Abstract
The neuronal correlate of perceptual decision making has been extensively studied in the monkey somatosensory system by using a vibrotactile discrimination task, showing that stimulus encoding, retention, and comparison are widely distributed across cortical areas. However, from a network perspective, it is not known what role oscillations play in this task. We recorded local field potentials (LFPs) from diverse cortical areas of the sensorimotor system while one monkey performed the vibrotactile discrimination task. Exclusively during stimulus presentation, a periodic response reflecting the stimulus frequency was observed in the somatosensory regions, suggesting that after initial processing, the frequency content of the stimulus is coded in some other way than entrainment. Interestingly, we found that oscillatory activity in the beta band reflected the dynamics of decision making in the monkey sensorimotor network. During the comparison and decision period, beta activity showed a categorical response that reflected the decision of the monkey and distinguished correct from incorrect responses. Importantly, this differential activity was absent in a control condition that involved the same stimulation and response but no decision making required, suggesting it does not merely reflect the maintenance of a motor plan. We conclude that beta band oscillations reflect the temporal and spatial dynamics of the accumulation and processing of evidence in the sensorimotor network leading to the decision outcome.
Perceptual decision making has been extensively studied in the monkey somatosensory system (1–3) by using a vibrotactile discrimination task (4). Various task aspects (e.g., stimulus encoding, retention, and comparison) turned out to be widely distributed across cortical areas. Notably, during the comparison and decision periods, spike rates in several premotor, motor, and prefrontal regions encoded both the result of the decision process and information on which it was based (2, 3, 5, 6).
Although important insight has been gained from these studies focusing on single-unit spikes, additional aspects of neuronal (population) dynamics are reflected by oscillatory activity. From a network perspective, it is still largely unknown what role oscillations in the LFPs play in perceptual decision making. Work in humans using magneto/electro-encephalography (M/EEG) suggests that oscillations in the beta and gamma bands play a significant role in perceptual working memory and decision making (reviewed in refs. 7 and 8), which was recently confirmed for the somatosensory system (9, 10). How these oscillations detected at the scalp level are reflected by intracranially recorded neuronal oscillatory activity remains largely unexplored (but see ref. 11).
Here, we asked how oscillatory activity contributes to the perceptual decision process. We recorded LFPs across five cortical areas within the sensorimotor network in a monkey performing a somatosensory discrimination task (12). LFPs reflect synchronized activity in a population of neurons, more specifically, they consist to a large degree of the postsynaptic potentials or input to a population (for a discussion, see ref. 13), whereas spikes reflect the output activity of single neurons. Therefore, studying somatosensory perceptual decision making at the LFP level provides insight in the spatiotemporal dynamics of the populations involved and offers a complementary view to what is known from spike recordings.
We show that oscillations in the beta band (15–30 Hz) reflect the dynamics of decision making in the monkey sensorimotor network. Although beta activity has been associated with motor processing, its functional role remains elusive. Some studies suggest that the beta rhythm serves to inhibit motor regions (14), whereas others suggest that beta activity plays a direct functional role in neuronal processing (15, 16), or reflects the “status quo” (17). Here, we find that differential beta activity reflects the decision of the monkey and distinguishes correct from incorrect responses. Importantly, this differential activity was absent in a control condition that involved the same stimulus and motor response but no decision making, suggesting this activity is context-dependent and not merely reflecting the maintenance of a motor plan. Therefore, we conclude that oscillations in the beta band reflect the temporal and spatial dynamics of the accumulation of evidence in the sensorimotor network leading to the final decision.
Results
We recorded the simultaneous neuronal activity from primary somatosensory cortex (S1), secondary somatosensory cortex (S2), dorsal premotor cortex (DPC), medial premotor cortex (MPC), and primary motor cortex (M1), while a monkey had to discriminate between the frequencies of two consecutive vibrotactile stimuli (Fig. 1). Here, we explored the role of oscillatory activity in the coding of the stimuli and the subsequent comparison process.
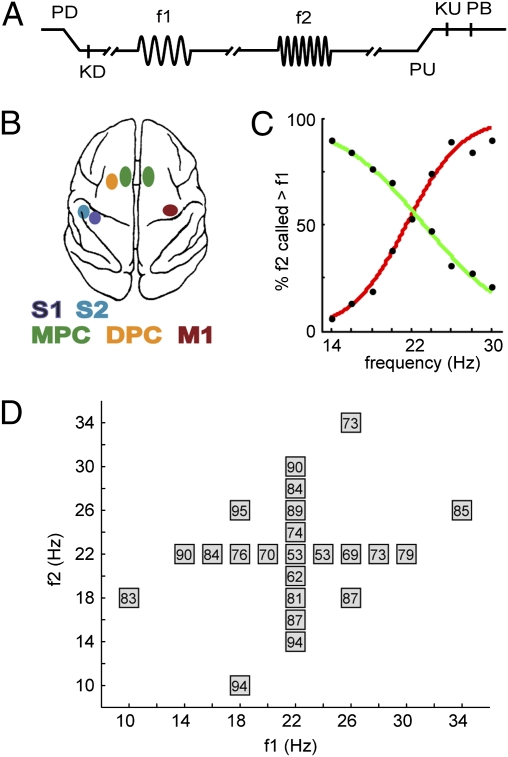
Fig. 1.
