Abstract
B-lymphocyte development is dictated by the protein products of functionally rearranged Ig heavy (H) and light (L) chain genes. Ig rearrangement begins in pro-B cells at the IgH locus. If pro-B cells generate a productive allele, they assemble a pre-B cell receptor complex, which signals their differentiation into pre-B cells and their clonal expansion. Pre-B cell receptor signals are also thought to contribute to allelic exclusion by preventing further IgH rearrangements. Here we show in two independent mouse models that the accumulation of a stabilized μH mRNA that does not encode μH chain protein specifically impairs pro-B cell differentiation and reduces the frequency of rearranged IgH genes in a dose-dependent manner. Because noncoding IgH mRNA is usually rapidly degraded by the nonsense-mediated mRNA decay machinery, we propose that the difference in mRNA stability allows pro-B cells to distinguish between productive and nonproductive Ig gene rearrangements and that μH mRNA may thus contribute to efficient H chain allelic exclusion.
Developing B lymphoid cells generate Ig genes by recombination of gene segments (1). This process is initiated in pro-B cells of the bone marrow with the assembly of diversity (D) and joining (J) gene segments at both IgH alleles. Subsequently, a variable (V) gene segment can be recombined to a preexisting DJ-joint to form a VDJ exon (1). Once a functional VH exon has been generated, a heavy (H) chain is produced, which assembles with the surrogate light (L) chain and the signal molecules Igα/Igβ to form the pre-B cell receptor complex (pre-BCR). The pre-BCR provides signals for clonal expansion, survival, and differentiation into pre-B cells (2). Of the two IgH alleles, only one contributes to the BCR—a phenomenon known as allelic exclusion. This process is thought to be regulated at the level of V-to-DJ recombination (3, 4) and ensures that each B cell produces a single clonotypic antibody. Monospecificity of a B cell is important, because only a monospecific BCR allows efficient generation of self-tolerant B cells during B cell ontogeny, whereas at later stages in B cell development allelic exclusion contributes to efficient antigen-specific antibody responses.
B cell ontogeny is characterized by a biphasic induction of the V(D)J recombinase [recombination activating gene (RAG)] and a sequential rearrangement of IgH and IgL chain alleles. RAG is turned off in B cells expressing a functional, self-tolerant Ig; although perhaps too simplistic, this by and large explains both allelic and isotypic exclusion at the L chain loci. Although it is tempting to propose analogous models for allelic exclusion of IgH and IgL chain genes, there are, in fact, great differences—not only in temporal sequence of gene assembly, but also in strictness of exclusion: a small percentage of B cells does express two different L chains (5), but only one in 104 cells expresses two H chains (6).
Various competing theories on the mechanism of IgH chain allelic exclusion have been proposed, and they are not necessarily mutually exclusive (7). In a stochastic model, allelic exclusion is considered to be a statistical consequence of a low frequency of rearrangements encoding functional H chains (8, 9). In its bare-bones form, the stochastic hypothesis seems to be disproven for the IgH locus, because pro-B cells expressing signaling-defective forms of the pre-BCR have a large proportion of μH chain double producers (10). Mice with defective Ig receptor signaling support a genetic model in which the pre-BCR controls allelic exclusion. First, only transgenes encoding the membrane but not the cytoplasmic form of the μH chain mediate allelic exclusion (11). Second, concomitant deletion of the (pre)BCR-associated Syk family kinases Syk and ZAP-70 resulted in allelic inclusion (12), as did mutations in Igα and Igβ (13–15), which either block their association with the μH chain or interfere with intracellular signaling cascades. Similarly, allelic inclusion occurred at the T cell receptor (TCR)-β locus in mice with disruptions of either the TCR adapter protein SLP-76 or the TCR-associated kinase p56lck (16, 17).
In the genetic regulation model of H chain allelic exclusion, μH chain protein (as part of the pre-BCR) inhibits further rearrangements at the IgH locus, so that a second, functional IgH gene cannot be assembled (18). However, how is this inhibition accomplished? Before the rearrangement of a V gene segment, both H chain alleles are in a DJ-rearranged configuration (19) and are indistinguishable with regard to germ line transcription (20), nuclear localization (21), and locus contraction (22). Therefore, V-to-DJ recombination must either be asynchronous to allow enough time for H chain surface expression and pre-BCR signaling, or a productive VDJ recombination event must halt recombination until pre-BCR signals have been initiated. Because the repair-checkpoint protein ATM is activated by recombination-induced DNA double-strand breaks, it is thought to play a role in this process (23). Afterward, both IgH loci are “decontracted” to suppress further V-to-DJ rearrangements, and the partially rearranged IgH allele is silenced by pericentromeric relocation, thereby making it inaccessible for Rag (21, 22, 24–27). Given that allelic exclusion of the IgH allele is quite effective, and double producers are less frequent than predicted in most models that interfere with H chain signaling, a feedback inhibition of V-to-DJ recombination by the pre-BCR alone seems insufficient.
