Abstract
The functional characteristics of membrane progesterone receptors (mPRs) have been investigated using recombinant mPR proteins over-expressed in MDA-MB-231 breast cancer cells. Although these cells do not express the full-length progesterone receptor (PR), it is not known whether they express N-terminally truncated PR isoforms which could possibly account for some progesterone receptor functions attributed to mPRs. In the present study, the presence of N-terminally truncated PR isoforms was investigated in untransfected and mPR-transfected MDA-MB-231 cells, and in MDA-MB-468 breast cancer cells. PCR products were detected in PR-positive T47D Yb breast cancer cells using two sets of C-terminus PR primers, but not in untransfected and mPR-transfected MDA-MB-231 cells, nor in MDA-MB-468 cells. Western blot analysis using a C-terminal PR antibody, 2C11F1, showed the same distribution pattern for PR in these cell lines. Another C-terminal PR antibody, C-19, detected immunoreactive bands in all the cell lines, but also recognized α-actinin, indicating that the antibody is not specific for PR. High affinity progesterone receptor binding was identified on plasma membranes of MDA-MB-468 cells which was significantly decreased after treatment with siRNAs for mPRα and mPRβ. Plasma membranes of MDA-MB-468 cells showed very low binding affinity for the PR agonist, R5020, ≤1% that of progesterone, which is characteristic of mPRs. Progesterone treatment caused G protein activation and decreased production of cAMP in MDA-MB-468 cells, which is also characteristic of mPRs. The results indicate that the progestin receptor functions in these cell lines are mediated through mPRs and do not involve any N-terminally truncated PR isoforms.
Key terms: membrane progesterone receptor, mPR, nuclear progesterone receptor, PR, truncated progesterone receptors, breast cancer cells
1. Introduction
In addition to the classic intracellular genomic mechanism of steroid action mediated by nuclear steroid receptors [1], there is extensive evidence that steroids also activate specific receptors on the surface of cells resulting in rapid induction of intracellular signaling transduction pathways and hormonal responses that are often nongenomic [2, 3]. However, despite extensive research over the last decade, the identities of the steroid membrane receptors that act as intermediaries for many of these nonclassical steroid actions remain unresolved and controversial. For example, nuclear progesterone receptors (PRs) have been implicated in progesterone’s rapid activation of second messengers in several cell models [4, 5], whereas the novel membrane progesterone receptors (mPRs) appear to mediate the nonclassical actions of progesterone in others [6, 7]. The mPRs are 7-transmembrane 40 kDa proteins that are unrelated to the nuclear steroid receptor and G protein coupled receptor superfamilies, but instead belong to the newly described progestin and adipoQ receptor (PAQR) family [8, 9].
The mPRs were discovered in spotted seatrout ovaries where an mPR subtype, named mPRalpha (mPRα), was shown to function as a progesterone membrane receptor and act as an intermediary in the progestin induction of oocyte maturation by a nongenomic mechanism [6]. Subsequently mPRα and two related proteins, mPRβ and mPRγ, were identified in other vertebrates, including humans, and were also shown to have the binding characteristics of progesterone membrane receptors [10]. The functional characteristics of mPRs, especially mPRα, have been extensively studied in various cell models since their discovery in 2003 [7]. Recombinant human, spotted seatrout and goldfish mPRα proteins expressed on PR-negative MDA-MB-231 breast cancer cell membranes display high-affinity, limited-capacity, specific progestin binding typical of membrane progestin receptors, with highest binding affinities for their endogenous progestin hormones, progesterone, 17,20β,21-trihydroxy-4-pregnen-3-one, and 17,20β-dihydroxy-4-pregnen-3-one, respectively [9, 11]. The mPRαs have very different progestin binding affinities from those of the PRs which have been exploited to investigate their specific functions in cells which express both types of progesterone receptors [12, 13]. The recombinant mPRαs are coupled to inhibitory G proteins (Gi) in MDA-MB-231 cell membranes and down-regulate adenylyl cyclase activity resulting in decreased cAMP levels [9]. Similar functional characteristics to those of the recombinant mPRα proteins have been reported for endogenous mPRα and mPRβ in human myometrial cells [13], human T lymphocytes and Jurkat cells [14], human SKBR3 breast cancer cells [15], a rodent GnRH neuronal cell line [16], in fish oocytes [17] and in fish granulosa/theca cells [18]. Taken together, these results suggest that the progestin binding and signaling characteristics of mPRs are fundamental functions of these proteins in vertebrate cells.
The progesterone receptor characteristics of mPRs need to be confirmed in vertebrate cells lacking any other progesterone receptors in order to provide definitive proof that these functions are solely attributable to mPRs. The MDA-MB-231 breast cancer cell line was selected for investigating the functions of recombinant mPRs because it lacks the full-length PR [19]. However, N-terminally truncated PR isoforms have been identified in breast cancer tissues and cell lines [20–22] as well as in other tissues [23, 24], which raises the possibility that they are also present in breast cancer cells lacking the full-length PR, but would have not been detected using the commonly used PR primers and antibodies directed against the N-terminus of the receptor. An N-terminally truncated variant of the estrogen receptor (ER), named ERα-36, has recently been detected by Kang and coworkers in SKBR3 breast cancer cells [25] which lack the full-length ER, but express the 7-transmembrane membrane estrogen receptor, GPR30 [26]. They also detected ERα-36 in HEK-293 cells [25] which have been used to investigate the estrogen receptor functions of recombinant GPR30 [27]. On the basis of their results showing that membrane ERα-36 expression was up-regulated in the HEK293 cells after transfection with human GPR30, Kang et al. proposed that the increased estrogen receptor functions in the GPR30-transfected cells were associated with the increase in ERα-36 protein levels and not with the increase in GPR30 levels [25]. While these conclusions are inconsistent with our findings with endogenous fish GPR30s [28], and are not supported by the results of our recent studies on human GPR30, they do indicate a need to investigate the potential role of truncated forms of nuclear steroid receptors in nonclassical steroid signaling proposed to be mediated through novel steroid membrane receptors.
