Abstract
Like MTL-heterozygous (a/α) cells, white MTL-homozygous (a/a or α/α) cells of Candida albicans, to which a minority of opaque cells of opposite mating type have been added, form thick, robust biofilms. The latter biofilms are uniquely stimulated by the pheromone released by opaque cells and are regulated by the mitogen-activated protein kinase signal transduction pathway. However, white MTL-homozygous cells, to which opaque cells of opposite mating type have not been added, form thinner biofilms. Mutant analyses reveal that these latter biofilms are self-induced. Self-induction of a/a biofilms requires expression of the α-receptor gene STE2 and the α-pheromone gene MFα, and self-induction of α/α biofilms requires expression of the a-receptor gene STE3 and the a-pheromone gene MFa. In both cases, deletion of WOR1, the master switch gene, blocks cells in the white phenotype and biofilm formation, indicating that self-induction depends upon low frequency switching from the white to opaque phenotype. These results suggest a self-induction scenario in which minority opaque a/a cells formed by switching secrete, in a mating-type-nonspecific fashion, α-pheromone, which stimulates biofilm formation through activation of the α-pheromone receptor of majority white a/a cells. A similar scenario is suggested for a white α/α cell population, in which minority opaque α/α cells secrete a-pheromone. This represents a paracrine system in which one cell type (opaque) signals a second highly related cell type (white) to undergo a complex response, in this case the formation of a unisexual white cell biofilm.
INTRODUCTION
Approximately 90% of Candida albicans isolates are a/α and 10% either a/a or α/α (24, 25, 50). For a/α strains to mate, they must undergo homozygosis to a/a or α/α (20, 21, 29). Then, a/a and α/α strains must switch from the white to opaque phenotype (46) in order to be mating competent (27, 30). Opaque cells secrete a cell type-specific pheromone, which stimulates the mating response in cells of opposite mating type (5, 26, 38). However, in a fashion unique to C. albicans, these pheromones also induce mating-incompetent white cells, but not mating-competent opaque cells, of opposite mating type to form robust biofilms, similar morphologically to those formed by a/α cells (13, 42–44, 48, 53–55). These MTL-homozygous white cell biofilms have been demonstrated in vitro to facilitate mating between opaque a/a and α/α cells (13, 48).
Although the addition of minority opaque cells of opposite mating type (1 to 10%) to a population of white cells increases the thickness of the final white cell biofilm by more than 50%, single-sex white a/a or α/α cell populations, to which cells of opposite mating types have not been added, also form robust biofilms composed of a basal layer of yeast cells and a thick upper layer of hyphae and matrix (13, 42–44, 53–55). We previously showed that deletion of the α-receptor gene STE2 results in highly reduced, abnormal white a/a cell biofilms in the absence of minority opaque α/α cells, suggesting that the basic unisexual biofilm formed by white a/a cells is self-induced through the release of α-pheromone, which activates the biofilm pathway through the α-pheromone receptor (53, 54). Alby et al. (1) subsequently demonstrated that C. albicans a/a cells could undergo low-frequency same-sex mating that was also dependent on STE2, as well as MFα expression, indicating that a/a cells released α-pheromone for self-mating in an autocrine-like fashion. Our original observation (53, 54) and that of Alby et al. (1) clearly showed that a major rule in the sexual strategy of the hemiascomycetes was breached in C. albicans, namely, that a/a cells could secrete pheromone of opposite mating type (i.e., α-pheromone) that was self-inducing. However, our observation (53, 54) also suggested that a second rule might also have been breached, namely, that white a/a cells secreted pheromone. In experiments on the C. albicans mating system, it was clearly established through crosses that opaque cells, not white cells, released pheromone for the mating process (5, 26).
We have therefore explored three questions related to self-induced, same-sex biofilm formation. First, we tested whether self-induction is indeed based on the release by a/a cells of α-pheromone by testing whether deletion of the MFα gene results in the same defects in white a/a cell biofilm formation as deletion of the gene for the α-pheromone receptor, STE2. Second, we tested whether deletion of STE3 and MFa in α/α cells results in similar white α/α cell biofilm defects. Third, we tested whether a minority of cells must switch to opaque in a same-sex white cell population in order to form a basic white cell biofilm. To accomplish the last of these tests, we generated deletion mutants of the WOR1 master switch gene (18, 49, 56) in an a/a and α/α strain. Our results indicate that for both a/a and α/α cells, a similar paracrine system regulates self-induction of same-sex biofilm formation. In a single-sex population of either white a/a or white α/α cells, a minority must undergo low-frequency, spontaneous switching to the opaque phenotype, and these minority same-sex opaque cells release α-pheromone or a-pheromone, respectively, to activate the α-pheromone receptor or a-pheromone receptor, respectively, of majority white a/a or α/α cells.
MATERIALS AND METHODS
Strains and media.
