Abstract
Various ovarian cell types including granulosa cells and ovarian surface epithelial cells express the progesterone (P4) binding protein, Progesterone Receptor Membrane Component-1 (PGRMC1). PGRMC1 is also expressed in ovarian tumors. PGRMC1 plays an essential role in promoting the survival of both normal and cancerous ovarian cell in vitro. Given the clinical significance of factors that regulate the viability of ovarian cancer, this review will focus on the role of PGRMC1 in ovarian cancer, while drawing insights into the mechanism of PGRMC1’s action from cell lines derived from healthy ovaries as well as ovarian tumors.
Studies using PGRMC1 siRNA demonstrated that P4’s ability to inhibit ovarian cells from undergoing apoptosis in vitro is dependent on PGRMC1. To confirm the importance of PGRMC1, the ability of PGRMC1-deplete ovarian cancer cell lines to form tumors in intact nude mice was assessed. Compared to PGRMC1-expressing ovarian cancer cells, PGRMC1-deplete ovarian cancer cells formed tumors in fewer mice (80% compared to 100% for controls). Moreover, the number of tumors derived from PGRMC1-deplete ovarian cancer cells was 50% of that observed in controls. Finally, the tumors that formed from PGRMC1-deplete ovarian cancer cells were about a fourth the size of tumors derived from ovarian cancer cells with normal levels of PGRMC1. One reason for PGRMC1-deplete tumors being smaller is that they had a poorly developed microvasculature system. How PGRMC1 regulates cell viability and in turn tumor growth is not known but part of the mechanism likely involves the regulation of genes that promote cell survival and inhibit apoptosis.
Keywords: Progesterone, PGRMC1, Ovary, Ovarian Cancer
1. Introduction
Ovarian cancer kills more women than all the other gynecologic cancers combined and is the fourth leading cause of cancer death among women in the United States. In fact 1 in 57 women will be ultimately diagnosed with ovarian cancer. When this cancer is detected early, the 5-year survival rate is greater than 90%. However, only 24% of the cancers are detected early. As a result most ovarian cancers are detected in more advanced stages in which the cancer cells have spread outside the ovary. Once the ovarian cancer has spread, the 5-year survival rate decreases to less than 25% (See the Johns Hopkins web site for details; http://ovariancancer.jhmi.edu/menu) [1].
Treatment of patients with ovarian cancer consists of surgery to remove the ovary, uterus and the tumor(s). This is usually followed by platinum-based (carboplatin and cisplatin) chemotherapy. In spite of these intense surgical and chemotherapeutic treatments, the ovarian cancer more often than not recurs. At this point the patients are given salvage chemotherapy and possibly de-bulking surgery to remove the tumors that are usually distributed throughout the peritoneum. Again platinum-based chemotherapy is often used to treat the recurrent ovarian cancers but many of the ovarian cancer cells are resistant to these platinum-based agents and thus these drugs are relatively ineffective. Alternatively, higher doses of platinum-based drugs can be tried but the platinum drugs are very toxic so increasing their dosage is not an effective approach (http://ovariancancer.jhmi.edu/menu) [1].
Clearly, the present chemotherapeutic agents are useful but not sufficient to effectively treat ovarian cancer. To improve the effectiveness of traditional chemotherapy, a genetic approach needs to be developed that can be used either alone or in conjugation with traditional chemotherapy. The target of this type of gene-based treatment should be a gene that 1) is highly expressed in ovarian cancers compared to normal tissue, 2) can be depleted by various gene-silencing techniques, such as siRNA and 3) is essential for ovarian cancer cell proliferation and/or survival. Finally, the gene-silencing reagent (i.e. siRNA) should be able to be delivered specifically to the tumor. Based on these criteria, we believe that progesterone receptor membrane component 1 (PGRMC1) is an excellent candidate for the development of such a gene-based treatment for ovarian cancer. The data that supports this concept is outlined in the following sections of this review.
2. Progesterone (P4) and Ovarian Cancers
The first implication that the P4 might be involved in regulating ovarian cancer comes from observations that progestin-containing oral contraceptive usage seems to protect against ovarian cancer (See review by Ho [2]). Moreover, the incidence of ovarian cancer is increased in women with P4 deficiency, while the high levels of serum P4 during pregnancy is associated with lower risks of ovarian cancer. In addition, women with a history of twin pregnancies exhibit a lower risk for developing ovarian cancer, and this may be related to the higher serum levels of P4 in maternal circulation during twin pregnancies compared to singleton pregnancies.
