Abstract
Although bacterial-fungal interactions shape microbial virulence during polymicrobial infections, only a limited number of studies have evaluated this interaction on a genetic level. We report here that one interaction is mediated by sopB, an effector of a type III secretion system (TTSS) of Salmonella enterica serovar Typhimurium. In these studies, we screened 10 TTSS effector-related mutants and determined their role in the killing of C. albicans filaments in vitro during coinfection in planktonic environments. We found that deleting the sopB gene (which encodes inositol phosphatase) was associated with a significant decrease in C. albicans killing at 25°C after 5 days, similar to that caused by the deletion of sipB (which encodes TTSS translocation machinery components). The sopB deletion dramatically influenced the killing of C. albicans filaments. It was associated with repressed filamentation in the Caenorhabditis elegans model of C. albicans-S. Typhimurium coinfection, as well as with biofilm formation by C. albicans. We confirmed that SopB translocated to fungal filaments through SipB during coinfection. Using quantitative real-time PCR assays, we found that the Candida supernatant upregulated the S. Typhimurium genes associated with C. albicans killing (sopB and sipB). Interestingly, the sopB effector negatively regulated the transcription of CDC42, which is involved in fungal viability. Taken together, these results indicate that specific TTSS effectors, including SopB, play a critical role in bacterial-fungal interactions and are important to S. Typhimurium in order to selectively compete with fungal pathogens. These findings highlight a new role for TTSS of S. Typhimurium in the intestinal tract and may further explain the evolution and maintenance of these traits.
INTRODUCTION
Microbial survival is based on diverse bacterial-bacterial, fungal-fungal, and bacterial-fungal interactions. These interactions are ubiquitous in nature, as well as in clinical environments, but very little is known about the genetic mechanism(s) associated with these interactions (51). Most of the previous studies have focused on Candida albicans, the opportunistic fungal pathogen that can exist as both yeast and filamentous cells according to its growing circumstances and conditions (59). C. albicans is the most common pathogenic fungus and may cause mucosal and systemic infections in immunosuppressed and immunocompetent hosts. The morphological transition from a yeast to a filamentous cell is critical for C. albicans pathogenesis (34, 55). Interestingly, the ability of C. albicans to develop filaments is also impacted by the presence of bacterial pathogens (8), and this association was extensively described during the interaction between C. albicans and various pathogenic bacteria, including Streptococcus gordonii (5), Staphylococcus epidermidis (2), Pseudomonas aeruginosa (25), Burkholderia cenocepacia (8), and Acinetobacter baumannii (52). However, limited work has been done on the interaction of C. albicans with intestinal bacterial pathogens. These studies are particularly important because in the human intestinal tract there exists a remarkable microbial community that includes C. albicans in essentially all humans (28, 51, 54).
Salmonella enterica serovar Typhimurium is one of the most common food-borne pathogens and can cause intestinal inflammation leading to diarrheal diseases in humans and animals (58). The type III secretion systems (TTSS) encoded by Salmonella pathogenicity island 1 (SPI-1) and SPI-2 on the bacterial chromosome are important virulence factors for Salmonella pathogenesis (74). Simply put, TTSS represents a molecular syringe allowing the bacteria to deliver effector proteins directly into the host cell cytosol (35, 74). To date, more than 30 SPI-1- and SPI-2-regulated effectors in Salmonella are known to use these systems to translocate these proteins into the host cell cytoplasm (37). Despite the importance of the TTSS in pathogenesis, the recognition and targeting of TTSS effectors remains poorly understood (17).
In previous work we found that the human intestinal pathogen S. Typhimurium significantly influenced the survival of C. albicans filaments (67). We found in the present study that the sopB effector is essential for competing with C. albicans filaments, and we show that S. Typhimurium influences the survival and filamentation of C. albicans in a Caenorhabditis elegans model and biofilm formation through the TTSS sopB effector. Moreover, the sopB effector can repress TEC1 (which encodes a transcription factor for filamentation and biofilm formation), HWP1 and ALS3 (which encode filament specific cell wall proteins), and CDC42 (which encodes a Rho-type GTPase that is related to viability) in C. albicans.
MATERIALS AND METHODS
Fungal and bacterial strains, plasmids, and growth conditions.
The strains and plasmids used in the present study are listed in Table 1. C. albicans and S. Typhimurium strains were routinely cultured in yeast-peptone-dextrose (YPD) at 30°C and Luria-Bertani (LB) medium (Difco, Detroit, MI) at 37°C, respectively. When necessary, kanamycin (45 μg/ml), ampicillin (100 μg/ml), and erythromycin (300 μg/ml) were used for selective culture of mutants.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype and/or relevant characteristicsa | Source or reference |
|---|---|---|
| Bacterial strains | ||
| HB101 | Nonpathogenic E. coli; normal food source for C. elegans | 11 |
| 14028 | S. Typhimurium wild-type | ATCCb |
| ΔsopB | ΔsopB Ω Kmr; Salmonella outer protein; homologue to ipgD of Shigella; regulated by SPI-1 | 58 |
| ΔsopD | ΔsopD Ω Kmr; secreted protein in the Sop family; transferred to eukaryotic cells; regulated by SPI-1 | 58 |
| ΔsopE2 | ΔsopE2 Ω Kmr; type III secreted protein effector; regulated by SPI-1 | 58 |
| ΔsipB | ΔsipB Ω Kmr; cell invasion protein; regulated by SPI-1 | 58 |
| ΔinvA | ΔinvA Ω Kmr; invasion protein; regulated by SPI-1 | 58 |
| ΔssaE | ΔssaE Ω Kmr; secretion system effector; regulated by SPI-2 | 58 |
| ΔsseB | ΔsseB Ω Kmr; secretion system effector; regulated by SPI-2 | 58 |
| ΔsseJ | ΔsseJ Ω Kmr; Salmonella translocated effector; regulated by SPI-2 | 58 |
| ΔadrA | ΔadrA Ω Kmr; putative diguanylate cyclase/phosphodiesterase domain 1 | 58 |
| ΔluxS | ΔluxS Ω Kmr; quorum-sensing protein, produces autoinducer signaling molecules | 58 |
| ΔphoP | ΔphoP Ω Kmr; response regulator in two-component regulatory system | 58 |
| ΔssrA | ΔssrA Ω Kmr; two-component regulatory system | 58 |
| 14028-GFP | 14028 wild type containing pCM18 | This study |
| ΔsopB-GFP | ΔsopB containing pCM18 | This study |
| 14028/SopB | 14028 wild type containing pSB2908 | This study |
| ΔsipB/SopB | ΔsipB containing pSB2908 | This study |
| ΔsopB/SopB | ΔsopB containing pSB2908 | This study |
| Candida strains | ||
| DAY185 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/HIS1::his1::hisG arg4::hisG/URA3::ARG4::arg4::hisG | 13 |
| Δtup1 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/tup1::hisG-URA3-hisG | 10 |
| Δsuv3 | ura3Δ::λimm434/ura3Δ::λimm434 suv3::Tn7-URA3/suv3::Tn7-UAU1 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | 55 |
| SC5314 | Clinical isolate | 19 |
| Δcph1/Δefg1 | ura3Δ::λimm434/ura3Δ::λimm434 cph1Δ::hisG/cph1Δ::hisG efg1Δ::hisG/efg1Δ::hisG-URA3-hisG | 34 |
| SSY50-B | ura3Δ::λimm434/tet-NRG1/URA3 | 59 |
| Plasmids | ||
| pCM18 | Emr; pTRKL2-PCP25-RBSII-gfpmut3; broad host | 21 |
| pSB2908 | Amr; pBAD24 Plac::FLAG-tagged SopB+; arabinose inducible | 50 |
Amr, Emr, and Kmr represent ampicillin, erythromycin, and kanamycin resistance, respectively.
