Abstract
Integrated livestock-fish aquaculture utilizes animal excreta, urine, and feed leftovers as pond fertilizers to enhance the growth of plankton and other microorganisms eaten by the fish. However, antimicrobial-resistant bacteria may be transferred and develop in the pond due to selective pressure from antimicrobials present in animal feed, urine, and feces. In an experimental pig-fish farm located in periurban Hanoi, Vietnam, nine piglets were provided feed containing 5 μg of tetracycline (TET)/kg pig weight/day and 0.45 μg of enrofloxacin (ENR)/kg pig weight/day during the second and fourth (last) months of the experiment. The aim of this study was to determine the association between the provision of pig feed with antimicrobials and the development of antimicrobial resistance, as measured in a total of 520 Escherichia coli and 634 Enterococcus strains isolated from pig manure and water-sediment pond samples. MIC values for nalidixic acid (NAL) and ENR showed that E. coli and Enterococcus spp. overall exhibited significant higher frequencies of resistance toward NAL and ENR during the 2 months when pigs were administered feed with antimicrobials, with frequencies reaching 60 to 80% in both water-sediment and manure samples. TET resistance for both indicators was high (>80%) throughout the study period, which indicates that TET-resistant E. coli and Enterococcus spp. were present in the piglets before the initiation of the experiment. PCR-based identification showed similar relative occurrences of Enterococcus faecium, Enterococcus faecalis, and other Enterococcus spp. in the water-sediment and manure samples, suggesting that Enterococcus spp. isolated in the ponds originated mainly from the pig manure. The development of antimicrobial resistance in integrated animal husbandry-fish farms and possible transfers and the impact of such resistance on food safety and human health should be further assessed.
INTRODUCTION
The use of animal manures as fertilizers of aquaculture ponds is practiced widely in Southeast Asia (12). Livestock manure is disposed of into fish ponds, and the release of nutrients supports the growth of photosynthetic organisms (10). In Vietnam, the VAC system (Vuon = garden, Ao = pond, and Chuong = pigpen) is a traditional household-based integrated farming system which was developed to improve and increase yields from livestock keeping (15). Pigs are fed mainly commercial feed, often containing antimicrobials added as growth promoters to improve the feed conversion rate and to control and prevent diseases (6). Manure, urine, and surplus feed are continuously discharged directly into the fish ponds.
The discharge of animal wastes containing antimicrobial residues and resistant bacteria into fish ponds may favor selection and growth of antimicrobial-resistant bacteria, with a potential risk of resistance genes being disseminated into a wide range of aquatic environmental bacteria (12). A previous study in Thailand of the impact of integrated aquaculture on the development of antimicrobial resistance in the pond microflora demonstrated a significant increase of resistance among ubiquitous Acinetobacter spp. obtained from the water-sediment interphase toward six different classes of antimicrobials during the first 2 months of production at a newly started integrated fish farm. Overall, a higher prevalence of bacterial resistance was found in integrated farms than that in control farms (12). That study was impaired by a lack of knowledge about the type and amount of antimicrobials added to the animal feed as growth promoters and therapeutics at the farms studied (12). Enterococcus spp. and Escherichia coli are common in environments contaminated by human and animal feces and therefore are often used as indicators of fecal contamination and to monitor antimicrobial resistance (12, 13, 14).
The aim of the study was to determine the association between the provision of pig feed with antimicrobials and the development of antimicrobial resistance, as measured in E. coli and Enterococcus strains isolated from pig manure and water-sediment pond samples at an experimental integrated VAC farm.
(Part of this work was presented at FEMS 2009—3rd Congress of European Microbiologists, Gothenburg, Sweden, 2009.)
MATERIALS AND METHODS
Experimental farm and feed containing antimicrobials.
