Abstract
Thrombopoietin (TPO) is the cytokine that is chiefly responsible for megakaryocyte production but increasingly attention has turned to its role in maintaining hematopoietic stem cells (HSCs). HSCs are required to initiate the production of all mature hematopoietic cells, but this differentiation needs to be balanced against self-renewal and quiescence to maintain the stem cell pool throughout life. TPO has been shown to support HSC quiescence during adult hematopoiesis, with the loss of TPO signaling associated with bone marrow failure and thrombocytopenia. Recent studies have shown that constitutive activation mutations in Mpl contribute to myeloproliferative disease. In this review, we will discuss TPO signaling pathways, regulation of TPO levels and the role of TPO in normal hematopoiesis and during myeloproliferative disease.
Key words: thrombopoietin, TPO, Mpl, hematopoietic stem cell, hematopoiesis, Jak2, MPLW515K, MPLW515L
Introduction
Hematopoietic stem cells (HSCs) are a rare multipotent cell type that are required to initiate blood cell production throughout life. As well as having the ability to produce all the mature hematopoietic cell types, HSCs can self renew, which allows HSCs to be maintained at constant levels in the adult bone marrow, and to expand during fetal development and post transplantation. During steady-state hematopoiesis, HSCs are predominately quiescent and only cycle very rarely. It is likely that the maintenance of HSC quiescence is vital to sustaining the HSC pool throughout life. HSCs are regulated by intrinsic and extrinsic factors that control their ability to self-renew, proliferate and replenish the hematopoietic system. The cytokine thrombopoietin (TPO), as well as having a lineage specific role in the regulation of megakaryopoiesis, is vital for HSC regulation. In this review, we will examine recent work exploring how TPO is involved in maintenance of HSC quiescence.
Thrombopoietin
Thrombopoietin is the chief cytokine that regulates megakaryocyte production, signaling through its receptor Mpl. The expression pattern of Mpl provides clues to the dual functions of TPO, with Mpl expressed predominantly on megakaryocytes, platelets, hemangioblasts and hematopoietic stem cells.1–3 TPO's important role in platelet production was shown when it was found that TPO-/- and Mpl-/- mice have an ∼85% reduction in platelets and megakaryocytes, while the mature cells of other lineages remain unaffected.4–6 TPO acts early in megakaryocyte lineage commitment to promote the proliferation of megakaryocyte progenitors,7 and also increases the ploidy of megakaryocytes.6 However, TPO has little or no effect on platelet shedding from megakaryocytes as TPO does not promote proplatelet formation by megakaryocytes in vitro8 or alter platelet morphology and their ability to become activated,9 and after TPO injection, it takes mice three or more days to increase their platelet count.7
Stem Cell Role for TPO
While megakaryocytes are the only hematopoietic cell lineage that are affected by loss of TPO signaling, less than half the wild-type levels of erythroid and myeloid progenitors are found in TPO-/- and Mpl-/- mice.5,10 The reduction in multipotent progenitor and HSC function is even more severe, with competitive transplant assays revealing that Mpl-/- bone marrow, even when ten-fold in excess, was unable to effectively reconstitute hematopoietic organs.11 This suggested that TPO signaling could be involved in maintaining HSC number or in enabling expansion of stem cells after transplantation. To investigate HSC number in Mpl-/- mice, competitive transplant assays into TPO-/- hosts were used. This demonstrated that the Mpl-/- bone marrow was more able to compete with wild-type bone in a TPO-free environment but was still required in excess to effectively compete against wild-type bone marrow in a primary transplant.12 To investigate HSC expansion after transplantation, wild-type bone marrow was transplanted into irradiated wild-type and TPO-/- mice. It was found that at least four times more bone marrow cells were required to protect the TPO-/- mice.13 Both these results suggest that TPO facilitates the expansion and self renewal of HSCs after transplantation.
During adult hematopoiesis, TPO has a role in maintaining the quiescence of HSCs as is demonstrated by the age-progressive loss of HSCs in TPO-/- mice, the increased cycling observed in HSCs in TPO-/- mice and mice treated with an anti-Mpl antibody and the increased proportion of quiescent HSCs found after TPO stimulation.15,16 Investigation of the expression of cell cycle genes found that loss of TPO signaling was associated with downregulation of cell cycle inhibitors Cdkn1c (p57Kip2) and Cdkn2d (p19Ink4d) in HSCs.15,16 Cyclophosphamide/granulocyte colony stimulating factor (G-CSF) treatment, which is commonly used in human clinical transplantation to drive HSC self-renewing proliferation and to induce HSC migration into the blood stream,17–20 resulted in the downregulation of both of these genes as HSCs proliferate.21 Additionally, mouse embryonic day 12 (E12) aorta-gonads-mesonephros (AGM) cells that had lost Cdkn1c were less able to repopulate the bone marrow of irradiated adult mice than wild-type AGM cells.22 Genes from the Hox family have also been found to be important for the expansion and self-renewal of HSCs.23,24 Several genes in this family, Hoxb4, Hoxa5, Hoxa9, Hoxa10 and Meis1, a Hox family cofactor, were downregulated in the HSCs of Tpo-/- mice or are induced by TPO.15,25,26 Together, these modulations of genes that are involved in the cell cycle or are important hematopoietic transcription factors may explain some of the phenotypes observed in HSCs when TPO signaling is impaired.
In humans, TPO signaling has also been linked to HSC regulation, because patients with congenital amegakaryocytic thrombocytopenia (CAMT), caused by loss-of-function mutations in Mpl,27,28 progressively develop bone marrow failure.29 As in Mpl-/- mice, these patients are born with normal numbers of hematopoietic cells except for a megakaryocyte and platelet deficiency. CAMT patients can develop bone marrow failure as early as two months of age and by a median age of two years.29 Mpl-/- mice do not develop bone marrow failure, but, by one year of age, their myeloid cells are reduced compared to wild-type mice,15 suggesting the onset of exhaustion of the stem cell pool, analogous to that seen in CAMT patients.
It was thought that some of the HSC defects found in CAMT patients might be rescued with restoration of Mpl expression, if they are due to continued lack of TPO signaling. A study using tissue-appropriate promoters, such as Mpl and Gp1ba promoter, was able to restore Mpl expression in Mpl-/- cells to near physiological levels in megakaryocytes and platelets using lentiviral vectors.14 This resulted in an amelioration of the stem cell defects, with mice transplanted with the Mpl-transduced cells having an increase of Lin− Sca-1+ c-Kit+ (LSK) cells compared to mice transplanted with Mpl-/- GFP transduced cells and the Mpl transduced cells having increased long-term repopulating potential after secondary transplant and showing correction of transcriptional changes in cell cycle genes observed in LSK cells.14 Encouragingly, this study indicates that the HSC loss observed in CAMT patients is not irreversible and points towards the development of gene therapies for this disease.
