Abstract
TREK-1, TREK-2 and TRAAK are mechanosensitive two-pore domain K+ (K2P) channels thought to be involved in the attenuation of mechanotransduction. Because colon inflammation is associated with colon mechanohypersensitivity, we hypothesized that the role of these channels in colon sensory (dorsal root ganglion, DRG) neurons would be reduced by colon inflammation. Accordingly, we studied the functional expression of mechanosensitive K2P channels in colon sensory neurons in both thoracolumbar (TL) and lumbosacral (LS) DRG that represent the splanchnic and pelvic nerve innervations of the colon, respectively. In colon DRG neurons identified by retrograde tracer previously injected into the colon wall, 62% of TL neurons and 83% of LS neurons expressed at least one of three K2P channel mRNAs; the proportion of neurons expressing the TREK-1 gene was greater in LS than in TL DRG. In electrophysiological studies, single-channel activities of TREK-1a, TREK-1b, TREK-2, and TRAAK-like channels were detected in cultured colon DRG neuronal membranes. After trinitrobenzene sulfonic acid-induced colon inflammation, we observed significant decreases in the amount of TREK-1 mRNA, in the response of TREK-2-like channels to membrane stretch, and in the whole cell outward current during osmotic stretch in LS colon DRG neurons. These findings document that the majority of DRG neurons innervating the mouse colon express mechanosensitive K2P channels and suggest that a decrease in their expression and activities contributes to the increased colon mechanosensitivity that develops in inflammatory bowel conditions.
Keywords: TREK channels, mechanosensitive channels, dorsal root ganglia neurons
two-pore domain k+ (k2p) channels are recently identified, structurally distinct K+ channels that have four transmembrane and two pore-forming domains. These channels are active across a range of physiological membrane potentials, which suggests they are background K+ channels playing an important role in regulating cellular excitability by setting and shaping the resting membrane potential and action potential. Six subfamilies of K2P channels have been described to date (27). TREK-1 (K2P2.1), TREK-2 (K2P10.1), and TRAAK (K2P4.1) are TREK subfamily members activated by a variety of physical and chemical stimuli such as membrane stretch, heat, intracellular acidosis (TREK-1 and -2), or alkalosis (TRAAK) and lipids (5, 24, 30–33), implicating these channels in mechano/thermo/chemosensation.
All three K2P channels are found in sensory ganglia neurons. In human and rat dorsal root ganglia (DRG), gene expression of TREK-1 and -2 was reported to be moderate and restricted, whereas TRAAK expression was strong and abundant by whole ganglia RT-PCR and in situ hybridization, respectively (35, 48). Functionally, TREK-2-like channel activity was more frequently observed in single-channel recordings from cultured neonatal rat DRG neurons than the other two channels (26). In mouse DRG, in contrast, 60% of neurons were found to express TREK-1 mRNA (2), and its immunoreactivity was mostly observed in small- and medium-sized neurons (31). All three TREK subfamily channels were also immunohistologically detected in small-sized neurons in rat trigeminal ganglia, where they often colocalized with thermosensitive transient receptor potential vanilloid (TRPV)1 channels (53). TREK-1 and TRAAK gene transcripts (TREK-2 was not examined) are also present in nodose ganglia neurons innervating the stomach and proximal duodenum with TRAAK mRNA preferentially detected in TRPV1-negative neurons (54). The inhibitory role of these K2P channels in mechano/thermosensation is supported by studies in TREK-1 and/or TRAAK knockout mice in which sensitivity is increased to von Frey probing of the hindpaw and to tail immersion temperatures between 46 and 50°C (2, 39).
Although the presence and role of mechano/thermosensitive K2P channels in sensory neurons have been documented, their expression profile in DRG neurons related to an innervated target has not been widely investigated. Moreover, little information is available regarding changes in their expression and properties in conditions where alterations in mechano/thermosensation occur. Accordingly, we studied the expression of TREK-1, TREK-2, and TRAAK in DRG neurons innervating the mouse colon, an organ physiologically subjected to stretch during normal function. Because the colon is innervated by two nerves with overlapping but also physiologically distinct functions (6, 8, 52), DRG neurons at thoracolumbar (TL) (lumbar splanchnic nerve) and lumbosacral (LS) (pelvic nerve) levels were studied separately. Second, to test the hypothesis of the inhibitory role of the channels in mechanosensation, we examined the expression and properties of K2P channels in colon DRG neurons after colon inflammation that produces colon mechanohypersensitivity. Portions of these data have been presented in abstract form (28a).
