Astract
Several amino acids were found to undergo progressive age-dependent racemisation in the lifelong proteins of normal human lenses. The two most highly racemised were Ser and Asx. By age 70, 4.5% of all Ser residues had been racemised, along with >9% of Asx residues. Such a high level of inversion, equivalent to between 2 and 3 d - amino acids per polypeptide chain, is likely to induce significant denaturation of the crystallins in aged lenses. Thr, Glx and Phe underwent age-dependent racemisation to a smaller degree. In model experiments, d - amino acid content could be increased simply by exposing intact lenses to elevated temperature. In cataract lenses, the extent of racemisation of Ser, Asx and Thr residues was significantly greater than for age-matched normal lenses. This was true, even for cataract lenses removed from patients at the earliest ages where age-related cataract is observed clinically. Racemisation of amino acids in crystallins may arise due to prolonged exposure of these proteins to ocular temperatures and increased levels of racemisation may play a significant role in the opacification of human lenses.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-010-9171-7) contains supplementary material, which is available to authorized users.
Keywords: Temperature, Age-related cataract, Posttranslational modification, Blindness, Human lens, Racemisation
Introduction
Little quantitative data are available for the raft of changes that long-lived proteins undergo, although it is likely that age-dependent degradation of abundant proteins will result in measurable changes in the tissues of older people (Ritz-Timme and Collins 2002; Truscott 2010). Several sites in the human body, including lung (Shapiro et al. 1991), heart (Powell et al. 1992), brain (Shapira et al. 1988), teeth (Helfman and Bada 1975), skin (Verzijl et al. 2000), intervertebral discs (Ritz and Schutz 1993) and cartilage (Maroudas et al. 1992) contain abundant proteins that are very long-lived, with half-lives measured in decades.
At other sites in the human body, proteins do not turnover at all. The most well-known tissue with such life-long proteins is the lens (Lynnerup et al. 2008). The lens grows throughout life, with the accumulation of fibre cells over pre-existing cells that, during differentiation, lose all cellular organelles. In order for the lens to remain transparent, these lifelong proteins must retain their structure throughout the lifetime of the individual.
A number of post-translational modifications (PTMs) have been described in the proteins isolated from older human lenses (Nagaraj et al. 1991; MacCoss et al. 2002). Some PTMs appear to be present at high concentrations (mol/mol protein). In older human lenses these include deamidation (Hains and Truscott 2010), phosphorylation (Spector et al. 1985), methylation (Lapko et al. 2003) and truncation (Srivastava and Srivastava 2003). Oxidation of Cys and Met residues, while extensive in age-related nuclear cataract lenses (Truscott and Augusteyn 1977b; Garner and Spector 1980), is a minor process in aged normal lenses (Wells-Knecht et al. 1993; Truscott 2005).
Another PTM of lens proteins, about which little quantitative information is available, is racemisation. Asn residues can racemise readily due to the formation of a five-membered succinimide ring intermediate (Clarke 1987). The other amide-containing amino acid, Gln, undergoes a similar mechanism but at a slower rate (Robinson and Robinson 2004). Racemisation is analysed using acid hydrolysis of proteins followed by the separation of the d- and l-forms of amino acids. During hydrolysis Asn is converted to Asp, so a combined figure for Asp + Asn (Asx) is obtained. d-Asx was reported to accumulate in normal lens proteins at a rate of 0.14% per year (Masters et al. 1977). In addition, the D/L Asp ratio was higher in the water insoluble proteins (Masters et al. 1978), which was interpreted as evidence for a loss of the native protein conformation. Subsequently Fujii’s group (Fujii et al. 1999a) identified selected sites on proteins, such as alpha crystallin, as being particularly prone to racemisation.
In this paper, we examined racemisation of lens crystallins as a function of age, as part of a programme to quantify the major processes involved in age-dependent denaturation of long-lived proteins. In addition, the data from normal-aged lenses were compared with those from age-matched cataract lenses in order to provide a better understanding of the changes involved in the formation of cataract: the major cause of world blindness.
Materials and methods
Normal human lenses were obtained from the Sydney Eye Bank, with ethical approval from the University of Sydney and foetal lenses from the Endocrinology Department, Prince of Wales Hospital, Randwick, NSW, Australia. Cataractous lenses were obtained from the K.T. Seth Eye Hospital, Rajkot, Gujarat, India. Two normal lenses were examined per decade over the age range 0–86 years. Twelve cataractous lenses were graded according to the Pirie classification system (Pirie 1968) as follows: type I (40, 45, 55 years), type II/III (46, 50, 50, 60, 65, 65, 70 and 75 years) and type IV (80 years). All cataract lenses were stored at −80°C prior to analysis.
For the heating experiments, lenses dissected from fresh pig eyes (Crown pork, Strathfield, NSW, Australia) were incubated at 60°C in sealed humidified tubes as described previously (Heys et al. 2007) for 16 days, with two lenses removed at intervals. This temperature was used previously to promote racemisation of Asx in peptides (Fujii et al. 1996).
For all lenses, the nucleus was separated from the cortex by coring through the visual axis with a 4.5 mm diameter trephine and removing ∼0.5 mm of newly synthesised lens material from each end of the core. In this study, only the nuclear regions were examined. Normal human and pig lens samples were homogenised with 7 M guanidine-HCl, 100 mM Tris, pH 8 while the cataract lenses were homogenised with the same buffer containing 50 mM DTT to ensure all proteins were solubilised (Truscott and Augusteyn 1977a). The presence of DDT was shown not to affect racemisation of Ser or Thr but made a small reproducible difference for Asx (+0.95%) and the figures shown have been corrected for this. Overnight dialysis against four changes of distilled water was carried out at 4°C and the samples were freeze dried.