ATCC, American Type Culture Collection.
Nematode strains.
C. elegans glp-4(bn2);sek-1(km4) strain was used for all experiments as described previously (11, 43). The C. elegans glp-4 mutant animals are suited for liquid assay experiments since worms are unable to produce progeny at 25°C; however, sterile animals have enhanced life span compared to wild-type animals (41). C. elegans sek-1 encodes a conserved mitogen-activated protein kinase kinase involved in innate immunity (29), expediting the time of experiments. Worms were cultured and maintained on Escherichia coli HB101 by using standard procedures (11).
In vitro coinfection assay under planktonic environments.
In vitro coculture assays were performed in 2 ml of LB broth and incubated in a roller drum at 25°C for 5 days (52). A starting inoculum of ca. 106 CFU/ml of S. Typhimurium strain 14028 wild type or its isogenic mutants (58) (Table 1) and ca. 5 × 105 cells/ml of C. albicans DAY185 were used for all experiments. To quantify the viability of C. albicans in coinfection conditions, we used CFU analysis as previously described (22, 25, 67). YPD agar plates containing kanamycin (45 μg/ml), ampicillin (100 μg/ml), and streptomycin (100 μg/ml) were used to select for C. albicans strains. The CFU were determined by diluting cells by 100 to 107 via 10-fold serial dilution steps in 0.85% NaCl solution that was applied as 10-μl drops on agar plates (14). The YPD agar plates were incubated at 30°C for 48 h. Two independent cultures were used for each strain.
In vitro coinfection assay for filament specific killing.
In order to monitor the killing and inhibition of C. albicans filaments via the sopB effector, we evaluated C. albicans filaments from C. albicans Δtup1 and C. albicans SSY50-B. C. albicans Δtup1 is a strain that constitutively produces filaments at 25°C, while the Δsuv3 mutant produces no filaments under the same conditions (10, 55). In addition, we studied the genetically engineered strain C. albicans SSY50-B (tetracycline-regulatable tet-NRG1) that constitutively forms filaments in the presence of 20 μg of doxycycline (DOX)/ml at 37°C but does not produce filaments in the absence of DOX (59). Using these strains, we evaluated the viability of C. albicans filaments infected with S. Typhimurium strains by using the XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] assay as described earlier (38) with slight modifications. More specifically, C. albicans Δtup1 and Δsuv3 were cultured at 25°C for 48 h in order to produce filaments and yeast cells, respectively. In addition, C. albicans SSY50-B was grown at 37°C for 24 h with or without 20 μg of DOX/ml to facilitate the formation of filaments and yeast-type cells, respectively. Filaments and normal yeast cells were washed three times with phosphate-buffered saline (PBS) and incubated with 106 CFU of S. Typhimurium/ml in LB medium for 24 h. After incubation, the C. albicans filaments were recovered by centrifugation at 5,000 rpm for 3 min, washed three times by PBS, and then the XTT-menadione solution, consisting 10 ml of XTT (0.5 mg/ml in PBS) and 1 μl of menadione (10 mM menadione in acetone) was added. After incubation for 2 h at 37°C and centrifugation, 80 μl of the XTT-menadione supernatants was transferred to the wells of a 96-well microtiter plate, and measured by using a microtiter plate reader (Molecular Devices, Sunnyvale, CA) at 490 nm. All results were normalized based on the E. coli HB101 control.
C. elegans coinfection assay for filamentation.
The C. elegans coinfection assays for C. albicans filamentation were performed as previously described (67). In brief, synchronized, young adult nematodes were preinfected for 4 h on lawns of C. albicans DAY185 and then transferred into wells of a six-well microtiter dish (40 worms per well), followed by three washes with M9 medium. Each well contained 2 ml of liquid assay medium (20% brain heart infusion and 80% M9). S. Typhimurium strains were inoculated at the concentration of ca. 106 CFU/ml before the addition of the C. albicans-infected worms. The plates were then incubated at 25°C and examined at 24-h intervals for 6 days for viability and the formation of penetrative filaments by using a Nikon SMZ645 dissecting microscope. In addition, to evaluate whether Salmonella strains influence filament elongation of C. albicans in C. elegans, worms with initial filamentation (worms with filaments after 1 day) were moved to new wells of 96-well plates (Corning no. 3882), including S. Typhimurium strains expressing green fluorescent protein (GFP) (Table 1; ca. 106 CFU/ml) in liquid assay medium and then incubated at 25°C for an additional 5 days. The qualitative observation of C. albicans filamentation and elongation in C. elegans was performed by using a Discovery-1 microscope (Molecular Devices, Sunnyvale, CA) using a fluorescein isothiocyanate (FITC) filter set or with bright-field transmitted light.
S. Typhimurium attachment to C. albicans filaments.
To evaluate the filamentation in vitro, C. albicans SSY50-B (59) organisms were inoculated into YPD with 20 μg of DOX/ml in 96-well plates (Corning, Inc., Corning, NY; no. 3882) and incubated for 8 h at 37°C with shaking at 150 rpm. After incubation, the wells were washed five times with PBS, and ca. 106 CFU/ml of Salmonella strains expressing GFP in LB containing erythromycin (300 μg/ml) were added, followed by incubation for 15 h at 37°C. After six washings with PBS to remove unattached Salmonella strains, GFP-expressing Salmonella strains on C. albicans filaments were observed by using a Discovery-1 microscope under FITC and bright-field light. In addition, S. Typhimurium cells attached to C. albicans filaments were counted by using the CFU assay, following recovery of C. albicans filaments from 96-well plates using vigorous pipetting. LB agar plates containing fluconazole (32 μg/ml) were used to select for S. Typhimurium. Plates were incubated at 37°C for 24 h.
Silicone pad biofilm assay.
The effect of S. Typhimurium on C. albicans biofilm growth was evaluated by using a polymicrobial silicone pad assay as described previously (52). Spider medium (32) was used as the medium for C. albicans biofilm development. The quantitative biofilm mass was calculated by subtracting the original weight of the silicone pad from its postincubation (60 h) weight and adjusting for the weight of control silicone pads exposed without fungal cells.
Monitoring of SopB in C. albicans.
To monitor the SopB delivered into fungal filaments from S. Typhimurium, filamentous C. albicans SSY50-B cells (i.e., the genetically engineered Candida strain with constitutive filaments under DOX) cultured in the presence of 20 μg of DOX/ml were coinfected with wild type or the S. Typhimurium sipB mutant (TTSS translocation machinery component deficient) expressing FLAG epitope-tagged SopB (50) under 0.05% l-arabinose for 15 h. After gentamicin treatment (100 μg/ml) for 1 h to kill exterior filament-binding Salmonella, fungal cells were processed for immunofluorescence by using monoclonal anti-FLAG M2 FITC antibody (Sigma-Aldrich Corp., St. Louis, MO) based on the manufacturer's protocol. Fluorescent observation of SopB in filaments was performed by using confocal laser microscopy (TCS-NT; Leica Microsystems).
qRT-PCR.
