Abstract
We hypothesized that nitric oxide synthase (NOS) isoforms may be regulated by dynamin (DNM) in the inner medullary collecting duct (IMCD). The aims of this study were to determine which DNM isoforms (DNM1, DNM2, DNM3) are expressed in renal IMCDs, whether DNM interacts with NOS, whether a high-salt diet alters the interaction of DNM and NOS, and whether DNM activates NO production. DNM2 and DNM3 are highly expressed in the rat IMCD, while DNM1 is localized outside of the IMCD. We found that DNM1 interacts with NOS1α, NOS1β, and NOS3 in the inner medulla of male Sprague-Dawley rats on a 0.4% salt diet. DNM2 interacts with NOS1α, while DNM3 interacts with both NOS1α and NOS1β. DNM2 and DNM3 do not interact with NOS3 in the rat inner medulla. We did not observe any change in the DNM/NOS interactions with rats on a 4% salt diet after 7 days. Furthermore, NOS1α interacts with DNM2 in mIMCD3 and COS7 cells transfected with NOS1α and DNM2-GFP constructs and the NOS1 reductase domain is necessary for the interaction. Finally, COS7 cells expressing NOS1α or NOS1α/DNM2-GFP had significantly higher nitrite production compared with DNM2-GFP only. Nitrite production was blocked by the DNM inhibitor dynasore or the dominant negative DNM2K44A. Ionomycin stimulation further increased nitrite production in the NOS1α/DNM2-GFP cells compared with NOS1α only. In conclusion, DNM and NOS1 interact in the rat renal IMCD and this interaction leads to increased NO production, which may influence NO production in the renal medulla.
Keywords: rat nephron, protein-protein interactions
nitric oxide (NO) is a critical regulator of several physiological pathways in the kidney. Mattson's laboratory previously demonstrated that the highest NOS activity was in the inner medullary collecting duct (IMCD) in the rat (37). All NO synthase (NOS) isoforms are expressed in various segments of the rat nephron (24, 25, 37), specifically NOS1 (neuronal NOS) is expressed in the macula densa, cortical and medullary collecting duct, thick ascending limb, and vasa recta (3, 18, 37); NOS2 (inducible NOS) is constitutively expressed in the thick ascending limb of the nephron (12, 19); and NOS3 is expressed in endothelial cells throughout the kidney, thick ascending limb, and collecting duct cells (3, 33) . The NOS1 gene has many alternative promoters (4, 10) and reports showed that there are three NOS1 splice variants expressed in the rat nephron termed NOS1α, NOS1β, and NOS1γ (17, 22, 28). The reductase domain of the three NOS isoforms and the NOS1 splice variants are all highly conserved (1, 39).
Protein-protein interactions and subcellular localization are important regulators of NOS activity and consequently NO production. Several proteins have been shown to interact with NOS and regulate NO production (1, 39). We previously reported that NOS activity within the renal inner medulla is over fourfold higher in the intracellular membrane fraction relative to the cytosolic and plasma membrane fractions (31). Of the known NOS-interacting proteins that activate NOS and may be relevant in various subcellular compartments (23), dynamin (DNM) would be considered one of the top candidate proteins.
DNMs belong to the large GTPase family of proteins and are comprised of several domain structures that facilitate interactions, specifically a lipid-binding pleckstrin homology domain, a GTPase effector domain, and a proline-rich COOH-terminal domain (e.g., 13). DNMs are essential in the endocytotic pathway, whereby these proteins are involved in the scission of invaginated clathrin-coated vesicles (35) and caveloae (38). There are three members that are differentially expressed; dynamin-1 (DNM1) is predominately expressed in the nervous system (21), while dynamin-2 (DNM2) and dynamin-3 (DNM3) are ubiquitously expressed (9, 16, 29). Additionally, up to 25 splice variants have been described (5). Cao et al. (7) previously demonstrated that DNM/NOS3 interact in bovine aortic endothelial cells and determined that the proline-rich COOH-terminal domain of DNM2 directly interacts with the FAD region of the reductase domain of NOS3. This direct interaction was demonstrated to increase NOS3 activity (7). Wang et al. (36) further showed that DNM1 interacts with NOS3 in HEK 293 cells, although it is unclear whether DNM1 also activates NOS3 in these cells.
Little is known about whether NOS1 isoform interacts with DNM, and specifically whether these proteins interact in the kidney. Given the high degree of homology between the reductase domains of NOS1 and NOS3, and the high NOS activity in the rat renal inner medulla and IMCD (37), we hypothesized that DNM interacts with NOS1 and/or NOS3 in the renal inner medulla and regulates NO production. The initial aims of this study were to determine the expression of DNM isoforms in the renal inner medulla and whether DNM and NOS interact in renal inner medullary tissue. These studies revealed that indeed DNM and NOS interact in the renal tissue, thus we further elucidated whether the interaction is influenced by a high-salt diet and/or whether the DNM/NOS1 interaction activates NO production.
EXPERIMENTAL DESIGN AND METHODS
Animals.
All animal protocols were in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Utilization Committee at the Medical College of Georgia. Male Sprague-Dawley rats (230–250 g) were purchased from Harlan Labs and maintained at the lab animal services facility. Animals were fed ad libitum a 0.4% NaCl (normal salt, Harlan Teklad, Indianapolis, IN) diet with free access to tap water. After acclimation to the animal facility (7 days), rats were maintained on 0.4% NaCl diet (normal salt) or given 4.0% NaCl (high salt, Harlan Teklad) diet for 7 days. Rats were anesthetized with pentobarbital sodium (Sigma, St. Louis, MO) and killed by thoracotomy. The left and right kidneys were immediately excised, decapsulated, and the inner medulla was dissected and snap-frozen in liquid nitrogen. All tissues were kept at −80°C until analysis. For immunohistochemical analysis, rats were anesthetized as described and the kidneys were perfusion-fixed with 10% neutral-buffered formalin (NBF; Fisher Scientific, Pittsburg, PA) for 10 min. The kidneys were excised, decapsulated, sectioned sagitally into thirds, and further fixed in 10% NBF for 24 h at room temperature. The kidneys were then embedded in paraffin and sectioned.
Immunohistochemistry.
