Abstract
This study characterizes the contribution of bone marrow-derived cells (BMDCs) to Barrett's adenocarcinoma of the esophagus using a mouse surgical model of disease and human specimens. Transplantation of bone marrow expressing beta galactosidase into a wild-type mouse, followed by surgical esophagojejunostomy, allowed tracking of BMDCs into the surgical anastomosis and resulting Barrett's metaplasia. Human tissue from a male patient who had been transplanted with female bone marrow and later developed esophageal adenocarcinoma allowed us to tract donor-derived cells into the tumor. Using a combination of antibodies directed against beta-galactosidase (animal studies) and X/Y fluorescent in situ hybridization (FISH) (human studies), combined with specific lineage staining directed against epithelial, fibroblast, endothelial, and leukocyte markers, we show that bone marrow cells contribute to both the epithelial and stromal component of esophageal adenocarcinoma. These findings demonstrate that BMDCs can generate cancer-associated fibroblasts as well as contribute directly to epithelial cells in cancer of the esophagus.
Introduction
The notion and controversy that bone marrow-derived cells (BMDCs) contribute to peripheral tissues began about 10 years ago when it was noted that BMDCs may incorporate into solid organs as epithelial, fibroblast, endothelial, and fat cells [1,2]. Under usual conditions, reports suggest that BMDC incorporation is minimal, whereas other reports fail to find any contribution of marrow stem cells to peripheral tissues [3]. Reports differ in the type of cells studies (total marrow, hematopoietic stem cells, mesenchymal cells, etc.), which may explain the disparate findings. However, when found, the level of engraftment of BMDC into the peripheral tissue increases with injury and inflammation, and it is thought the influx of these cells into injured tissues may be a reparative attempt by the body [4–6]. Usually, these BMDC reside as terminally differentiated cells; however, under some situations, they may take up residence in the stem cell niche and function as peripheral stem cells [7], which may lead to clonal repopulation of tissue. Because of the strong association between inflammation, chronic tissue injury/repair, and cancer, it seemed logical to pursue a role for BMDC and cancer. Animal models transplanted with tagged bone marrow have been used to address the association between chronic inflammatory conditions that predispose to cancer and the presence of BMDCs, including Helicobacter-related stomach cancer [7], chronic reflux-related Barrett's metaplasia [8], and genetic- and chemical-induced colonic inflammation [9–11]. Sex chromosome mismatched allogeneic bone marrow or solid organ transplant recipients who later develop malignancy are the pool of human patients usually studied. Indeed, several recent reports support the notion that BMDC contribute to human malignancies, though the contribution ranges widely and includes tumors that appear to be clonally derived from a bone marrow source [13–16], tumors whose epithelium is largely (on the order of 20%) BMDC [17], and tumors that contain clusters of BM cells, though the bulk of the tumor is host derived [18]. The significance of each of these scenarios is not yet clear, and may depend upon a multitude of factors, including the organ involved, the time course of tumor growth, and the factors predisposing to cancer. In addition, stromal cells within gastric cancer and colonic polyps have recently been shown to arise from bone marrow sources [19,20], implicating bone marrow cells as important players in the critical signaling environment of tumors.
The aim of this study was to examine the contribution of BMDC to the stroma and the epithelium of Barrett's associated adenocarcinoma of the esophagus, using both a mouse model of Barrett's metaplasia, and tissue obtained from a patient with Barrett's adenocarcinoma who had been transplanted previously with sex-mismatched total bone marrow.
Materials and Methods
Animal studies
All studies were conducted at the University of Massachusetts Medical School under Institutional Animal Care and Use Committee approval. Eight-week-old C57BL/6 mice were irradiated and transplanted with total bone marrow from C57BL/6JGtrosa26 (ROSA 26) mice (Jackson laboratories) according to published protocol [7]. Four weeks later, mice underwent esophagojejunostomy with an end-to-side anastomosis between the esophagus and the proximal jejunum 1.5 cm distal to the ligament of Treitz as previously published [21]. The gastric remnant remained in place. Twenty weeks after surgery, mice were euthanized, esophagi removed, and tissue processed for histology and specific bacterial beta-galactosidase (Promega) E-cadherin or alpha smooth muscle actin (αSMA) immunohistochemistry according to published protocols [7].