Somatosensory discrimination task. (A) Sequence of events: Mechanical probe is lowered (PD), monkey places response hand on key (KD), after a variable prestimulus delay first vibrotactile stimulus is presented (f1), after a 3-s fixed delay the second stimulus is presented (f2), after another 3-s fixed delay the probe is lifted (PU), the monkey releases the key (KU), and pushes either a lateral or medial button (PB) to indicate whether f2 was of higher or lower frequency than f1, respectively. The monkey was rewarded with a drop of liquid for correct discriminations. (B) Overview of recording sites. During each recording session, up to seven electrodes were individually placed in each of five cortical regions: S1, S2, DPC, MPC, and M1. (C) Psychometric curves showing the percentage of trials in which f2 was assessed as higher than f1, when f1 was maintained at 22 Hz and f2 was variable (red curve), and when f2 was maintained at 22 Hz and f1 was variable (green curve). The varied stimulus frequency is presented on the x axis. (D) Stimulus set used during recordings. Each box represents a stimulus pair and shows the percentage of correct responses for the comparison of that pair.
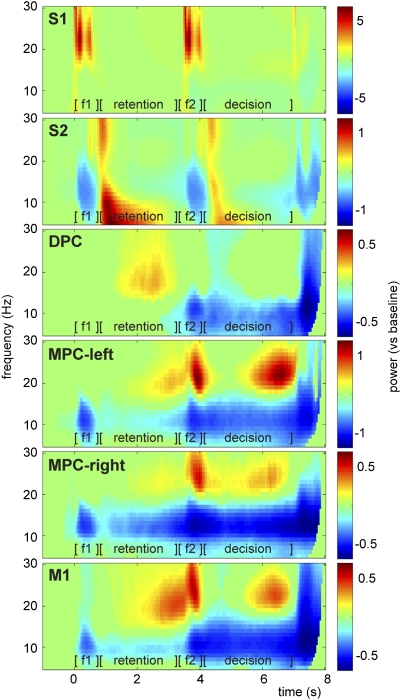
Time-frequency analysis of the LFPs revealed task-related modulation of the alpha (8–14 Hz), beta (15–30 Hz), and gamma (40–100 Hz) frequency bands in somatosensory, premotor, and motor regions (Fig. 2 and Fig. S1A). Generally, alpha power decreased and beta and gamma power increased as a response to the task. The somatosensory regions showed a stimulus-evoked response, reflected in a power increase in the beta and gamma range. The beta increase was sustained into the early delay periods for S2. The premotor and motor regions presented a shift in modulation from the stimulus periods toward the retention and decision periods. Furthermore, during the motor response, both alpha and beta decreased, while gamma power increased in most regions. Activity during f1 and f2 most likely reflects stimulus-evoked activity, whereas the modulation during the retention and decision intervals reflects changes in the oscillations intrinsic to these networks. A cluster-based randomization test was performed to assess statistical significance, comparing the task-related activity (t = 0–8 s) with the baseline activity (t = −1 to −0.5 s). Only time-frequency samples with significant power modulations (P < 0.05) are presented in Fig. 2 and Fig. S1A. Note that both correct and incorrect response trials were included in this analysis.
Fig. 2.
Oscillatory activity in the LFPs during the somatosensory discrimination task. Time-frequency representations showing oscillatory activity in alpha (8–14 Hz) and beta (15–30 Hz) band related to different aspects of the discrimination task. Presentation of first stimulus (f1, t = 0–0.5 s), retention period (t = 0.5–3.5 s), presentation of second stimulus (f2, t = 3.5–4 s), and decision period (t = 4–7 s), followed by the delayed motor response. Showing significant power modulations only (tested vs. baseline activity with cluster-based permutation statistics, P < 0.05), averaged over all recording sessions and channels within each region. S1, S2, DPC, and MPC-left were recorded contralateral to the stimulated hand; MPC-right and M1 were recorded contralateral to the response hand.
Regarding the higher frequency responses, note that only in MPC-left we observed a significant band-limited gamma power increase (≈50 Hz) during the decision making delay, which was differently modulated for correct than incorrect responses (cluster-based test, P < 0.05; Fig. S1B). In DPC, we observed a weak but significant increase of gamma band activity during the retention delay, which did not differ for correct vs. incorrect trials. Because of the relatively weak effects in the gamma band, we will focus the rest of this report on the periodic stimulus responses and the beta band modulation.