Five kidney sections, of 5-μm thickness, per animal were analyzed as previously described (30). The tissue sections were incubated overnight at 4°C with the isoform-specific antibodies to DNM1–3 (1:2,000; Abcam, Cambridge, MA). The secondary antibody was goat anti-rabbit, horseradish peroxidase polymer (Biocare, Concord, CA) incubated for 20 min at room temperature in a humidified chamber. Immunoreactivity was detected by diaminobenzidine (Dako, Carpinteria, CA). Slides were visualized with the Olympus BX40 microscope, affixed with a DP70 Camera.
IMCD3 and COS7 transfections.
Mouse IMCD-3 (mIMCD3) and COS7 cells were purchased from ATCC (Manassas, VA). One day before transfection, 2 × 105 cells were plated in growth medium (DMEM or DMEM:F-12, respectively) containing 5% fetal bovine serum (Atlanta Biologicals, Atlanta, GA) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) resulting in cells 90–95% confluent at time of transfection in 100-mm plates. Fugene 6 transfection agent (Roche, Indianapolis, IN) was added to serum- and antibiotic-free media (for COS7) and Opti-MEM (Invitrogen; for mIMCD-3) for 15 min. Plasmid DNA (NOS1α, NOS1 reductase domain, DNM2-GFP constructs, or dominant negative DNM2-K44A) was added to this media at a concentration of 1 μg in 6 μl of Fugene and incubated for 15 min at room temperature. The fugene/DNA media was added to each plate and gently rocked back and forth. The cells were incubated at 37°C in a 5% CO2 incubator for 48 h with the medium changed after 6 and 24 h of transfection. All transfection combinations were repeated four to five independent times.
Immunoprecipitation protocol.
Cerebellar or inner medullary tissues were homogenized in 500 μl of lysis buffer (20 mM Tris, 150 mM NaCl, 0.5% Triton X-100, 1 mM EDTA, 1 mM EGTA, 10 μM leupeptin, 2 μM pepstatin A, 1 mM phenylmethylsulfonyl fluoride, pH 7.5) centrifuged at 4,000 g. Protein concentration of the supernatant determined with the Bio-Rad BCA kit (Carlsbad, CA). Protein (750 μg) was incubated with Protein A/G beads conjugated with pan-dynamin (Upstate Millipore, Billerica, MA) or dynamin isoform-specific antibodies (10 μg antibody/immunoprecipitate; Abcam).
Transfected cells were harvested, washed twice in 10 mM PBS (Invitrogen), and lysed in lysis buffer. Cell lysates were centrifuged at 4,000 g for 10 min at 4°C. The supernatant was retained and protein concentration was determined (BCA assay, Bio-Rad). Three micrograms of green fluorescent protein (GFP) antibody or 10 μg of NOS1-specific antibody was incubated with 50 μl of preblocked protein A/G agarose bead slurry (Santa Cruz Biotechnologies, Santa Cruz, CA) for 1 h at 4°C.
Excess antibody from the immunoprecipitate complex was removed by washing the beads 3× with lysis buffer. Next, 500 μg of protein were mixed with 50 μl antibody-conjugated beads in an empty Bio-spin disposable column (Bio-Rad) overnight at 4°C. The beads were then washed 3× with lysis buffer + protease inhibitors. Proteins were eluted by boiling the beads in 2× Laemmli buffer (Bio-Rad) with 5% beta-mercaptoethanol for 5 min at 90°C. Immunoprecipitated proteins were run on 8% SDS-PAGE, blotted to PVDF membranes, and detected with anti-GFP (1:10,000; Clontech, Mountainview, CA), monoclonal, anti-COOH-terminus NOS1 (1:1,000; BD Scientific, San Jose, CA), or polyclonal anti-NOS3 (1:100; Santa Cruz Biotechnologies) antibodies and visualized with goat anti-mouse secondary antibody (Invitrogen) or goat anti-rabbit secondary antibody (Rockland, Gilbertsville, PA) using the Odyssey Infrared Imaging System (Licor Biosciences).
Cellular fractionation of rat IMCDs.
Rat IMCDs were freshly isolated following the method of Kohan et al. (15). The rat IMCDs were fractionated into a plasma membrane-enriched, intracellular membrane-enriched, and cytosolic or soluble fractions through differential centrifugation as described by Tiwari et al. (32). Protein (5 μg) was separated by SDS-PAGE, and Western blots were probed for NOS1, NOS3, DNM1, DNM2, and DNM3 expression. Additionally, the DNM isoform-specific antibodies were preabsorbed with their respective peptides, and rat inner medulla or cerebellum immunoblots were probed.
Nitrite measurement.
COS7 cells were cultured in 12-well plates and transfected with combinations of NOS1α and DNM2-GFP as described above. Forty eight hours posttransfection, the cells were serum starved for 4 h, at which point they were washed with 10 mM PBS and incubated in 1 ml of 1× HBSS (no phenol red, Cellgro, Manassas, VA), 20 U/ml superoxide dismutase, and 250 μM l-arginine. In addition, cells were stimulated with 3 μM ionomycin (dissolved in DMSO) or vehicle [0.1% DMSO]final for 1 h at 37°C in 5% CO2. Following this incubation, the HBSS was removed and cells were then solubilized in 0.1 N NaOH for 20 min at room temperature, and total protein was measured by BCA assay (Bio-Rad). Nitrite analysis of the HBSS was measured by HPLC (ENO20, Eicom, San Diego, CA) and all values were normalized to total protein.
Inhibition of DNM.
COS7 cells transfected with NOS1α and DNM2-GFP were grown to confluency, serum-starved 4 h, and treated with the DNM-specific inhibitor dynasore (14) (Sigma). Dynasore was dissolved in DMSO. Transfected COS7 were incubated in HBSS+SOD+l-arginine (see above) and vehicle [0.8% DMSO]final or dynasore (20–80 μM) for 1 h and nitrite production was determined. To ensure that the dynasore was not cytotoxic at the concentrations used, we incubated COS7 cells with the various dynasore concentrations for 1 h, washed the cells twice with 10 mM PBS, trypsinized the cells, and counted with a hemocytometer. Additionally, freshly isolated rat IMCDs were incubated with 0.8% DMSO (vehicle) or 80 μM dynasore and nitrite production was measured. Finally, IMCD3 cells, which endogenously express DNM2 and DNM3, were either untransfected, transfected with DNM2-GFP or the dominant negative DNM2-K44A (Addgene, Cambridge, MA) and were also assayed for nitrite production, following the aforementioned method.