The clinical correlations between P4 and lower incidences of ovarian cancer are also supported to some extent by in vitro studies that demonstrate the ability of high P4 doses to suppress growth and induce apoptosis (See review by Ho [2] and recently confirmed by Fauvet et al [3]). How P4 induces apoptosis is not completely known but one study suggests that P4 makes ovarian cancer cells more sensitive to the killing effects of Tumor Necrosis Factor-related Apoptosis-inducing Ligand [4]. These clinical and basic science studies therefore suggest that P4 might be useful as an adjunct treatment for ovarian cancer. In fact one study using a xenograph ovarian tumor model has shown that high doses of P4 enhance the apoptotic action of cisplatin [5], which is consistent with a very limited clinical trial data [6]. Unfortunately other trials in both animal and humans have been relatively unsuccessful (See review by Ho [2]). The reasons for these variable outcomes is not clear but may be related to our lack of understanding of how P4 mediates its action. For example it is generally assumed that P4 mediates its protective action through the classic nuclear progesterone receptors (PGR-A; PGR-B). This assumption is based in part on the observations that PGR is expressed in some ovarian cancers (Figure 1A, B). In addition cAMP mediated activation of the PGR-B induces cell cycle arrest, cellular senescence and suppresses tumorigenicity of ovarian cancer cells [7]. Moreover, the loss of heterozygosity and/or polymorphisms of PGR are associated with increased ovarian cancer risks, which implies that PGR activation protects against the development of ovarian cancers [8, 9]. In addition PGR is not detected in most ovarian cancers with its absence correlated with a poor prognosis [7,10].
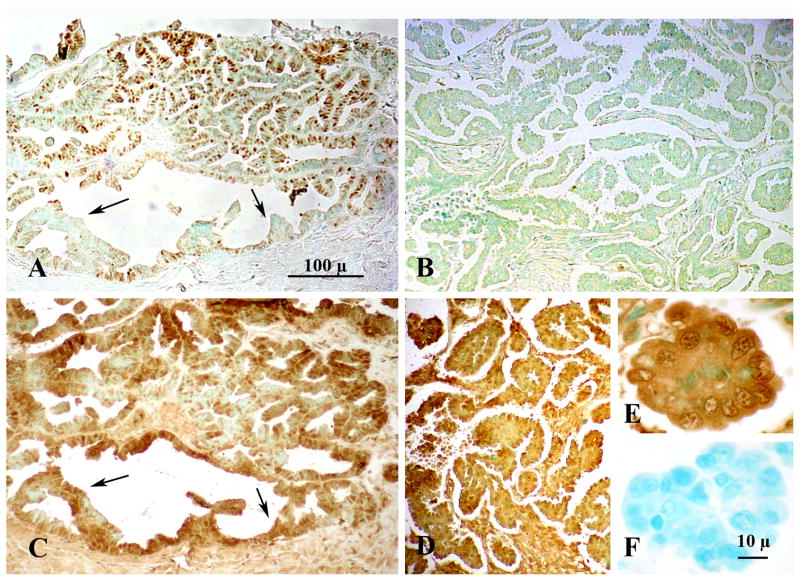
Figure 1.
Immunohistochemical localization of Progesterone Receptor (PGR) (A,B) and Progesterone Receptor Membrane Component-1 (PGRMC1) (C,D) in Stage IIIc Grade 2 (A,C) and Stage IIIc Grade 3 (B,D,E) ovarian tumors. Images from panels A and C and B and D are taken from adjacent sections from respective tumors. Panels A, B C and D are shown at the same magnification. The arrows in panels A and C mark the location of ovarian cancer cells that do not express PGR but do express PGRMC1. The image in panel E is a higher magnification of the image shown in panel D. Panel F is a negative control. Both panel E and F are shown at the same magnification. Data taken from Peluso et al [20].
However, P4’s actions are more complex as revealed by recent studies that show that P4 can inhibit ovarian cancer cells from undergoing cisplatin-induced apoptosis both in vitro and in vivo [11]. P4’s ability to either induce or inhibit apoptosis could explain why the clinical trials have failed to show a beneficial effect of P4 on ovarian cancer progression. The reason for the opposite effect of P4 on ovarian cancer cell survival is unknown but it may be that the anti-apoptotic actions of P4 are not mediated by PGRs. There are other putative mediators of P4’s actions that are expressed in ovarian cancers such as the progestin and adipoQ receptors (PAQR) [12] and progesterone receptor membrane component-1 (PGRMC1) [13]. Both of these progestin receptors have been detected in ovarian cancer cells [11]. Their respective roles are just beginning to be revealed with most of the work being focused on PGRMC1.
3. PGRMC1 Expression in Human Ovarian Tumors
PGRMC1 is small protein with an apparent molecular weight of 28 kDa. It is generally detected in western blots as a band between 22–27 kDa and a band of ≈ 55 kDa. The ≈ 55 kDa band appears to be a dimer, since high concentrations of DTT reduces the amount of this high molecular weight band [14].