Human studies
The study protocol was approved by the University of Massachusetts Medical School institutional review board. Tissue obtained at the time of esophagogastroduodenoscopy was processed according to standard laboratory protocol. To image the specific lineage markers in the same tissue section as the X and Y chromosomes, the following protocol was used. Monoclonal antibodies against CD45 (DAKO #M0701), CD31 (DAKO #M0823), smooth muscle actin (SMA; DAKO #M0851), vimentin (DAKO #M0725), and E-cadherin (BD Transduction Laboratories #610182) were used, observed with a polymer based detection system, and stained with hematoxylin. Slides were scanned to capture all material using the Duet scanning system (BioView). Slides were decoverslipped, destained, and hybridized with CEP X probe (SpectrumGreen, DXZ1, Alpha Satellite, Abbott Molecular) and CEP Y probe (SpectrumOrange or SpectrumAqua, DYZ1, Satellite III, Abbott Molecular). Two fluorescent scans were performed with the Duet scanning system using automated algorithms for X/Y enumeration. A target scan was performed to determine the X/Y status of immuno-stained cells followed by an area scan of all DAPI-stained nuclei to determine the overall distribution of male (XnYn) and female (XnXn) cells in the specimen. Total percentage of male and female cells were calculated by computer algorithm. Percent male and female E-cadherin-positive (n = 467) and SMA-positive (n = 541) cells were counted manually. Counts were modeled using generalized linear mixed models [22–24] assuming underlying binomial distribution and a logit link function; a form of logistic regression modeling [25] without the necessity of an assumption of independent observations. Fishclass was modeled as a random factor and patient versus control samples as a fixed factor, and the data were further dichotomized into groups where there were either any y chromosomes versus no chromosomes as another fixed factor and included the interaction between patient/control group and y chromosome group. In addition, a second classification was changed to dichotomize on whether or not there was 2 X and zero Y chromosomes. The total number of cells was used as the number of trials in the model. Modeling was performed using the GLIMIX procedure [26] in the SAS statistical software package [27]. The Y chromosome in captured images was pseudocolored red for clarity and ease of detection in the figures.
Results
Animal studies
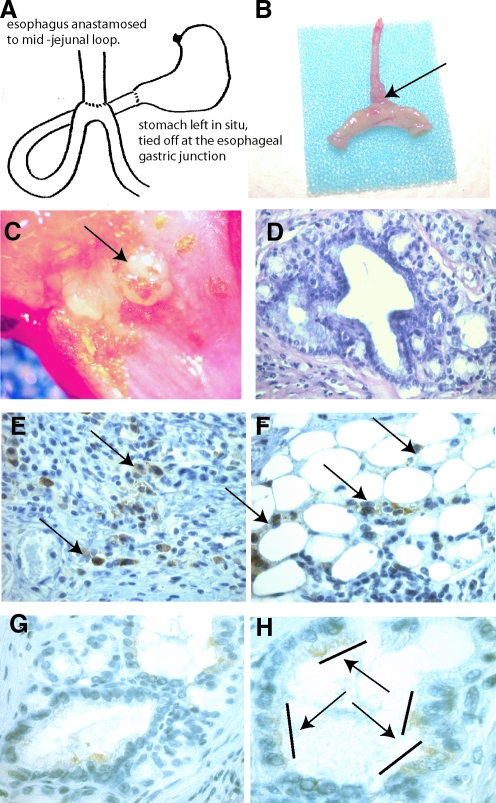
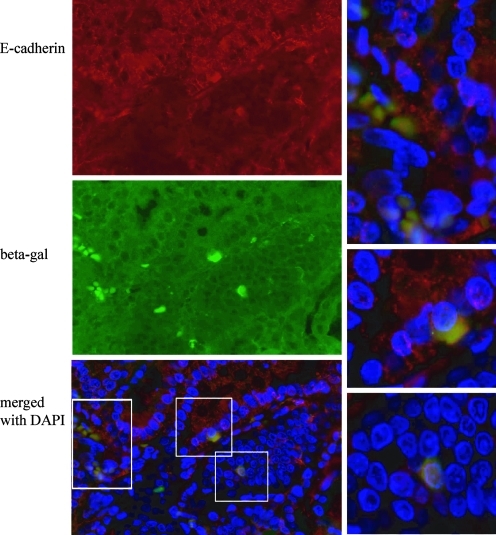
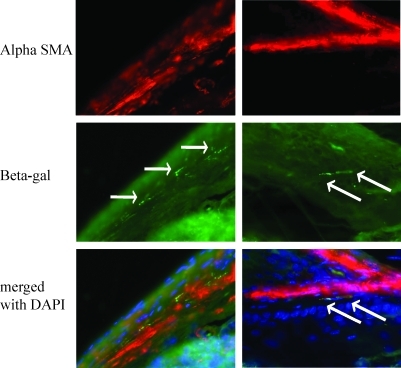
Twenty mice underwent lethal irradiation followed by bone marrow transplantation with total marrow from C57BL/6JGtrosa26 (ROSA 26) mice followed by esophagojejunsotomy as diagramed in Fig. 1A. Twelve of these mice survived for the time of the study; 3 died during surgery or within 24 h, and 5 died over the ensuing weeks. Mice were hand-fed 3 times a day for 20 weeks. These mice maintained weight comparable with control mice. At euthanasia, the esophagojejunal anastomosis was removed, opened, photographed, and processed for routine histology and immunohistochemistry. Eight mice (40%) had thickening at the surgical anastomosis, unrelated to the suture line (Fig. 