The occurrence of N-terminally truncated forms of the PR in MDA-MB-231 cells and in MDA-MB-231 cells transfected with mPRα or mPRβ was investigated in the present study. Progesterone membrane receptor binding and G protein activation through endogenous mPRs has not been investigated in a full-length PR-negative breast cancer cell line, such as MDA-MB-468 cells. Therefore, progesterone binding and signaling through endogenous mPRs and the effects of mPR knockdowns were investigated in MDA-MB-468 cells, as well as the presence of N-terminally truncated PR isoforms in these breast cancer cells.
2. Materials and Methods
Expression of recombinant mPRs and cell culture
Human mPRα or mPRβ cDNA was amplified, ligated into a pBK-CMV expression vector and transfected into MDA-MB-231 human breast cancer cells with Lipofectamine as described previously [9]. Control MDA-MB-231 cells were transfected with an empty pBK-CMV vector at the same time. Stably-transfected mPRα, mPRβ, or empty vector cell lines were established by continued selection with geneticin (G-418, Invitrogen, Carlsbad, CA) over several weeks. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS) as described previously [9]. T47D Yb and MDA-MB-468 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in L-15 medium supplemented with 10% FBS and antibiotics. .
Preparation of plasma membranes and cell lysates
Cells were grown in 15 cm culture dishes to 80% confluence, washed 3 times with ice-cold PBS and then harvested with a cell scraper in HAED (25 nM Hepes, 10 mM NaCl, 1 mM dithioerythritol, 1 mM EDTA, pH 7.6) buffer [9]. Cells were homogenized using a handheld glass tissue grinder (Wheaton Tenbroek, Fisher Scientific, Pittsburgh, PA) and centrifuged at 1000 × g for 7 min to remove nuclei. The supernatant was centrifuged at 20,000 × g for 20 min to pellet the plasma membranes. The pellets were reconstituted in HAED buffer and the membranes were enriched by layering them on a 1.2M sucrose pad and centrifugation for 40 min at 9600 × g. The enriched plasma membrane layers were pelleted by centrifugation at 20,000 × g for 20 min and reconstituted in HAED for subsequent analysis. Cells were lysed in the culture dish by incubation with RIPA buffer (Pierce, Rockford, IL) for 30 min at 4°C. The cell lysate sample was transferred to a tube, shaken for 30 min at 4°C, centrifuged to pellet the cellular debris, and the supernatant was used for Western blot analysis.
RT-PCR
Primers were designed according to the sequence of human progesterone receptor (GenBank access number M15716) within the region of 2233–2977 base pairs, which corresponds to the ligand binding domain (LBD, amino acids 687–933, Fig. 1A) in the C terminus of PR; sense1: 5′-GAG CTT AAT GGT GTT TGG TC-3′ (2443); antisense1: 5′-GTT TGA CTT CGT AGC CCT T-3′ (2692); sense2: 5′-GAA GGG CTA CGA AGT CAA A-3′ (2672); antisense2: 5′GCA GCA ATA ACT TCA GAC ATC-3′ (2919). Total RNA was extracted from the cell lines using Tri-reagent (Sigma-Aldrich) following the manufacturer’s instructions. Reverse transcription (RT) of five micrograms of total RNA from each cell line was conducted with SuperScript III transcriptase (Invitrogen) at 50°C for 1 hour. The RT products (0.5 μl/cell line) were amplified through PCR in 20 μl Mastermix (Promega, Madison WI) containing 200 nM of each sense and antisense primer. The PCR amplification protocol consisted of 35 cycles of 95°C for 30 sec, 55°C for 30 sec and 72°C for 1 min, followed by a 72°C, 10 min extension step. The PCR products were separated by 1% agarose electrophoresis and then visualized by ethidium bromide staining.
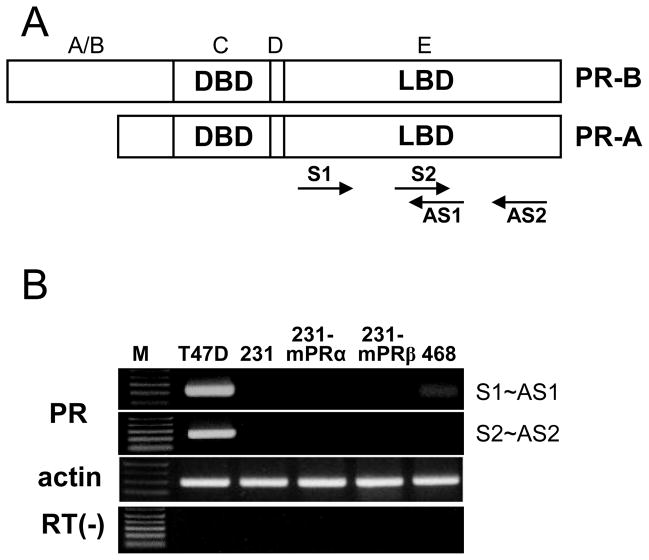
Fig. 1.
C terminus PR primer design and RT-PCR detection of C terminus PR mRNA expression in breast cancer cell lines. A, Schematic diagram of encoded protein showing DNA binding domain (DBD) and ligand binding domain (LBD). Two pairs of primers were designed within the range of LBD. S1 and S2, sense primer 1 and 2; AS1 and AS2, antisense primer 1 and 2. B, RT-PCR results. M: DNA size makers; T47D: PR positive T47D Yb cells; 231: MDA-MB-231 cells; 231-mPRα: human mPRα transfected MDA-MB-231 cells; 231-mPRβ: human mPRβ transfected MDA-MB-231 cells; 468: MDA-MB-468 cells.
Western blot analysis
Western blot analyses were performed as described previously [9]. Briefly, plasma membranes or cell lysates were solubilized by incubation in 5 × reducing sample buffer (Pierce) for 15 min at room temperature, loaded onto a 10% poly-acrylamide gel and the proteins were separated by PAGE. Recombinant α-actinin, obtained from PROSPEC (Rehovot, Israel), was also loaded onto some of the gels. The proteins were transferred from the gels onto nitrocellulose membranes, washed 3 times with TBS and blocked with 5% non fat milk, prior to incubation overnight at 4°C in PBS + 5% milk containing the primary antibodies (1:1000). Two C-terminal PR antibodies, C-19 and 2C11F11, purchased from Santa Cruz Biotechnology (Santa Cruz, CA), were evaluated. The membrane was then incubated with secondary antibody (LI-COR, fluorescent conjugated) for 1 hr at room temperature. The membranes were washed 3 times and scanned on an Odyssey® Infrared Imaging System (LI-COR Biosciences. Lincoln, Nebraska).