MATERIALS AND METHODS
Adult male C57BL/6 (Taconic, Germantown, NY) mice (25–30 g) were used in this study. Mice were housed under a 12-h:12-h light/dark cycle. Water and food were provided ad libitum. All procedures were approved by and in accordance with the guidelines of the Institutional Animal Care and Use Committee, University of Pittsburgh.
Cell labeling.
The colon was exposed surgically through laparotomy (2% isoflurane anesthesia; Hospira, Lake Forest, IL), and a 2% solution of retrograde tracer, 1,1′-dioctadecyl-3,3,3′,3-tetramethylindocarbocyanine perchlorate (DiI; Invitrogen, Carlsbad, CA) in absolute dimethyl sulfoxide was injected into the organ wall using a 30-gauge needle. Three to five sites were injected into the distal colon wall, each in a volume of ∼5 μl. Mice were used for experiments 14–21 days after DiI injection.
Colon inflammation.
2,4,6-Trinitrobenzene sulfonic acid (TNBS, 1%; Sigma-Aldrich, St. Louis, MO) in 50% ethanol was instilled intracolonically (200 μl) under 2% isoflurane anesthesia using a 22-gauge, 24-mm-long stainless-steel feeding needle. Mice were then kept vertical for 3 min and subsequently returned to their home cages. Nutrients and water were supplied in a gel form (ClearH2O; DietGel, Portland, ME), and their nest area was covered with a hut to keep them warm until euthanasia at day 2 after TNBS instillation.
DRG neuron culture.
Mice were euthanized with overdose of pentobarbital sodium (Nembutal; Ovation Pharmaceutical, Deerfield, IL) followed by decapitation. TL (T10-L1) or LS (L6-S1) DRG were dissected out and enzymatically digested with collagenase IV (200 U/ml; Worthington Biochemical, Lakewood, NJ) and dispase (7.5 U/ml, Worthington) in serum-free, advanced DMEM/F12 containing 1% penicillin/streptomycin (Invitrogen) at 37°C in 5% CO2 for 40 min. After trituration with a fire-polished, large-bore glass Pasteur pipette, the cell suspension (∼2 ml) was transferred to a conical tube containing 8 ml of advanced DMEM/F12 with 10% fetal bovine serum (Sigma) to stop the digestion. After centrifugation at 280 g for 10 min, the pellet was dissociated in fresh medium, cells were plated on poly-d-lysine-coated coverslips (Becton Dickinson Labware, Bedford, MA), and incubated overnight at 37°C in 5% CO2. Only DiI-labeled neurons were used for subsequent experiments within 30 h after plating.
Single-cell RT-PCR.
DiI-labeled neurons in culture were collected individually with glass pipettes and expelled into microcentrifuge tubes containing reverse transcriptase mix (15), and their first-strand cDNAs were synthesized using Oligo (dT)12–18 primer (Invitrogen) through a series of incubations: 65°C for 1.5 min, room temperature for 2 min, 37°C for 20 min after adding 20 U SuperScript II (Invitrogen), and 65°C for 10 min. Successfully processed cells were screened by examining a transcript of a housekeeping gene GAPDH. Reverse-transcription-negative controls (cells processed without SuperScript II or a cell-free bath aspirate) were included in every screening. The first-round multiplex PCR was performed using two-fifths of the original first-strand cDNA sample as a template in a 25-μl solution containing 1xGoTaq reaction buffer (Promega, Madison, WI), 0.4 μM external primers mix, 0.2 mM dNTPs, and 0.2 μl GoTaq DNA polymerase (Promega); primer sequences are listed in Table 1. Reactions consisted of initialization at 95°C for 10 min, 35 cycles at 94°C/30 s, 52°C/30 s, and 72°C/30 s before a final extension step at 72°C for 10 min. Each first-round PCR product served as template in the second-round PCR using a channel-specific internal primer pair. The second-round PCR product was electrophoresed on 2% agarose gel, stained with ethidium bromide, and photographed.
Table 1.