1B). Upon opening the esophagus, 4 mice (20%) had nodular exophytic lesions (Fig. 1C, arrow) arising at the distal esophageal anastomosis. Lesions were hard, fixed, and nonulcerated. Microscopically, lesions consisted of columnar epithelium with markedly distorted columnar gland structures, loss of nuclear polarity, and an altered nuclear-to-cytoplasmic ratio. These areas of columnar metaplasia were surrounded by stratified squamous epithelium, consistent with Barrett's metaplasia in the mouse model (Fig. 1D). Immunohistochemistry (IHC) directed against bacterial beta-galactosidase revealed donor-derived inflammatory cells within the esophagus, directly underlying metaplastic tissue (Fig. 1E, arrows), and donor-derived adipose cells (Fig. 1F, arrows) within peri-glandular stroma. Interestingly, roughly half of the Barrett's glands contained beta-galactosidase expressing epithelial cells. These cells were found in groups of 3–4 cells within glands, and never comprised entire glands (Fig. 1H. bars define positive cells, highlighted by arrows). Additional staining using immunofluroescence (IF) to detect betagalactosidase and E-cadherin revealed colocalization of signal, thus confirming the epithelial phenotype of the beta galactosidase-positive cells (Fig. 2). Additional IF staining using antibody directed against alpha SMA demonstrates bone marrow-derived activated fibroblasts at the edge of connective tissue bands as well as within stroma separating cords of tumor cells (Fig. 3). Sex-mismatched transplants were not performed; therefore, we were unable to identify transplanted cells by additional nuclear markers.
FIG. 1.
Surgical mouse model of Barrett's metaplasia. (A) Cartoon depiction of the surgical esophagojejunostomy. (B) Actual excised esophagus and anastomosed jejunum from a mouse at 20 weeks. Arrow points to the thickened esophagus proximal to the anastomosis. (C) Opening of the surgical specimen reveals nodular thickened area proximal to the anastomosis, not involving the suture line. (D) H&E staining of a histological section through the anastomosis. Poorly organized glands lined by columnar epithelium with interspersed stromal tissue surrounded by squamous epithelial cells. Immunohistochemical staining directed against beta-galactosidase (brown cytoplasmic staining) reveals bone marrow cells within (E) inflammatory infiltrate (arrows). (F) Adipose tissue surrounding tumor (arrows) and (G, H) within gland structures. Bars and arrows show 2 and 3 contiguous cells that are marrow derived.
FIG. 2.
Epithelial cells of Barrett's metaplasia in the mouse are marrow derived. Immunofluorescence staining of tissue sections through the lower esophagus. E-cadherin (red), beta-galactosidase (green), and nuclei (blue) as labeled (40 ×). Merged images of bone marrow-derived cells expressing E-cadherin that are boxed are shown at higher magnification (60 ×).
FIG. 3.
Stromal cells within Barrett's metaplasia in the mouse are marrow derived. Immunofluorescence staining of tissue sections through the lower esophagus. Alpha smooth muscle actin (alpha SMA) (red), beta-galactosidase (green), and nuclei (blue) as labeled (40×). Arrows highlight bone marrow-derived cells expressing alpha SMA and beta galactosidase.