Periodic Stimulus Response in the Somatosensory Regions.
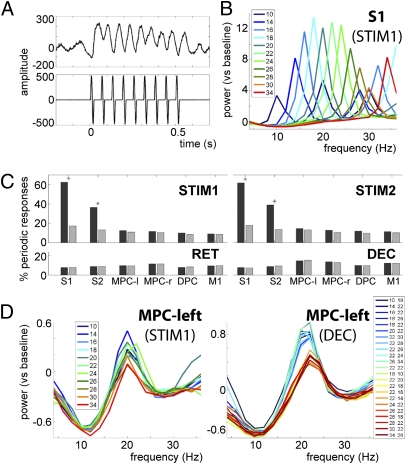
During vibrotactile stimulation, the raw single-trial LFPs as recorded in S1 (low-pass filtered at 250 Hz) clearly reflected the stimulus frequency (Fig. 3A). To evaluate for each of the regions whether there was a periodic stimulus response, we computed the single-trial power spectra during stimulus presentation. A periodic stimulus response was defined as an exact match between the peak frequency in the single-trial power spectrum and the actual stimulus frequency for that trial (Fig. 3B). The total percentage of trials showing a periodic stimulus response was computed for each region and each recording session separately. We then repeated this approach for the delay windows (retention and decision making periods). To assess significance, we performed a two-proportion z-test comparing the experimentally observed proportion of trials against a chance-level proportion based on 1,000 randomizations of the data. Entrainment to the stimulus frequency was exclusively observed in the somatosensory regions during stimulus presentation (P < 0.05, Bonferroni corrected for multiple comparisons; Fig. 3C) and was more prominent in S1 (≈60% of the trials) than in S2 (40%).
Fig. 3.
Periodic stimulus response in somatosensory regions. (A) Trace of single-trial LFPs during stimulus presentation (t = 0–0.5 s), recorded with one electrode in S1 (Upper) and the respective stimulus (16 Hz) as recorded from the trigger channel (Lower). (B) Power spectrum during stimulus presentation in S1, sorted by stimulus frequency. The peak frequencies exactly reflect the actual stimulus frequencies (averaged over trials). (C) S1 and S2 showed a significant periodic response to the stimulus (P < 0.05, Bonferroni corrected for multiple comparisons, denoted by *). The analysis was performed for the stimulus windows (STIM1, t = 0–0.5; STIM2, t = 3.5–4), and the delay windows (RET, t = 0.5–3.5 s; DEC, t = 4–7), contrasting experimental data (dark gray bars) with randomized data (light gray bars). (D) Power spectra during stimulus presentation (Left) and decision period (Right) in MPC-left, sorted by stimulus frequency. During the decision period, the beta band activity is modulated in a categorical fashion, reflecting the two decision outcomes (i.e., f2 > f1, blue traces, vs. f2< f1, red traces).
However, in MPC-left the beta power (but not frequency) was modulated by the stimulus frequency during stimulus presentation (Fig. 3D; showing f1, similar response for f2). No such modulation was observed during the retention delay. To assess whether the beta power reflected the comparison process, we sorted the trials for each stimulus frequency pair and computed the corresponding power spectra. We observed a modulation of beta power by the stimulus frequency in all regions during the decision period (shown here for MPC-left). The peak beta frequency was independent of stimulus frequency and observed at 22 Hz in all regions except S1, where it was observed at 16 Hz. The beta power modulation appeared to be categorical and to reflect the comparison of the stimuli (i.e., f2 > f1 or f2 < f1).
Beta Power Modulation Categorically Reflects the Decision Outcome.
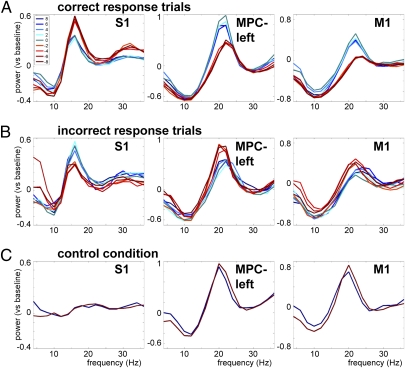
To further explore the categorical modulation in the beta band, we then sorted the trials according to the difference between f2 and f1. If the beta power modulation truly reflects the outcome of the comparison process, it should differentiate correct from incorrect response trials. We compared the beta power modulation during the decision period (averaged over a 3-s window: t = 4–7 s) for correct (Fig. 4A) and incorrect trials (Fig. 4B). For correct trials, beta power was higher for f2 > f1 and lower for f2 < f1, whereas for incorrect trials this modulation was inversed, suggesting that the beta power predicts the decision outcome and, hence, the mistake the monkey is about to commit. High beta power leads to pressing the button corresponding to f2 > f1, whereas low beta power corresponds to f2 < f1, regardless of the actual stimulus properties. Note that in S1, this effect is reversed.