Quantitative reverse transcription-PCR (qRT-PCR) was performed using the CHROMO4 real-time PCR system (MJ Research, Inc., Waltham, MA). After disruption with glass beads (Sigma-Aldrich), total RNA was isolated according to the protocol of an RNeasy minikit (Qiagen, Valencia, CA), including an on-column DNase digestion with RNase-free DNase (Qiagen). After the RNA was isolated, 50 ng of total RNA was used for the qRT-PCR using a SuperScript III Platinum SYBR Green One-Step qRT-PCR kit (Invitrogen, Carlsbad, CA). Primers were designed by using Primer3Input Software (v0.4.0; http://frodo.wi.mit.edu/primer3/) and are listed in Table 2. Relative expression levels were calculated by using the 2−ΔΔCT method (33). The control genes 18S rRNA and 16S rRNA were used to normalize the expression data for Candida and Salmonella, respectively. The annealing temperature was 60°C for all of the genes in the present study. To investigate the transcriptional regulations of sopB and sipB by C. albicans supernatants, overnight cultures of C. albicans DAY185 Δtup1 versus Δsuv3 (at 25°C) and C. albicans SC5314 versus Δcph1/Δefg1 (at 37°C) were inoculated in YPD and cultured for 48 and 24 h, respectively. Supernatants were quickly recovered, filtered, and stored at −80°C before use. The sopB and sipB transcript levels were investigated after exposure to prepared supernatants from C. albicans or farnesol (Sigma-Aldrich; 100 and 200 μM concentrations) for 2 h. In contrast, C. albicans DAY185 filamentous cells on silicone pads (18 h) were exposed to the S. Typhimurium wild type and mutants for 3 h. After infection by the Salmonella strains, the C. albicans filamentous cells on pads were washed twice with PBS and then quickly recovered by vigorous pipetting. The results were normalized using the E. coli strain HB101, which has no impact on C. albicans filamentation and biofilm formation.
Table 2.
Oligonucleotides used for qRT-PCR
| Organism and gene | Orientationa | Sequence (5′-3′) |
|---|---|---|
| Salmonella | ||
| sopB | F | CTTATACAACGGAATGCAGATTCTC |
| R | AGTTATAGAGGTTATGCAGCGAGTG | |
| sipB | F | ATTACTGCTTGGCAAGTTAATGACC |
| R | TTTGATACTGGCTTCATAGAGATCC | |
| 16S rRNA | F | TGTAGCGGTGAAATGCGTAG |
| R | CAAGGGCACAACCTCCAAG | |
| Candida | ||
| TEC1 | F | TGGTGCTTATTCACGTGTCC |
| R | TTCTGAATTTCCCGGTTTTG | |
| HWP1 | F | CTCCAGCTGGCTCAAGTGGT |
| R | TGGCAGATGGTTGCATGAGT | |
| ALS3 | F | ACTTCCACAGCTGCTTCCAC |
| R | TGCAGATGGAGCATTACCAC | |
| CDC42 | F | AGGGTGAAAAATTGGCTAAGGA |
| R | TGCAGCTACTATAGCCTCGTCA | |
| 18S rRNA | F | GTGCCAGCAGCCGCGGTA |
| R | TGGACCGGCCAGCCAAGC |
F, forward primer; R, reverse primer.
Statistical analysis.
C. elegans survival was examined by using the Kaplan-Meier method, and differences were determined by using the log-rank test (STATA6; STATA, College Station, TX). Differences in the number from each experiment were determined by using a Student t test. Each result is a representative experiment of at least two independent biological replicates. A P value of 0.05 in all replicate experiments was considered statistically significant.
RESULTS
Role of SPI-1 effectors in the viability of C. albicans.
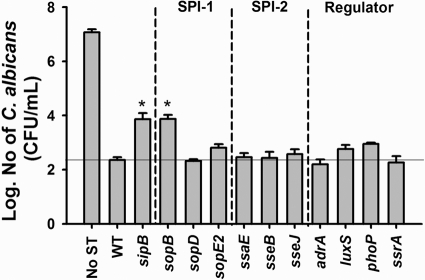
In order to evaluate the hypothesis that TTSS is involved in the S. Typhimurium-C. albicans interaction, we selected 10 mutant strains that have a mutation involving different TTSS effectors. They can be categorized into three groups: (i) strains with a mutation involving the SPI-1 system effectors, including SopB (48), SopD (69), and SopE2 (4); (ii) strains with a mutation involving the SPI-2 system effectors, including SsaE (40), SseB (23), and SseJ (49); and (iii) strains with a mutation involving TTSS-related regulators such as AdrA (18), LuxS (66), PhoP (20), and SsrA (7). Of note is that there was no significant difference in the growth rates in vitro among these S. Typhimurium mutants (data not shown).
Using this collection of S. Typhimurium strains, we compared the viability of C. albicans exposed to the S. Typhimurium wild-type (WT) strain or isogenic mutants using in vitro coinfection conditions. At 25°C after 5 days, the deletion of sopB strongly enhanced the survival of C. albicans by 16.6-fold compared to the WT strain (Fig. 1). In contrast, deletion of SPI-2 gene ssaE, sseB, or sseJ or global regulator gene adrA, luxS, phoP, or ssrA still repressed the fungal viability similar to the WT. Previous reports demonstrated that translocases (including SipB) controlled by SPI-1 are required for translocation of SopB into the host cell cytosol (71). To explore whether the SipB translocation machinery component is also linked to killing of C. albicans, we examined the viability of C. albicans coinfected with the sipB mutant. Consistent with the results observed with the sopB mutant, the viability of C. albicans was also increased as a result of the sipB deletion (16.8-fold; Fig. 1). To summarize the analysis of the S. Typhimurium mutant strains, the sopB effector and the sipB translocase influence the anticandidal activity of the bacterium.
Fig. 1.
The S. Typhimurium sopB effector influences the viability of C. albicans under an in vitro planktonic environment. The viability of C. albicans in the presence of S. Typhimurium wild type (WT) or various mutants was determined at 25°C after 5 days. The error bars represent the standard errors of the mean for two independent biological replicates. Asterisks indicate significantly different values (P < 0.05; Student t test).
The sopB effector selectively represses the viability of C. albicans.
Gram-negative bacteria, such as A. baumannii (52) and P. aeruginosa (25) bind to and kill C. albicans filaments without affecting yeast cells, and our group showed that S. Typhimurium may preferentially kill filamentous C. albicans cells over yeast cells (67). However, the viability measured using CFU assays does not accurately reflect the number of viable cells for filamentous fungi (9). Therefore, in order to evaluate the effect of the sopB effector against C. albicans filaments, we also used XTT assays (38). As expected, C. albicans Δtup1 cells grown under conditions that promote filament development were highly susceptible to S. Typhimurium WT, whereas deleting sopB and sipB limited the effect of S. Typhimurium (Fig. 2A). Consistently, we verified a similar activity using the C. albicans SSY50-B filaments (Fig. 2B). Importantly, the effect was limited in yeast-type cells from C. albicans Δsuv3 or from strain SSY50-B grown without DOX (Fig. 2). These results indicate that the sopB effector is important for the killing of C. albicans filaments.
Fig. 2.