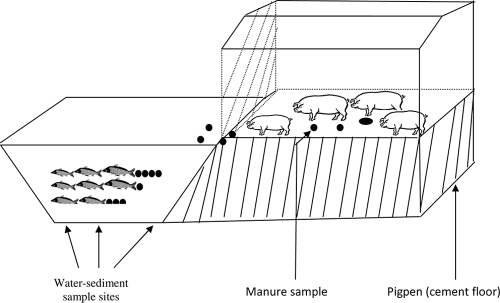
This study was conducted from January through June 2008 at an experimental farm located in the Gia Lam district in periurban Hanoi, Vietnam. The integrated pig-fish (VAC) farm consisted of a small farmhouse build on the dike of a fish pond sized 250 m2 and with a depth of 1.4 m (Fig. 1). Before the experiment, fecal material was removed from each of the three pig pens in the farmhouse, which were then disinfected by spraying all surfaces with 5% sodium hypochlorite solution and washed repeatedly with clean water. The fish pond was emptied of water by pumping, and CaCO3 (5 kg/100 m2) was subsequently applied not only to disinfect the bottom sediment but also to fertilize the pond. Following 2 weeks of drying, the pond was filled up with river water and stocked with fingerlings of common carp (Cyprinus carpio), silver carp (Hypophthalmichthys molitrix), and Nile tilapia (Oreochromis niloticus), which are the fish species typically stocked in household-based Vietnamese VAC farming systems (9). Nine piglets, each weighing approximately 15 kg, were purchased and introduced into the pens. The piglets had been fed only locally produced feed, mainly rice bran and vegetables, and did not receive any antimicrobials before being introduced into the pens. The floors of the pig pens were cleaned twice a day with well water from a hose, and the mixture of water, pig feces, urine, and surplus feed was discharged through pipes into the pond (Fig. 1). During the first and third months of the experiment, the pigs were fed pelleted commercial feed without any antimicrobials (Table 1). The commercial feed was produced by a local animal feed manufacturing company. During the second and fourth months of the experiment, pigs were fed the same type of commercial pig feed but now containing tetracycline (TET; 5 μg/kg pig weight/day) and enrofloxacin (ENR; 0.45 μg/kg pig weight/day), which are commonly used as growth promoters in pig feed in Vietnam (15). These concentrations are typically used in feed as growth promoters in small-scale pig farming in the Gia Lam district of Hanoi, Vietnam. Each morning during the study period, an amount of feed equal to the total daily ration to be used at the farm was prepared and fed to the pigs in three meals.
Fig. 1.
Schematic illustration of an experimental integrated pig-fish farm showing sample types and sites.
Table 1.
Sampling scheme for fecal and water-sediment samples collected from the integrated pig-fish farm
| Sampling period | Day of sampling |
|---|---|
| Mo 1, feed without antimicrobials | 1 |
| 15 | |
| 30 | |
| Mo 2, feed with TET (5 μg/kg pig wt/day) and ENR (0.45 μg/kg pig wt/day) | 33 |
| 48 | |
| 60 | |
| Mo 3, feed without antimicrobials | 63 |
| 75 | |
| 90 | |
| Mo 4, feed with TET (5 μg/kg pig wt/day) and ENR (0.45 μg/kg pig wt/day) | 93 |
| 108 | |
| 120 | |
| 135 |
Collection of water-sediment and pig manure samples.
Water-sediment and fecal samples were collected at 13 sampling times during the experiment (Table 1). At each sampling time, water-sediment samples were collected with a sterile large spoon placed at the end of a bamboo stick. Five subsamples of approximately 100 g from the top 1- to 3-cm water-sediment interface were carefully collected from five different locations in the pond. One subsample was collected at the point where wastes from the pigs were discharged into the pond. The four other subsamples were collected from each corner of the pond (Fig. 1). All subsamples were placed in a sterile plastic bag, mixed well into a composite sample which was kept on ice in an insulated box, and transported to the laboratory.
Fecal samples obtained from pigs were collected by hand using sterilized gloves. Five subsamples, each weighing about 100 g, were collected from the floor of the pens and mixed well in a sterile plastic bag. This composite fecal sample was transferred to the laboratory in an insulated box (4 to 5°C). The bacteriological analyses were initiated on the same day that the fecal and water-sediment samples were collected.
Isolation of E. coli from sediment and fecal samples.