Little is known about how PGRMC1 functions. As indicated in the review by Cahill [15], bacterially-expressed PGRMC1 failed to bind P4. However, partially purified PGRMC1-GFP fusion protein, which was expressed in mammalian cells, binds P4 with high affinity (≈ 35 nM for rat PGRMC1 [16] and ≈ 11 nM for human PGRMC1 [17]). Moreover, serum P4 levels are elevated in patients with ovarian cancer compared to aged-matched controls [18]. P4 levels are also higher in the peritoneal fluid of ovarian cancer patients [19]. These findings support the idea that ovarian cancer cells synthesize P4. Therefore in the presence of these elevated levels of P4, PGRMC1 and PGR if present are likely to be continuously active.
As previously indicated some ovarian cancers express PGR but PGRMC1 is present in virtually every cell within all the ovarian tumors that were examined [20] (Figure 1C,D). Quantitative analysis revealed that PGRMC1 mRNA levels increase nearly 2 fold in Stage III and IV ovarian cancers compare to that of non-cancerous ovarian tissue [20], while the mRNA levels of PGR decrease with advance stages of ovarian cancer. As a result the ratio of PGRMC1 mRNA to the PGR mRNA dramatically increases as PGR mRNA declines [20]. Thus changes in the ratio of PGRMC1 to PGR could explain changes in the response of ovarian cancer cells to P4 with the higher PGRMC1 to PGR promoting tumor growth and development.
4. PGRMC1 and Its Role in Regulating Ovarian Cancer Cell Biology In Vitro
To test this hypothesis we used two different ovarian cancer cell lines, Ovcar-3 and SKOV-3 cells. While neither of these cell lines expressed PGR, they both expressed PGRMC1 [20]. As such, these ovarian cancer cell line mimic the progestin receptor profile of more advanced cancer. Interestingly, in these cell lines P4 inhibited cisplatin’s ability to induce apoptosis. P4’s actions were mediated by PGRMC1 since treatment with PGRMC1 siRNA was effectively attenuated P4’s anti-apoptotic action.
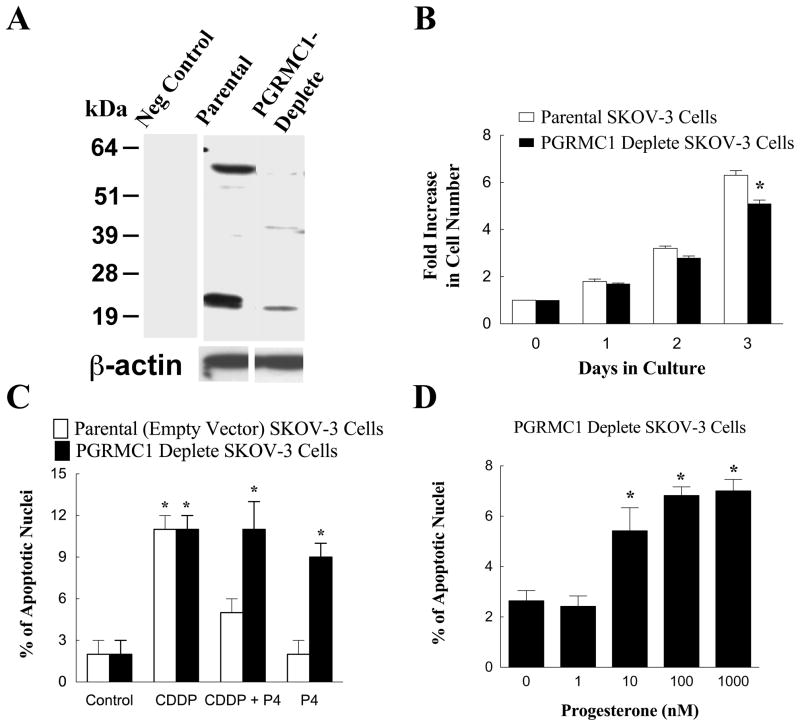
Our next step was to generate an ovarian cancer cell line whose expression of PGRMC1 was permanently depleted. For this approach we used the ovarian cancer cell line SKOV-3. As shown in Figure 2A we were able to generate a cell line whose PGRMC1 levels were reduced by greater than 80% compared to their parental controls. PGRMC1-deplete SKOV-3 cells had an impaired ability to undergo mitosis in vitro compared to SKOV-3 cells (Figure 2B). Surprisingly depleting PGRMC1 also changed the response to P4 from one that antagonizes cisplatin’s apoptotic action to one that induces apoptosis at a rate similar to that of cisplatin (Compare Figure 2C with 2D). Taken together our in vitro studies suggest that PGRMC1 promotes ovarian tumor cell proliferation and depleting PGRMC1 slows cellular proliferation and enhances apoptosis even in the presence of P4.
Figure 2.
