2006;125:1041–1053. doi: 10.1016/j.cell.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Antonin W, Ellenberg J, Dultz E. Nuclear pore complex assembly through the cell cycle: regulation and membrane organization. FEBS Lett. 2008;582:2004–2016. doi: 10.1016/j.febslet.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 5.D'Angelo MA, Hetzer MW. Structure, dynamics and function of nuclear pore complexes. Trends Cell Biol. 2008;18:456–466. doi: 10.1016/j.tcb.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim RY, Ullman KS, Fahrenkrog B. Biology and biophysics of the nuclear pore complex and its components. Int Rev Cell Mol Biol. 2008;267:299–342. doi: 10.1016/S1937-6448(08)00632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strambio-De-Castillia C, Niepel M, Rout MP. The nuclear pore complex: bridging nuclear transport and gene regulation. Nat Rev Mol Cell Biol. 2010;11:490–501. doi: 10.1038/nrm2928. [DOI] [PubMed] [Google Scholar]
- 8.Iino H, Maeshima K, Nakatomi R, Kose S, Hashikawa T, Tachibana T, et al. Live imaging system for visualizing nuclear pore complex (NPC) formation during interphase in mammalian cells. Genes Cells. 2010;15:647–660. doi: 10.1111/j.1365-2443.2010.01406.x. [DOI] [PubMed] [Google Scholar]
- 9.Maeshima K, Iino H, Hihara S, Funakoshi T, Watanabe A, Nishimura M, et al. Nuclear pore formation but not nuclear growth is governed by cyclin-dependent kinases (Cdks) during interphase. Nat Struct Mol Biol. 2010;17:1065–1071. doi: 10.1038/nsmb.1878. [DOI] [PubMed] [Google Scholar]
- 10.D'Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136:284–295. doi: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doucet CM, Talamas JA, Hetzer MW. Cell cycle-dependent differences in nuclear pore complex assembly in metazoa. Cell. 2010;141:1030–1041. doi: 10.1016/j.cell.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fichtman B, Ramos C, Rasala B, Harel A, Forbes DJ. Inner/Outer nuclear membrane fusion in nuclear pore assembly: biochemical demonstration and molecular analysis. Mol Biol Cell. 2010;21:4197–4211. doi: 10.1091/mbc.E10-04-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funakoshi T, Clever M, Watanabe A, Imamoto N. Localization of Pom121 to the inner nuclear membrane is required for an early step of interphase NPC assembly. Mol Biol Cell. 2010;22:1058–1069. doi: 10.1091/mbc.E10-07-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dultz E, Ellenberg J. Live imaging of single nuclear pores reveals unique assembly kinetics and mechanism in interphase. J Cell Biol. 2010;191:15–22. doi: 10.1083/jcb.201007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doudna JA. Chemical biology at the crossroads of molecular structure and mechanism. Nat Chem Biol. 2005;1:300–303. doi: 10.1038/nchembio1105-300. [DOI] [PubMed] [Google Scholar]
- 16.Bach S, Knockaert M, Reinhardt J, Lozach O, Schmitt S, Baratte B, et al. Roscovitine targets, protein kinases and pyridoxal kinase. J Biol Chem. 2005;280:31208–31219. doi: 10.1074/jbc.M500806200. [DOI] [PubMed] [Google Scholar]
- 17.Gray NS, Wodicka L, Thunnissen AM, Norman TC, Kwon S, Espinoza FH, et al. Exploiting chemical libraries, structure and genomics in the search for kinase inhibitors. Science. 1998;281:533–538. doi: 10.1126/science.281.5376.533. [DOI] [PubMed] [Google Scholar]
- 18.Malumbres M, Barbacid M, Cell cycle. CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 19.Chao SH, Price DH. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem. 2001;276:31793–31799. doi: 10.1074/jbc.M102306200. [DOI] [PubMed] [Google Scholar]
- 20.Lam LT, Pickeral OK, Peng AC, Rosenwald A, Hurt EM, Giltnane JM, et al. Genomic-scale measurement of mRNA turnover and the mechanisms of action of the anti-cancer drug flavopiridol. Genome Biol. 2001;2:41. doi: 10.1186/gb-2001-2-10-research0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whittaker SR, Te Poele RH, Chan F, Linardopoulos S, Walton MI, Garrett MD, et al. The cyclin-dependent kinase inhibitor seliciclib (R-roscovitine; CYC202) decreases the expression of mitotic control genes and prevents entry into mitosis. Cell Cycle. 2007;6:3114–3131. doi: 10.4161/cc.6.24.5142. [DOI] [PubMed] [Google Scholar]