A series of 10-fold dilutions (10−1 to 10−4) of the composite fecal and water-sediment samples were made in 1% peptone diluents containing 0.9% NaCl (BO0471; Oxoid, Cambridge, England). Aliquots of 100 μl of dilution solutions were spread in duplicate on selective E. coli-coliform chromogenic medium agar plates (CM1046; Oxoid). Plates were incubated at 37°C for 24 h, and then 20 individual purple colonies were randomly selected, streaked onto tryptone soy agar (CM0131; Oxoid), and incubated at 37°C overnight to obtain pure cultures. Colonies were subsequent grown in brain heart infusion (BHI) (CM1032; Oxoid) broth overnight and stored for further characterization at −80°C in Eppendorf tubes containing 30% glycerin.
Isolation of Enterococcus spp. and identification of Enterococcus faecium and Enterococcus faecalis by PCR.
Sample dilutions of water-sediment and feces were spread onto duplicate Slanetz & Bartley agar plates (CM0377; Oxoid) and incubated at 44°C for 48 h. Up to 30 presumptive Enterococcus colonies with a red, maroon, or pink color were randomly selected from each sample and stored at −80°C as previously described.
Identification of E. faecium and E. faecalis was done by multiplex PCR using the following primer sequences (5′–3′): for E. faecalis, ATCAAGTACAGTTAGTCTT (forward) and ACGATTCAAAGCTAACTG (reverse); and for E. faecium, GCAAGGCTTCTTAGAGA (forward) and CATCGTGTAAGCTAACTTC (reverse) (7). For one PCR, the following was added: 2 μl of purified DNA (Qiagen Nordic, Copenhagen, Denmark), 2.5 μl of 10× PCR buffer, 0.25 μl deoxynucleoside triphosphate (dNTP), 0.5 μl of each primer (10 pmol/μl), and distilled H20 to a total volume of 25 μl. The PCR program was 94°C/5 min for the first cycle, 94°C for 1 min, 54°C for 1 min and 72°C for 2 min for the next 35 cycles, and 72°C for 10 min for the last cycle. PCR was done on a DNA thermal cycler (GeneAmp PCR system 2400; Applied Biosystems, Nieuwerkerk a/d IJssel, Holland). The PCR product was separated by electrophoresis in a 1% agarose gel (SeaKem GTG agarose; Cambrex, ME), stained in 5 μl ethidium bromide (Sigma, St. Louis, MO), and visualized under UV light by Gel Doc 2000 (Bio-Rad, Sweden). A 100-bp molecular mass standard (100-bp DNA ladder; Invitrogen, Carlsbad, CA) was used as size marker during electrophoresis of the PCR products.
Antimicrobial susceptibility testing.
In addition to monitoring resistance toward enrofloxacin and tetracycline that was supplied to the pigs through the medicated feed, resistance to nalidixic acid (NAL) was also determined in E. coli, as resistance to fluoroquinolones is generally believed to be a two-step process, in which the first step is NAL resistance caused be a single mutation in gyrA (4). The MICs of NAL, ENR, and TET were determined for Enterococcus spp. and E. coli according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (5) on Mueller-Hinton agar plates (PO0152; Oxoid). The antimicrobials and tested ranges included the following: for Enterococcus spp., ENR from 0.25 to 32 μg/ml and TET from 1 to 32 μg/ml, and for E. coli, ENR from 0.25 to 32 μg/ml, TET from 4 to 32 μg/ml, and NAL from 2 to 128 μg/ml. A 10-μl culture suspension in BHI broth was adjusted to a McFarland standard of 0.5 so that it contained approximately 104 CFU, then inoculated with a micropipette as a spot on the surface of Mueller-Hinton agar plates containing the test antimicrobials, and subsequently incubated at 37°C for 24 h. The plates were read to score the MICs and were categorized as resistant using the following MIC breakpoints, as stated by the CLSI (5) (for ENR, values for ciprofloxacin were used [11]): for Enterococcus spp., ENR of ≥4 μg/ml and TET of ≥16 μg/ml, and for E. coli, ENR of ≥4 μg/ml, TET of ≥16 μg/ml, and NAL of ≥32 μg/ml. The reference strains E. coli ATCC 25922 and Enterococcus faecalis ATCC 29212 were used as controls.