Statistical analysis.
For nitrite determination, data were plotted as means ± SE. To test the significance of overexpressing NOS1 and DNM2 in COS7 cells on nitrite production, a one-way ANOVA with Tukey post hoc test was performed with an α = 0.05 considered significant. To test the significance of ionomycin on nitrite production in these cells, a two-way ANOVA with unpaired Student's t-test as the post hoc test was performed. To account for the multiple comparisons, an α = 0.017 was considered statistically significant.
RESULTS
DNM isoform expression in the renal inner medulla and CD.
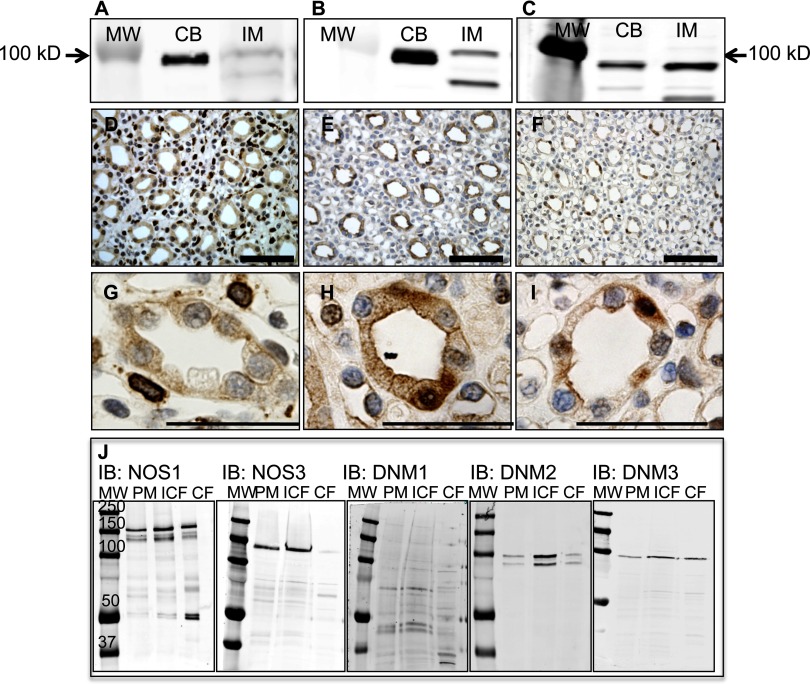
DNM1 (95 kDa), DNM2 (95 kDa), and DNM3 (83 kDa) were expressed in the rat renal inner medullary homogenates as shown by Western blot (Fig. 1, A–C). Rat brain cerebellar homogenate was utilized as a positive control. Antibody specificity was confirmed with peptide antigen blockade in Western blots (Supplemental Fig. S1; the online version of this article contains supplemental data). Immunohistochemical analysis within the inner medulla localized DNM1 in cells surrounding the collecting ducts and with low expression localized in the collecting ducts (Fig. 1, D and G). DNM2 (Fig. 1, E and H) and DNM3 (Fig. 1, F and I) appeared to be exclusively expressed in the collecting duct with other structures demonstrating very low or no immunoreactivity. Finally, DNM isoforms, NOS1, and NOS3 subcellular localization was determined in isolated rat IMCD. As seen in Fig. 1J, NOS1, DNM2, and DNM3 are expressed in the plasma membrane, intracellular membrane, and soluble fractions, while NOS3 was expressed in the plasma membrane and intracellular membrane fractions only. DNM1 was expressed at very low levels in the rat IMCD subcellular fractions.
Fig. 1.
Dynamin isoforms are expressed in the rat renal inner medulla (IM) and rat brain cerebellum (CB). Representative immunoblots of dynamin-1 (DNM1; A), DNM2 (B), and DNM3 (C). Immunohistochemical analysis of the rat renal IM (D−F: lower magnification; G−I: higher magnification; scale bar = 50 μm): D and G: DNM1 expression observed outside the collecting duct; E and H: DNM2 expression localized to the collecting duct; F and I: DNM3 expression observed within the collecting duct. J: immunoblots of the subcellular fractions [plasma membrane (PM), intracellular membrane (ICF), and soluble or cytosolic (CF) fractions] of isolated rat inner medullary collecting ducts for NOS1, NOS3, DNM1, DNM2, and DNM3.
DNM/NOS interactions in vivo.
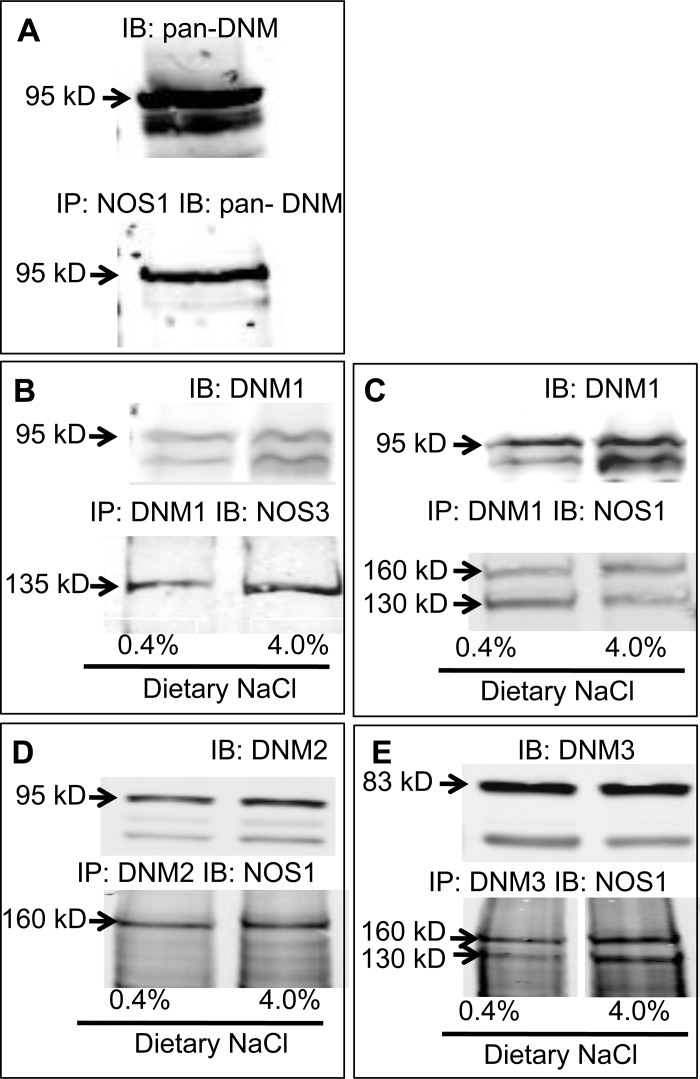
The rat cerebellum expresses high levels of NOS1α and DNM so initially we probed for NOS1/DNM interactions in the cerebellum using a pan-DNM antibody and found that NOS1 interacts with DNM in the cerebellum (Fig. 2A).