The names, genotypes, and origins of the C. albicans strains used in the present study are listed in Table S1 in the supplemental material. All strains were maintained at 25°C on agar plates containing YPD medium or modified Lee's medium (4, 23) supplemented with phloxine B (5 μg/ml), which distinguishes between white and opaque colonies (3). For experimental purposes, cells from 5-day colonies were inoculated into fresh liquid modified Lee's medium and grown at 25°C in a water bath with vigorous shaking until they reached stationary phase.
Mutant construction and complementation.
Mutants were derived from the natural a/a strain P37005 (25) or the natural α/α strain WO-1 (46). The plasmid pSFS2A (40), harboring a recyclable flipper cassette SAT1-2A with a dominant nourseothricin resistance (SATr) marker, was used for mutant construction. This plasmid was a generous gift from Joachim Morschhäuser at the University of Würzburg, Würzburg, Germany. All of the primers used to create gene deletions are provided in Table S2 in the supplemental material. To generate the homozygous deletion mutant of a given gene, a two-step PCR disruption strategy was used. A deletion cassette was constructed by amplifying the 5′ and 3′ flanking regions of each target gene by the PCR using primers listed in Table S2 in the supplemental material. The 5′ and 3′ regions were then digested with SmaI and ligated together using T4 ligase. The 5′-3′ fusion product was amplified by PCR and subcloned into the pGEM-T Easy vector (Promega, Madison, WI). The SAT1-2A fragment was then inserted into the SmaI-digested, dephosphorylated plasmid. This plasmid was digested with SacI plus SphI to generate the deletion cassette, which was then used for C. albicans transformation by electroporation (14). For each gene, two independent transformants were confirmed as heterozygous by both PCR and Southern analysis. The heterozygotes were then subjected to a popout strategy in the maltose-containing medium YPM (1% yeast extract, 2% Bacto peptone, and 2% maltose) to excise the CaSAT1 marker. A second deletion cassette was then constructed in a similar manner. The new 5′ and 3′ flanking regions that contained sequences deleted in the first step were amplified by PCR, using the primers noted for each gene in Table S2 in the supplemental material. The resulting plasmid was digested with SacI and SphI and used to transform the heterozygous mutant derivatives. Null mutants for each gene were confirmed by both PCR and Southern analysis.
For complementation of a homozygous deletion mutant, the CaSAT1 marker was deleted from each null mutant by a popout protocol described for heterozygous mutants (42, 53, 54). The 5′ and 3′ regions flanking the stop codon were amplified by PCR with the primers noted for each gene in Table S2 in the supplemental material. The 5′-3′ fusion product was amplified by PCR and subcloned into pGEM-T Easy (Promega). The SAT1-2A fragment was then inserted into the SmaI-digested, dephosphorylated plasmid. The resulting plasmid was digested with SacI and SphI and used for transformation of the null mutant of each gene. Transformants were verified by both PCR sequencing and Southern analysis.
Characteristics of biofilm formation.
Methods for measuring the biomass of a biofilm (35, 55), the release of β-glucan from the biofilm matrix (36, 42, 55), biofilm thickness (13, 55), and the cell density at the substrate of a biofilm (55) have previously been described in detail. Biofilms grown for 48 h on an elastomer surface were developed according to methods previously described (13, 55). To quantitate safranin O staining (11, 45), 48-h biofilms grown on elastomer squares were stained with 1% safranin O solution. After 20 min of incubation at 25°C, the wells were washed with distilled water. The safranin O bound to a biofilm was then eluted with 95% ethanol, and the optical density of the extract at 540 nm was measured by using a microplate reader (MDS Analytical Technologies, Ontario, Canada).
RT-PCR.
Biofilms were treated with 0.05% trypsin-EDTA solution (Invitrogen) to release them from the substrate. Total RNA was extracted by using an RNeasy minikit (Qiagen, Valencia, CA). Reverse transcription-PCR (RT-PCR) was used to assess gene expression levels according to methods previously described (28, 55). The primers used are listed in Table S3 in the supplemental material.
RESULTS
MFα is necessary for self-stimulation of biofilm formation by white a/a cells.
