Abstract
A multiple nucleopolyhedrovirus (MNPV) was isolated from Trichoplusia ni (Hübner) (Lepidoptera: Noctuidae) larvae that had been stung by the parasitoid Cotesia marginiventris (Cresson) (Hymenoptera: Braconidae). The wild type virus was plaque purified by infecting a Heliothis subflexa (BCIRL- HsAM1) cell line and isolating several clones. The mean estimated genomic size of this virus based on PstI, BstEII, StyI, HindIII restriction profiles was estimated to be 106 ± 2.5 kbp (mean±SE). A clone designated as TnMNPV/CmBCL9 was used in bioassays against several lepidopteran pests and in comparative studies with the baculoviruses AcMNPV, AgMNPV, AfMNPV, PxMNPV and HzSNPV of Autographa califomica, Anticarsia gemmatalis, Anagrapha falcifera, Plutella xylostella, and Helicoverpa zea, respectively. Infectivity studies showed that TnMNPV/CmBCL9 was highly infectious for Heliothis subflexa and T. ni, with an LC50 value 0.07 occlusion bodies/mm2 in both species and also infectious for H. zea and Heliothis virescens with LC50 values of 0.22 and 0.27 occlusion bodies/mm2, respectively. Restriction endonuclease analysis of the isolate and selected baculoviruses revealed profiles that were very similar to AfMNPV but different from the restriction endonuclease profiles of the other baculoviruses. Hybridization studies suggest that the TnMNPV/CmBCL9 was closely related to AfMNPV and AcMNPV-HPP. Further support for this comes from a phylogenetic analysis employing a split-graphs network, comparing the polh, egt, and p10 genes from TnMNPV/CmBCL9 with those from other baculoviruses and suggests that this virus is closely related to the AcMNPV variants, AfMNPV and RoMNPV of Rachiplusia ou.
Keywords : TnMNPV/CmBCL9, polh, egt, p10, AfMNPV, Anagrapha falcifera, split-graphs network
Introduction
Baculoviruses are double stranded DNA viruses belonging to the family Baculoviridae that infect members of the phylum Arthropoda, mainly insects from the order Lepidoptera, but also other insect orders including Hymenoptera, Diptera, Coleoptera, Neuroptera, Thysanura and Trichoptera. They have also been reported to occur in the order Decapoda (shrimp) (Tanada and Kaya 1993; Possee 1993; Murphy et al. 1995). Baculoviruses have been successfully used worldwide to control Lepidopteran and Hymenopteran insect pests of agriculture and forestry importance (Granados and Federici 1986; Miller 1997; Moscardi 1999) and thus help reduce the need for chemical insecticides.
The Baculoviridae family is comprised of two genera, the Nucleopolyhedrovirus and the Granulovirus (Murphy et al. 1995). Members of these genera, such as the multiple and single nucleopolyhedroviruses (MNPV, SNPV), and granuloviruses, have a unique biphasic replicative cycle in which budded virus is produced early in the infection, and later, when viral particles are produced, they become occluded into proteinacious occlusion bodies formerly referred to as polyhedral inclusion bodies. The budded virus is responsible for the systemic spread of the virus within the host and is the entity used for infecting cell culture. The occlusion bodies are the main means by which the virus is disseminated in the environment between susceptible larvae. This is achieved through cell lysis of infected larvae resulting in contamination of the leaf surfaces and subsequent consumption of leaf tissue by healthy larvae.
There are many reports on the association of insect viruses with parasitoid wasps belonging to the families Braconidae and Ichneumonidae (Stoltz and Vinson 1977, 1979; Stoltz and Faulkner 1978; Vinson and Iwantsch 1980 A; Vinson and Iwantsch 1980 B; Fleming et al. 1983; Styer et al. 1987; Strand and Pech 1995; Doucet and Cusson 1996; Ferrarese et al. 2005). Such association may be as a contaminant on the parasitoid, or the virus may be internalized in the host tissues as is the case with the polydnaviruses that are the most studied (Kroemer and Webb 2004; Webb and Strand 2005).
The objectives of the present report were to establish the identity of the baculovirus isolated from parasitized T. ni larvae, to determine the relationship of this isolate to other well known baculoviruses, and to attempt to determine the possible origin of the newly isolated baculovirus.
Materials and Methods
History of the parasitoid
The braconid parasitoid, Cotesia marginiventris was originally obtained from the USDA, ARS, Stoneville, MS facility where it was reared on Spodoptera exigua. At the time, no indication of a possible baculovirus infection in the colony was reported. After receivership the parasitoid was then initially reared on Spodoptera frugiperda larvae obtained periodically from the USDA, ARS, Starkville, MS with no observable baculovirus symptoms reported either from that facility nor later at our laboratory. The parasitoid was then reared on Tnchoplusia ni larvae available inhouse from our insectary and there was no report of an observed baculovirus infection in the T. ni colony subsequent to exposure to the parasitoid.
Recovery and propagation of a baculovirus from Cotesia marginiventris
In the course of immunological studies employing C. marginiventris, it was found that several T. ni larvae that were stung by this parasitoid displayed typical baculovirus symptoms resulting in lysis of the larvae. Examination by light microscopy of the liquid contents from T. ni cadavers revealed the presence of occlusion bodies. T. ni larvae displaying typical baculovirus infection were consistently observed on other occasions following parasitization. Occlusion bodies from collected dead larvae were fed to 3rd instar T. ni by topical application to a wheatsoy diet (Bio-Serv, www.bio-serv.com) surface in order to amplify occlusion bodies as well as serve as a source of infectious hemolymph for inoculation of cell cultures.
Determination of possible latent viral infection in T. ni larvae
To investigate the possibility that individuals in the T. ni colony might harbor TnMNPV/CmBCL9 as a latent virus, 35 early 3rd instar T. ni larvae from the laboratory colony were stressed by incubating them at 37°C for six days to monitor for any pathogenic signs of an infection that would indicate a possible latent virus.
Viral source originating from the adult parasitoid interior
To investigate a possible viral source originating internally from the parasitoid, ten C. marginiventris from an exteriorly washed group of 40 insects resulting in T. ni infection were macerated in 2 ml Hanks' Balanced Salt Solution (HBSS) (Sigma, Co., www.sigmaaldrich.com), spun at 10,000 rpm in a tabletop centrifuge for 5 min to remove insect debris and then passed through a 0.22 µn filter. 30 µl undiluted samples of this filtrate were added to each of 15 wells of a 50-well tray each containing artificial diet and a 2nd instar T. ni larva. An equivalent number of larvae were used as controls. They were then incubated at 28°C to monitor for larval pathogenicity. Another 30 µl sample of undiluted filtrate was also used to inoculate three T-25 cm2 flasks (5 ml) containing 1 × 105 cells/ml to determine possible budded virus presence in the parasitoid. Another flask containing the same TN-CLl cell concentration was mock infected to act as a control.
