Abstract
In this study we investigated if peroxisome proliferator-activated receptor β/δ activation protects from copper-induced acute liver damage. Mice treated with copper had significant body weight loss, serum alanine aminotransferase increase, modest changes in liver histology, increase of tumor necrosis factor α and macrophage inflammatory protein 2 mRNA and 8-hydroxy-2'-deoxyguanosine. Mice treated with copper and peroxisome proliferator-activated receptor β/δ agonist GW0742 had significantly less body weight loss, less serum alanine aminotransferase increase, less tumor necrosis factor α, macrophage inflammatory protein-2 and 8-hydroxy-2'-deoxyguanosine upregulation than copper treated mice. The opposite effect was observed in mice treated with copper and peroxisome proliferator-activated receptor β/δ antagonist GSK0660. In vitro, copper induced reactive oxygen species, which was lower in cells treated with GW0742 or transfected with peroxisome proliferator-activated receptor β/δ expression vector; together, transfection and GW0742 had an additive reactive oxygen species-reducing effect. Copper also upregulated Fas ligand and Caspase 3/7 activity, effects that were significantly lower in cells also treated with GW0742. In conclusion, peroxisome proliferator-activated receptor β/δ activation reduced copper-induced reactive oxygen species, pro-inflammatory and acute phase reaction cytokines in mice liver. Peroxisome proliferator-activated receptor β/δ agonists could become useful in the management of copper-induced liver damage.
Keywords: liver damage, apoptosis, copper, reactive oxygen species, Wilson’s disease
Introduction
Copper toxicity is a rare but important cause of liver failure. Copper toxicity occurs only on acute accidental poisoning with copper sulfate or upon an insidious onset as in idiopathic copper toxicity, which is an even more rare condition whose pathogenesis is not quite well understood yet; or in Wilson’s disease (WND)(1,2) which is the model disease for copper toxicity. WND is an autosomal recessive disorder in which copper transport from the hepatocytes to bile flow and incorporation of copper into ceruloplasmin are impaired.(3) This situation leads to excess copper accumulation in several organs, particularly liver and brain; however, liver damage is the common condition in copper toxicity. It has been proposed that the mechanism of copper toxicity is via generation of free radicals which are strongly linked to inflammation, fibrosis and apoptosis that eventually leads to liver failure.(4) One of WND’s form of clinical presentation is fulminant WND; condition for which plasmapheresis and chelation therapy have no demonstrated benefit in mortality, but remain the only bridge to transplantation in these patients.(5)
Peroxisome proliferator-activated receptor β/δ (PPARβ/δ) is the last member of the PPAR’s family to be carefully studied. It is ubiquitously expressed in most tissues, with higher expression in liver, intestine and keratinocytes.(6,7) Its activation improves glucose homeostasis, increases serum HDL cholesterol, increases fatty acid catabolism in muscle, mediates terminal differentiation and inhibits cell proliferation in different models.(8–10) PPARβ/δ also has anti-inflammatory effects including inhibition of cytokine production, modulation of cell adhesion molecules and inhibition of nuclear factor κB (NFκB) signaling. Besides the mentioned characteristics and many other metabolic functions, PPARβ/δ has been described to ameliorate carbon tetrachloride induced liver toxicity.(9,11)
Recent reports suggest that PPARβ/δ has an important role in oxidative stress; for example, PPARβ/δ activation protects cardiomyoblasts(12) and umbilical vein endothelial cells from oxidative stress-induced apoptosis(13) and promotes reversal of metabolic abnormalities, reduces oxidative stress and increases fatty acid oxidation in obese men.(14) PPARβ/δ null mice have larger histological damage and higher levels of damage markers [Alanine aminotransferase (ALT)] than wild type mice when treated with known hepatotoxicants.(9,11) Since PPARβ/δ appears to have a protective role in oxidative stress-induced injury and prevents carbon tetrachloride (CCl4)-induced liver damage, we investigated if PPARβ/δ protects from copper-induced liver damage through reduction of oxidative stress, inflammation and apoptosis.
Materials and Methods
Reagents
The following reagents were purchased from Sigma Aldrich Co. (St. Louis, MO) unless otherwise stated. The highly specific PPARβ/δ agonist GW0742 and the antagonist GSK0660 were diluted first in dimethylsulfoxide (DMSO) and later in culture media for in vitro studies (DMSO less than 1:100 000) and in NaCl 0.9% for in vivo studies (DMSO less than 1%). Copper sulfate and Nitriloacetate (NTA) were diluted in saline solution into 0.08 mol/L and 0.16 mol/L respectively and mixed at a molar ratio of 1:4 (modified from Toyokuni et al.)(15) for in vivo studies. Copper sulfate was diluted in distilled water and then in culture media for in vitro experiments. Fresh dilutions of all reagents were prepared prior to each experiment.
In vivo study
Animal experiments were approved by the Ethics Committee for Animal Experimentation of Shimane University and its rules were followed, assuring human care to all the animals used. Six weeks old BALB/c mice were purchased form CLEA Japan Inc. and divided into groups of 5 under day:night cycles of 12:12 h, fed NMF chow and water ad libitum. At least one week after their arrival to the animal experimentation facilities, mice were treated for 3 days in a row and sacrificed on day 4. Group #1 served as control, receiving the corresponding oral and intraperitoneal vehicles of the substances given to the other groups. Group #2 was treated with 10 mg/kg/day of the PPARβ/δ agonist GW0742 by oral gavage. Group #3 was treated with 1 mg/kg/day I.P. of PPARβ/δ antagonist GSK0660. Group #4 was treated with 10 mg/kg/day I.P. of copper in form of copper-nitriloacetate (NTA). Group #5 was treated with 10 mg/kg/day of copper I.P. and with 10 mg/kg/day of GW0742 P.O. Group #6 was treated with 10 mg/kg/day of copper I.P. and 1 mg/kg/day of GSK0660 I.P.
