Abstract
The antimicrobial activity of organic acids in combination with nonchemical treatments was evaluated for inactivation of Salmonella enterica serotype Typhimurium within 1 min. It was observed that the effectiveness of the multiple-hurdle treatments was temperature (P ≤ 0.05) and pH (P ≤ 0.05) dependent and corresponded to the degree of organic acid lipophilicity (sodium acetate being least effective and sodium propionate being the most effective). This led to the hypothesis that the loss in viability was due at least in part to cell membrane disruption. Evaluation of osmotic response, potassium ion leakage, and transmission electron micrographs confirmed treatment effects on the cell membrane. Interestingly, all treatments, even those with no effect on viability, such as with sodium acetate, resulted in measurable cellular stress. Microarray experiments explored the specific response of S. Typhimurium to sodium acetate and sodium propionate, the most similar of the tested treatments in terms of pKa and ionic strength, and found little difference in the changes in gene expression following exposure to either, despite their very different effects on viability. Taken together, the results reported support our hypothesis that treatment with heated, acidified, organic acid salt solutions for 1 min causes loss of S. Typhimurium viability at least in part by membrane damage and that the degree of effectiveness can be correlated with lipophilicity of the organic acid. Overall, the data presented here indicate that a combined thermal, acidified sodium propionate treatment can provide an effective antimicrobial treatment against Salmonella.
INTRODUCTION
Salmonella is a major food-borne pathogen contributing to thousands of cases of food-borne illness each year, with more than 40,000 confirmed cases reported in 2006 (9). Since 1996, Salmonella enterica serotypes Typhimurium and Enteritidis have continued to be the most common serotypes associated with illness (9). Moreover, the most recent CDC figures show that 34.2% and 21.9% of Salmonella Typhimurium isolates are resistant to two and five antibiotic subclasses, respectively (10). The United States Department of Agriculture (USDA) Food Safety and Inspection Service (FSIS) 2008 progress report found that, of the inspected industries, poultry processors were still the most common industrial segment to be Salmonella positive, with 7.3% of broilers positive for Salmonella (61, 62). Improved antimicrobial treatments are thus needed to improve poultry safety and lower the incidence of salmonellosis.
Researchers have explored a variety of antimicrobial techniques, including chemical and thermal treatments, to reduce Salmonella contamination of poultry carcasses. Chlorine in chill tanks is one of the most universally employed interventions (46), but it has several disadvantages, including production of toxic by-products (40) and loss of efficacy in the presence of organic debris (46). In addition, consumer interest in organic food products is increasing and chlorine (above the level found in drinking water) is not approved for use in processing of organic foods (11). For these reasons, there is interest in reducing the reliance on chlorine in poultry processing.
Organic acids and their salts have long been used as food additives and preservatives but also have food processing applications (45). Numerous organic acids and their salts, such as lactic acid/lactate and acetic acid/acetate, have generally recognized as safe (GRAS) status (12). Another benefit to organic acids is their ability to maintain antimicrobial efficacy even in the presence of extraneous organic matter (13), unlike chlorine (46). Organic acids have several different putative antimicrobial mechanisms, including osmotic stress, disruption of intracellular pH, and newer concepts like membrane perturbation (for a review, see reference 25). Much research on the antimicrobial nature of organic acids focuses on their accumulation in the cytoplasm, potentially leading to both osmotic stress and cytoplasmic acidification, but scientists are moving away from this in light of developments suggesting that bacteria do not always require a pH-neutral cytoplasm (28, 36). Furthermore, the existence of organic acid-specific effects has been confirmed by recent research demonstrating divergent changes in gene expression following exposure to various organic acids. For example, it has been observed that acetate (17, 18, 30) and formate (26) stimulate virulence gene expression in Salmonella while propionate and butyrate can repress it (17, 18, 23).
Thermal treatments are another common antimicrobial intervention. Thermal treatments of poultry carcasses have been shown to lower carcass contamination with the food-borne pathogen Campylobacter (7). Thermal treatments have also been studied for efficacy against Salmonella (37, 38). The treatment temperatures explored range from 55°C up to 70°C (7, 37, 38), but it has been noted that temperatures around 70°C can lead to a partially cooked appearance (15). Reducing the length of thermal treatment application can help reduce adverse effects on the carcasses. Purnell and colleagues (43) showed that a thermal treatment of 75°C for 30 s can lead to tearing of the skin, while a modified thermal treatment of 70°C for 40 s followed by an immediate cool-down had no adverse effect.
Multiple-hurdle antimicrobial intervention treatments are often investigated for their potential to reduce microbial populations by use of combined mild treatments (31). When designing interventions for application in food processing, multiple-hurdle interventions are particularly advantageous because they can lead to effective reductions in food-borne pathogens using treatments at low intensities, which can maximize the likelihood that food quality will be maintained (39, 53). We have strategically designed a multiple-hurdle intervention employing select organic acid salts (sodium acetate [SA], sodium lactate [SL], and sodium propionate [SP]), pH, and temperature hurdles combined into one treatment. Previous research in our lab has shown similar treatments to be highly effective against S. Typhimurium (36). Here, we investigate the range of treatment effectiveness in vitro as well as the treatment's mechanism of action using viability, osmotic response, potassium leakage, and transmission electron microscopy (TEM) studies.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Salmonella enterica subsp. enterica serotype Typhimurium strain LT2 (ATCC 19585) (35) was used for the duration of the study. S. Typhimurium LT2 was stored as a glycerol stock at −80°C. Prior to an experiment, S. Typhimurium LT2 was streaked for isolation on brain heart infusion (BHI; Difco BD Diagnostics, Sparks, MD) agar and incubated at 37°C overnight. For growth, a single isolated colony was inoculated into a culture tube containing 5 ml of sterile BHI broth and incubated without aeration at 37°C. After 12 h, the culture was transferred 1:100 into fresh BHI broth and again incubated without aeration at 37°C. Following 12 h of incubation, the culture was transferred 1:1,000 into fresh BHI and incubated without aeration at 37°C until stationary phase (defined as 12 h postinoculation).
Application of heated, acidified organic acid salt treatments.