Primer pairs used for PCR (5′-3′)
| Gene (expected size) | External Primers | Internal Primers | Accession No. |
|---|---|---|---|
| GAPDH (493, 242 bp) | GCTGAGTATGTCGTGGAGTCTA | GTTTGTGATGGGTGTGAACCAC | NM_008084 |
| CATACTTGGCAGGTTTCTCCAG | TGGATGCAGGGATGATGTTCTG | ||
| TREK-1 (557, 229 bp) | CACATGGAGAGATACAGACTGC | GAGATACAGACTGCTGGCATAG | NM_010607 |
| GAGATGGGTGGAGCTTTCTTTG | GTAGATGTAAGTACGGGCACAG | ||
| TREK-2 (438, 254 bp) | CAGTGGCAACGCTATAGTTCTC | GTGATAGGTGGTGCACAGATAG | NM_029911 |
| CCACCTACACTACCTATCCCAT | GCCATTGGTTAAGGAGATAGCC | ||
| TRAAK (427, 109 bp) | CAGTGAGAATCTGGCCTTCATC | ATTTGACCAAAGAGCCGTCC | NM_008431 |
| TTTGAGGCACAGTTGTGAGG | TTGGATGTGAAGAACCAGCC |
Quantitative real-time PCR.
The quantity of K2P channel transcripts in DiI-labeled colon neurons was measured adopting a single-cell real-time RT-PCR protocol (1, 42). Specifically, the first-strand cDNA sample of an individual neuron was preamplified by 26 cycles, which was chosen on the basis of the linear amplification range of the reference standard GAPDH in single cells, under the multiplex PCR condition described above. After identification of the channel transcripts expressed in each neuron, the 26-cycle preamplification product from neurons expressing the channel transcript(s) was diluted six times and pooled according to the channel transcript and DRG level (TL vs. LS) of each animal. The pooled product served as template in quantitative PCR separately for a specific channel transcript and the reference standard on the ABI PRISM 7000 system (Applied Biosystems, Foster City, CA) using ABsolute QPCR CYBR Green ROX Mix (ABgene, Rochester, NY). Threshold cycle (CT) and PCR efficiency were determined within the linear amplification range. The quantity of the channel transcript was expressed relative to that of the reference standard by raising the averaged PCR efficiency to the power of the CT difference (ΔCT) between the reference standard and the channel transcript. Preamplification uniformity of the target transcripts was also assessed by calculating the difference of ΔCT(ΔΔCT) in the same samples preamplified by two different cycle numbers (20 vs. 26 cycles). The ΔΔCT of all channel transcripts were within ±0.51.
Electrophysiological recordings.
Single-channel and whole cell recordings were performed with an Axopatch 200B patch-clamp amplifier (Axon Instruments, Union City, CA). Cells on coverslips were continuously superfused with normal bath solution containing (in mM): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose (pH 7.4, 310 mOsm) in a recording chamber. For single-channel recording, borosilicate glass micropipettes (Sutter Instrument, Novato, CA) with tip resistance greater than 5 MΩ were fabricated and filled with pipette solution containing (in mM): 140 KCl, 1 MgCl2, 5 EGTA, 10 HEPES, 10 tetraethylammonium chloride (TEA-Cl), 1 4-aminopyridine (4-AP), and 0.01 glibenclamide (pH 7.3). The tip was coated with silicon film using Sigmacote (Sigma) to improve signal/noise ratio. Single-channel activities were recorded in an inside-out mode under symmetrical K+ conditions by superfusing the pipette solution without TEA-Cl, 4-AP, and glibenclamide to the cytosolic side of the membrane patch. The recorded signal was low-pass filtered at 2 kHz (80 dB/decade) at a sampling rate of 10 kHz and digitized (Digidata 1320A, Axon Instruments). For single-channel analysis, any events shorter than the filter rise time (Tr = 0.17 ms) were ignored, and those shorter than 2.5 Tr (0.42 ms) were excluded from the current amplitude histogram. NPo (N is the number of channels in the patch, and Po is the channel open probability) was analyzed as a measure of channel activity, and its changes during any stimuli were expressed relative to the activity before the stimulus application. The membrane patch was stretched by applying negative pressure inside the patch pipette through a water-filled U-shape manometer, and drugs were applied using a fast-step SF-77B superfusion system (Warner Instruments, Hamden, CT) with a three-barrel pipette placed in close proximity (100 μm) to the cell. Openings of multiple ion channels were frequently recorded in one membrane patch, hampering clear identification of single-channel activity. Therefore, for channel identification experiments, we only used patches that contained no more than three types of channels distinct in their amplitude and kinetics. Changes in channel activities upon stimulation were quantified only when the patches contained one type of TREK subfamily channel.
