Abstract
Background
Uterine Serous Papillary Carcinoma (USPC), is an aggressive and chemotherapy resistant variant of endometrial cancer. We evaluated the expression of human –trophoblast-cell-surface-marker (Trop-2) and the potential of hRS7, a humanized anti-Trop-2 monoclonal antibody, as a novel therapeutic strategy against USPC.
Methods
Trop-2 expression was evaluated by immunohistochemistry (IHC) in a total of 23 USPC. Six primary USPC cell lines were assessed by flow cytometry and real-time-PCR for Trop-2 expression. Sensitivity to hRS7 (Immunomedics, Inc.) antibody-dependent-cellular-cytotoxicity (ADCC) and complement-dependent-cytotoxicity (CDC) was tested in standard 5-hrs-51Cr-release-assays against primary USPC cell lines.
Results
Expression of Trop-2 was found in 15 out of 23 (65%) of the tumor tissues tested by IHC and in 50% (3/6) of the USPC cell lines tested by real-time-PCR and flow-cytometry [Trop-2 expression in USPC versus normal-endometrial-cells (NEC)(p < 0.005)]. USPC cell lines overexpressing Trop-2, regardless of their intrinsic resistance to natural killer cytotoxicity, were highly sensitive to hRS7-mediated ADCC in vitro (range of killing 28.2% to 64.4%) (p< 0.001). Negligible cytotoxicity against USPC was seen in the absence of hRS7 or in the presence of Rituximab control-antibody (range of killing 1.1% to 12.4%). Incubation with interleukin-2 (50 IU/ml) in addition to hRS7 further increased the cytotoxic activity against USPC cell lines overexpressing Trop-2 (p= 0.008).
Conclusion
Trop-2 is highly expressed in uterine serous carcinoma at mRNA and protein levels. Primary USPC cell lines are highly sensitivity to hRS7-mediated-cytotoxicity in vitro. hRS7 may represent a novel therapeutic agent for USPC refractory to standard treatment modalities.
Keywords: Endometrial neoplasms, Uterine Serous Papillary Carcinoma, Trop-2, trophoblast cell-surface marker, hRS7, antibody dependent cellular cytotoxicity
INTRODUCTION
Cancer of the uterine corpus is the most prevalent gynecologic tumor in women, with an estimated 43,470 cases and 7,950 deaths in the United States in 2010 (1). Uterine serous papillary carcinoma (USPC) is an aggressive variant of endometrial cancer (2). Although it accounts for less than 10% of all endometrial tumors, it causes up to 39% of all endometrial cancer-related deaths (3-6). USPC, referred to as a Type II endometrial cancer, is characterized by a high-grade, complex histology and carries a poor prognosis as it is often spread beyond the uterine corpus at the time of diagnosis (2-6). Type I and II endometrial cancers appear to have a different pattern of molecular alterations that underlie pathogenesis and progression. While endometrioid carcinomas tend to have alterations in the tumor suppressor gene, PTEN, these are uncommon in USPC, with p53 mutations and HER2/neu expression occurring more commonly in this tumor subtype (7-9). USPC has a tendency toward early invasion of the lymphatic and vascular spaces and lymph nodes and to microscopically involve other intraperitoneal structures, despite minimal or no invasion present within the uterus. These tumor characteristics lead to high recurrence rates and a poor prognosis for USPC patients (2-9).
Large-scale gene expression analysis, using techniques such as high-density oligonucleotide and cDNA microarrays, represents a powerful new tool to identify genes involved in endometrial carcinogenesis. Using this technology, our group has recently identified the intronless gene encoding for Trophoblast cell-surface marker (Trop-2, also termed TACSTD2, GA733-1, M1S1, EGP-1) as one of the top differentially expressed genes in USPC when compared to normal human endometrial cells (NEC) (10). Trop-2 is a surface glycoprotein originally identified in human placental trophoblast (11) and subsequently reported to be highly expressed by various types of human carcinomas, but rarely in normal adult tissues (11-15). Although the biological role of Trop-2 is still unclear, its overexpression has been found to correlate with invasive behavior and poor prognosis in various types of human carcinomas (16-19). Consistent with this view, our group has recently reported Trop-2 as an independent marker for poor overall survival in ovarian carcinoma patients (20). Importantly, its overexpression by epithelial tumor cells and its transmembrane localization render Trop-2 an attractive target for cancer immunotherapy. hRS7 is a humanized IgG1 monoclonal antibody developed against Trop-2 using complementary-determining-region (CDR) and transfection techniques of the murine RS7-3G11 antibody (Immunomedics, Inc., Morris Plains, NJ, USA) (21-23). Since RS7-3G11 has been shown to rapidly internalize into target cells (21-23), hRS7 has been initially tested labeled with 131I-IMP-R4 to evaluate its effectiveness in preclinical radioimmunotherapy (RAIT) studies on breast cancer xenograft models (23). In these studies, nude mice bearing subcutaneous MDA-MB-468 human breast cancer were treated with a single administration of 131I-IMP-R4-hRS7. MTVs (mean tumor volumes) 8 weeks post-treatment were 20% and 280% of the starting MTVs in 131I-IMP-R4-hRS7-treated and untreated control groups, respectively. Moreover, complete remission was reported in 5 out of 11 mice treated with 131I-IMP-R4-hRS7 (23). Although these results suggest a promising use of hRS7 as a carrier for radiometabolic therapy after labeling with suitable radionuclides, to our knowledge, the ability of hRS7 in inducing antibody dependent cellular cytotoxicity (ADCC) against primary human USPC cell lines has not been previously studied.
To fill this gap in knowledge, in this study we investigated the expression of Trop-2 in multiple USPC specimens and evaluated the in vitro potential of hRS7 as a novel immunotherapeutic agent against biologically aggressive and chemotherapy resistant USPC cell lines overexpressing Trop-2.