The recent discovery of noncoding RNA as a critical regulator of gene expression led us to consider an additional mechanism for H chain allelic exclusion, in which the mRNA that encodes a productive μH chain is sensed by the pro-B cell. With the intronic IgH enhancer in close proximity to the VH promoter, an immediate consequence of any V-to-DJ recombination is a high transcription rate of the rearranged locus and the appearance of μH transcripts. Although transcription rates of productively and nonproductively rearranged IgH loci are similar (28), only transcripts from a productively rearranged (coding) allele are stable and accumulate. In contrast, noncoding (nonsense) mRNA from a nonproductively rearranged allele is rapidly degraded by the nonsense-mediated mRNA decay (NMD) mechanism (29–31). Thus, stable coding μH mRNA could indicate the presence of a productive IgH allele and exclude the rearrangement of the other allele, whereas unstable μH mRNA encoded by a nonproductively rearranged IgH gene would be degraded and have no effect. To experimentally uncouple an effect mediated by the μH mRNA from any signal transmitted by its product of translation, the μH chain protein, we used an exception in NMD activity: When located close to the translation start site, a premature termination codon is not recognized by the NMD (32). This exception allowed us to create IgH alleles that are transcribed into stable untranslated μH mRNAs and to assay their effect on B-cell development.
Results
Mice Expressing Nonsense μH mRNA That Is Not Degraded.
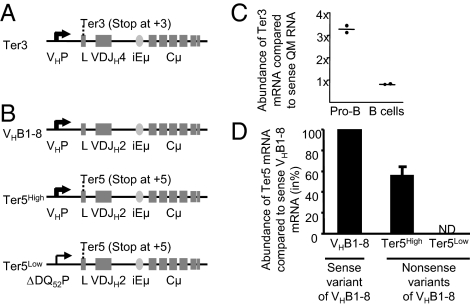
To determine the effects of a stable μH mRNA on B cell development and/or VDJ recombination, we established three mouse lines in which stable μH mRNA production is separated from translation into μH chain protein. One line (designated Ter3) expresses mRNA from the productively rearranged H chain transgene VH17.2.25, which has been rendered nonproductive by converting codon 3 of the leader exon into a translational stop codon (Fig. 1A). In contrast to the premature termination codons found in most nonproductive H chain transcripts, the nonsense codon in Ter3 transcripts triggers only a weak NMD response, resulting in stable and abundant μH Ter3 transcripts (32). The Ter3 line presented here has ≈10 transgene copies integrated into a genomic region of chromosome 5 without annotated features and is representative of lines established from three independent founders. In addition, we used two H chain gene knockin lines, in which the endogenous DQ52JH cluster was replaced by a VHB1-8 VDJ exon that was rendered nonproductive by the introduction of a termination codon at position 5 (designated the Ter5 allele; Fig. 1B). In one Ter5 line, transcription of the targeted H chain gene is driven by its physiological H chain promoter, whereas in the other, transcription is driven by a weak truncated DQ52 promoter (33). This results in high (Ter5High mouse) and low (Ter5Low mouse) amounts of noncoding H transcripts, respectively (see below).
Fig. 1.
Construction of mice expressing stabilized noncoding (nonsense) μH mRNA. (A) The Ter3 transgene consists of a mutated VH17.2.25 VDJ exon, in which codon +3 in the leader has been changed to a stop codon (Ter3), followed by the intronic IgH enhancer (iEμ) and the complete genomic Cμ region. (B) Schematic organization of the targeted IgH locus in three strains of knockin mice expressing VHB1-8 μH mRNA either as a sense variant (VHB1-8, Top) or as nonsense variants with a translational stop codon at position 5 (Ter5High and Ter5Low, Middle and Bottom) under the control of either the endogenous VH promoter (VHP, Top and Middle) or a weak truncated DQ52 promoter (DQ52P, Bottom). (C) Ter3 mRNA abundance was determined in FACS-sorted pro-B and splenic B cells from Ter3, IgHQM/wt mice that express both nonsense VH17.2.25 μH mRNA from the Ter3 transgene and sense VH17.2.25 μH mRNA from the knockin IgH locus of the QM mouse. Both μH transcripts were amplified in one reaction by RT-PCR, and their abundances were estimated from the fragment intensities after a Ter3 transgene-specific restriction digest (SI Appendix, Fig. S1). (D) Quantification of VHB1-8 μH mRNA in splenocytes from heterozygous VHB1-8, Ter5High, and Ter5Low mice by quantitative TaqMan RT-PCR using primers specific for the VHB1-8 sequence. ND = not detected.