Heated, acidified organic acid salt treatments were applied as previously reported and with the following modifications (36). For each treatment, 50 μl of stationary-phase S. Typhimurium (approximately 105 CFU) was exposed to 450 μl of one of the following solutions: 1.25% or 2.5% sodium lactate (SL), sodium acetate (SA), or sodium propionate (SP) (all prepared in deionized H2O [dH2O] and adjusted to pH 4 or 7) or sterile dH2O adjusted to pH 4. The two pH levels were selected to evaluate the effect of the proportion of undissociated weak organic acid (Table 1). All organic acid salts were obtained from Sigma-Aldrich (St. Louis, MO), and solution percentages were selected to be within the range documented in current literature and federal regulations (22, 37). Prior to use, all solutions were sterilized by autoclaving for 20 min at 121°C and 15 lb/in2. The organic acid salt treatments were preheated to 50°C, 55°C, or 60°C prior to exposure to S. Typhimurium. Following addition of the solution, the treatments were placed in a water bath at the appropriate temperature for 1 min. Room-temperature treatments were applied for 5 min. These four test temperatures were chosen such that they were below the point (i.e., 70°C) at which poultry carcass quality has been shown to decline (15). Immediately following exposure, appropriate serial dilutions in phosphate-buffered saline (PBS, pH 7.4) were spread-plated onto BHI agar and the plates were incubated for 18 h at 37°C prior to enumeration of recovered Salmonella. Results were recorded as log10 CFU/ml with a detection limit of 1 log10 CFU/ml. All experiments were performed at least three independent times.
Table 1.
Relevant characteristics of pH 4 organic acid salt solutions and controls
| Treatment | Molecular formula | pH | % HAc | Estimated ionic strengthd | Salinity (ppt)d |
|---|---|---|---|---|---|
| Organic acid salt solutionsa | |||||
| 2.5% sodium acetate | C2H3NaO2 | 4 | 85 | 0.27 | 9.6 |
| 2.5% sodium lactate | C3H5NaO3 | 4 | 42 | 0.24 | 8.2 |
| 2.5% sodium propionate | C3H5NaO2 | 4 | 88 | 0.32 | 12.3 |
| Controls | |||||
| Deionized H2O | NAb | 4 | NA | 0.0074 | 0.4 |
| PBS | NA | 7.4 | NA | 0.32 | 12 |
Organic acid salt percentages were selected to be consistent with current literature and federal regulations (22, 38).
NA, not applicable.
% HA, percent undissociated weak organic acid at pH 4 as calculated by the Henderson-Hasselbach equation [pH = pKa + log10(A−/HA)] using the pKa values 4.76, 3.86, and 4.87 for SA, SL, and SP, respectively. For comparison purposes, at pH 7 the % HA values are 0.3, 0.1, and 0.7 for SA, SL, and SP, respectively.
Specific conductance (used in estimated ionic strength calculation) and salinity were measured using an Orion conductivity meter at 25°C. Ionic strength was calculated using the equation I = ∑[i] × zi2 (where i is each ion and z is its charge), and the Russell equation (I = 1.6 × 10−5 × specific conductance [in mmhos/cm] [49]) was used for calculating the estimated ionic strength.
Osmotic response assays.
The osmotic response ability of S. Typhimurium following exposure to the organic acid salt solution treatments was assessed. A loss of osmotic response has been correlated with a loss of membrane integrity (55, 56). Osmotic response was estimated by measuring the ability of Salmonella exposed to a given treatment to respond to PBS + 0.75 M NaCl relative to PBS alone. In hypertonic solutions (i.e., PBS + 0.75 M NaCl), cells will plasmolyze, resulting in an increase in optical density (OD) relative to the OD of the control (i.e., PBS) (32); this change in OD will here be referred to as the osmotic response. Osmotic response assays were performed essentially as described previously (52). Briefly, 1 ml of stationary-phase S. Typhimurium (approximately 109 CFU) was exposed to one of the following 50°C or 55°C treatments: 2.5% solutions of SA, SL, and SP (pH 4 or 7). At this high concentration of cells, all treatments were sublethal (data not shown), ensuring that the stress on only viable cells was measured. After 1 min of incubation, the bacterial suspensions were centrifuged for 5 min to remove the treatment and then resuspended in either PBS or PBS containing 0.75 M NaCl (PBS + 0.75 M NaCl). After 4 min of room-temperature incubation, optical density at 680 nm (OD680) of the PBS or PBS + 0.75 M NaCl suspensions was measured. Osmotic response data are reported as the percent OD increase ([ODPBS+NaCl/ODPBS] × 100) and represent the averages of at least three independent experiments.
Potassium measurements.
Potassium is an important intracellular ion in many bacteria, including Salmonella, and is actively imported into cells (59). Potassium leakage from Salmonella exposed to the organic acid salt treatments was used to estimate the treatments' membrane-damaging effects. Potassium was measured using a Genesis inductively coupled plasma optical emission spectrometer (ICP-OES; Spectro Analytical Instruments Inc.). For ICP-OES analysis, S. Typhimurium was grown as described to stationary phase. One milliliter of stationary-phase S. Typhimurium (approximately 109 CFU) was exposed to 9 ml of each of the following treatments at 55°C for 1 min: 2.5% solutions of SA, SL, and SP (pH 4 or 7) or dH2O (pH 4). This high concentration of cells was used to ensure that all treatments were sublethal (data not shown) and that only leakage from viable cells was measured. Immediately following treatment, bacterial suspensions were centrifuged for 10 min to pellet the cells and the supernatants were filter sterilized (0.2 μm). Filtered supernatants from treated cells were collected three independent times and held at 4°C until analysis. Potassium levels are presented as parts per million (ppm).
TEM.
Transmission electron microscopy (TEM) was used to visualize the effects of the organic acid salt treatments on the cell wall and membrane. Prior to TEM analysis, S. Typhimurium was grown as described to stationary phase. One milliliter of S. Typhimurium (approximately 109 CFU) was exposed to 9 ml of each of the following at 55°C for 1 min: 2.5% solutions of SA, SL, and SP (pH 4 or 7) and dH2O (pH 4). Immediately following, bacterial suspensions were centrifuged for 10 min to collect the cells. Cell pellets were fixed, sectioned, and stained as previously described (33). Briefly, pellets were fixed in a modified Karnovsky fixative (2% paraformaldehyde and 2.5% glutaraldehyde in 0.5 M cacodylate buffer, pH 7.2) and postfixed in 1% osmium tetroxide in 0.5 M cacodylate buffer, pH 7.2 (27). The cell pellets were stained overnight in 0.5% uranyl acetate at 4°C and then dehydrated with a graded ethanol series. The cell pellets were embedded in Spurr's medium at 70°C overnight (54). The embedded pellets were sectioned using a diamond knife and then stained with 2% uranyl acetate and Reynolds' lead citrate (44) for 4 min each. The stained cell pellet sections were viewed at 80 kV with a JEM 100 CX transmission electron microscope (JEOL, Tokyo, Japan), and all images were taken at 16,000×. All chemicals used in TEM specimen preparation were purchased from Electron Microscopy Supply (Hatfield, PA).