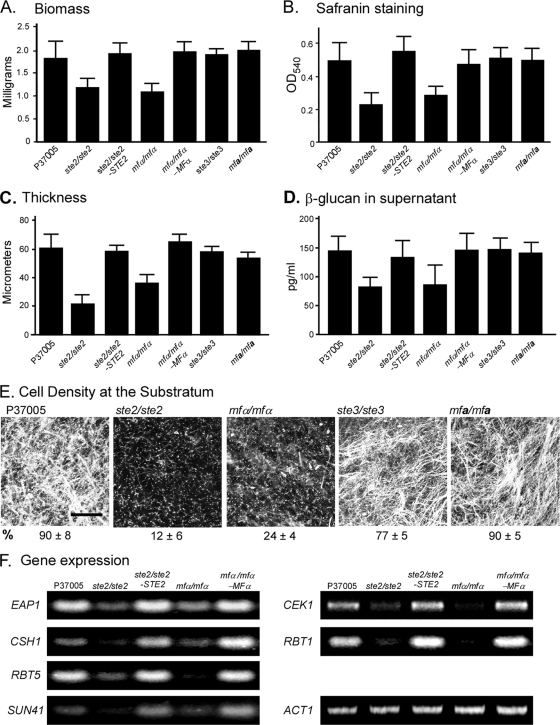
We previously demonstrated that STE2, which encodes the α-pheromone receptor, is essential for biofilm formation in a white a/a cell population to which no α/α cells were added (53, 54). Self-induction could be the result of spontaneous activation by the receptor without a ligand or activation by α-pheromone released from the same a/a cells, as has been shown to be the case for low-frequency homothallic mating (1). To test between these alternatives, biofilm formation was compared among the deletion mutant of MFα, which encodes the α-pheromone protein, the deletion mutant of STE2, the natural parental a/a strain P37005, and the complemented mfα1/mfα1-MFα1 and ste2/ste2-STE2 mutant strains. The mfα1/mfα1 deletion mutant exhibited defects in biofilm formation similar to those of the ste2/ste2 deletion mutant, including a decrease in biofilm biomass (Fig. 1A), a decrease in safranin staining of biofilms (Fig. 1B), a decrease in biofilm thickness (Fig. 1C), a decrease in β-glucan released into the supporting medium by the biofilm matrix (Fig. 1D), and a decrease in the cell density of the biofilm at the substratum (Fig. 1E). The ste2/ste2-STE2 and mfα/mfα-MFα complemented strains formed biofilms with characteristics similar to those of biofilms formed by the parental strain (Fig. 1A, B, C, and D).
Fig. 1.
Self-induction of a same-sex white a/a biofilm depends upon the α-pheromone receptor STE2 and the α-pheromone gene MFα, but not the a-pheromone receptor STE3 or the a-pheromone gene MFa. Analysis was performed on 48-h biofilms of the parental strain P37005; the ste2/ste2, mfα/mfα, ste3/ste3, and mfa/mfa deletion mutants; and the ste2/ste2-STE2, mfα/mfα-MFα, ste3/ste3-STE3, and mfa/mfa-MFa complemented mutant strains. (A) Biofilm biomass; (B) safranin O straining of biofilms, a reflection of biomass; (C) biofilm thickness; (D) β-glucan released into the supernatant; (E) cell density of biofilms at the substratum; (F) gene expression, measured by RT-PCR. ACT1 expression serves as a control for equal loading. Bar in panel E, 100 μm.
Since the genes EAP1, CSH1, RBT5, SUN41, CEK1, and RBT1 have been shown to be upregulated by the addition of α-pheromone to white cells (42), we tested whether upregulation of the six genes was defective in the ste2/ste2 and mfα/mfα mutants. All six tested genes exhibited dramatically reduced levels of expression in both mutants (Fig. 1F). Expression of both was restored in the complemented strains (Fig. 1F). Together, these results demonstrate that deletion of either STE2 or MFα results in similar defects in white a/a cell biofilm formation, suggesting that self-induction is mediated by the activation of α-pheromone receptors through the release of α-pheromone by the same a/a cells.
Ste3 and a-pheromone are not involved in self-induction of white a/a biofilms.
Deleting STE3, which encodes the a-pheromone receptor, or MFa, which encodes the a-pheromone, in the a/a strain P37005 had no measurable effect on self-induction of white cell biofilm formation. The biofilms formed by white cells of the ste3/ste3 and mfa/mfa mutants exhibited biofilm biomass (Fig. 1A), safranin staining (Fig. 1B), thickness (Fig. 1C), β-glucan release (Fig. 1D), and cell densities at the substratum (Fig. 1E) similar to that of biofilms formed by white a/a cells of the parental wild-type strain P37005.
Self-stimulation of white a/a biofilms requires white to opaque switching.
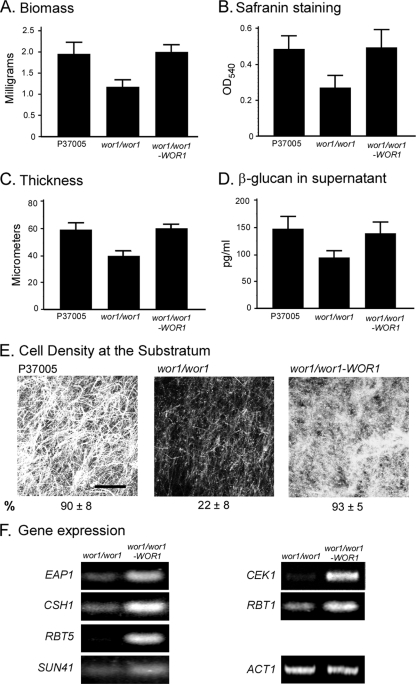
The preceding results indicate that self-stimulation depends upon the release of α-pheromone in a white a/a cell population. However, studies have indicated that it is opaque cells, not white cells, that release pheromone during mating (5, 26, 38). We therefore entertained the hypothesis that it is the minority of opaque a/a cells formed in white a/a cell populations through spontaneous switching (3, 6, 41, 46) that produces the α-pheromone that activates the α-pheromone receptors of white cells in same-sex biofilm formation. To test this hypothesis, we generated a deletion mutant of WOR1, the master switch gene essential for the white to opaque transition (18, 49, 56), in the a/a strain P37005. Cells of the wor1/wor1 mutant are blocked in the white phenotype (18, 49, 56). Plating experiments revealed that white wor1/wor1 cell populations contained no detectable opaque cells (<10−4). The frequencies of opaque cells in the parental strain P37005 and the complemented wor1/wor1-WOR1 strain ranged between 10−2 and 10−3. White a/a cells of the wor1/wor1 mutant exhibited reductions in biofilm biomass (Fig. 2A), safranin staining of biofilms (Fig. 2B), biofilm thickness (Fig. 2C), β-glucan release from biofilms (Fig. 2D), and the density of cells in the biofilm at the substratum (Fig. 2E). These parameters were restored in the wor1/wor1-WOR1 complemented strain. The genes EAP1, CSH1, RBT5, SUN41, CEK1, and RBT1 were also expressed at lower levels in the wor1/wor1 mutant than in the wor1/wor1-WOR1 complemented strain (Fig. 2F). The decreases in wor1/wor1 cells for all tested parameters were similar to those observed for the ste2/ste2 and mfα/mfα mutants (Fig. 1). These results support the hypothesis that self-induction of biofilm formation in a white a/a cell population depends upon the capacity of a minority of white cells to switch to the opaque phenotype, the latter presumably responsible for the release of α-pheromone.