To determine the abundance of the nonsense VH17.2.25-Ter3 mRNA, we bred Ter3 mice to quasi-monoclonal (QM) mice, in which the endogenous DQ52JH cluster is replaced with the functional VH17.2.25 exon (34) (i.e., QM mice express “Ter3” mRNA without a nonsense codon) (SI Appendix, Fig. S1A). The mice used for our determination thus had the genotype Ter3Tg, IgHQM/wt, and they expressed both sense and nonsense VH17.2.25 μH mRNA. From these mice we FACS-sorted pro-B and splenic B cells and amplified both H chain transcripts in one reaction by RT-PCR. Although the promoter and VDJ exons are nearly identical, a codon change in the Ter3 transgene created a specific restriction site, which enabled us to estimate the respective mRNA abundances from the fragment intensities (SI Appendix, Fig. S1B). Compared with coding QM mRNA, the amount of noncoding Ter3 mRNA in splenic B cells was almost identical (Fig. 1C and SI Appendix, Fig. S1B); in pro-B cells, for unknown reasons the amount was three times higher.
For the Ter5 mice we compared the abundance of μH mRNA in splenocytes from heterozygous Ter5High mice, Ter5Low mice, and mice with the same VDJ knockin allele but no premature termination codon. In Ter5Low mice, we detected no transcripts; in Ter5High mice, the Ter5 mRNA accumulated up to 60% of the transcripts encoded by the sense allele (Fig. 1D). Therefore, the abundance of nonsense μH mRNA in the Ter3 and Ter5High mice was by and large within the physiological range of μH mRNA that is translated.
Ter3 and Ter5 μH mRNAs Are Not Translated into μH Chain Protein.
We also confirmed that no truncated μH chain was produced in any of these mice. Accordingly, we bred the Ter3 mouse to a Rag2-deficient mouse. In the resulting recombination-deficient Ter3 mice, the further differentiation of pro-B cells ought to be completely blocked. Indeed, this was the case: just as in the Rag2-deficient mouse, none of the (c-kit+, CD19+) pro-B cells produced any intracellular μH chain. In the wild-type control mouse, however, almost 40% of all cells stained with a polyclonal anti-IgM antibody (SI Appendix, Fig. S2A). We also analyzed lysates from bone marrow cells of homozygous Ter5Low and Ter5High mice by Western blotting with a polyclonal anti-IgM serum. Again, although the wild-type produced large amounts of μH chain, there was none present in the Ter5 mice or the Rag-deficient mice (SI Appendix, Fig. S2B). Nonsense μH mRNA from both Ter3 and Ter5 mice also lack larger out-of-frame ORFs, which could result in the accumulation of a stress-inducing polypeptide.
Because they are neither degraded nor translated, the noncoding Ter3 and Ter5 μH mRNAs serve as a valid surrogate of a μH mRNA stabilized by translation; therefore, they enable us to study their effect on B cell development in the absence of μH chain protein and pre-BCR signals. Especially the Ter5High mouse with its physiological IgH promoter starts with pro-B cells that closely mimic wild-type pro-B cells that have just productively rearranged their IgH locus. The two types of cells differ only in the translatability of their in-frame rearrangement.
Pro-B Cell Differentiation Is Impaired by Stable Noncoding μH mRNA.
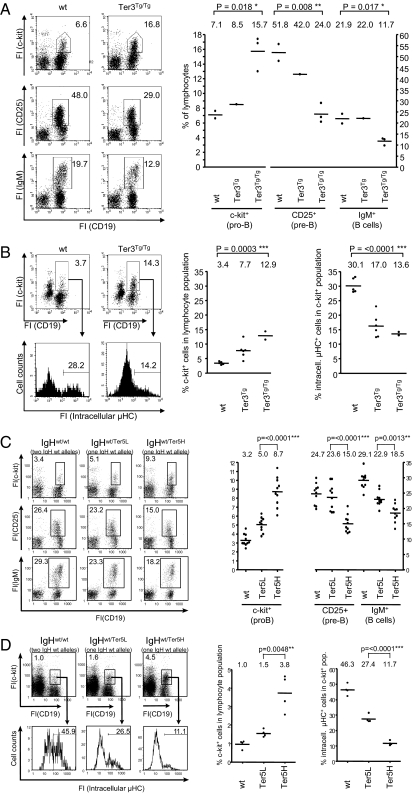
If the accumulation of a stable μH mRNA interfered with B cell differentiation and/or VDJ rearrangement, we would expect to find an impaired transition of B lymphoid precursors from the μH chain-negative pro-B to the μH chain-positive pre-B cell stage. Indeed, the number of c-kit+ pro-B cells was increased, and the number of subsequent developmental stages was decreased in Ter3 and Ter5High mice, both relatively and in absolute numbers (Fig. 2 A and C and SI Appendix, Table S1). The impairment of early B cell development was dose dependent (i.e., more noncoding mRNA inhibited B cell development to a greater degree) (Ter3Tg/Tg vs. Ter3Tg). In addition, the frequency of cells with newly synthesized intracellular μH chains in the c-kit+ pro-B population was decreased in both Ter3Tg and Ter5High mice (Fig. 2 B and D), indicating that stable noncoding μH mRNA interferes with B cell development before or at the stage of V-to-DJ rearrangement. We note that IgH rearrangement in Ter5 mice is restricted to a single IgH allele and, therefore, is only half as likely to be productive as rearrangement in wild-type mice. This may explain the phenotypic difference between Ter5Low and wild-type mice, but it does not account for the pronounced difference between Ter5Low and Ter5High mice. The Ter3 allele, on the other hand, was introduced as a conventional transgene; in Ter3 mice, both endogenous IgH alleles are thus unchanged and can rearrange. Therefore, the differences in pro-B cell differentiation between wild-type, Ter3Tg, and Ter3Tg/Tg mice ought to be due to the amount of noncoding μH mRNA transcribed from the transgene.