Amino acid analysis
Samples (∼0.5 mg) of the lyophilised lens proteins were weighed into glass vials and dissolved in 6 M HCl to a final concentration of ∼125 μM. Tubes were flushed with nitrogen, sealed and placed in an oven at 110°C. After 30 min, they were removed and the caps re-tightened (Kaufman and Manley 1998). Samples were hydrolysed for 6 and 22 h. During acid hydrolysis, traces of metals can promote racemisation (Van Den Oetelaar et al. 1986). In order to reduce this background racemisation, lens proteins underwent extensive dialysis against Milli Q water and tubes were pre-extracted for 48 h with 6 M HCl at 100°C.
For high-performance liquid chromatography (HPLC) analysis, 25 μL of the hydrolysates were evaporated to dryness in a dessicator and dissolved in 400 μL of 0.01 M HCl containing 0.01 mM homoarginine (l-hArg) and 0.77 mM sodium azide. Four separate HPLC runs were carried out for all samples with two injections each from duplicate vials. Separation of d- and l-amino acids was obtained using HPLC (Agilent 1100) with derivatization of the amino acids performed on-line prior to injection, using the automated features of the Chemstation LC-3D software (Agilent Technologies, Victoria, Australia). The o-phthaldialdehyde/N-isobutyryl-l-cysteine derivatizing reagent was drawn into the sample loop both before and after the sample, and solutions were mixed by a motorised plunger at room temperature before injection onto the reversed phase C18 column (Sigma #58355C30). Eluent A consisted of 23 mM sodium acetate, 1.5 mM sodium azide and 2.6 mM EDTA pH 6. Eluent B was 100% HPLC grade methanol. The amino acids were eluted using a linear gradient of 95% A and 5% B to 95% B and 5% A at a flow rate of 560 μL/min over 115 min. A fluorescence detector with excitation 230 nm and emission 445 nm was used and Chemstation LC-3D software used to calculate peak areas for quantification.
Statistical analysis
Linear regression analysis and Mann–Whitney U tests were performed using StatsDirect software Version 2.7.7 (StatsDirect Software Inc., San Diego, CA, USA). Simple linear regression analysis was performed on both normal and cataract samples to determine the amino acid racemisation as a function of age. Since Asx and Ser racemised rapidly in the first decade, the lines of best fit were determined using lens aged >12 years. The non-parametric Mann–Whitney U test was used to evaluate if the levels of d-amino acids in normal lenses were significantly different from those in cataract lenses (Harding 1984). Since age-related cataract only becomes evident above the age of 40, only lenses >40 years were used for both data sets. A two-sided P value of less than 0.05 was considered statistically significant.
Results
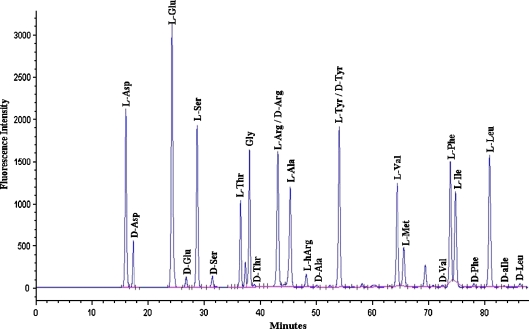
Racemisation of amino acids was monitored by reversed phase HPLC (Kaufman and Manley 1998) that allows resolution of the d- and l-forms, and acid was used to hydrolyse the peptide bonds prior to HPLC. As depicted in Fig. 1, good separations of d- and l-Asp, Glu, Ser, Thr, Ala, Val, Phe, Leu, and Ile were obtained. Retention times were highly reproducible and this analytical method allowed us to quantify the levels of eight amino acids.
Fig. 1.
HPLC profile of amino acids in the acid digest of proteins from a 65-year-old human cataract lens
Each sample was hydrolysed for both 6 and 22 h. Hydrolysis for 6 h minimises artefactual racemisation, and is used for young fossils, (Kaufman and Manley 1998) however, this time may not permit hydrolysis of all peptide bonds, so 22 h at 110°C was also utilised. Amino acid racemisation results for normal human lens proteins from the 6- and 22-h hydrolyses showed very similar trends for the percentage of d-amino acids as a function of age for Asx, Glx, Ser, Phe, Thr and Ala although, as expected, values from the 22 h digests were higher. Graphs for the 6 h hydrolyses are depicted in Figs 2, 3, 4, 5, 6, 7 and 8. Those from the 22-h hydrolyses are shown in Supplementary data. All values for racemisation of proteins from the centre of adult lenses were compared to those from foetal lenses as a baseline, since the amino acids in these proteins will be all in the l-form prior to hydrolysis. Such a procedure is valid because human lenses grow continuously throughout life with cells laid over the lens that was present at birth. The lens at birth can be considered as the nucleus of the adult lens and there is no compaction of the adult nucleus. (Fagerholm et al. 1981; Heys and Truscott 2008)
Fig. 2.
Racemisation of Asx as a function of age, in normal and cataract lens proteins. Racemisation expressed as % 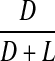 . (normal;
. (normal; 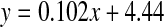 , R2 = 0.916, two-sided P < 0.0001: cataract;
, R2 = 0.916, two-sided P < 0.0001: cataract;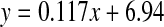 , R2 = 0.491, two-sided P = 0.0111). Mann–Whitney U test, two-sided P = 0.0067
, R2 = 0.491, two-sided P = 0.0111). Mann–Whitney U test, two-sided P = 0.0067
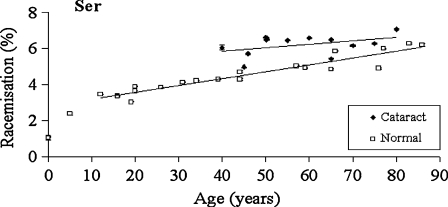
Fig. 3.