RNA isolation.
RNA was isolated in order to analyze S. Typhimurium gene expression after exposure to SA and SP at pH 4. These two treatments were specifically chosen because (i) acetic and propionic acids are similar in pKa and the corresponding pH 4 organic acid salt treatments have similar ionic strength and salinity (Table 1) and (ii) Salmonella itself produces SA (16) and therefore may have specific mechanisms in place for countering increased SA, possibly contributing to its observed enhanced survival following SA exposure (Fig. 1). In preparation for total RNA extraction, 1 ml of stationary-phase S. Typhimurium (approximately 109 CFU) was exposed to 9 ml of each of the following at 55°C for 1 min: 2.5% solutions of SA and SP and dH2O (all pH 4). All treatments were sublethal (data not shown) at this high concentration of cells, ensuring that RNA was collected only from viable cells. The treated S. Typhimurium cells were then centrifuged for 5 min to remove the treatment, and the cell pellets were resuspended in 1 ml of RNAProtect (Qiagen, Valencia, CA). After 5 min of incubation at room temperature, the RNAProtect was removed by centrifugation and the cell pellets were resuspended in 1 ml of Trizol (Invitrogen, Grand Island, NY). The cells were broken using the FastPrep system (Qbiogene, Irvine, CA) for 40 s at 6.0 m/s (rapid oscillating reciprocal motion/s). From the broken cell lysate, RNA was extracted per the manufacturer's instructions. Extracted RNA was purified using the RNeasy minikit with DNase treatment (Qiagen). RNA quantity and quality were determined by spectroscopy (NanoDrop 2000; NanoDrop Products, Wilmington, DE) and electrophoresis (Experion automated electrophoresis system using the Experion RNA StdSens analysis kit; Bio-Rad, Hercules, CA).
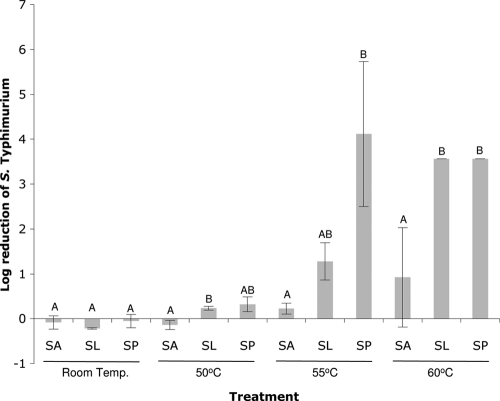
Fig. 1.
Reduction of S. Typhimurium viability following exposure to solutions of 2.5% sodium acetate (SA), sodium lactate (SL), and sodium propionate (SP) at pH 4 relative to the control treatment (dH2O, pH 4) at the four temperatures indicated. Error bars indicate standard deviations. Each of the experimental factors temperature and organic acid contributed significantly to the reduction of S. Typhimurium (P ≤ 0.05). Letters above the bars represent statistically significant differences within each temperature.
Microarray hybridization and processing.
cDNA was generated by using random hexamers (Invitrogen) as primers for reverse transcription. The primers were annealed (70°C for 10 min, followed by snap-freezing in ice for 1 min) to total RNA (2.5 μg) and were extended with SuperScript III reverse transcriptase (Invitrogen) with 0.1 M dithiothreitol, 12.5 mM deoxynucleoside triphosphate (dNTP)/5-(3-aminoallyl)-2′-dUTP, trisodium salt (aa-UTP; Ambion, Austin, TX) mix at 42°C overnight. Residual RNA was removed by alkaline treatment followed by neutralization, and cDNA was purified with a QIAquick PCR purification kit (Qiagen). Purified aminoallyl-modified cDNA was subsequently labeled with Cy3 or Cy5 monofunctional N-hydroxysuccinimide (NHS) ester cyanogen dyes (GE-Amersham Pharmacia Biotech, Piscataway, NJ) according to the manufacturer's instructions. Labeled cDNA was purified using a Qiagen PCR purification kit, and the purified labeled cDNA was hybridized with Salmonella Typhimurium genome microarrays version 8.0 provided by the Pathogen Functional Genomics Resource Center (PFGRC). The array design information is available at http://pfgrc.jcvi.org/index.php/microarray/array_description/salmonella_typhimurium/version8.html.
Microarray data analysis.
Hybridization signals were scanned using an Axon 4000B scanner (Axon Instruments) with Acuity 4.0 software, and scans were saved as a TIFF image. Scans were analyzed using TIGR-Spotfinder (www.tigr.org/software/) software, and the local background was subsequently subtracted. The data set was normalized by applying the LOWESS algorithm using TIGR-MIDAS (www.tigr.org/software/) software. The normalized log2 ratio of test/reference signal for each spot was recorded. The averaged log2 ratio for each gene on the six replicate slides was ultimately calculated. Significant changes of gene expression were identified with SAM (significance analysis of microarrays) software using one class mode (60). The SAM program was used to assign a score to each gene on the basis of change in gene expression relative to the standard deviation of repeated measurements. For genes with scores greater than the adjustable threshold, permutations of the repeated measurements were used to estimate the percentage of genes identified by chance, i.e., the false discovery rate (FDR) (60). A cutoff of 2-fold for over- and underexpressed open reading frames (ORFs) was used to identify differentially expressed genes. The differentially regulated genes were further classified according to the functional categories described in the comprehensive microbial resource of TIGR (http://cmr.tigr.org/tigr-scripts/CMR/shared/Genomes.cgi). To ensure the quality of the microarray experimental data, three independent biological replicates were used to prepare RNA samples. RNA samples obtained from each biological replicate were used to make Cy-dye-labeled probes for at least two separate arrays in which the incorporated dye was reversed (dye swap).
Statistical analyses.
For all experiments except microarrays, statistical analyses were performed using JMP 7.0. When performing two or more mean comparisons within a data set, one-way analysis of variance (ANOVA) or Tukey-Kramer honestly significant difference (HSD) was used. In all cases, statistical significance was set at α ≤ 0.05.