Fig. 4.
Beta power reflects decision and predicts mistakes. (A) Power spectra during the decision period for correct response trials in S1 (Left), MPC-left (Center) and M1 (Right), sorted by the difference between f2 and f1 (blue traces: f2 > f1; red traces: f2 < f1). (B and C) Same as A but for incorrect response trials (B) and the control condition (C).
To test the statistical significance of the decision-related activity, a within-sessions paired-sample t test was used, comparing the beta power averaged over trials for the f2 > f1 vs. the f2 < f1 case (Table 1). Significant beta power modulation for correct trials was observed in S1, S2, MPC-left, DPC, and M1 (P < 0.05), and a trend was observed in MPC-right (P = 0.107). For incorrect trials, the beta power modulation was significantly inversed in S1, MPC-left, and M1 (P < 0.05), and a trend was observed in DPC (P = 0.166). Thus, for somatosensory, premotor, and motor cortex, beta activity reflects the decision outcome and distinguishes (for S1, MPC-left, and M1) correct from incorrect responses.
Table 1.
Modulation of beta power by the decision outcome
| Region | Correct | Incorrect | Controls |
| S1 | t(46) = −4.856* | t(46) = 5.108* | t(37) = −0.406 |
| P = 0.000 | P = 0.000 | P = 2.061 | |
| S2 | t(46) = 5.614* | t(46) = −1.263 | t(37) = 1.528 |
| P = 0.000 | P = 0.638 | P = 0.405 | |
| DPC | t(33) = 2.527* | t(33) = −1.987 | t(18) = 0.140 |
| P = 0.049 | P = 0.166 | P = 2.671 | |
| MPC-left | t(32) = 5.957* | t(32) = −2.539* | t(28) = −1.926 |
| P = 0.000 | P = 0.049 | P = 0.193 | |
| MPC-right | t(12) = 2.366 | t(12) = −1.328 | — |
| P = 0.107 | P = 0.626 | ||
| M1 | t(38) = 8.807* | t(38) = −2.978* | t(23) = −1.437 |
| P = 0.000 | P = 0.015 | P = 0.493 |
*Significant effects are marked in bold text; reported P values are Bonferroni corrected for multiple comparisons.
For each of the regions and each of the conditions (correct, incorrect and control trials), a paired-sample t test was performed; testing within sessions whether beta power in the f2 > f1 case was significantly different from that in the f2 < f1 case.
Furthermore, to demonstrate that this effect is directly related to the discrimination task and not merely reflecting the preparation for the upcoming motor response, we analyzed the beta power modulation in a control condition. In this condition, the monkey was presented with the tactile stimuli, but during the entire trial, a light indicated which button to press. Thus, this condition contains the same stimulation and same eventual motor movement, but no comparison process. Therefore, it allows us to study whether the observed beta power modulation reflects actual decision making rather than maintenance of a motor plan. (Note that fewer frequency pairs were used in the control condition: Only pairs with a difference of ± 8 Hz were used, allowing for sufficient number of trials to compare with the experimental condition, while keeping the monkey attentive.) All regions except S1 showed a prominent beta band increase compared with baseline, however, no beta power modulation was observed in the control condition: None of the regions showed a significant difference between f2 > f1 and f2 < f1 (P > 0.05; Fig. 4C and Table 1). The lack of significant effects in the control condition demonstrates that the reported differential beta activity is context-dependent and not merely reflecting the maintenance of a motor plan.
Beta Power Time Course Reflects Decision Dynamics.
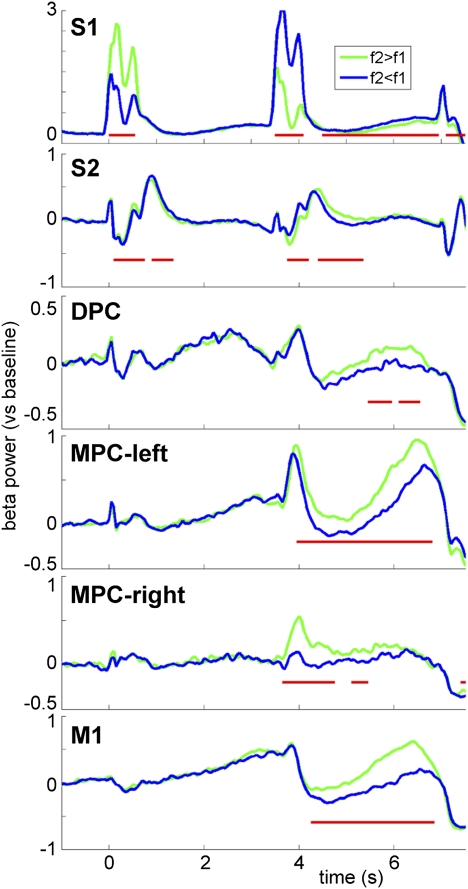
To assess the dynamics of the decision process across regions and time, we analyzed the time course of the beta power modulation for each of the regions (Fig. 5). Beta power time courses for f2 > f1 and f2 < f1 showed sustained differential activity for parts of the decision delay in all regions. (Note: Differential activity in the somatosensory regions during stimulus presentation is likely due to the periodic stimulus response, hence cannot be attributed to “decision making.”) A nonparametric permutation test clustering neighboring time samples, while frequency was averaged over the beta band, revealed the time samples for which the beta power modulation was significant (P < 0.05; Fig. 5).