Studies of TPO signaling in HSCs have uncovered two contrasting roles for TPO in adult hematopoiesis: (1) maintaining stem cells in a quiescent state to preserve them with age and (2) expanding HSCs in times of crisis, such as post transplant. While these two roles are in opposition, in both states TPO regulates cell cycle transition. The microenvironment of HSCs is also important for regulating the balance between quiescence and proliferation, with stem cells residing in a specialized niche in the bone marrow.30–32 Interactions between TPO and signals from the niche could be responsible for balancing TPO's dual stem cell roles. Long-term HSCs, which are Mpl+, are closely associated with TPO-producing osteoblasts in the niche.16 TPO stimulates the expression of Tie2 on HSCs, which is the receptor for angiopoietin-1 (Ang-1), and helps keep HSCs adhered to the osteoblastic niche.33 This suggests that TPO is actively involved in maintaining the association of HSCs with the niche.
It was thought that TPO's role in the regulation of HSCs only began postnatally, because Mpl-/- and TPO-/- fetal livers were found to have the same number of E12.5 progenitor cells as found in wild-type mice, with an equivalent ability to repopulate the bone marrow with E14.5 fetal liver cells after transplant.5,15 However, Mpl mRNA expression can be detected in the AGM, fetal liver and yolk sac as early as E10.5,34 and E14.5 AA4+ Sca-1+ Mpl+ fetal liver cells have superior repopulating ability compared to AA4+ Sca-1+ Mpl− fetal liver cells.35 Loss of TPO signaling may cause a developmental delay in hematopoiesis, as Mpl-/- E11.5 fetal livers were unable to reconstitute mice, even in larger numbers than wild-type cells.34 Transplants using limited numbers of Lin− Sca-1+ AA4+ fetal liver cells identified defects in the repopulation of ability of Mpl-/- cells at both E12.5 and E14.5, but transplants using unfractionated E12.5 and E14.5 fetal livers showed similar ability of wild-type and Mpl-/- cells to reconstitute irradiated animals.34 Secondary transplants of these cells resulted in lower reconstitution by the Mpl-/- cells, but it was unclear if this was due to an intrinsic defect of the cells, or if this was due to continued lack of TPO signaling compared to the wild-type cells. Transplants using TPO-/- mice as recipients might be useful to investigate this in an equivalent TPO-null environment for both genotypes.
Use of TPO in Ex Vivo HSC Culture
HSC transplantation is an important therapeutic procedure, but its application is often restricted by difficulties in ex vivo expansion and maintenance of HSCs.36,37 Similar to its ability to expand HSCs after transplantation, TPO can augment ex vivo expansion of HSCs to increase the pool available for transplantation, but it is far more effective when used in combination with other cytokines.38 Most cytokine combinations proposed include stem cell factor (SCF), fms-like tyrosine kinase 3 ligand (FL) and TPO.39,40 A nonpeptidyl small molecle agonist of MPL, NR-101, was found to be more efficient than TPO in expanding HSCs.41 Interestingly, this effect seemed to be specific to HSCs, as it was not more efficient than TPO in inducing megakaryocyte expansion. Further investigation into its downstream signaling could help elucidate the pathways that TPO regulates in HSCs as opposed to megakaryocytes.
Regulation of TPO Levels
In order to produce platelets when required, TPO concentration is linked to platelet levels. This was first noted when elevated TPO levels were observed in thrombocytopenic animals.42 Similar high levels of TPO were also observed in response to chemotherapy-induced thrombocytopenia.43 Further studies showed that TPO levels are inversely proportional to platelet mass, which suggested that there was a feedback mechanism that detected decreased platelet mass and caused a subsequent increase in circulating TPO levels.44
TPO is predominately produced by the liver45 but is also produced by the kidney, spleen and stromal cells in the bone marrow.45–47 Because levels of erythropoietin (EPO), the cytokine most closely related to TPO, had been found to be regulated at a transcriptional level in response to anemia,48–50 it was expected that levels of TPO transcription would be regulated by a similar mechanism. Surprisingly, it was found that the kidney and liver produce thrombopoietin constitutively, with their TPO mRNA levels unresponsive to thrombocytopenia,51 and that platelets were able to absorb TPO from TPO-conditioned medium in a dose-dependent manner.51
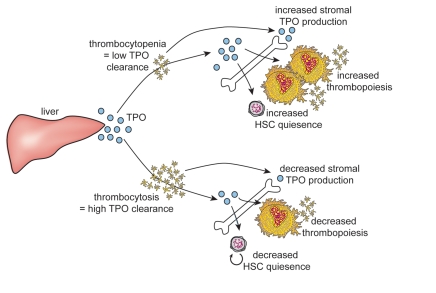
This led to the discovery that TPO is removed from circulation primarily by binding to Mpl. Platelets and megakaryocytes account for the bulk of Mpl receptors.1,35,52,53 In situations such as thrombocytopenia, there are insufficient platelets to remove excess TPO from the circulation (Fig. 1). The high levels of TPO remaining result in the stimulation of megakaryocyte and platelet production. This mechanism of control allows TPO levels to be directly regulated by available platelet mass. Supporting this observation is the high level of TPO found in Mpl-/- mice where TPO is unable to be receptor-internalized and the thrombocytosis observed in Mpl-/- mice with partial transgene rescue of Mpl expression, where the low levels of Mpl allow for increased circulating TPO.54 In contrast, despite the high levels of circulating TPO seen in these mice, the procedure only resulted in partial restoration of ability of Mpl-/- HSCs to repopulate the bone marrow after transplantation. This may be because TPO signaling in HSCs requires a high level of Mpl receptor expression, making these cells relatively less responsive to high levels of circulating TPO.
Figure 1.
TPO levels are regulated by platelet mass. The liver constitutively produces TPO. Upon binding to Mpl receptors on platelets, TPO is internalized and removed from circulation. A smaller proportion of total TPO is produced by stromal cells in the bone marrow and their TPO mRNA production is sensitive to factors produced by platelets such as PF4 and thus also linked to platelet number. TPO then stimulates thrombopoiesis and HSC quiescence in the bone marrow. In the case of thrombocytosis, much TPO is removed from circulation, resulting in low levels of TPO signaling, while during thrombocytopenia, little TPO is cleared from circulation, resulting in high levels of TPO signaling. This feedback system allows thrombopoiesis to be regulated by the available platelet mass but also links HSC quiescence to platelet numbers.
Further details of this pathway have recently been described. Upon Mpl stimulation with TPO, the receptor complex rapidly undergoes clearance from the circulation by clatharin-dependent endocytosis.55 Mpl is then ubiquinated and degraded by Cbl through its E3 ubiquitin ligase activity.56 Cbl's important role as a negative regulator of TPO signaling is shown, because it is one of the few negative regulators that has a significant effect on steady state megakaryopoiesis; Cbl-/- mice have a thrombocytosis and excessive numbers of splenic megakaryocytes.57 Not all Mpl significantly contributes to clearance of TPO. Transplantation of wild-type bone marrow into Mpl-/- mice created a mouse with Mpl expressed on its hematopoietic system but not on endothelial cells, showing that endothelial cell Mpl does not measurably affect TPO levels, despite such cells having relatively high numbers of receptors.53 Thus, the presence of Mpl does not ensure adequate thrombopoietin regulation, and further work is needed to see if endothelial cells might lack crucial components of the TPO degradation pathway.