Microarray data accession number.
The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO series accession number GSE27922 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE27922) (19).
RESULTS
Heated, acidified solutions of SL and SP are effective against S. Typhimurium within 1 min.
The heated, acidified organic acid salt treatments were performed by exposing stationary-phase S. Typhimurium to 1.25 and 2.5% solutions of different organic acid salts (SA, SL, or SP) at four different temperatures (room temperature and 50, 55, and 60°C) and two pHs (4 and 7) as well as dH2O (pH 4). Overall, treatment effectiveness was found to be pH (P ≤ 0.05), temperature (P ≤ 0.05), and organic acid (P ≤ 0.05) dependent but the effect of organic acid concentration was not statistically significant (P ≥ 0.05). No statistically significant differences between any of the pH 7 treatments at any of the tested temperatures were observed (P ≥ 0.05, data not shown). Thus, results discussed below refer to the pH 4 2.5% organic acid treatments only (Fig. 1).
In general, increasing effectiveness of the pH 4 treatments, in terms of greater reduction in viability relative to the dH2O control, was observed with increasing temperature (Fig. 1) and organic acid lipophilicity, as approximated by the chemical structure (Table 1). At 55°C, S. Typhimurium viability following exposure to 2.5% SP resulted in a statistically significant reduction (4.12 log CFU/ml) relative to all other 55°C treatments except SL (1.28 logs). Interestingly, while reduction of S. Typhimurium exposed to 2.5% SA did increase from 0.23 logs at 55°C to 0.94 logs at 60°C, SA was still significantly less effective than SL and SP at 60°C (P ≤ 0.05). Thus, the synergistic reduction in S. Typhimurium survival observed with the 1-min combination treatment of 55 or 60°C heat and either of the acidic SL or SP treatments was not observed for SA, which had virtually no effect on survival, regardless of temperature.
Osmotic response measurements suggest that heated, acidified SL and SP treatments affect the S. Typhimurium membrane.
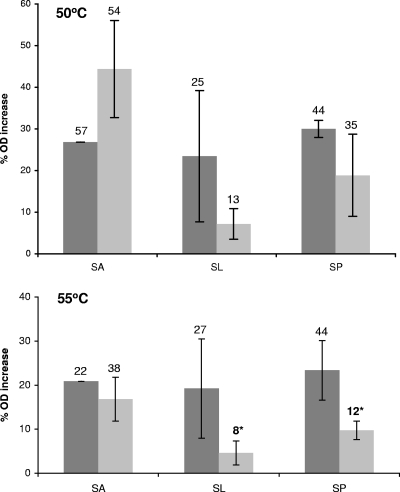
To better characterize the cause behind the observed loss of viability, S. Typhimurium was assessed for its osmotic response ability following exposure to pH 4 solutions of 2.5% SA, SL, or SP for 1 min. The organic acid salt solutions at pH 7 served as control treatments. At 50°C, effects on S. Typhimurium's osmotic response were apparent for the pH 4 organic acid salt treatments (Fig. 2), despite a lack of effect on viability at 50°C (Fig. 1). Compared to their pH 7 counterparts at 50°C, both SL and SP exhibited a reduced osmotic response with only 16 and 11% increases in OD, respectively. At 55°C, the reduced osmotic response following SL and SP pH 4 treatments compared with SL and SP at pH 7 was also observed (14.5 and 13.9%, respectively), and this reduction was significant (P < 0.05, Fig. 2).
Fig. 2.
Osmotic response of stationary-phase S. Typhimurium to PBS and PBS + 0.75 M NaCl as measured by the percent change in OD680. S. Typhimurium was exposed for 1 min to 50°C (top) or 55°C (bottom) solutions of 2.5% sodium lactate (SL), sodium propionate (SP), or sodium acetate (SA) at pH 7 (dark gray) or pH 4 (light gray). Error bars represent standard deviations, and numbers above each bar show the highest % OD increase recorded. The asterisks indicate pH 4 treatments that were significantly reduced compared to their pH 7 counterparts.
Potassium leakage and TEM analyses indicate that heated SL and SP treatments influence the S. Typhimurium membrane regardless of pH.
The results of the osmotic response assays were further supported by ICP-OES measurements of potassium loss from S. Typhimurium exposed to pH 4 solutions of 2.5% SA, SL, or SP at 55°C for 1 min (Table 2). The organic acid salt solutions at pH 7, pH 4 dH2O, and PBS (pH 7.4) served as controls. As expected, pH 4 dH2O as well as PBS resulted in minimal potassium leakage (approximately 2 ppm). These data are consistent with the robust osmotic response displayed by S. Typhimurium following exposure to 55°C pH 4 dH2O (data not shown). Surprisingly, SL and SP at pH 7 yielded potassium leakage values nearly identical to those observed at pH 4 (Table 2). Even exposure to SA had a modest effect, which was more pronounced at pH 4 (6.9 ppm) than pH 7 (3.5 ppm). Furthermore, the potassium leakage resulting from exposure to any of the organic acid salt treatments was significantly greater than that from either pH 4 dH2O or pH 7.4 PBS (P < 0.05).
Table 2.
Potassium leakage from S. Typhimurium exposed to multiple-hurdle treatments at 55°C
| Treatment | pH | Potassium lost (SD) (ppm)a |
|---|---|---|
| Organic acid salt solutions | ||
| 2.5% sodium acetate | 4 | 6.9 (0.17)D |
| 7 | 3.5 (0.21)E | |
| 2.5% sodium lactate | 4 | 16.5 (0.51)A |
| 7 | 14.3 (0.35)B | |
| 2.5% sodium propionate | 4 | 12.1 (0.01)C |
| 7 | 11.5 (0.12)C | |
| Controls | ||
| Deionized H2O | 4 | 2.4 (0.09)F |
| PBS | 7.4 | 2.2 (0.27)F |
Capital letters indicate statistical groupings.