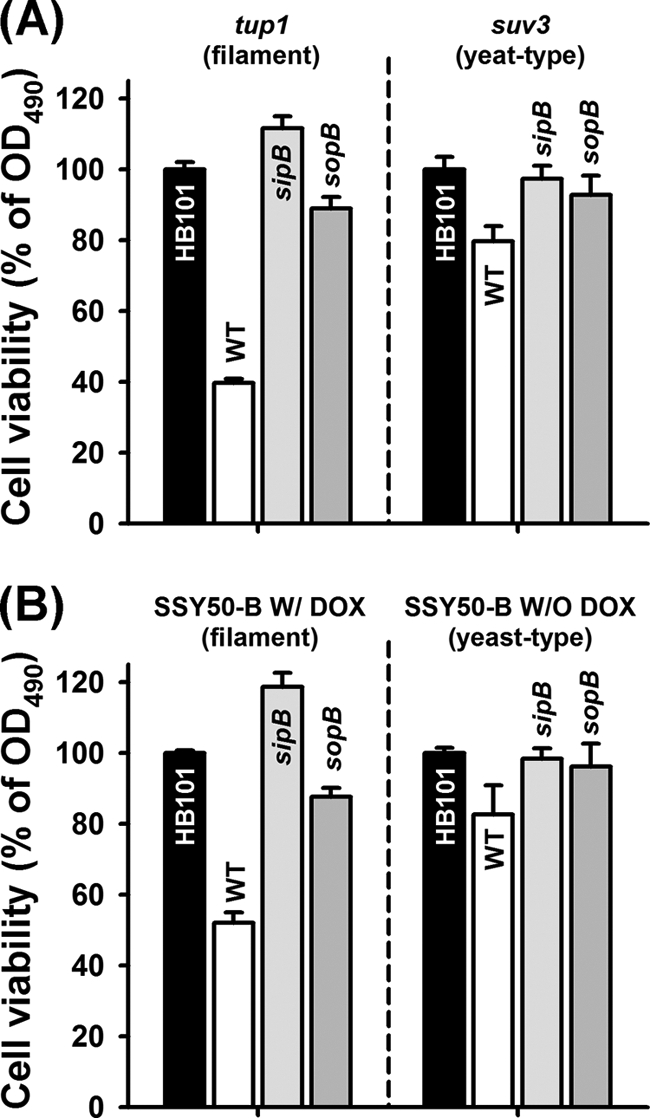
S. Typhimurium kills C. albicans filaments via the sopB effector. Viability of C. albicans filaments using the XTT assay. (A) C. albicans Δtup1 or Δsuv3 strains were cultured at 25°C for 48 h for constitutive filament production or normal yeast cells, respectively. (B) In addition, the experiments with C. albicans SSY50-B were performed in the presence or absence of DOX (20 μg/ml) for abundant filaments or yeast form cells. Filaments or yeast cells were exposed to S. Typhimurium wild-type (WT) and mutant strains for 24 h. The cell viability was evaluated and normalized against the HB101 control. Bars represent the standard errors of the mean for two independent biological replicates.
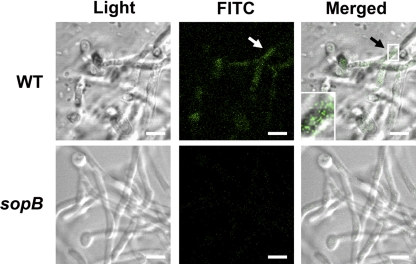
SopB is translocated into filaments of C. albicans through SipB.
To visualize the translocation of SopB into filaments, C. albicans cells were coinfected with S. Typhimurium strains expressing FLAG-tagged SopB that enable immunodetection of the protein (Table 1). We found that the fluorescent SopB protein shifts into the interior of the C. albicans filaments; however, there was no signal or SopB translocation when we used the sipB mutant (merged images in Fig. 3). In addition, we detected weak fluorescent SopB signals in yeast-type cells (data not shown). This finding demonstrates that, similar to what happens during the interaction of mammalian cells with S. Typhimurium (50), the SopB effector protein translocates into fungal filaments via SipB.
Fig. 3.
Translocation of SopB into C. albicans filaments via SipB. SopB translocation in S. Typhimurium wild-type (top row) and sipB mutant (bottom row) was visualized by confocal microscopy. Scale bars, 4.2 μm. The inset shows an enlarged view of the area indicated by the arrow. An immunofluorescence assay was performed as described in Materials and Methods.
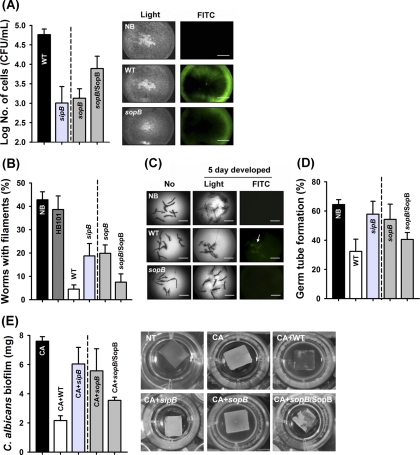
sopB is essential for the attachment to C. albicans filaments.
We hypothesize that the sopB effector can also influence S. Typhimurium attachment to C. albicans filaments, since SPI-1 effectors have been shown to be required for Salmonella attachment to eukaryotic host cells (31). To evaluate this hypothesis, we investigated the attachment of S. Typhimurium WT and the sopB mutant to filaments produced by C. albicans. In this experiment, C. albicans SSY50-B filaments were prepared in the presence of 20 μg of DOX/ml and then added to either the GFP-expressing S. Typhimurium WT or the sopB mutant. As expected, fluorescent GFP intensity was decreased in the sopB deletion mutant on C. albicans filaments compared to the WT (Fig. 4A). Moreover, by direct CFU counting, we verified that the deletion of either sopB or sipB resulted in a significant reduction in bacterial attachment to C. albicans filaments consistently by >10-fold, and this repression was abolished by complementation of SopB (Fig. 4A; P < 0.05). These results suggest that, at least in part, the sopB effector share the function for attachment on fungal filaments with mammalian cells.
Fig. 4.
S. Typhimurium strongly inhibits C. albicans filamentation and biofilm formation via the sopB effector. (A) Attachment of S. Typhimurium wild-type (WT) and sopB mutant strains to the C. albicans filaments. C. albicans SSY50-B filaments were formed in 96-well plates with 20 μg of DOX/ml and infected with GFP-expressing Salmonella strains for 15 h at 37°C. Salmonella attachment on C. albicans filaments was visualized by using fluorescence microscopy (scale bars, 1 mm) and counted by using the CFU assay. (B) Inhibition of C. albicans DAY185 filamentation in the C. elegans coinfection model. (C) Repression of filament elongation in the C. elegans coinfection model. An arrow indicates the GFP expression of S. Typhimurium cells on the C. albicans filaments (scale bars, 1 mm). (D) Inhibition of germ tube formation. (E) C. albicans biofilm formation on silicone squares in spider medium for 60 h at 37°C (NT, no treatment; CA, C. albicans alone; CA+WT, C. albicans cocultured with S. Typhimurium wild type; CA+sopB or sipB, C. albicans cocultured with the S. Typhimurium sopB or sipB mutants; CA+sopB/SopB, C. albicans cocultured with the S. Typhimurium sopB mutant overproducing SopB).
The sopB effector mediates inhibition of C. albicans filaments in vivo.
To explore whether sopB plays a direct role in the inhibition of C. albicans filaments in vivo, we utilized the C. elegans coinfection assay. Using this assay we previously reported that S. Typhimurium (but not nonpathogenic E. coli strains) can inhibit C. albicans filamentation (67), even though S. Typhimurium alone is toxic in C. elegans (data not shown). Corroborating the viability tests, deleting sopB and sipB significantly increased the number of filament-coated worms compared to the WT (Fig. 4B), but filamentation in the presence of the sopB mutant was less than that observed for E. coli HB101 or C. albicans alone (P < 0.05). Corroborating these results, we found that the number of worms with filaments was restored by complementing SopB. This finding using the model host C. elegans indicates that the sopB effector is an important factor that inhibits C. albicans filaments in vivo.