Fig. 2.
Self-induction of a same-sex white a/a cell biofilm depends upon WOR1, the master switch gene necessary for the white-to-opaque transition. Biofilms (48 h) were analyzed for the parental strain P37005, the deletion wor1/wor1 mutant, and the wor1/wor1-WOR1 complemented mutant. See the legend to Fig. 1 for explanations of the panels.
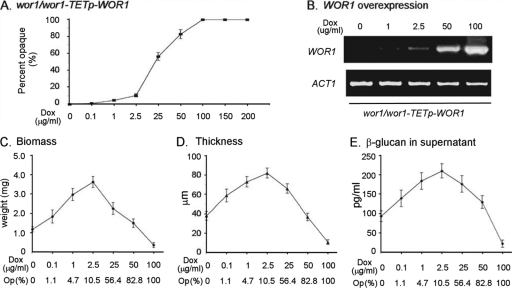
The proportion of white cells that spontaneously switch to opaque can vary according to the culture conditions (2, 19, 32, 39, 46). Under the conditions used here to culture white cells, plating experiments revealed that the frequency of opaque cells in five day colonies of the parental train P37005 was approximately 10−2 to 10−3. If white cell biofilm formation requires low-frequency switching to the opaque phenotype, then incrementally increasing the percentage of minority opaque cells in a white cell population should result in incremental increases in biofilm parameters, until opaque cells, which do not form biofilms (13), interfere with white cell biofilm formation. To test this prediction, we generated the wor1/wor1 derivative, wor1/wor1-TETp-WOR1, in which WOR1 is under the tetracycline (doxycycline)-inducible promoter TETp. By increasing incrementally the concentration of doxycycline (49), we were able to increase incrementally the proportion of opaque cells and assess biofilm parameters with each incremental increase.
When doxycycline was increased from 0 to 2.5 μg/ml, the proportion of opaque cells that formed in white wor1/wor1-TETp-WOR1 cell populations increased from less than 0.1 to 10% (Fig. 3A). When doxycycline was increased from 2.5 to 25 μg/ml, the proportion of opaque cells increased from 10 to 55%, and when doxycycline was increased to 50 μg/ml, the proportion reached 80% (Fig. 3A). The level of WOR1 expression, measured by the RT-PCR, increased as a function of doxycycline concentration (Fig. 4B). Incremental increases of doxycycline from 0 to 2.5 μg/ml caused incremental increases in biofilm biomass, biofilm thickness, and the level of β-glucan released by the biofilm into the supernatant. (Fig. 4C, D, and E, respectively). At 2.5 μg of doxycycline/ml, the biofilm biomass, biofilm thickness, and released β-glucan reached levels approximately 4-fold, 2-fold, and 2-fold, respectively, that of unstimulated (i.e., 0 μg of doxycycline/ml) populations (Fig. 4C, D, and E, respectively). When doxycycline was increased incrementally from 2.5 to 100 μg/ml, the three assessed biofilm characteristics decreased incrementally from the peak values at 2.5 μg of doxycycline/ml (Fig. 4C, D, and E, respectively). These declines were due to interference by opaque cells when their proportion was raised above 10% (13). These results add further weight to the conclusion that self-induction requires that a minority of white cells switch to opaque.
Fig. 3.