Progesterone membrane receptor binding assay
Binding of [3H]-progesterone ([3H]-P4, [2,4,6,7-3H]-progesterone, 102.1Ci/mmol, GE Healthcare, Piscataway, NJ) to plasma membranes prepared from MDA-MB-468 cells was conducted as described previously [9]. Plasma membrane pellets reconstituted in ice-cold HAED buffer (0.5 mg protein/ml) were added to one set of tubes containing [3H]-P4 alone (total binding) and another set of tubes containing [3H]-P4 and 100-fold excess non-radiolabeled P4 (non specific binding) and incubated at 4°C for 30 min. Progesterone bound to the plasma membranes was separated from free by rapid filtration through Whatman GF/B glass fiber filters presoaked in assay buffer using a Brandel Semi Auto Harvester (Gaithersberg, MD). The filters were washed twice with 12.5 ml assay buffer and the bound radioactivity was measured by scintillation counting. For saturation analysis [3H]-P4 over the range of 0.5–8.0 nM was incubated with the membrane fractions. For competition assays, competitors over the concentration range of 10−5 to 10−10 M were incubated with 2 nM [3H]-P4 and plasma membrane preparations and the displacement of [3H]-P4 binding by the steroid competitors was expressed as a percentage of the maximum specific binding of progesterone. Saturation and Scatchard analyses were conducted by nonlinear regression using the Prism GraphPad program (GraphPad Software, San Diego, CA). Affinity (Kd) and Bmax of [3H]-P4 binding was calculated from nonlinear curve fitting.
GTPγS binding to cell membranes
Activation of G proteins after progesterone treatment was determined by measuring the increase in specific binding of [35S]-GTPγS to plasma membranes as described previously [9]. Membranes (~10 μg membrane protein) were incubated for 30 min at 25°C in the presence of 5, 20 and 100 nM of progesterone with 0.5 nM [35S]-GTPγS (12,000 cpm, 1 Ci/mol; GE Healthcare) in the absence (TB) or presence (NSB) of 100 μM GTPγS. Bound [35S]-GTPγS was separated from free by filtering the incubation mixture through Whatman GF/B glass fiber filters followed by several washes. The radioactivity on each filters were measured using a scintillation counter.
Adenylyl cyclase activity in MDA-MB-468 cells
The production of cAMP by MDA-MB-468 cells over a 15 min incubation period at 37°C was measured as a indication of adenylyl cyclase activity in response to progesterone treatments (20, 100 and 200 nM). Cellular cAMP concentrations were measured with cAMP EIA kit (Cayman Chemical, Ann Arbor, MI) following the manufacturer’s instructions.
mPRα siRNA transfection of MDA-MB-468 cells
Human mPRα (GenBank access number: NM_178422), human mPRβ (NM_133367), and non-target control siRNA oligos (ON-TARGET plus SMARTpool, Dharmacon, Lafayette, CO) were transiently transfected into MDA-MB-468 cells as described previously [7]. Briefly, MDA-MB-468 cells, cultured in 15 cm culture dishes until 60% confluent, were transfected twice with 15 ml siRNA mix containing Opti-mem solution (Invitrogen), 3% Lipofectamine 2000 (Invitrogen) and 75 μl of mPRα or mPRβ siRNA oligo solution (20 μM, Invitrogen). The cells were co-incubated with the siRNA mix (100 nM siRNA oligos) for 36–48 hr before they were harvested for analysis. Knockdown of mPR mRNAs was confirmed by RT-PCR using the following primers: mPRα sense 5′-CTT TGC CCT GCT GTG TGA TC-3′, antisense 5′-CAC TGC CAA ACT GGT ACA CG-3′; mPRβ sense 5′-CGC TAC TAC TTC TTC AGC CT-3′, antisense 5′-GCA ACA GCC AGC ACA AGA T-3′.
3. Results
Detection of the PR C terminus in breast cancer cell lines by RT-PCR
PCR products were only detected in PR-positive T47D Yb cells (Fig. 1B) using primers within the LBD of PR (Fig. 1A). A strong band was detected in the T47D Yb cells after 35 cycles of PCR. In contrast, no PCR products were obtained with the PR C-terminus primers from RT-PCR of mRNA extracted from the PR-negative MDA-MB-231 and MDA-MB-468 cell lines, or in human mPRα- and mPRβ-transfected MDA-MB-231 cells (Figure 1B).
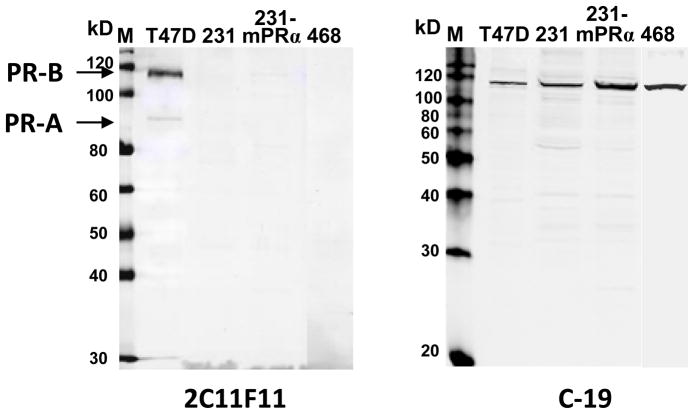
Western blot analyses of breast cancer cell lines using two C-terminal PR antibodies
Different results were obtained on Western blots of the breast cancer cell lines with the two C-terminal PR antibodies. Strong immunoreactive bands at approximately 110kD were detected in all the cell lines using the Santa Cruz C-19 PR antibody, whereas the Santa Cruz 2C11F11 antibody did not detect any bands in the PR-negative cell lines. The 2C11F11 antibody detected two strong bands in the PR-positive T47D cell samples at~95 and ~115 kD, corresponding to the molecular masses of PR-A (95 kD) and PR-B (116 kD) [29].