We also evaluated whether the sopB effector is involved in the inhibition of preformed filaments. After the forming filaments of C. albicans in nematodes for 1 day, we transferred C. elegans into fresh liquid medium containing the GFP expressing S. Typhimurium WT and the sopB mutant (Table 1). Consistently, we found that exposure to S. Typhimurium WT inhibits filament elongation, but the effect of the sopB mutant was significantly less (Fig. 4C). In addition, we introduced GFP-expressing Salmonella strains to visualize the binding of S. Typhimurium to C. albicans filaments and inhibit their elongation (Fig. 4C, white arrow). We found that, similar to our in vitro studies reported above, sopB is essential for the association of S. Typhimurium with C. albicans filaments. Moreover, as in the in vitro and in vivo studies detailed above, our findings also indicated that sopB is essential for the initiation of the fungal germ tubes (Fig. 4D). Hence, we conclude that the sopB effector is directly involved in an antagonistic effect against C. albicans filaments at different stages of hyphal development.
The sopB effector mediates the S. Typhimurium effect on C. albicans biofilm.
The morphological transition from yeast to filaments is a major requirement for biofilm formation, as well as virulence (16, 55). Therefore, we considered the possibility that the sopB effector may influence C. albicans biofilm formation. This hypothesis is an extension of our finding above in which S. Typhimurium attaches to filaments (Fig. 4A), and this inhibited filament formation and elongation (Fig. 4B and C) and selectively killed C. albicans filaments (Fig. 1 and 2) via the sopB effector. We evaluated the effect of S. Typhimurium on the C. albicans biofilm using silicone pads (67). As shown in Fig. 4E, the robust C. albicans biofilm was dramatically repressed by the S. Typhimurium WT. This profound reduction was depleted when the C. albicans biofilm was exposed to the sipB or sopB mutants (P < 0.05 compared to the WT), while they were still statistically different with the biofilms of C. albicans alone. Accordingly, complementing SopB restored the inhibition of biofilm formation. Our observations confirm that the killing of filaments medicated by sopB effector affects filamentation, as well as fungal biofilm formation.
The S. Typhimurium sopB effector negatively regulates the transcription of TEC1, HWP1, ALS3, and CDC42 in C. albicans.
Using qRT-PCR, we found that sopB is upregulated by supernatant obtained from C. albicans filaments [Fig. 5A; (2.5 ± 0.4)-fold in Δtup1 versus Δsuv3 strains and (8.9 ± 0.3)-fold in SC5314 versus Δcph1/Δefg1 strains]. Also, under these conditions, sipB was slightly induced by the supernatant from filamentous C. albicans cells. At least in part, these findings are in agreement with our results on the role of sopB and sipB in competing C. albicans filaments (Fig. 1). We further evaluated whether the C. albicans quorum-sensing signal farnesol influenced the regulation of sopB and sipB, but farnesol at 100 and 200 μM did not alter the transcription of either of these genes (data not shown).
Fig. 5.
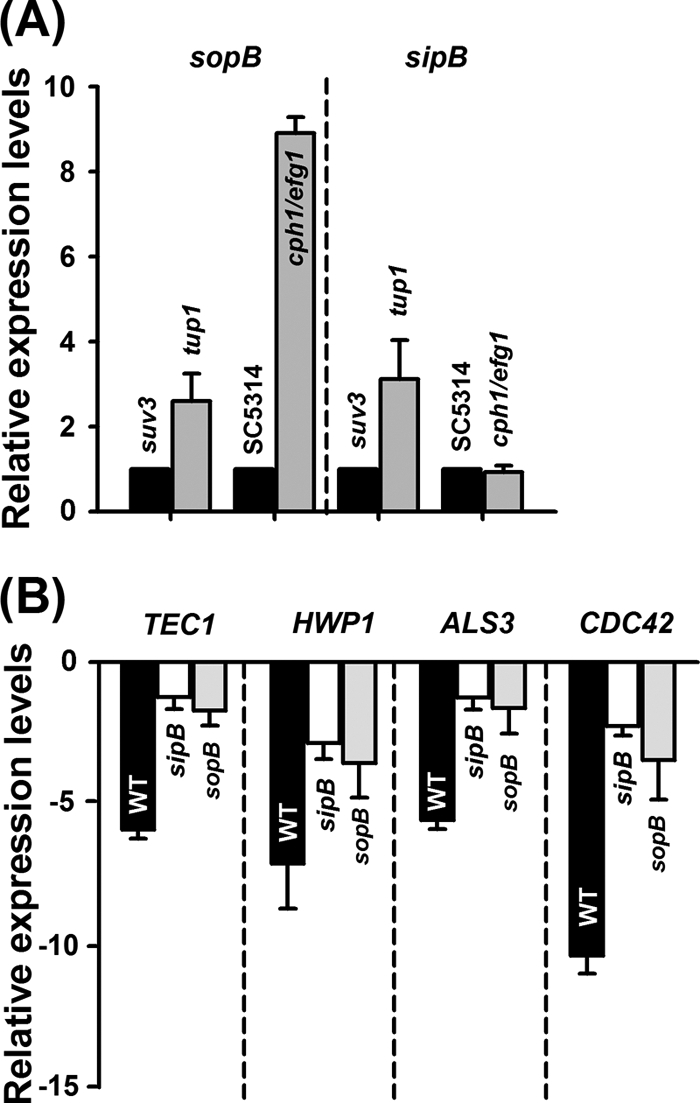
Gene expression in S. Typhimurium and C. albicans. (A) Transcription of sopB and sipB of the S. Typhimurium wild type in the presence of supernatants from filaments or yeast-form C. albicans. (B) Transcriptional levels of TEC1, HWP1, ALS3, and CDC42 in the presence or absence of sopB or sipB. The fold change was normalized by comparison to an HB101 control.
Initially, we hypothesized that S. Typhimurium TTSS effectors, including SopB and SipB, regulate specific components associated with the C. albicans morphological transition. Preliminary results among essential virulence factors, including NRG1 (which encodes a transcriptional repressor and regulates filament formation and virulence) (44), TUP1 (which encodes a transcriptional corepressor and represses filamentous growth) (10), TEC1 (which encodes a TEA/ATTS transcription factor involved in the pheromone response pathway in white cells and the regulation of filament-specific genes) (60), EFG1 (which encodes a transcriptional repressor) (64), HWP1 (which encodes a filament-specific cell wall protein) (47), ALS3 (which encodes a filament-specific adhesion) (72), SOD2 (which encodes superoxide dismutase) (27), and CDC42 (which encodes a Rho-type GTPase) (56), indicate that S. Typhimurium supernatants selectively repressed transcriptional levels of TEC1, HWP1, ALS3, and CDC42 (data not shown). Moreover, we investigated whether the transcription of TEC1, HWP1, ALS3, and CDC42 genes in C. albicans are influenced by the direct contact between the fungus and S. Typhimurium and the translocation of the sopB effector. The qRT-PCR assay showed that the transcriptional levels of TEC1 [(−5.9 ± 0.3)-fold], HWP1 [(−7.1 ± 1.6)-fold], and ALS3 [(−5.6 ± 0.3)-fold], were significantly repressed by S. Typhimurium WT, whereas these reductions were restored by the deletion of sopB effector or the sipB translocase gene (Fig. 5B). It is reasonable that the function of TEC1, HWP1, and ALS3 is linked to hyphal development and biofilm formation in C. albicans as described previously (47, 60, 72). More importantly, the transcription of CDC42 was strongly decreased by (10.4 ± 0.6)-fold in the presence of S. Typhimurium. Critically, CDC42 is essential for Candida viability (39). Therefore, our qRT-PCR results suggest that, at least in part, the sopB effector translocated into filaments through SipB may kill C. albicans filaments through associating with CDC42.