For whole cell current recording, glass micropipettes with tip resistance 2∼3 MΩ were filled with pipette solution containing (in mM): 120 K gluconate, 20 KCl, 0.2 CaCl2, 2 MgATP, 10 EGTA, and 10 HEPES (pH 7.2, 302 mosM). After establishing a stable whole cell configuration, the normal bath solution was exchanged with an isotonic recording solution containing (in mM): 85 choline chloride, 5 KCl, 3 MgCl2, 10 glucose, 10 HEPES, 10 TEA-Cl, 1 4-AP, and 90 d-mannitol (pH 7.4, 310 mosM). We omitted Na+ and Ca2+ from the recording solution to avoid the contamination of K+ outward current by inward flux of these two ions through stretch-sensitive cation channels. Neurons were held at −60 mV and subjected to slow ramp depolarization from −110 mV to 20 mV (20 mV/s). Series resistance was compensated by >80%, and leak currents were not subtracted. Membrane stretch was done by swelling the cell with hypotonic recording solution (220 mosM) containing a volume-activated chloride channel blocker 5-Nitro-2-(3-phenylpropylamino)benzoic acid (100 μM) and measured by increase in neuronal size (μm2). Neurons under electrodes were photographed using a microscope-mounted digital camera (DFC340FX; Leica Microsystems, Bannockburn, IL). Data were sampled at 10 kHz and low-pass filtered at 5 kHz. All membrane potentials noted in this study are liquid junction potential corrected.
Data analysis.
Data are presented as means ± SE with n, the number of samples and N, the number of mice. The software packages pClamp 10.0 (Axon Instruments), Origin 8 (Originlab Corporation, Northampton, MA) and SigmaPlot 9.0 (Systat Software, San Jose, CA) were used for data analyses. Chi-square tests were used for analyses of contingency tables with more than three categories. A set of independent contrasts was specified a priori for comparing the proportion of cells expressing each channel between two groups (e.g., TL vs. LS), and Fisher's exact test was used for analysis of the 2 × 2 contingency table. For analysis of differences between means, the Mann-Whitney U-test or Wilcoxon signed rank test was used for comparing two groups, and two-way ANOVA with the Holm-Sidak post hoc multiple-comparisons test was used for groups with two independent variables (e.g., TL vs. LS in naïve vs. TNBS-treated mice). Results were considered statistically significant when P < 0.05.
RESULTS
Gene expression of TREK subfamily channels in colon DRG neurons.
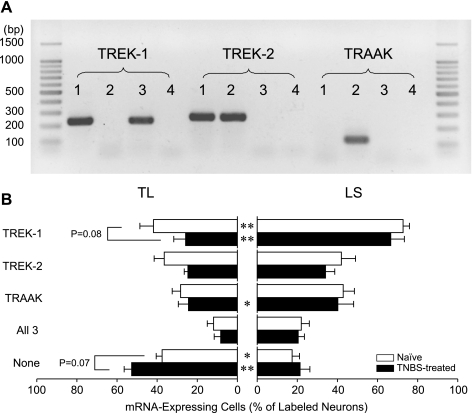
In 77 TL colon DRG neurons (N = 6), 62% expressed at least one of the TREK subfamily channel gene transcripts. The TREK-1 gene transcript was detected in 42%, TREK-2 in 36%, and TRAAK in 27% of TL cells; these proportions of K2P channel gene expression did not differ within the sample of TL neurons (P > 0.17, χ2 test). Among all TL neurons, 12% were found to express all three channel mRNAs (Fig. 1). Cells with two TREK channel gene transcripts were also frequently encountered; 9% of colon TL DRG neurons had both TREK-1 and TREK-2, 6% had TREK-1 and TRAAK, and 4% had TREK-2 and TRAAK.
Fig. 1.
Differential gene expression of TREK subfamily K2P channels between thoracolumbar (TL) and lumbosacral (LS) colon DRG neurons from naïve and 2,4,6-trinitrobenzene sulfonic acid (TNBS)-treated mice. A: representative photographs of RT-PCR products of TREK subfamily channels from 3 colon dorsal root ganglia (DRG) neurons (lanes 1–3, no template in lane 4). The expected amplicon sizes were 229 bp (TREK-1), 254 bp (TREK-2), and 109 bp (TRAAK). B: in both naïve and TNBS-treated mice, the proportion of cells expressing the TREK-1 gene was significantly greater in LS than in TL colon DRG neurons, whereas the percentage of neurons not expressing any of these three channels (None) was greater in TL than in LS colon neurons. Error bars (SE) represent animal-to-animal variations in percentage. *P < 0.05 and **P < 0.001 between TL and LS colon DRG neurons by Fisher's exact test.