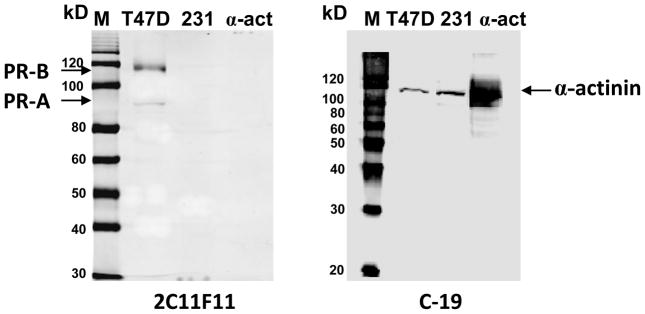
The specificity of the two antibodies for PR and its isoforms was evaluated by comparing the location on Western blots of the immunoreactive bands from breast cancer cell line extracts with that of recombinant human α-actinin. The C-19 antibody detected a strong, diffuse recombinant human α-actinin band on the Western blot which corresponded to the positions of the ~110 kD immunoreactive bands detected in extracts of MDA-MB-231 plasma membranes and T47D cell lysates (Fig. 3). In contrast, the 2C11F11 antibody did not detect human recombinant α-actinin by Western blot analysis (Fig. 3). In agreement with the results in Fig. 2, the 2C11F11 PR antibody did not show any immunoreactive bands in a plasma membrane extract of MDA-MB-231 cells, but recognized two bands in T47D cell samples corresponding to PR-A and PR-B (Fig. 3).
Fig. 3.
Western blot analyses of α-actinin and breast cancer cell lines using the two C-terminal PR antibodies. M: prestained (left) and Western (right) protein size marker; T47D: T47D Yb cell lysate (40 μg); 231: MDA-MB-231 cell membrane (12 μg); α-act: α-actinin protein (~0.5 μg). Left panel, detected with 2C11F11 anti PR C-terminus antibody. Right panel, detected with C-19 antibody.
Fig. 2.
Western blot analyses of PR-positive and PR-negative breast cancer cell lines using two C-terminal PR antibodies. M: prestained (left) and Western (right) protein size marker; T47D: T47D Yb cell lysate; 231: MDA-MB-231 cell lysate; 231-mPRα: mPRα transfected MDA-MB-231 cell lysate; 468: MDA-MB-468 cell lysate. Sample proteins: 40μg/lane. Left panel, detected with 2C11F11 anti PR C-terminus antibody from Santa Cruz. Right panel, detected with C-19 antibody from same source.
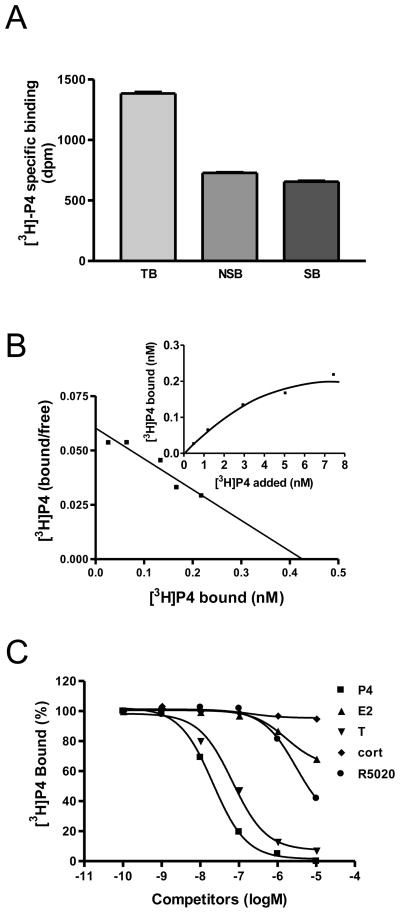
Specific progesterone binding to plasma membranes of MDA-MB-468 cells
A single point binding assay showed high amounts of specific [3H]-P4 binding (~ 50% of total binding) to plasma membranes of MDA-MB-468 cells (Fig. 4A). Saturation and Scatchard analyses of specific [3H]-P4 binding demonstrated the presence of a high affinity (Kd=6.03 nM) and limited-capacity (Bmax=0.387 nM) single binding site (Fig. 4B). Among the steroids tested, progesterone showed highest binding affinity for the membrane progesterone receptor (Fig. 4C). Testosterone displayed 5-fold lower binding affinity than progesterone, whereas estradiol-17β and cortisol showed negligible binding. The potent PR agonist, R5020, showed very low binding affinity for the membrane progesterone receptor, approximately 500 times lower than that of progesterone, displacing ~50% [3H]-P4 at a concentration of ~10 μM (Fig. 4C).
Fig. 4.
Steroid binding characteristics of membrane progesterone receptor on MDA-MB-468 cells. A, specific [3H]-P4 binding to plasma membranes in a single point radioreceptor assay. TB: total binding; NSB: non-specific binding; SB: specific binding. B, Saturation and Scatchard analyses of [3H]-P4 binding to plasma membranes. C, Competition curves for steroid binding to plasma membranes of MDA-MB-468 cells expressed as a percentage of maximum specific [3H]-P4 binding. P4: progesterone; E2: estradiol; T: testosterone; cort: cortisol; R5020: promegestone.
Effects of progesterone treatment on G protein activation and cAMP production by MDA-MB-468 cells
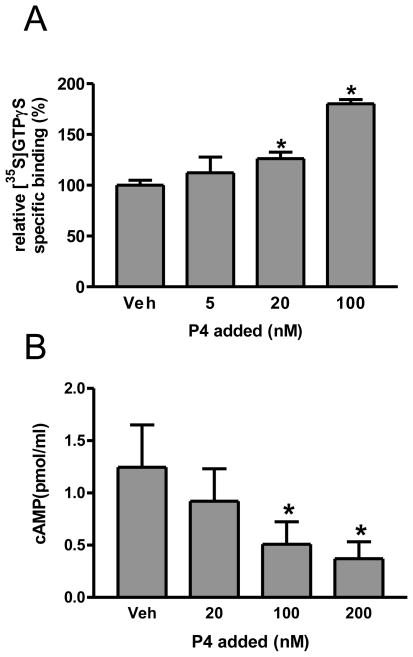
Treatment of MDA-MB-468 cells with 20 and 100 nM progesterone significantly increased [35S]-GTPγS binding to the cell membranes in a concentration-dependent manner compared to the vehicle controls (Fig. 5A). Treatment with 100 and 200 nM progesterone also caused a concentration-dependent significant decrease in intracellular cAMP levels compared to the vehicle controls (Fig. 5B).