Mice body weight was assessed before treatment on day 1 and on day 4 before being sacrificed by inhalation of diethyl ether. Blood sample was taken by cardiac puncture to later assess the concentration of serum ALT. Mice were dissected and the liver was removed, part of the liver was snap frozen in liquid nitrogen and kept at −80°C for protein assays, other part was placed in abundant volume of RNAlater (Ambion, Inc.) and other part was preserved for histology.
Histology
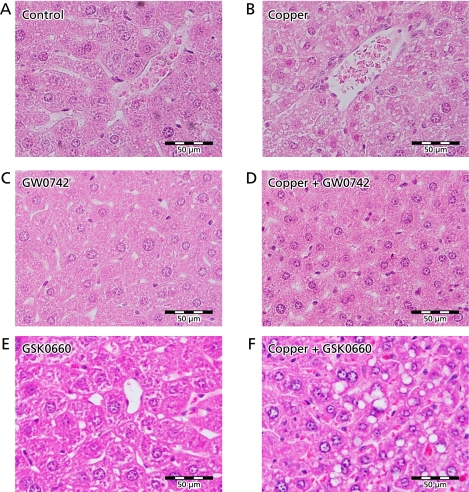
Liver tissue was fixed in 10% formalin and embedded in paraffin. Sections were mounted and stained with hematoxylin and eosin and examined under light microscopy. As histological changes induced by copper and other reagents were expected to occur diffusely in the liver, we examined the largest section of each sample and representative changes observed in the sections were selected and included in Fig. 1.
Fig. 1.
Liver histology. Mice were treated for 3 days with 10 mg/kg/day of copper and 10 mg/kg/day of PPARβ/δ agonist (GW0742) and/or 1 mg/kg/day of the PPARβ/δ antagonist (GSK0660) and sacrificed at day #4. (A) Control mice were treated with the corresponding vehicles. (B) Modest vacuolization in cells around the central vein and atrophic degeneration was observed in the liver of mice treated with copper. No obvious changes were observed in mice that received GW0742 (C) or copper+GW0742 (D). Mice treated with GSK0660 alone show some vacuolization of cells and changes in nucleus: cytoplasm ratio (E). The mentioned changes were more noticeable in the mice that received copper and GSK0660 (F). Liver of all mice in each group were processed, assessing all fields in one section of each; a representative field of each group was selected for this figure. Hematoxylin and eosin staining.
RNA analysis
Total RNA was isolated from liver tissue using the RNeasy mini Kit (QIAGEN) and from cultured cells using Isogen (Nippon Gene Co., Toyama, Japan) as described in their protocols. RNA quantity and quality were assessed by spectrophotometry with NanoDrop® ND-1000; upon doing this, 1 µg of total RNA was reverse-transcriptased with the StrataScript® First Strand Synthesis System (Stratagene, La Jolla, CA) according to the manufacturer’s instructions. RT-PCR was performed by SYBR® Green (Applied Biosystems, Foster City, CA) method with STEPONEPLUSTM system (Applied Biosystems, Foster City, CA). The expression of tumor necrosis factor α (TNFα), macrophage inflammatory protein 2 (MIP-2) and the house keeping gene GAPDH in mice (Mus musculus) tissue were evaluated using the following primers: TNFα, forward primer, 5'-GAC AAG GCT GCC CCG ACT A-3'; reverse primer, 5'-CGG CAG AGA GGA GGT TGA CT-3'; MIP-2, forward primer, 5'-GGC CCA AGA TCC GCT ACA-3'; reverse primer, 5'-GTG TGG GTA CTT TGG CTT CAT TT-3; GAPDH, forward primer 5'-CAT GGC CCT TCC GTG TTC CTA-3', reverse primer RV: 5'-GCG GCA CGT CAG ATC CA-3'. The expression of Fas ligand (FasL) and GAPDH genes in HepG2 cells (Homo sapiens) were assessed with the following primers: FasL, forward primer: 5'-AGG AAA GTG GCC CAT TTA ACA G-3', reverse primer: 5'-CCA GAA AGC AGG ACA ATT CCA TAG-3'; human GAPDH, forward primer 5'-CCA CAT CGC TCA GAC ACC-3', reverse primer RV: 5'-TGA CCA GGC GCC CAA TA-3'.
In vitro study
The human hepatoma cell line HepG2 was obtained from ATCC (Manassas, VA), cultured and maintained in DMEM media (GIBCO Products, Carlsbad, CA) supplemented with Fetal Bovine Serum (ATCC Inc., Manassas, VA) to 10% of the final volume and penicillin-streptomycin (GIBCO Products, Carlsbad, CA) to a 1:100 dilution. Experiments were carried out with complete growth media unless otherwise stated. Maintenance and experimental cell cultures were kept at 37°C and 5% CO2.
Cell proliferation assays
The CellTiter96®AQUEOUS Non-Radioactive Cell Proliferation Assay (Promega Corp. Madison, WI) method was used to assess cells in proliferation and chemo sensitivity. The protocol was followed and adapted to the needs of this study. HepG2 cells were seeded in 96 well plates and treated with different concentrations of CuSO4 and GW0742 for 24 to 48 h; the formation of formazan was measured with an ELISA plate reader (BioRad, Hercules, CA) at 490 nm 4 h after adding the PMS/MTS solution.