Human studies
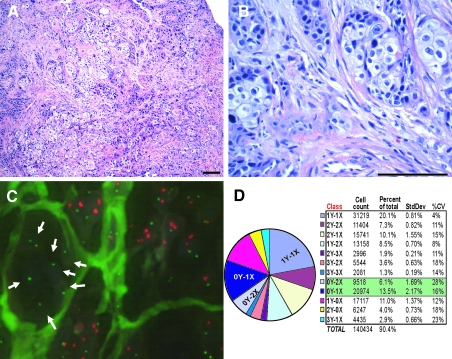
A 45-year-old man presented with severe dysphagia. At age 35 he was found to have acute myloblastic leukemia (AML M2) and was treated with etoposide and cytarabine followed by allogenic bone marrow transplantation from his sister. After his initial treatment, he was largely noncompliant to follow-up. At the time of his most recent hospitalization (10 years after transplantation) he presented with dysphagia and underwent a diagnostic endoscopic esophagogastroduodenoscopy and computed tomography scan, which revealed a locally extensive 8 cm circumferential lower esophageal lesion. Histological analysis revealed poorly differentiated carcinoma with mixed features of adenocarcinoma and squamous cell carcinoma within a reactive myofibroblastic stroma (Fig. 4A, B). Biopsies of the esophagus taken before any therapy were processed for X/Y fluorescent in situ hybridization (FISH) and IHC directed against epithelial, stromal, and immune markers. Overall, the tumor contained 6.1% 0Y, 2X cells, and 13.5% 0Y, 1X cells, suggesting that at least 6.1% of the cells within the tumor were derived from the donor, and as many as 19.6% of cells could be donor derived (Fig. 4C, D). To rule out the possibility that the tumor cells containing a 2X0Y chromosomes were a truncation artifact of thin sections, Barrett's metaplasia (n = 2) and adenocarcinoma (n = 2) control specimens from nontransplanted men (ie, 1X1Y) were analyzed using the same technique and compared to a second set of slides taken from our patient and processed at the same time. On average 0.9% (±0.25% SD) of the control esophagus nuclei exhibited the 2X0Y pattern, whereas 3.8% (±0.16% SD) of the nuclei in the patients esophageal adenocarcinoma had an 2X0Y pattern. The patient's esophageal adenocarcinoma specimens were 4.39 times more likely to have nuclei with 2 X and zero Y chromosomes than the control specimens (P < 0.0001), indicating that nuclei with a 2X0Y chromosome pattern are likely derive from the stem cell transplant. Conversely, control specimens were slightly more likely (1.039 times, P = 0.146) to have nuclei with less than 2 X or more than zero Y chromosomes (eg, 1X1Y, 0X1Y) than the patient specimen. We also examined the percentage of nuclei having any Y chromosome in the patient tumor or control specimens (eg, 1X0Y, 2X0Y, and 4X0Y). For instance, a 1X0Y nucleus could result from truncation effects because the enumeration probe for chromosome X is approximately one-third the size of the Y probe. In addition, overlapping nuclei could produce a 3X0Y, or 4X0Y nucleus given the limitations of automated counting systems. On average 10.1% (±2.4% SD) of the control nuclei exhibited the 0Y pattern (1X0Y = 8.9%, 2X0Y = 0.9%, 3X0Y = 0.2%, 4X0Y =0.0%), whereas 24% (±0.7% SD) of the nuclei in the patients esophageal adenocarcinoma contained the 0Y pattern (1X0Y = 18.3%, 2X0Y = 3.8%, 3X0Y = 1.2%, 4X0Y = 0.4%). When a nucleus contained no Y chromosomes, the patient esophageal adenocarcinoma specimens were 2.64 times more likely to exhibit the 0Y pattern than the controls (P < 0.0001). In nuclei with any Y chromosome the patient specimens were 1.23 times less likely to have a Y chromosome than the control (ie, the controls were 1.23 times more likely, P < 0.0001). The distribution of X/Y chromosomes within the patient tumor is shown in Fig. 4D.
FIG. 4.
Human esophageal tumor arising in a transplant patient. (A) H&E-stained sections show a poorly differentiated adenosquamous carcinoma composed of islands and small clusters of tumor cells within a dense desmoplastic stroma; 10× magnification, scale bar = 100 μm. (B) On higher power the tumor shows mixed features of adenocarcinoma and squamous cell carcinoma with high-grade pleomorphic nuclei and mitotic activity. A reactive myofibroblastic stromal component and vessels are evident within the tumor; 40× magnification, scale bar = 100 μm. (C) Fluor-escent in situ hybridization (FISH) for X (green) and Y (red) chromosomes. A cluster of XX female tumor cells can be seen surrounded by stroma (arrows). (D) Enumeration of XY chromosome counts are shown in pie chart form and table.