In LS colon DRG neurons, however, the number of cells expressing TREK-1 mRNA was greater (P < 0.0001, χ2 test, standardized residual >2.5) than those expressing TREK-2 or TRAAK mRNA. In 81 samples (N = 6), the TREK-1 gene transcript was found in 73% of neurons, TREK-2 in 42%, and TRAAK in 43%. Seventeen percent of LS neurons were found to be devoid of any TREK channel gene transcripts, whereas 22% expressed all three channel mRNAs. Fourteen percent of LS colon DRG neurons were positive to both TREK-1 and -2, 16% to TREK-1 and TRAAK, and 1% to TREK-2 and TRAAK.
Two major differences were observed between TL and LS colon DRG neurons in the proportion of channel-expressing cells. More LS colon DRG neurons expressed TREK-1 mRNA than their TL counterparts (P < 0.0001, Fisher's exact test), and the proportion of neurons not expressing any TREK subfamily channel mRNA was significantly less in the LS population (P < 0.005, Fisher's exact test). When quantified in neurons grouped by the channels expressed, TREK-1 mRNA was most abundant, and no difference was detected between TL and LS colon DRG neurons in the content of channel mRNAs (Fig. 2).
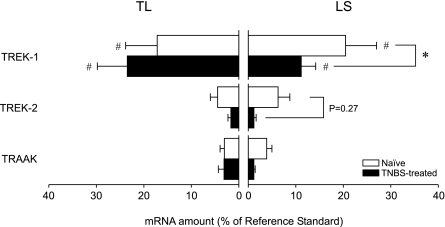
Fig. 2.
Quantity of TREK subfamily two-pore domain K+ (K2P) channel mRNAs in TL and LS colon DRG neurons from naïve and TNBS-treated mice. TREK-1 mRNA was most abundant in both TL and LS colon neurons, but the relative amount of channel mRNA was not different between TL and LS colon neurons from naïve mice. Two days after intracolonic treatment with TNBS, the quantity of TREK-1 mRNA was significantly reduced in LS colon DRG neurons. #P < 0.05 between channel transcripts. *P < 0.05 between naïve and TNBS-treated mice (N = 5 each).
We tested commercial antibodies to immunolocalize the three channel proteins in DRG tissue sections and culture, but none of them provided convincing results in our experiment conditions (data not shown).
TREK subfamily-like single-channel activities in colon DRG neurons.
To examine the functional expression of TREK subfamily channels in colon DRG neurons, single-channel activity was recorded in inside-out patches under symmetrical K+ conditions (140 mM) at membrane potentials ranging from −80 mV to 80 mV. Isolation of the TREK subfamily channels was facilitated by including TEA, 4-AP, and glibenclamide in the pipette solution, all of which suppress the activities of KV channels and KCa channels without inhibition of TREK subfamily channels activities (27).
We observed four mechanosensitive K+ channels with distinct single-channel current-voltage (I-V) relationships that allowed us to differentiate them by their chord conductances at ±60 mV (Fig. 3). The first and second type showed relatively linear I-V relationships with 140 pS and 40 pS conductances, respectively, at ±60 mV. The third and fourth type showed weak inward rectification. The chord conductance of the third type was 50 pS at 60 mV and 110 pS at −60 mV, and that of the fourth was 55 pS at 60 mV and 80 pS at −60 mV. At least one of the four channels was encountered in 39% of patches in TL and 40% in LS colon DRG neurons.
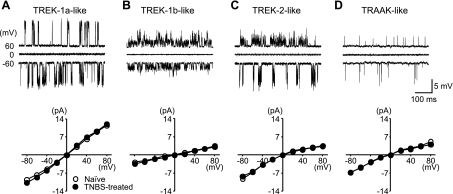
Fig. 3.
Single-channel activities of TREK subfamily-like channels in cultured colon DRG neurons. TREK-1a (A), TREK-1b (B), TREK-2 (C), and TRAAK-like (D) channel activities were detected in inside-out membrane patches under symmetrical K+ (140 mM) condition. The I-V relationship of each channel did not differ between naïve and TNBS-treated mice.