Intracellular reactive oxygen species (ROS) determination
Intracellular ROS were determined by fluorometry using the oxidation-sensitive probe 5-(and-6)-carboxy-2',7'-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) (Molecular Probes, Inc. Eugene, OR) according to the manufacturer’s instructions. Briefly, HepG2 cells were seeded in 96 well plates and transfected with PPARβ/δ expression vector or empty vector; 6 h later media was changed to conditioned media (containing copper sulfate and/or GW0742). Twenty four hours after adding conditioned media cells were loaded carboxy-H2DCFDA probe and fluorescence was read 4 h later.
Caspase 3/7 activity determination
Caspase 3/7 activity was measured using Apo-ONE® Homogeneous Caspase-3/7 Assay (Promega Corp. Madison, WI) according to instructions. Briefly, HepG2 cells were seeded in white 96-well plates and treated with different concentrations of copper and GW0742. After 18 h of treatment, Apo-ONE® Caspase-3/7 reagent was added to each well and fluorescence was read 6 h later (Fluoroskan Ascent FL). Each experiment was performed in triplicate.
Plasmids and transient transfection assays
Full-length human PPARβ/δ cDNA (Genbank accession number NM_006238) was amplified by PCR and cloned into an eukaryotic TA cloning pCR3.1 expression vector (Invitrogen Corporation, Carlsbad, CA). HepG2 cells were seeded in 6 or 24-well plates and transfected when cultures reached 50–70% confluency using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Eighteen to twenty four hours later, transfected cells were treated with conditioned media as required.
Western blot
Protein was extracted from cultured cells using RIPA buffer plus HALT Protease and phosphatase inhibitor cocktail (Thermo Scientific, Rockford, IL) according to the manufacturer’s protocol. Sixty micrograms of protein per lane were loaded and fractionated by 10% SDS-PAGE gel (TEFCO, Tokyo, Japan). After fractionation, proteins were transferred to a polyvinylidene difluoride membrane (Amersham Biosciences Inc., UK) and later blocked with 5% milk/TTBS. Incubation with specific first antibodies was done overnight at 4°C and secondary antibodies for 2 h at room temperature. PPARδ protein was detected with PPARδ polyclonal antibody (Cayman Chemical, Ann Arbor, MI) and the β-actin protein was detected with monoclonal anti-β-actin clone AC-15 (Sigma, St. Louis, MO) and reacted with Polyclonal Rabbit anti-mouse immunoglobulins/HRP (Dako, Denmark) respectively.
8-hydroxy-2'-deoxy-Guanosine (8-OHdG) EIA
First, DNA purification from mouse liver tissue was performed with QIAamp® DNA Mini kit (QIAGEN, Alameda, CA) according to manufacturer’s protocol. After purification, DNA was denatured by boiling for 10 min and digested with nuclease P1 (Sigma, Japan) at 37°C for 30 min. pH was adjusted to 8.0, alkaline phosphatase was added and inactivated by boiling after 30 min of incubation at 37°C. Samples were immediately kept in ice for at least 10 min before performing 8-OHdG enzyme immunoassay.
8-OHdG ELISA kit was obtained from Cayman Chemical (Ann Arbor, MI) and ran on samples after DNA purification and digestion as mentioned above. ELISA assay was performed according to manufacturer’s protocol. Data analysis was performed with Cayman’s data analysis spread sheet (www.caymanchem.com/analysis/eia) and later each sample was normalized to the concentration of DNA.
Statistical analysis
Data are presented as mean ± SEM. Student’s t test was used to examine the differences between experimental groups of the in vitro studies and the non-parametric Mann-Whitney-Wilcoxon U test was used to examine the differences between experimental groups of the in vivo studies. A value of p<0.05 was considered significant.
Results
PPARβ/δ agonism protects from copper-induced liver damage
To test the hypothesis that PPARβ/δ activation protects from copper-induced liver damage, BALB/c mice were treated for 3 days with intraperitoneal injections of copper and the highly specific PPARβ/δ agonist GW0742 or the PPARβ/δ antagonist GSK0660. Normal histology is observed in the liver of control mice that received the corresponding vehicles P.O. and I.P. (Fig. 1A). As well, mice treated with GW0742 alone (Fig. 1C) did not show obvious histological changes. Mice treated with GSK0660 alone (Fig. 1E) show some vacuolization of cells and changes in nucleus:cytoplasm ratio. In mice treated with copper nitriloacetate it was possible to observe modest vacuolization in cells around the central vein and atrophic degeneration in the liver (Fig. 1B). The latter histological changes were not observed in the mice that received copper and PPARβ/δ agonist GW0742 (Fig. 1D), but were more severe in mice treated with copper and the PPARβ/δ antagonist GSK0660 (Fig. 1F).