Racemisation of Ser as a function of age in normal and cataract lens proteins. Racemisation expressed as % 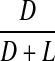 . (normal;
. (normal; 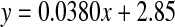 , R2 = 0.901, two-sided P < 0.0001: cataract;
, R2 = 0.901, two-sided P < 0.0001: cataract; 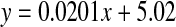 , R2 = 0.194, two-sided P = 0.152). Mann–Whitney U test, two-sided P = 0.0044
, R2 = 0.194, two-sided P = 0.152). Mann–Whitney U test, two-sided P = 0.0044
Fig. 4.
Racemisation of Thr as a function of age in normal and cataract lens proteins. Racemisation expressed as % 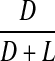 . (normal;
. (normal;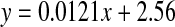 , R2 = 0.482, two-sided P = 0.0007: cataract;
, R2 = 0.482, two-sided P = 0.0007: cataract; 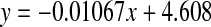 , R2 = 0.0341, two-sided P = 0.566). Mann–Whitney U test, two-sided P = 0.016
, R2 = 0.0341, two-sided P = 0.566). Mann–Whitney U test, two-sided P = 0.016
Fig. 5.
Racemisation of Glx as a function of age, in normal lens proteins. Racemisation expressed as % 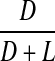 . (normal;
. (normal; 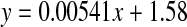 , R2 = 0.636, two-sided P < 0.0001)
, R2 = 0.636, two-sided P < 0.0001)
Fig. 6.
Racemisation of Phe as a function of age, in normal lens proteins. Racemisation expressed as % 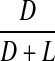 . (normal;
. (normal; 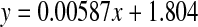 , R2 = 0.581, two-sided P < 0.0001)
, R2 = 0.581, two-sided P < 0.0001)
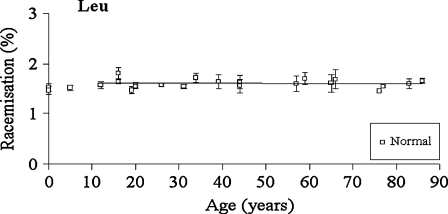
Fig. 7.
Racemisation of Leu as a function of age, in normal lens proteins. Racemisation expressed as % 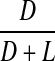 . (normal;
. (normal; 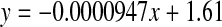 , R2 = 0.000763, two-sided P = 0.908)
, R2 = 0.000763, two-sided P = 0.908)
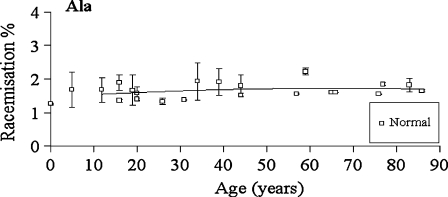
Fig. 8.
Racemisation of Ala as a function of age, in normal lens proteins. Racemisation expressed as % 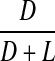 . (normal;
. (normal; 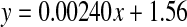 , R2 = 0.0675, two-sided P = 0.269)
, R2 = 0.0675, two-sided P = 0.269)
Asx
The values shown in Fig 2 reflect the racemisation of both Asn and Asp in proteins, since the side chain amide of Asn is hydrolysed with acid. In a pattern that was also shown with another amino acid that racemised significantly (Ser), there was a more rapid increase in racemisation in the early years, with 4% inversion occurring between ages 0 and 12. After this time, the rate of racemisation was linear. When cataract lenses were compared with normal lenses, it was apparent that there was a significant increase in the amount of d-Asx present at all ages. The slopes of the normal and cataract lines of best fit were almost identical (normal: 0.102; cataract: 0.117), however, the absolute values for cataract lenses were greater by approximately 4% at every age. As a consequence, cataract lens proteins from people aged 40, showed equivalent racemisation of Asn/Asp to those of 80-year-old normal lenses.
Ser
In normal lenses, the pattern for conversion of l-Ser to d-Ser matched closely that for Asx (Fig 3). Half of the total inversion occurred in the first ∼12 years of life. After that, the rate of conversion of l- to d-Ser increased linearly at ∼0.4% per decade. The outcome of this process is that approximately 4.5% of all Ser residues in a 70-year-old normal lens nucleus are racemised.
By comparison with the normal lenses, the increase in the amount of d-Ser as a function of age in cataract proteins was smaller, and therefore the linear regression lines for cataract and normal lenses appear to converge at about age 120. In the earliest years at which age-related cataract occurs, between 40 and 50, there was approximately 1.5% more d-Ser in the proteins in the centre of cataract lenses compared with normal. This equates to about 40% increase in Ser racemisation in the cataract lens proteins when compared to the normals in this decade.
Thr
d-Thr showed a significant increase with age in the normal lenses at a rate of 0.13% per decade (Fig 4). As was found with Ser and Asx, there was a significant increase in the d-form in the first decade of life which subsequently became linear. The data for the cataract lenses was more scattered, possibly due to the fact that d-Thr was less well resolved than the other amino acids and it eluted as a shoulder on Gly. As indicated by the line of best fit, there was a statistically significant difference in the levels in cataract lenses compared with normal lenses.
For the following amino acids, no significant difference between cataract and normal lenses was observed and only the d-amino acid data from normal lenses are shown.
Glx
The values for racemisation of Gln and Glu in lens proteins were much lower than for those of Asx (Fig 5). No significant racemisation was observed from birth to the early forties in normal lenses, with only a very small increase detected thereafter (0.017% per decade).
Phe
There was a very slight increase in the proportion of d-Phe with age in normal lenses (Fig 6).
Leu and Ala
There was no significant increase in the proportion of d-Leu or d-Ala with age in normal (two sided p = 0.908 and p = 0.269, respectively) lenses (Figs 7 and 8).