Fig. 2.
Noncoding μH mRNA impairs pro-B cell differentiation. (A) Bone marrow cells of 6-wk-old Ter3 mice and wild-type littermates were membrane stained with the indicated antibodies, and fluorescence intensities (FI) of cells in the lymphocyte gate were determined by flow cytometry. Percentages of cells in the individual gates are indicated. (B) Pro-B cells (c-kit+/CD19+) analyzed for intracellular μH chain. Bone marrow cells were membrane stained with antibodies against c-kit and CD19, permeabilized, and restained with antibodies against μH chain. (C and D) Bone marrow cells of 6-wk-old wild-type and heterozygous Ter5Low and Ter5High mice were stained as described above. Right: Results of an entire litter (A and B) or of multiple litters (C and D) are summarized in these diagrams; one dot represents one mouse.
Cell Specificity of Stable μH mRNA Effect.
The question arose as to whether the effect of stable μH mRNA on B cell development is nonspecific. However, μH mRNA itself is a transcript unique to B cells; as long as the stable μH mRNA contains a regular VDJ exon and is expressed in the same amounts as in wild-type cells, its activity ought to faithfully reflect that of translatable μH mRNA in wild-type B cells. Nevertheless, we ruled out some explanations for the impairment in pro-B cell differentiation. According to one hypothesis, increased competition for ribosomes or transcription factors (like Pax5) in the transgenic mice would reduce the expression of μH chain from the productively rearranged endogenous locus and/or the Pax5 target gene CD19, thereby inhibiting pro-B cell development. However, there was no difference in the expression of intracellular μH chain between pro-B cells from wild-type, Ter3tg (Fig. 2B, histogram), and heterozygous Ter5High mice (Fig. 2D, histogram), or between total bone marrow cells from Ter3Tg and wild-type mice, or from IgHQM/wt and IgHQM/wt/Ter3Tg mice (SI Appendix, Fig. S3A). Nor was there any difference in membrane expression of CD19 between pro-B cells from JH−/− and JH−/−/Ter3Tg mice (SI Appendix, Fig. S3B), in which B cell development is arrested at the pro-B cell stage. Therefore, the expression of nonsense μH mRNA from a single endogenous locus (Ter5 mouse) or multiple transgenes (Ter3 mouse) does not reduce the availability of transcription factors involved in the expression of the endogenous IgH and CD19 locus.
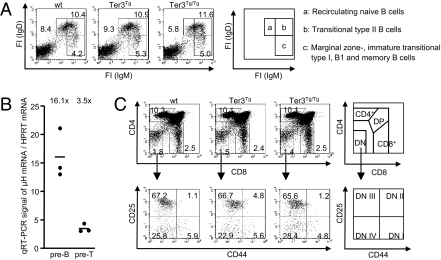
In another experiment, we differentiated between the splenic B cell populations in Ter3 transgenic mice and found them unaltered, compared with wild-type. These populations included recirculating naive B cells (population a in Fig. 3A), transitional type II B cells (population b in Fig. 3A), and marginal zone, immature transitional type I, B1 and memory B cells (population c in Fig. 3A). Finally, we looked at T cell development, which requires recombination of the TCR-β locus at the pro-T cell stage (the double-negative DN III stage). Pre-T cells of Ter3Tg/Tg mice express μH mRNA at levels approximately one fifth of that in pre-B cells (Fig. 3B) and two fifths of that in Ter3 heterozygous pre-B cells. VDJ recombination at the TCR-β locus occurs in the DN III population (pro-T cell stage), and impaired recombination should increase this population (35). However, this and the other thymic cell populations were unaltered (Fig. 3C).
Fig. 3.