The development of a dsRed-SKOV-3 cell line that was depleted in PGRMC1. Panel A is a western blot showing PGRMC1 levels in parental and PGRMC1-deplete dsRed SKOV-3 cells. β-actin western blot is shown to demonstrate equal protein loading. The − sign indicates a lane of a western blot in which the primary antibody was omitted and replaced with IgG (i.e. a negative control). The rate of cell proliferation of parental and PGRMC1-deplete SKOV-3 cells is shown in panel B. The effect of cisplatin (CDDP; 30 nM) and progesterone (P4; 1 μM) on the rate of apoptosis of parental empty vector and PGRMC1-deplete SKOV-3 cells is shown in panel C. The effect of increasing concentrations of P4 on the percentage of PGRMC1-deplete SKOV-3 cells undergoing apoptosis is shown in panel D. Data taken from Peluso et al [11].
5. PGRMC1 and Ovarian Tumor Development in Nude Mice
These in vitro studies are encouraging in that they suggest that tumors derived from PGRMC1-deplete SKOV-3 cells would grow at a much slower rate. To test this we modified our parental and PGRMC1-deplete SKOV-3 cells lines so that both cell lines expressed the fluorescent protein, dsRed. This was done to allow us to inject these cells into the peritoneum of intact nude mice and then be able to detect the tumors derived from these cells by monitoring their fluorescence. It is important to appreciate that it is more difficult to grow and subsequently detect ovarian tumors in the peritoneum as opposed to tumors generated by a subcutaneous injection of tumor cells under the flank skin. However, ovarian tumors do not metastasize to subcutaneous sites but rather form secondary sites throughout the peritoneum [21]. Based on this, we believe that it is more relevant to generate and study peritoneal ovarian tumors even through they are require more sophisticated techniques to monitor their development and function.
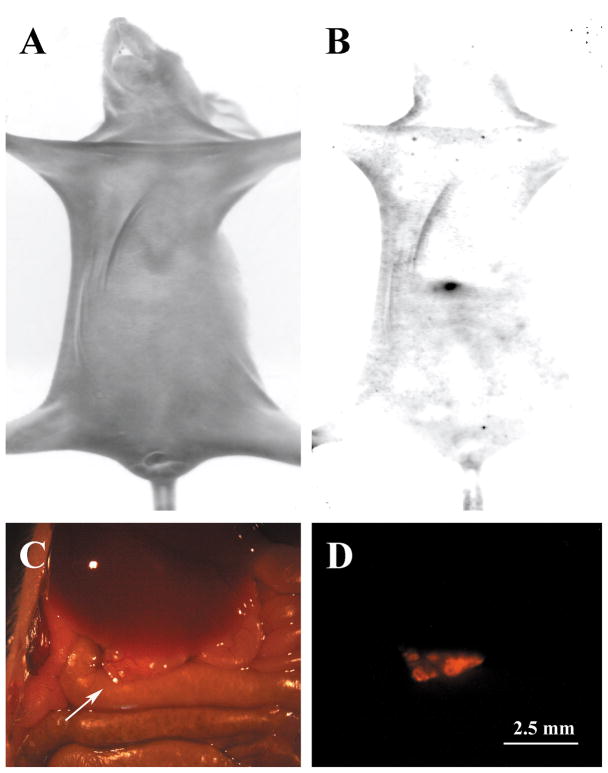
We observed that 5 weeks were required for dsRed SKOV-3 cells to form ovarian tumors that could be observed within the peritoneum of intact nude mice, whose serum P4 levels are approximately 4 ng/ml [22]. Those tumors that formed near the ventral surface of the abdominal wall could be detected in intact living mice using the Kodak Image system (Figure 3A,B). That the fluorescent body observed in Figure 3B corresponds to an ovarian tumor was confirmed by exposing the abdominal cavity and observing the peritoneum using a dissecting microscope equipped with fluorescent optics. Although difficult to detect under brightfield optics (Figure 3C), the tumor was readily detectable when observed under epi-fluorescence (Figure 3D).
Figure 3.
Detection of ovarian tumors within intact living nude mice. The mouse shown in these images was injected with 10 million dsRed SKOV-3 cells and the tumors were allowed to develop for 5 weeks. In panel A, a bright field image of a nude mouse is shown. When observed under dsRed fluorescent filters (B), a tumor was observed (black arrow). This animal was dissected to expose the peritoneum and a tumor (arrow) was observed under bright field (C) and fluorescent optics (D).