Heating of intact lenses
In order to determine if the increase in the racemisation of amino acid residues observed in human lenses as a function of age could be reproduced in the laboratory, intact pig lenses were exposed to thermal stress. Lenses were incubated at 60°C for a total of 16 days and the results for d-Asx and d-Ser are depicted in Fig 9. As can be seen, both amino acids racemised over time in a linear manner with the percentage of d-Asx reaching approximately 3% after 16 days. Ser racemised more slowly and reached approximately 0.7% after 16 days.
Fig. 9.
Thermal stress and racemisation of amino acid residues in intact lenses. Pig lenses were incubated at 60°C. ad-Asx as a function of time of incubation (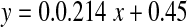 , R2 = 0.929, two-sided P = 0.0082). bd-Ser as a function of time of incubation. (
, R2 = 0.929, two-sided P = 0.0082). bd-Ser as a function of time of incubation. (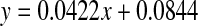 , R2 = 0.924, two-sided P = 0.0091)
, R2 = 0.924, two-sided P = 0.0091)
Discussion
One of the major findings of this study was that several amino acids were racemised in proteins from normal human lenses and that the extent increased progressively with age. Furthermore, the levels were significantly elevated in cataract lenses. Asx and Ser, in particular, showed substantial age-dependent racemisation. This is the first report of d-Ser in lenses, although it increases with age in articular cartilage and dentin (Stabler et al. 2009). On average, the total number of racemised Asx, Ser and Thr residues in crystallins from a 70-year-old normal lens, amounted to two or three d-amino acids per polypeptide chain. Glx and Phe were also racemised, but to a smaller degree. This extent of inversion is very likely to induce significant denaturation of crystallins in aged lenses. These data are summarised in Table 1. By contrast, Leu and Ala (Figs. 7 and 8), as well as Val and Ile (data not shown), displayed no significant age-dependent racemisation. In cartilage, levels of d-Leu were reported to increase slightly with age (Stabler et al. 2009).
Table 1.
An estimate of the number of racemised amino acid residues per polypeptide chain for the major crystallins of a normal lens at age 70 years
| Crystallin | No. of amino acids | Asx | Ser | Thr | d-Asx | d-Ser | d-Thr | Total d-amino acidsa |
|---|---|---|---|---|---|---|---|---|
| Alpha A | 173 | 17 | 19 | 9 | 1.6 | 0.8 | 0.1 | 2.5 |
| Alpha B | 175 | 13 | 17 | 9 | 1.2 | 0.8 | 0.1 | 2.1 |
| Beta A3 | 215 | 16 | 20 | 11 | 1.5 | 0.9 | 0.2 | 2.6 |
| Beta-A4 | 196 | 12 | 15 | 8 | 1.1 | 0.7 | 0.1 | 1.9 |
| Beta-B1 | 252 | 19 | 24 | 12 | 1.8 | 1.1 | 0.2 | 3.1 |
| Beta-B2 | 205 | 19 | 19 | 6 | 1.8 | 0.8 | 0.1 | 2.7 |
| Gamma-C | 174 | 14 | 13 | 5 | 1.3 | 0.6 | 0.1 | 2.0 |
| Gamma-D | 174 | 19 | 17 | 3 | 1.8 | 0.8 | 0.0 | 2.6 |
| Gamma-S | 178 | 15 | 11 | 7 | 1.4 | 0.5 | 0.1 | 2.0 |
The following values for d-amino acids were used: Asx (9.27%), Ser (4.43%) and Thr (1.39%) as determined by the linear regression lines of each graph (Figs 2 and 4), taking as a baseline the value obtained for foetal lenses. Human crystallin sequence data from ExPASy proteomics server of the Swiss Institute of Bioinformatics. Individual crystallins were not purified for these analyses and the values shown are estimates since different crystallins, and regions within them, will likely behave differently in terms of their susceptibility to racemisation
aSum of Asx, Ser and Thr
Asn/Asp displayed the greatest level of racemisation. The amount of d-Asp in normal human lens proteins increased linearly with age, from teenage years onwards, and thus could be used indirectly to date human lenses. It is therefore one of few indices that can be shown to change in a constant manner with human age. In normal lenses, the level of d-Asp increased until it reached more than 9% of total Asp/Asn content at age 70. Our data agree well with those of Masters et al. (1977) who found d-Asx to accumulate in proteins of normal lenses at a rate of 0.14% per year. The data are also in agreement with studies from Fujii’s laboratory who elucidated sites on alpha crystallins (Fujii et al. 1999b; Nakamura et al. 2008) that are particularly susceptible to racemisation of Asp. Since the vast bulk of protein in the lens is made up of crystallins (Harding 1991), it was possible to convert this figure to an average number of residues per polypeptide chain, assuming that all crystallins were affected equally. This calculation showed that 1.1–1.8 Asp/Asn residues were racemised per polypeptide in a 70-year-old person (Table 1). This factor alone is likely to lead to significant protein denaturation, especially since the process which leads to racemisation of Asp via a cyclic intermediate can also generate three isoaspartyl residues for each altered Asp residue (Capasso and Di Cerbo 2000). The values shown in Table 1 illustrate the quantitative importance of this process for the aged lens; however, it should be noted that different proteins, and regions within them, very likely behave differently in terms of their susceptibility to racemisation, so the values must be considered as estimates only.
Although Asp, as indicated by the literature (Ritz-Timme and Collins 2002), was the most highly racemised amino acid, significant levels of other amino acid residues were also racemised. Serine was the next most abundant d-amino acid. It might have been predicted that d-Glu levels would have been substantial (Cloos and Christgau 2002) since there are mechanisms in the literature for cyclisation of Glu that are analogous to those of Asn (Robinson and Robinson 2004). Such processes involve the formation and cleavage of cyclic amides that incorporate the peptide bond immediately N-terminal to the Asn or Gln residue. This results in deamidation as well as racemisation and the formation of isomers such as isoAsp (Geiger and Clarke 1987). Recent data show that approximately equal numbers of deamidated Asn and Gln residues are present in older lens crystallins (Hains and Truscott 2010), so such a cyclisation pathway may operate in human crystallins. Measurable levels of d-Glx were detected and they increased with age (Fig 5) in normal lenses, however the levels were much lower than those for Asx. d-Phe was also quantified however its levels were also tiny by comparison with Ser and Asx.