Changes in TPO produced by stromal cells in the marrow appear to be an additional variable in the overall production of TPO. TPO mRNA in the bone marrow can increase in response to thrombocytopenia and experimental manipulation.46,58 Several platelet specific granule proteins, including platelet factor 4 (Pf4), transforming growth factor (TGF)β and thrombospondin (Tsp), can suppress the production of TPO mRNA in stromal cells.46 Paracrine hormone production can have a stronger effect on local cells than endocrine production by the liver, so potentially this mechanism may have significant effects on those HSCs and megakaryocytes that interact directly with the stroma. Transplantation of Tpo-/- liver cells into wildtype mice resulted in fewer than half the platelet numbers seen after wildtype liver cell transplants into wildtype mice.45 While no data on HSCs were reported, this would be a useful model to measure how the relative importance of TPO produced by the liver compared to TPO produced locally by endothelial cells for the regulation of HSCs.
The regulation of circulating TPO levels appears to be primarily driven by platelets and megakaryocytes, as these cells have the majority of expressed Mpl in the body. However, TPO is also important for maintenance of the function of HSCs. Non physiological numbers of platelets may result in HSC perturbation. Our group has investigated this in MybPlt4/Plt4 mice, which have a mutation in the transcription factor Myb, which results in several hematopoietic perturbations, including severe thrombocytosis.59 This thrombocytosis is independent of TPO signaling, and the high numbers of platelets remove much of the TPO from the system. This means the HSCs are exposed to sub physiological levels of TPO.60 There are several phenotypes characteristic of TPO starvation of HSCs in these mice,15,16 such as increased cycling of HSCs and loss of LT-HSCs with age.60 There is a TPO response signature common between Mpl-/- and MybPlt4/Plt4 mice containing genes downregulated in the absence of TPO, suggesting that genes such as Ndn,61 Cdkn1c62 and Maged163 could be involved in maintaining the balance between HSC quiescence and activity regulated by TPO. In support of this regulatory pathway, changes in HSC Cdkn1c expression have independently been associated with administration of TPO in mice.16
This suggests that there is a feedback loop in which platelet numbers, through regulation of available TPO levels, regulate the entry of HSCs into cycle. Such a feedback mechanism may provide protection in times of hematopoietic stress associated with bleeding. After the loss of platelets both via both bleeding and wound healing, the resulting increase in TPO levels will drive production of megakaryocytes and platelets. However, as this stress on the hematopoietic system runs the risk of exhausting the HSC compartment and compromising future hematopoietic production, the action of TPO to promote the quiescence of the most primitive stem cells may represent the mechanism by which acute demand for new blood cells can be met without compromising long term production.
Signaling Targets
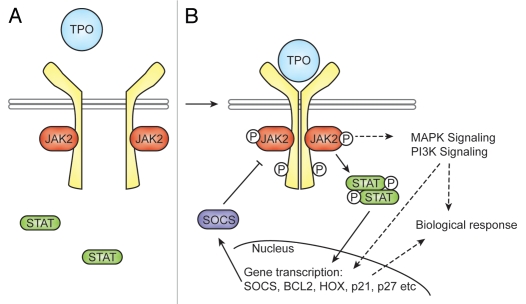
Mpl, the receptor for TPO, is a member of the hematopoietic cytokine receptor superfamily.64 The binding of TPO to Mpl, induces the dimerization of Mpl.65 This allows the associated Janus Kinase 2 (Jak2) molecules to transphosphorylate, leading to their activation65–67 (Fig. 2). Demonstrating that Jak2 is vital for TPO signaling, Jak2-/- fetal liver cells are unable to produce megakaryocytes in response to TPO.68 Phosphorylation of another Janus family protein kinase, Tyk2, also occurs upon TPO binding, but unlike Jak2, has not been shown to be essential for TPO signaling.66,69 Activated Jak2 then phosphorylates the distal portion of Mpl and leads to the recruitment of signaling proteins to the receptor via their SH2 domains.70,71 This includes the Stat (Signal Tranducers and Activators of Transcription) family of latent transcription factors (reviewed in ref. 71). Under basal conditions, Stats are found in the cytoplasm and are unable to bind DNA.72 Tyrosine phosphorylation of the Stats by Janus kinases causes dimerization, allowing translocation to the nucleus and binding to specific promoter regions.73 Stat3 and Stat5 are phosphorylated in response to TPO.74,75 Among the targets that Stats induce in response to TPO signaling are genes involved in proliferation and survival, such as cyclin D1, p27, p21 and Bcl-xL.76–78 STAT proteins also contribute to the negative regulation of TPO signaling by upregulating expression of members of the SOCS (suppressors of cytokine signaling) family.79 SOCS proteins can then directly bind with their SH2 domains to phosphotyrosine residues in cytokine receptors and JAKs to inhibit their tyrosine kinase activity. While functional redundancy between the eight members of the SOCS family makes it difficult to ascribe precise roles to them all, TPO has been shown to induce the expression of CIS80 and be inhibited by SOCS179,81 and SOCS3.82 Potentially, other SOCS proteins also act in the TPO signaling pathway.
Figure 2.
TPO signaling pathway. In unstimulated cells (A), Mpl (in yellow) exists as a monomer, and signaling molecules, such as JAK2 and STATs, are inactive. Upon TPO binding (B), Mpl dimerizes. This conformational change results in phosphorylation of its associated JAK2 molecules, which leads to phosphorylation of Mpl and activation of downstream signaling pathways, including STATs, the MAPK pathway and the PI3K pathway. JAK2 can phosphorylate STAT3 and STAT5 in response to TPO signaling, which results in their dimerization and translocation to the nucleus. There transcription is initiated, and the integration of these pathways leads to the downstream effect of TPO. STATs also induce transcription of SOCS proteins, which are generally not expressed, resulting in negative feedback and downregulation of JAK2 and Mpl activation.