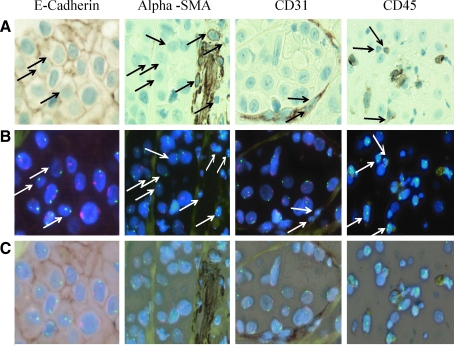
Comparing the standard H&E sections, and overlaying with the X, Y FISH, donor cells were clearly seen as clusters of female epithelial cells (Fig. 4C) sharply demarcated from XY tumor cells by tumor stroma, as well as XX female epithelial cells interspersed with male tumor cells in a seemingly random pattern. Slides that had been analyzed by FISH were then subjected to specific immunostaining for epithelial (E-cadherin and cytokeratin), endothelial (CD31), stromal (vimentin and alpha smooth muscle actin), or immune cell (CD45)-specific protein expression and target areas matched by computer. E-cadherin-positive female epithelial tumor cells (Fig. 5 and Supplementary Fig. S1, available online at www.liebertonline.com/scd) comprised up to 31.47% of the E-Cadherin (E-CAD)-positive tumor cells (2X0Y; 11.13%, 1X0Y; 20.34%), whereas 20.5% of the E-CAD-negative epithelial cells had a 2X0Y karyotype. The tumor contained a moderate amount of stromal tissue. Evaluation of cancer-associated fibroblasts (CAFs) by specific anti-alpha smooth muscle actin (Fig. 5 and Supplementary Fig. S2, available online at www.liebertonline.com/scd) and antivimentin (data not shown) immunohistochemistry revealed that 12.57% of CAF cells were 2X0Y and 4.07% were 1X0Y (Fig. 5). Female (2X0Y) CD31+ endothelial cells were found interspersed with male 1X1Y CD31+ endothelial cells and comprised <2% of the endothelial cells (Fig. 5 and Supplementary Fig. S3, available online at www.liebertonline.com/scd). Specific staining for donor-derived inflammatory cells using anti-CD45 antibody showed the patient to be about 75% donor/host chimera. Inflammatory cells were found throughout the tumor at low numbers interspersed with host- and donor-derived larger epithelial (CD45−) tumor cells (Fig. 5 and Supplementary Fig. S4, available online at www.liebertonline.com/scd). Many (50%) cells within the tumor were aneuploid. Of these, about 12% carried a karyotype consistent with fusion between host and donor cells (Fig. 4D).
FIG. 5.
Specific immunohistochemistry and X/Y FISH analysis performed in tandem on the same slide. (A) Immunohistochemistry as indicated (B) X (green) and Y (red) FISH followed by (C) an overlay for each grouping. Epithelial cells stained with anti-E-cadherin antibody and fibroblast cells within the stroma are stained with antibody against alpha smooth muscle actin; endothelial cells are stained with antibody directed against CD31 and leukocytes are stained with anti-CD45. Arrows point out 1X0Y and 2X0Y cells.
Discussion
BM contribution to Barrett's metaplasia has been shown in a rat model of disease [8] though the cell phenotype has not been defined. This is the first time a possible role for BM cells in Barrett's metaplasia in a mouse model and Barrett's adenocarcinoma in a human model has been demonstrated. Here we show that BMDC contribute to Barrett's metaplasia and adenocarcinoma as epithelial tumor cells, stromal myofibroblast, and endothelial cells. In both a mouse model of metaplasia and a human model of Barrett's adenocarcinoma, we found a significant number of tumor and stromal cells to be directly of donor origin, and others to be possibly a product of fusion between the donor and the host cells. Of interest is the pattern of 2–3 contiguous BMDC within the mouse Barrett's metaplastic glands rather than entire glands derived from a BM source. This is consistent with the recent finding from human studies that demonstrate glands within Barrett's metaplasia contain more than 1 stem cell giving rise to ribbons of daughter cells up the length of the gland, and over time, presumably, 1 dominant stem cell prevails [28]. Although this study did not address the origin of the stem cells giving rise to Barrett's metaplasia, it supports our findings that we interpret as a BMDC replacing a peripheral stem cell within the gland and thus giving rise to several contiguous cells within the gland, but not the entire gland unit.