Fig. 2.
Representative immunoblots of coimmunoprecipitation experiments with rat brain cerebellar tissue (A) and renal inner medullary tissue from rats on normal salt (0.4% NaCl) or 7-day high-salt (4.0%) diet (B–E). A: nitric oxide synthase type 1 (NOS)1 and DNM interacts. B: DNM1 interacts with NOS3. C: DNM1 interacts with NOS1α and NOS1β. D: DNM2 interacts with NOS1α. E: DNM3 interacts with NOS1α and NOS1β. IP, immunoprecipitation; IB, immunoblot.
Lysates from inner medulla of the rat kidney were immunoprecipitated with DNM isoform-specific antibodies and immunoblotted with a COOH terminus NOS1-specific antibody to determine which DNMs may interact with NOS1α and/or NOS1β. As observed in Fig. 2, C and E, DNM1 and DNM3 interact with NOS1α and NOS1β (n = 6–7), while DNM2 interacts with NOS1α (Fig. 2D; n = 6). The DNM/NOS1 interactions were similar in tissues from rats on normal- and high-salt diets (Fig. 2, B−E; n = 6–7).
Similar to a previous report in endothelial cells (36), we found that DNM1 and NOS3 interact in the rat renal inner medulla (Fig. 2B; n = 6). However, DNM2 and DNM3 do not appear to interact with NOS3 in the rat renal inner medulla (data not shown; n = 10). The DNM1/NOS3 interaction was similar in tissues from rats on a normal-salt and high-salt diet (Fig. 2B; n = 7).
DNM2/NOS1 interaction in vitro.
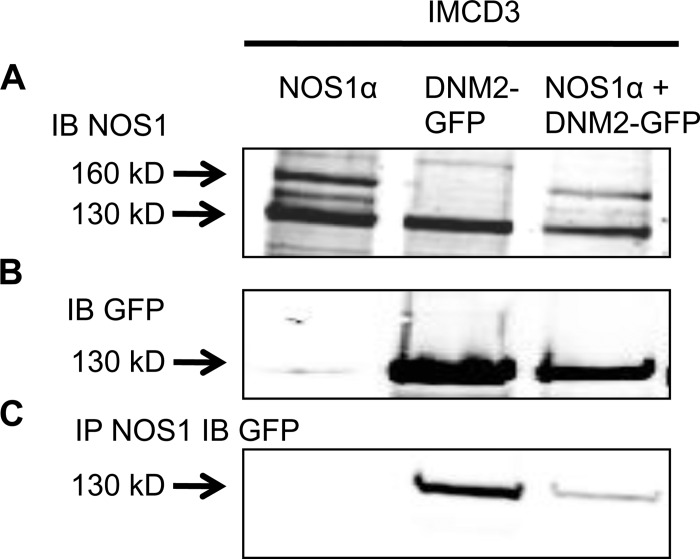
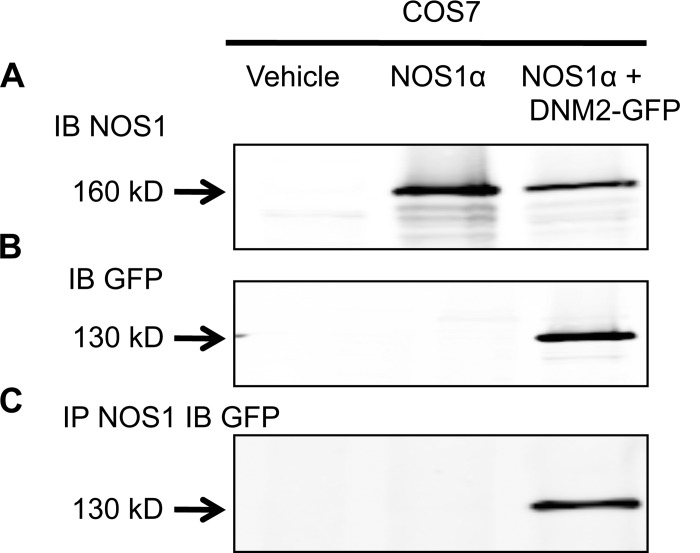
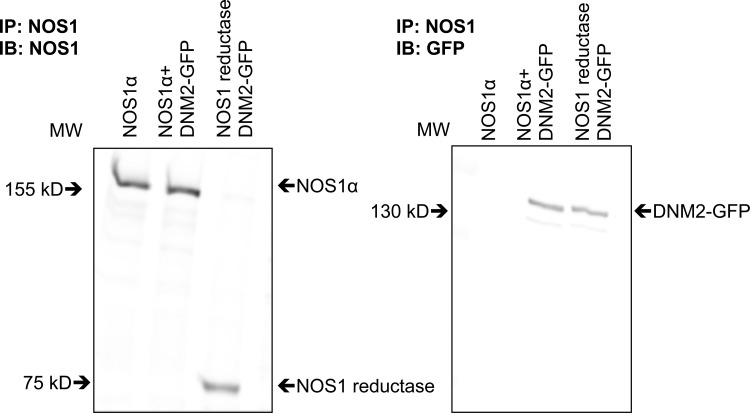
mIMCD3 and COS7 cultured cells were transfected with NOS1α and DNM2-GFP constructs (Figs. 3 and 4). mIMCD3 cells express NOS1β endogenously (Fig. 3A), whereas COS7 cells do not express NOS isoforms. We confirmed that DNM2 and NOS1 interact in these in vitro settings (Figs. 3C and 4C; n = 4). In addition, we found an interaction between endogenous NOS1β expressed in mIMCD3 cells and DNM2-GFP (Fig. 3C; n = 4). In addition, COS7 cells were transfected with the NOS1 reductase domain (identical domain in NOS1α and NOS1β) and/or DNM2-GFP and immunoprecipitated with a COOH terminus NOS1-specific antibody. We determined that DNM2 interacts with the reductase domain of NOS1 (Fig. 5).