As noted above, d-Ser levels were second only to those of d-Asp. By age 70, almost 5% of total Ser in lens proteins was in the d-form (Fig 3). The mechanism for this is not yet known. It could involve dehydroalanine as an intermediate (Cloos and Jensen 2000) and there is indirect evidence that dehydroalanine is formed in older lens proteins (Linetsky et al. 2004). Another process could potentially involve the hydroxyl side chain of Ser in a nucleophilic attack on the adjacent peptide bond (Paulus 2000). We are currently exploring this with model peptides. Interestingly, the rate of formation of d-Ser (Fig 3) was not linear across the whole age range and in this regard, the appearance of the graph mirrored that of Asp and Thr. Half of the increases in the d-forms occurred in the first 12 years of life. The reasons for this are not yet clear, but may suggest that some Ser, Thr and Asx residues are more susceptible to inversion than others. Asp 151 in alpha A crystallin, which has been identified by Fujii’s group as being particularly susceptible to racemisation in human lenses (Fujii et al. 1999b) may be one such site, since our initial data suggest that it is approximately 25% racemized by age 20. Conformationally, flexible regions, those in the terminal regions of proteins (Di Salvo et al. 1999) and those that contain favourable sequences, such as Asx-Gly, may be particularly susceptible to inversion (Radkiewicz et al. 2001).
Of major importance was the finding that the levels of racemisation were significantly higher in cataract lenses than in age-matched normal lenses. This was true for Ser, Thr and Asx. d-Thr levels were also higher in cataract lenses, however, the data were more scattered. Previously, there has been conflict in the literature concerning the levels of d-Asx in cataract lenses. Initially, Masters et al. (1977) reported elevated levels of Asp in cataract lenses; however, later investigations did not support this (Van den Oetelaar and Hoenders 1989).
It is clear from the graphs (Figs 2, 3 and 4) that even at the earliest ages at which age-related cataract occurs (i.e. age 40–50 years), that there are statistically elevated concentrations of racemised amino acids compared with normal lenses. Since the overall levels are highest for d-Asp, it is most clearly evident in this case. The percentage of racemisation of Asp/Asn in 40-year-old cataract lenses is approximately the same as that in 80-year-old normal lenses. A similar, but not identical, pattern was seen in the case of Ser. This is a remarkable finding and the d-amino acid graphs (Figs 2 and 3) match those seen for stiffness of cataract and normal lenses (Heys and Truscott 2008). One might conclude that this correlation is evidence for the involvement of racemisation in the aetiology of cataract. In our study, the type of cataract did not appear to influence the degree of racemisation.
One factor which governs the rate of racemisation is temperature (Fujii et al. 1996; Collins et al. 1999). In model studies using intact lenses, we showed that exposure to elevated temperatures caused significantly raised concentrations of d-amino acid residues (Fig 9). Thermal energy increases the rate of processes such as keto-enol tautomerisation (Schwass and Finley 1984) that can convert l- to d-amino acids. The 3D structure of individual proteins will affect the rate of racemisation at particular sites. For instance, Asp residues inserted into alpha helical peptides are more prone to racemization than those in beta-sheets, (Kuge et al. 2007) and there are conformational constraints on aspartic acid racemization in structured regions of proteins like the triple-helix of collagen (van Duin and Collins 1998). In intact lenses, elevating the temperature could potentially promote other processes, such as proteolysis, that may in turn affect racemisation. As yet, we do not have information on whether some proteins may be more susceptible to racemisation than others, or the sites involved.
Exposure to body temperature over a period of decades may be responsible for the age-related increase in d-amino acids in normal lenses, but it is less clear what could cause the increased levels of racemisation in cataract lenses. The answer is unknown, but could be related to somatic factors, or to the environment within the lens. Could, for example, the pH be slightly elevated in cataract lenses, since a higher pH is known to increase the rate of racemisation be implicated? It is interesting that body temperature varies significantly between individuals (Sund-Levander et al. 2002), is raised during infection, and can be influenced by caloric restriction (Roth et al. 2002). Body temperature at the high end of the normal range, diet and frequency of fevers could be predisposing factors. It is also known that the temperature in the eye can vary dramatically. For example, the lens temperature of a monkey placed in the midday sun rose from 35°C to almost 42°C within 5 min (Al-Ghadyan 1986), whereas in other animals maintained at 4°C, the lens temperature dropped to 28°C (Schwartz 1965). Such ambient and somatic temperature effects, possibly in combination with the incidence of infection, may explain the much earlier age of onset of cataract in India (Harding 1991). These aspects will be explored in future studies.
Substantial deamidation of lens proteins has been documented (Wilmarth et al. 2006; Hains and Truscott 2010) and, when coupled with racemisation, is likely to induce significant crystallin denaturation in older human lenses. In agreement with this, the insoluble crystallins that form from soluble proteins in the human lens over time, show elevated levels of both d-Asx (Masters et al. 1977; Garner and Spector 1978) and deamidation (Wilmarth et al. 2006). In the case of racemisation, the finding of increased denaturation in insoluble proteins may not be surprising considering the number of d-amino acids present in the crystallins of older human lenses (Table 1). The total number ranged from 1.9 to 3.1 per polypeptide chain. It is also possible that other amino acids that we were unable to measure with our assay system may also show age-dependent increases in l- to d-transformation with age.