Despite strong and convincing reports, there remains controversy regarding the contribution of BM cells to tumor epithelium and tumor stroma in humans. Controversial and conflicting data likely result from several factors. First, the identification of transplanted cells within recipient human tissue requires that the transplanted cells carry a trackable marker such as a difference in the sex chromosomes. Second, tumor growth often takes several years to become clinically apparent and it is often not clear if tumors arising in transplanted patients initiated before or after the transplant. Third, there is no uniformity with regard to treatment of patients before transplant, the type of cells/tissues transplanted (total marrow, mobilized stem cells, organ transplant, etc.), and immunosuppressive treatment after transplantation. These factors and others often limit the pool of patients that can provide meaningful data. Also, different tumor types may behave differently with regard to the contribution of BM cells to the tumor and tumor stroma. As in the case presented here, prior chemotherapy, severe graft versus host disease, and other confounding variables may alter the usual activity of BMDC. Extrapolation of findings to patients without these variables must be done cautiously. This patient presented with advanced adenosquamous carcinoma of the esophagus. The clinical impression was of a tumor arising in Barrett's metaplasia; however, without prior endoscopic evidence of metaplasia, and with concurrent squamous features, it remains possible that the lesion may not have arisen via the usual Barrett's sequence of events.
Our patient (male) received a bone marrow transplant from a female donor. Cells carrying 2 X-chromosomes and lacking a Y-chromosome are most probably of donor origin; however, cells with 1 X and lacking a Y chromosome are of indeterminate origin. Tissue sectioning and the specificity of the X-probe allows a majority of the X chromosomes to be detected; however, some XX cells may only have 1 X chromosome evident. The Y-probe, on the other hand, recognizes a larger target on the Y chromosome, identifying a high percentage of male cells. While it is tempting to assign these cells to donor origin, we must be aware that tumors can selectively eliminate the Y-chromosome (reviewed in ref. [29]); thus, XO cells may be host-derived tumor cells with the Y-chromosome eliminated. For this reason, we have presented most probable 2X female cell counts, and enumerated the 1XOY cells as potential female cells. Nevertheless, a significant number of tumor cells are donor derived. Lack of E-CAD expression within epithelial tumor cells is associated with a worse prognosis in Barrett's adenocarcinoma, and lack of E-CAD expression has been associated with a more aggressive behavior. Interestingly, a large number of 2X0Y epithelial tumor cells were E-CAD negative, consistent with an aggressive cancer cell phenotype.
Tumor stroma contains activated fibroblasts termed CAFs, endothelial cells, fat cells, and inflammatory cells embedded in extracellular matrix. Tumor stroma has been shown to promote growth and invasive behavior of tumor cells through secretion of growth factors and chemokines/cytokines, and by promoting tissue remodeling. The origin of these stromal cells has been a topic of debate with evidence supporting their recruitment from local stores, their origination from tumor cells undergoing epithelial to mesenchymal transition, and recruitment from bone marrow stem cell sources (reviewed in ref. [30]). Of those cells originating from bone marrow sources, it is not clear if one stem cell type is responsible for different cell lineages or if multiple tissue-specific progenitor cells are involved. Our findings in the mouse model and human Barrett's adenocarcinoma demonstrate that stromal cells within Barrett's adenocarcinoma can be bone marrow derived. In the human samples, 12.57% of the CAFs originate from donor cells, and rare cells could be found as endothelial cells. These studies confirm findings from other tumor types such as breast, colon, lung, and stomach, where both epithelial cells and stromal components of tumors have been shown to be marrow derived (reviewed in refs. [30,31]). While we are assigning these findings to transdifferentiation of marrow cells, we have not investigated a role for fusion of BMDC to host cells, and therefore cannot exclude this possibility. Phagocytosis of dead cell fragments by epithelial cells is an unlikely explanation, especially in the human samples where nuclear incorporation of an ingested X chromosome as well as elimination of the native Y chromosome would need to occur to produce our findings. It is also not clear at this point what role these cells play in cancer initiation, progression, or metastasis. Understanding the dynamic nature of cancer initiation and progression, which often times involves recruitment of BMDCs, will be essential for targeted therapies aimed at disrupting these cell–cell interactions.