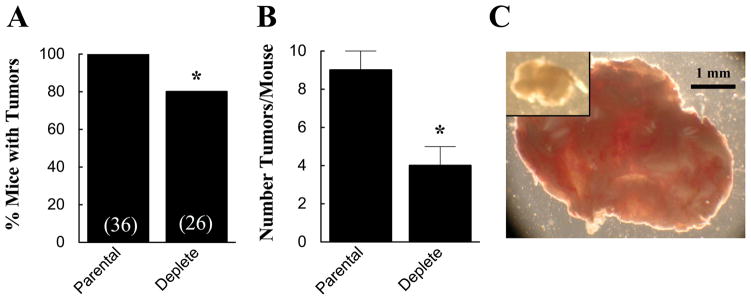
Using this xenograph model, we assessed the role of PGRMC1 in ovarian tumor formation and development by injecting intact nude mice with either dsRed (wild-type) or dsRed PGRMC1-deplete SKOV-3 cells [11]. After 5 weeks, only 80% of the mice injected with dsRed PGRMC1-deplete SKOV-3 cells developed tumors compared to 100% of the mice injected with dsRed (wild-type) SKOV-3 cells (Figure 4A). In those mice with tumors, the number of tumors that formed was about half the number observed in mice injected with dsRed (wild-type) SKOV-3 cells (Figure 4B). Finally, the size of the tumors derived from PGRMC1-deplete SKOV-3 cells was about one fourth that of tumors derived from dsRed (wild-type) SKOV-3 cells (Figure 4C). These in vivo findings clearly demonstrate that PGRMC1 is a major regulator of tumor formation and growth.
Figure 4.
The effect of depleting PGRMC1 on the percentage of mice that develop tumors (A) and the number of tumors, which develop in mice that form tumors (B). The numbers in parentheses in A are the number of mice observed. The effect of depleting PGRMC1 on the relative size of the tumors is shown in panel C. The tumor derived from PGRMC1-deplete SKOV-3 cells is show in the inset of panel C. Both images are shown at the same magnification. The data in panel A and B is taken from Peluso et al [11].
Unfortunately the mechanism through which PGRMC1 regulates tumor growth is unknown. However, we have shown that PGRMC1-deplete ovarian tumors have a fewer blood vessels compared to tumors derived from wild-type SKOV-3 cells [11]. Specifically, our quantitative image analysis revealed that microvasculature comprises 2.80 ± 0.75% of the wild-type tumors and only 0.40 ± 0.11% of tumors derived from PGRMC1-depleted tumors (n= 4 per group; p < 0.05) [11]. This finding suggests that PGRMC1 is essential for the vascular development and without the ability to expand its vascular network the tumor cannot continue to grow and may even regress [23]. These observations strongly support our hypothesis that depleting PGRMC1 in existing tumors would dramatically slow their growth and reduce their viability.
6. PGRMC1 as a Regulator of Gene Transcription
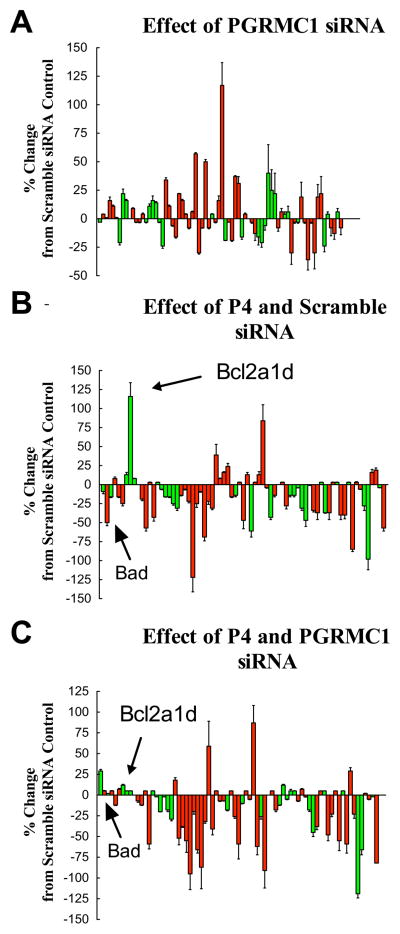
Interestingly, PGRMC1 is localized to the plasma membrane, cytoplasm and nucleus of both ovarian granulosa cells and ovarian cancer cells [13, 24]. The nuclear localization suggests that PGRMC1 could be involved in regulating gene expression and this is consistent with the altered gene profile observed in tumors derived from PGRMC1-deplete SKOV-3 cells [11]. In addition, recent studies have shown that P4’s anti-apoptotic action in rat spontaneously immortalized granulosa cells (SIGCs) is dependent on de novo RNA and protein synthesis [25]. In an attempt to identify some of the genes whose expression might be part of the putative genomic action of PGRMC1, mRNA levels of numerous genes involved in apoptosis were assessed using real-time PCR microarrays using RNA isolated from cultured SIGCs [25]. This approach yielded several interesting findings. First, depletion of PGRMC1 in the absence of P4 significantly altered that pattern of gene expression such that the overall effect was to increase the mRNA levels of many genes that are involved in promoting apoptosis (Figure 5A). This suggests that PGRMC1, which is not bound to P4, regulates gene expression in a manner that would make the cells more susceptible to undergoing apoptosis.