Methods
Trop-2 immunostaining of formalin-fixed tumor tissues
A total of 23 USPC specimens (18 primary and 5 metastatic obtained from uterine serous tumors with single cell differentiation, i.e., pure USPC), and 5 normal endometrium control tissues obtained from similar age women were evaluated by standard immunohistochemical staining (IHC) on formalin-fixed tumor tissue for Trop-2 surface expression. USPC specimens were derived from patients harboring Stage I disease (5 patients), stage II (2 patients), stage III (7 patients) and stage IV (9 patients). Briefly, IHC stains were performed on 4-μm-thick sections of formalin-fixed, paraffin-embedded tissue as previously described (20). The purified goat polyclonal antibody against the recombinant human Trop-2 extracellular domain (R&D Systems, Inc., Minneapolis, MN; diluted 1:100) was applied for 1 hour. A secondary biotinylated anti-goat antibody (Vector Laboratories, Burlingame, CA; diluted 1:250) and the streptavidin-biotin complex (StreptABComplex/HRP, Dako, CA, USA) were applied, then 3′3-diaminobenzidine (Dako, CA, USA) was used as chromogen and the sections were counterstained by hematoxylin (Dako). Cases with less than 10% membranous staining in tumor cells were considered negative for Trop-2 expression. The intensity of membranous immunoreactivity for Trop-2 in tumor cells was subjectively scored as follow: (a) 0, negative; (b) 1+, weak membrane staining; (c) 2+, medium staining; and (d) 3+, strong membrane staining. Appropriate negative and positive controls were performed with each case. Trop-2 immunoreactivity scores were performed blindly by two pathologists. The inter-observer variability in sample scoring was less than 10%.
Establishment of USPC Cell Lines
Primary USPC tumor cell lines from 6 patients with invasive USPC were obtained from fresh tumor biopsies collected at the time of surgery, under approval of the Institutional Review Board. Tumors were staged according to the International Federation of Gynecologists and Obstetricians 1988 operative staging system. Six primary USPC cell lines (USPC ARK-1, USPC ARK-2, USPC ARK-3, USPC ARK-4, USPC ARK-5, and USPC ARK-6) were established after sterile processing of the tumor samples from surgical biopsies as described previously (24). Source-patient characteristics of these 6 USPC cell lines are described in Table 1. The amplification of the c-erbB2 gene by FISH, expression levels of HER-2/neu receptor by immunohistochemistry (IHC) and mRNA expression levels by quantitative RT-PCR for these primary USPC cell lines have been recently reported (24).
Table 1.
Patient characteristics from which the six USPC cell lines were established
| Patient | Age (years) |
Race* | FIGO^ Stage |
USPC Histopathology |
Year of Diagnosis |
|---|---|---|---|---|---|
| USPC ARK-1 | 62 | AA | IVA | Pure | 1997 |
| USPC ARK-2 | 63 | AA | IVB | Pure | 1998 |
| USPC ARK-3 | 59 | AA | IVB | Mixed | 2006 |
| USPC ARK-4 | 73 | C | IVB | Pure | 2004 |
| USPC ARK-5 | 73 | AA | IIIC | Pure | 2006 |
| USPC ARK-6 | 62 | C | IB | Mixed | 2005 |
AA, African American; C, Caucasian;
FIGO, International Federation of Gynecology and Obstetrics
Quantitative real-time polymerase chain reaction
RNA isolation from all the 6 primary USPC cell lines used in the cytotoxicity experiments and normal endometrium control tissues obtained from the same healthy donors used for the IHC staining experiments were performed using TRIzol Reagent (Invitrogen) according to the manufacturer's instructions as previously described (20). Since Trop-2 is an intronless gene, all RNA samples were treated with TURBO DNase enzyme (TURBO DNA-free Kit; Ambion, Inc., Applied Biosystem Business, CA) to remove the contaminating DNA eventually present. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed in duplicate by using a primer set and probe specific for Trop-2 (i.e., Trop2-EX56, forward: CGCCTTGGGTTTAAATTATTTGATGAGT; reverse: GCTACTACATAGGCCCAGTTAACAA). The endogenous control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Assay on Demand Hs99999905_m1 (Applied Biosystems, Foster City, CA, USA) was used to normalize variations in cDNA quantities from different samples. The comparative threshold cycle (CT) method was used for the calculation of amplification fold as specified by the manufacturer.
Flow cytometry
The humanized anti-Trop-2 MAb hRS7 (Immunomedics, Inc., Morris Plains, NJ, USA) was used for flow cytometry studies. Briefly, 6 primary USPC cell lines obtained from the above described patients were stained with 2 μg/ml of hRS7. 2.5 μg/ml of the chimeric anti-CD20 MAb Rituximab (Rituxan, Genentech, San Francisco, CA, USA) was used as a negative control. A goat anti-human F(ab′)2 immunoglobulin (BioSource International, Camarillo, CA, USA) was used as a secondary reagent. Analysis was conducted with a FACScan, using Cell Quest software (Beckton Dickinson, Franklin Lakes, NJ, USA).
Tests for ADCC
A standard five-hours chromium (51Cr) release assay was performed to measure the cytotoxic reactivity of Ficoll-Paque™ PLUS (GE Healthcare, Uppsala, Sweden) separated peripheral blood lymphocytes (PBL) obtained from several healthy donors against all 6 USPC cell lines. The release of 51Cr from the target cells was measured as evidence of tumor cell lysis after exposure of tumor cells to 2 μg/ml of hRS7. Controls included the incubation of target cells alone or with PBL or mAb separately. The chimeric anti-CD20 MAb Rituximab was used as a negative control for hRS7 in all bioassays. ADCC was calculated as the percentage of killing of target cells observed with hRS7 plus effector cells compared with 51Cr release from target cells incubated alone.