Viral source originating from surface contact with a contaminated adult parasitoid
The question of whether or not the virus could have been transmitted through surface contact with an exteriorly contaminated parasitoid was also investigated. Forty adult parasitoids were collected and initially stored at -80°C. One ml of HBSS was added to the sample and then stored at 4°C for several days. The intention was to have the solution gently remove any potential parasitoidsurface virus so that it could be used as inoculum for both in vitro and in vivo assays. For the in vitro assay, 1 ml inoculum sterilized through a 0.22 µm filter was added to a T-25 cm2 flask containing about 1 × 105 TN-CL1 cells/ ml. The inoculum was removed after 2 h and replaced with 5 ml ExCell-401 (10% FBS) medium and incubated at 28°C. Another flask containing the same TN-CL1 cell concentration was mock infected to act as a control. To test whether the virus was present as occlusion bodies attached to the parasitoid body surface, 30 µl of surfacewashed parasitoid solution was added to each of 15 wells of a 50-well tray each containing artificial diet and a 2nd instar T. ni larva. An equivalent number of larvae were used as controls. Trays were then incubated at 28°C to monitor for larval pathogenesis.
Plaque purification of wild type virus
Infectious hemolymph was collected from five 3rd instar T. ni larvae fed approximately 105 occlusion bodies and hemolymph collected on ice 48 h after exposure by snipping several prolegs. The infectious hemolymph was diluted at a ratio of 1:2 with ExCell 401 (SAFC Biosciences, www.sigmaaldrich.com/SAFC/Biosciences.htm1) and passed through a 0.45 µm millipore filter. A T-25 cm2 flask of Heliothis subflexa cells (BCIRL-HS-AM1, McIntosh 1991) at 4 × 105 cells/ml in 5 ml of ExCell 401 containing 10% inactivated fetal bovine serum and antibiotics (McIntosh et al. 2005) were inoculated with 0.5ml of the filtered infectious hemolymph and incubated at 28°C for 5 days. Supernatant fluid was recovered by centrifugation at 1500 × g for 10 min and the cell pellet containing occlusion bodies were re-suspended in 5 ml of purified water and stored at -20°C. The supernatant from the infected BCIRL-HS-AM1 cell line was used to plaque purify the virus as previously described (McIntosh et al. 1997) and clones isolated. Selected clones and wild type virus were produced in 3 T-225 cm2 flasks in BCIRL-HS-AM1 cells and the budded virus collected for DNA extraction (McIntosh et al. 2005).
Restriction enzyme analysis
Restriction endonuclease analyses were performed on the wild type virus as well as on selected clones that gave identical profiles. One of the clones, (TnMNPV/ CmBCL9), was selected for a comparative study of its restriction endonuclease profile with those of several other baculoviruses and was used in all the remaining studies. The nucleopolyhedroviruses employed were: Autographa californica (AcMNPV) (McIntosh and Ignofo 1989), Anagrapha falcifera (AfMNPV), (McIntosh 1991), Anticarsia gemmatalis (AgMNPV), (Grasela and McIntosh 1998), Plutella xylostella (PxMNPV), (Kariuki et al. 2000) and the single nucleopolyhedrovirus from Helicoverpa zea (HzSNPV), (McIntosh et al. 2001). These baculoviruses were produced in cell culture as described and DNA extracted from budded virus as previously reported (McIntosh and Grasela 2006). The restriction enzymes used included HindIII, StyI, VspI, BstEII, XhoI and PstI and digestion of the DNA was carried out according to the manufacturers instructions.
Hybridization studies
Hybridization studies were conducted with the 5 named baculoviruses as well as TnMNPV/CmBCL9 to determine the relationship if any, of the latter with the known baculoviruses. A previously described protocol (Kariuki and McIntosh 1999) using random primed DNA labeling of a VspI probe with digoxigenin-dUTP was followed for this comparative study.
Electron microscopy
Samples of TnMNPV/CmBCL9 occlusion bodies were prepared for transmission electron microscopy as previously described (Kariuki and McIntosh 1999) to determine whether the virus was a SNPV or MNPV, and were processed by the Electron Microscopy Core facility at the University of Missouri-Columbia.
In vivo infectivity studies of TnMNPV/ CmBCL9 occlusion bodies
Occulsion bodies produced in BCIRL-HS-AM1 were used in infectivity studies against 24h old larvae from T. ni, S. frugiperda, S. exigua, Helicoverpa zea, Heliothis virescens, and H. subflexa. Both T. ni and H. subflexa larvae were obtained from the insectary at the Biological Control of Insects laboratory and the remaining larvae were obtained commercially (Bio-Serv, www.bio-serv.com). For each virus tested, the sample size consisted of three groups of 25 larvae per dosage replicated twice. Larvae were incubated at 28°C for 7 days, and mortalities were recorded daily for all insects and the LC50 values calculated employing PoloPlus v.l (LeOra Software, leorasoftware.com).
Determining the DNA sequences of the polyhedrin, egt, and p10 genes
Polyhedrin protein sequences from Autographa califomica MNPV (AcMNPV) (GenBank No. NC_001623), Anticarsia gemmatalis MNPV (AgMNPV) (GenBank No. NC_008520), Bombyx mori MNPV (BmMNPV) (GenBank No. L33180), Spodoptera frugiperda MNPV (SfMNPV) (Genbank No. AY250076), and Orgyia pseudotsugata MNPV (OpMNPV) (GenBank No. U75930) were used with the web-based software Block Marker and the algorithm MOTIF (Smith et al. 1990) (http://blocks.fhcrc.org/blocks/make_blocks.html) to find conserved blocks in the related five unaligned protein sequences. The blocks were then used with the algorithm CODEHOP (COnsensus-DEgenerate Hybrid Oligonucleotide Primers) (Rose et al. 1998) (http://blocks.fhcrc.org/codehop.html) to generate the following pair of degenerate primers used to PCR amplify a predicted 651 bp fragment of the TnMNPV/CmBCL9 polyhedrin gene:
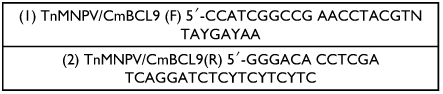 |
A similar approach was also taken to determine theTnMNNPV/CmB egt sequence employing degenerate primers designed from the following protein sequences: AcMNPV, AgMNPV, Helicoverpa armigera MNPV (HaMNPV) (Genbank No. NC_003094), OpMNPV, Rachiplusia ou (RoMNPV) 4 (GenBank No. NC_004323), and SfMNPV. A predicted 1629 bp egt fragment was generated using the following primer pair:
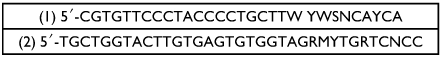 |
Likewise, a partial p10 sequence was determined by employing the following degenerate primers based on the p10 amino acid sequences of AcMNPV, AgMNPV, BmMNPV, OpMNPV, and RoMNPV to generate a 204 bp p10 fragment:
 |
TnMNPV/CmBCL9 DNA (100 –200 ng/µl) was amplified using puReTaq Ready-To-Go PCR beads (Amersham Biosciences, www.apbiotech.com) under the following conditions: 95°C, 3 min (1X); 94°C, 45 s, 60°C, 1 min, 72°C, 2 min (40X); 72°C, 5 min; held 15°C. The reaction products were run on a 2% Metaphor gel containing 1 µg/µl ethidium bromide and visualized with a VersaDoc imaging system (Bio-Rad Laboratories, Inc., www.bio-rad.com). The expected amplicon product was gel extracted using QIAEX II gel extraction kit (Qiagen, Inc., www.qiagen.com). Purified amplicon products were then cloned into the pCR4-TOPO plasmid according to the protocol provided by the manufacturer (Invitrogen, Corp., www.invitrogen.com). To obtain a more reliable nucleotide sequence read of the putative TnMNPV/ CmBCL9 polyhedrin gene, two clones, labeled TnMNPV/ CmBCL1 and TnMNPV/CmBCL2, containing the amplicon from two separate PCR reactions were sequenced from the 5′- and the 3′-end of the amplicon insert using M13 Forward (-20) and M13 Reverse primers by the DNA Core facility at the University of Missouri. The four sequence reads were subsequently used to generate a consensus sequence of the TnMNPV/CmBCL9 polyhedrin gene employing the BioEdit Sequence Editor (Hall 1999).