Figure 5.
The effects of PGRMC1 siRNA treatment of the expression profile of various apoptosis-related genes is shown in panel A. The effects of 1 μM P4 plus scramble siRNA or 1 μM P4 plus PGRMC1 siRNA treatment are shown in panels B and C, respectively. In each graph the mRNA levels of apoptosis-related genes were assessed by real-time PCR and shown as a percentage change from scramble control values. The values are means (± standard error) of three experiments. Genes associated with the induction of apoptosis are shown in red and the anti-apoptotic genes shown in green. Data is taken from Peluso et al [25].
Second, P4 alters the gene expression profile such that the mRNAs that encode many genes involved in promoting apoptosis are suppressed. Some of suppressed genes include caspase 1,2,3 and 14 as well as Bad (Figure 5B). The only anti-apoptotic gene showing an increase in expression is Bcl2a1d, a BCL2 family member that functions like BCL2 to prevent apoptosis [26, 27]. The increase in the ratio of BCL2 to BAD is known to promote granulosa cell survival [28], which would explain in part P4’s anti-apoptotic action. Moreover, the overall effect of P4 is to promote the genes known to promote cell survival, which would make the cells more resistant to apoptosis. This fits with P4’s well-characterized anti-apoptotic action [15].
Finally, P4-regulated expression of Bcl2a1d and Bad is dependent on PGRMC1, since P4’s ability to induce Bcl21d mRNA and to suppress Bad mRNA is completely attenuated by pretreatment with PGRMC1 siRNA (Figure 5C). However other P4-induced changes in the gene profile do not appear to be dependent on PGRMC1. Most noticeably the changes related to the mRNA levels of several caspase family members. The mechanism through which P4 influences the expression of these genes is unknown but does not involve the nuclear P4 receptor (PGR), since SIGCs do not express these receptors [13, 20]
7. Future Research and Summary
It is clear from our in vitro and in vivo data that PGRMC1 is an excellent candidate for gene therapy. Although numerous issues remain to be resolved, two are pressing. The first involves developing a method to deplete PGRMC1’s action in existing tumors. Since ovarian tumors are almost exclusively found within the peritoneal cavity [21], it is possible to treat these tumors by injecting therapeutic agents directly into the peritoneal cavity. A few groups have had some success in depleting specific genes within peritoneal tumors by simply injecting siRNAs mixed with various transfection reagents into the peritoneum. For example Verma et al [29] injected siRNA targeted to β-catenin and showed that this slowed the growth of colon cancer xenographs. A database of some papers that have utilized siRNA to silence gene expression in vivo can be found at http://www.ambion.com/techlib/resources/invivo/index.html.
However, the effectiveness of PGRMC1 siRNA treatment would be greatly improved if the siRNA could be directed or targeted specifically to the ovarian tumors. One way to deliver chemotherapeutic drugs is to link them to nanoparticles [30]. The drug-laden nanoparticles can be labeled with tumor-specific markers, so that the nanoparticles would preferentially concentrate to the tumors. While considerable effort is being made in this area, targeting nanoparticles to ovarian tumors may be a difficult goal to achieve. However, ovarian tumors express high levels of PGRMC1 [20, 24] and some PGRMC1 is localized to the exterior of the tumor cell surface [31]. There are at least two ligands that bind PGRMC1 and either could be used to target nanoparticles to ovarian tumors. The first ligand is P4. P4-labeled nanoparticles would specifically bind to PGRMC1 [31], thus targeting the ovarian tumor. The use of P4 to target ovarian tumors would potentially have the additional advantage of triggering apoptosis (death) of ovarian cancer cells once PGRMC1 levels are depleted (See Figure 2D). Alternatively, a monoclonal antibody that binds to the extracellular surface of PGRMC1 could also be linked to nanoparticles. Like P4, this antibody would bind to the extracellular component of PGRMC1, thereby selectively targeting the ovarian tumor.
The second issue relates to the relative lack of information about the mechanism through which ligand activation of PGRMC1 mediates its anti-apoptotic action. Simply based on the observation at that PGRMC1 localizes to the plasma membrane, cytoplasm and nucleus, it appears that PGRMC1 will likely have multiple sites of action. Defining of the cellular and biochemical events associated with each putative site of action will be essential in order to fully develop PGRMC1 as a therapeutic agents.
In summary, we have shown the P4-PGRMC1 interaction regulates the viability of normal and cancerous ovarian cells both in vitro and in vivo. Moreover, our data suggests that agonizing PGRMC1’s action could have therapeutic uses particularly as it relates to ovarian cancer. Before these putative therapeutic uses can be advanced considerably more details regarding PGRMC1’s mechanism of action must be elucidated. Finally, it is important to appreciate that at least three families of progestin binding proteins, PGRs, PGRMCs and progestin and adipoQ receptors (PAQRs) are expressed in ovarian cancers (for review see Peluso [24]). The level of expression of each of these receptor families varies with the developmental stage of the cancer. It is likely then that the precise combination of receptors present at each stage of ovarian cancer development could account for different responses to endogenous P4, since each of these receptor families activate different signaling pathways [13]. Thus the complexities involved in the interaction between these mediators of P4’s action in ovarian cancer cells merits further investigation.