Interleukin-2 enhancement of ADCC
To investigate the effect of interleukin-2 (IL-2) on hRS7-mediated ADCC, effector PBL were incubated for 5 hours at 37°C at a final concentration of IL-2 (Aldesleukin; Chiron Therapeutics, Emeryville, CA, USA) ranging from 50-100 IU/ml in 96-well microtiter plates. Target cells were primary USPC cell lines exposed to 2 μg/ml of hRS7, whereas controls included the incubation of target cells alone or with PBL in the presence or absence of IL-2 or mAb, respectively. Rituximab was used as a control mAb. ADCC was calculated as the percentage of killing of target cells observed with mAb plus effector PBL, as compared with target cells incubated alone. Each experiment was performed with PBL obtained from at least 2 healthy donors.
Test for complement-mediated target cell lysis and γ-globulin inhibition
A standard 5-hours chromium (51Cr) release assay identical to those performed for ADCC assays was used, except that human serum in a dilution of 1:2 was added in place of the effector cells. This human serum was used as a source of complement to test for complement-mediated target cell lysis. To evaluate the eventual inhibition of ADCC against USPC cell lines by physiological human serum concentrations of γ-globulin, human serum diluted 1:2 was added in the presence or absence of effector PBL. In some experiments, heat-inactivated human serum (56°C for 60 minutes) was added in the presence of effector PBL. Controls included the incubation of target cells alone or with either lymphocytes or mAb separately. Rituximab was used as a control mAb.
Statistical analysis
For qRT-PCR data, the right skewing was removed by taking copy number ratios relative to the lowest-expressing NEC (normal human endometrial cells) sample (“relative copy number”), log2 transforming them to ΔCTs, and comparing the results via unequal-variance t-test for USPC-versus-NEC. Group means with 95% confidence intervals (CIs) were calculated by computing them on the ΔCTs and then reverse-transforming the results to obtain means (with 95% CIs) of relative copy numbers. Differences in Trop-2 expression by flow cytometry were analyzed by the unpaired t-test, and a p-value of < 0.05 among samples was considered to be significant. The Wilcoxon rank-sum (WRS) test was used to compare USPC types to normal endometrium for differences in IHC staining intensities. Sample-type differences were expressed as odds ratios accompanied by 95% confidence limits. Kruskal-Wallis test and chi-square analysis were used to evaluate differences in hRS7-induced ADCC levels in primary tumor cell lines. Statistical analysis was performed using SPSS version 17 (SPSS, Chicago, IL, USA).
RESULTS
Trop-2 expression by Immunohistochemistry on USPC versus normal endometrial tissue
To determine whether the previously reported high expression of Trop-2 mRNA in gene expression profiling USPC studies (10) also result in high expression of the protein on the surface of tumor cells, we performed immunohistochemical analysis on formalin-fixed, paraffin-embedded tumor tissue from a set of 23 USPCs. As representatively shown in Figure 1, we found membranous positivity for Trop-2 in 15 out of the 23 (65%) tumor samples tested by IHC. The intensity of Trop-2 staining was significantly higher among the tumor specimens compared to normal endometrial controls (WRS p value = <0.005). In these positive samples, 3 out of the 23 specimens were found to have a low positivity (1+) for Trop-2 protein, while the remaining specimens available for IHC testing showed moderate (i.e., 2+ : 7 samples) or strong (i.e., 3 + : 5 samples) Trop-2 positivity.
Figure 1.
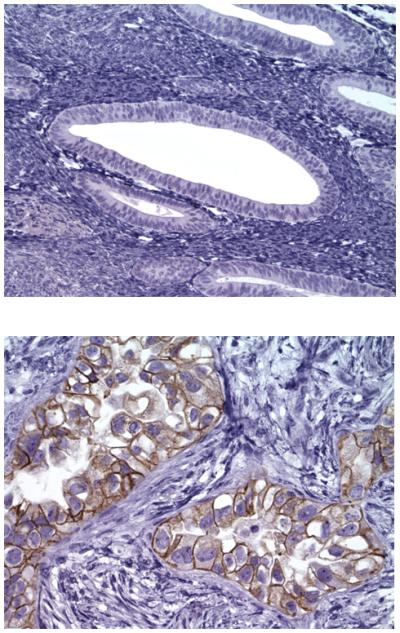
Representative IHC localization analyses of Trop-2 in USPC specimens. Upper panel: normal endometrium negative for Trop-2. Lower panel: USPC specimen showing 3+ positivity for Trop-2 expression. Original magnification: x 200.
Trop-2 transcript levels in USPC carcinomas
A total of six primary USPC lines were tested for Trop-2 expression by qRT-PCR . Table 1 shows the histopathological characteristics of the USPC patients. Among the 6 tumors tested, 3 carcinomas (i.e., USPC ARK-2, USPC ARK-3 and USPC ARK-6) showed a high mRNA copy number, ranging from 410.0 to 1628.6 (Table 2). Trop-2 expression was significantly higher in the USPC cell lines compared to the normal endometrial cells (NEC) (p = 0.005). In contrast, low Trop-2 expression by qRT-PCR was detected in the other 3 cell lines, ranging from 0.1 to 30.2 mRNA copy numbers (Table 2). The difference between Trop-2 expression in USPC cell lines with low Trop-2 expression (i.e., USPC-ARK-1, USPC-ARK-4, and USPC-ARK-5) and high Trop-2 expression (i.e., USPC ARK-2, USPC ARK-3 and USPC ARK-6) was statistically significant at p= 0.005.
Table 2.