Resistance prevalence was determined for all 4 months (months 1 and 3, without antimicrobials added to the feed, and months 2 and 4, with feed containing antimicrobials), each consisting of three and four (month 4 only) individual samplings (Table 1). The percentage of resistance for each month consists of data obtained from analyses of strains collected at three or four sampling points. Changes in antimicrobial resistance were determined between consecutive months by 2-by-2 contingency tables and Fischer's exact test. A P value of <0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Development of antimicrobial resistance in E. coli and Enterococcus spp. isolated from pig manure.
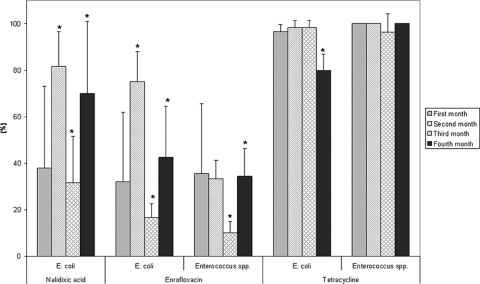
Feed without antimicrobials was given to the pigs during the first and third months of the experiment, whereas feed with antimicrobials was administered at 5 μg TET/kg pig weight/day and 0.45 μg ENR/kg pig weight/day in the second and fourth months of the study. Figure 2 shows the frequency of antimicrobial resistance seen among E. coli and Enterococcus strains isolated from pig manure samples. The resistances are based on MIC values determined for a total of 260 E. coli isolates (60, 60, 60, and 80 isolates for each study month, respectively) and 330 Enterococcus isolates (76, 78, 80, and 96 isolates, respectively). Differences in the numbers of Enterococcus isolates obtained were due to contamination and occasionally low numbers of colonies. E. coli showed significantly higher frequencies of resistance toward NAL and ENR during months 2 and 4, when pigs were administered feed with antimicrobials, than during months 1 and 3, when the feed did not contain any antimicrobials (Fig. 2). For NAL, 82% and 70% of E. coli strains isolated during months 2 and 4 were resistant, compared with resistance in 38% and 32% of E. coli strains found in months 1 and 3, respectively (P < 0.001). A similar trend was seen for ENR, in which the frequency of E. coli strains resistant to ENR was 75% in month 2 and 43% in month 4, compared with 32% and 17% in months 1 and 3, respectively (P < 0.01). The frequency of ENR-resistant Enterococcus spp. was lower than that of E. coli. Although the prevalence of ENR resistance in month 3 was lower than those in months 2 and 4 (P < 0.001), there was not a clear association between the development of resistance and provision of feed containing ENR.
Fig. 2.
The frequency (percentage) of tetracycline- and quinolone-resistant E. coli and Enterococcus spp. isolated from pig manure during four monthly samplings. The asterisks placed above the bars indicate significant differences in average resistance compared to that from the previous month.
The frequency of resistance to TET in E. coli and Enterococcus spp. was high throughout the study period, with an initial frequency of more than 95% before the piglets were exposed to any antimicrobials (Fig. 2). These findings are supported by an experimental study of feedlots, in which cattle that had not previously been administered antimicrobials were shown to carry E. coli with a high frequency of TET resistance (2). The piglets used in our study were fed only rice bran and vegetables and did not receive any antimicrobials before being introduced into the pens. Thus, it is likely that TET-resistant E. coli and Enterococcus spp. were transmitted to the piglets from the sow, other animals, or the external environment with which the piglets had contact before they were delivered to our experimental farm.
Antimicrobial resistance in E. coli and Enterococcus spp. isolated from water-sediment samples.