Nucleotide sequence accession numbers
The polh, egt, and p10 sequences described in this study have been deposited in GenBank; the accession numbers are EF418027 EF418026, and EF418025, respectively.
Analysis of sequence data
Multiple-sequence alignmenst of the nucleotide sequences were performed using T-Coffee (Notredame et al. 2000), which generates a library of the best global and local alignments based on the Sim algorithm from the Lalign package (Huang and Miller 1991). The CORE index was employed to evaluate the consistency between a multiple alignment and every pair of aligned residues contained in the library. To obtain a more accurate picture of the relationship between the newly isolated TnMNPV/CmBCL9 and other viruses in the Baculoviridae family, a network-based tool was employed for deciphering the evolutionary relationships in molecular sequence data. The method builds a network primarily constructed from distances determined from splitdecomposition theory and can be implemented using the SplitsTree4 (v.4.6) program that generates a Split-graphs network (Huson 1998; Huson and Bryant 2006). A network-based approach has several advantages, one of which avoids the implicit assumption of a tree-like evolutionary process. This allows one the flexibility to determine if the data follow a tree-like evolutionary path or to identify some other underlying pattern the typical tree representation might not discern. The significance of the topology of the split-graphs was verified by bootstrap resampling (1000 replicates). The program allows one to choose among various distance matrixes to construct a graphical representation of the phylogenetic relationship.
Results and Discussion
Electron microscopy
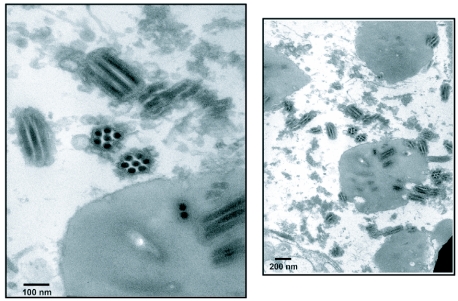
Transmission electron microscopy of TnMNPV/ CmBCL9 occlusion bodies are depicted in Figure 1. It shows the virus to be of the MNPV type because they contain many buddle-like structures embedded within a polyhedrin protein matrix each containing a multiple number of smaller virus particles.
Figure 1.
Transmission electron micrograph of the TnMNPV/CmBCL9 in BCIRL-HS-AM1 cells.
Latent viral presence in the T. ni larvae
Of the 35 3rd instar larvae reared at 37°C, only five larvae died after six days of exposure. None of these larvae as well as the remaining insect showed any pathology typical of a baculovirus infection. This suggests that the source of the virus might come more from contamination rather than a possible latent virus.
Viral source originating from the adult parasitoid interior
All 15 2nd instar larvae treated with a solution from macerated parasitoid showed no sign of pathogenicity after 7 days incubation. It is recognized that this test would only detect budded virus because the sample was passed through a 0.22 µm filter. It is also known that early instar larvae are not the most sensitive system for assaying for budded virus. There was no observed mortality in an equivalent number of control larvae. TN-CL1 cells inoculated with the filtered macerated parasitoid solution showed no signs of occlusion bodies formation after 7 days, other than general deterioration of cells probably from some component in the HBSS wash. Control cells were normal and almost confluent after 5 days.