DISCUSSION
Bacterial-fungal encounters are common in nature, as well as in clinical settings, and shape microbial pathogenesis. The interactions between fungal and bacterial pathogens have been previously extensively investigated (2, 5, 8, 25, 52, 67). Of importance is the diverse population of bacteria and fungi that coexist within the human intestinal tract. However, very little is known about the specific mechanisms underlying these interactions. In the present study we demonstrate that TTSS and especially the sopB effector are essential for S. Typhimurium to survive the interaction with intestinal fungi, such as C. albicans. The role of the sopB effector, as well as that of the associated translocase SipB, is based on the following five findings. (i) The deletion of sopB significantly increased the survival of C. albicans in vitro (Fig. 1). (ii) The sopB effector translocates into filaments via SipB (Fig. 3) and kills C. albicans filaments (Fig. 2). (iii) Similar to the findings in vitro, sopB of S. Typhimurium diminished the viability of C. albicans filaments during C. elegans infection (Fig. 4). (iv) The sopB effector repressed the elongation of filaments and germ tubes, as well as Candida biofilm formation (Fig. 4). (v) Remarkably, the sopB effector is associated with the transcriptional repression of CDC42 in C. albicans (Fig. 5). Based on these findings, we report that the sopB effector of S. Typhimurium is an important weapon for competing against fungi, and this constitutes a novel role for bacterial TTSS effectors.
We recently utilized C. elegans to study monomicrobial infection due to C. albicans (11, 53) or S. Typhimurium (1), as well as to study the C. albicans-S. Typhimurium coinfection (67). This model of intestinal infection is particularly relevant for the study of microbial infections (45). In our previous observation (67), the S. Typhimurium-C. albicans competition in polymicrobial infection seemed to be mediated by direct adhesion to filaments, as well as growth-dependent molecules secreted by the bacteria. Therefore, we investigated the hypothesis that TTSS effectors may play a role in S. Typhimurium competition against C. albicans. The TTSS apparatus and its effector proteins are exclusively expressed and secreted at the stationary phase of growth (61) and are strongly linked to virulence traits, such as contact, invasion, and biofilm formation (37). Interestingly, we found that the antagonistic interaction between S. Typhimurium and C. albicans is multifactorial and that the viability of C. albicans is associated with the S. Typhimurium sopB and sipB TTSS translocation machinery units (Fig. 1).
We show that the TTSS genes regulated by SPI-1 (including sopB and sipB) are involved in competing with fungal pathogens. TTSS that are regulated by SPI-1 mainly mediate the initial attachment of S. Typhimurium on mammalian cells and the initiation of mammalian cell death is SPI-1 dependent (30), whereas the SPI-2 component of TTSS involve postinvasion processing, including vacuole maturation (15). Importantly, sopB and sipB are associated with SPI-1 regulation. The Salmonella SopB (also known as SigD) is a homologue of IpgD from Shigella flexneri (46) and is an inositol phosphate that acts on the phospholipids in the host cell membrane (30, 57). SopB is the SPI-1-regulated effector for TTSS that is quickly translocated into the mammalian host after contact (50) and is linked to enteropathogenesis of mammalian cells since sopB defective mutants of Salmonella are attenuated (48). Therefore, we suggest that SopB may modify membrane phospholipids of C. albicans filaments and that this modification results in filament death. In addition, SipB is required for the assembly of the TTSS needle complex and is one of the effectors regulated by SPI-1. Interestingly, SipB helps the translocation of SPI-1 effectors, including SopB into host cells (71, 74), and triggers mammalian cell death directly (24). Of note is that the regulatory system encoded by ssrA, which controls the SPI-2 system (7), had no effect in the competition with C. albicans (Fig. 1). Consistent with these observations, the sopB effector is essential for the S. Typhimurium attachment to filaments of C. albicans (Fig. 4A). Furthermore, after translocating into the filaments via the SipB translocase (Fig. 3), the sopB effector can inhibit filament elongation (Fig. 4C) in C. elegans, as well as fungal biofilm formation on silicone squares (Fig. 4E).
Generally, bacteria, including S. Typhimurium, produce quorum-sensing (QS) signaling molecules to regulate expression of a number of genes, including the luxS gene, which is important for the synthesis of this QS signal in S. Typhimurium (66). Importantly, the QS signaling molecule is also resistant to acid and heat (65). Boone et al. (8) identified a novel signaling molecule in B. cenocepacia that is a structural homologue of a QS molecule that inhibited germ tube and filament formation of C. albicans. Thus, we postulated that a QS-related gene is also essential in interacting with fungi. Unexpectedly, in the present study, the deletion of luxS had no impact on the viability of C. albicans (Fig. 1), which was also true with another other QS sensor gene sdiA that did not inhibit C. albicans filaments (65). Of note is that the activity of this signal molecule is maximal at mid-exponential phase and dramatically decreases at the stationary phase (65). Although bacterial QS signals were not responsible for the antifungal effects of S. Typhimurium on C. albicans directly, previous work has shown that 3-oxo-C12 homoserine lactone, the QS signaling molecule produced by P. aeruginosa, was involved in C. albicans filamentation (26), and our group has reported that QS is involved in the Acinetobacter-Candida interaction (52). These results suggest that different strategies to fight C. albicans may be dependent on the specific genus of bacteria.
Even though TTSS-related components were not directly linked in the Pseudomonas-Candida interaction, previous reports demonstrate that effector proteins such as ExoS are toxic in Saccharomyces cerevisiae (63) and clearly contribute to the toxicity of Pseudomonas toward amoebae (36). Importantly, it has been reported that SopB strongly influences the viability of S. cerevisiae (3). Interestingly, ExoS and SopB share a function in mammals by modulating GTPase and actin polymerization (3, 36). Importantly, we show that the sopB effector strongly repressed CDC42 mRNA levels (Fig. 5B). Notably, silencing of CDC42 is lethal in Candida (39) and inhibits the growth of mammalian cells (70). More importantly, Cdc42p (cell division cycle 42) is involved in C. albicans filament-specific characteristics, including hyphal growth and filament-specific gene transcripts (12, 68, 73). A recent report describes that the SopB effector protein strongly binds with the small G-protein Cdc42p in yeast (56), as well as in mammalian cells (57). Therefore, it seems reasonable to assume that, at lease in part, the SopB effector of S. Typhimurium affects C. albicans filaments through association with Cdc42p. In addition, because, Cdc42p is also required for invasion via hyphal growth of C. albicans (6), our future goal is to establish the pathway that inhibits or kills filaments through the SopB effector-Cdc42p association.