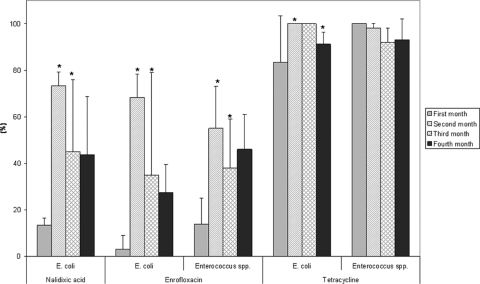
Pig feces, urine, and feed surplus were washed and discharged daily into the fish pond. Thus, the pond environment received continuously resistant fecal microorganisms and antimicrobials as well as their metabolites. Antimicrobial resistance was determined by the MIC method in a total of 260 E. coli isolates (60, 60, 60, and 80 isolates for each study month, respectively) and 304 Enterococcus isolates (79, 83, 52, and 90 isolates) obtained from water-sediment samples. Similar to what was seen from the analysis of pig manure, the frequency of NAL and ENR resistance in E. coli and that of ENR resistance in Enterococcus spp. in general were significant higher in months 2 and 4 than in the previous months (1 and 3), when feed did not contain antimicrobials. Resistances were higher (P < 0.001) in month 2 compared to those in the first month and lower (P < 0.001) in month 3 compared to those in month 2 (Fig. 3). The frequencies of resistance to TET for both indicators were high throughout the study period, ranging from 80 to 100%. For E. coli, the prevalence was significantly higher in month 2 than in the first month (P < 0.01) and significantly lower in month 4 than in month 3 (P < 0.05).
Fig. 3.
The frequency (percentage) of tetracycline- and quinolone-resistant E. coli and Enterococcus spp. isolated from water-sediment samples during four monthly samplings. The asterisks placed above the bars indicate significant differences in average resistance compared to that from the previous month.
The increase in NAL and ENR resistance seen in E. coli between sampling months 1 and 2 for both sample types and the subsequent decrease in resistance between months 2 and 3 suggest that E. coli recovered from the water-sediment samples originated mainly from the pig manure. Thus, it is unlikely that other sources, e.g., contaminated surface runoff water or other animals (like companion animals and birds), were a main source of the E. coli isolated. Closely related genotypes, e.g., from ribotyping or pulsed-field gel electrophoresis (PFGE) typing, among E. coli isolates from manure and water-sediment samples would support that the E. coli isolates originated from the pig manure.
The increases in resistance to NAL and ENR for isolates from water-sediment samples between months 1 and 2 (Fig. 3) were most likely associated with the use of these antimicrobials, and the subsequent excretion of resistant bacteria and their presence could have been favored due to selection pressure exerted by the presence of antimicrobials or antimicrobial residues (12). It is unlikely that the resistant bacteria may have acted as donors of quinolone resistance genes upon release into the fish ponds, as resistance to quinolones are rarely transferred horizontally. The significant increase in NAL and ENR resistance prevalence for E. coli and Enterococcus spp. from sampling month 1 (with no antimicrobials added to the pig feed) to sampling month 2 (during which antimicrobials were added to the pig feed) confirms previous observations that a relatively pristine aquatic environment responds quickly to an input of antimicrobials, antimicrobial residues, and antimicrobial-resistant bacteria with an increase in the prevalence of antimicrobial-resistant bacteria (12). The subsequent reduction in antimicrobial resistance in sampling month 3 indicates that the response may be temporary and disappears when the selection/input is withdrawn. Even though resistance prevalence in pig feces increased during the last sampling period, this was surprisingly not associated with a significant increase in resistance among bacteria from water-sediment samples.
Distribution of E. faecium, E. faecalis, and other Enterococcus spp.
A total of 330 presumptive Enterococcus strains were obtained from the Slanetz & Bartley agar plates following subculture of the pig manure samples. Among these, the species-specific PCR identified 78 E. faecalis (23.6%) and 48 E. faecium (14.5%) strains, with the remaining 204 (61.8%) strains considered to belong to other Enterococcus species. A previous study covering several European Union countries showed that E. faecalis and E. faecium constituted 50 to 75% of isolated enterococci in pig feces samples (8). We are not able to explain why the two species constituted such a surprisingly low proportion of Enterococcus spp. found in the fecal samples. The pigs studied were initially fed mainly rice bran and vegetables before being introduced into the pens, where they subsequently were fed commercial pelleted feed only. It is uncertain to what extent such feed may have been the source of Enterococcus species. Identification to the species level and further characterization of the Enterococcus isolates are needed to describe the distribution of different Enterococcus spp. in the pigs.