Viral source originating from contact with parasitoid
TN-CL1 cells inoculated with the parasitoid-surface wash showed no sign of occlusion bodies formation after 7 days other than general deterioration of cells probably from some component in the HBSS wash. Control cells were normal and almost confluent after 5 days. When the supernatant collected from these cells was used to inoculate a fresh batch of TN-CL1 cells, no signs of viral infection were observed. Additionally, all 2nd instar larvae fed on diet treated with the parasitoid-surface wash also showed no pathogenesis nor did the untreated controls after 7 days. It is highly unlikely that budded virus would remain stable on the surface of an insect long enough to be somehow transmitted. Occlusion bodies attached to the parasitoid body that contaminate the diet surface would be the more plausible source of infection
Restriction endonuclease analysis and molecular weight of TnMNPV/CmBCL9
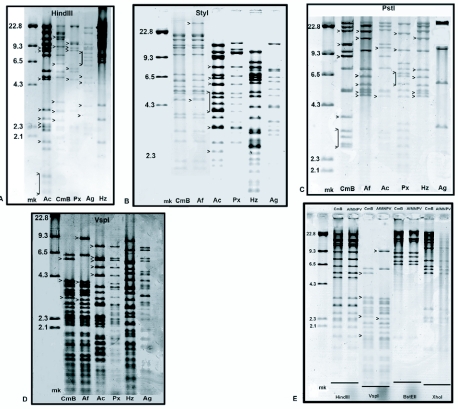
Two of the plaque purified clones (TnMNPV/CmBCL9 and TnMNPV/CmBCL14) and wt TnMNPV/CmB gave identical HindIII restriction patterns (data not shown). TnMNPV/CmBCL9 was employed in all other studies. A comparison of the HindIII restriction pattern between TnMNPV/CmBCL9 and four other baculoviruses revealed a number of differences both in the number as well as molecular size of the DNA fragments (Figure 2A). Six major bands ranging from 10.0 kb to 2.3 kb were found unique to TnMNPV/CmBCL9, whereas several HindIII restriction fragments present in AcMNPV-HPP (from 2.1 kb to 9.3 kb), PxMNPVCL3 (4.5 kb, 5.5 kb, 9.1 kb, 9.2 kb, 9.4 kb, 9.5 kb), AgMNPCL4-3A1 (from 6.0 kb to 9.3 kb) were absent in TnMNPV/CmBCL9. Most of the smaller HindIII bands (< 4.3 kb) of HzSNPV were absent in TnMNPV/ CmBCL9 as well as in the other viruses examined. A comparison of the StyI fragmentation profile showed a significant number of differences between TnMNPV/ CmBCL9 and AcMNPV-HPP, PxMNPVCL3, AgMNPCL4-3A1, and HzSNPV (Figure 2B). In contrast, the overall StyI restriction profile between TnMNPV/CmBCL9 and AfMNPV were indistinguishable except for the presence of two unique restriction fragments in AfMNPV. No further restriction pattern differences were detected on examination of these two viruses with the enzymes HindIII, BstEII, and XhoI in terms of identical number and molecular size distribution of fragments (Figure 2C). However, the VspI restriction pattern revealed major differences between TnMNPV/ CmBCL9 and AfMNPV especially in the higher molecular size region (> 6.5 kb) as well as three unique bands between the 4.3 – 6.5 kb region of AfMNPV (Figure 2D). A number of major band differences in the PstI restriction profiles were also evident among TnMNPV/CmBCL9, AcMNPV-HPP, PxMNPVCL3, AgMNPV-CL1-3A1, and HzSNPV (Figure 2E). In particular, there were six unique PstI fragments in AfMNPV that were absent in TnMNPV/CmBCL9, whereas eight unique bands were detected in TnMNPV/CmBCL9 that were absent in AfMNPV. In contrast, two unique PstI fragments that were detected in AcMNPV were absent in TnMNPV/CmBCL9. The molecular weight of TnMNPV/CmBCL9 was estimated to be 106 ± 2.5 kbp (mean ± SE) based on PstI, BstEII, StyI, HindIII restriction profiles. This compares with an estimated mean genomic size for AfMNPV of 118.1 Kbp S.E. ± 6.9 in the host T. ni (Vail et al. 1993).
Figure 2.
Comparison of five restriction profile patterns between TnMNPV/CmBCL9 and other baculoviruses. (A) HindIII restriction profile: (mk) DNA marker in kb; (Ac) AcMNPV-HPP; (CmB) TnMNPV/CmBCL9; (Px) PxMNPVCL3; (Ag) AgMNPV-CL4-3A1; and (Hz) HzSNPV. (B) SfyI restriction profile: (mk) DNA marker in kb; (CmB) TnMNPV/CmBCL9; (Af) AfMNPV; (Ac) AcMNPV-HPP; (Px) PxMNPVCL3; (Hz) HzSNPV; and (Ag) AgMNPV-CL4-3A1. (C) PstI restriction profile: (mk) DNA marker in kb; (CmB )TnMNPV/ CmBCL9; (Af) AfMNPV; (Ac) AcMNPV-HPP; (Px) PxMNPVCL3; (Hz) HzSNPV; and (Ag) AgMNPV-CL4-3A1; (D) VspI restriction profile: (mk) DNA marker in kb; (CmB) TnMNPV/CmBCL9; (Af) AfMNPV; (Ac) AcMNPV-HPP; (Px) PxMNPVCL3; (Hz) HzSNPV; and (Ag) AgMNPV-CL4-3A1: (E) Comparison of four different restriction profile patterns between TnMNPV/CmBCL9 and AfMNPV.
Hybridization studies
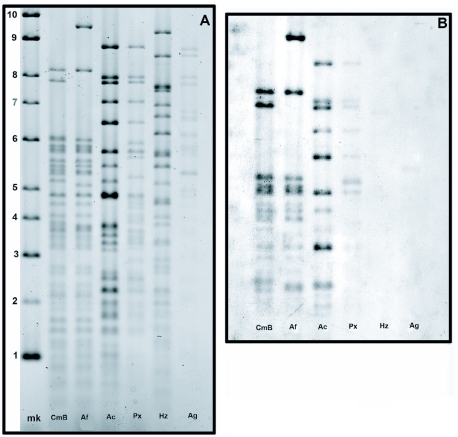
The VspI restriction profile showed some clearly distinct band differences specifically within the 6–10 kb range between TnMNPV/CmBCL9 and the other viruses. Hybridization analysis employing a VspI probe constructed from genomic TnMNPV/CmBCL9 revealed that the restriction pattern of the TnMNPV/CmB isolate appears to be more genetically similar to AcMNPV-HPP and AfMNPV than to PxMNPV, AgMNPV-CL1-3A1, or HzSNPV (Figure 3A, B). Federici and Hice (1997), also showed AfMNPV to be a genomic variant of AcMNPV based on Southern hybridization, the organization of the polyhedrin gene region, and nucleotide and deduced amino acid sequences of eight other viral genes in this region.
Figure 3.
(A) Comparison of VspI restriction patterns between TnMNPV/CmBCL9 and five other baculoviruses. (mk) DNA marker in kb; (CmB) TnMNPV/CmBCL9; (Af) AfMNPV; (Ac) AcMNPV-HPP; (Px) PxMNPV-C13; (Hz) HzSNPV; and (Ag) AgMNPV-CL4-3A1. (B) Southern hybridization using a dig-labeled VspI probe from TnMNPV/CmBCL9. (CmB) TnMNPV/CmBCL9; (Af) AfMNPV; (Ac) AcMNPV-HPP; (Px) PxMNPVCL3; (Hz) HzSNPV; (Ag) and AgMNPV-CL4-3A1.