In the elderly, racemisation of amino acids in crystallins of normal human lenses is clearly of major quantitative significance. It is probable that such degrees of inversion may play a role in alterations to the physical properties of older normal lenses such as the huge increase in stiffness that accompanies ageing (Heys et al. 2007). It is likely that every polypeptide chain in the centre of older lenses is altered and that this will result in conformational changes. Exposure of formerly buried hydrophobic regions in human crystallins may lead to aggregation and the formation of high molecular weight proteins (Buckingham 1972; Truscott and Augusteyn 1977a) and ultimately insolubilisation of crystallins. It is known that the levels of insoluble protein are much higher in nuclear cataract lenses (Pirie 1968; Truscott and Augusteyn 1977a) and this is probably involved in the induction of the lens opacity. It is obviously important to discover why the levels of racemisation are higher in cataract lenses. In future experiments, we plan to investigate sites of racemisation in individual crystallins and in other long-lived proteins (Ritz-Timme and Collins 2002).
Conclusions
Racemisation appears to be the single most abundant modification in the lifelong proteins isolated from older human lenses, and cataract lenses have significantly higher levels of d-amino acids than age-matched normal lenses. This is the first report of racemisation of Ser, Thr, Glu/Gln and Phe in the human lens.
A number of conclusions flow from this discovery. Firstly, because cataract and age-matched normal lens proteins differed significantly, racemisation measurements should be used with caution as an indicator of age. For each of the three most highly-racemised amino acid residues, d-Ser, d-Asx and d-Thr, the first decade of life was characterised by a significantly greater rate of racemisation than in later years.
Since exposure of intact lenses to elevated temperature promoted racemisation of amino acids, thermal stress may play a key role in determining the extent of racemisation. Factors, which influence ocular temperature, should be considered as risk factors for age-related cataract formation.
Supplementary material
Below is the link to the electronic supplementary material.
(DOC 25 kb)
(DOC 25 kb)
(DOC 25 kb)
(DOC 24 kb)
(DOC 24 kb)
(DOC 24 kb)
(DOC 24 kb)
Acknowledgements
The co-operation of Terry Lachlan and Colin Murray-Wallace at the University of Wollongong is gratefully acknowledged; in particular, assistance from Terry in running the HPLC was greatly appreciated. This study was supported by grants from the NIH (EY013570) and National Health and Medical Research Council (NHMRC) Grant 512334. RJWT is a NHMRC Senior Research Fellow. MH is the recipient of a Save Sight Institute PhD scholarship.
References
- Al-Ghadyan C. Rise in lens temperature on exposure to sunlight or high ambient temperature. Br J Ophthalmol. 1986;70:421–426. doi: 10.1136/bjo.70.6.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham RH. The behaviour of reduced proteins from normal and cataractous lenses in highly dissociating media: a cross-linked protein in cataractous lenses. Exp Eye Res. 1972;14:123–129. doi: 10.1016/0014-4835(72)90057-7. [DOI] [PubMed] [Google Scholar]
- Capasso S, Cerbo P. Kinetic and thermodynamic control of the relative yield of the deamidation of asparagine and isomerization of aspartic acid residues. J Pept Res. 2000;56:382–387. doi: 10.1034/j.1399-3011.2000.00778.x. [DOI] [PubMed] [Google Scholar]
- Cheng R, Feng Q, Argirov OK, Ortwerth BJ. Structure elucidation of a novel yellow chromophore from human lens proteins. J Biol Chem. 2004;279:45441–45449. doi: 10.1074/jbc.M405664200. [DOI] [PubMed] [Google Scholar]
- Clarke S. Propensity for spontaneous succinimide formation from aspartyl and asparaginyl residues in cellular proteins. Int J Pept Protein Res. 1987;30:808–821. doi: 10.1111/j.1399-3011.1987.tb03390.x. [DOI] [PubMed] [Google Scholar]
- Cloos P, Christgau S. Non-enzymatic covalent modifications of proteins: mechanisms, physiological consequences and clinical applications. Matrix Biol. 2002;21:39–52. doi: 10.1016/S0945-053X(01)00188-3. [DOI] [PubMed] [Google Scholar]
- Cloos P, Jensen A. Age-related de-phosphorylation of proteins in dentin: a biological tool for assessment of protein age. Biogerontology. 2000;1:341–356. doi: 10.1023/A:1026534400435. [DOI] [PubMed] [Google Scholar]
- Collins MJ, Waite ER, Duin ACT. Predicting protein decomposition: the case of aspartic acid racemization kinetics. Philos Trans R Soc Lond. 1999;354:51–64. doi: 10.1098/rstb.1999.0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvo ML, Delle Fratte S, Maras B, Bossa F, Wright HT, Schirch V. Deamidation of asparagine residues in a recombinant serine hydroxymethyltransferase. Arch Bioch Biophys. 1999;372:271–279. doi: 10.1006/abbi.1999.1512. [DOI] [PubMed] [Google Scholar]
- Fagerholm P, Philipson B, Lindström B. Normal human lens—the distribution of protein. Exp Eye Res. 1981;33:615–620. doi: 10.1016/S0014-4835(81)80101-7. [DOI] [PubMed] [Google Scholar]
- Fujii N, Momose Y, Harada K. Kinetic study of racemization of aspartyl residues in model peptides of alpha A-crystallin. Int J Pept Protein Res. 1996;48:118–122. doi: 10.1111/j.1399-3011.1996.tb00821.x. [DOI] [PubMed] [Google Scholar]
- Fujii N, Harada K, Momose Y, Ishii N, Akaboshi M. d-Amino acid formation induced by a chiral field within a human lens protein during aging. Biochem Biophys Res Commun. 1999;263:322–326. doi: 10.1006/bbrc.1999.1279. [DOI] [PubMed] [Google Scholar]