From a total of 304 presumptive Enterococcus spp. that were obtained from the Slanetz & Bartley agar plates following subculture of water-sediment samples, the species-specific PCR identified 43 E. faecalis (14.1%) and 62 E. faecium (20.4%) strains, with the remaining 199 (65.5%) strains considered to be other Enterococcus spp. Although some variations were seen in the relative occurrence of E. faecium, E. faecalis, and other Enterococcus spp. when comparing isolates from pig manure and water-sediment samples, the similar relative distributions suggest that the Enterococcus spp. isolated in the ponds originated mainly from the pig manure. We did not see any major variation in the proportion of E. faecalis, E. faecium, and other Enterococcus spp. between sampling periods that may have explained differences in resistance prevalence, but there may have been changes in the distribution of species within the group of other Enterococcus spp.
Our findings are in agreement with a related study in Thailand of Enterococcus spp. isolated from integrated poultry-fish farms (13). Here, a total of 410 enterococcus isolates from integrated and traditional fish farms were collected to assess whether the input of manure from chickens receiving feed containing growth promoters and antimicrobial treatments influenced the species composition and the bacterial antimicrobial resistance in the fish pond environment. Overall, E. faecium and E. faecalis constituted 54% of the enterococcus population, compared to 30 to 35% in this study. In the Thai study, E. faecium and E. faecalis were the predominant species isolated from water-sediment samples collected at the integrated farms, whereas Enterococcus casseliflavus and Enterococcus mundtii isolates were most prevalent in traditional farms with no inputs of animal manure. E. faecalis and E. faecium demonstrated the highest prevalences of resistance, whereas E. mundtii isolates were susceptible to all antimicrobials tested (13). All the enterococcus species isolated from the integrated farms generally demonstrated higher prevalences of resistance to the tested antimicrobials than the same species from traditional farms (13). In the present study, no differences in resistance prevalence between E. faecalis, E. faecium, and other Enterococcus spp. were found (data not shown), and resistance data were therefore presented as combined resistance for all isolated enterococci. The results from Thailand and our findings suggest that the Enterococcus species composition and antimicrobial resistance in tropical integrated aquaculture environments are influenced by fecal and antimicrobial pollution. However, at the same time, the frequent isolation on selective Slanetz & Bartley agar plates of several Enterococcus spp. that are ubiquitous to the external environment and not likely of fecal origin (13) questions the usefulness of this agar medium to assess levels of recent fecal pollution in tropical aquatic environments.
Integrated aquaculture has not been shown to be a major source of antimicrobial-resistant bacteria pathogenic to humans, and it is currently unknown as to what extent integrated aquaculture may contribute to the problems of antimicrobial resistance encountered in human medicine. Theoretically, however, antimicrobial-resistant bacteria selected for in integrated aquaculture settings may be human pathogens, e.g., zoonotic bacteria, or donate resistance genes to human pathogens (1). Further, the discharge of antimicrobial residues and resistant bacteria into fish ponds and the associated change in bacterial biodiversity may negatively affect the pond productivity through changes in the composition of ubiquitous microorganisms that are important in the breakdown of organic matter and as a feed source for fish fry. The study by Petersen and Dalsgaard (13) did show that the input of poultry manure into fish farms was associated with a significantly higher occurrence of antimicrobial-resistant enterococci in the fish intestine than the level of resistance found in fish from control ponds without animal manure inputs. Similar knowledge should be obtained for the small-scale household-based VAC aquaculture systems that are very popular and widely promoted in Vietnam. It is, however, unknown to what extent such enterococci or other resistant fecal bacteria may be transferred to the fish meat by, e.g., contamination through transfer from the gut or cross-contamination with gut content when cleaning the fish.