Fig. 5.
Beta power time courses reflect decision dynamics. Time courses of beta power (S1: 12–20 Hz; all other regions: 18–26 Hz) for each region separately. Trials (correct responses only) were averaged according to the outcome of the comparison of f2 and f1 (green traces: f2 > f1; blue traces: f2 < f1). Significant differences between the two traces are indicated by a red line (cluster-based permutation test, P < 0.05).
Discussion
We explored the role of oscillatory activity in the coding and comparison of vibrotactile stimuli. During stimulus presentation, a periodic response reflecting the stimulus frequency was observed in the somatosensory regions. In none of the other regions or time windows periodicity was observed, suggesting that after initial processing, the frequency content of the stimulus is coded in some other way than entrainment. In fact, during the comparison and decision period, beta power in the sensorimotor network showed a categorical response, reflecting the decision of the monkey and dissociating correct from incorrect responses. Importantly, this differential activity was not observed in a control condition where a light indicated the correct response. Although the vibrotactile stimuli and motor responses in this control condition were the same as in the discrimination task, there was no comparison taking place. Therefore, we conclude that the beta power modulation is context-dependent and reflects the actual decision process, and not merely the maintenance of a motor plan.
Extensive previous studies on the same paradigm demonstrated that spikes in S1 show a strong periodic response to the stimulus, whereas in downstream regions (S2 and further), there are minimal or no traces of periodic activity (1, 18). Even within S1, periodicity diminishes significantly when comparing area 3b to area 1 (18, 19). Previous work in humans using M/EEG recordings reported steady-state somatosensory evoked potentials (SSSEPs): rhythmic activity at the vibrotactile stimulus frequency (20, 21), which has been localized by source modeling to S1 (10, 22, 23). However, to obtain SSSEPs with M/EEG, it is required to average over several dozens of trials to get sufficient signal-to-noise ratio.
Here, we show that a periodic stimulus response can be detected in the single-trial LFPs. Contrary to the aforementioned M/EEG and spike work, we observe periodic stimulus responses not only in S1, but also in S2. This discrepancy could be explained by better signal-to-noise ratio compared with M/EEG data and direct recordings of the actual neuronal activity. Note that the (weaker) S2 entrainment is unlikely to be caused by common pickup due to volume conduction, because we rereferenced the data per region. It is possible that feedforward projections from S1 (or direct thalamic inputs) send periodically coded stimulus information toward S2. This input would then be visible in the LFPs of S2 but not necessarily in its spike activity, because the LFPs mainly reflect the input to a region (i.e., the postsynaptic potentials) whereas spikes reflect the region's output. Further, as we observed the periodic response only during stimulus presentation and only in somatosensory regions, it confirms that the beta power modulations we report are not in any way confounded by sensory entrainment (even though stimulus frequencies are in the beta range).
Studying the time course of the beta modulation, we observed different latencies in different areas. The differential activity was significantly sustained throughout the entire decision period in MPC-left. In M1, the onset was slightly later and we observed a gradual build-up of the differential activity. Further, in MPC-right, S2, S1, and DPC, the differential activity was significant for parts of the decision period. Comparing MPC-left (recorded contralateral to the stimulus hand) and MPC-right (recorded contralateral to the response hand), it seems that the beta activation in MPC-left is sustained longer and actually increases toward the response, which might be due to different functional roles of these regions (also note that fewer recordings were made in MPC-right, which could explain the weaker/noisier power estimates). The various observed patterns could reflect either the dynamics of contribution of the various regions to the actual decision making process and/or feedback signals from the “decision network” reaching these regions. With the current approach, it is not possible to distinguish between these possibilities. Future research, addressing interactions between these and other frontal regions should further address this issue. Here, we propose that the beta band activity reflects the temporal and spatial dynamics of the accumulation and processing of evidence in the sensorimotor network, resulting in the decision outcome.