Ter3 mRNA does not affect later B cell stages and thymic development. (A) Splenocytes from 6-wk-old mice membrane stained with the indicated antibodies. Fluorescence intensities (FI) of cells in the lymphocyte gate were determined. Percentages of cells in the individual gates are indicated. The following populations are marked clockwise by squares in the dot plot diagram and explained in the schematic diagram to the right: population a, recirculating naive B cells (IgMdull, IgDhigh); population b, transitional type II B cells (IgMhigh, IgDhigh); and population c, IgMhigh, IgDdull cells including marginal zone, immature transitional type I, B1 and memory B cells. (B) Ter3 μH mRNA and hypoxanthine phophoribosyltransferase (HPRT) mRNA abundances were measured by quantitative RT-PCR in sorted pre-B and pre-T cells from three Ter3Tg/Tg mice. (C) Thymocytes from 6-wk-old mice were membrane stained with the indicated antibodies, and fluorescence intensities of cells in the lymphocyte gate were determined. Percentages of cells in the individual gates are indicated. Upper: Cells were analyzed for CD4 and CD8 expression. Lower: Double-negative (DN) population indicated in the upper diagrams was divided into stages DN I–IV, according to CD25 and CD44 expression.
Frequency of Recombined IgH Alleles Is Decreased by Stable μH mRNA.
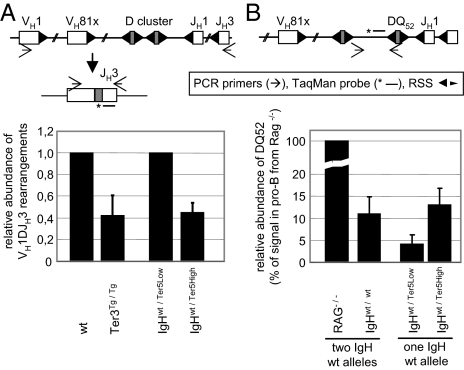
As far as we could determine, stable μH mRNA affected only the numbers of pro-B and pre-B cells. In pro-B cells, it also reduced the number of μH chain-expressing cells (Fig. 2 B and D), presumably because of a reduced frequency of recombined IgH alleles. To measure this frequency directly, we isolated DNA from pro-B cells and determined the ratio of VDJ rearrangements vs. germ line configuration by quantitative TaqMan PCR. The forward primer was specific for the most abundant VH1 (J558) family, and the reverse primer was specific for the JH3 segment, which is used in neither the Ter3 nor the Ter5 transgene. Both homozygous Ter3Tg/Tg and heterozygous Ter5High mice had approximately half as many VH1-DJH3 rearrangements as wild-type and heterozygous Ter3Low mice, respectively (Fig. 4A). These results correlate closely with the decreased frequency of μH chain-positive cells in the pro-B cell populations of the respective mice (Fig. 2 B and D).
Fig. 4.
VDJ recombination frequency is decreased by stable μH mRNA. (A) Quantification of VH1DJH3 rearrangements in pro-B cells by TaqMan PCR. Genomic DNA from FACS-sorted CD19+/c-kit+ pro-B cells of the indicated genotypes was analyzed using the indicated primers and probe. Signals in Ter3Tg/Tg and IgHwt/Ter5High mice were normalized to those in wild-type and IgHwt/Ter5Low mice, respectively. (B) Quantification of germ line or DQ52JH-rearranged IgH loci by TaqMan PCR. Amplification of the sequence 5′ of the DQ52 gene segment is possible only on germ line or DQ52JH-rearranged IgH loci; it is deleted by all other D-to-JH rearrangements or a VH-to-DQ52 rearrangement. Genomic DNA from sorted CD19+/c-kit+/surface IgM− pro-B cells of the indicated mice was analyzed using the indicated primers and probe. Results (mean ± SD) are from one of two independent experiments.
In a second TaqMan PCR, we assessed the relative frequency of DQ52 elements that still contained their upstream recombination signal (i.e., IgH alleles that were either in the germ line configuration or had a DQ52JH rearrangement) (Fig. 4B). In this assay, DNA from heterozygous Ter5High mice gave a threefold stronger signal than DNA from heterozygous Ter5Low mice, indicating a higher proportion of pro-B cells with either a nonrearranged H allele or an allele with a DQ52-to-JH rearrangement in Ter5High mice. Furthermore, the frequency of immature B cells with a VH-replaced QM allele was lower in QM mice with a transgenic Ter3 allele than in QM mice without it (SI Appendix, Fig. S4). These observations support our hypothesis that developmental inhibition occurs before or at the stage of V-to-DJ recombination.
VDJ Recombinase Is Unaffected by Stable μH mRNA.