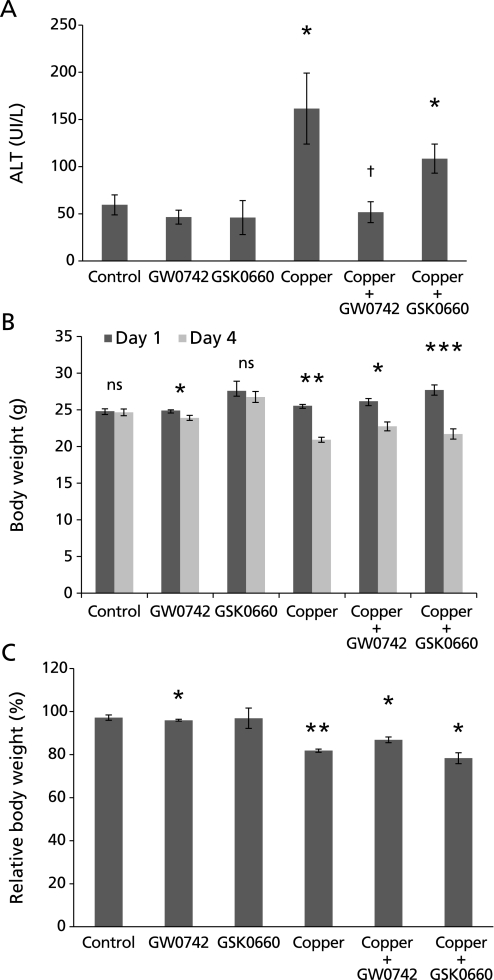
Average serum ALT in the control group was 59.5 IU/L, average that increased to 161.6 IU/L (p<0.05) in the group treated with copper. Mice treated with copper and GW0742 had an average ALT of 46.5 IU/L, which is not significantly different from the serum ALT of the control group but significantly lower than ALT of mice treated with copper alone (p<0.05). Mice treated with copper and PPARβ/δ antagonist GSK0660 had a serum ALT of 108.5 IU/L which is comparable to those treated with copper and significantly higher than that in mice that received only GSK0660 (46.0 IU/L) or control (p<0.05) (Fig. 2A).
Fig. 2.
Serum ALT and mice body weight. (A) Control mice had an average ALT concentration of 59.5 UI/L, concentration that significantly increased to 161 UI/L in mice treated with copper. Serum ALT in mice treated with copper and GSK0660 was 108 UI/L, which is not significantly different to the increase observed in mice treated with copper alone. ALT in mice treated with copper and GW0742 was 46.5 UI/L, what is significantly lower than in mice treated with copper alone and comparable to control group (*p<0.05 vs control; †p<0.05 vs copper). (B, C) Body weight was assessed before treatment on day 1 and before sacrifice on day 4. Average body weight and relative changes in body weight between day 1 (100%) and day 4 can be observed in B and C respectively. Copper alone induced a significant 18% decrease in body weight, decrease that was still significant but less important in those that also received GW0742 (13%). Mice treated with copper and GSK0660 lost a significant 20% of body weight. GW0742 induced a small but significant 3% body weight loss. Error bars represent SEM and p value was assessed by Mann-Whitney-Wilcoxon U Test (*p<0.05, **p<0.01, ***p<0.001).
Mice body weight was assessed as a measure of mice general condition and by the end of the experiment, important changes in body weight were observed in the different groups. Control group did not gain weight during the 4 experimental days probably due to the administration of DMSO (GW0742’s vehicle) by oral gavage. Mice treated with GW0742 alone had a small but significant weight loss of 3% (p<0.05). The group of mice treated with copper alone lost a significant 18% of body weight (p<0.01), decrease that was less severe (13%) in the group treated with copper and GW0742 (p<0.05). The group treated with copper and GSK0660 lost 20% of body weight (p<0.05) (Fig. 2 B and C).
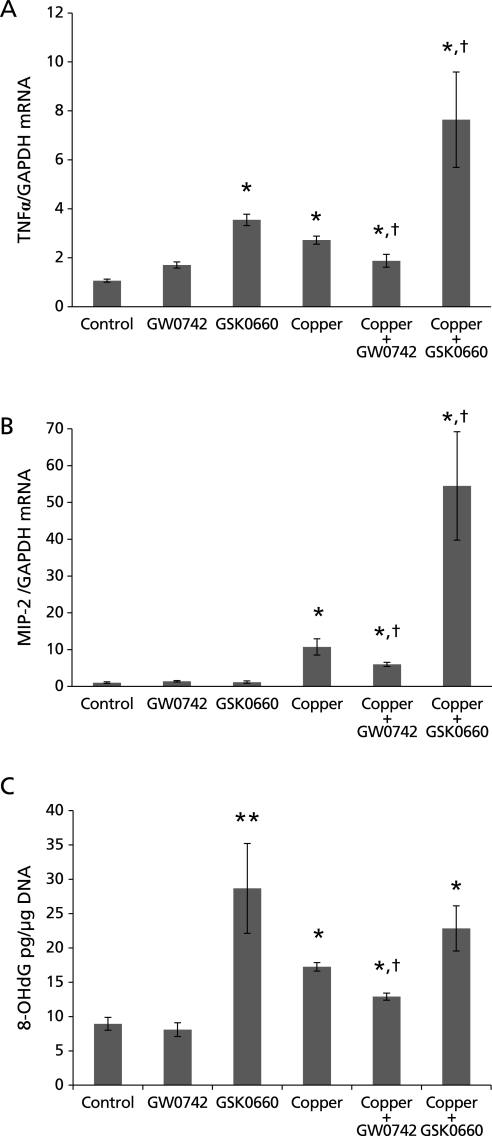
mRNA expression of the acute phase reaction stimulant TNFα in the liver of these mice was evaluated. TNFα mRNA significantly increased to 2.72 fold in mice treated with copper (p<0.05). TNFα mRNA expression was 1.85 fold in the mice treated with copper and PPARβ/δ agonist GW0742, which is significantly lower than in those treated with copper alone (p<0.05). The mice that received PPARβ/δ antagonist GSK0660 alone had a 3.54 fold expression of TNFα mRNA compared with the control, expression that rose to 7.64 fold in mice that received copper and GSK0660 (p<0.05) (Fig. 3A).