Trop-2 mRNA and protein expression in USPC cell lines
| Sample | Flow cytometry | qRT-PCR | |
|---|---|---|---|
| MFI* ± SD | Cells (%) ± SD | m-RNA Copy Number |
|
| USPC ARK-1** | 18.7 ± 3.9 | 55.1± 3.4 | 5.1 |
| USPC ARK-2** | 234.7 ± 36.0 | 99.7 ± 0.2 | 1628.6 |
| USPC ARK-3** | 202.9 ± 62.2 | 98.2 ± 10.0 | 1581.0 |
| USPC ARK-4 | 17.9 ± 6.2 | 3.5 ± 0.3 | 0.1 |
| USPC ARK-5 | 16.2 ± 6.1 | 12.1 ± 10.5 | 30.2 |
| USPC ARK-6 | 81.0 ± 30.2 | 96.2 ± 4.5 | 410.0 |
MFI: Mean Fluorescence Intensity
USPC cell lines showing c-erbB2 gene amplification by FISH assay (24).
Trop-2 surface expression by flow cytometry in USPC primary cell lines
To determine whether the high expression of Trop-2 mRNA detected by qRT-PCR in 50% of our USPC cell lines also resulted in high expression of the protein on the surface of tumor cells, we performed flow cytometry on all primary tumors. USPC ARK-2, USPC ARK-3 and USPC ARK-6 cell lines showed once again high Trop-2 surface expression by flow cytometry (Figure 2; Table 2). Trop-2 surface expression results from flow cytometric analysis were found to be in good agreement with Trop-2 expression results found by qRT-PCR in all six primary USPC cell lines. The difference in Trop-2 surface protein expression tested by flow cytometry between the cell lines with low and high Trop-2 expression was statistically significant (p< 0.01).
Figure 2.
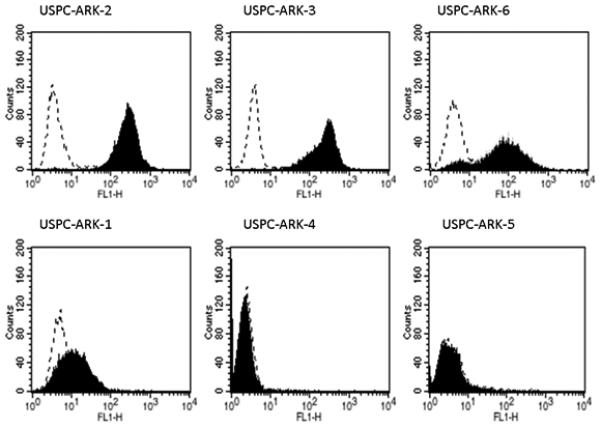
Flow cytometry histograms of primary USPC cell lines showing high (USPC ARK-2, USPC ARK-3 and USPC ARK-6), and low (USPC ARK-1, USPC ARK-4 and USPC ARK-5) expression of Trop-2. Rituximab (dashed line); hRS7 (solid black).
USPC cell lines are highly resistant to NK cell activity but sensitive to hRS7-mediated ADCC
Six primary USPC cell lines were evaluated for their sensitivity to natural killer cells (NK). These cell lines were exposed to PBL collected from multiple healthy donors and the cytotoxicity was measured using a standard 5-hrs 51Cr release assay. Using dose titration experiments with different doses of hRS7, killing of the USPC cells was found to plateau at a hRS7 concentration of 2 μg/ml (data not shown). Thus, this dose was used in all following experiments. USPC cell lines were found highly resistant to NK-mediated killing with exposure to PBL as effectors to target at 2 ratios (E/T; 25:1 and 50:1) (mean killing 5.8% ± 3.5 SD) (Table 3). In contrast, significant killing was demonstrated against high Trop-2 expressing cell lines after incubation with hRS7 to mediate ADCC (range of killing 28.2-64.4%, mean 46.3%; p< 0.001) (Figure 3, upper and middle panel, Table 3), while low Trop-2 expressing cell lines were resistant to hRS7 (Figure 3, lower panel, Table 3). As expected by qRT-PCR and flow cytometry results of Trop-2 expression, high Trop-2 expressing cell lines were killed at a higher level by hRS7 than low Trop-2 expressing cell lines (Table 3, p<0.001). All cell lines were resistant to incubation with Rituximab (2.5 μg/ml) control antibody in the presence of PBL (Table 3).
Table 3.
hRS7-dependent cytotoxicity results in USPC cell lines
| Cell line | Control ± SD | Rituximab ± SD | hRS7 ± SD | p-value* | |
|---|---|---|---|---|---|
| USPC ARK-1 | 6.3 ± 1.0 | 5.9 ± 2.9 | 7.6 ± 3.9 | ||
| USPC ARK-2 | 3.4 ± 1.6 | 3.5 ± 1.0 | 49.5 ± 7.0 | ||
| USPC ARK-3 | 2.5 ± 1.1 | 2.8 ± 1.5 | 52.2 ± 9.7 | ||
| USPC ARK-4 | 8.2 ± 2.6 | 10.5 ± 0.1 | 8.6 ± 4.0 | ||
| USPC ARK-5 | 3.1 ± 2.5 | 6.5 ± 2.1 | 6.5 ± 3.2 | ||
| USPC ARK-6 | 11.3 ± 1.2 | 8.9 ± 1.9 | 32.4 ± 4.4 | ||
| Average | 5.8 ± 3.5 | 6.3 ± 3.0 | ο 46.3 ± 11.0 | a 7.6 ± 3.3 | < 0.001 |
Cytotoxicity results in Trop-2-positive cell lines versus controls
Average of 16 experiments in Trop-2-positive cell lines
Average of 9 experiments in Trop-2-negative cell lines
Figure 3.