In conclusion, our experimental study of the integrated pig-fish farm showed that development of resistance to NAL and ENR, but not to TET, among E. coli and Enterococcus spp. isolated from manure and water-sediment samples was associated with the provision of feed containing the two antimicrobials. Further studies should assess the environmental and human health importance of increased levels of bacterial resistance in integrated animal-fish farm environments.
ACKNOWLEDGMENTS
This work received financial support from the Danida (Danish International Development Assistance)-funded SUSANE research capacity building project (J.nr.104. Dan.8.L.722).
We thank Sang Nguyen Quang and Gia Hung Bui for their support when conducting the experiment and collecting samples at the farm. We also thank the technical staff at the Department of Veterinary Disease Biology, University of Copenhagen, for valuable instructions in the laboratory.
Footnotes
Published ahead of print on 20 May 2011.
REFERENCES
- 1. Alderman D. J., Hastings T. S. 1998. Antibiotic use in aquaculture: development of antibiotic resistance potential for consumer health risks. Int. J. Food Sci. Tech. 33:139–155 [Google Scholar]
- 2. Alexander T. W., et al. 2008. Effect of subtherapeutic administration of antibiotics on the prevalence of antibiotic-resistant Escherichia coli bacteria in feedlot cattle. Appl. Environ. Microbiol. 74:4405–4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reference deleted.
- 4. Cavaco L. M., et al. 2008. Prevalence of quinolone resistance mechanisms and associations to minimum inhibitory concentrations in quinolone-resistant Escherichia coli isolated from humans and swine in Denmark. Microb. Drug Resist. 14:163–169 [DOI] [PubMed] [Google Scholar]
- 5. CLSI/NCCLS 2001. Performance standards for antimicrobial susceptibility testing: 11th informational supplement. CLSI/NCCLS, Wayne, PA.
- 6. Cromwell G. L., et al. 1996. Efficacy of the antimicrobial compound U-82,127 as a growth promoter for growing-finishing pigs. J. Anim. Sci. 74:1284–1287 [DOI] [PubMed] [Google Scholar]
- 7. Dutka-Malen S., Evers S., Courvalin P. 1995. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 33:24–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kühn I., et al. 2003. Comparison of enterococcal populations in animals, humans, and the environment: a European study. Int. J. Food Microbiol. 88:133–145 [DOI] [PubMed] [Google Scholar]
- 9. Lan N. T. P., Dalsgaard A., Cam P. D., Mara D. 2007. Microbiological quality of fish grown in wastewater-fed and non-wastewater-fed fishponds in Hanoi, Vietnam: influence of hygiene practices in local retail markets. J. Water Health 5:209–218 [PubMed] [Google Scholar]
- 10. Little D. C., Edwards P. 1999. Alternative strategies for livestock-fish integration with emphasis on Asia. Ambio 28:118–124 [Google Scholar]
- 11. Peters J., Mac K., Wichmann-Schauer H., Klein G., Ellerbroek L. 2003. Species distribution and antibiotic resistance patterns of enterococci isolated from food of animal origin in Germany. Int. J. Food Microbiol. 88:311–314 [DOI] [PubMed] [Google Scholar]
- 12. Petersen A., Andersen J. S., Kaewmak T., Somsiri T., Dalsgaard A. 2002. Impact of integrated fish farming on antimicrobial resistance in a pond environment. Appl. Environ. Microbiol. 68:6036–6042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petersen A., Dalsgaard A. 2003. Species composition and antimicrobial resistance genes of Enterococcus spp. isolated from integrated and traditional fish farms in Thailand. Environ. Microbiol. 5:395–402 [DOI] [PubMed] [Google Scholar]
- 14. Poeta P., Costa D., Rodrigues J., Torres C. 2006. Antimicrobial resistance and the mechanisms implicated in faecal enterococci from healthy human, poultry, and pets in Portugal. J. Antimicrob. Agents 27:131–137 [DOI] [PubMed] [Google Scholar]
- 15. Vu T. K. V., Tran M. T., Dang T. T. S. 2007. A survey of manure management on pig farms in Northern Vietnam. Livest. Sci. 112:288–297 [Google Scholar]