Another negative regulator of TPO signaling is Lnk. The Lnk-/- HSC pool has been shown to continue to expand postnatally, and this expansion is dependent on TPO signaling, as Lnk-/- TPO-/- mice have a similar reduction in HSCs as TPO-/- mice.52 Lnk-/- HSCs also have decelerated cell cycle kinetics and an increased quiescent fraction,83 perhaps because the larger HSC pool means that a smaller proportion of the HSCs are required to undergo proliferation in order to maintain hematopoiesis. Lnk mediates its affect through Jak2, binding directly to phosphorylated tyrosine residues in Jak2 following TPO stimulation to attenuate Jak2 signaling.83
In addition to the JAK/STAT pathway, TPO signaling activates the phosphatidylinositol-3-kinase (PI3K) pathway and the mitogen activated protein kinase (MAPK) pathways. In the PI3K pathway, TPO activates PI3K, which leads to the phosphorylation and activation of its downstream effector, AKT.84 This activates the transcription factor FOXO3a, which then induces p27Kip1 expression.85 TPO stimulation of the PI3K/AKT pathway is required for cell cycle progression in megakaryocyte progenitors.84,85 It is unclear whether TPO stimulation of this pathway plays a similar role in HSC functions. The MAPK pathway involves a protein kinase cascade, with two of the MAPKs, extracellular signal-related protein kinase 1 (ERK1) and ERK2, having been shown to be involved in TPO signaling.86–88 Thrombopoietin has been shown to activate this pathway in human CD34+ cells, a population that includes HSCs, as well as in megakaryocytes and platelets.89 MAPK signaling is required for TPO induced Hox activation.26
While few studies have attempted to distinguish TPO's signaling pathway in HSCs from that in megakaryocytes, one study has provided insight into the function of the Mpl receptor in HSCs. The distal region of Mpl receptor contains three major signaling tyrosines. A truncated Mpl receptor lacking the last 60 amino acids, Δ60, removes this region but retains the domains required for Jak2 activation.90 Bone marrow from the Δ60 mouse line was found to produce normal numbers of megakaryocytes, albeit with a reduced ploidy, and these mice had compromised long-term stem cell activity, as observed after serial transplantation.90,91 This was a far milder reduction in stem cell activity than was observed in Mpl-/- mice, suggesting that the retained Box1 and Box2 motifs, required to activate Jak2, are the most vital parts of Mpl for its HSC activity. The distal region acts to activate Stat, Akt and Mapk, but perhaps the residual levels of activation that occur without it are sufficient to maintain HSC function, except in stress situations.
Signaling in Myeloproliferative Disorders
Regulation of signaling pathways is vital for the healthy function of hematopoietic progenitors. It was recently found that constitutive activation mutations in both Mpl and Jak2 result in myeloproliferative disorder (MPD). MPLW515L and MPLW515K mutations are found in 1% of essential thrombocythaemia (ET) cases and 5% of primary myelofibrosis (PMF).92–95 These mutations result in constitutive cytokine-independent activation of the JAK-STAT pathway.93 Activating Jak2 mutations are even more prevalent in MPD with 95% of polycythaemia vera (PV) cases and 50% of ET and PMF cases having the mutation JAK2V617F.96–98
When mice were transplanted with bone marrow transduced with MPLW515L mutation, they quickly developed a lethal PMF-like disease with thrombocytosis and leucocytosis.93 Expression of MPLW515L in cell lines resulted in cytokine-independent growth and the constitutive activation of JAK, STAT3, STAT5, AKT and ERK.93,99 These cells were also hyperresponsive to TPO, with increased phosphorylation of signaling molecules and increased proliferation in response to TPO.93 MPLW515 mutations affect cell cycle transition, promoting G1-S-phase transition in the absence of TPO.99
Investigation of the clonal consequences of the MPLW515 mutations in patients with MPD has found that these mutations involve early stem cell-derived events that can be detected in hematopoietic lineages from myeloid to B and T cells, and thus involve both myeloid and lymphoid progenitors.100,101 They have been found to be associated with higher platelet counts and lower hemoglobin levels than in MPB patients with the JAK2V617F mutation.94,95 Despite the pathogenic activity of the mutation, when tested in cell lines and in transplant studies, the presence of MPLW515 mutations in MPD patients provides no prognostic information for survival, thrombosis or myelofibrotic transformation.94
Before these mutations had been identified, it was observed that Mpl was downregulated on platelets from MPD patients.102 Recent investigation has found that Mpl expression on platelets is negatively correlated with JAK2V617F allele burden.103 MiR-28, a microRNA that targets Mpl, appears to play a role in this downregulation, with overexpression of JAK2V617F leading to induction of MiR-28 expression.104 It is unclear if this contributes to the pathology of the disease, or if it is a negative feedback mechanism resulting from the constitutive activation of the JAK-STAT pathway.
Summary and Future Prospects
Since its isolation, thrombopoietin has been of tremendous clinical interest due to its role as a potent stimulator of thrombopoiesis. First generation recombinant forms of TPO were developed over a decade ago and increased platelet counts in patients with a range of thrombocytopenias and in healthy platelet donors.105–108 After this promising beginning, its use was discontinued because some subjects developed autoantibody formation with cross reactivity to endogenous TPO.109,110 The second generation of thrombopoietic agents has been developed using techniques such as TPO peptides mimetics, TPO non-peptide mimetics and Mpl antibodies.111 Two of these, Romiplostin, a TPO peptide mimetic, and Eltombopag, a non-peptide TPO mimentic, have been successfully used for the treatment of immune thrombocytopenic purpura (ITP) and are now approved by the US Food and Drug Administration.112–114 The use of TPO and its mimetics to regulate HSCs is less advanced, but TPO signaling in HSCs may be as clinically relevant as in megakaryocytes, with a role in HSC expansion both before and after transplantation. Future work will help clarify which effects of TPO are shared by both HSCs and megakaryocytes and which are specific to each cell type.
Acknowledgements
Our work was supported by grants from the National Health and Medical Research Council and the National Institutes of Health (CA22556). C.d.G. was the recipient of the Sydney Parker Smith Postdoctoral Research Fellowship from the Cancer Council, Victoria, and D.M. is supported by the Carden Fellowship Fund of the Cancer Council, Victoria.