Fig. 3.
Representative immunoblots of mouse inner medullary collecting duct (mIMCD)-3 cells transfected with NOS1α or DNM2-green fluorescent protein (GFP) constructs and coimmunoprecipitation with anti-NOS1. A: transfected NOS1α (160 kDa) and endogenous NOS1β (130 kDa) are expressed in mIMCD-3 cells. B: GFP was only expressed in the DNM2-GFP-transfected mIMCD-3 cells. C: mIMCD-3 cell lysates immunoprecipitated (IP) with anti-NOS1 and immunoblotted (IB) with anti-GFP demonstrating NOS1 and DNM2 interact in vitro.
Fig. 4.
Representative immunoblots of COS7 cells transfected with NOS1α or DNM2-GFP constructs and coimmunoprecipitation with anti-NOS1. A: expression of transfected NOS1α in COS7 cells. B: GFP was only expressed in the DNM2-GFP-transfected cells. C: COS7 cell lysates IP with anti-NOS1 and IB with anti-GFP demonstrating NOS1 and DNM2 interact in vitro. The vehicle treatment was transfection agent fugene only.
Fig. 5.
Representative immunoblots of COS7 cells transfected with NOS1α, NOS1 reductase domain, and/or DNM2-GFP constructs. Left: expression of the transfected NOS1α and NOS1 reductase domains in COS7 cells. Right: COS7 cell lysates IP with anti-NOS1 and IB with anti-GFP demonstrating DNM2 interacts with the reductase domain of NOS1.
DNM2/NOS1 interaction increases nitrite production.
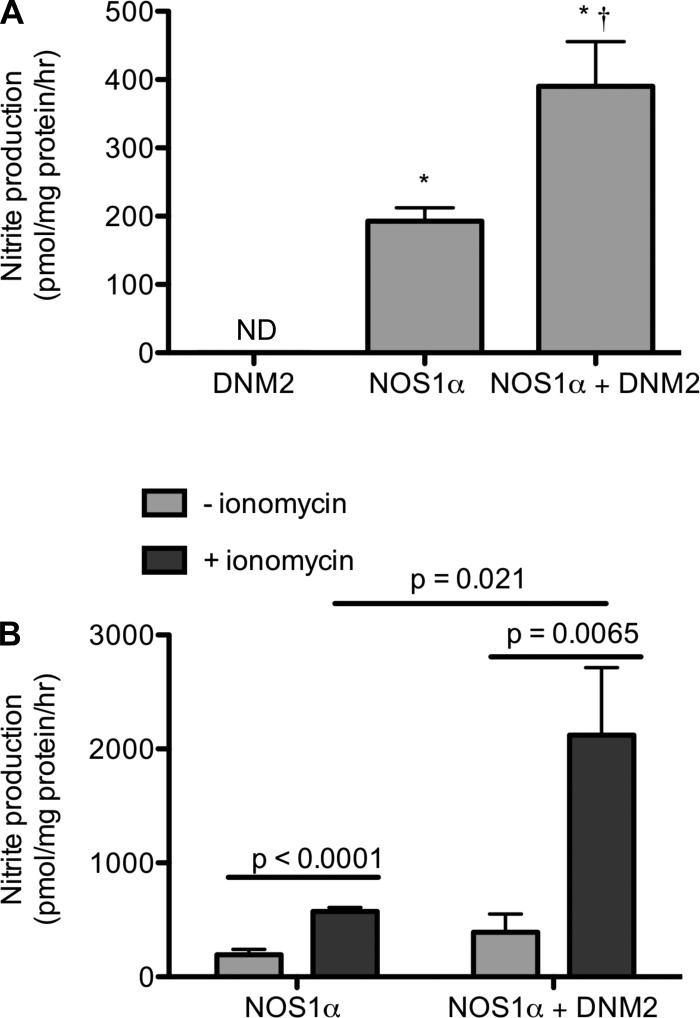
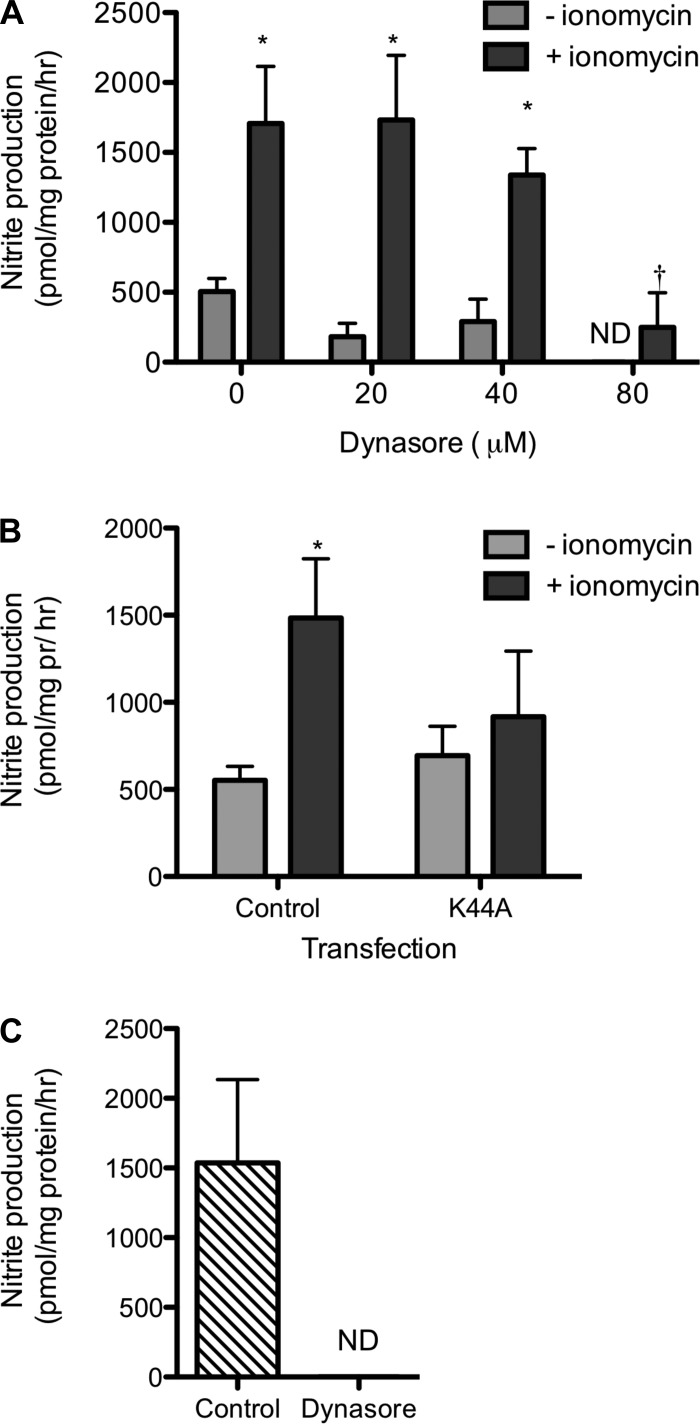
COS7 cells were transfected with DNM2-GFP, NOS1, or NOS1 and DNM2-GFP to determine nitrite production as an index of NOS1 activity. Nitrite production was below detection in the COS7 cells transfected with DNM2-GFP alone (Fig. 6A). Nitrite production significantly increased in cells transfected with NOS1α (193 ± 20 pmol·mg protein−1·h−1; n = 6) and further increased in the NOS1α + DNM2-transfected cells (390 ± 65 pmol·mg protein−1·h−1; n = 6; P < 0.001; Fig. 6A). Stimulation with ionomycin increased nitrite production in both the NOS1α (574 ± 34 pmol·mg protein−1·h−1; n = 5) and NOS1 + DNM2-transfected cells (2,122 ± 592 pmol·mg protein−1·h−1; n = 4; Fig. 6B). Basal and ionomycin-stimulated nitrite production was inhibited by incubation with dynasore for 1h (Fig. 7A; n = 3, ionomycin P < 0.0001, dynasore P = 0.007, interaction P = 0.11). We confirmed that incubation with dynasore was not cytotoxic (Supplemental Fig. S2). Additionally, the DNM2-specific contribution to nitrite production by mIMCD3 cells was inhibited by the dominant negative DNM2-K44A as well as the ionomycin-stimulated increase in nitrite (Fig. 7B; n = 3–5, ionomycin P = 0.015, transfection P = 0.48, interaction P = 0.42). Finally, nitrite production was completely abolished by incubation of freshly isolated rat IMCDs with 80 μM dynasore (Fig. 7C; n = 4).
Fig. 6.
Nitrite production by COS7 cells overexpressing DNM2, NOS1α, or NOS1α + DNM2. A: nitrite production is significantly increased when DNM2 is coexpressed with NOS1. Statistical analysis by 1-way ANOVA. *P < 0.05 compared with DNM2. †P < 0.05 NOS1α vs. NOS1α+DNM2. B: nitrite production is significantly increased in cells transfected with NOS1α or NOS1α + DNM2 constructs were incubated in the presence of 3 μM ionomycin for 1 h. Statistical analysis by 2-way ANOVA. The experiment was repeated 4–6 times.