In vivo infectivity studies of TnMNPV/ CmBCL9 occlusion bodies
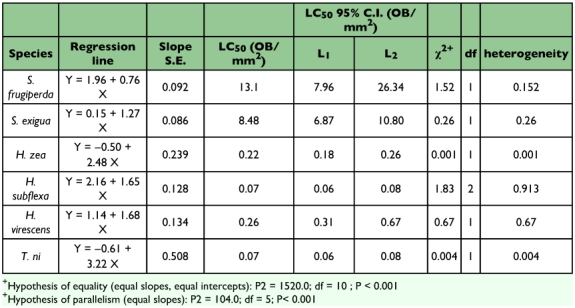
Analysis of the hypothesis of equality and parallelism showed that changes in TnMNPV/CmBCL9 infectivity per unit change in dosage rate were significantly different among the six species tested (P< 0.001) (Table 1). However, viral activity at a specific response level may still be similar between some comparisons. For example, the TnMNPV/CmB CL9 virus was equally effective against H. zea (LC50 = 0.22 occlusion bodies/mm2) and H. virescens (LC50 = 0.26 occlusion bodies/mm2). TnMNPV/CmBCL9 showed a 4.2 -12.2X lower infectivity against S. exigua larvae in comparison to reported LC50s of Plutella xylostella MNPV (CL3) (0.70 occlusion bodies/ mm2), AcMNPV (2.01 occlusion bodies/mm2), and AfMNPVCL1 (1.67 occlusion bodies/mm2) (Kariuki and McIntosh 1999). The LC50 of TnMNPV/CmBCL9 in S.Jrugiperda was 13.1 occlusion bodies/mm2. Although they used 2nd-instar larvae incubated at 26°C and recorded an accumulative mortality after a 10-day period, Berretta et al. (1997) reported that S. frugiperda larvae infected with SfMNPV-AR and SfMNPV-ME isolates from Argentina had LC50s (13.9 and 14.0 occlusion bodies/mm2, respectively) similar to the TnMNPV/CmB CL9 LC50 reported here. Of the six species tested, the TnMNPV/CmBCL9 virus was most virulent against H. subflexa (LC50 = 0.07 occlusion bodies/mm2) and T. ni (LC50 = 0.07 occlusion bodies/mm2). Given that TnMNPV/CmB CL9 appears to be a variant of both AcMNPV and AfMNPV some further comparison can be made between TnMNPV/CmBCL9 and these two viruses. Although they used neonates instead of 24 h-old larvae, Hostetter and Puttler (1991) found AcMNPV and AfMNPV LC50 values to be somewhat higher for H. virescens (0.45 occlusion bodies/mm2 and 0.35 occlusion bodies/mm2, respectively) and T. ni (0.39 occlusion bodies/mm2 and 0.15 occlusion bodies/mm2, respectively) relative to TnMNPV/CmBCL9. Finally, a major difference can be seen in TnMNPV/CmB CL9 where this virus is more infectious in H. zea (LC50 = 0.22 occlusion bodies/mm2) than AcMNPV (10.3 occlusion bodies/mm2) as reported by Hostetter and Putler (1991).
Table 1.
Probit analysis results of larval mortalities from six lepidopteran species infected with TnMNPV/CmBCL9.
Analysis of the polh, egt, and p10 sequences
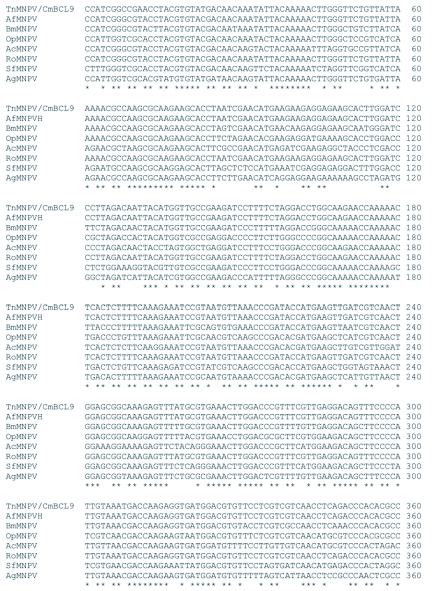
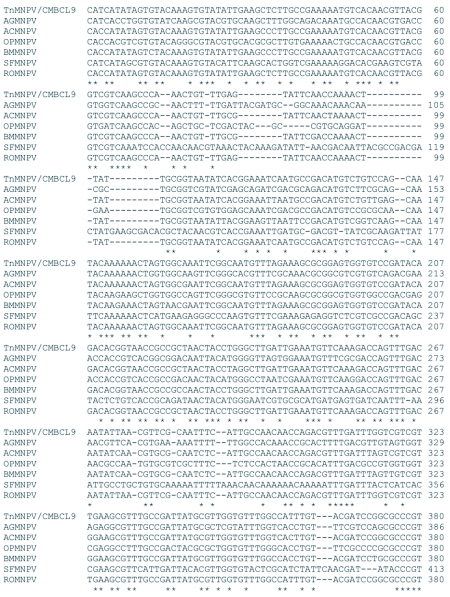
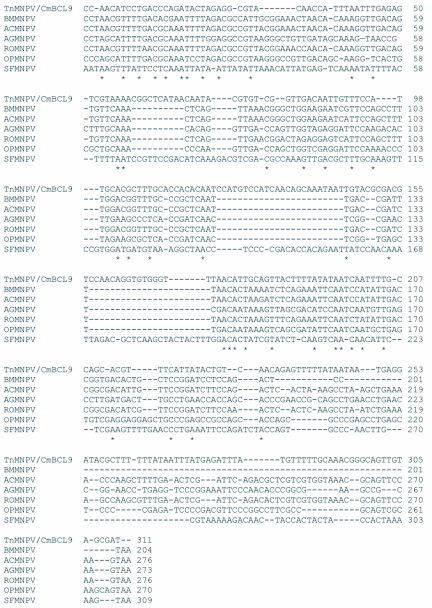
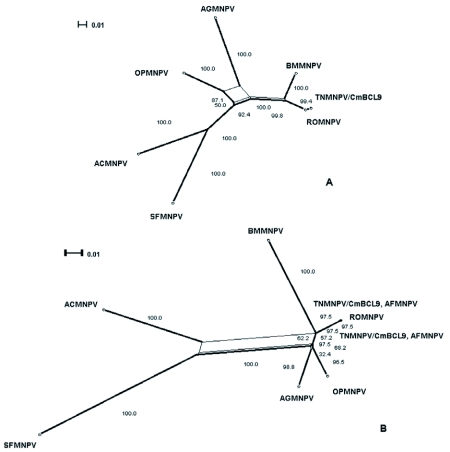
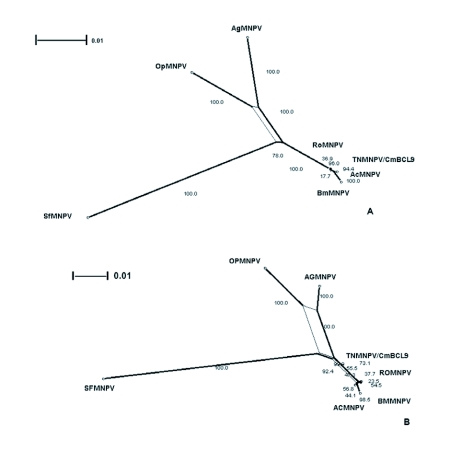
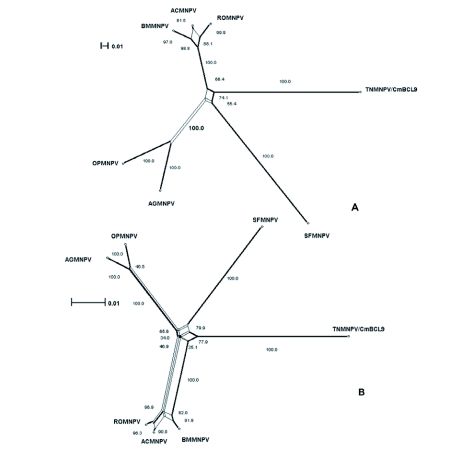
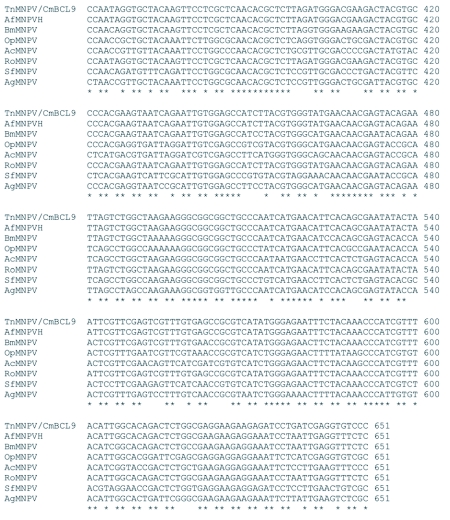
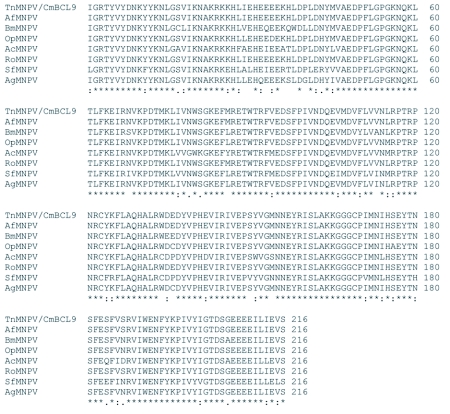
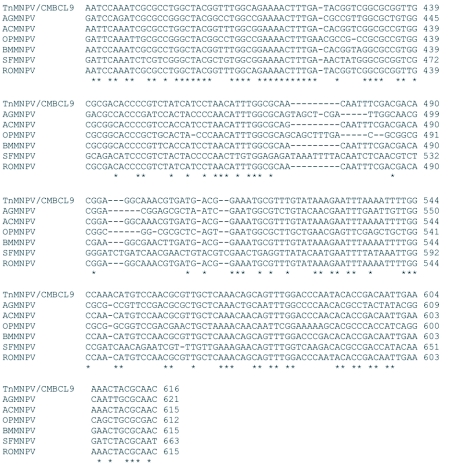
The distribution of BLAST hits showed that the TnMNPV/CmB CL9 polyhedrin nucleotide sequence had the best E values when aligned with RoMNPV (E = 0.0) and AfMNPV (E = 0.0). The TnMNPV/CmB CL9 polyhedrin sequence has a GC content of 46.7%, while the AfMNPV polh gene (Genbank no. AFU64896) was reported to have a GC content of 46.1%. The multiplesequence alignment of the TnMNPV/CmB CL9 polyhedrin nucleotide and predicted protein sequences with seven viruses selected from the Genbank (BmMNPV [U75359], AfMNPV [AAB53357], OpMNPV [M14885], AcMNPV [KO1149], RoMNPV [DQ345451], SfMNPV [JO4333], AgMNPV [NC_008520]) is depicted in Figure 4A. If in the alignment only the partially determined TnMNPV/CmBCL9 sequence based on 651 nucleotides is considered, then