We also report the observation that the C. albicans supernatant induces sopB transcripts, whereas the sopB effector repressed TEC1, HWP1, and ALS3 transcripts (Fig. 5). It was extensively established that filamentation and biofilm formation are involved in C. albicans virulence and that TEC1, HWP1, and ALS3 are important key genes for the hyphal development and biofilm formation of C. albicans (47, 60, 72). Equally important, other investigators confirmed that deletion of TEC1 and HWP1 resulted in a significant reduction of C. albicans virulence in Galleria mellonella (16) and mice (62). Moreover, our qRT-PCR results are consistent with our biofilm data that the C. albicans biofilm was critically repressed by the sopB effector (Fig. 4E). Therefore, these results indicate that in addition to its effect in mammals, SopB may influence the transcriptions of genes involved in hypha- and biofilm-associated fungal pathogenesis during killing events.
Bacterial-fungal interactions play a significant role in human health since they may enhance, modulate, or decrease microbial pathogenesis (42). Intestinal bacteria need to overcome the other intestinal microflora, and the interaction of C. albicans with intestinal Gram-negative bacteria may control filamentation. The sopB effector plays a critical role in allowing the bacteria to attach and kill C. albicans filaments, and the viability and filamentation of C. albicans is influenced by sopB. Our working model suggests that in the presence of C. albicans filaments, the sopB effector is activated. The SopB effector translocates into the fungal filaments via SipB translocase and clearly kills filaments. This killing event may trigger the repression of biofilm formation. Our results propose a novel role for TTSS effectors in bacterial-fungal interactions.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institutes of Health (P01 AI083214, R01 AI075286, and R21 AI079569) to E.M.
We are grateful for the pSB2908 and pCM18 plasmids provided by Jorge E. Galán (Yale University) and Thomas K. Wood (Texas A&M University), respectively. We thank Michael McClelland (Sidney Kimmel Cancer Center) for providing the S. Typhimurium 14028 wild-type and the isogenic mutants. We also thank Beth B. Fuchs for help with the confocal microscopy and Jeffrey J. Coleman for useful discussions.
Footnotes
Published ahead of print on 15 April 2011.
REFERENCES
- 1. Aballay A., Ausubel F. M. 2001. Programmed cell death mediated by ced-3 and ced-4 protects Caenorhabditis elegans from Salmonella typhimurium-mediated killing. Proc. Natl. Acad. Sci. U. S. A. 98:2735–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adam B., Baillie G. S., Douglas L. J. 2002. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J. Med. Microbiol. 51:344–349 [DOI] [PubMed] [Google Scholar]
- 3. Alemán A., et al. 2005. The amino-terminal non-catalytic region of Salmonella typhimurium SigD affects actin organization in yeast and mammalian cells. Cell Microbiol. 7:1432–1436 [DOI] [PubMed] [Google Scholar]
- 4. Bakshi C. S., et al. 2000. Identification of SopE2, a Salmonella secreted protein which is highly homologous to SopE and involved in bacterial invasion of epithelial cells. J. Bacteriol. 182:2341–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bamford C. V., et al. 2009. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect. Immun. 77:3696–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bassilana M., Hopkins J., Arkowitz R. A. 2005. Regulation of the Cdc42/Cdc24 GTPase module during Candida albicans hyphal growth. Eukaryot. Cell 4:588–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bohez L., et al. 2008. The Salmonella pathogenicity island 2 regulator ssrA promotes reproductive tract but not intestinal colonization in chickens. Vet. Microbiol. 126:216–224 [DOI] [PubMed] [Google Scholar]
- 8. Boone C., et al. 2008. A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J. 2:27–36 [DOI] [PubMed] [Google Scholar]
- 9. Bowman J. C., et al. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Braun B. R., Johnson A. D. 1997. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science 277:105–109 [DOI] [PubMed] [Google Scholar]
- 11. Breger J., et al. 2007. Antifungal chemical compounds identified using a Caenorhabditis elegans pathogenicity assay. PLoS Pathog. 3:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Court H., Sudbery P. 2007. Regulation of Cdc42 GTPase activity in the formation of hyphae in Candida albicans. Mol. Biol. Cell 18:265–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davis D., Wilson R. B., Mitchell A. P. 2000. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol. Cell. Biol. 20:971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donegan K., Matyac C., Seidler R., Porteous A. 1991. Evaluation of methods for sampling, recovery, and enumeration of bacteria applied to the phylloplane. Appl. Environ. Microbiol. 57:51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drecktrah D., Knodler L. A., Galbraith K., Steele-Mortimer O. 2005. The Salmonella SPI1 effector SopB stimulates nitric oxide production long after invasion. Cell Microbiol. 7:105–113 [DOI] [PubMed] [Google Scholar]
- 16. Fuchs B. B., et al. 2010. Role of filamentation in Galleria mellonella killing by Candida albicans. Microbes Infect. 12:488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Galán J. E., Wolf-Watz H. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444:567–573 [DOI] [PubMed] [Google Scholar]
- 18. García B., et al. 2004. Role of the GGDEF protein family in Salmonella cellulose biosynthesis and biofilm formation. Mol. Microbiol. 54:264–277 [DOI] [PubMed] [Google Scholar]
- 19. Gillum A. M., Tsay E. Y., Kirsch D. R. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of Saccharomyces cerevisiae ura3 and Escherichia coli pyrF mutations. Mol. Gen. Genet. 198:179–182 [DOI] [PubMed] [Google Scholar]
- 20. Groisman E. A., Chiao E., Lipps C. J., Heffron F. 1989. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc. Natl. Acad. Sci. U. S. A. 86:7077–7081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hansen M. C., Palmer R. J. J., Udsen C., White D. C., Molin S. 2001. Assessment of GFP fluorescence in cells of Streptococcus gordonii under conditions of low pH and low oxygen concentration. Microbiology 147:1383–1391 [DOI] [PubMed] [Google Scholar]
- 22. Harriott M. M., Noverr M. C. 2010. Ability of Candida albicans mutants to induce Staphylococcus aureus vancomycin resistance during polymicrobial biofilm formation. Antimicrob. Agents Chemother. 54:3746–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hensel M., et al. 1998. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol. Microbiol. 30:163–174 [DOI] [PubMed] [Google Scholar]
- 24. Hersh D., et al. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. U. S. A. 96:2396–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hogan D. A., Kolter R. 2002. Pseudomonas-Candida interactions: an ecological role for virulence factors. Science 296:2229–2232 [DOI] [PubMed] [Google Scholar]
- 26. Hogan D. A., Vik A., Kolter R. 2004. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 54:1212–1223 [DOI] [PubMed] [Google Scholar]
- 27. Hwang C. S., Baek Y. U., Yim H. S., Kang S. O. 2003. Protective roles of mitochondrial manganese-containing superoxide dismutase against various stresses in Candida albicans. Yeast 20:929–941 [DOI] [PubMed] [Google Scholar]
- 28. Keeney K. M., Finlay B. B. 2011. Enteric pathogen exploitation of the microbiota-generated nutrient environment of the gut. Curr. Opin. Microbiol. 14:92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim D. H., et al. 2002. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297:623–626 [DOI] [PubMed] [Google Scholar]
- 30. Knodler L. A., Finlay B. B., Steele-Mortimer O. 2005. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J. Biol. Chem. 280:9058–9064 [DOI] [PubMed] [Google Scholar]
- 31. Lara-Tejero M., Galán J. E. 2009. Salmonella enterica serovar typhimurium pathogenicity island 1-encoded type III secretion system translocases mediate intimate attachment to nonphagocytic cells. Infect. Immun. 77:2635–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu H., Köhler J., Fink G. R. 1994. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 266:1723–1726 [DOI] [PubMed] [Google Scholar]