- Fujii N, Takemoto LJ, Momose Y, Matsumoto S, Hiroki K, Akaboshi M. Formation of four isomers at the Asp-151 residue of aged human [alpha]A-crystallin by natural aging. Biochem Biophys Res Commun. 1999;265:746–751. doi: 10.1006/bbrc.1999.1748. [DOI] [PubMed] [Google Scholar]
- Garner WH, Spector A. Racemization in human lens: evidence of rapid insolubilization of specific polypeptides in cataract formation. Proc Natl Acad Sci USA. 1978;75:3618–3620. doi: 10.1073/pnas.75.8.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner WH, Spector A. Selective oxidation of cysteine and methionine in normal and senile cataractous lenses. Proc Natl Acad Sci USA. 1980;77:1274–1277. doi: 10.1073/pnas.77.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T, Clarke S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J Biol Chem. 1987;262:785–794. [PubMed] [Google Scholar]
- Hains PG, Truscott RJW. Age-dependent deamidation of life-long proteins in the human lens. Invest. Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.09-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding EF. An efficient, minimal storage procedure for calculating the Mann–Whitney U, generalized U and similar distributions. J Roy Statist Soc Ser C. 1984;33:1–6. [Google Scholar]
- Harding J. Cataract: biochemistry, epidemiology and pharmacology. London: Chapman and Hall; 1991. [Google Scholar]
- Helfman PM, Bada JL. Aspartic acid racemization in tooth enamel from living humans. Proc Natl Acad Sci USA. 1975;72:2891–2894. doi: 10.1073/pnas.72.8.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heys KR, Truscott RJW. The stiffness of human cataract lenses is a function of both age and the type of cataract. Exp Eye Res. 2008;86:701–703. doi: 10.1016/j.exer.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Heys KR, Friedrich MG, Truscott RJW. Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat shock protein, alpha-crystallin, in maintaining lens flexibility. Aging Cell. 2007;6:807–815. doi: 10.1111/j.1474-9726.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- Heys KR, Friedrich MG, Truscott RJW. Free and bound water in normal and cataractous human lenses. Invest Ophthalmol Vis Sci. 2008;49:1991–1997. doi: 10.1167/iovs.07-1151. [DOI] [PubMed] [Google Scholar]
- Kaufman DS, Manley WF. A new procedure for determining dl amino acid ratios in fossils using reverse phase liquid chromatography. Quatern Sci Rev. 1998;17:987–1000. doi: 10.1016/S0277-3791(97)00086-3. [DOI] [Google Scholar]
- Korlimbinis A, Truscott RJW. Identification of 3-hydroxykynurenine bound to proteins in the human lens. A possible role in age-related nuclear cataract. Biochem J. 2006;45:1950–1960. doi: 10.1021/bi051744y. [DOI] [PubMed] [Google Scholar]
- Kuge K, Brack A, Fujii N. Conformation-dependent racemization of aspartyl residues in peptides. Chem Eur J. 2007;13:5617–5621. doi: 10.1002/chem.200601677. [DOI] [PubMed] [Google Scholar]
- Lapko VN, Smith DL, Smith JB. In vivo carbamylation and acetylation of water-soluble human lens alphaB-crystallin lysine 92. Protein Sci. 2001;10:1130–1136. doi: 10.1110/ps.40901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapko VN, Smith DL, Smith JB. Methylation and carbamylation of human gamma-crystallins. Protein Sci. 2003;12:1762–1774. doi: 10.1110/ps.0305403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linetsky M, Hill JMW, LeGrand RD, Hu F. Dehydroalanine crosslinks in human lens. Exp Eye Res. 2004;79:499–512. doi: 10.1016/j.exer.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C, Heinemeier J (2008) Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout Life. PLoS ONE :1529-1531 [DOI] [PMC free article] [PubMed]
- MacCoss MJ, McDonald WH, Saraf A, Sadygov R, Clark JM, Tasto JJ, Gould KL, Wolters D, Washburn M, Weiss A, Clark JI, Yates JR., III Shotgun identification of protein modifications from protein complexes and lens tissue. Proc Natl Acad Sci USA. 2002;99:7900–7905. doi: 10.1073/pnas.122231399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A, Palla G, Gilav E. Racemization of aspartic acid in human articular cartilage. Connect Tissue Res. 1992;28:161–169. doi: 10.3109/03008209209015033. [DOI] [PubMed] [Google Scholar]
- Masters P, Bada J, Zigler J. Aspartic acid racemisation in the human lens during ageing and in cataract formation. Nature. 1977;268:71–73. doi: 10.1038/268071a0. [DOI] [PubMed] [Google Scholar]
- Masters PM, Bada JL, Zigler JS. Aspartic acid racemization in heavy molecular weight crystallins and water insoluble protein from normal human lenses and cataracts. Proc Natl Acad Sci USA. 1978;75:1204–1208. doi: 10.1073/pnas.75.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj RH, Sell DR, Prabhakaram M, Ortwerth BJ, Monnier VM. High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc Natl Acad Sci USA. 1991;88:10257–10261. doi: 10.1073/pnas.88.22.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Sakai M, Sadakane Y, Haga T, Goto Y, Kinouchi T, Saito T, Fujii N. Differential rate constants of racemization of aspartyl and asparaginyl residues in human alpha A-crystallin mutants. Biochem Biophys Res Commun. 2008;1784:1192–1199. doi: 10.1016/j.bbapap.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Paulus H. Protein splicing and related forms of protein autoprocessing. Annu Rev Biochem. 2000;69:447–496. doi: 10.1146/annurev.biochem.69.1.447. [DOI] [PubMed] [Google Scholar]