the nucleotide composition between the polyhedrin sequence of TnMNPV/CmB CL9 and the other viruses (BmMNPV, AfMNPV, OpMNPV, AcMNPV, RoMNPV, SfMNPV, AgMNPV) consist of sequences having a 21.7 identity. If one makes a similar comparison using aligned amino acids sequences, then the residue composition between the TnMNPV/CmB CL9 polh gene and the other viruses consist of sequences having a 74.2% identity, and a 2.2%, 12.4% semi-conserved and conserved substitution level, respectively. Comparatively, the BLAST distribution is somewhat incomplete as there are no records of either the egt or the p10 genes from AfMNPV in the GenBank database, and, as such, these comparisons are tentative. The TnMNPV/CmBCL9 egt has a GC content of 45.1% and the p10 a GC content of 36.7%. For the partial aligned egt and p10 nucleotide sequences, 33.0% and 4.8 identities, respectively, were found when TnMNPV/CmBCL9 was compared with six different viruses: BmMNPV, OpMNPV, AcMNPV, RoMNPV, SfMNPV, AgMNPV (Figure 5A, Figure 6A). For the partial egt and p10 aligned protein sequences the semi-conserved substitution levels were 10.9% and 1.2%, respectively and conserved substitutions were 20.6% and 13.1%, respectively. Initial split-graph analysis of the polh nucleotide and translated protein sequences clearly placed TnMNPV/CmB CL9 into a group consisting of RoMNPV/AfMNPV/BmMNPV and this relationship can be explained for the most part as a split-graph network rather than a tree-like network (Figure 7). The central portions of the egi (Figure 8) and p10 (Figure 9) graphs also show box-like structures that indicate incompatible data suggesting a network-like rather than a simple evolutionary tree structure. The PHI test of recombination showed significant evidence for recombination in the polh aligned sequences (p = 1.894 × 10-5). In contrast, no evidence was found to indicate a recombination signal in either the egt (p = 0.1052) nor p10 (p = 0.1549) sequences. The split graphs of the egt and p10 DNA sequences are illustrated in Figure 8 and Figure 9, respectively. Parallel evolution, model heterogeneity, and sampling error along with recombination can result in misleading interpretation of phylogenetic histories. Baculoviruses are known for exhibiting recombination and their evolutionary histories may not be best represented by a bifurcating or multifurcating trees. We chose the use of a split-graph network as its premise is that it can represent a relationship among lineages without assuming a tree-like evolutionary process. Also a major difference between a network and a tree is that cycles are permitted in which paths start and end at the same node. In general, similar network patterns were generated between each of the DNA and protein aligned sequences of the three genes (Figures 7, 8, 9). Based on the polh and egt sequence alignments TnMNPV/CmB CL9 was grouped with RoMNPV, BmMNPV, AfMNPV, and AcMNPV.
Figure 4A.
Multiple-sequence alignment of the TnMNPV/CmBCL9 polh nucleotide sequence with seven baculoviruses employing the T-Coffee package, (*) indicate identical residues.
Figure 5A.
Multiple-sequence alignment of the TnMNPV/CmBCL9 egt nucleotide sequence with six baculoviruses employing the TCoffee package, (*) indicate identical residues.
Figure 6A.
Multiple-sequence alignment of the TnMNPV/CmBCL9 p10 nucleotide sequence with five baculoviruses employing the T-Coffee package. (*) indicate identical residues.