Fig. 5.
Effects of progesterone treatment on G protein activation and cAMP levels in MDA-MB-468 cells A, Specific [35S]GTPγS binding to plasma membranes after 30 min treatment with progesterone(5–100 nM). Veh: vehicle control; *: P<0.05 compared to Veh. N=3. B, Intracellular cAMP levels after 15 min treatment with progesterone (20–200 nM). Veh: vehicle control; *: P<0.05 compared to Veh, N=3.
Effects of transfection of MDA-MB-468 cells with siRNAs for mPRα and mPRβ on [3H]-P4 binding and G protein activation
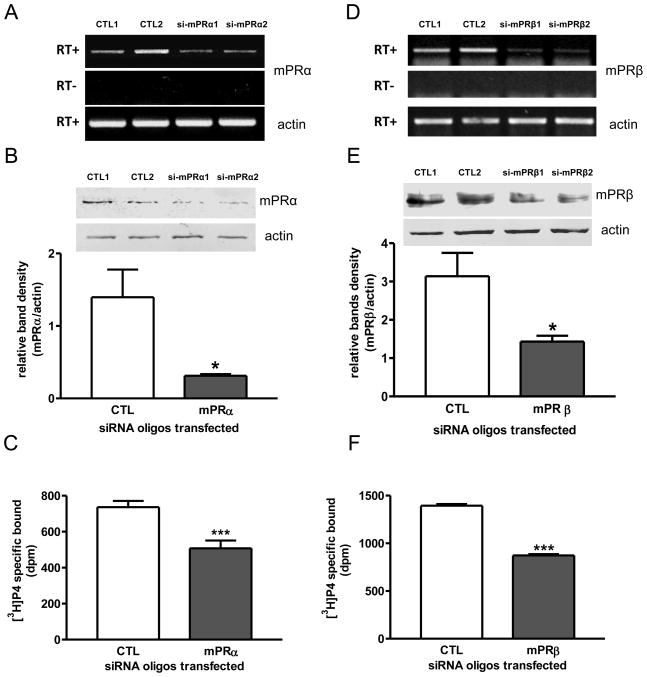
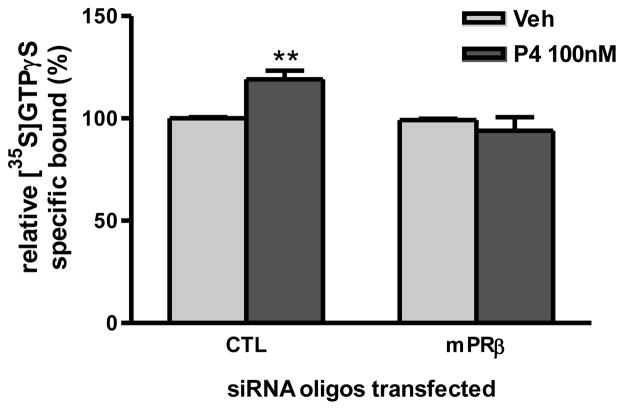
Transfection of MDA-MB-468 cells with mPRα siRNA oligos caused decreases in both mRNA and membrane protein mPRα expression in the cells (Fig. 6A, 6B). The decrease in mPRα expression in the cells after the mPRα siRNA oligo treatment was accompanied by a significant decrease in specific [3H]-P4 binding to plasma membranes prepared from the cells (Fig. 6C). Similarly, transfection of mPRβ siRNA oligos caused decreased mPRβ expression (Fig. 6D, 6E) and a significant decrease in specific [3H]-P4 binding (Fig. 6F). In addition, the G protein activation response to progesterone treatment was lost after knockdown of mPRβ expression by transfection with mPRβ siRNA (Fig. 7).
Fig. 6.
Effects of transfection of human mPRα or mPRβ siRNAs into MDA-MB-468 cells on mPR expression and plasma membrane [3H]-P4 binding. A, D, RT-PCR detection of mPRα (A) and mPRβ (D) mRNA levels in control (CTL) and mPRα siRNA (si-mPRα) (A) or mPRβ siRNA (si-mPRβ) (D) -transfected cells. RT+ and −: positive and negative reverse transcription. B, E, Plasma membrane mPRα (B) and mPRβ (E) protein levels of cells transfected with CTL and mPR siRNA oligos. *: p<0.05 compared to CTL. C, F, [3H]-P4 specific binding to plasma membrane of CTL and mPR siRNA oligo-transfected cells. ***: p<0.001 compared to CTL. N=3.
Fig. 7.
Effects of transfection of human mPRβ siRNA into MDA-MB-468 cells on G protein activation in control (CTL) and mPRβ siRNA (si-mPRβ)-transfected cells. A single large batch of cells was transfected with siRNA for both the G protein activation experiments and for steroid binding studies shown in Fig.6. The effects of siRNA treatment on mPRβ mRNA and plasma membrane protein levels are shown in Fig, 6D and 6F. ***: p<0.001 compared to CTL. N=3.
4. Discussion
The results demonstrate that N-terminal truncated isoforms of the PR are not present in the MDA-MB-231 and MDA-MB-468 breast cancer cell lines. MDA-MB-231 cells have been used extensively to over-express mPRs for detailed investigations of their functional characteristics [6, 7, 9, 11, 12, 30], whereas progesterone binding and signaling through endogenous mPRs were investigated in MDA-MB-468 cells in the present study. The finding that no C terminus PR or full-length PR transcripts and proteins could be detected by RT-PCR or with the specific 2C11F11 C-terminal PR antibody in either of these breast cancer cell lines, or in mPRα- or mPRβ-transfected MDA-MB-231 cells, indicates that none of the purported receptor functions of mPRs in these cells can be explained by the presence of any form of the PR. In contrast, the results of the mPR siRNA transfection studies, showing that down-regulation of mPRα or mPRβ protein expression in the MDA-MB-468 cells is associated with significant decreases in membrane [3H]-P4 binding and loss of G protein activation, clearly implicate the mPRs in progesterone signaling in these cells. The present results confirming that progesterone signaling in these PR-negative breast cancer cell lines is mediated solely through mPRs provide further evidence that the mPRs function as a distinct class of membrane steroid receptors mediating nonclassical progestin actions in vertebrate cells.