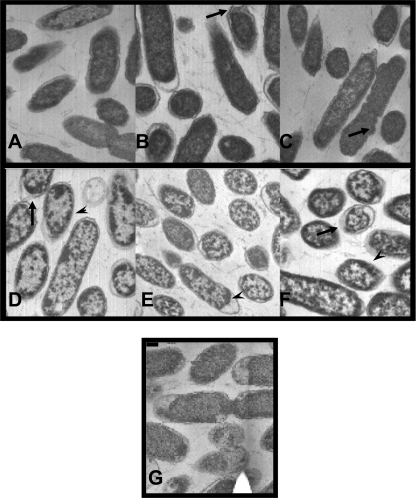
In order to visualize the treated cells, stationary-phase S. Typhimurium exposed to 55°C solutions of 2.5% SA, SL, or SP at pH 7 (Fig. 3A, B, and C, respectively) and pH 4 (Fig. 3D, E, and F) for 1 min was visualized using TEM. Aberrances in the cell wall/membrane were observed in cells exposed to all treatments, but cells exposed to the pH 4 organic acid salt solutions showed visible clumping of the cytoplasmic contents compared to cells exposed to the pH 7 solutions or the water control (Fig. 3G).
Fig. 3.
In preparation for TEM, stationary-phase S. Typhimurium cells were exposed to organic acid salt treatments at 55°C for 1 min. Images A, B, and C represent S. Typhimurium exposed to pH 7 solutions of 2.5% sodium acetate, sodium lactate, and sodium propionate, respectively, while images D, E, and F show cells following exposure to pH 4 solution of 2.5% sodium acetate, sodium lactate, and sodium propionate, respectively. Image G shows cells exposed to the control (pH 4 dH2O). Circular shapes depict cell cross sections, and long oval shapes show cells lengthwise. Cell wall/membrane aberrances are indicated by the large arrows, and clumping of the cytoplasmic contents is shown by arrowheads.
Microarray results suggest minimal differences in the response of S. Typhimurium to either 55°C pH 4 SA or SP exposure.
The viability, osmotic response, potassium leakage, and TEM data indicated existence of an organic acid-specific treatment effect on S. Typhimurium. We sought to better characterize the response of S. Typhimurium to these treatments by comparing its patterns of gene expression after exposure to SA and SP at pH 4. These treatments were specifically chosen because, despite the chemical similarities between the SA and SP treatments, the survival of S. Typhimurium following exposure to SP was generally significantly less compared to SA (Fig. 1).
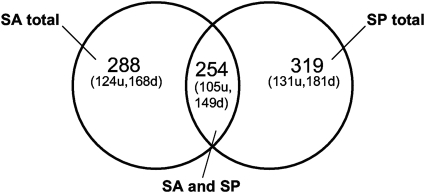
Stationary-phase S. Typhimurium was exposed to each of the following at 55°C for 1 min: 2.5% solutions of SA and SP and dH2O (all pH 4). Total RNA from SA- and SP-treated cells was competitively hybridized against that from the pH 4 dH2O-treated cells, providing an internal control for temperature and pH. Overall, it was found that 353 S. Typhimurium genes were differentially expressed in SA- and/or SP-treated cells, including 14 plasmid-associated genes (Fig. 4; see Table S1 in the supplemental material). For genes differentially expressed following treatment with SA and SP (n = 254), no opposing changes in gene expression were observed (i.e., if gene expression was up in SA it was also up in SP and vice versa). Transcription of 203 (59%) of the genes was repressed in at least one treatment, and 150 (41%) genes were induced. Seventeen percent of genes were differentially expressed in response to SP only (n = 65), while only 10% of genes were differentially expressed in response to SA only (n = 34). Expression of the transcriptional regulator rpoS was repressed in response to pH 4 SA or SP exposure (−2.23 and −2.17, respectively; Table S1). It was observed that numerous genes with functions in heat shock response or as molecular chaperones were repressed following either pH 4 SA or pH 4 SP treatment (e.g., dnaK, hptJ, dnaJ, grpE, clpP, and hscAB; Table S1). It was also found that genes related to regulation of attachment and/or motility (e.g., fimW, fimZ, and fliZ) were induced (Table S1).
Fig. 4.
Comparison of Salmonella Typhimurium gene expression in response to pH 4 sodium acetate (SA) and sodium propionate (SP) treatments (u, upregulated genes; d, downregulated genes).
DISCUSSION
Salmonella contamination of poultry is an important contributor to food-borne illness. In this study, multiple-hurdle treatments, combining organic acid salt, pH, and temperature hurdles, were examined for their mechanism of action against Salmonella in vitro. It was observed that a synergistic reduction of S. Typhimurium could be achieved with the combination of heat (>50°C), acidic pH, and at least two of the organic acid salts tested. Specifically, at 55°C both SL and SP, the more lipophilic organic acids, led to significant reductions in S. Typhimurium numbers within 1 min of treatment, but it was not until 60°C that SA, the least lipophilic, began to cause a substantial reduction.
Both organic acids and temperature are known to influence membranes by diffusion through them and by altering their fluidity, respectively (2, 25). This led us to suspect that the mechanism of efficacy of the thermal, organic acid salt treatments was through membrane disruption. In addition to diffusing through membranes, undissociated organic acids have been shown to partition into the bilayer in correlation with their lipophilicity and cause membrane perturbation (21, 57, 58). A study by Stratford et al. (58) examined the effects of the acetic and sorbic acid treatments (pH 4) on various spoilage yeasts at 28°C over the course of 28 days. They concluded that acetic acid can inhibit Aspergillus niger by cytoplasmic acidification, while the more lipophilic (and more toxic) sorbic acid did not cause a substantial drop in pHi. The researchers hypothesized that sorbic acid inhibited A. niger through perturbation of the membrane. The osmotic response and potassium leakage data shown here support the hypothesis that lipophilic organic acids (in this case lactic and propionic) affect the integrity of the cell membrane. The osmotic response data showed an effect of pH 4 SL and SP on S. Typhimurium at 50°C and 55°C. Sampathkumar et al. (52) also observed a similar reduction in the ability of S. Typhimurium to respond to a change in osmotic pressure following exposure to alkaline pH. Their data showed comparable osmotic responses at pH 7 (27%), while at pH 10 the response was reduced to approximately 16%, suggesting that alkaline pH also affects S. Typhimurium membrane integrity. Our potassium leakage results support the osmotic response data, clearly demonstrating that SL and SP caused the greatest potassium loss of the tested treatments.