Fig. 3.
TNFα and MIP-2 mRNA and 8-OHdG in liver of mice treated with copper and PPARβ/δ agonist (GW0742) or antagonist (GSK0660). (A) TNFα was upregulated in all experimental groups; in mice treated with copper it increased to 2.72 fold and to 1.85 fold in the group treated with copper and GW0742, significantly lower than copper alone group. The group treated with copper and GSK0660 experienced a steep upregulation of TNFα, 7.64 fold, significantly higher than in the group treated with copper only. (*p<0.05 vs control; †p<0.05 vs copper) (B) MIP-2 expression increased to 10.71 fold in mice treated with copper, increase that was reduced to 5.99 fold in mice treated with copper and GW0742 and steeply increased to 54.51 fold in those treated with copper and GSK0660. (*p<0.05 vs control; †p<0.05 vs copper) (C) 8-OHdG was assessed in liver DNA; mice treated with copper had almost double 8-OHdG than control (8.95 vs 17.25 pg/µg of DNA). Copper-induced increase of 8-OHdG was reduced by half in mice also treated with GW0742. GSK0660 alone and in combination with copper significantly increased 8-OHdG 3 and 2 fold respectively. Error bars represent SEM and p value was assessed by Mann-Whitney-Wilcoxon U Test (*p<0.05, **p<0.005 vs control; †p<0.05 vs copper).
The mRNA expression of the inflammatory cytokine MIP-2 was also evaluated in the liver of the different groups. The liver of mice treated with copper alone had a 10.71 fold upregulation of MIP-2 mRNA (p<0.05). The upregulation of MIP-2 induced by copper was reduced by half in mice that also received the PPARβ/δ agonist (5.99 fold, p<0.05). Mice treated with copper and PPARβ/δ antagonist had an upregulation of MIP-2 of 5 fold compared with mice treated with copper alone, this corresponds to 54.51 fold when compared with control mice (p<0.05) (Fig. 3B).
8-OHdG is a product of oxidative stress damage to DNA; we assessed its presence in liver DNA of mice treated with copper, GW0742 and GSK0660. Liver of mice treated with GW0742 alone had almost same amount of 8-OHdG as control group, 8.10 and 8.95 picogram per microgram (pg/µg) of DNA respectively. The liver of mice treated with GSK0660 had over 3 times the concentration of control group, 28.68 pg/µg of DNA (p<0.005) while liver of mice treated with copper alone had almost double concentration of 8-OHdG than control mice, 17.25 pg/µg of DNA (p<0.05). Administration of GW0742 to mice challenged with copper reduced the increase of 8-OHdG by half, to 12.90 pg/µg of DNA (p<0.05). The combination of copper and GSK0660 induced 8-OHdG to 22.83 pg/µg of DNA which is comparable to the concentration observed in mice treated with copper and with GSK0660 alone (p<0.05 vs control) (Fig. 3C).
PPARβ/δ reduces ROS
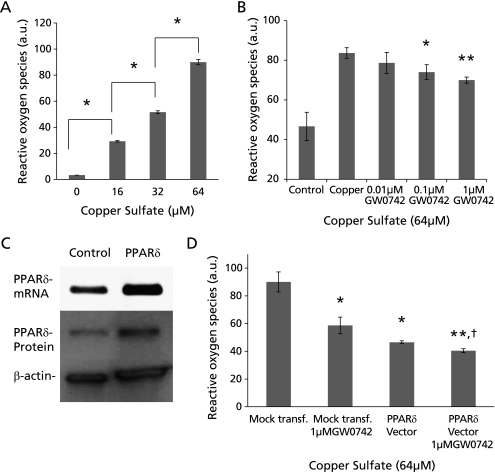
HepG2 cells were treated with copper sulfate and GW0742 to evaluate ROS production. First we determined that copper, at concentrations of 16, 32 and 64 µM, induces ROS formation in a dose-dependent manner (Fig. 4A). ROS induced by 64 µM of copper sulfate in HepG2 cells was significant and dose dependently lower in cells also treated with 0.1 and 1 µM of GW0742 (p<0.05 and <0.01 vs copper, respectively) (Fig. 4B).
Fig. 4.
Copper-induced reactive oxygen species formation in HepG2 cells. (A) Copper at concentrations of 16, 32 and 64 µM induced ROS formation in a dose dependent manner (*p<0.0005). (B) ROS induced by 64 µM of copper were significantly lower in HepG2 cells co-treated with 0.1 and 1 µM of GW0742 (*p<0.05, **p<0.01 vs copper). (C) Transfection of HepG2 cells with our PPARδ expression vector effectively increases PPARδ mRNA and protein. (D) HepG2 cells transfected with PPARδ expression vector and exposed to 64 µM of copper had significantly less ROS than those transfected with an empty vector and exposed to 64 µM of. Cells transfected with PPARδ expression vector and treated with 1 µM GW0742 had an additive effect decreasing copper-induced ROS formation copper (*p<0.05, **p<0.01 vs mock transfection; †p<0.05 vs PPARδ vector). Error bars represent SEM and p value was assessed by Student’s t test.
To further evaluate the role of PPARβ/δ in the reduction of ROS, we transfected HepG2 cells with PPARβ/δ full length expression vector. In Fig. 4C it is possible to observe the expression of PPARβ/δ at mRNA and protein levels in control and in transfected HepG2 cells. Cells overexpressing PPARβ/δ had significantly less ROS formation than mock transfected cells (p<0.05). Furthermore, cells over expressing PPARβ/δ and treated with GW0742 had an enhanced reduction of ROS when compared with those only transfected with PPARβ/δ expression vector (p<0.05) and those mock transfected (p<0.01) (Fig. 4D).