Figure 7.
The split-graph of the polh, egt, and p10 genes shows that the phylogenetic relationship among the viruses is more appropriately depicted by a non-tree like data set (box-like structures) rather than the typical bifurcating tree. The Hamming distances (dh), which are computed by giving equal weight to the number of nucleotide differences as well as insertions-deletions between each sequence pair between taxa are accurately represented by the split-graph based on a fit index of 91.7. The number besides each branch is the percent of computed graphs in which a split corresponding to the branch occurred by bootstrap re-sampling.
Figure 8.
The split-graph of aligned egt DNA sequences from seven baculovirus 33 including TNMNPV/CmBCL9. This network is based on a logDet character transformation matrix and split-decomposition distance transformation. The fit index 35 indicates that 95% of the original distance is represented by the split-graph. (B) The split-graph of the aligned egt protein translated DNA sequences from seven 37 baculovirus including TNMNPV/CmBCL9. The split index is 98.1%. The splits were calculated employing the ProteinMLdist character transformation and split- 39 decomposition distance transformation.
Figure 9.
The split-graph of aligned p10 DNA sequences from seven baculovirus including TNMNPV/CmBCL9. This network is based on a uncorrected_P character 43 transformation matrix and split-decomposition distance transformation. The fit index is 13 indicates that 90.4% of the original distance is represented by the split-graph. (B) I The split-graph of the aligned p10 protein translated DNA sequences from seven baculovirus including TNMNPV/CmBCL9. The split index is 98.7%. The splits were 3 calculated employing the uncorrected_P character transformation and splitdecomposition distance transformation.
continued.
Figure 4B.
The putative amino acid sequence of the TnMNPV/CmBCL9 polh gene: (*) indicate identical residues; (:) indicate semiconserved residues; (.) designate conserved residues.
Specifically, the AfMNPV node in Figure 7A might be interpreted as the ancestor of TnMNPV/CmBCL9. In the absence of a published AfMNPV egt gene sequence, analysis without AfMNPV still grouped TnMNPV/ CmBCL9 with RoMNPV, AcMNPV, and BmMNPV. On the other hand, based on the p10 gene network, the relation of TnMNPV/CmBCL9 with the other viruses was counter to what the polh and egt gene network showed by placing TnMNPV/CmBCL9 as a distantly unique virus (Figure 9). When all three gene sequences were concatenated using all the viruses except AfMNPV, TnMNPV/CmBCL9 was still grouped with RoMNPV and BmMNPV.
continued.
In this study, information is presented on a new multiple nucleopolyhedrovirus variant, TnMNPV/CmB CL9 found in T. ni larvae following parasitization with the parasitoid C. marginiventris. Larval mortality studies showed that the virus is highly infectious for 24 h-old T. ni and H. subflexa larvae and infectious for both 24 h-old H. zea and H. virescens larvae. Restriction DNA and hybridization profiles indicated that TnMNPV/CmB CL9 is genetically similar to AfMNPV, but appears to be a new isolate of the multiple nucleopolyhedrovirus type. The partially determined polh, egt, and p10 nucleotide sequences in a split-graph analysis further demonstrated a close relationship to AfMNPV, BMNPV, and RoMNPV, previously determined genetic variants of AcMNPV. Various possible sources of the virus that were examined in this study included (1) surface contamination of parasitoid, (2) virus sequestered by the parasitoid, and (3) a latent virus present in the T. ni colony. The latter possibility seems unlikely since no infection of larvae was observed in the stock colony of T. ni and attempts to activate latency were unsuccessful. The actual origin of the virus at this time remains unknown.
Figure 5B.
The putative amino acid sequence of the TnMNPV/CmBCL9 polh gene: (*) indicate identical residues; (:) indicate semiconserved residues; (.) designate conserved residues.
Figure 6B.
The putative amino acid sequence of the TnMNPV/CmBCL9 polh gene: (*) indicate identical residues; (:) indicate semiconserved residues; (.) designate conserved residues.
Abbreviations
- HzSNPV -
Helicoverpa zea single nucleopolyhedroviruses
- TnMNPV -
Trichoplusia ni multiple nucleopolyhedroviruses
- wt -
wildtype
References
- Berretta M, Rios ML, Sciocco de Cap A. Characterization of a nuclear polyhedrosis virus of Spodoptera frugiperda from Argentina. Journal of Invertebrate Pathology. 1997;71:280–282. doi: 10.1006/jipa.1997.4731. [DOI] [PubMed] [Google Scholar]
- Bruen TC, Herve P, Bryant D. A simple and robust statistical test for detecting the presence of recombination. Genetics. 2006;172:2665–2681. doi: 10.1534/genetics.105.048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet D, Cusson M. Role of the calyx fluid in alterations of immunity in Choristoneura fumiferana larvae parasitized by Tranosema rostrale. Comparative Biochemistry and Physiology. 1996;114A:31–317. [Google Scholar]
- Federici BA, Hice RH. Organization and molecular characteriization of genes in the polyhedrin region of the Anagrapha falcifera multinucleocapsid NPV. Archives of Virology. 1997;142:333–348. doi: 10.1007/s007050050080. [DOI] [PubMed] [Google Scholar]
- Ferrarese R, Brivio M, Congiu T, Falabella P, Grimaldi A, Mastore M, Perlett G, Pennacchio F, Sciacca L, Tettamanti G, Valvassori R, de Eguileor M. Early suppression of immune response in Heliothis virescens larvae by the endophagous parasitoid Toxoneuron nigriceps. Invertebr Surv J. 2005;2:60–68. [Google Scholar]
- Fleming JGW, Blissard GW, Summers MD. Expression of Campoletis sonorensis virus in the parasitoid host. Journal of Virology. 1983;48:74–78. doi: 10.1128/jvi.48.1.74-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasela JJ, McIntosh AH. In vitro and in vivo host range of Anticarsia gemmatalis multiple nuclear polyhedrosis virus. In Vitro Cellular and Developmental Biology. 1998;34:79–83. doi: 10.1007/s11626-998-0057-2. [DOI] [PubMed] [Google Scholar]
- Granados RR, Federici BA. The Biology of Bacubviruses. 1 & 2. CRC Press; 1986. [Google Scholar]
- Hall T. BioEdit: a user-friendly biological sequence alignment and analysis program for Windows 95/98/NT. Nucleic Acid Sym Ser. 1999;41:95–98. [Google Scholar]