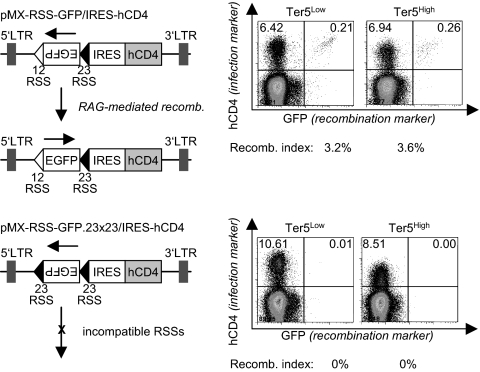
To determine whether stable μH mRNA interferes with VDJ recombination by directly inhibiting the RAG recombinase, we transduced sorted pro-B cells from the bone marrow of homozygous Ter5High and Ter5Low mice with a VDJ recombination plasmid that contains an inverted EGFP gene. The EGFP cassette is flanked by recombination signal sequences (RSS) and can be activated upon RAG-mediated reversion (35). RAG activities in pro-B cells from both wild-type and Ter5High mice were similar (Fig. 5). Hence, the accumulation of noncoding μH mRNA does not directly affect the VDJ recombinase, a finding consistent with the normal development of B cells beyond the pre-B cell stage.
Fig. 5.
VDJ recombinase activity is unaffected by stable μH mRNA. Total bone marrow cells from homozygous Ter5Low and Ter5High mice were isolated and transduced with two viral vectors containing an inverted EGFP flanked by RSS. The first vector contained two compatible RSS, which allows inversion and expression of the EGFP. The second variant contained two incompatible RSS and served as negative control. Frequency of recombined GFP-positive cells in the infected hCD4-positive population was measured 48 h after infection and is depicted as recombination activity index.
Discussion
Currently, allelic exclusion at the IgH locus is explained by a feedback mechanism that presumes signaling via the pre-BCR containing the μH chain encoded by a productive allele. An analysis of mice with a deletion including the μ membrane exons (μMT mice) shows that sequences spanning and flanking the membrane exons are needed for IgH allelic exclusion (10). However, from this experiment it is not clear whether these sequences are only necessary to generate the μH chain protein or whether the (stable) μH mRNA, or even the untranslated regions of the mRNA, also contribute to allelic exclusion.
In this study, we investigated the potential role of μH mRNA in the remarkable strictness of exclusion at the IgH locus. Our data show that a stable μH mRNA impairs pro-B cell development in the absence of a μH chain signal. We cannot exclude that a nontranslatable but stable mRNA other than that encoding μH chain would have the same effect. However, with the many stable translated mRNAs encoding functional proteins already present in a cell, just adding any other one might not be expected to make a difference. At any rate, because we did not detect an effect on the development of pre-B and immature B cells into mature B cell subsets (Fig. 3A), or on T cell development in the thymus (Fig. 3C), the effect would have to be restricted to pro-B cells. One could also argue that a well-transcribed IgH allele will sequester transcription factors for target genes whose expression levels are critical at an early stage in B cell development, like the genes for μH chain and CD19. However, because the expression levels of μH chain and CD19 were not affected by the expression of Ter3 and Ter5 mRNA (Fig. 2 B and D and SI Appendix, Fig. S3), we think that the observed phenotype is not caused by transcription factor sequestration.
Our results rather fit the observations in mice with decreased NMD, which are reported in the accompanying article by Frischmeyer-Guerrerio et al. (36). Their study demonstrated a critical role for the NMD in T and B lymphocyte development. B cells may thus distinguish between productively and nonproductively rearranged IgH alleles at the RNA level, and this distinction may help in allelic exclusion. However, how can μH mRNA contribute to allelic exclusion? We demonstrated the effect of a μH mRNA on B cell differentiation in mice with either a conventional IgH transgene (the Ter3 mouse) or a VDJ exon knockin (the Ter5 mouse). One explanation for the observed phenotype could be that expression of a μH mRNA simply confers a growth disadvantage to pro-B cells. In this case, pro-B cells with a productive IgH gene, and thus expressing a stable coding μH mRNA, would grow more slowly than cells that have not yet rearranged; but cells without a productive VDJ rearrangement do not progress anyway, because they do not receive a differentiation signal from a μH chain protein. A cell with two productive VDJ rearrangements would grow even more slowly and would be at a disadvantage compared with a cell with only one productive VDJ rearrangement.
Alternatively, we propose a model in which stable μH mRNA has a suppressive effect on VDJ recombination, even in the absence of μH chain protein. Because NMD selectively degrades noncoding transcripts from nonproductive IgH alleles, only sense μH transcripts accumulate and are capable of inhibiting, directly or indirectly, V-to-DJ rearrangements. Because we excluded a direct effect on RAG activity, μH mRNA might interfere with the opening and/or the accessibility of the second IgH allele, for example, in combination with antisense RNA transcribed during VDJ recombination from the IgH locus (20). Alternatively, abundant μH transcripts could act similarly to Xist, which mediates X chromosome inactivation (37). In this model, suppression of recombination at the other IgH allele is followed by the strong feedback signal from the pre-BCR, which would shut it down permanently. Such an adapted feedback model of allelic exclusion could bridge the time gap between a productive VDJ rearrangement and the initiation of an H chain-dependent pre-BCR signal.