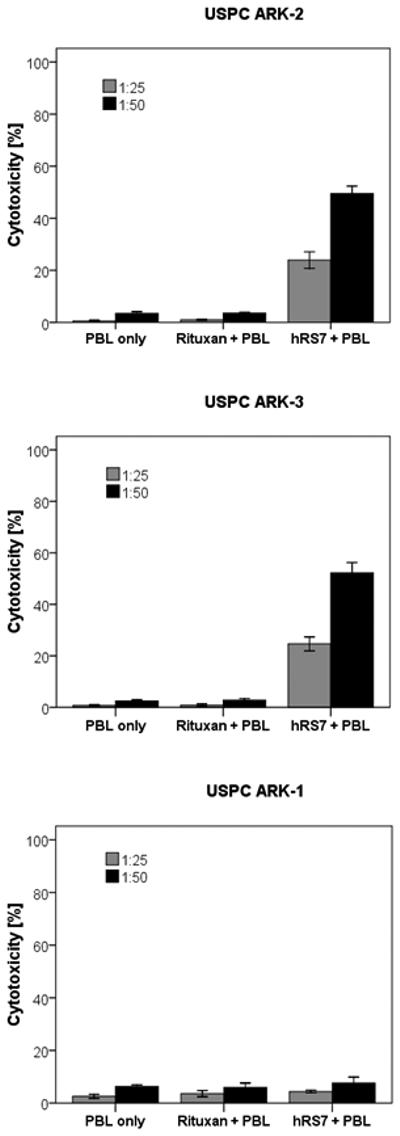
Representative cytotoxicity experiments (Effector to target: E/T; 25:1 and 50:1) using hRS7 against high Trop-2 expressing [i.e., USPC ARK-2 (upper panel), USPC ARK-3 (middle panel)] and low Trop-2 expressing USPC cell lines [i.e., USPC ARK-1 (lower panel)]. High levels of hRS7 induced cytotoxicity were evident against USPC ARK-2 and USPC ARK-3 primary cell lines expressing high levels of Trop-2. In contrast, negligible cytotoxicity was detectable against USPC ARK-1 (i.e., a low Trop-2 expressor cell line). In all cell lines tested, no significant cytotoxicity was detected in the absence of hRS7 or in the presence of Rituximab control mAb.
IL-2 enhancement of ADCC against USPC
To investigate the effect of interleukin-2 (IL-2) in combination with hRS7 (2 μg/ml) on ADCC against USPC cell lines overexpressing Trop-2, PBL from healthy donors were incubated with 50-100 IU/ml of IL-2 for 5 hours. As representatively shown in Figure 4, hRS7-mediated ADCC was significantly increased in the presence of IL-2 in all primary cell lines tested (p= 0.008). While the stimulation of PBL with IL-2 leads to a significantly higher ADCC in the presence of hRS7, it did not significantly increase tumor killing in the absence of hRS7 or in the presence of Rituximab control antibody (Figure 4).
Figure 4.
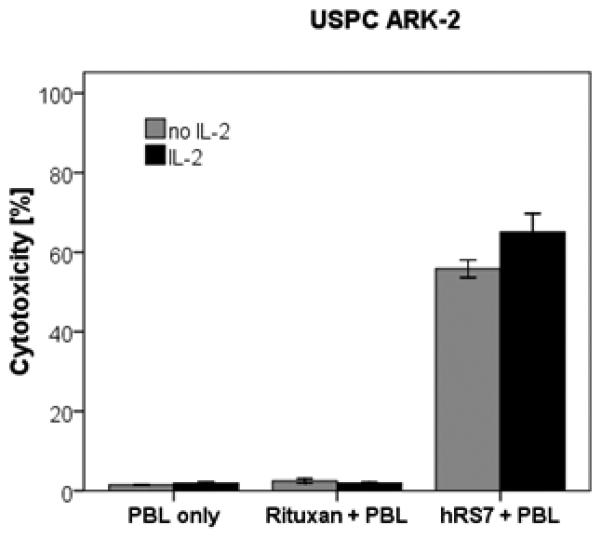
Representative effect of low doses of interleukin-2 (IL-2) in combination with hRS7 (2 μg/ml) on ADCC against USPC ARK-2 primary cell line (Effectors to target ratio 50 : 1). PBL from healthy donors were incubated for 5 hours in the presence of 100 IU/ml of IL-2. hRS7-mediated ADCC was significantly increased in the presence of low doses of IL-2. No significant increase in cytotoxicity was detected after 5 hours IL-2 treatment in the absence of hRS7 or in the presence of the Rituximab isotype control mAb.
Effect of complement and physiological concentrations of IgG on hRS7-mediated ADCC
In order to evaluate the effect of complement on the hRS7-mediated ADCC as well as its potential inhibition by physiological IgG serum concentrations, human serum diluted 1 : 2 (with and without heat inactivation) was added to high Trop-2 expressing USPC cell lines (i.e., USPC ARK-2, USPC ARK-3 and USPC ARK-6) during standard 5-hrs 51Cr release assays in the presence of PBL. As representatively demonstrated in Figure 5 for USPC ARK-2, in two primary cell lines (i.e., USPC ARK-2 and USPC ARK-6), no significant decrease in killing after incubation with serum compared to incubation without serum was noted. In contrast, in USPC ARK-3 cell line, the addition of serum led to a significant increase in killing (p=0.02) (Figure 5). These results are consistent with a higher sensitivity of USPC ARK-3 cell line to complement in vitro when compared to USPC ARK-2 and USPC ARK-6.
Figure 5.