Our beta band findings are in line with the firing rate modulation observed in spike recordings on the same paradigm (reviewed in ref. 1). In S2, premotor, and motor cortex, several types of neurons were found, including ones that reflect either f1 or f2, and neurons that respond as a function of f2–f1. Some cells respond stronger when f2 > f1, whereas others have a preference for f2 < f1 (2, 3, 5, 6). Further, analysis of the control condition confirmed that these responses are really specific to the discrimination task, because the differential spike activity virtually disappeared when a light indicated the to-be-pressed button.
We believe that the beta activity reflects the processing of evidence in the network, revealing the decision process building up to the final outcome. It shows the winner-takes-all result of the combined individual neuron responses, i.e., the net population response. Interestingly, we find stronger beta for f2 > f1 (and for f2 = f1) than for f2 < f1. Perhaps the first has the monkey's preference and there is a bias to this decision unless available evidence leads to the alternative choice. Beta oscillations reflect synchronization of activity within a population rather than single unit activity, which might explain why we do not see the more particular dynamics as observed with the spikes that can distinguish between different types of responses (i.e., neurons favoring f2, f1, f2 > f1, f2 < f1, or more complex combinations thereof). Studying the interaction between spikes and fields, Pesaran et al. report decision making related spike-field coherence in the beta band (15 Hz) between monkey dorsal premotor cortex and parietal cortex (24). The authors suggest this beta band coherence reflects a decision circuit that influences the selection of a movement goal, which fits with our interpretation of beta activity reflecting the decision process.
Further, our interpretation is in line with a recent discussion by Siegel et al. (8), who propose that beta band activity in a widespread network, including prefrontal and parietal regions, is involved in linking sensory evidence to motor plans (the latter both reflected by gamma band oscillations). They argue for a continuous input of accumulating sensory evidence to the cortical motor system. Our thinking is also consistent with the interpretation of frontal-parietal beta oscillations recorded in humans during visual decision making (25). In this magnetoencephalography (MEG) study, Donner et al. (25) concluded that large-scale beta oscillations support the persistent activity required for evidence accumulation. They used a decision making task involving visual motion detection and found increased beta band activity (narrowband, 12–24 Hz) in a widespread network, including posterior parietal and dorsolateral prefrontal cortex, which predicted the correctness of a subject's upcoming choice (25). Beta activity dissociated the accuracy of the response (hits and correct rejections vs. misses and false alarms) but did not reflect the content of the subject's decision (absence vs. presence of target). Donner et al. (25) argued that in these regions, the beta band activity reflects the computations underlying the decision process, rather than the neural representation of the choice itself. This conclusion is consistent with our current results, although we find narrowband beta power in a widespread network reflecting the decision outcome, rather than the correctness of the response.
The role of beta in decision making is further supported by a human MEG study on a visual motion detection task, where a beta band decrease (broadband, 12–36 Hz, accompanied by a similar effect in the alpha range) in addition to a gamma band increase in M1 built up gradually to predict whether the subject was going to report presence or absence of a visual motion stimulus, regardless of correctness of this response (26). We too report beta band modulation in M1 reflecting the decision, however, we find a narrowband increase in beta rather than a more general, broadband decrease. The broadband decrease in humans might reflect the activity combined at the scalp level from several regions participating in the task.
Regarding the role of gamma band activity, which has been associated with neuronal processing (7) and communication (27), our results remain inconclusive. We observed a strong evoked broadband gamma component both during stimulation and motor response. However, during the delay periods, we observed significant gamma modulation in DPC (during the retention period) and MPC-left (decision period). Only the latter showed a significant difference for correct vs. incorrect response trials. Rather, it seems that in our somatosensory decision making paradigm, the beta band rhythm is the most prominent sensorimotor rhythm involved.
Recently, Spitzer et al. used EEG to study the somatosensory discrimination task in humans (10). They reported a modulation of prefrontal beta power (20–25 Hz, source-localized to the inferior frontal gyrus) that reflected the stimulus frequency held in working memory. This beta power modulation during stimulus retention was related to successful frequency discrimination. In the current study, we did not record from PFC, however, these results offer further support for the idea that oscillations in the beta band are task-related and reflect gradual accumulation of evidence in an extended network, ultimately reflecting the decision outcome.
Modeling work suggests that beta band synchronization might be involved in functional coupling of networks over larger distances (because of the longer cycle supporting longer conduction delays), whereas faster gamma band oscillations are more optimal for relatively local computations (28). It is suggested that beta rhythms are used for higher level interactions involving more distant structures (16). This view is compatible with our current findings, and future work looking into the functional role of beta synchronization between regions would be highly relevant.
To conclude, several studies relate beta band modulation to aspects of decision making. Although the reported spectral, spatial, and functional aspects vary (possibly due to differences in the paradigms used), the limited available evidence points toward an extended cortical network involved in decision making, reflected in (parts of) the beta band. Here, we show that oscillations in the beta band reflect the dynamics of decision making in the monkey sensorimotor network. The study of oscillatory activity offers a complementary view to what is known from spike recordings, because the oscillations reflect synchronized population activity rather than single cell responses.