In summary, we have identified a specific μH mRNA-dependent process that—independent of a μH chain signal—distinguishes between a productive and a nonproductive IgH allele and thereby may contribute to the establishment of allelic exclusion at the IgH locus.
Materials and Methods
Standard procedures and methods, such as animal handling, flow cytometry, and RNA analyses, as well as statistical methods, are described in SI Appendix, SI Materials and Methods.
RAG Activity Assay in Primary Pro-B Cells.
The viral vectors pMX-RSS-GFP/IRES-hCD4 and pMX-RSS-GFP.23x23/IRES-hCD4 (35) (kindly provided by M. Schlissel, Department of Molecular and Cell Biology, University of California, Berkeley, CA) were packaged in PlatE cells and used to infect total bone marrow cells from homozygous Ter5L and Ter5H mice. Frequency of GFP-positive cells in the hCD4-positive population was measured by flow cytometry 48 h after infection and is depicted as recombination activity index.
Supplementary Material
Acknowledgments
We thank C. Vettermann and J. Borst for helpful comments and J. Wittmann and T. Winkler for discussion. This research was supported by the Interdisciplinary Center for Clinical Research and the Deutsche Forschungsgemeinschaft (SFB 466 and FOR 832 Research Grant JA 968/4 to H.-M.J.), the Netherlands Organization for Scientific Research and the Dutch Cancer Foundation (VIDI program 917.56.328 and KWF 2008-4112 to H.J.), and the National Institutes of Health (Grant R01AI041570 to M.W.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019224108/-/DCSupplemental.
References
- 1.Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu Rev Immunol. 2006;24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 2.Vettermann C, Herrmann K, Jäck HM. Powered by pairing: the surrogate light chain amplifies immunoglobulin heavy chain signaling and pre-selects the antibody repertoire. Semin Immunol. 2006;18:44–55. doi: 10.1016/j.smim.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Pernis B, Chiappino G, Kelus AS, Gell PG. Cellular localization of immunoglobulins with different allotypic specificities in rabbit lymphoid tissues. J Exp Med. 1965;122:853–876. doi: 10.1084/jem.122.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiler E. Differential activity of allelic gamma-globulin genes in antibody-producing cells. Proc Natl Acad Sci USA. 1965;54:1765–1772. doi: 10.1073/pnas.54.6.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerdes T, Wabl M. Autoreactivity and allelic inclusion in a B cell nuclear transfer mouse. Nat Immunol. 2004;5:1282–1287. doi: 10.1038/ni1133. [DOI] [PubMed] [Google Scholar]
- 6.Barreto V, Cumano A. Frequency and characterization of phenotypic Ig heavy chain allelically included IgM-expressing B cells in mice. J Immunol. 2000;164:893–899. doi: 10.4049/jimmunol.164.2.893. [DOI] [PubMed] [Google Scholar]
- 7.Vettermann C, Schlissel MS. Allelic exclusion of immunoglobulin genes: Models and mechanisms. Immunol Rev. 2010;237:22–42. doi: 10.1111/j.1600-065X.2010.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perry RP, et al. Transcription of mouse kappa chain genes: Implications for allelic exclusion. Proc Natl Acad Sci USA. 1980;77:1937–1941. doi: 10.1073/pnas.77.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleclough C, Perry RP, Karjalainen K, Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981;290:372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura D, Rajewsky K. Targeted disruption of mu chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 1992;356:154–156. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- 11.Nussenzweig MC, et al. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin mu. Science. 1987;236:816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- 12.Schweighoffer E, Vanes L, Mathiot A, Nakamura T, Tybulewicz VL. Unexpected requirement for ZAP-70 in pre-B cell development and allelic exclusion. Immunity. 2003;18:523–533. doi: 10.1016/s1074-7613(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 13.Papavasiliou F, Jankovic M, Gong S, Nussenzweig MC. Control of immunoglobulin gene rearrangements in developing B cells. Curr Opin Immunol. 1997;9:233–238. doi: 10.1016/s0952-7915(97)80141-0. [DOI] [PubMed] [Google Scholar]
- 14.Papavasiliou F, Jankovic M, Suh H, Nussenzweig MC. The cytoplasmic domains of immunoglobulin (Ig) alpha and Ig beta can independently induce the precursor B cell transition and allelic exclusion. J Exp Med. 1995;182:1389–1394. doi: 10.1084/jem.182.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papavasiliou F, Misulovin Z, Suh H, Nussenzweig MC. The role of Ig beta in precursor B cell transition and allelic exclusion. Science. 1995;268:408–411. doi: 10.1126/science.7716544. [DOI] [PubMed] [Google Scholar]