- 22.Maul GG. Nuclear pore complexes. Elimination and reconstruction during mitosis. J Cell Biol. 1977;74:492–500. doi: 10.1083/jcb.74.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodoor K, Shaikh S, Salina D, Raharjo WH, Bastos R, Lohka M, et al. Sequential recruitment of NPC proteins to the nuclear periphery at the end of mitosis. J Cell Sci. 1999;112:2253–2264. doi: 10.1242/jcs.112.13.2253. [DOI] [PubMed] [Google Scholar]
- 24.Rasala BA, Orjalo AV, Shen Z, Briggs S, Forbes DJ. ELYS is a dual nucleoporin/kinetochore protein required for nuclear pore assembly and proper cell division. Proc Natl Acad Sci USA. 2006;103:17801–17806. doi: 10.1073/pnas.0608484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franz C, Walczak R, Yavuz S, Santarella R, Gentzel M, Askjaer P, et al. MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly. EMBO Rep. 2007;8:165–172. doi: 10.1038/sj.embor.7400889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasala BA, Ramos C, Harel A, Forbes DJ. Capture of AT-rich chromatin by ELYS recruits POM121 and NDC1 to initiate nuclear pore assembly. Mol Biol Cell. 2008;19:3982–3996. doi: 10.1091/mbc.E08-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maul GG, Maul HM, Scogna JE, Lieberman MW, Stein GS, Hsu BY, et al. Time sequence of nuclear pore formation in phytohemagglutinin-stimulated lymphocytes and in HeLa cells during the cell cycle. J Cell Biol. 1972;55:433–447. doi: 10.1083/jcb.55.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeshima K, Yahata K, Sasaki Y, Nakatomi R, Tachibana T, Hashikawa T, et al. Cell cycle-dependent dynamics of nuclear pores: pore-free islands and lamins. J Cell Sci. 2006;119:4442–4451. doi: 10.1242/jcs.03207. [DOI] [PubMed] [Google Scholar]
- 29.Narita M, Lowe SW. Senescence comes of age. Nat Med. 2005;11:920–922. doi: 10.1038/nm0905-920. [DOI] [PubMed] [Google Scholar]
- 30.Narita M. Cellular senescence and chromatin organisation. Br J Cancer. 2007;96:686–691. doi: 10.1038/sj.bjc.6603636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann FR, Nurse P. Nuclear size control in fission yeast. J Cell Biol. 2007;179:593–600. doi: 10.1083/jcb.200708054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jorgensen P, Edgington NP, Schneider BL, Rupes I, Tyers M, Futcher B. The size of the nucleus increases as yeast cells grow. Mol Biol Cell. 2007;18:3523–3532. doi: 10.1091/mbc.E06-10-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Xu HP, Riggs M, Rodgers L, Wigler M. byr2, a Schizosaccharomyces pombe gene encoding a protein kinase capable of partial suppression of the ras1 mutant phenotype. Mol Cell Biol. 1991;11:3554–3563. doi: 10.1128/mcb.11.7.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toda T, Dhut S, Superti-Furga G, Gotoh Y, Nishida E, Sugiura R, et al. The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol Cell Biol. 1996;16:6752–6764. doi: 10.1128/mcb.16.12.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiozaki K, Russell P. Cell cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature. 1995;378:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- 36.Huber MD, Gerace L. The size-wise nucleus: nuclear volume control in eukaryotes. J Cell Biol. 2007;179:583–584. doi: 10.1083/jcb.200710156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nat Rev Cancer. 2004;4:677–687. doi: 10.1038/nrc1430. [DOI] [PubMed] [Google Scholar]
- 38.Maul GG, Deaven LL, Freed JJ, Campbell GL, Becak W. Investigation of the determinants of nuclear pore number. Cytogenet Cell Genet. 1980;26:175–190. doi: 10.1159/000131439. [DOI] [PubMed] [Google Scholar]