Conclusion
BMDC contribute to the cancer epithelium, fibroblasts, and endothelium of esophageal tumors. The contribution is important given the recent evidence that critical stromal signaling may emanate from a subset of cells recruited to a tumor, suggesting that even in small numbers, BMDC may have a substantial impact on tumor growth.
Supplementary Material
Acknowledgments
This study was funded by R01CA119061 to J.H.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Jiang Y. Jahagirdar BN. Reinhardt RL. Schwartz RE. Keene CD. Gonzalez Ortiz-XR. Reyes M. Lenvik T. Lund T. Blackstad M. Du J. Aldrich S. Lisberg A. Low WC. Largaespada DA. Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–46. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 2.Krause DS. Theise ND. Collector MI. Henegariu O. Hwang S. Gardner R. Neutzel S. Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2002;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 3.Wagers AJ. Sherwood RI. Christensen JL. Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 4.Brittan M. Chance V. Elia G. Poulsom R. Alison MR. MacDonald TT. Wright NA. A regenerative role for bone marrow following experimental colitis: contribution to neovasculogenesis and myofibroblasts. Gastroenterol. 2005;128:1984–1995. doi: 10.1053/j.gastro.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki M. Abe R. Fujita Y. Ando S. Inokuma D. Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto R. Yajima T. Yamazaki M. Kanai T. Mukai M. Okamoto S. Ikeda Y. Hibi T. Inazawa J. Watanabe M. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8:1011–1017. doi: 10.1038/nm755. [DOI] [PubMed] [Google Scholar]
- 7.Houghton J. Stoicov C. Nomura S. Rogers AB. Carlson J. Li H. Cai X. Fox JG. Goldenring JR. Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 8.Sarosi G. Brown G. Jaiswal K. Feagins LA. Lee E. Crook TW. Souza RF. Zou YS. Shay JW. Spechler SJ. Diseases of the Esophagus. 2008;21:43–50. doi: 10.1111/j.1442-2050.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 9.Makoto MT. Role of bone marrow-derived cells in colon cancer: lessons from mouse model studies. J Gastroenterol. 2009;44:93–102. doi: 10.1007/s00535-008-2321-3. [DOI] [PubMed] [Google Scholar]
- 10.Komori M. Tsuji S. Tsujii M. Murata H. Iijima H. Yasumaru M. Nishida T. Irie T. Kawano S. Hori M. Involvement of bone marrow-derived cells in healing of experimental colitis in rats. Wound Repair Regen. 2005;13:109–118. doi: 10.1111/j.1067-1927.2005.130114.x. [DOI] [PubMed] [Google Scholar]
- 11.Brittan M. Chance V. Elia G. Poulsom R. Alison MR. MacDonald TT. Wright NA. A regenerative role for bone marrow following experimental colitis: contribution to neovasculogenesis and myofibroblasts. Gastroenterology. 2005;128:1984–1995. doi: 10.1053/j.gastro.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi Y. Tsuji S. Tsujii M. Nishida T. Ishii S. Nakamura T. Eguchi H. Kawano S. The transdifferentiation of bone-marrow-derived cells in colonic mucosal regeneration after dextran-sulfate-sodium-induced colitis in mice. Pharmacology. 2007;80:193–199. doi: 10.1159/000104148. [DOI] [PubMed] [Google Scholar]
- 13.Golfinopouloss V. Pentheroudakis G. Kamakari S. Metaxa-Mariatou V. Pavlidis N. Donor derived breast cancer in a bone marrow transplantation recipient. Breast Cancer Res Treat. 2009;113:211–213. doi: 10.1007/s10549-008-9922-7. [DOI] [PubMed] [Google Scholar]
- 14.Boix R. Sanz C. Mora M. Quer A. Beyer K. Musulen E. Gonzalez C. Bayona S. Saladie JM. Ariza A. Primary renal cell carcinoma in a transplanted kidney: genetic evidence of recipient origin. Transplantation. 2009;87:1057–1061. doi: 10.1097/TP.0b013e31819d1e5f. [DOI] [PubMed] [Google Scholar]