- 16.Aifantis I, et al. Allelic exclusion of the T cell receptor beta locus requires the SH2 domain-containing leukocyte protein (SLP)-76 adaptor protein. J Exp Med. 1999;190:1093–1102. doi: 10.1084/jem.190.8.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson SJ, Levin SD, Perlmutter RM. Protein tyrosine kinase p56lck controls allelic exclusion of T-cell receptor beta-chain genes. Nature. 1993;365:552–554. doi: 10.1038/365552a0. [DOI] [PubMed] [Google Scholar]
- 18.Alt FW, Rosenberg N, Enea V, Siden E, Baltimore D. Multiple immunoglobulin heavy-chain gene transcripts in Abelson murine leukemia virus-transformed lymphoid cell lines. Mol Cell Biol. 1982;2:386–400. doi: 10.1128/mcb.2.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alt FW, et al. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984;3:1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolland DJ, et al. Antisense intergenic transcription in V(D)J recombination. Nat Immunol. 2004;5:630–637. doi: 10.1038/ni1068. [DOI] [PubMed] [Google Scholar]
- 21.Roldán E, et al. Locus ‘decontraction’ and centromeric recruitment contribute to allelic exclusion of the immunoglobulin heavy-chain gene. Nat Immunol. 2005;6:31–41. doi: 10.1038/ni1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuxa M, et al. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 2004;18:411–422. doi: 10.1101/gad.291504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hewitt SL, et al. RAG-1 and ATM coordinate monoallelic recombination and nuclear positioning of immunoglobulin loci. Nat Immunol. 2009;10:655–664. doi: 10.1038/ni.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haines BB, Brodeur PH. Accessibility changes across the mouse Igh-V locus during B cell development. Eur J Immunol. 1998;28:4228–4235. doi: 10.1002/(SICI)1521-4141(199812)28:12<4228::AID-IMMU4228>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 25.Skok JA, et al. Nonequivalent nuclear location of immunoglobulin alleles in B lymphocytes. Nat Immunol. 2001;2:848–854. doi: 10.1038/ni0901-848. [DOI] [PubMed] [Google Scholar]
- 26.Kosak ST, et al. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science. 2002;296:158–162. doi: 10.1126/science.1068768. [DOI] [PubMed] [Google Scholar]
- 27.Sayegh CE, Jhunjhunwala S, Riblet R, Murre C. Visualization of looping involving the immunoglobulin heavy-chain locus in developing B cells. Genes Dev. 2005;19:322–327. doi: 10.1101/gad.1254305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eberle AB, Herrmann K, Jäck HM, Mühlemann O. Equal transcription rates of productively and nonproductively rearranged immunoglobulin mu heavy chain alleles in a pro-B cell line. RNA. 2009;15:1021–1028. doi: 10.1261/rna.1516409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumann B, Potash MJ, Köhler G. Consequences of frameshift mutations at the immunoglobulin heavy chain locus of the mouse. EMBO J. 1985;4:351–359. doi: 10.1002/j.1460-2075.1985.tb03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jäck HM, Berg J, Wabl M. Translation affects immunoglobulin mRNA stability. Eur J Immunol. 1989;19:843–847. doi: 10.1002/eji.1830190510. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Wilkinson MF. Nonsense surveillance in lymphocytes? Immunity. 1998;8:135–141. doi: 10.1016/s1074-7613(00)80466-5. [DOI] [PubMed] [Google Scholar]
- 32.Buzina A, Shulman MJ. Infrequent translation of a nonsense codon is sufficient to decrease mRNA level. Mol Biol Cell. 1999;10:515–524. doi: 10.1091/mbc.10.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukita Y, Jacobs H, Rajewsky K. Somatic hypermutation in the heavy chain locus correlates with transcription. Immunity. 1998;9:105–114. doi: 10.1016/s1074-7613(00)80592-0. [DOI] [PubMed] [Google Scholar]
- 34.Cascalho M, Ma A, Lee S, Masat L, Wabl M. A quasi-monoclonal mouse. Science. 1996;272:1649–1652. doi: 10.1126/science.272.5268.1649. [DOI] [PubMed] [Google Scholar]
- 35.Liang HE, et al. The “dispensable” portion of RAG2 is necessary for efficient V-to-DJ rearrangement during B and T cell development. Immunity. 2002;17:639–651. doi: 10.1016/s1074-7613(02)00448-x. [DOI] [PubMed] [Google Scholar]
- 36.Frischmeyer-Guerrerio PA, et al. Perturbation of thymocyte development in nonsense-mediated decay (NMD)-deficient mice. Proc Natl Acad Sci USA. 2011;108:10638–10643. doi: 10.1073/pnas.1019352108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagano T, Fraser P. Emerging similarities in epigenetic gene silencing by long noncoding RNAs. Mamm Genome. 2009;20:557–562. doi: 10.1007/s00335-009-9218-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.