It was notable that, while the 55°C pH 7 SL- and SP-exposed S. Typhimurium maintained an osmotic response and remained viable, these treatments still caused significant potassium leakage. It is commonly thought that it is the undissociated organic acids that cross or partition into the cell membrane and are responsible for the antimicrobial effects of organic acids (25). However, our pH 7 potassium leakage and TEM data suggest that even solutions of highly dissociated lipophilic organic acids (i.e., lactate and propionate at pH 7) are capable of weakening the membrane. TEM further revealed that cells exposed to any of the pH 4 organic acid salt treatments showed obvious cytoplasmic clumping. This clumping may indicate denatured proteins and ribosomes resulting from an acidic cytoplasmic pH facilitated by the now-leaky membrane. Similar compaction of the S. Typhimurium cytoplasm has previously been observed following exposure to 2.5% trisodium phosphate (pH 11), a treatment which was concluded to affect membrane integrity (52). Moreover, lactic acid has been shown to permeabilize the outer membrane of S. Typhimurium (1, 24). Helander and Mattila-Sandholm (24) observed that the presence of either lactic or citric acids increased cellular uptake of the fluorescent dye 1-N-phenylnaphthylamine when exposed to nisin (compared with uptake following exposure to nisin alone). The authors also noted that acetic, lactic, citric, and propionic acids at pH 4 were not sufficient to cause significant cellular leakage by the methods used in that study (i.e., measurement of nucleic acids lost following room-temperature exposure). This is consistent with our data, which showed no substantial reduction in viability caused by the room-temperature treatments.
The microarray data comparing the effects on gene expression of pH 4 SA and SP treatments further confirm the TEM results demonstrating that S. Typhimurium responses to any of the given treatments are very similar. This may be a result of the short exposure time (1 min), which may not allow for substantial changes in gene transcription. While collecting RNA following a longer exposure may have revealed more organic acid-specific changes in gene expression, it was important to perform the analysis using the short exposure time to reflect what would be most likely to occur in a processing setting (43). Following either treatment, no increases in transcription of rpoS or phoPQ, both genes known to play key roles in response to organic and inorganic acid stress in Salmonella (3, 6, 20, 29), were observed. Likewise, while acetate and propionate have been shown previously to influence virulence gene expression (17, 18, 23, 30), no significant changes were observed in transcription of virulence factors. There may be the potential for repression of at least one of the Salmonella pathogenicity islands (SPI1), as it was found that dnaK expression was repressed in response to either pH 4 SA or pH 4 SP treatment. dnaK has recently been demonstrated to contribute to SPI1 regulation via a series of intermediate genes that ultimately affect the SPI1 transcriptional regulator HilD (34). dnaK expression has also been shown to be inhibited by gamma irradiation (8), demonstrating that a variety of environmental conditions affect dnaK regulation. In addition to the SPI1 genes themselves, fimbrial genes also have important roles in Salmonella pathogenicity (14). FliZ has been shown to posttranslationally regulate FimZ, leading to a repression of type 1 fimbrial genes (51). The regulators fliZ and fimZ were induced following both pH 4 SA and SP exposure. It has also been demonstrated that FimZ can induce hilE, which is a negative regulator of SPI1 (5, 50). These data together support the hypothesis that the pH 4 SA and pH 4 SP treatments may in fact lead to a repression of virulence gene expression. The microarray analysis also revealed that numerous heat shock genes were repressed following either pH 4 SA or pH 4 SP treatment. These data support a recent study that found S. Typhimurium to be impaired in the synthesis of heat shock genes following membrane damage (41). Together, these data suggest that S. Typhimurium relies heavily on its membrane integrity in order to accurately respond to its surrounding environment.
Membrane destabilization weakens the cell against a variety of factors that can lead to cell death (e.g., changes in intracellular pH, loss of cellular contents, loss of the electrochemical gradient [in turn causing loss of motility and ATP production]), but it is not the only mechanism by which organic acids have been shown to work against Salmonella. A study from 2005 suggested that an intracellular pH drop following exposure to various organic acids can cause destabilization of cytoplasmic enzymes in Salmonella (42), and Barua et al. (4) determined that S. Typhimurium lipopolysaccharide (LPS) mutants were less fit to grow in the presence of acetic acid than the wild-type strain. These researchers suggested that LPS specifically may contribute to acid resistance. However, another explanation might be that any outer membrane damage (to LPS or otherwise) may also result in increased acid sensitivity, as is supported by our potassium leakage and TEM results. While our study cannot exclude other mechanisms of antimicrobial efficacy for the tested multiple-hurdle treatments (i.e., organic acid anion accumulation [47]) as contributing factors, the data support the conclusion that membrane destabilization is a critical component during exposure to the brief (1-min) SL and SP treatments tested.
Conclusions.
The significant reduction of S. Typhimurium that occurs following a multiple-hurdle treatment of select heated, acidified organic acid salt solutions can be attributed at least in part to the membrane-damaging effects of the combined thermal and organic acid treatment, with effectiveness increasing as organic acid lipophilicity increases. The membrane disruption then leaves Salmonella vulnerable to the acidic pH of the treatment. Existing literature has documented several possible mechanisms of action for organic acids, and these data further suggest that the mechanism may vary according to the accompanying conditions of exposure. Our results highlight that in spite of chemical similarity (i.e., SA and SP) antimicrobial effectiveness of organic acids can vary. Therefore, it may be important to take into account organic acid-specific effects prior to application of organic acid intervention treatments in a commercial setting. Overall, the data presented indicate that a combined thermal, acidified SP treatment may provide a simple yet effective antimicrobial treatment to combat Salmonella.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the University of Arkansas Water Quality Lab (Brian Haggard, director, and Stephanie Williamson, program associate) for performing the ICP-OES analysis. We especially thank M. G. Johnson and K. E. Gibson for their critical review of the manuscript.
This research was funded by a USDA NRI postdoctoral fellowship (award 2008-35201-04637) to S. R. Milillo and a USDA NIFSI grant (award 406-2008 51110) to S. C. Ricke. The Salmonella Typhimurium microarray slides were obtained through NIAID's Pathogen Functional Genomics Resource Center, managed and funded by the Division of Microbiology and Infectious Diseases, NIAID, NIH, DHHS, and operated by the J. Craig Venter Institute.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 8 April 2011.