- 15.Chakraborty A. Lazova R. Davies S. Backvall H. Ponten F. Brash D. Pawelek J. Donor DNA in a renal cell carcinoma metastasis from a bone marrow transplant recipient. Bone Marrow Transplant. 2004;34:183–186. doi: 10.1038/sj.bmt.1704547. [DOI] [PubMed] [Google Scholar]
- 16.Janin A. Murata H. Leboeuf C. Cayuela JM. Gluckman E. Legrès L. Desveaux A. Varna M. Ratajczak P. Soulier J. de Thé H. Bertheau P. Socié G. Donor-derived oral squamous cell carcinoma after allogeneic bone marrow transplantation. Blood. 2009;113:1834–1840. doi: 10.1182/blood-2008-07-171702. [DOI] [PubMed] [Google Scholar]
- 17.Avital I. Moreira AL. Klimstra DS. Leversha M. Papadopoulos EB. Brennan M. Downey RJ. Donor-derived human bone marrow cells contribute to solid organ cancers developing after bone marrow transplantation. Stem Cells. 2007;25:2903–2909. doi: 10.1634/stemcells.2007-0409. [DOI] [PubMed] [Google Scholar]
- 18.Cogle CR. Theise ND. Fu D. Ucar D. Lee S. Guthrie SM. Lonergan J. Rybka W. Krause DS. Scott EW. Bone marrow contributes to epithelial cancers in mice and humans as developmental mimicry. Stem Cells. 2007;25:1881–1887. doi: 10.1634/stemcells.2007-0163. [DOI] [PubMed] [Google Scholar]
- 19.Brittan M. Hunt T. Jeffery R. Poulsom R. Forbes SJ. Hodivala-Dilke K. Goldman J. Alison MR. Wright NA. Bone marrow derivation of pericryptal myofibroblasts in the mouse and human small intestine and colon. Gut. 2002;50:752–757. doi: 10.1136/gut.50.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Worthley DL. Ruszkiewicz A. Favies R. Moore S. Nivison-Smith I. To LB. Browett P. Western R. Durrant S. So J. Young GP. Mullighan CG. Bardy PG. Michael MZ. Human gastrointestinal neoplasia associated myofibroblasts can develop from bone marrow derived cells following allogeneic stem cell transplantation. Stem Cells. 2009;27:1463–1468. doi: 10.1002/stem.63. [DOI] [PubMed] [Google Scholar]
- 21.Stenstrom B. Furnes MW. Tømmerås K. Syversen U. Zhao CM. Chen D. Mechanism of gastric bypass–induced body weight loss: one-year follow-up after micro–gastric bypass in rats. J Gastrointest Surg. 2006;10:1384–1391. doi: 10.1016/j.gassur.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Liang KY. Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 23.McCullagh P. Nelder JA. Generalized Linear Models. 2nd. Chapman and Hall; London: 1989. [Google Scholar]
- 24.Wolfinger R. O'Connell M. Generalized linear mixed models: a pseudo-likelihood approach. J Stat Comput Simul. 1993;48:233–243. [Google Scholar]
- 25.Hosmer DW. Lemeshow S. Applied Logistic Regression. John Wiley and Sons, Inc.; New York: 1989. [Google Scholar]
- 26.The GLIMMIX Procedure. SAS Institute, Inc.; Cary, NC: 2005. [Google Scholar]
- 27.SAS 9.2. Version 9.2. SAS Institute, Inc.; Cary, NC: 2008. [Google Scholar]
- 28.Nicholson AM. Graham TA. Simpson AI. Humphries A. Wright NA. McDonald SA. Jankowski JA. Analysis of the clonality of Barrett's esophagus glands reveals they are clonal units and establishes a common stem cell for glandular and squamous epithelium. Gastroenterology. 2010;138:S1–S138. [Google Scholar]
- 29.Nestor O. Bianchi Y. Chromosome structural and functional changes in human malignant diseases. Mutat Res Rev Mutat Res. 2009;682:21–27. doi: 10.1016/j.mrrev.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Li H. Fan X. Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem. 2007;101:805–815. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- 31.Houghton J. Li H. Fan X. Liu Y. Liu J. Rao VP. Poutahidis T. Taylor C. Jackson E. Hewes C. Lyle S. Cerny A. Bowen G. Cerny J. Moore N. Kurt-Jones E. Erdman S. Mutations in bone marrow derived stromal cells unmask latent malignancy. Stem Cells Dev. 2010 doi: 10.1089/scd.2009.0439. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.