- Pirie A. Color and solubility of the proteins of human cataracts. Invest Ophthalmol Vis Sci. 1968;7:634–650. [PubMed] [Google Scholar]
- Powell J, Vine N, Crossman M. On the accumulation of d-aspartate in elastin and other proteins of the ageing aorta. Atherosclerosis. 1992;97:201–208. doi: 10.1016/0021-9150(92)90132-Z. [DOI] [PubMed] [Google Scholar]
- Radkiewicz JL, Zipse H, Clarke S, Houk KN. Neighboring side chain effects on asparaginyl and aspartyl degradation: an Ab initio study of the relationship between peptide conformation and backbone NH acidity. J Am Chem Soc. 2001;123:3499–3506. doi: 10.1021/ja0026814. [DOI] [PubMed] [Google Scholar]
- Ritz-Timme S, Collins MJ. Racemization of aspartic acid in human proteins. Ageing Res Rev. 2002;1:43–59. doi: 10.1016/S0047-6374(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Ritz S, Schutz HW. Aspartic acid racemization in intervertebral discs as an aid to postmortem estimation of age at death. J Forensic Sci. 1993;38:633–640. [PubMed] [Google Scholar]
- Robinson NE, Robinson AB. Molecular clocks: deamidation of asparaginyl and glutaminyl residues in peptides and proteins. Cave Junction, OR: Althouse Press; 2004. [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter JE. Biomarkers of caloric restriction may predict longevity in humans. Sci. 2002;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- Schwartz B. Environmental temperature and the ocular temperature gradient. Arch Ophthalmol. 1965;74:237–243. doi: 10.1001/archopht.1965.00970040239022. [DOI] [PubMed] [Google Scholar]
- Schwass DE, Finley JW. Heat and alkaline damage to proteins: racemization and lysinoalanine formation. J Agric Food Chem. 1984;32:1377–1382. doi: 10.1021/jf00126a040. [DOI] [Google Scholar]
- Shapira R, Wilkinson KD, Shapira G. Racemization of individual aspartate residues in human myelin basic protein. J Neurochem. 1988;50:649–654. doi: 10.1111/j.1471-4159.1988.tb02960.x. [DOI] [PubMed] [Google Scholar]
- Shapiro S, Endicott S, Province M, Pierce J, Campbell E. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of d-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87:1828–1834. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A, Chiesa R, Sredy J, Garner W. Camp-dependent phosphorylation of bovine lens alpha-crystallin. Proc Natl Acad Sci USA. 1985;82:4712–4716. doi: 10.1073/pnas.82.14.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava OP, Srivastava K. Beta B2-crystallin undergoes extensive truncation during aging in human lenses. Biochim Biophys Acta. 2003;301:44–49. doi: 10.1016/s0006-291x(02)02975-3. [DOI] [PubMed] [Google Scholar]
- Stabler TV, Byers SS, Zura RD, Kraus VB. Amino acid racemization reveals differential protein turnover in osteoarthritic articular and mensical cartilages. Arthritis Res Ther. 2009;11:34–42. doi: 10.1186/ar2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sund-Levander M, Forsberg C, Wahren LK. Normal oral, rectal, tympanic and axillary body temperature in adult men and women: a systematic literature review. Scand J Caring Sci. 2002;16:122–128. doi: 10.1046/j.1471-6712.2002.00069.x. [DOI] [PubMed] [Google Scholar]
- Truscott RJW. Age-related nuclear cataract—oxidation is the key. Exp Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Truscott RJW. Are ancient proteins responsible for the age-related decline in health and fitness? Rejuvenation Res. 2010;13:83–89. doi: 10.1089/rej.2009.0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott RJW, Augusteyn RC. Changes in human lens proteins during nuclear cataract formation. Exp Eye Res. 1977;24:159–170. doi: 10.1016/0014-4835(77)90256-1. [DOI] [PubMed] [Google Scholar]
- Truscott RJW, Augusteyn RC. Oxidative changes in human lens proteins during senile nuclear cataract formation. Biochim Biophys Acta. 1977;492:43–52. doi: 10.1016/0005-2795(77)90212-4. [DOI] [PubMed] [Google Scholar]
- Oetelaar PJM, Beijsterveldt LEC, Beckhoven JRCM, Hoenders HJ. Detection of aspartic acid enantiomers by chiral capillary gas chromatography: determination of in vivo racemization and reduction of metal induced background. J Chromatogr. 1986;368:135–143. doi: 10.1016/S0021-9673(00)91054-9. [DOI] [PubMed] [Google Scholar]
- Oetelaar PJM, Hoenders HJ. Racemization of aspartyl residues in proteins from normal and cataractous human lenses: an aging process without involvement in cataract formation. Exp Eye Res. 1989;48:209–214. doi: 10.1016/S0014-4835(89)80070-3. [DOI] [PubMed] [Google Scholar]
- Duin ACT, Collins MJ. The effects of conformational constraints on aspartic acid racemization. Org Geochem. 1998;29:1227–1232. doi: 10.1016/S0146-6380(98)00098-9. [DOI] [Google Scholar]
- Verzijl N, DeGroot J, Oldehinkel E, Bank RA, Thorpe SR, Baynes JW, Bayliss MT, Bijlsma JW, Lafeber FP, Tekoppele JM. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem J. 2000;350:381–387. doi: 10.1042/0264-6021:3500381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells-Knecht MC, Huggins TG, Dyer DG, Thorpe SR, Baynes JW. Oxidized amino acids in lens protein with age. Measurement of o-tyrosine and dityrosine in the aging human lens. J Biol Chem. 1993;268:12348–12352. [PubMed] [Google Scholar]
- Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, Pevzner PA, David LL. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystalline insolubility? J. Proteome Res. 2006;5:2554–2566. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
(DOC 25 kb)
(DOC 25 kb)
(DOC 25 kb)
(DOC 24 kb)
(DOC 24 kb)
(DOC 24 kb)
(DOC 24 kb)