REFERENCES
- 1. Alakomi H. L., et al. 2000. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 66:2001–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvarez-Ordonez A., Fernandez A., Lopez M., Arenas R., Bernardo A. 2008. Modifications in membrane fatty acid composition of Salmonella Typhimurium in response to growth conditions and their effect on heat resistance. Int. J. Food Microbiol. 123:212–219 [DOI] [PubMed] [Google Scholar]
- 3. Baik H. S., Bearson S., Dunbar S., Foster J. W. 1996. The acid tolerance response of Salmonella Typhimurium provides protection against organic acids. Microbiology 142:3195–3200 [DOI] [PubMed] [Google Scholar]
- 4. Barua S., et al. 2002. Involvement of surface polysaccharides in the organic acid resistance of Shiga toxin-producing Escherichia coli O157:H7. Mol. Microbiol. 43:629–640 [DOI] [PubMed] [Google Scholar]
- 5. Baxter M. A., Jones B. D. 2005. The fimYZ genes regulate Salmonella enterica serovar Typhimurium invasion in addition to type 1 fimbrial expression and bacterial motility. Infect. Immun. 73:1377–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bearson B. L., Wilson L., Foster J. W. 1998. A low-pH-inducible, PhoPQ-dependent acid tolerance response protects Salmonella Typhimurium against inorganic acid stress. J. Bacteriol. 180:2409–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berrang M. E., Dickens J. A., Musgrove M. T. 2000. Effects of hot water application after defeathering on the levels of Campylobacter, coliform bacteria, and Escherichia coli on broiler carcasses. Poult. Sci. 79:1689–1693 [DOI] [PubMed] [Google Scholar]
- 8. Caillet S., Millette M., Dussault D., Shareck F., Lacroix M. 2008. Effect of gamma radiation on heat shock protein expression of four foodborne pathogens. J. Appl. Microbiol. 105:1384–1391 [DOI] [PubMed] [Google Scholar]
- 9. CDC 2008. Salmonella surveillance: annual summary, 2006. U.S. Department of Health and Human Services, Atlanta, GA [Google Scholar]
- 10. CDC 2009. National antimicrobial resistance monitoring system for enteric bacteria (NARMS): 2006 human isolates final report. U.S. Department of Health and Human Services, Atlanta, GA [Google Scholar]
- 11. CFR 21 March 2011, accession date Code of Federal Regulations. Title 7, part 205, subpart G. U.S. Government Printing Office, Washington, DC: http://ecfr.gpoaccess.gov/cgi/t/text/text-idx?c=ecfr&sid=5ff47df8d096db4da5855c9fe7a6c239&rgn=div8&view=text&node=7:3.1.1.9.32.7.354.6&idno=7 [Google Scholar]
- 12. CFR 2 November 2010, accession date Code of Federal Regulations. Title 21, part 184, subpart B. U.S. Government Printing Office, Washington, DC: http://ecfr.gpoaccess.gov/cgi/t/text/text-idx?c=ecfr&sid=786bafc6f634363 4fbf79fcdca7061e1&rgn=div5&view=text&node=21:3.0.1.1.14&idno=21 [Google Scholar]
- 13. Cherrington C. A., Allen V., Hinton M. 1992. The influence of temperature and organic matter on the bactericidal activity of short-chain organic acids on salmonellas. J. Appl. Bacteriol. 72:500–503 [DOI] [PubMed] [Google Scholar]
- 14. Clegg S., Gerlach G. F. 1987. Enterobacterial fimbriae. J. Bacteriol. 169:934–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cox N. A., Mercuri A. J., Thomson J. E., Gregory D. W. 1974. Quality of broiler carcasses as affected by hot water treatments. Poult. Sci. 53:1566–1571 [Google Scholar]
- 16. Dunkley K. D., et al. 2009. Cell yields and fermentation responses of a Salmonella Typhimurium poultry isolate at different dilution rates in an anaerobic steady state continuous culture. Antonie Van Leeuwenhoek 96:537–544 [DOI] [PubMed] [Google Scholar]
- 17. Durant J. A., Corrier D. E., Ricke S. C. 2000. Short-chain volatile fatty acids modulate the expression of the hilA and invF genes of Salmonella Typhimurium. J. Food Prot. 63:573–578 [DOI] [PubMed] [Google Scholar]
- 18. Durant J. A., Corrier D. E., Stanker L. H., Ricke S. C. 2000. Expression of the hilA Salmonella Typhimurium gene in a poultry Salm. Enteritidis isolate in response to lactate and nutrients. J. Appl. Microbiol. 89:63–69 [DOI] [PubMed] [Google Scholar]
- 19. Edgar R., Domrachev M., Lash A. E. 2002. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30:207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foster J. W., Moreno M. 1999. Inducible acid tolerance mechanisms in enteric bacteria. Novartis Found. Symp. 221:55–69 [DOI] [PubMed] [Google Scholar]
- 21. Freese E., Sheu C. W., Galliers E. 1973. Function of lipophilic acids as antimicrobial food additives. Nature 241:321–325 [DOI] [PubMed] [Google Scholar]
- 22. FSIS 4 January 2011. Directive 7120.1, safe and suitable ingredients used in the production of meat, poultry, and egg products, revision 5. Food Safety and Inspection Service, Washington, DC: http://fsis.usda.gov/OPPDE/rdad/FSISDirectives/7120.1.pdf [Google Scholar]
- 23. Gantois I., et al. 2006. Butyrate specifically down-regulates Salmonella pathogenicity island 1 gene expression. Appl. Environ. Microbiol. 72:946–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Helander I. M., Mattila-Sandholm T. 2000. Permeability barrier of the gram-negative bacterial outer membrane with special reference to nisin. Int. J. Food Microbiol. 60:153–161 [DOI] [PubMed] [Google Scholar]
- 25. Hirshfield I. N., Terzulli S., O'Byrne C. 2003. Weak acids: a panoply of effects on bacteria. Sci. Prog. 86:245–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang Y., Suyemoto M., Garner C. D., Cicconi K. M., Altier C. 2008. Formate acts as a diffusible signal to induce Salmonella invasion. J. Bacteriol. 190:4233–4241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karnovsky M. J. 1965. Abstr. 5th Annu. Meet. Am. Soc. Cell Biol., abstr. 270 [Google Scholar]
- 28. Kashket E. R. 1987. Bioenergetics of lactic-acid bacteria—cytoplasmic pH and osmotolerance. FEMS Microbiol. Rev. 46:233–244 [Google Scholar]
- 29. Kwon Y. M., Ricke S. C. 1998. Induction of acid resistance of Salmonella Typhimurium by exposure to short-chain fatty acids. Appl. Environ. Microbiol. 64:3458–3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lawhon S. D., Maurer R., Suyemoto M., Altier C. 2002. Intestinal short-chain fatty acids alter Salmonella Typhimurium invasion gene expression and virulence through BarA/SirA. Mol. Microbiol. 46:1451–1464 [DOI] [PubMed] [Google Scholar]