PPARβ/δ reduces copper-induced apoptosis
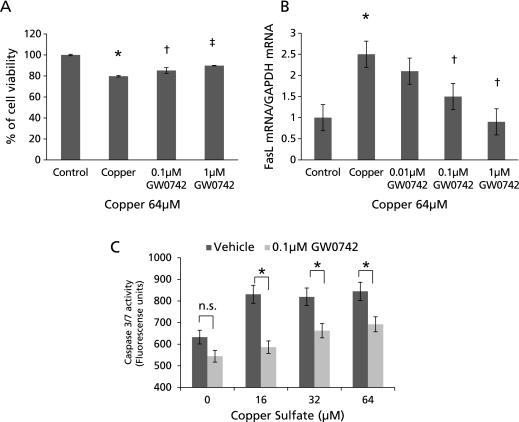
The effect of GW0742 and copper sulfate on HepG2 cells was assessed using the MTS assay for chemosensitivity and cytotoxicity. First, we determined that 24 h of GW0742 at concentrations ranging from 0.1 µM to 10 µM did not induce changes in HepG2 viability (data not shown). Next, cells were treated with 64 µM of copper and 0.1–1 µM GW0742 for 24 h. Copper alone reduced cell viability by 20% (p<0.00005), reduction that was significantly and dose-dependently lower in cells co-treated with 0.1 and 1 µM of GW0742 (p<0.005, p<0.0001, respectively) (Fig. 5A). To further analyze the effect of copper and PPARβ/δ agonism on cell viability, the apoptosis inducer FasL mRNA was assessed by QPCR. Under the same conditions mentioned above, copper alone upregulated FasL expression in HepG2 cells by 2.5 fold (p<0.05); co-treatment with 0.01, 0.1 and 1 µM GW0742 reduced FasL upregulation to 2.1, 1.5 (p<0.05) and 0.91 (p<0.05) fold respectively (Fig. 5B).
Fig. 5.
Apoptosis in HepG2 cells treated with copper and PPARβ/δ agonist. (A) Copper at concentrations of 64 µM significantly reduced HepG2 cells viability, reduction that was significantly lower in cells co-treated with 0.1 and 1 µM of GW0742. (*p<0.00005 vs control; †p<0.005, ‡p<0.0001 vs copper) (B) Fas ligand (FasL) mRNA expression significantly increased to 2.5 fold in cells treated with copper, expression that was reduced dose dependently when cells were co-treated with GW0742. (*p<0.05 vs control; †p<0.05 vs copper) (C) Caspase 3/7 activity also increased significantly in cells treated with different concentrations of copper, activation that was significantly lower in cells co-treated with 0.1 µM of GW0742. (*p<0.05 vs control) Error bars represent SEM and p value was assessed by Student’s t test.
Downstream in the apoptosis pathway, caspase 3/7 activity was significantly upregulated by 16, 34 and 64 µM copper. Copper-induced caspase 3/7 activity was significantly reduced in cells also treated with 0.1 µM of GW0742 (p<0.05). This reduction was observed even in cells that were not challenged with copper (Fig. 5C).
Discussion
In this study, PPARβ/δ ligand activation protected BALB/c mice from liver damage induced by copper while the use of a novel PPARβ/δ antagonist aggravated the outcome of mice challenged with copper. Liver damage and failure induced by copper is rare and presents mainly as WND, which is a slow progressive accumulation of copper that affects the liver, brain, kidneys and in rare cases presents as acute liver failure with hemolysis.(5,16) Even though WND is a condition that, if treated appropriately, confers the patient a quite normal life style and survival there is no therapy with proven benefit to treat patients that present with acute liver failure and hemolysis. However, in isolated cases, plasmapheresis plus copper chelants have successfully been used as a bridge to transplantation.(5) Some reports describe survival of independent cases where antioxidants like vitamin A and E have been used as ROS scavengers against copper and CCl4, but these are usually case reports that lack of further research or long term effects.(17,18)
A study in rats by Toyokuni et al.(15) caught our attention as 9 mg/kg/day in the form of copper nitriloacetate induced hepatomegaly, hemoglobinuria and jaundice in Wistar rats within 12 h and death within 72 h. This condition of hipercupremia and its consequent liver damage, hemolysis and death within a short time could be used to simulate an important part of the physiopathology of fulminant WND. Furthermore, copper distribution and histological changes achieved with long term administration of copper nitriloacetate were comparable to those observed in WND.(15) We reproduced their findings as mice died within 96 h from the initiation of copper therapy and significant weight loss was observed in the first 4 days. The short duration of our study and the lack of chronic copper storage and inflammation resulted in a non-florid histology; as well, it must be considered that hemolysis played a key role in death of rats in the previously mentioned study, condition that is likely to be reproduced in our study. However, the type and severity of changes in the group that received copper alone and copper plus GSK0660 resemble those seen in WND patients(19) and help us understand the protective role of PPARβ/δ activation in copper-challenged mice.
The PPARβ/δ antagonist GSK0660 was recently described(20) and reports using this antagonist have just started to emerge; therefore, reports of in vivo use are very few and no description of its specificity at this level has been reported. GSK0660 is known for lacking bioavailability, therefore, first we had to develop a pilot study to determine an adequate dosage of GSK0660 for our main study. Doses as high as 10 mg/kg/day were safe for mice if used alone, but doses higher than the ones used in this study plus copper resulted in severe illness and death of the mice well before the end point.