Increasing the frequency of switching incrementally by increasing the level of expression of WOR1, the master switch gene, incrementally, causes incremental increases in biofilm parameters of a same-sex a/a white cell biofilm, until the proportion of opaque cells, which do not form biofilms, interferes. The wor1/wor1 mutant was transformed with WOR1 under the regulation of a tetracycline (doxycycline)-controlled promoter, generating the wor1-wor1-TETp-WOR1 strain. The biofilms were then treated with increasing concentrations of the inducer doxycycline. (A) Percentage of opaque cell formation in a 48-h biofilm as a function of doxycycline concentration, as assessed by plating experiments. (B) WOR1 expression as a function of doxycycline concentration, as assessed by RT-PCR. ACT1 expression levels are included as a loading control. (C) Biomass of biofilms as a function of doxycycline concentration. (D) Thickness of biofilms as a function of doxycycline concentration. (E) β-Glucan released into the supernatant as a function of doxycycline concentration. The mean percentage of opaque cells measured by plating experiments is provided at the bottom of panels C through E. The data from three-independent experiments were pooled and analyzed for the data in panels A and C through E. Error bars represent standard deviations.
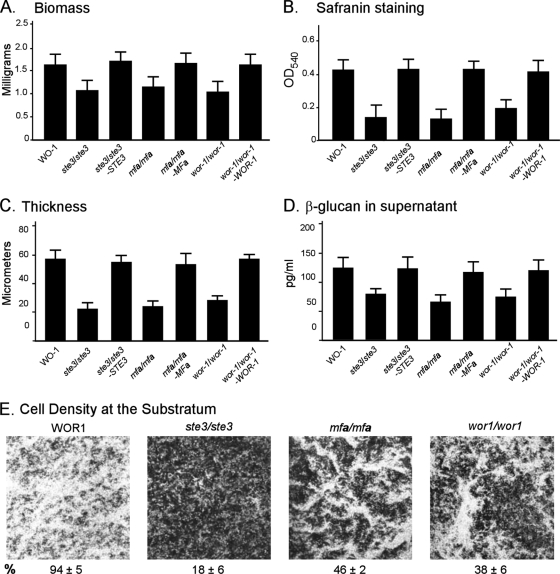
Fig. 4.
Self-induction of a same-sex white α/α biofilm depends upon the a-pheromone receptor STE3, the a-pheromone gene MFa, and the master switch gene WOR1. Biofilms (48 h) were analyzed for the parental α/α strain WO-1, the ste3/ste3, mfa/mfa, and wor1/wor1 deletion mutants, and the ste3/ste3-STE3, mfa/mfa-MFa, and wor1/wor1-WOR1 complemented mutants. See the legend to Fig. 1 for explanations of the panels.
Self-induction of white α/α cell biofilm formation.
Neither our previous study on white a/a cell biofilm formation (53, 54) nor the study by Alby et al. (1) on self-mating by a/a cells tested whether α/α cells underwent self-induction by secreting a-pheromone. We therefore generated deletion mutants in the natural α/α strain WO-1 for STE3, which encodes the a-pheromone receptor, MFa, which encodes the a-pheromone, and WOR1, the master switch gene. The resultant α/α ste3/ste3 and mfa/mfa mutants exhibited reductions in white α/α biofilm biomass (Fig. 4A), safranin staining (Fig. 4B), biofilm thickness (Fig. 4C), the release of β-glucan (Fig. 4D) and the density of cells in biofilms at the substratum (Fig. 4E). The reductions were similar to those of the ste2/ste2 and mfα/mfα a/a mutants (Fig. 1A, B, C, D, and E, respectively). Biofilm parameters were restored in the complemented ste3/ste3-STE3 and mfa/mfa-MFa strains (Fig. 4A, B, C, and D, respectively). These results indicate that self-induction of biofilm formation in white α/α cell populations to which no a-cells were added involves a-pheromone stimulation of the a-pheromone receptor. The wor1/wor1 mutant generated in the α/α strain WO-1 exhibited reductions in the same four biofilm parameters similar to those of the ste3/ste3 and mfa/mfa mutants (Fig. 4A through D). These latter results indicate that a minority of cells in a white α/α cell population must spontaneously switch to opaque in order to self-stimulate biofilm formation, just as a minority of white a/a cells must switch to opaque.
DISCUSSION
C. albicans, which is ca. 90% a/α in nature, undergoes homozygosis to a/a or α/α in order to mate (20, 21, 29). The latter must, however, then switch from the white to opaque phenotype in order to achieve mating competence (30, 27). MTL-heterozygous (a/α) cells and MTL-homozygous (a/a or α/α) white cells to which no cells of opposite mating type are added, and both form robust biofilms on elastomer surfaces that are morphologically similar, containing an adhesive basal layer of yeast cells and a thick upper region of hyphae and matrix (13, 42–44, 53–55). Opaque cells do not similarly form robust biofilms (13). The addition of a minority (1 to 10%) of opaque cells of opposite mating type increases the thickness of a MTL-homozygous white cell biofilm by >50%, presumably by acting as a source for pheromone of opposite mating type (13, 42–44, 53–55). We previously demonstrated that deleting STE2, the α-pheromone receptor, results in a highly defective white a/a cell biofilm in the absence of minority opaque α/α cells (54). This result indicated that homogeneous white a/a cell populations released α-pheromone that activated α-pheromone receptors on the same cells to generate a basic same-sex biofilm.