The existence of a various isoforms of the full-length PR, PR-B, lacking different portions of the N-terminal region have been proposed over the past 20 years since the identification of a PR, named PR-A, lacking part of the N-terminal A/B domain [21, 29]. Additional PR variants were predicted from the extensive 5′ heterogeneity in human PR transcripts seen in Northern blots [31]. An ~60 kDa N-terminally truncated PR isoform, PR-C, lacking the A/B, DNA binding (C domain) and hinge region (D domain) of the PR was detected in T47D breast cancer cells [21], and as well as evidence that it caused enhanced progestin-induced transcriptional activity and was capable of forming heterodimers with PR-B [32, 33]. The PR-C isoform was also detected in myometrial cells and Western blot analysis using the C-19 antibody suggesting that PR-C levels were upregulated during labor led the authors to propose that this truncated PR was associated with progesterone withdrawal at term [24]. However, the existence of PR-C and the specificity of the C-19 antibody have been challenged by other investigators [34, 35]. Sequencing of the proteins in the C-19 antibody immunoreactive bands in Western blots of human myometrial cell extracts by Madson et al. revealed that they were not PR isoforms but instead were the highly abundant cytoskeletal protein α-actinin, as well as the less abundant desmin and vimentin [34]. The present results are consistent with these findings indicating that the C-19 antibody is not specific for PR isoforms. The single immunoreactive band detected in both in T47D Yb and in two PR-negative breast cancer cell lines using the C-19 antibody corresponds to the position on the Western blot of recombinant human α-actinin which we confirmed was also detected with the antibody.
Other N-terminally truncated PR isoforms have been reported in breast cancer cells and tissues. A smaller PR isoform than PR-A, PR78KDa, has been detected in human breast tumor tissue samples using monoclonal antibodies, but its functional significance is unknown [20]. Another truncated PR, cloned from human adipose and aortic cDNA libraries, PR-M, encodes a 38 kDa protein and has been detected in T47D breast cancer cells using the C-19 C-terminus PR antibody [22]. PR-M has been proposed to mediate non-genomic actions of progesterone, but its receptor functions have not been investigated [22]. In contrast, only two immunoreactive protein bands, corresponding to the apparent molecular weights of PR-B and PR-A were detected on Western blots of lysates of T47D cells using the 2C11F11 C-terminal PR antibody in the present study. No evidence was obtained for the existence of PR-C, PR78kDa, PR-M or any other N-terminally truncated PR isoforms in T47D and the other breast cancer cells using this antibody. A large number of faint nonspecific bands were detected with the C-19 antibody after overloading the lanes (40μg/lane) with lysates from MDA-MB-231 cells, but none of them corresponded to the positions of any previously described truncated PR isoforms. These results are consistent with those of Samaleco and Gellersen who conducted an extensive evaluation of all the commercially available C-terminal PR antibodies with T47D and MDA-MB-231 cells after systematic expression of all the possible truncated PRs [35]. They concluded that their results do not support the existence of naturally occurring PR-C, PR-M or other truncated PR isoforms in vivo or in these breast cancer cells and that the C-19 antibody is not specific and should not be used to screen for truncated PRs [35].
Recent studies showing increased abundance of mPRs in malignant breast tissue [15] and inhibitory effects of progesterone treatment on epithelial to mesenchymal transition (EMT) in MDA-MB-468 cells resulting in a more invasive phenotype [36], suggest mPRs have important roles in breast cancer. Zuo and coworkers have shown that progesterone activates PI3K, EGFR and down-stream signals in MDA-MB-468 cells [36], but [3H]-P4 membrane binding and G protein activation through endogenous mPRs have not been demonstrated in these cells or in any other PR-negative breast cancer cells. The present results demonstrate that plasma membranes of MDA-MB-468 cells have a high affinity, limited-capacity, single binding site for progesterone with a Kd (6.03 nM), similar to those human (4.17 nM), spotted seatrout (7.58 nM), goldfish (3.9 nM), and zebrafish (7 nM) recombinant mPRs [9, 11, 30]. The binding affinities of mPRs are considerably lower than those of PRs in human uterus and oviduct (Kd 0.82 nM and 0.75 nM), ovine uterus (1.6 nM), seatrout ovary (1.89 nM) and zebrafish recombinant PR (1.70 nM) [37–40]. The steroid specificity of the progesterone receptor on MDA-MB-468 cells is also characteristic mPRs, showing a very low binding affinity (≤1%) for the potent PR agonist, R5020 [9, 12]. The results of the mPR siRNA transfection experiments clearly demonstrate that the [3H]-P4 binding to MDA-MB-468 cells membranes is associated with the presence of both mPRα and mPRβ, since decreased protein expression of either mPR subtype resulted in a decrease in specific [3H]-P4 binding. However, the other mPR subtypes, such as mPRγ which is also expressed in these cells, may also contribute to membrane [3H]-P4 binding.