Mice treated with the PPARβ/δ antagonist alone showed a significant upregulation of 8-OHdG and TNFα in liver, what translates as an increase in liver injury probably due to the reduced protection against ROS. It is important to recall that TNFα and oxidative stress can induce each other,(21) what could result in vicious circle of injury (Fig. 6). Moreover, the steep increase of TNFα and MIP-2 in mice challenged with copper and GSK0660 and the opposite effect observed in mice challenged with copper and GW0742, show the protective role of PPARβ/δ.
Fig. 6.
In this study, we show that copper induces ROS, TNFα and MIP-2 in liver of BALB/c mice and ROS and FasL in HepG2 cells. These effects were reverted by the administration of PPARβ/δ agonist and enhanced by the administration of a PPARβ/δ antagonist. PPARβ/δ’s protective role could be used in clinical settings of liver damage as agonists of this receptor are already being tried in humans.
Copper’s toxicity appears to be via ROS formation and the oxidative stress explained by the Harber-Weiss reaction.(19,21,22) Our results showing that HepG2 cells treated with concentrations of copper comparable to those found in WND patients go through important oxidative stress that eventually induces apoptosis, coincides with reports from other researchers.(21,22) We found that PPARβ/δ activation reduced copper-induced ROS and apoptosis in the same way it does in cardiomyoblasts exposed to hydrogen peroxide(12) or in the liver of mice with iron overload.(23) Therefore, we believe that PPARβ/δ activation reduces copper-induced FasL and caspase 3/7 activity to induce a recovery in cell viability in vitro. Transfection of PPARβ/δ expression vector by itself reduced ROS formation, an effect that was additive when transfected hepatoma cells were treated with GW0742. This indicates that PPARβ/δ has a role in reduction of ROS and apoptosis.
PPARβ/δ is also known to inhibit reactive phase and pro-inflammatory cytokines, apoptosis and have an important role in liver metabolism.(9,24) These roles have been proven once again under the particularity of copper-induced toxicosis (Fig. 6). In this study we show that PPARβ/δ agonism reduces oxidative stress in vitro and in vivo; moreover, reduction of oxidative stress-induced DNA damage in liver tissue could be the reason of reduction of apoptosis and recovery of cell viability in vitro. Since our findings are comparable to that in which PPARβ/δ protected from CCl4 induced hepatotoxicity, PPARβ/δ agonists have the potential of becoming an important tool in the management of some patients with acute onset of WND and probably other causes of liver damage and failure.(11,25) Furthermore, another PPARβ/δ agonist named GW501516 is already being used in clinical trials with favorable outcome;(14) however, more studies in humans are necessary to assess the safety of PPARβ/δ agonism in the long term. In the meanwhile, the evaluation of PPARβ/δ agonist in animal models of WND, like ATP7B−/− mice or Long-Evans-Cinnamon rats, could give us a clearer idea of the effect of PPARβ/δ agonism in WND-like settings. Studies with large numbers of animals in order to have identifiable cases of fulminant hepatitis and the waiting-time to reproduce chronic copper storage in tissue represent an important challenge to the researchers.
In conclusion, the present study demonstrates that PPARβ/δ activation protects mice from liver damage induced by copper through reduction of TNFα, MIP-2 and oxidative stress-induced DNA damage. The high expression of PPARβ/δ receptors in liver, PPARβ/δ’s anti-inflammatory and anti-oxidative stress activity and the fact that a PPARβ/δ agonist is already being used in humans,(14) lead us to believe that the use of PPARβ/δ agonists could become an important tool in the management of copper-induced liver damage and probably other causes of liver damage and failure where few therapeutic options are available. It is, of course, mandatory to further study the safety of the agonist in humans and its effect in specific models of liver damage and failure.
Acknowledgments
The authors want to thank Ms. Keiko Masuzaki for the valuable technical help with histology preparations.
Abbreviations
- MIP-2
macrophage inflammatory protein 2
- 8-OHdG
8-hydroxy-2'-deoxyguanosine
- PPAR
Peroxisome Proliferator Activated Receptor
- ROS
reactive oxygen species
- TNFα
tumor necrosis factor alpha
- WND
Wilson’s Disease
References
- 1.Scheinberg IH, Sternlieb I. Wilson disease and idiopathic copper toxicosis. Am J Clin Nutr. 1996;63:842S–845S. doi: 10.1093/ajcn/63.5.842. [DOI] [PubMed] [Google Scholar]
- 2.Pankit AN, Bhave SA. Copper metabolic defects and liver disease: environmental aspects. J Gastroenterol Hepatol. 2002;17:S403–S407. doi: 10.1046/j.1440-1746.17.s3.35.x. [DOI] [PubMed] [Google Scholar]
- 3.Nagasaka H, Inoue I, Inui A, et al. Relationship between oxidative stress and antioxidant systems in the liver of patients with Wilson disease: hepatic manifestation in Wilson disease as a consequence of augmented oxidative stress. Pediatr Res. 2006;60:472–477. doi: 10.1203/01.pdr.0000238341.12229.d3. [DOI] [PubMed] [Google Scholar]