Fig. 7.
Inhibition of DNM decreases nitrite production. A: nitrite production from COS7 cells overexpressing NOS1α + DNM2 was inhibited by the dynamin inhibitor dynasore in the presence and absence of ionomycin. Statistical analysis by 2-way ANOVA. *P < 0.05 compared with 3 μM ionomycin. †P < 0.05 compared with 0 μM dynasore. B: nitrite production from control (untransfected) mIMCD-3 cells or the dominant negative DNM2-K44A in the presence and absence of ionomycin. Statistical analysis by 2-way ANOVA. *P < 0.05 compared with 3 μM ionomycin. C: nitrite production by freshly isolated rat IMCDs was abolished by incubation with 80 μM dynasore for 1 h. ND, not detected.
DISCUSSION
The major findings of this study show that NOS1 interacts with DNM isoforms in rat cerebellar and renal inner medullary tissues as well as the renal IMCD. We also found that NOS3 interacts with DNM1 in the renal inner medulla. We found that DNM2 and DNM3 are highly expressed within the IMCD, while DNM1 has a relative low level of expression in the CD. Furthermore, we demonstrated that DNM2 activates NOS1 and stimulates NO production with a specific interaction of DNM2 with the reductase domain of NOS1. Moreover, inhibition of DNM blocked NO production in rat renal IMCDs.
DNMs play an integral role in the endocytotic pathway (13) as well as being involved in the regulation of protein functions through protein-protein interactions (34). DNMs contain proline-rich and pleckstrin homology domains that interact with numerous proteins and subcellular components including tubulin of the microtubules (27), clathrin of the plasma membrane (5), and TGN38 of the trans-golgi network (5). In addition, DNM1 preferentially regulates endocytosis of apical membrane proteins, while DNM2 regulates endocytosis of both apical and basolateral proteins (2). Taken together, these studies, and others, suggest that DNM targets and functions within various subcellular components. Very little is known about DNM expression and function in IMCD and the renal inner medulla. mRNA for DNM2 and DNM3 is expressed in the kidney (9, 16, 29); however, expression in cellular structures has not been identified. Here, we present that DNM1 is expressed in cells in the inner medullary interstitium, with little expression in the IMCD. Given the comparatively high expression of DNM1 in neurons and low expression in other tissues (21, 29), it seems logical to speculate that these structures are related to nerves. However, the inner medulla is not innervated (11), thus further analysis is necessary to confirm the identification of the DNM1-positive cells. In contrast, DNM2 and DNM3 are highly expressed in the IMCD with little expression in the interstitium or vasa recta. In addition, we found expression of DNM2- and DNM3-truncated proteins (85 and 73 kDa, respectively), which may represent inner medulla-specific splice variants of these DNMs. As many as 25 DNM mRNA splice variants have been sequenced, and DNM2 splice variants are expressed in the whole kidney (5). However, comparatively little is known about DNM3 splice variants or its expression in the kidney.