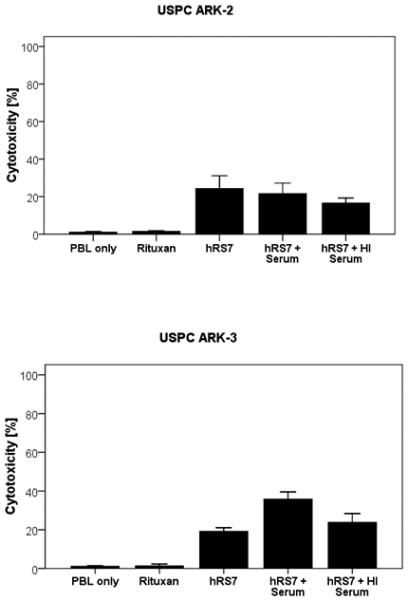
Representative cytotoxicity experiments adding human serum (diluted 1:2) to hRS7 against USPC ARK-2 and USPC ARK-3 cell lines. USPC cell lines were treated with serum (with or without heat inactivation) in the presence or absence of the effector cells and hRS7 in a standard 5-hours 51Cr release assays. Incubation with physiological concentrations of IgG (i.e., heat-inactivated serum diluted 1:2) to PBL in the presence of hRS7 did not significantly reduce the degree of ADCC achieved in the presence of hRS7 against USPC ARK-2 (Upper Panel). Addition of untreated serum (diluted 1:2) to PBL in the presence of hRS7 consistently increased hRS7-mediated cytotoxicity against USPC-ARK-3 (p < 0.02) (lower panel).
DISCUSSION
In this study, we have investigated Trop-2 expression and localization by immunohistochemistry in normal endometrium, and compared such expression to primary and metastatic uterine serous tumors. In addition we have evaluated the in vitro potential of hRS7 MAb as a new form of therapy against biologically aggressive and chemotherapy resistant USPC cell lines overexpressing Trop-2 in vitro. Our findings demonstrate that (i) Trop-2 mRNA and protein are significantly up-regulated in USPC compared to normal endometrial tissues; (ii) three out of six freshly established tumor cell lines derived from patients harboring advanced stages USPC and experiencing progression of disease on chemotherapy expressed high levels of Trop-2 on their cell surfaces as measured by flow cytometry, and (iii) primary USPC cell lines overexpressing Trop-2 are highly susceptible to ADCC mediated by hRS7, a human mAb recently developed against Trop2-expressing cancers. Our results may therefore have important implications for the treatment of USPC patients harboring tumors resistant to chemotherapy.
Of interest, in high grade ovarian serous carcinoma, a tumor resembling USPC, we have recently reported a significant association between increased expression of Trop-2 and poor overall survival (20). Although the relationship between high Trop-2 expression and aggressiveness of epithelial neoplasms remains unclear, it has been suggested that Trop-2, possessing cytoplasmic serine and tyrosine phosphorylation sites, might function as a cell signal transducer and regulator of tumor cell growth and increase tumor cell resistance to apoptosis (25). Consistent with this view, a recent paper has evidenced that a large fraction of human cancers express a bicistronic CYCLIN D1-TROP2 mRNA chimera, acting as an oncogene able to induce aggressive tumor growth (26). Another recent study has reported that Trop-2 is necessary for tumorigenesis in colon carcinoma cell lines, showing that Trop-2 targeting with specific antibodies may result in inhibition of tumor cell migration and invasion (27). Taken together, these observations support the possibility that aberrant expression of Trop-2 may account for the enhanced invasive behavior and increased biologic aggressiveness of multiple human cancers including USPC.
Although in vivo data will ultimately be necessary to validate the potential of hRS7 against Trop-2 expressing USPC, our in vitro experimental results suggest that targeting cancer cells with high surface expression of Trop-2, may be a novel, potentially effective option to treat residual/resistant USPC after standard adjuvant chemotherapy. Consistent with this view, in this study we have tested the ability of hRS7, a human anti-Trop-2 antibody (20), for its ability to kill in vitro multiple primary USPC cell lines expressing different levels of Trop-2. In this regard, three out of six of the primary USPC cell lines available to this study were found to express significant levels of Trop-2 by flow cytometry, and this expression did not correlate with HER2/neu expression (Table 2) (24). Therefore, USPC cell lines may potentially respond to anti-Trop-2 therapy, regardless of their different sensitivity/resistance to various chemotherapy or immunotherapy (i.e. anti-HER2/neu-targeted) agents (24, 28). In agreement with our qRT-PCR and protein expression results, all high Trop-2 expressing primary USPC cell lines were highly sensitive to ADCC in the presence of hRS7. These data, therefore, demonstrate that although these tumor cells are extremely resistant to multiple clinically available chemotherapeutic agents, they remain highly sensitive to lysis by NK cells when these are engaged by the Trop-2-specific antibody hRS7.
To test the feasibility of hRS7-induced ADCC in the in vivo setting, ADCC experiments were performed in the presence of human serum as a source of high concentrations of irrelevant human IgG that could potentially block the ability of effector cells to interact with the antibody at the target site. In this study, we show that ADCC against USPC cell lines was not significantly decreased by high concentrations (up to 50%) of human serum. Indeed, in some USPC cell lines (i.e., USPC-ARK-3) an increase in cell death was detected in the presence of effector cells and non-heat inactivated human serum. These results indicate that the binding of hRS7 to the Fc receptor on mononuclear effector cells is likely enabled in the in vivo situation.
Treatment of cancer patients with combinations of MAbs and cytokines does not amount to a mere addition to the benefit of each treatment modality alone, but has been demonstrated to have synergistic potential (29,30). Recently, low doses of rIL-2 have been given by continuous infusion or subcutaneously, with remarkable immunologic results coupled with negligible toxicity (30-31). Indeed, subcutaneous IL-2 increased the absolute number of circulating NK cells by approximately 10-fold and induced an increase in the function of these lymphocytes against a series of cancer targets (29-30). This point is noteworthy because oncology patients have been noted to have suppressed ADCC responses (30). However, cytotoxicity levels in such patients can be increased in vitro to levels similar to those of normal donors by exposing effector cells to IL-2 (31). Our findings further support this view, as a significant increase in ADCC against USPC was detected after exposure of effector cells to low doses of IL-2 in vitro for a brief time (i.e., for 5 h). These data suggest that the administration of low (i.e., non toxic) doses of IL-2 in vivo may enhance the function of NK cells and may significantly increase the efficacy of hRS7 therapy in USPC patients. Furthermore, given the aggressive and highly resistant nature of USPC to currently available cytotoxic anti-cancer therapies, these combined therapies might be particularly important in the treatment of USPC patients.