Acknowledgments
The author would like to acknowledge the excellent technical assistance of Xiufang Liu and Anna Gawkowska without which this work could not have been completed. This work was supported in part by grants from the Connecticut Department of Public Health (BCH-2007-0919) and the National Institute of Child Health and Development (RO1 HD 052740)
Footnotes
Disclosure Statement
The author does not have any actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Salzberg M, Thurlimann B, Bonnefois H, Fink D, Rochlitz C, von Moos R, Senn H. Current concepts of treatment strategies in advanced or recurrent ovarian cancer. Oncology. 2005;68:293–298. doi: 10.1159/000086967. [DOI] [PubMed] [Google Scholar]
- 2.Ho SM. Estrogen, progesterone and epithelial ovarian cancer. Reprod Biol Endocrinol. 2003;1:73. doi: 10.1186/1477-7827-1-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fauvet R, Dufournet Etienne C, Poncelet C, Bringuier AF, Feldmann G, Darai E. Effects of progesterone and anti-progestin (mifepristone) treatment on proliferation and apoptosis of the human ovarian cancer cell line, OVCAR-3. Oncol Rep. 2006;15:743–748. [PubMed] [Google Scholar]
- 4.Syed V, Mukherjee K, Godoy-Tundidor S, Ho SM. Progesterone induces apoptosis in TRAIL-resistant ovarian cancer cells by circumventing c-FLIPL overexpression. J Cell Biochem. 2007;102:442–452. doi: 10.1002/jcb.21304. [DOI] [PubMed] [Google Scholar]
- 5.Murdoch WJ, Van Kirk EA, Isaak DD, Shen Y. Progesterone facilitates cisplatin toxicity in epithelial ovarian cancer cells and xenografts. Gynecol Oncol. 2008;110:251–255. doi: 10.1016/j.ygyno.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Feng Y. Effect of progesterone combined with chemotherapy on epithelial ovarian cancer. Chin Med J (Engl) 2003;116:388–391. [PubMed] [Google Scholar]
- 7.Takahashi A, Kato K, Kuboyama A, Inoue T, Tanaka Y, Kuhara A, Kinoshita K, Takeda S, Wake N. Induction of senescence by progesterone receptor-B activation in response to cAMP in ovarian cancer cells. Gynecol Oncol. 2009;113:270–276. doi: 10.1016/j.ygyno.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Davis M, Hitchcock A, Foulkes WD, Campbell IG. Refinement of two chromosome 11q regions of loss of heterozygosity in ovarian cancer. Cancer Res. 1996;56:741–744. [PubMed] [Google Scholar]
- 9.Gabra H, Langdon SP, Watson JE, Hawkins RA, Cohen BB, Taylor L, Mackay J, Steel CM, Leonard RC, Smyth JF. Loss of heterozygosity at 11q22 correlates with low progesterone receptor content in epithelial ovarian cancer. Clin Cancer Res. 1995;1:945–953. [PubMed] [Google Scholar]
- 10.Hogdall EV, Christensen L, Hogdall CK, Blaakaer J, Gayther S, Jacobs IJ, Christensen IJ, Kjaer SK. Prognostic value of estrogen receptor and progesterone receptor tumor expression in Danish ovarian cancer patients: from the ‘MALOVA’ ovarian cancer study. Oncol Rep. 2007;18:1051–1059. [PubMed] [Google Scholar]
- 11.Peluso JJ, Gawkowska A, Liu X, Shioda T, Pru JK. Progesterone receptor membrane component-1 regulates the development and Cisplatin sensitivity of human ovarian tumors in athymic nude mice. Endocrinology. 2009;150:4846–4854. doi: 10.1210/en.2009-0730. [DOI] [PubMed] [Google Scholar]
- 12.Romero-Sanchez M, Peiper SC, Evans B, Wang Z, Catasus L, Ribe A, Prat J, Giri JG. Expression profile of heptahelical putative membrane progesterone receptors in epithelial ovarian tumors. Hum Pathol. 2008;39:1026–1033. doi: 10.1016/j.humpath.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Peluso JJ. Multiplicity of progesterone’s actions and receptors in the mammalian ovary. Biol Reprod. 2006;75:2–8. doi: 10.1095/biolreprod.105.049924. [DOI] [PubMed] [Google Scholar]
- 14.Falkenstein E, Eisen C, Schmieding K, Krautkramer M, Stein C, Losel R, Wehling M. Chemical modification and structural analysis of the progesterone membrane binding protein from porcine liver membranes. Mol Cell Biochem. 2001;218:71–79. doi: 10.1023/a:1007269507856. [DOI] [PubMed] [Google Scholar]