- 31. Leistner L. 2000. Basic aspects of food preservation by hurdle technology. Int. J. Food Microbiol. 55:181–186 [DOI] [PubMed] [Google Scholar]
- 32. Mager J., Kuczynski M., Schatzberg G., Avi-Dor Y. 1956. Turbidity changes in bacterial suspensions in relation to osmotic pressure. J. Gen. Microbiol. 14:69–75 [DOI] [PubMed] [Google Scholar]
- 33. Martin E. M., et al. 2004. Novel cytopathological structures induced by mixed infection of unrelated plant viruses. Phytopathology 94:111–119 [DOI] [PubMed] [Google Scholar]
- 34. Matsui M., Takaya A., Yamamoto T. 2008. σ32-mediated negative regulation of Salmonella pathogenicity island 1 expression. J. Bacteriol. 190:6636–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McClelland M., et al. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852–856 [DOI] [PubMed] [Google Scholar]
- 36. Milillo S. R., Ricke S. C. 2010. Synergistic reduction of Salmonella in a model raw chicken media using a combined thermal and acidified organic acid salt intervention treatment. J. Food Sci. 75:M121–M125 [DOI] [PubMed] [Google Scholar]
- 37. Morrison G. J., Fleet G. H. 1985. Reduction of Salmonella on chicken carcasses by immersion treatments. J. Food Prot. 48:939–943 [DOI] [PubMed] [Google Scholar]
- 38. Murphy R. Y., Osaili T., Duncan L. K., Marcy J. A. 2004. Effect of sodium lactate on thermal inactivation of Listeria monocytogenes and Salmonella in ground chicken thigh and leg meat. J. Food Prot. 67:1403–1407 [DOI] [PubMed] [Google Scholar]
- 39. Nam K. C., Ahn D. U. 2003. Use of antioxidants to reduce lipid oxidation and off-odor volatiles of irradiated pork homogenates and patties. Meat Sci. 63:1–8 [DOI] [PubMed] [Google Scholar]
- 40. Odabasi M. 2008. Halogenated volatile organic compounds from the use of chlorine-bleach-containing household products. Environ. Sci. Technol. 42:1445–1451 [DOI] [PubMed] [Google Scholar]
- 41. Porta A., et al. 2010. Genetic modification of the Salmonella membrane physical state alters the pattern of heat shock response. J. Bacteriol. 192:1988–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Price-Carter M., Fazzio T. G., Vallbona E. I., Roth J. R. 2005. Polyphosphate kinase protects Salmonella enterica from weak organic acid stress. J. Bacteriol. 187:3088–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Purnell G., Mattick K., Humphrey T. 2004. The use of ‘hot wash’ treatments to reduce the number of pathogenic and spoilage bacteria on raw retail poultry. J. Food Eng. 62:29–36 [Google Scholar]
- 44. Reynolds E. S. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17:208–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ricke S. C. 2003. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 82:632–639 [DOI] [PubMed] [Google Scholar]
- 46. Ricke S. C., Kundinger M. M., Miller D. R., Keeton J. T. 2005. Alternatives to antibiotics: chemical and physical antimicrobial interventions and foodborne pathogen response. Poult. Sci. 84:667–675 [DOI] [PubMed] [Google Scholar]
- 47. Russell J. B. 1992. Another explanation for the toxicity of fermentation acids at low pH—anion accumulation versus uncoupling. J. Appl. Bacteriol. 73:363–370 [Google Scholar]
- 48. Reference deleted.
- 49. Russell L. L. 1976. Chemical aspects of groundwater recharge with wastewaters. Ph.D. thesis University of California, Berkeley, CA [Google Scholar]
- 50. Saini S., Pearl J. A., Rao C. V. 2009. Role of FimW, FimY, and FimZ in regulating the expression of type I fimbriae in Salmonella enterica serovar Typhimurium. J. Bacteriol. 191:3003–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saini S., Slauch J. M., Aldridge P. D., Rao C. V. 2010. Role of cross talk in regulating the dynamic expression of the flagellar Salmonella pathogenicity island 1 and type 1 fimbrial genes. J. Bacteriol. 192:5767–5777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sampathkumar B., Khachatourians G. G., Korber D. R. 2003. High pH during trisodium phosphate treatment causes membrane damage and destruction of Salmonella enterica serovar Enteritidis. Appl. Environ. Microbiol. 69:122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sommers C., Kozempel M., Fan X., Radewonuk E. R. 2002. Use of vacuum-steam-vacuum and ionizing radiation to eliminate Listeria innocua from ham. J. Food Prot. 65:1981–1983 [DOI] [PubMed] [Google Scholar]
- 54. Spurr A. R. 1969. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26:31–43 [DOI] [PubMed] [Google Scholar]
- 55. Strange R. E. 1964. Effect of magnesium on permeability control in chilled bacteria. Nature 203:1304–1305 [DOI] [PubMed] [Google Scholar]
- 56. Strange R. E., Postgate J. R. 1964. Penetration of substances into cold-shocked bacteria. J. Gen. Microbiol. 36:393–403 [DOI] [PubMed] [Google Scholar]
- 57. Stratford M., Anslow P. A. 1998. Evidence that sorbic acid does not inhibit yeast as a classic ‘weak acid preservative’. Lett. Appl. Microbiol. 27:203–206 [DOI] [PubMed] [Google Scholar]
- 58. Stratford M., Plumridge A., Nebe-von-Caron G., Archer D. B. 2009. Inhibition of spoilage mould conidia by acetic acid and sorbic acid involves different modes of action, requiring modification of the classical weak-acid theory. Int. J. Food Microbiol. 136:37–43 [DOI] [PubMed] [Google Scholar]
- 59. Su J., Gong H., Lai J., Main A., Lu S. 2009. The potassium transporter Trk and external potassium modulate Salmonella enterica protein secretion and virulence. Infect. Immun. 77:667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tusher V. G., Tibshirani R., Chu G. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U. S. A. 98:5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. USDA/FSIS 2008. Isolation and identification of Salmonella from meat, poultry, and egg products. MLG 4.02, rev. 10/25/02 USDA/FSIS microbiology laboratory guidebook, 3rd ed U.S. Department of Agriculture, Food Safety Inspection Service, Washington, DC [Google Scholar]
- 62. USDA/FSIS 2008. Progress report on Salmonella testing of raw meat and poultry products, 1998–2008. Food Safety and Inspection Service, Washington, DC [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.