In conclusion, this is the first report on Trop-2 protein expression and hRS7 antibody-dependent cellular cytotoxicity in USPC, the most aggressive and chemotherapy-resistant variant of endometrial cancer. Our study has demonstrated that Trop-2 expression is highly and consistently expressed at protein levels in 65% of primary and metastatic USPC tested by IHC, as well as at both mRNA and protein levels in 50% of primary cell lines established from patients harboring chemotherapy resistant tumors tested by qRT-PCR and flow cytometry. The high density and the membranous localization of Trop-2 on USPC cells, combined with its negative expression in mesothelial type cells in the abdominal cavity (data not shown), suggests that this protein could represent an accessible tumor target antigen for both intravenous (i.v.) and intraperitoneal (i.p.) antibody-based therapies. This holds particularly true for USPC patients where other potentially effective targeted in vivo therapies (i.e., trastuzumab) (32,33) are not indicated due to the lack of expression of its target HER2/neu on USPC cells.
Condensed Abstract.
The treatment of advanced/metastatic USPC is challenging and the options are very limited. hRS7 is a humanized monoclonal antibody targeting Trop-2. In this study we demonstrate that Trop-2 is overexpressed in USPC and that hRS7 induces a high level of antibody-dependent cellular cytotoxicity (ADCC) against multiple primary USPC cell lines resistant to chemotherapy. Trop-2 may represent an attractive novel target for the treatment of patients harboring USPC refractory to standard treatment modalities.
ACKNOWLEDGMENTS
Supported in part by NIH R01 CA122728-01A2 to AS, and grants 501/A3/3 and 0027557 from the Italian Institute of Health (ISS) to AS. This investigation was also supported by NIH Research Grant CA-16359 from the National Cancer Institute. The Authors thank Immunomedics, Inc., Morris Plains, NJ, USA for providing hRS7 Mab free of charge for our studies.
Abbreviations
- USPC
uterine serous papillary carcinoma
- Trop-2
human trophoblast-cell-surface marker
- RT-PCR
reverse transcriptase-polymerase chain reaction
- SEM
standard error of the mean
- NEC
normal endometrial controls
- ADCC
antibody dependent cellular cytotoxicity
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6:93–108. doi: 10.1097/00000478-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Creasman W, Kohler M, Odicino F, et al. Prognosis of papillary serous, clear cell, and Grade 3 Stage I carcinoma of the endometrium. Gynecol Oncol. 2004;95:593–596. doi: 10.1016/j.ygyno.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz PE. The management of serous papillary uterine cancer. Curr Opin Oncol. 2006;18(5):494–9. doi: 10.1097/01.cco.0000239890.36408.75. Schwartz PE. [DOI] [PubMed] [Google Scholar]
- 5.Carcangiu ML, Chambers JT. Uterine papillary serous carcinoma: a study on 108 cases with emphasis on prognostic significance of associated endometrioid carcinoma, absence of invasion, and concomitant ovarian cancer. Gynecol Oncol. 1992;47:298–305. doi: 10.1016/0090-8258(92)90130-b. [DOI] [PubMed] [Google Scholar]
- 6.Goff BA, Kato D, Schmidt RA, et al. Uterine papillary serous carcinoma: pattern of metastatic spread. Gynecol Oncol. 1994;54:264–8. doi: 10.1006/gyno.1994.1208. [DOI] [PubMed] [Google Scholar]
- 7.Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol. 2006;24:4783–91. doi: 10.1200/JCO.2006.06.7173. [DOI] [PubMed] [Google Scholar]
- 8.Santin AD, Bellone S, Van Stedum S, et al. Determination of HER-2/neu Status in Uterine Serous Papillary Carcinoma: Comparative Analysis of Immunohistochemistry and Fluorescence in situ Hybridization. Gynecol Oncol. 2005;98:24–30. doi: 10.1016/j.ygyno.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 9.Santin AD, Bellone S, Van Stedum S, et al. Amplification of c-erbB2 Oncogene: a Major Prognostic Indicator in Uterine Serous Papillary Carcinoma. Cancer. 2005;104(7):1391–7. doi: 10.1002/cncr.21308. [DOI] [PubMed] [Google Scholar]
- 10.Santin AD, Zhan F, Cane' S, et al. Gene expression fingerprint of uterine serous papillary carcinoma: identification of novel molecular markers for uterine serous cancer diagnosis and therapy. Brit J Cancer. 2005;92(8):1561–73. doi: 10.1038/sj.bjc.6602480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipinski M, Parks DR, Rouse RV, Herzenberg LA. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(8):5147–50. doi: 10.1073/pnas.78.8.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miotti S, Canevari S, Ménard S, et al. Characterization of human ovarian carcinoma-associated antigens defined by novel monoclonal antibodies with tumor-restricted specificity. Int J Cancer. 1987;15(39):297–303. doi: 10.1002/ijc.2910390306. [DOI] [PubMed] [Google Scholar]
- 13.Alberti S, Miotti S, Stella M, et al. Biochemical characterization of Trop-2, a cell surface molecule expressed by human carcinomas: formal proof that the monoclonal antibodies T16 and MOv-16 recognize Trop-2. Hybridoma. 1992;50:1330–36. doi: 10.1089/hyb.1992.11.539. [DOI] [PubMed] [Google Scholar]