Here we demonstrate that, as is the case for self-mating of a/a cells (1), self-stimulation of biofilm formation in a white a/a cell population is dependent upon expression of both STE2, the α-pheromone receptor gene, and MFα, the α-pheromone gene. Hence, self-stimulation of a white a/a biofilm appears to involve the release by a/a cells of α-pheromone, which activates the α-pheromone receptor on the same cell. Unlike self-mating, which is a rare event (1), self-induction of biofilm formation occurs in a majority of white a/a cells. The lack of a need to delete BAR1, the gene encoding the extracellular protease that digests α-pheromone, is presumably because we are assessing a mass population response, rather than a rare event. We also demonstrate here for the first time that white cells of the opposite mating type, α/α, undergo the same general scenario for self-stimulation, presumably releasing a-pheromone, which activates the a-pheromone receptor Ste3 of the same cells. Most importantly, however, we show here that unlike self-mating (1), which appears to represent an autocrine-like system, involving one cell phenotype (opaque) (8, 9, 17, 31, 33, 34, 37, 47, 51), self-induction of MTL-homozygous biofilms is a paracrine system, involving two closely related cell phenotypes, signaling opaque cells and responding white cells (12, 15, 17, 52).
We tested whether self-stimulated white cell a/a and α/α biofilms required a switch to opaque, because in the mating system only opaque cells have been shown to release pheromone (5, 26, 38). Using WOR1 deletion mutants generated in a/a and α/α strains, we provide evidence suggesting that a minority of cells in white a/a or α/α cell populations must be able to switch to opaque in order to induce white cells to form a biofilm. Our combined results support the conclusion that opaque a/a and α/α cells release α-pheromone and a-pheromone, respectively, in a mating-type-nonspecific manner. This result is surprising given that the maturation and release of α-pheromone and a-pheromone by α and a cells of Saccharomyces cerevisiae, respectively, have been demonstrated to involve a number of accessory proteins, some of which have previously been shown to be expressed in a mating-type-specific manner (7, 10, 16, 22). Our results suggest that opaque a/a and α/α cells must express these accessory molecules in a mating-type-nonspecific manner, presumably at basal levels sufficient to process and secrete the low levels of the pheromone of the opposite mating type.
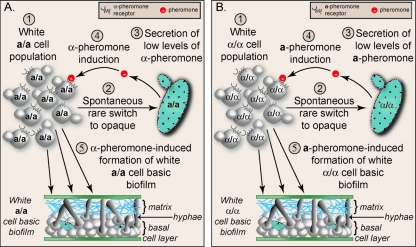
Our results, therefore, suggest that same-sex white a/a biofilm formation, in the absence of minority opaque cells of opposite mating type, occurs through the following scenario (Fig. 5 A). In a white cell population homogeneous for the a/a mating type, a minority of white cells spontaneously switch to opaque (3, 6, 41, 46). The minority opaque a/a cells then produce, process, and secrete low levels of α-pheromone in a mating-type-specific fashion (Fig. 5A). Secreted α-pheromone binds to the α-pheromone receptor, Ste2, of the majority white a/a cells. Receptor occupancy activates the mitogen-activated protein kinase signal transduction pathway, which targets the transcription factor Tec1 in white cells (Fig. 5A) (42–44, 53, 54). Tec1 then binds to the cis-acting activation motif WPRE of genes both encoding the components of the mitogen-activated protein kinase pathway and genes directly involved in biofilm formation (Fig. 5A) (42, 44). A similar scenario is presented for self-activation of white α/α cell populations, which involves mating-type-nonspecific synthesis of a-pheromone (Fig. 5B).
Fig. 5.
Steps in self-activation of a same-sex white a/a biofilm (A) and a white α/α biofilm (B).
Supplementary Material
ACKNOWLEDGMENTS
We thank Claude Pujol for helpful discussions; Deborah Wessels for help with the figures; Joachim Morschhäuser from the University of Würzburg, Würzburg, Germany, for generously providing key plasmids; and Tania Toulabi and Sandra Beck for assistance in manuscript assembly.
This study was funded by the Developmental Studies Hybridoma Bank, a National Resource initiated under the auspices of National Institutes of Health.
S.Y., N.S., K.J.D., and D.R.S. conceived and designed the experiments; S.Y., N.S., K.J.D., K.L.L., G.H., and T.S. performed the experiments; S.Y., N.S., K.J.D., and D.R.S. analyzed the data; D.R.S., S.Y., and N.S. wrote the paper; and K.J.D., S.Y., and N.S. made the figures.
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
Published ahead of print on 15 April 2011.