- 33. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 34. Lo H. J., et al. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939–949 [DOI] [PubMed] [Google Scholar]
- 35. Ly K. T., Casanova J. E. 2007. Mechanisms of Salmonella entry into host cell. Cell Microbiol. 9:2103–2111 [DOI] [PubMed] [Google Scholar]
- 36. Matz C., et al. 2008. Pseudomonas aeruginosa uses type III secretion system to kill biofilm-associated amoebae. ISME J. 2:843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McGhie E. J., Brawn L. C., Hume P. J., Humphreys D., Koronakis V. 2009. Salmonella takes control: effector-driven manipulation of the host. Curr. Opin. Microbiol. 12:117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meshulam T., Levitz S. M., Christin L., Diamond R. D. 1995. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanilide (XTT). J. Infect. Dis. 172:1153–1156 [DOI] [PubMed] [Google Scholar]
- 39. Michel S., et al. 2002. Generation of conditional lethal Candida albicans mutants by inducible deletion of essential genes. Mol. Microbiol. 46:269–280 [DOI] [PubMed] [Google Scholar]
- 40. Miki T., Shibagaki Y., Danbara H., Okada N. 2009. Functional characterization of SsaE, a novel chaperone protein of the type III secretion system encoded by Salmonella pathogenicity island 2. J. Bacteriol. 191:6843–6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miyata S., Begun J., Troemel E. R., Ausubel F. M. 2008. DAF-16-dependent suppression of immunity during reproduction in Caenorhabditis elegans. Genetics 178:903–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morales D. K., Hogan D. A. 2010. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog. 6:e1000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moy T. I., et al. 2006. Identification of novel antimicrobials using a live-animal infection model. Proc. Natl. Acad. Sci. U. S. A. 103:10414–10419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murad A. M., et al. 2001. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 20:4742–4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mylonakis E., Casadevall A., Ausubel F. M. 2007. Exploiting amoeboid and non-vertebrate animal model systems to study the virulence of human pathogenic fungi. PLoS Pathog. 3:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Niebuhr K., et al. 2000. IpgD, a protein secreted by the type III secretion machinery of Shigella flexneri, is chaperoned by IpgE and implicated in entry focus formation. Mol. Microbiol. 38:8–19 [DOI] [PubMed] [Google Scholar]
- 47. Nobile C. J., Nett J. E., Andes D. R., Mitchell A. P. 2006. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot. Cell 5:1604–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Norris F. A., Wilson M. P., Wallis T. S., Galyov E. E., Majerus P. W. 1998. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc. Natl. Acad. Sci. U. S. A. 95:14057–14059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ohlson M. B., Fluhr K., Birmingham C. L., Brumell J. H., Miller S. I. 2005. SseJ deacylase activity by Salmonella enterica serovar Typhimurium promotes virulence in mice. Infect. Immun. 73:6249–6259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Patel J. C., Hueffer K., Lam T. T., Galán J. E. 2009. Diversification of a Salmonella virulence protein function by ubiquitin-dependent differential localization. Cell 137:283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peleg A. Y., Hogan D. A., Mylonakis E. 2010. Medically important bacterial-fungal interactions. Nat. Rev. Microbiol. 8:340–349 [DOI] [PubMed] [Google Scholar]
- 52. Peleg A. Y., et al. 2008. Prokaryote-eukaryote interactions identified by using Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 105:14585–14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pukkila-Worley R., Peleg A. Y., Tampakakis E., Mylonakis E. 2009. Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryot. Cell 8:1750–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Qin J., et al. 2010. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Richard M. L., Nobile C. J., Bruno V. M., Mitchell A. P. 2005. Candida albicans biofilm-defective mutants. Eukaryot. Cell 4:1493–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rodríguez-Escudero I., Rotger R., Cid V. J., Molina M. 2006. Inhibition of Cdc42-dependent signaling in Saccharomyces cerevisiae by phosphatase-dead SigD/SopB from Salmonella typhimurium. Microbiology 152:3437–3452 [DOI] [PubMed] [Google Scholar]
- 57. Rogers L. D., et al. 2008. Identification of cognate host targets and specific ubiquitylation sites on the Salmonella SPI-1 effector SopB/SigD. J. Proteomics 71:97–108 [DOI] [PubMed] [Google Scholar]
- 58. Santiviago C. A., et al. 2009. Analysis of pools of targeted Salmonella deletion mutants identifies novel genes affecting fitness during competitive infection in mice. PLoS Pathog. 5:e1000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Saville S. P., Lazzell A. L., Monteagudo C., Lopez-Ribot J. L. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 2:1053–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schweizer A., Rupp S., Taylor B. N., Röllinghoff M., Schröppel K. 2000. The TEA/ATTS transcription factor CaTec1p regulates hyphal development and virulence in Candida albicans. Mol. Microbiol. 38:435–445 [DOI] [PubMed] [Google Scholar]
- 61. Song M., et al. 2004. ppGpp-dependent stationary phase induction of genes on Salmonella pathogenicity island 1. J. Biol. Chem. 279:34183–34190 [DOI] [PubMed] [Google Scholar]
- 62. Staab J. F., Bradway S. D., Fidel P. L., Sundstrom P. 1996. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 283:1535–1538 [DOI] [PubMed] [Google Scholar]
- 63. Stirling F. R., Evans T. J. 2006. Effects of the type III secreted pseudomonal toxin ExoS in the yeast Saccharomyces cerevisiae. Microbiology 152:2273–2285 [DOI] [PubMed] [Google Scholar]
- 64. Stoldt V. R., Sonneborn A., Leuker C. E., Ernst J. F. 1997. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 16:1982–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Surette M. G., Bassler B. L. 1998. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. U. S. A. 95:7046–7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Surette M. G., Miller M. B., Bassler B. L. 1999. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. U. S. A. 96:1639–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tampakakis E., Peleg A. Y., Mylonakis E. 2009. Interaction of Candida albicans with an intestinal pathogen, Salmonella enterica serovar Typhimurium. Eukaryot. Cell 8:732–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. VandenBerg A. L., Ibrahim A. S., Edwards J. E. J., Toenjes K. A., Johnson D. 2004. Cdc42p GTPase regulates the budded-to-hyphal-form transition and expression of hypha-specific transcripts in Candida albicans. Eukaryot. Cell 3:724–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wood M. W., et al. 2004. Structural analysis of Salmonella enterica effector protein SopD. Biochim. Biophys. Acta 1698:219–226 [DOI] [PubMed] [Google Scholar]
- 70. Wu F., et al. 2008. RNA-interference-mediated Cdc42 silencing downregulates phosphorylation of STAT3 and suppresses growth in human bladder-cancer cells. Biotechnol. Appl. Biochem. 49:121–128 [DOI] [PubMed] [Google Scholar]
- 71. Zhang S., et al. 2002. The Salmonella enterica serotype typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infect. Immun. 70:3843–3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhao X., et al. 2006. Candida albicans Als3p is required for wild-type biofilm formation on silicone elastomer surfaces. Microbiology 152:2287–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zheng X. D., Lee R. T., Wang Y. M., Lin Q. S., Wang Y. 2007. Phosphorylation of Rga2, a Cdc42 GAP, by CDK/Hgc1 is crucial for Candida albicans hyphal growth. EMBO J. 26:3760–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhou D., Galán J. 2001. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect. 3:1293–1298 [DOI] [PubMed] [Google Scholar]