Brosens and coworkers failed to express recombinant mPRs on the plasma membranes of transfected cells or detect specific [3H]-P4 binding, and on the basis of their negative findings have strongly asserted that the mPRs do not function as membrane progesterone receptors [41]. Some possible reasons for the discrepancies between their results and those obtained by several other laboratories with mammalian and bacterial expression systems have been discussed previously [7]. Recently, it has been demonstrated that mPRs heterologously expressed in third expression system, yeast, can be activated through a reporter system by low concentrations of progestins [42]. In addition, a molecular model describing the physicochemical requirements for ligand binding utilizing comparative molecular field analysis (CoMFA) has shown that mPRα, like PR, has a well defined single binding site [12]. Moreover, specific [3H]-P4 binding has been shown to plasma membranes of PR-negative cells expressing endogenous mPRs such as Jurkat cells, GT1-7 cells, and MDA-MB-468 cells in the present study [14, 16]. As a result, the ability of mPRs to bind progestins is no longer seriously questioned. However, the ability of mPRs to associate and activate G proteins is still disputed [43]. The present finding that progesterone can activate G proteins in PR-negative MDA-MB-468 cells and that this response is abrogated when mPRβ protein expression is down-regulated provides clear evidence that mPRs activate G proteins. Activation of G proteins and co-immunoprecipitation of G proteins with mPRs have been demonstrated with both recombinant and endogenous mPRs in a variety of cells lines [9, 12, 13, 14, 16, 44], but the present study is the first to demonstrate that decreasing mPR expression impairs G protein activation by progestins. The observation that treatment of MDA-MB-468 cells with progesterone causes concentration-dependent decreases in cAMP production is consistent with the results obtained with endogenous an recombinant human, rodent and fish mPRs in a variety of cell types [7, 13, 14, 16], where it has been shown the mPRs are coupled to an inhibitory G protein.
In summary, the absence of detectable N-terminally truncated isoforms of PR in MDA-MB-231 breast cancer cells, in mPRα- or mPRβ-transfected MDA-MB-231 cells, and in MDA-MB-468 cells, indicates no PR isoforms are involved in the progesterone binding and signaling ascribed to mPRs in these cells. On the other hand, mPRα and mPRβ were shown to be involved in membrane progesterone binding, G protein activation and signaling in MDA-MB-468 cells. Additional studies such as site-directed mutagenesis will be required to determine the structural requirements for steroid binding and G protein coupling to this novel class of 7-transmembrane steroid receptors.
Acknowledgments
We thank Jing Dong for technical assistance and Rebecca Alyea for reading an earlier version of the manuscript. This research was supported by National Institutes of Health grant ESO 12961 to P.T.
Abbreviations
- mPRα
membrane progesterone receptor alpha
- mPRβ
membrane progesterone receptor beta, PR-A, nuclear progesterone receptor A
- PR-B
nuclear progesterone receptor B, PR-C, nuclear progesterone receptor C
- PR-M
nuclear progesterone receptor M
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–86. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 2.Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3:27–41. doi: 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- 3.Revelli A, Massobrio M, Tesarik J. Nongenomic actions of steroid hormones in reproductive tissues. Endocr Rev. 1998;19:3–17. doi: 10.1210/edrv.19.1.0322. [DOI] [PubMed] [Google Scholar]
- 4.Boonyaratnakornkit V, Scott MP, Ribbon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinase. Mol Cell. 2001;8:269–80. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 5.Migliaccio A, Piccolo D, Castoria G, Dimenico MD, Biliancio A, Lombardi M, Gong W, Beato M, Aurichio F. Activation of Src/p21ras. Erk pathway by progesterone via cross-talk with the estrogen receptor. EMBO. 1998;17:2008–118. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A. 2003;100(5):2231–6. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas P. Characteristics of membrane progestin receptor alpha (mPRα) and progesterone membrane receptor component one (PGRMC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol. 2008;29:292–312. doi: 10.1016/j.yfrne.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61:372–80. doi: 10.1007/s00239-004-0375-2. [DOI] [PubMed] [Google Scholar]
- 9.Thomas P, Pang Y, Dong J, Groenen P, Kelder J, de Vlieg J, Zhu Y, Tubbs C. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins. Endocrinology. 2007;148(2):705–18. doi: 10.1210/en.2006-0974. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci U S A. 2003;100(5):2237–42. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tokumoto T, Tokumoto M, Thomas P. Interactions of diethylstilbestrol (DES) and DES analogs with membrane progestin receptor-alpha and the correlation with their nongenomic progestin activities. Endocrinology. 2007;148(7):3459–67. doi: 10.1210/en.2006-1694. [DOI] [PubMed] [Google Scholar]
- 12.Kelder J, Azevedo R, Pang Y, de Vlieg J, Dong J, Thomas P. Comparison between steroid binding to membrane progesterone receptor alpha (mPRalpha) and to nuclear progesterone receptor: correlation with physicochemical properties assessed by comparative molecular field analysis and identification of mPRalpha-specific agonists. Steroids. 2010;75(4–5):314–22. doi: 10.1016/j.steroids.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P. Progesterone signaling in human myometrium through two membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006;20(7):1519–34. doi: 10.1210/me.2005-0243. [DOI] [PubMed] [Google Scholar]
- 14.Dosiou C, Hamilton AE, Pang Y, Overgaard MT, Tulac S, Dong J, Thomas PP, Giudice LC. Expression of membrane progesterone receptors on human T lymphocytes and Jurkat cells and activation of G-proteins by progesterone. J Endocrinol. 2008;196(1):67–77. doi: 10.1677/JOE-07-0317. [DOI] [PubMed] [Google Scholar]