REFERENCES
- 1. Alby K., Schaefer D., Bennett R. J. 2009. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature 460:890–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alby K., Bennett R. J. 2009. Stress-induced phenotypic switching in Candida albicans. Mol. Biol. Cell 20:3178–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson J. M., Soll D. R. 1987. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J. Bacteriol. 169:5579–5588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bedell G. W., Soll D. R. 1979. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect. Immun. 26:348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bennett R. J., Uhl M. A., Miller M. G., Johnson A. D. 2003. Identification and characterization of a Candida albicans mating pheromone. Mol. Cell. Biol. 23:8189–8201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergen M. S., Voss E., Soll D. R. 1990. Switching at the cellular level in the white-opaque transition of Candida albicans. J. Gen. Microbiol. 136:1925–1936 [DOI] [PubMed] [Google Scholar]
- 7. Berkower C., Michaelis S. 1991. Mutational analysis of the yeast a-factor transporter STE6, a member of the ATP binding cassette (ABC) protein superfamily. EMBO J. 10:3777–3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bodel P. 1976. Colchicine stimulation of pyrogen production by human blood leukocytes. J. Exp. Med. 143:1015–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cantrell D. A., Collins M. K., Crumpton M. J. 1988. Autocrine regulation of T-lymphocyte proliferation: differential induction of IL-2 and IL-2 receptor. Immunology 65:343–349 [PMC free article] [PubMed] [Google Scholar]
- 10. Chen P., Sapperstein S. K., Choi J. D., Michaelis S. 1997. Biogenesis of the Saccharomyces cerevisiae mating pheromone a-factor. J. Cell Biol. 136:251–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cousins B. G., et al. 2007. Effects of a nanoparticulate silica substrate on cell attachment of Candida albicans. J. Appl. Microbiol. 102:757–765 [DOI] [PubMed] [Google Scholar]
- 12. Cunha G. R., et al. 2000. Paracrine mechanisms of mouse mammary ductal growth. Adv. Exp. Med. Biol. 480:93–97 [DOI] [PubMed] [Google Scholar]
- 13. Daniels K. J., Srikantha T., Lockhart S. R., Pujol C., Soll D. R. 2006. Opaque cells signal white cells to form biofilms in Candida albicans. EMBO J. 25:2240–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Backer M. D., et al. 1999. Transformation of Candida albicans by electroporation. Yeast 15:1609–1618 [DOI] [PubMed] [Google Scholar]
- 15. Furie B., Furie B. C. 1988. The molecular basis of blood coagulation. Cell 53:505–518 [DOI] [PubMed] [Google Scholar]
- 16. He B., et al. 1991. RAM2, an essential gene of yeast, and RAM1 encode the two polypeptide components of the farnesyltransferase that prenylates a-factor and Ras proteins. Proc. Natl. Acad. Sci. U. S. A. 88:11373–11377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hooper J. E. 1994. Distinct pathways for autocrine and paracrine Wingless signaling in Drosophila embryos. Nature 372:461–464 [DOI] [PubMed] [Google Scholar]
- 18. Huang G., et al. 2006. Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 103:12813–12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang G., Srikantha T., Sahni N., Yi S., Soll D. R. 2009. CO2 regulates white-to-opaque switching in Candida albicans. Curr. Biol. 19:330–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hull C. M., Johnson A. D. 1999. Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans. Science 285:1271–1275 [DOI] [PubMed] [Google Scholar]
- 21. Hull C. M., Raisner R. M., Johnson A. D. 2000. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 289:307–310 [DOI] [PubMed] [Google Scholar]
- 22. Julius D., Blair L., Brake A., Sprague G., Thorner J. 1983. Yeast alpha factor is processed from a larger precursor polypeptide: the essential role of a membrane-bound dipeptidyl aminopeptidase. Cell 32:839–852 [DOI] [PubMed] [Google Scholar]
- 23. Lee K. L., Rega M. E., Watson R. R., Campbell C. C. 1975. Identification of yeast phase of pathogenic fungi by the specificity of their aminopeptidase(s). Sabouraudia 13:132–141 [DOI] [PubMed] [Google Scholar]
- 24. Legrand M., et al. 2004. Homozygosity at the MTL locus in clinical strains of Candida albicans: karyotypic rearrangements and tetraploid formation. Mol. Microbiol. 52:1451–1462 [DOI] [PubMed] [Google Scholar]
- 25. Lockhart S. R., et al. 2002. In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics 162:737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lockhart S. R., Zhao R., Daniels K. J., Soll D. R. 2003. Alpha-pheromone-induced “shmooing” and gene regulation require white-opaque switching during Candida albicans mating. Eukaryot. Cell 2:847–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lockhart S. R., Daniels K. J., Zhao R., Wessels D., Soll D. R. 2003. Cell biology of mating in Candida albicans. Eukaryot. Cell 2:49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lockhart S. R., Wu W., Radke J. B., Zhao R., Soll D. R. 2005. Increased virulence and competitive advantage of a/α over a/a or α/α offspring conserves the mating system of Candida albicans. Genetics 169:1883–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Magee B. B., Magee P. T. 2000. Induction of mating in Candida albicans by construction of MTLa and MTLα strains. Science 289:310–313 [DOI] [PubMed] [Google Scholar]