- 15.Cahill MA. Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol. 2007;105:16–36. doi: 10.1016/j.jsbmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Peluso JJ, Romak J, Liu X. Progesterone receptor membrane component-1 (PGRMC1) is the mediator of progesterone’s antiapoptotic action in spontaneously immortalized granulosa cells as revealed by PGRMC1 small interfering ribonucleic acid treatment and functional analysis of PGRMC1 mutations. Endocrinology. 2008;149:534–543. doi: 10.1210/en.2007-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peluso JJ, Liu X, Gawkowska A, Johnston-MacAnanny E. Progesterone activates a progesterone receptor membrane component 1-dependent mechanism that promotes human granulosa/luteal cell survival but not progesterone secretion. J Clin Endocrinol Metab. 2009;94:2644–2649. doi: 10.1210/jc.2009-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien ME, Dowsett M, Fryatt I, Wiltshaw E. Steroid hormone profile in postmenopausal women with ovarian cancer. Eur J Cancer. 1994;30A:442–445. doi: 10.1016/0959-8049(94)90414-6. [DOI] [PubMed] [Google Scholar]
- 19.Halperin R, Hadas E, Langer R, Bukovsky I, Schneider D. Peritoneal fluid gonadotropins and ovarian hormones in patients with ovarian cancer. Int J Gynecol Cancer. 1999;9:502–507. doi: 10.1046/j.1525-1438.1999.99075.x. [DOI] [PubMed] [Google Scholar]
- 20.Peluso JJ, Liu X, Saunders MM, Claffey KP, Phoenix K. Regulation of ovarian cancer cell viability and sensitivity to cisplatin by progesterone receptor membrane component-1. J Clin Endocrinol Metab. 2008;93:1592–1599. doi: 10.1210/jc.2007-2771. [DOI] [PubMed] [Google Scholar]
- 21.Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118:1367–1379. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonnel AC, Van Kirk EA, Isaak DD, Murdoch WJ. Effects of progesterone on ovarian tumorigenesis in xenografted mice. Cancer Lett. 2005;221:49–53. doi: 10.1016/j.canlet.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Spannuth WA, Sood AK, Coleman RL. Angiogenesis as a strategic target for ovarian cancer therapy. Nat Clin Pract Oncol. 2008;5:194–204. doi: 10.1038/ncponc1051. [DOI] [PubMed] [Google Scholar]
- 24.Peluso JJ. Non-genomic actions of progesterone in the normal and neoplastic mammalian ovary. Semin Reprod Med. 2007;25:198–207. doi: 10.1055/s-2007-973432. [DOI] [PubMed] [Google Scholar]
- 25.Peluso JJ, Liu X, Gawkowska A, Lodde V, Wu CA. Progesterone inhibits apoptosis in part by PGRMC1-regulated gene expression. Mol Cell Endocrinol. 320:153–161. doi: 10.1016/j.mce.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasooly R, Schuster GU, Gregg JP, Xiao JH, Chandraratna RA, Stephensen CB. Retinoid x receptor agonists increase bcl2a1 expression and decrease apoptosis of naive T lymphocytes. J Immunol. 2005;175:7916–7929. doi: 10.4049/jimmunol.175.12.7916. [DOI] [PubMed] [Google Scholar]
- 27.Yin W, Raffelsberger W, Gronemeyer H. Retinoic acid determines life span of leukemic cells by inducing antagonistic apoptosis-regulatory programs. Int J Biochem Cell Biol. 2005;37:1696–1708. doi: 10.1016/j.biocel.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Kaipia A, Hsu SY, Hsueh AJ. Expression and function of a proapoptotic Bcl-2 family member Bcl-XL/Bcl-2-associated death promoter (BAD) in rat ovary. Endocrinology. 1997;138:5497–5504. doi: 10.1210/endo.138.12.5588. [DOI] [PubMed] [Google Scholar]
- 29.Verma UN, Surabhi RM, Schmaltieg A, Becerra C, Gaynor RB. Small interfering RNAs directed against beta-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin Cancer Res. 2003;9:1291–1300. [PubMed] [Google Scholar]
- 30.Yallapu MM, Jaggi M, Chauhan SC. Scope of nanotechnology in ovarian cancer therapeutics. J Ovarian Res. 3:19. doi: 10.1186/1757-2215-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peluso JJ, Pappalardo A, Losel R, Wehling M. Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone’s antiapoptotic action. Endocrinology. 2006;147:3133–3140. doi: 10.1210/en.2006-0114. [DOI] [PubMed] [Google Scholar]