The NOS1 splice variants, NOS1α and NOS1β, and NOS3 are highly expressed in the rat IM (22, 28). Within the rat IMCD, we present that NOS1α and NOS1β are expressed in the plasma membrane, intracellular membrane fraction, and cytosolic or soluble fraction, while NOS3 was only found in the plasma membrane and intracellular membrane fractions. DNM2 and DNM3 are also expressed in all three subcellular fractions of IMCDs. We observed that NOS1, DNM2, and DNM3 are expressed in the same subcellular compartments and that NOS1 interacts with DNM2 as well as DNM3. Although we only observed NOS1α interacting with DNM2 in the rat IM tissue lysates, NOS1β expressed in the mIMCD3 cells was found to interact with DNM2. This may reflect a species-specific difference or an in vivo vs. in vitro difference. In the conditions of our study, NOS3 only interacts with DNM1 in the renal tissue lysates. Since DNM1 is expressed at very low levels in the IMCD, we concluded that DNM-dependent regulation of NOS in the IMCD is predominately with the NOS1 variants over NOS3. It is unclear whether the DNM/NOS1 interactions result in translocation among different subcellular compartments. Within the vascular endothelium, DNM2 is proposed to regulate NOS3 through direct activation of the enzyme via stimulation of the reductase domain (7), but also through facilitating trafficking and translocation (6, 8). The dominant negative DNM2 (K44A) anchors NOS3 to the plasma membrane and in various endothelial cell lines shown to inhibit NO production (8, 26). The mechanism of inhibition by DNM2 K44A is through an effect on NOS3 trafficking and not due to the mutation itself; the K44A mutation is distant from the region that directly binds to NOS3 (8, 26). We propose that a similar mechanism may occur between DNM2 and/or DNM3 with NOS1 within the IMCD. Future studies will determine whether the interaction of the DNMs and NOS1 splice variants is regulated differentially among the subcellular compartments.
Renal medullary NOS1-dependent NO is a mediator of natriuresis and diuresis (20, 25). These new findings indicate that DNM regulates NOS1-derived NO production in the IMCD, thus validating the physiological relevance of the DNM/NOS1 interaction. We found that the DNM/NOS interactions in the inner medullary tissue of rats fed a normal- and a high-salt diet were similar. Previously, we reported that NOS expression and activity were the highest in the intracellular membrane fraction in the renal inner medulla under both normal-salt and high-salt diet conditions (31). Taken together, these observations suggest that under high-salt conditions, NO production in the renal inner medulla may not be regulated by differential NOS subcellular compartmentalization or interaction with DNM. Thus, we reasoned that high-salt conditions may stimulate intracellular Ca2+ signaling within the IMCD leading to an increase in NO production. Cao et al. (6) reported that the DNM2/NOS3 interaction is more sensitive to intracellular Ca2+ signaling in endothelial NO production. Therefore, we determined whether the DNM2/NOS1 interaction is sensitive to increases in intracellular Ca2+. Indeed, we demonstrated that ionomycin led to a greater than threefold increase in nitrite production in the NOS1α + DNM2-transfected cells, while only a twofold increase in nitrite production in the NOS1α-transfected cells, thus indicating that an increase in intracellular Ca2+ significantly enhances NO production when DNM2 is bound to NOS1 as opposed to NOS1 in the absence of DNM2. Clearly, more research is needed to understand how DNM may regulate NO production in the IMCD during a high-salt intake.
In conclusion, DNM regulates NO production in rat IMCD via a protein-protein interaction with NOS1. Future studies will elucidate whether this interaction results in an increase in NOS trafficking and NO production in subcellular compartments necessary to regulate sodium excretion.
GRANTS
This work was supported by National Institutes of Health Grant HL60653 (to J. S. Pollock) and the National Kidney Foundation Post Doctoral Fellowship (to K. A. Hyndman).
Present address of J. Xue: Cell Signaling Unit, Children's Medical Research Institute, The University of Sydney, NSW 2145, Australia.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
Plasmids containing full-length NOS1α were a generous gift from Dr. Tatsuo Suzuki, Department of Neuroplasticity, Institute on Aging and Adaptation, Shinshu University Graduate School of Medicine, Matsumoto 390-8621, Japan. Full-length dynamin-2-GFP was a generous gift from Dr. Phillip J. Robinson, Children's Medical Research Institute, The University of Sydney, Sydney, NSW 2145, Australia. NOS1 reductase domain was a generous gift of Dr. Stephen Black, Vascular Biology Center, Medical College of Georgia at Georgia Health Sciences University, Augusta, GA 30912.
REFERENCES
- 1. Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 357: 593–615, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Altschuler Y, Barbas SM, Terlecky LJ, Tang K, Hardy S, Mostov KE, Schmid SL. Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. J Cell Biol 143: 1871–1881, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bachmann S, Bosse HM, Mundel P. Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. Am J Physiol Renal Fluid Electrolyte Physiol 268: F885–F898, 1995. [DOI] [PubMed] [Google Scholar]
- 4. Brenman JE, Xia H, Chao DS, Black SM, Bredt DS. Regulation of neuronal nitric oxide synthase through alternative transcripts. Dev Neurosci 19: 224–231, 1997. [DOI] [PubMed] [Google Scholar]
- 5. Cao H, Garcia F, McNiven MA. Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell 9: 2595–2609, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao S, Yao J, McCabe TJ, Yao Q, Katusic ZS, Sessa WC, Shah V. Direct interaction between endothelial nitric oxide synthase and dynamin-2. Implications for nitric oxide synthase function. J Biol Chem 276: 14249–14256, 2001. [DOI] [PubMed] [Google Scholar]
- 7. Cao S, Yao J, Shah V. The proline-rich domain of dynamin-2 is responsible for dynamin-dependent in vitro potentiation of endothelial nitric oxide synthase activity via selective effects on reductase domain function. J Biol Chem 278: 5894–5901, 2003. [DOI] [PubMed] [Google Scholar]