References
- 1.Debili N, Wendling F, Cosman D, Titeux M, Florindo C, Dusanter-Fourt I, et al. The Mpl receptor is expressed in the megakaryocytic lineage from late progenitors to platelets. Blood. 1995;85:391–401. [PubMed] [Google Scholar]
- 2.Forsberg EC, Prohaska SS, Katzman S, Heffner GC, Stuart JM, Weissman IL. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1:28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Methia N, Louache F, Vainchenker W, Wendling F. Oligodeoxynucleotides antisense to the proto-oncogene c-mpl specifically inhibit in vitro megakaryocytopoiesis. Blood. 1993;82:1395–1401. [PubMed] [Google Scholar]
- 4.Gurney AL, Carver-Moore K, de Sauvage FJ, Moore MW. Thrombocytopenia in c-mpl-deficient mice. Science. 1994;265:1445–1447. doi: 10.1126/science.8073287. [DOI] [PubMed] [Google Scholar]
- 5.Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood. 1996;87:2162–2170. [PubMed] [Google Scholar]
- 6.de Sauvage FJ, Carver-Moore K, Luoh SM, Ryan A, Dowd M, Eaton DL, et al. Physiological regulation of early and late stages of megakaryocytopoiesis by thrombopoietin. J Exp Med. 1996;183:651–656. doi: 10.1084/jem.183.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaushansky K, Lok S, Holly RD, Broudy VC, Lin N, Bailey MC, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994;369:568–571. doi: 10.1038/369568a0. [DOI] [PubMed] [Google Scholar]
- 8.Ito T, Ishida Y, Kashiwagi R, Kuriya S. Recombinant human c-Mpl ligand is not a direct stimulator of proplatelet formation in mature human megakaryocytes. Br J Haematol. 1996;94:387–390. doi: 10.1046/j.1365-2141.1996.d01-1813.x. [DOI] [PubMed] [Google Scholar]
- 9.Bunting S, Widmer R, Lipari T, Rangell L, Steinmetz H, Carver-Moore K, et al. Normal platelets and megakaryocytes are produced in vivo in the absence of thrombopoietin. Blood. 1997;90:3423–3429. [PubMed] [Google Scholar]
- 10.Carver-Moore K, Broxmeyer HE, Luoh SM, Cooper S, Peng J, Burstein SA, et al. Low levels of erythroid and myeloid progenitors in thrombopoietin-and c-mpl-deficient mice. Blood. 1996;88:803–808. [PubMed] [Google Scholar]
- 11.Kimura S, Roberts AW, Metcalf D, Alexander WS. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci USA. 1998;95:1195–1200. doi: 10.1073/pnas.95.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abkowitz JL, Chen J. Studies of c-Mpl function distinguish the replication of hematopoietic stem cells from the expansion of differentiating clones. Blood. 2007;109:5186–5190. doi: 10.1182/blood-2006-08-044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox N, Priestley G, Papayannopoulou T, Kaushansky K. Thrombopoietin expands hematopoietic stem cells after transplantation. J Clin Invest. 2002;110:389–394. doi: 10.1172/JCI15430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heckl D, Wicke DC, Brugman MH, Meyer J, Schambach A, Büsche G, et al. Lentiviral gene transfer regenerates hematopoietic stem cells in a mouse model for Mpl-deficient aplastic anemia. Blood. 2011;117:3737–3747. doi: 10.1182/blood-2010-09-308262. [DOI] [PubMed] [Google Scholar]
- 15.Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Månsson R, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1:671–684. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci USA. 1997;94:1908–1913. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright DE, Cheshier SH, Wagers AJ, Randall TD, Christensen JL, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor causes selective mobilization of bone marrow hematopoietic stem cells into the blood after M phase of the cell cycle. Blood. 2001;97:2278–2285. doi: 10.1182/blood.v97.8.2278. [DOI] [PubMed] [Google Scholar]
- 19.Neben S, Marcus K, Mauch P. Mobilization of hematopoietic stem and progenitor cell subpopulations from the marrow to the blood of mice following cyclophosphamide and/or granulocyte colony-stimulating factor. Blood. 1993;81:1960–1967. [PubMed] [Google Scholar]
- 20.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 21.Passegué E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202:1599–1611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mascarenhas MI, Parker A, Dzierzak E, Ottersbach K. Identification of novel regulators of hematopoietic stem cell development through refinement of stem cell localization and expression profiling. Blood. 2009;114:4645–4653. doi: 10.1182/blood-2009-06-230037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thorsteinsdottir U, Sauvageau G, Humphries RK. Enhanced in vivo regenerative potential of HOXB4-transduced hematopoietic stem cells with regulation of their pool size. Blood. 1999;94:2605–2612. [PubMed] [Google Scholar]
- 24.Lawrence HJ, Helgason CD, Sauvageau G, Fong S, Izon DJ, Humphries RK, et al. Mice bearing a targeted interruption of the homeobox gene HOXA9 have defects in myeloid, erythroid and lymphoid hematopoiesis. Blood. 1997;89:1922–1930. [PubMed] [Google Scholar]
- 25.Kirito K, Fox N, Kaushansky K. Thrombopoietin stimulates Hoxb4 expression: an explanation for the favorable effects of TPO on hematopoietic stem cells. Blood. 2003;102:3172–3178. doi: 10.1182/blood-2003-03-0944. [DOI] [PubMed] [Google Scholar]
- 26.Kirito K, Fox N, Kaushansky K. Thrombopoietin induces HOXA9 nuclear transport in immature hematopoietic cells: Potential mechanism by which the hormone favorably affects hematopoietic stem cells. Mol Cell Biol. 2004;24:6751–6762. doi: 10.1128/MCB.24.15.6751-6762.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballmaier M, Germeshausen M, Schulze H, Cherkaoui K, Lang S, Gaudig A, et al. c-mpl mutations are the cause of congenital amegakaryocytic thrombocytopenia. Blood. 2001;97:139–146. doi: 10.1182/blood.v97.1.139. [DOI] [PubMed] [Google Scholar]
- 28.Ihara K, Ishii E, Eguchi M, Takada H, Suminoe A, Good RA, et al. Identification of mutations in the c-mpl gene in congenital amegakaryocytic thrombocytopenia. Proc Natl Acad Sci USA. 1999;96:3132–3136. doi: 10.1073/pnas.96.6.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ballmaier M, Germeshausen M, Krukemeier S, Welte K. Thrombopoietin is essential for the maintenance of normal hematopoiesis in humans: Development of aplastic anemia in patients with congenital amegakaryocytic thrombocytopenia. Ann NY Acad Sci. 2003;996:17–25. doi: 10.1111/j.1749-6632.2003.tb03228.x. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Xie T. Stem cell niche: Structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 31.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 32.Wilson A, Laurenti E, Trumpp A. Balancing dormant and self-renewing hematopoietic stem cells. Curr Opin Genet Dev. 2009;19:461–468. doi: 10.1016/j.gde.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Petit-Cocault L, Volle-Challier C, Fleury M, Peault B, Souyri M. Dual role of Mpl receptor during the establishment of definitive hematopoiesis. Development. 2007;134:3031–3040. doi: 10.1242/dev.001818. [DOI] [PubMed] [Google Scholar]