Materials and Methods
General.
One monkey (Macaca mulatta) was trained to perform a somatosensory discrimination task (Fig. 1A). Both spikes and LFPs were recorded simultaneously from somatosensory, premotor, and motor areas (Fig. 1B). The animal was handled in accordance with the standards of the National Institutes of Health and the Society for Neuroscience.
Experimental Paradigm.
Vibrotactile stimuli (500-ms pulse trains, 10–34 Hz) were delivered to the right hand. After presentation of the first stimulus (f1), a 3-s retention period was followed by presentation of the second stimulus (f2). The monkey's task was to indicate whether f2 was of lower or higher frequency than f1, by means of a left hand button press after a 3-s forced delay. Task performance of the monkey was 74% correct responses (mean over 47 sessions; see also Fig. 1 C and D).
Data Acquisition.
Neuronal recordings (47 sessions with up to 240 trials per session) were acquired with an array of seven independent, movable microelectrodes inserted in each of five cortical areas simultaneously. These areas included S1 (47 sessions), S2 (47), DPC (34), and MPC (33) in the hemisphere contralateral to the stimulated hand, and MPC (13) and M1 (39) in the hemisphere contralateral to the response hand. A more extensive description of the task and procedures can be found in previous publications (4, 12).
Data Analysis.
For data analysis we used custom-build Matlab code and the FieldTrip toolbox (www.ru.nl/neuroimaging/fieldtrip). The data were down-sampled offline to a sampling frequency of 1 kHz. A band-stop filter was applied to remove line noise (60 Hz and harmonics) caused by the power net. To remove further recording artifacts, the data were rereferenced per region: For each recording site, the average signal from electrodes in that same region was subtracted (per trial). Trials containing remaining artifacts (e.g., because of movement or electronic interference) were removed based on visual inspection of the data.
Spectral Analysis.
Trials were segmented into 500-ms epochs and multiplied with a Hanning taper to improve spectral estimation. Power spectra were computed by using a fast Fourier transform approach. Further, we computed time-frequency representations of the power spectra by using an adaptive sliding time window of five cycles length (Δt = 5/f) and a Hanning taper for lower frequencies (5–30 Hz), and a sliding time window of fixed length (200 ms) and five orthogonal Slepian tapers resulting in ±15 Hz smoothing (29) for higher frequencies (30–100 Hz). Power was averaged over trials within each recording session, normalized by a relative baseline correction (t = −1 to −0.5 s), and then averaged over electrodes within the same region. This procedure gives average power spectra per region for each session, which were used for statistical analysis.
For analysis of beta power modulation (Results), the peak beta frequency was established for each region during the decision delay period (S1, 16 Hz; all other regions, 22 Hz). For subsequent analysis of beta power, a ±4 Hz window around this peak frequency was used (i.e., 12–20 Hz for S1; 18–26 Hz for other regions).
Statistical Analysis.
To establish whether the difference between two conditions was significantly different from 0, a cluster-based nonparametric randomization test was applied within sessions. By clustering neighboring samples (i.e., time-frequency points) that show the same effect, this test deals with the multiple comparisons problem (method described in ref. 30).
Supplementary Material
Acknowledgments
R.R. was supported by an International Research Scholars Award from the Howard Hughes Medical Institute and grants from Consejo Nacional de Ciencia y Tecnología and Dirección del Personal Académico de la Universidad Nacional Autónoma de México. S.H. was supported by the Division for the Earth and Life Sciences (ALW) Open Competition Grant 817.02.010 and O.J. was supported by Vici Grant 453.09.002, both from the Netherlands Organization for Scientific Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107297108/-/DCSupplemental.