- 15.Dressing GE, Thomas P. Identification of membrane progestin receptors in human breast cancer cell lines and biopsies and their potential involvement in breast cancer. Steroids. 2007;72(2):111–6. doi: 10.1016/j.steroids.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE. Progesterone receptor A (PRA) and PRB-independent effects of progester. doi: 10.1210/en.2008-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pace MC, Thomas P. Activation of a pertussis toxin-sensitive, inhibitory G-protein is necessary for steroid-mediated oocyte maturation in spotted seatrout. Dev Biol. 2005;285:70–9. doi: 10.1016/j.ydbio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Dressing G, Pang Y, Dong J, Thomas P. Progestin signaling through mPRα in Atlantic croaker granulosa/theca cell co-cultures and its involvement in progestin inhibition of apoptosis. Endocrinology. doi: 10.1210/en.2010-0165. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horwitz K, Zava D, Thilangar A, Jensen E, McGuire W. Steroid receptor analyses of nine human breast cancer cell lines. Cancer Res. 1978;38:2434–2437. [PubMed] [Google Scholar]
- 20.Yeates C, Hunt SM, Balleine RL, Clarke CL. Characterization of a truncated progesterone receptor protein in breast tumors. J Clin Endocrinol Metab. 1998;83(2):460–7. doi: 10.1210/jcem.83.2.4531. [DOI] [PubMed] [Google Scholar]
- 21.Wei LL, Miner R. Evidence for the existence of a third progesterone receptor protein in human breast cancer cell line T47D. Cancer Res. 1994;54(2):340–3. [PubMed] [Google Scholar]
- 22.Saner KJ, Welter BH, Zhang F, Hansen E, Dupont B, Wei Y, Price TM. Cloning and expression of a novel, truncated, progesterone receptor. Mol Cell Endocrinol. 2003;200(1–2):155–63. doi: 10.1016/s0303-7207(02)00380-5. [DOI] [PubMed] [Google Scholar]
- 23.Younglai EV, Wu Y, Foster WG, Lobb DK, Price TM. Binding of progesterone to cell surfaces of human granulosa-lutein cells. J Steroid Biochem Mol Biol. 2006;101(1):61–7. doi: 10.1016/j.jsbmb.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Condon JC, Hardy DB, Korvaric K, Mendelson CR. Upregulation of progesterone receptor (PR) C isoform in laboring myometrium by nuclear factor-κ-B may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2002;20:764–75. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- 25.Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, Wang ZY. Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24(4):709–21. doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–60. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 27.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146(2):624–32. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 28.Pang Y, Dong J, Thomas P. Estrogen signaling characteristics of Atlantic croaker G protein-coupled receptor 30 (GPR30) and evidence it is involved in maintenance of oocyte meiotic arrest. Endocrinology. 2008;149(7):3410–26. doi: 10.1210/en.2007-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. Embo J. 1990;9(5):1603–14. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanna R, Pang Y, Thomas P, Zhu Y. Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors alpha and beta in transfected cells. J Endocrinol. 2006;190(2):247–60. doi: 10.1677/joe.1.06694. [DOI] [PubMed] [Google Scholar]
- 31.Wei LL, Gonzalez-Aller C, Wood WM, Miller LA, Horwitz KB. 5′-Heterogeneity in human progesterone receptor transcripts predicts a new amino-terminal truncated “C”-receptor and unique A-receptor messages. Mol Endocrinol. 1990;4(12):1833–40. doi: 10.1210/mend-4-12-1833. [DOI] [PubMed] [Google Scholar]
- 32.Wei LL, Hawkins P, Baker C, Norris B, Sheridan PL, Quinn PG. An amino-terminal truncated progesterone receptor isoform, PRc, enhances progestin-induced transcriptional activity. Mol Endocrinol. 1996;10(11):1379–87. doi: 10.1210/mend.10.11.8923464. [DOI] [PubMed] [Google Scholar]
- 33.Wei LL, Norris BM, Baker CJ. An N-terminally truncated third progesterone receptor protein, PR(C), forms heterodimers with PR(B) but interferes in PR(B)-DNA binding. J Steroid Biochem Mol Biol. 1997;62(4):287–97. doi: 10.1016/s0960-0760(97)00044-7. [DOI] [PubMed] [Google Scholar]
- 34.Madsen G, Macintyre DA, Mesiano S, Smith R. Progesterone receptor or cytoskeletal protein? Reprod Sci. 2007;14(3):217–22. doi: 10.1177/1933719107302380. [DOI] [PubMed] [Google Scholar]
- 35.Samalecos A, Gellersen B. Systematic expression analysis and antibody screening do not support the existence of naturally occurring progesterone receptor (PR)-C, PR-M, or other truncated PR isoforms. Endocrinology. 2008;149(11):5872–87. doi: 10.1210/en.2008-0602. [DOI] [PubMed] [Google Scholar]
- 36.Zuo L, Li W, You S. Progesterone reverses the mesenchymal phenotypes of basal phenotype breast cancer cells via a membrane progesterone receptor mediated pathway. Breast Cancer Res. 2010;12(3):R34. doi: 10.1186/bcr2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukola A, Punnonen R. Estrogen and progesterone receptors in human uterus and oviduct. J Endocrinol Invest. 1983;6(3):179–83. doi: 10.1007/BF03350604. [DOI] [PubMed] [Google Scholar]
- 38.Bauer MA, Gorell TA. Analysis of progesterone receptor binding in the ovine uterus. Steroids. 1980;36(5):581–91. doi: 10.1016/0039-128x(80)90080-x. [DOI] [PubMed] [Google Scholar]
- 39.Pinter J, Thomas P. The ovarian progestogen receptor in the spotted seatrout, Cynoscion nebulosus, demonstrates steroid specificity different from progesterone receptors in other vertebrates. J Steroid Biochem Mol Biol. 1997;60(1–2):113–9. doi: 10.1016/s0960-0760(96)00177-x. [DOI] [PubMed] [Google Scholar]
- 40.Hanna RN, Daly SC, Pang Y, Anglade I, Kah O, Thomas P, Zhu Y. Characterization and expression of the nuclear progestin receptor in zebrafish gonads and brain. Biol Reprod. 2010;82(1):112–22. doi: 10.1095/biolreprod.109.078527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krietsch T, Fernandes MS, Kero J, Losel R, Heyens M, Lam EW-F, Huhtaniemi I, Brosens JJ, Gellersen B. Human homologs of the putative G protein-coupled membrane progestin receptors mPR{alpha}, {beta}, and {gamma} localize to the endoplasmic reticulum and are not activated by progesterone. Mol Endocrinol. 2006;20:3146–64. doi: 10.1210/me.2006-0129. [DOI] [PubMed] [Google Scholar]
- 42.Smith JL, Kupchak BR, Garitaonandia I, Haong LK, Maina AS, Regall LM, Lyons LK. Heterologous expression of human mPRα, mPRβ and mPRγ in yeast confirms their ability to function as membrane progesterone receptors. Steroids. 2008;73:1160–73. doi: 10.1016/j.steroids.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gellersen B, Fernandes MS, Brosens JJ. Non-genomic progesterone actions in female reproduction. Humnan Reproduction Update. 2009;15:119–38. doi: 10.1093/humupd/dmn044. [DOI] [PubMed] [Google Scholar]
- 44.Tubbs C, Thomas P. Progestin signaling through an olfactory G protein and membrane progestin receptor-alpha in Atlantic croaker sperm: potential role in induction of sperm hypermotility. Endocrinology. 2009;150(1):473–84. doi: 10.1210/en.2008-0512. [DOI] [PubMed] [Google Scholar]