- 30. Miller M. G., Johnson A. D. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293–302 [DOI] [PubMed] [Google Scholar]
- 31. Moore M. A., et al. 1980. Continuous human bone marrow culture: Ia antigen characterization of probable pluripotential stem cells. Blood 55:682–690 [PubMed] [Google Scholar]
- 32. Morrow B., Anderson J., Wilson J., Soll D. R. 1989. Bidirectional stimulation of the white-opaque transition of Candida albicans by ultraviolet irradiation. J. Gen. Microbiol. 135:1201–1208 [DOI] [PubMed] [Google Scholar]
- 33. Nadell C. D., Xavier J. B., Levin S. A., Foster K. R. 2008. The evolution of quorum sensing in bacterial biofilms. PLoS Biol. 6:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Navarro-Tableros V., Sanchez-Soto M. C., Garcia S., Hiriart M. 2004. Autocrine regulation of single pancreatic beta-cell survival. Diabetes 53:2018–2023 [DOI] [PubMed] [Google Scholar]
- 35. Nobile C. J., et al. 2006. Critical role of Bcr1-dependent adhesins in Candida albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nobile C. J., et al. 2009. Biofilm matrix regulation by Candida albicans Zap1. PLoS Biol. 7:e1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Palamakumbura A. H., Sommer P., Trackman P. C. 2003. Autocrine growth factor regulation of lysyl oxidase expression in transformed fibroblasts. J. Biol. Chem. 278:30781–30787 [DOI] [PubMed] [Google Scholar]
- 38. Panwar S. L., Legrand M., Dignard D., Whiteway M., Magee P. T. 2003. MFα1, the gene encoding the alpha mating pheromone of Candida albicans. Eukaryot. Cell 2:1350–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ramirez-Zavala B., Reuss O., Park Y. N., Ohlsen K., Morschhauser J. 2008. Environmental induction of white-opaque switching in Candida albicans. PLoS Pathog. 4:e1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reuss O., Vik A., Kolter R., Morschhauser J. 2004. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341:119–127 [DOI] [PubMed] [Google Scholar]
- 41. Rikkerink E. H., Magee B. B., Magee P. T. 1988. Opaque-white phenotype transition: a programmed morphological transition in Candida albicans. J. Bacteriol. 170:895–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sahni N., et al. 2009. Genes selectively up-regulated by pheromone in white cells are involved in biofilm formation in Candida albicans. PLoS Pathog. 5:e1000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sahni N., Yi S., Pujol C., Soll D. R. 2009. The white cell response to pheromone is a general characteristic of Candida albicans strains. Eukaryot. Cell 8:251–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sahni N., et al. 2010. Tec1 mediates the pheromone response of the white phenotype of Candida albicans: insights into the evolution of new signal transduction pathways. PLoS Biol. 8:e1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seidler M., Salvenmoser S., Muller F. M. 2006. In vitro effects of micafungin against Candida biofilms on polystyrene and central venous catheter sections. Int. J. Antimicrob. Agents 28:568–573 [DOI] [PubMed] [Google Scholar]
- 46. Slutsky B., et al. 1987. “White-opaque transition”: a second high-frequency switching system in Candida albicans. J. Bacteriol. 169:189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith J. J., Derynck R., Korc M. 1987. Production of transforming growth factor alpha in human pancreatic cancer cells: evidence for a superagonist autocrine cycle. Proc. Natl. Acad. Sci. U. S. A. 84:7567–7570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Soll D. R. 2010. Evolution of a new signal transduction pathway in Candida albicans. Trends Microbiol. 19:8–13 [DOI] [PubMed] [Google Scholar]
- 49. Srikantha T., et al. 2006. TOS9 regulates white-opaque switching in Candida albicans. Eukaryot. Cell 5:1674–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tavanti A., Gow N. A., Maiden M. C., Odds F. C., Shaw D. J. 2004. Genetic evidence for recombination in Candida albicans based on haplotype analysis. Fungal Genet. Biol. 41:553–562 [DOI] [PubMed] [Google Scholar]
- 51. Toribio M. L., Gutierrez-Ramos J. C., Pezzi L., Marcos M. A., Martinez C. 1989. Interleukin-2-dependent autocrine proliferation in T-cell development. Nature 342:82–85 [DOI] [PubMed] [Google Scholar]
- 52. Vrana J. A., Stang M. T., Grande J. P., Getz M. J. 1996. Expression of tissue factor in tumor stroma correlates with progression to invasive human breast cancer: paracrine regulation by carcinoma cell-derived members of the transforming growth factor beta family. Cancer Res. 56:5063–5070 [PubMed] [Google Scholar]
- 53. Yi S., et al. 2008. The same receptor, G protein, and mitogen-activated protein kinase pathway activate different downstream regulators in the alternative white and opaque pheromone responses of Candida albicans. Mol. Biol. Cell 19:957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yi S., et al. 2009. A Candida albicans-specific region of the alpha-pheromone receptor plays a selective role in the white cell pheromone response. Mol. Microbiol. 71:925–947 [DOI] [PubMed] [Google Scholar]
- 55. Yi S., et al. 2011. Utilization of the mating scaffold protein in the evolution of a new signal transduction pathway for biofilm development. MBio 2:e00237–e00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zordan R. E., Galgoczy D. J., Johnson A. D. 2006. Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc. Natl. Acad. Sci. U. S. A. 103:12807–12812 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.