- Hostetter DL, Puttier B. A new broad host spectrum nuclear polyhedrosis virus isolated from a celery looper: Anagrapha falcifera (Kirby) (Lepidoptera: Noctuidae). Journal of Environmental Entomology. 1991;20(5):1480–1488. [Google Scholar]
- Huang X, Miller W. A time-efficient, linear-space local similarity algorithm. Advances in Applied Mathematics. 1991;12:337–357. [Google Scholar]
- Huson DH. SplitsTree: A program for analyzing and visualizing evolutionary data. Bioinformatics. 1998;14:68–73. doi: 10.1093/bioinformatics/14.1.68. [DOI] [PubMed] [Google Scholar]
- Huson DH, Bryant D. Application of Phylogenetic networks in evolutionary studies. Molecular Biology and Evolution. 2006;23(2):254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Kariuki CW, McIntosh AH, Goodman CL. In vitro host range studies with a new baculovirus isolate from the diamondback moth Plutella xylostella (L.) (Plutellidae: Lepidoptera). In Vitro Cellular and Developmental Biology. 2000;36:271–276. doi: 10.1290/1071-2690(2000)036<0271:IVHRSW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kariuki CW, McIntosh AH. Infectivity studies of a new baculovirus isolate for the control of the diamondback moth (Plutellidae: Lepidoptera). Journal of Economic Entomology. 1999;92(5):1093–98. doi: 10.1093/jee/92.5.1093. [DOI] [PubMed] [Google Scholar]
- Kroemer JA, Webb BA. Polydnavirus genes and genomes: emerging gene families and new insights into polydnavirus replication. Annual Review of Entomology. 2004;49:431–456. doi: 10.1146/annurev.ento.49.072103.120132. [DOI] [PubMed] [Google Scholar]
- LeOra Software. POLO-PC Probit and Logit Analysis. User's manual. Berkeley; California: 1997. p. 28. [Google Scholar]
- McIntosh AH, Ignoffo CM. Replication of Autographa califonica nuclear polyhedrosis virus in five lepidopteran cell lines. Journal of Invertebrate Pathology. 1989;54:97–102. [Google Scholar]
- McIntosh AH. In vitro infectivity of a clonal isolate of Syngrapha falcifera (celery looper) multiple nuclear polyhedrosis virus. Journal of Invertebrate Pathology. 1991;17:649–650. [Google Scholar]
- McIntosh AH, Goodman L, Grasela JJ. Virus production and plaque-forming ability of Helicoverpa zea (Lepidoptera: Noctuidae) clonal cell lines. Applied Entomology and Zoology. 1997;32(4):657–658. [Google Scholar]
- McIntosh AH, Grasela JJ, Goodman CL. Growth of a clonal cell line of Helicoverpa zea (Lepidoptera: Noctuidae) in suspension culture and replication of its homologous baculovirus HzSNPV. Applied Entomology and Zoology. 2001;36(3):349–352. [Google Scholar]
- McIntosh AH, Goodman CL, Grasela JJ. A simplified and rapid method for extraction of DNA from Baculovirus occlusion bodies. Bioprocessing Journal (July/August, Tech Rev) 2006;5(2):59–61. [Google Scholar]
- McIntosh AH, Grasela JJ, Popham HJR. AcMNPV in permissive, semipermissive, and nonpermissive cell lines from Arthropoda. In Vitro Cellular and Developmental Biology. 2005;41:298–304. doi: 10.1290/0412083R.1. [DOI] [PubMed] [Google Scholar]
- Miller LK. The Baculoviruses. Plenum Press; 1997. [Google Scholar]
- Moscardi F. Assessment of the application of baculoviruses for control of lepidoptera. Annual Review of Entomology. 1990;14:257–289. doi: 10.1146/annurev.ento.44.1.257. [DOI] [PubMed] [Google Scholar]
- Murphy FA, Fauquet CM, Bishop DHL, Ghabrial SA, Jarvis AW, Martelli GP, Mayo MA, Summers MD. Virus taxonomy. Classification and nomenclature of viruses. Sixth report of the International Committee on Taxonomy of Viruses; Springer-Verlag: 1995. [Google Scholar]
- Notredame C, Higgins D, Heringa J. T-Coffee: A novel method for multiple sequence alignments. Journal of Molecular Biology. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- Possee RD. Viral approaches for insect control. In: Kim L, editor. Advanced Engineered Pesticides. CRC Press; 1993. pp. 99–112. [Google Scholar]
- Rose T, Schultz ER, Henikoff JG, Pietrokovski S, McCallum CM, Henikoff S. Consensus-degenerate hybrid oligonucleotide primers for amplification of distantly-related sequences. Nucleic Acid Research. 1998;26(7):1628–1635. doi: 10.1093/nar/26.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HO, Annau TM, Chandrasegaran S. Finding sequence motifs in groups of functionally related proteins. Proceedings of the National Academy of Science USA. 1990;87:826–830. doi: 10.1073/pnas.87.2.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz DB, Vinson SB. Baculovirus-like particles in the reproductive tracts of female parasitoid wasps. II: The genus Apantales. Canadian Journal of Microbiology. 1977;23:28–37. doi: 10.1139/m76-148. [DOI] [PubMed] [Google Scholar]
- Stoltz DB, Vinson SB. Viruses and parasitism in insects. Advances in Virus Research. 1979;24:125–171. doi: 10.1016/s0065-3527(08)60393-0. [DOI] [PubMed] [Google Scholar]
- Stoltz DB, Faulkner G. Apparent replication of an unusual viruslike particle in both a parasitoid wasp and its host. Canadian Journal of Microbiology. 1978;24:1509–1514. doi: 10.1139/m78-241. [DOI] [PubMed] [Google Scholar]
- Strand MR, Pech LL. Immunological compatibility in parasitoidhost relationships. Annual Review of Entomology. 1995;40:31–56. doi: 10.1146/annurev.en.40.010195.000335. [DOI] [PubMed] [Google Scholar]
- Styer EL, Hamm JJ, Nordlund DA. A new virus associated with the parasitoid Cotesia marginiventris (Hymenoptera: Braconidae): Replication in Noctuid host larvae. Journal of Invertebrate Pathology. 1987;50:302–309. [Google Scholar]
- Tanada Y, Kaya HK. Insect Pathology. Academic Press; 1993. [Google Scholar]
- Vail PV, Hoffmann DF, Streett DA, Manning JS, Tebbets JS. Infectivity of a nuclear polyhedrosis virus isolated from Anagrapha falcifera (Lepidoptera: Noctuidae) against production and postharvest pests and homologous cell lines. Environmental Entomology. 1993;22(5):1140–1145. [Google Scholar]
- Vinson SB, Iwantsch GF. A. Host regulation by insect parasitoids. Quarterly Review of Biology. 1980;55:143–165. [Google Scholar]
- Vinson SB, Iwantsch GF. B. Host suitability for insect parasitoids. Annual Review of Entomology. 1980;25:397–419. [Google Scholar]
- Webb BA, Strand MR. The biology and genomics of polydnaviruses. In: Gilbert LL, Latrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Vol. 6. Elsevier; 2005. pp. 323–360. [Google Scholar]