- 4.Strand S, Hofmann WJ, Grambihler A, et al. Hepatic failure and liver cell damage in acute Wilson's disease involve CD95 (APO-1/Fas) mediated apoptosis. Nat Med. 1998;4:588–593. doi: 10.1038/nm0598-588. [DOI] [PubMed] [Google Scholar]
- 5.Craig DG, Lee A, Hayes PC, et al. Review article: the current management of acute liver failure. Aliment Pharmacol Ther. 2010;31:345–358. doi: 10.1111/j.1365-2036.2009.04175.x. [DOI] [PubMed] [Google Scholar]
- 6.Escher P, Braissant O, Basu-Modak S, Michalik L, Wahli W, Desvergne B. Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology. 2001;142:4195–4202. doi: 10.1210/endo.142.10.8458. [DOI] [PubMed] [Google Scholar]
- 7.Girroir EE, Hollingshead HE, He P, Zhu B, Perdew GH, Peters JM. Quantitative expression patterns of peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) protein in mice. Biochem Biophys Res Commun. 2008;371:456–461. doi: 10.1016/j.bbrc.2008.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters JM, Gonzalez FJ. Sorting out the functional role(s) of peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) in cell proliferation and cancer. Biochim Biophys Acta. 2009;1796:230–241. doi: 10.1016/j.bbcan.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters JM, Hollingshead HE, Gonzalez FJ. Role of peroxisome-proliferator-activated receptor beta/delta (PPARbeta/delta) in gastrointestinal tract function and disease. Clin Sci (Lond) 2008;115:107–127. doi: 10.1042/CS20080022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee CH, Olson P, Hevener A, et al. PPARdelta regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci USA. 2006;103:3444–3449. doi: 10.1073/pnas.0511253103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shan W, Nicol CJ, Ito S, et al. Peroxisome proliferator-activated receptor-beta/delta protects against chemically induced liver toxicity in mice. Hepatology. 2008;47:225–235. doi: 10.1002/hep.21925. [DOI] [PubMed] [Google Scholar]
- 12.Pesant M, Sueur S, Dutartre P, et al. Peroxisome proliferator-activated receptor delta (PPARdelta) activation protects H9c2 cardiomyoblasts from oxidative stress-induced apoptosis. Cardiovasc Res. 2006;69:440–449. doi: 10.1016/j.cardiores.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Jiang B, Liang P, Zhang B, Huang X, Xiao X. Enhancement of PPAR-beta activity by repetitive low-grade H(2)O(2) stress protects human umbilical vein endothelial cells from subsequent oxidative stress-induced apoptosis. Free Radic Biol Med. 2009;46:555–563. doi: 10.1016/j.freeradbiomed.2008.10.051. [DOI] [PubMed] [Google Scholar]
- 14.Risérus U, Sprecher D, Johnson T, et al. Activation of peroxisome proliferator-activated receptor (PPAR)delta promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes. 2008;57:332–339. doi: 10.2337/db07-1318. [DOI] [PubMed] [Google Scholar]
- 15.Toyokuni S, Okada S, Hamazaki S, Fujioka M, Li JL, Midorikawa O. Cirrhosis of the liver induced by cupric nitriloacetate in Wistar rats. An experimental model of copper toxicosis. Am J Pathol. 1989;134:1263–1274. [PMC free article] [PubMed] [Google Scholar]
- 16.El-Youssef M. Wilson disease. Mayo Clin Proc. 2003;78:1126–1136. doi: 10.4065/78.9.1126. [DOI] [PubMed] [Google Scholar]
- 17.Yachi R, Igarashi O, Kiyose C. Protective effects of vitamin E analogs against carbon tetrachloride-induced fatty liver in rats. J Clin Biochem Nutr. 2010;47:148–154. doi: 10.3164/jcbn.10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sokol RJ, Twedt D, McKim JM, Jr., et al. Oxidant injury to hepatic mitochondria in patients with Wilson’s disease and Bedlington terriers with copper toxicosis. Gastroenterology. 1994;107:1788–1798. doi: 10.1016/0016-5085(94)90822-2. [DOI] [PubMed] [Google Scholar]
- 19.Langner C, Denk H. Wilson disease. Virchows Arch. 2004;445:111–118. doi: 10.1007/s00428-004-1047-8. [DOI] [PubMed] [Google Scholar]
- 20.Shearer BG, Steger DJ, Way JM, et al. Identification and characterization of a selective peroxisome proliferator-activated receptor beta/delta (NR1C2) antagonist. Mol Endocrinol. 2008;22:523–529. doi: 10.1210/me.2007-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song MO, Li J, Freedman JH. Physiological and toxicological transcriptome changes in HepG2 cells exposed to copper. Physiol Genomics. 2009;38:386–401. doi: 10.1152/physiolgenomics.00083.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aston NS, Watt N, Morton IE, Tanner MS, Evans GS. Copper toxicity affects proliferation and viability of human hepatoma cells (HepG2 line) Hum Exp Toxicol. 2000;19:367–376. doi: 10.1191/096032700678815963. [DOI] [PubMed] [Google Scholar]
- 23.Asare GA, Kew MC, Mossada KS, Paterson AC, Siziba K, Kahler-Venter CP. Effects of exogenous antioxidants on dietary iron overload. J Clin Biochem Nutr. 2009;44:85–94. doi: 10.3164/jcbn.08-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bishop-Bailey D, Bystrom J. Emerging roles of peroxisome proliferator-activated receptor-beta/delta in inflammation. Pharmacol Ther. 2009;124:141–150. doi: 10.1016/j.pharmthera.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Shan W, Palkar PS, Murray IA, et al. Ligand activation of peroxisome proliferator-activated receptor β/δ (PPAR β/δ) attenuates carbon tetrachloride hepatotoxicity by downregulating proinflammatory gene expression. Toxicol Sci. 2008;105:418–428. doi: 10.1093/toxsci/kfn142. [DOI] [PMC free article] [PubMed] [Google Scholar]