- 14.Nakashima K, Shimada H, Ochai T, et al. Serological identification of Trop-2 by recombinant cDNA expression cloning using sera of patients with esophageal squamous cell carcinoma. Int J Cancer. 2004;112:1029–35. doi: 10.1002/ijc.20517. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Zhou W, Velculescu VE, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276(5316):1268–72. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 16.Mühlmann G, Spizzo G, Gostner J, et al. TROP2 expression as prognostic marker for gastric carcinoma. J Clin Pathol. 2009 Oct 17;62(2):152–58. doi: 10.1136/jcp.2008.060590. Epub 2008. [DOI] [PubMed] [Google Scholar]
- 17.Fong D, Moser P, Krammel C, et al. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer. 2008 Sep 23;99(8):1290–95. doi: 10.1038/sj.bjc.6604677. Epub 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong D, Spizzo G, Gostner JM, et al. TROP2: a novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod Pathol. 2008 Dec 14;21(2):186–91. doi: 10.1038/modpathol.3801001. Epub 2007. [DOI] [PubMed] [Google Scholar]
- 19.Fang YJ, Lu ZH, Wang GQ, et al. Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int J Colorectal Dis. 2009;24(8):875–84. doi: 10.1007/s00384-009-0725-z. [DOI] [PubMed] [Google Scholar]
- 20.Bignotti E, Todeschini P, Calza S, et al. Trop-2 overexpression as an independent marker for poor overall survival in ovarian carcinoma patients. European Journal of Cancer. 2010;46(5):944–53. doi: 10.1016/j.ejca.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Stein R, Basu A, Chen S, Shih LB, Goldenberg DM. Specificity and properties of MAb RS7-3G11 and the antigen defined by this pancarcinoma monoclonal antibody. Int J Cancer. 1993;55(6):938–46. doi: 10.1002/ijc.2910550611. [DOI] [PubMed] [Google Scholar]
- 22.Stein R, Govindan SV, Mattes MJ, et al. Targeting human cancer xenografts with monoclonal antibodies labeled using radioiodinated, diethylenetriaminepentaacetic acid-appended peptides. Clin Cancer Res. 1999;5(10 Suppl):3079s–87s. [PubMed] [Google Scholar]
- 23.Govindan SV, Stein R, Qu Z, et al. Preclinical therapy of breast cancer with a radioiodinated humanized anti-EGP-1 monoclonal antibody: advantage of a residualizing iodine radiolabel. Breast Cancer Research & Treatment. 2004;84(2):173–82. doi: 10.1023/B:BREA.0000018417.02580.ef. [DOI] [PubMed] [Google Scholar]
- 24.El-Sahwi K, Bellone S, Cocco E, Cargnelutti M. In vitro Activity of Pertuzumab in Combination with Trastuzumab in Uterine Serous Papillary Adenocarcinoma. Brit. J. Cancer. 2010;102(1):134–43. doi: 10.1038/sj.bjc.6605448. al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cubas R, Li M, Chen C, Yao Q. Trop2: a possible therapeutic target for late stage epithelial carcinomas. Biochimica et Biophysica Acta. 2009;1796(2):309–14. doi: 10.1016/j.bbcan.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Guerra E, Trerotola M, Dell' Arciprete R, et al. A bicistronic CYCLIN D1-TROP2 mRNA chimera demonstrates a novel oncogenic mechanism in human cancer. Cancer Research. 2008;68(19):8113–21. doi: 10.1158/0008-5472.CAN-07-6135. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Day R, Dong Y, Weintraub SJ, Michel L. Identification of Trop-2 as an oncogene and an attractive therapeutic target in colon cancers. Mol Cancer Ther. 2008;7(2):280–85. doi: 10.1158/1535-7163.MCT-07-2003. [DOI] [PubMed] [Google Scholar]
- 28.Cross SN, Cocco E, Bellone S, et al. Differential sensitivity to platinum-based chemotherapy in primary uterine serous papillary carcinoma cell lines with high vs low HER-2/neu expression in vitro. Am J Obstet & Gynecol. 2010;203(2):162.e1–8. doi: 10.1016/j.ajog.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caron PC, Lai LT, Scheinberg DA. Interleukin-2 enhancement of cytotoxicity by humanized monoclonal antibody M195 (anti-CD33) in myelogenous leukemia. Clin. Cancer Res. 1995;1:63–70. [PubMed] [Google Scholar]
- 30.Caligiuri MA, Murray C, Robertson MJ, et al. Selective modulation of human natural killer cells in vivo following prolonged infusion of low-doses recombinant interleuikin 2. J Clin Invest. 1993;91:123–128. doi: 10.1172/JCI116161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortaldo JR, Woodhouse CS, Morgan AC, Jr, Herberman RB, Cheresh DA, Reisfeld RA. Analysis of effector cells in human antibody-dependent cellular cytotoxicity with murine monoclonal antibodies. J Immunol. 1987;138:3566–3572. [PubMed] [Google Scholar]
- 32.Villella JA, Cohen S, Smith DH, Hibshoosh H, Hershman D. HER-2/Neu overexpression in uterine papillary serous cancers and its possible therapeutic implications. Int J Gynecol Cancer. 2006;16:1897–1902. doi: 10.1111/j.1525-1438.2006.00664.x. doi: 10.1111/j.1525-1438.2006.00664. [DOI] [PubMed] [Google Scholar]
- 33.Santin AD, Bellone S, Roman JJ, McKenney JK, Pecorelli S. Trastuzumab treatment in patients with advanced or recurrent endometrial carcinoma overexpressing HER2/neu. Inter J Gynecol Obstet. 2008;102:128–131. doi: 10.1016/j.ijgo.2008.04.008. doi: 10.1016//j.ijgo.2008.04.008. [DOI] [PubMed] [Google Scholar]