- 35.Solar GP, Kerr WG, Zeigler FC, Hess D, Donahue C, de Sauvage FJ, et al. Role of c-mpl in early hematopoiesis. Blood. 1998;92:4–10. [PubMed] [Google Scholar]
- 36.Sorrentino BP. Clinical strategies for expansion of haematopoietic stem cells. Nat Rev Immunol. 2004;4:878–888. doi: 10.1038/nri1487. [DOI] [PubMed] [Google Scholar]
- 37.Piacibello W, Sanavio F, Garetto L, Severino A, Bergandi D, Ferrario J, et al. Extensive amplification and self-renewal of human primitive hematopoietic stem cells from cord blood. Blood. 1997;89:2644–2653. [PubMed] [Google Scholar]
- 38.Sitnicka E, Lin N, Priestley GV, Fox N, Broudy VC, Wolf NS, et al. The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood. 1996;87:4998–5005. [PubMed] [Google Scholar]
- 39.Ohmizono Y, Sakabe H, Kimura T, Tanimukai S, Matsumura T, Miyazaki H, et al. Thrombopoietin augments ex vivo expansion of human cord blood-derived hematopoietic progenitors in combination with stem cell factor and flt3 ligand. Leukemia. 1997;11:524–530. doi: 10.1038/sj.leu.2400588. [DOI] [PubMed] [Google Scholar]
- 40.Pineault N, Cortin V, Boyer L, Garnier A, Robert A, Thérien C, et al. Individual and synergistic cytokine effects controlling the expansion of cord blood CD34(+) cells and megakaryocyte progenitors in culture. Cytotherapy. 2010;13:467–480. doi: 10.3109/14653249.2010.530651. [DOI] [PubMed] [Google Scholar]
- 41.Nishino T, Miyaji K, Ishiwata N, Arai K, Yui M, Asai Y, et al. Ex vivo expansion of human hematopoietic stem cells by a small-molecule agonist of c-MPL. Exp Hematol. 2009;37:1364–1377. doi: 10.1016/j.exphem.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Kuter DJ, Beeler DL, Rosenberg RD. The purification of megapoietin: a physiological regulator of megakaryocyte growth and platelet production. Proc Natl Acad Sci USA. 1994;91:11104–11108. doi: 10.1073/pnas.91.23.11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shinjo K, Takeshita A, Nakamura S, Naitoh K, Yanagi M, Tobita T, et al. Serum thrombopoietin levels in patients correlate inversely with platelet counts during chemotherapy-induced thrombocytopenia. Leukemia. 1998;12:295–300. doi: 10.1038/sj.leu.2400946. [DOI] [PubMed] [Google Scholar]
- 44.Kuter DJ, Rosenberg RD. The reciprocal relationship of thrombopoietin (c-Mpl ligand) to changes in the platelet mass during busulfan-induced thrombocytopenia in the rabbit. Blood. 1995;85:2720–2730. [PubMed] [Google Scholar]
- 45.Qian S, Fu F, Li W, Chen Q, de Sauvage FJ. Primary role of the liver in thrombopoietin production shown by tissue-specific knockout. Blood. 1998;92:2189–2191. [PubMed] [Google Scholar]
- 46.Sungaran R, Chisholm OT, Markovic B, Khachigian LM, Tanaka Y, Chong BH. The role of platelet alpha-granular proteins in the regulation of thrombopoietin messenger RNA expression in human bone marrow stromal cells. Blood. 2000;95:3094–3101. [PubMed] [Google Scholar]
- 47.Lok S, Kaushansky K, Holly RD, Kuijper JL, Lofton-Day CE, Oort PJ, et al. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature. 1994;369:565–568. doi: 10.1038/369565a0. [DOI] [PubMed] [Google Scholar]
- 48.Goldberg MA, Gaut CC, Bunn HF. Erythropoietin mRNA levels are governed by both the rate of gene transcription and posttranscriptional events. Blood. 1991;77:271–277. [PubMed] [Google Scholar]
- 49.Beru N, McDonald J, Lacombe C, Goldwasser E. Expression of the erythropoietin gene. Mol Cell Biol. 1986;6:2571–2575. doi: 10.1128/mcb.6.7.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bondurant MC, Koury MJ. Anemia induces accumulation of erythropoietin mRNA in the kidney and liver. Mol Cell Biol. 1986;6:2731–2733. doi: 10.1128/mcb.6.7.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoffel R, Wiestner A, Skoda RC. Thrombopoietin in thrombocytopenic mice: Evidence against regulation at the mRNA level and for a direct regulatory role of platelets. Blood. 1996;87:567–573. [PubMed] [Google Scholar]
- 52.Buza-Vidas N, Antonchuk J, Qian H, Månsson R, Luc S, Zandi S, et al. Cytokines regulate postnatal hematopoietic stem cell expansion: Opposing roles of thrombopoietin and LNK. Genes Dev. 2006;20:2018–2023. doi: 10.1101/gad.385606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geddis AE, Fox NE, Kaushansky K. The Mpl receptor expressed on endothelial cells does not contribute significantly to the regulation of circulating thrombopoietin levels. Exp Hematol. 2006;34:82–86. doi: 10.1016/j.exphem.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Lannutti BJ, Epp A, Roy J, Chen J, Josephson NC. Incomplete restoration of Mpl expression in the mpl-/-mouse produces partial correction of the stem cell-repopulating defect and paradoxical thrombocytosis. Blood. 2009;113:1778–1785. doi: 10.1182/blood-2007-11-124859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hitchcock IS, Chen MM, King JR, Kaushansky K. YRRL motifs in the cytoplasmic domain of the thrombopoietin receptor regulate receptor internalization and degradation. Blood. 2008;112:2222–2231. doi: 10.1182/blood-2008-01-134049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saur SJ, Sangkhae V, Geddis AE, Kaushansky K, Hitchcock IS. Ubiquitination and degradation of the thrombopoietin receptor c-Mpl. Blood. 2009;115:1254–1263. doi: 10.1182/blood-2009-06-227033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy MA, Schnall RG, Venter DJ, Barnett L, Bertoncello I, Thien CB, et al. Tissue hyperplasia and enhanced T-cell signalling via ZAP-70 in c-Cbl-deficient mice. Mol Cell Biol. 1998;18:4872–4882. doi: 10.1128/mcb.18.8.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCarty JM, Sprugel KH, Fox NE, Sabath DE, Kaushansky K. Murine thrombopoietin mRNA levels are modulated by platelet count. Blood. 1995;86:3668–3675. [PubMed] [Google Scholar]