References
- 1.Romo R, Salinas E. Flutter discrimination: Neural codes, perception, memory and decision making. Nat Rev Neurosci. 2003;4:203–218. doi: 10.1038/nrn1058. [DOI] [PubMed] [Google Scholar]
- 2.Hernández A, et al. Decoding a perceptual decision process across cortex. Neuron. 2010;66:300–314. doi: 10.1016/j.neuron.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 3.Lemus L, et al. Neural correlates of a postponed decision report. Proc Natl Acad Sci USA. 2007;104:17174–17179. doi: 10.1073/pnas.0707961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernández A, Salinas E, García R, Romo R. Discrimination in the sense of flutter: New psychophysical measurements in monkeys. J Neurosci. 1997;17:6391–6400. doi: 10.1523/JNEUROSCI.17-16-06391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernández A, Zainos A, Romo R. Temporal evolution of a decision-making process in medial premotor cortex. Neuron. 2002;33:959–972. doi: 10.1016/s0896-6273(02)00613-x. [DOI] [PubMed] [Google Scholar]
- 6.Romo R, Hernández A, Zainos A, Lemus L, Brody CD. Neuronal correlates of decision-making in secondary somatosensory cortex. Nat Neurosci. 2002;5:1217–1225. doi: 10.1038/nn950. [DOI] [PubMed] [Google Scholar]
- 7.Jensen O, Kaiser J, Lachaux J-P. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Siegel M, Engel A, Donner T. Taking sides with pain - Lateralization aspects related to cerebral processing of dental pain. Front Hum Neurosci. 2011;5:12. doi: 10.3389/fnhum.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haegens S, Osipova D, Oostenveld R, Jensen O. Somatosensory working memory performance in humans depends on both engagement and disengagement of regions in a distributed network. Hum Brain Mapp. 2010;31:26–35. doi: 10.1002/hbm.20842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spitzer B, Wacker E, Blankenburg F. Oscillatory correlates of vibrotactile frequency processing in human working memory. J Neurosci. 2010;30:4496–4502. doi: 10.1523/JNEUROSCI.6041-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller KJ, et al. Cortical activity during motor execution, motor imagery, and imagery-based online feedback. Proc Natl Acad Sci USA. 2010;107:4430–4435. doi: 10.1073/pnas.0913697107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernández A, et al. Procedure for recording the simultaneous activity of single neurons distributed across cortical areas during sensory discrimination. Proc Natl Acad Sci USA. 2008;105:16785–16790. doi: 10.1073/pnas.0808702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logothetis NK, Kayser C, Oeltermann A. In vivo measurement of cortical impedance spectrum in monkeys: Implications for signal propagation. Neuron. 2007;55:809–823. doi: 10.1016/j.neuron.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: Frequency-specific features and functional correlates. Int J Psychophysiol. 2001;43:41–58. doi: 10.1016/s0167-8760(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 15.Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci USA. 1992;89:5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brovelli A, et al. Beta oscillations in a large-scale sensorimotor cortical network: Directional influences revealed by Granger causality. Proc Natl Acad Sci USA. 2004;101:9849–9854. doi: 10.1073/pnas.0308538101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engel AK, Fries P. Beta-band oscillations—signalling the status quo? Curr Opin Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Salinas E, Hernández A, Zainos A, Romo R. Periodicity and firing rate as candidate neural codes for the frequency of vibrotactile stimuli. J Neurosci. 2000;20:5503–5515. doi: 10.1523/JNEUROSCI.20-14-05503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernández A, Zainos A, Romo R. Neuronal correlates of sensory discrimination in the somatosensory cortex. Proc Natl Acad Sci USA. 2000;97:6191–6196. doi: 10.1073/pnas.120018597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snyder AZ. Steady-state vibration evoked potentials: Descriptions of technique and characterization of responses. Electroencephalogr Clin Neurophysiol. 1992;84:257–268. doi: 10.1016/0168-5597(92)90007-x. [DOI] [PubMed] [Google Scholar]
- 21.Giabbiconi CM, Dancer C, Zopf R, Gruber T, Müller MM. Selective spatial attention to left or right hand flutter sensation modulates the steady-state somatosensory evoked potential. Brain Res Cogn Brain Res. 2004;20:58–66. doi: 10.1016/j.cogbrainres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Nangini C, Ross B, Tam F, Graham SJ. Magnetoencephalographic study of vibrotactile evoked transient and steady-state responses in human somatosensory cortex. Neuroimage. 2006;33:252–262. doi: 10.1016/j.neuroimage.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 23.Forss N, Narici L, Hari R. Sustained activation of the human SII cortices by stimulus trains. Neuroimage. 2001;13:497–501. doi: 10.1006/nimg.2000.0700. [DOI] [PubMed] [Google Scholar]
- 24.Pesaran B, Nelson MJ, Andersen RA. Free choice activates a decision circuit between frontal and parietal cortex. Nature. 2008;453:406–409. doi: 10.1038/nature06849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donner TH, et al. Population activity in the human dorsal pathway predicts the accuracy of visual motion detection. J Neurophysiol. 2007;98:345–359. doi: 10.1152/jn.01141.2006. [DOI] [PubMed] [Google Scholar]
- 26.Donner TH, Siegel M, Fries P, Engel AK. Buildup of choice-predictive activity in human motor cortex during perceptual decision making. Curr Biol. 2009;19:1581–1585. doi: 10.1016/j.cub.2009.07.066. [DOI] [PubMed] [Google Scholar]
- 27.Fries P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Percival DB, Walden AT. Spectral Analysis for Physical Applications: Multitaper and Conventional Univariate Techniques. Cambridge, UK: Cambridge Univ Press; 1993. [Google Scholar]
- 30.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.