- 8. Chatterjee S, Cao S, Peterson TE, Simari RD, Shah V. Inhibition of GTP-dependent vesicle trafficking impairs internalization of plasmalemmal eNOS and cellular nitric oxide production. J Cell Sci 116: 3645–3655, 2003. [DOI] [PubMed] [Google Scholar]
- 9. Diatloff-Zito C, Gordon AJ, Duchaud E, Merlin G. Isolation of an ubiquitously expressed cDNA encoding human dynamin II, a member of the large GTP-binding protein family. Gene 163: 301–306, 1995. [DOI] [PubMed] [Google Scholar]
- 10. Eliasson MJ, Blackshaw S, Schell MJ, Snyder SH. Neuronal nitric oxide synthase alternatively spliced forms: prominent functional localizations in the brain. Proc Natl Acad Sci USA 94: 3396–3401, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eppel GA, Malpas SC, Denton KM, Evans RG. Neural control of renal medullary perfusion. Clin Exp Pharmacol Physiol 31: 387–396, 2004. [DOI] [PubMed] [Google Scholar]
- 12. Foster JM, Carmines PK, Pollock JS. PP2B-dependent NO production in the medullary thick ascending limb during diabetes. Am J Physiol Renal Physiol 297: F471–F480, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hinshaw JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol 16: 483–519, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirchhausen T, Macia E, Pelish HE. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol 438: 77–93, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kohan DE, Padilla E, Hughes AK. Endothelin B receptor mediates ET-1 effects on cAMP and PGE2 accumulation in rat IMCD. Am J Physiol Renal Fluid Electrolyte Physiol 265: F670–F676, 1993. [DOI] [PubMed] [Google Scholar]
- 16. Loebel DA, Tsoi B, Wong N, Tam PP. A conserved noncoding intronic transcript at the mouse Dnm3 locus. Genomics 85: 782–789, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Lu D, Fu Y, Lopez-Ruiz A, Zhang R, Juncos R, Liu H, Manning RD, Jr, Juncos LA, Liu R. Salt-sensitive splice variant of nNOS expressed in the macula densa cells. Am J Physiol Renal Physiol 298: F1465–F1471, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mattson DL, Wu F. Nitric oxide synthase activity and isoforms in rat renal vasculature. Hypertension 35: 337–341, 2000. [DOI] [PubMed] [Google Scholar]
- 19. Morrissey JJ, McCracken R, Kaneto H, Vehaskari M, Montani D, Klahr S. Location of an inducible nitric oxide synthase mRNA in the normal kidney. Kidney Int 45: 998–1005, 1994. [DOI] [PubMed] [Google Scholar]
- 20. Nakano D, Pollock JS, Pollock DM. Renal medullary ETB receptors produce diuresis and natriuresis via NOS1. Am J Physiol Renal Physiol 294: F1205–F1211, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakata T, Iwamoto A, Noda Y, Takemura R, Yoshikura H, Hirokawa N. Predominant and developmentally regulated expression of dynamin in neurons. Neuron 7: 461–469, 1991. [DOI] [PubMed] [Google Scholar]
- 22. Oberbaumer I, Moser D, Bachmann S. Nitric oxide synthase 1 mRNA: tissue-specific variants from rat with alternative first exons. Biol Chem 379: 913–919, 1998. [PubMed] [Google Scholar]
- 23. Oess S, Icking A, Fulton D, Govers R, Muller-Esterl W. Subcellular targeting and trafficking of nitric oxide synthases. Biochem J 396: 401–409, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ortiz PA, Garvin JL. Cardiovascular and renal control in NOS-deficient mouse models. Am J Physiol Regul Integr Comp Physiol 284: R628–R638, 2003. [DOI] [PubMed] [Google Scholar]
- 25. Pollock JS, Pollock DM. Endothelin and NOS1/nitric oxide signaling and regulation of sodium homeostasis. Curr Opin Nephrol Hypertens 17: 70–75, 2008. [DOI] [PubMed] [Google Scholar]
- 26. Sanchez FA, Rana R, Kim DD, Iwahashi T, Zheng R, Lal BK, Gordon DM, Meininger CJ, Duran WN. Internalization of eNOS and NO delivery to subcellular targets determine agonist-induced hyperpermeability. Proc Natl Acad Sci USA 106: 6849–6853, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shpetner HS, Vallee RB. Dynamin is a GTPase stimulated to high levels of activity by microtubules. Nature 355: 733–735, 1992. [DOI] [PubMed] [Google Scholar]
- 28. Smith C, Merchant M, Fekete A, Nyugen HL, Oh P, Tain YL, Klein JB, Baylis C. Splice variants of neuronal nitric oxide synthase are present in the rat kidney. Nephrol Dial Transplant 24: 1422–1428, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sontag JM, Fykse EM, Ushkaryov Y, Liu JP, Robinson PJ, Sudhof TC. Differential expression and regulation of multiple dynamins. J Biol Chem 269: 4547–4554, 1994. [PubMed] [Google Scholar]
- 30. Sullivan JC, Pardieck JL, Hyndman KA, Pollock JS. Renal NOS activity, expression, and localization in male and female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 298: R61–R69, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sullivan JC, Smart EJ, Pollock DM, Pollock JS. Influence of salt on subcellular localization of nitric oxide synthase activity and expression in the renal inner medulla. Clin Exp Pharmacol Physiol 35: 120–125, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Tiwari S, Nordquist L, Halagappa VK, Ecelbarger CA. Trafficking of ENaC subunits in response to acute insulin in mouse kidney. Am J Physiol Renal Physiol 293: F178–F185, 2007. [DOI] [PubMed] [Google Scholar]
- 33. Ujiie K, Yuen J, Hogarth L, Danziger R, Star RA. Localization and regulation of endothelial NO synthase mRNA expression in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 267: F296–F302, 1994. [DOI] [PubMed] [Google Scholar]
- 34. Urrutia R, Henley JR, Cook T, McNiven MA. The dynamins: redundant or distinct functions for an expanding family of related GTPases? Proc Natl Acad Sci USA 94: 377–384, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol 122: 553–563, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang G, Moniri NH, Ozawa K, Stamler JS, Daaka Y. Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proc Natl Acad Sci USA 103: 1295–1300, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu F, Park F, Cowley AW, Jr, Mattson DL. Quantification of nitric oxide synthase activity in microdissected segments of the rat kidney. Am J Physiol Renal Physiol 276: F874–F881, 1999. [DOI] [PubMed] [Google Scholar]
- 38. Yao Q, Chen J, Cao H, Orth JD, McCaffery JM, Stan RV, McNiven MA. Caveolin-1 interacts directly with dynamin-2. J Mol Biol 348: 491–501, 2005. [DOI] [PubMed] [Google Scholar]
- 39. Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide 20: 223–230, 2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.