- 59.Carpinelli MR, Hilton DJ, Metcalf D, Antonchuk JL, Hyland CD, Mifsud SL, et al. Suppressor screen in Mpl-/- mice: c-Myb mutation causes supraphysiological production of platelets in the absence of thrombopoietin signaling. Proc Natl Acad Sci USA. 2004;101:6553–6558. doi: 10.1073/pnas.0401496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Graaf CA, Kauppi M, Baldwin T, D Hyland C, Metcalf D, Willson TA, et al. Regulation of hematopoietic stem cells by their mature progeny. Proc Natl Acad Sci USA. 2010;107:21689–21694. doi: 10.1073/pnas.1016166108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kubota Y, Osawa M, Jakt LM, Yoshikawa K, Nishikawa S. Necdin restricts proliferation of hematopoietic stem cells during hematopoietic regeneration. Blood. 2009;114:4383–4392. doi: 10.1182/blood-2009-07-230292. [DOI] [PubMed] [Google Scholar]
- 62.Umemoto T, Yamato M, Nishida K, Yang J, Tano Y, Okano T. p57Kip2 is expressed in quiescent mouse bone marrow side population cells. Biochem Biophys Res Commun. 2005;337:14–21. doi: 10.1016/j.bbrc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 63.Du Q, Zhang Y, Tian XX, Li Y, Fang WG. MAGE-D1 inhibits proliferation, migration and invasion of human breast cancer cells. Oncol Rep. 2009;22:659–665. doi: 10.3892/or_00000486. [DOI] [PubMed] [Google Scholar]
- 64.Vigon I, Mornon JP, Cocault L, Mitjavila MT, Tambourin P, Gisselbrecht S, et al. Molecular cloning and characterization of MPL, the human homolog of the v-mpl oncogene: Identification of a member of the hematopoietic growth factor receptor superfamily. Proc Natl Acad Sci USA. 1992;89:5640–5644. doi: 10.1073/pnas.89.12.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tortolani PJ, Johnston JA, Bacon CM, McVicar DW, Shimosaka A, Linnekin D, et al. Thrombopoietin induces tyrosine phosphorylation and activation of the Janus kinase, JAK2. Blood. 1995;85:3444–3451. [PubMed] [Google Scholar]
- 66.Sattler M, Durstin MA, Frank DA, Okuda K, Kaushansky K, Salgia R, et al. The thrombopoietin receptor c-MPL activates JAK2 and TYK2 tyrosine kinases. Exp Hematol. 1995;23:1040–1048. [PubMed] [Google Scholar]
- 67.Drachman JG, Griffin JD, Kaushansky K. The c-Mpl ligand (thrombopoietin) stimulates tyrosine phosphorylation of Jak2, Shc and c-Mpl. J Biol Chem. 1995;270:4979–4982. doi: 10.1074/jbc.270.10.4979. [DOI] [PubMed] [Google Scholar]
- 68.Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 69.Drachman JG, Millett KM, Kaushansky K. Thrombopoietin signal transduction requires functional JAK2, not TYK2. J Biol Chem. 1999;274:13480–13484. doi: 10.1074/jbc.274.19.13480. [DOI] [PubMed] [Google Scholar]
- 70.Drachman JG, Sabath DF, Fox NE, Kaushansky K. Thrombopoietin signal transduction in purified murine megakaryocytes. Blood. 1997;89:483–492. [PubMed] [Google Scholar]
- 71.Murray PJ. The JAK-STAT signaling pathway: Input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 72.Levy DE, Kessler DS, Pine R, Darnell J., Jr Cytoplasmic activation of ISGF3, the positive regulator of interferon-alpha-stimulated transcription, reconstituted in vitro. Genes Dev. 1989;3:1362–1371. doi: 10.1101/gad.3.9.1362. [DOI] [PubMed] [Google Scholar]
- 73.Li WX. Canonical and non-canonical JAK-STAT signaling. Trends Cell Biol. 2008;18:545–551. doi: 10.1016/j.tcb.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miyakawa Y, Oda A, Druker BJ, Miyazaki H, Handa M, Ohashi H, et al. Thrombopoietin induces tyrosine phosphorylation of Stat3 and Stat5 in human blood platelets. Blood. 1996;87:439–446. [PubMed] [Google Scholar]
- 75.Bacon CM, Tortolani PJ, Shimosaka A, Rees RC, Longo DL, O'Shea JJ. Thrombopoietin (TPO) induces tyrosine phosphorylation and activation of STAT5 and STAT3. FEBS Lett. 1995;370:63–68. doi: 10.1016/0014-5793(95)00796-c. [DOI] [PubMed] [Google Scholar]
- 76.de Groot RP, Raaijmakers JA, Lammers JW, Koenderman L. STAT5-Dependent CyclinD1 and Bcl-xL expression in Bcr-Abl-transformed cells. Mol Cell Biol Res Commun. 2000;3:299–305. doi: 10.1006/mcbr.2000.0231. [DOI] [PubMed] [Google Scholar]
- 77.Matsumura I, Ishikawa J, Nakajima K, Oritani K, Tomiyama Y, Miyagawa J, et al. Thrombopoietin-induced differentiation of a human megakaryoblastic leukemia cell line, CMK, involves transcriptional activation of p21(WAF1/Cip1) by STAT5. Mol Cell Biol. 1997;17:2933–2943. doi: 10.1128/mcb.17.5.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Koning JP, Ward AC, Caldenhoven E, de Groot RP, Lowenberg B, Touw IP. STAT3beta does not interfere with granulocyte colony-stimulating factor-induced neutrophilic differentiation. Hematol J. 2000;1:220–225. doi: 10.1038/sj.thj.6200041. [DOI] [PubMed] [Google Scholar]
- 79.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 80.Okabe S, Tauchi T, Morita H, Ohashi H, Yoshimura A, Ohyashiki K. Thrombopoietin induces an SH2-containing protein, CIS1, which binds to Mpl: Involvement of the ubiquitin proteosome pathway. Exp Hematol. 1999;27:1542–1547. doi: 10.1016/s0301-472x(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 81.Wang Q, Miyakawa Y, Fox N, Kaushansky K. Interferon-alpha directly represses megakaryopoiesis by inhibiting thrombopoietin-induced signaling through induction of SOCS-1. Blood. 2000;96:2093–2099. [PubMed] [Google Scholar]
- 82.Chaligné R, Tonetti C, Besancenot R, Marty C, Kiladjian JJ, Socié G, et al. SOCS3 inhibits TPO-stimulated, but not spontaneous, megakaryocytic growth in primary myelofibrosis. Leukemia. 2009;23:1186–1190. doi: 10.1038/leu.2009.22. [DOI] [PubMed] [Google Scholar]
- 83.Bersenev A, Wu C, Balcerek J, Tong W. Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2. J Clin Invest. 2008;118:2832–2844. doi: 10.1172/JCI35808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geddis AE, Fox NE, Kaushansky K. Phosphatidylinositol-3-kinase is necessary but not sufficient for thrombopoietin-induced proliferation in engineered Mpl-bearing cell lines as well as in primary megakaryocytic progenitors. J Biol Chem. 2001;276:34473–34479. doi: 10.1074/jbc.M105178200. [DOI] [PubMed] [Google Scholar]
- 85.Nakao T, Geddis AE, Fox NE, Kaushansky K. PI3K/Akt/FOXO3a pathway contributes to thrombopoietininduced proliferation of primary megakaryocytes in vitro and in vivo via modulation of p27(Kip1) Cell Cycle. 2008;7:257–266. doi: 10.4161/cc.7.2.5148. [DOI] [PubMed] [Google Scholar]
- 86.Rojnuckarin P, Drachman JG, Kaushansky K. Thrombopoietin-induced activation of the mitogen-activated protein kinase (MAPK) pathway in normal megakaryocytes: Role in endomitosis. Blood. 1999;94:1273–1282. [PubMed] [Google Scholar]
- 87.Nagata Y, Todokoro K. Thrombopoietin induces activation of at least two distinct signaling pathways. FEBS Lett. 1995;377:497–501. doi: 10.1016/0014-5793(95)01386-5. [DOI] [PubMed] [Google Scholar]
- 88.Yamada M, Komatsu N, Okada K, Kato T, Miyazaki H, Miura Y. Thrombopoietin induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in a human thrombopoietin-dependent cell line. Biochem Biophys Res Commun. 1995;217:230–237. doi: 10.1006/bbrc.1995.2768. [DOI] [PubMed] [Google Scholar]
- 89.Majka M, Ratajczak J, Villaire G, Kubiczek K, Marquez LA, Janowska-Wieczorek A, et al. Thrombopoietin, but not cytokines binding to gp130 protein-coupled receptors, activates MAPKp42/44, AKT and STAT proteins in normal human CD34+ cells, megakaryocytes and platelets. Exp Hematol. 2002;30:751–760. doi: 10.1016/s0301-472x(02)00810-x. [DOI] [PubMed] [Google Scholar]
- 90.Luoh SM, Stefanich E, Solar G, Steinmetz H, Lipari T, Pestina TI, et al. Role of the distal half of the c-Mpl intracellular domain in control of platelet production by thrombopoietin in vivo. Mol Cell Biol. 2000;20:507–515. doi: 10.1128/mcb.20.2.507-515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tong W, Ibarra YM, Lodish HF. Signals emanating from the membrane proximal region of the thrombopoietin receptor (mpl) support hematopoietic stem cell self-renewal. Exp Hematol. 2007;35:1447–1455. doi: 10.1016/j.exphem.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M, et al. MPL515 mutations in myelo-proliferative and other myeloid disorders: A study of 1,182 patients. Blood. 2006;108:3472–3476. doi: 10.1182/blood-2006-04-018879. [DOI] [PubMed] [Google Scholar]
- 93.Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beer PA, Campbell PJ, Scott LM, Bench AJ, Erber WN, Bareford D, et al. MPL mutations in myeloproliferative disorders: Analysis of the PT-1 cohort. Blood. 2008;112:141–149. doi: 10.1182/blood-2008-01-131664. [DOI] [PubMed] [Google Scholar]
- 95.Vannucchi AM, Antonioli E, Guglielmelli P, Pancrazzi A, Guerini V, Barosi G, et al. Characteristics and clinical correlates of MPL 515W>L/K mutation in essential thrombocythemia. Blood. 2008;112:844–847. doi: 10.1182/blood-2008-01-135897. [DOI] [PubMed] [Google Scholar]
- 96.James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 97.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 98.Levine RL, Belisle C, Wadleigh M, Zahrieh D, Lee S, Chagnon P, et al. X-inactivation-based clonality analysis and quantitative JAK2V617F assessment reveal a strong association between clonality and JAK2V617F in PV but not ET/MMM, and identifies a subset of JAK2V617F-negative ET and MMM patients with clonal hematopoiesis. Blood. 2006;107:4139–4141. doi: 10.1182/blood-2005-09-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chaligné R, Tonetti C, Besancenot R, Roy L, Marty C, Mossuz P, et al. New mutations of MPL in primitive myelofibrosis: only the MPL W515 mutations promote a G1/S-phase transition. Leukemia. 2008;22:1557–1566. doi: 10.1038/leu.2008.137. [DOI] [PubMed] [Google Scholar]
- 100.Pardanani A, Lasho TL, Finke C, Mesa RA, Hogan WJ, Ketterling RP, et al. Extending Jak2V617F and MplW515 mutation analysis to single hematopoietic colonies and B and T lymphocytes. Stem Cells. 2007;25:2358–2362. doi: 10.1634/stemcells.2007-0175. [DOI] [PubMed] [Google Scholar]
- 101.Chaligné R, James C, Tonetti C, Besancenot R, Le Couédic JP, Fava F, et al. Evidence for MPL W515L/K mutations in hematopoietic stem cells in primitive myelofibrosis. Blood. 2007;110:3735–3743. doi: 10.1182/blood-2007-05-089003. [DOI] [PubMed] [Google Scholar]
- 102.Moliterno AR, Hankins WD, Spivak JL. Impaired expression of the thrombopoietin receptor by platelets from patients with polycythemia vera. N Engl J Med. 1998;338:572–580. doi: 10.1056/NEJM199802263380903. [DOI] [PubMed] [Google Scholar]
- 103.Moliterno AR, Williams DM, Rogers O, Spivak JL. Molecular mimicry in the chronic myeloproliferative disorders: Reciprocity between quantitative JAK2 V617F and Mpl expression. Blood. 2006;108:3913–3915. doi: 10.1182/blood-2006-03-008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Girardot M, Pecquet C, Boukour S, Knoops L, Ferrant A, Vainchenker W, et al. miR-28 is a thrombopoietin receptor targeting microRNA detected in a fraction of myeloproliferative neoplasm patient platelets. Blood. 2010;116:437–445. doi: 10.1182/blood-2008-06-165985. [DOI] [PubMed] [Google Scholar]
- 105.Basser RL, Rasko JE, Clarke K, Cebon J, Green MD, Hussein S, et al. Thrombopoietic effects of pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) in patients with advanced cancer. Lancet. 1996;348:1279–1281. doi: 10.1016/S0140-6736(96)04471-6. [DOI] [PubMed] [Google Scholar]
- 106.Fanucchi M, Glaspy J, Crawford J, Garst J, Figlin R, Sheridan W, et al. Effects of polyethylene glycol-conjugated recombinant human megakaryocyte growth and development factor on platelet counts after chemotherapy for lung cancer. N Engl J Med. 1997;336:404–409. doi: 10.1056/NEJM199702063360603. [DOI] [PubMed] [Google Scholar]
- 107.Nomura S, Dan K, Hotta T, Fujimura K, Ikeda Y. Effects of pegylated recombinant human megakaryocyte growth and development factor in patients with idiopathic thrombocytopenic purpura. Blood. 2002;100:728–730. doi: 10.1182/blood.v100.2.728. [DOI] [PubMed] [Google Scholar]
- 108.Kuter DJ, Goodnough LT, Romo J, DiPersio J, Peterson R, Tomita D, et al. Thrombopoietin therapy increases platelet yields in healthy platelet donors. Blood. 2001;98:1339–1345. doi: 10.1182/blood.v98.5.1339. [DOI] [PubMed] [Google Scholar]
- 109.Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98:3241–3248. doi: 10.1182/blood.v98.12.3241. [DOI] [PubMed] [Google Scholar]
- 110.Basser RL, O'Flaherty E, Green M, Edmonds M, Nichol J, Menchaca DM, et al. Development of pancytopenia with neutralizing antibodies to thrombopoietin after multicycle chemotherapy supported by megakaryocyte growth and development factor. Blood. 2002;99:2599–2602. doi: 10.1182/blood.v99.7.2599. [DOI] [PubMed] [Google Scholar]
- 111.Kuter DJ. New thrombopoietic growth factors. Clin Lymphoma Myeloma. 2009;9:347–356. doi: 10.3816/CLM.2009.s.034. [DOI] [PubMed] [Google Scholar]
- 112.Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): A 6-month, randomised, phase 3 study. Lancet. 2011;377:393–402. doi: 10.1016/S0140-6736(10)60959-2. [DOI] [PubMed] [Google Scholar]
- 113.Bussel JB, Kuter DJ, George JN, McMillan R, Aledort LM, Conklin GT, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006;355:1672–1681. doi: 10.1056/NEJMoa054626. [DOI] [PubMed] [Google Scholar]
- 114.Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: A double-blind randomised controlled trial. Lancet. 2008;371:395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]