As shown in Fig. 4, the activities of these four different channels were increased upon membrane stretch, by arachidonic acid (AA, 20 μM), and by decrease in intracellular pH for the first, second, and third type or by increase in the intracellular pH for the fourth type, all of which are characteristic properties of TREK subfamily channels. The first type was similar to classical TREK-1 (TREK-1a), the third to TREK-2, and the fourth to TRAAK recorded in rat neurons and COS-7 cells transfected with each channel gene (20, 26). The second type resembled the TREK-like channel iK,AA3 originally reported in rat supraoptic nucleus magnocellular neurons (20), and TREK-1b, the splice variant of TREK-1, observed in rat cardiac ventricular muscle and TREK-1 gene-transfected HEK293 cells (51).
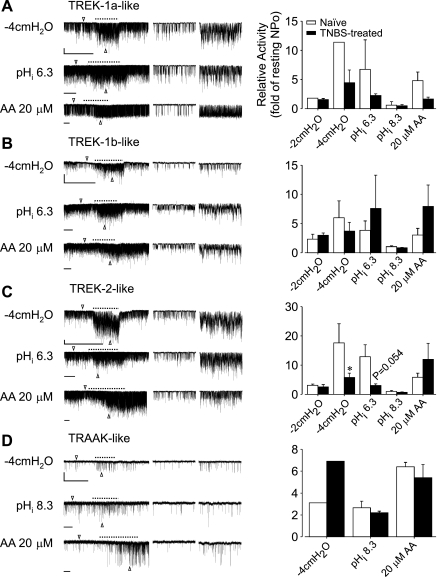
Fig. 4.
Single-channel responses of TREK subfamily-like channels to membrane stretch, changes in intracellular pH, and arachidonic acid (AA). TREK-1a (A), TREK-1b (B), TREK-2 (C), and TRAAK-like (D) channel activities were increased by membrane stretch and AA (cytosolic side superfusion) at −60 mV membrane potential. Intracellular acidosis (pH 6.3) activated TREK-1- and TREK-2-like channels, whereas alkalosis (pH 8.3) stimulated TRAAK-like channels. In the examples shown in the left traces, the vertical bars indicate 5 pA and horizontal bars, 10 s. The middle and the right traces are 1-s segments of the left traces marked by arrow heads that represent the channel activities before and during stimulus application (dashed line). The responses of TREK-2-like channels to membrane stretch (−4 cmH2O negative pressure) and intracellular acidosis (pH 6.3) were decreased in TNBS-treated mice (C, right). The difference in the responses of the other channels between naïve and TNBS-treated mice was not statistically tested because of limited sample size. *P < 0.05 by Mann-Whitney U-test. NPo, N is the number of channels in the patch, and Po is the channel open probability.
Channel activities of two or more of these TREK subfamily-like channels were often observed in a single patch, especially in LS colon DRG neurons. The observation frequency of each channel is summarized in Table 2. Because of the limited number of patches that contained only one channel type, necessary for an unconfounded analysis, no statistical comparison of channel activities was made between TL and LS neurons, and the responses of each channel found in both TL and LS DRG neurons were pooled for presentation.
Table 2.
Observation frequencies (% of patched neurons) of TREK subfamily-like channels in colon DRG neurons
| TREK-1a-like | TREK-1b-like | TREK-2-like | TRAAK-like | |
|---|---|---|---|---|
| TL | ||||
| Naïve (26) | 8 | 15 | 19 | 0 |
| TNBS-treated (38) | 13 | 0* | 26 | 0 |
| LS | ||||
| Naïve (35) | 9 | 14 | 23 | 6 |
| TNBS-treated (36) | 17 | 17 | 39 | 11 |
N = 6 for naïve and 8 for 2,4,6-trinitrobenzene sulfonic acid (TNBS)-treated mice. Numbers in parentheses indicate the number of total cells analyzed in corresponding group.
P < 0.05 vs. naïve mice by Fisher's exact test.
DRG, dorsal root ganglion; TL, thoracolumbar; LS, lumbosacral.
Effect of colon inflammation.
When applied into the lumen of mouse colon, TNBS dissolved in ethanol induces inflammation accompanied by mechanical hypersensitivity to colorectal distension (29, 49).
The percentage of colon DRG neurons expressing each channel gene was not significantly changed 2 days after intracolonic instillation of TNBS (Fig. 1) although there was a strong tendency toward a decrease in the proportion of TREK-1 gene-expressing cells (P = 0.0796, Fisher's exact test; P < 0.02 by χ2 test) and an increase in the proportion of cells not expressing any of the three TREK channel mRNAs (P = 0.0689, Fisher's exact test; P < 0.01 by χ2 test) in TL neurons. The differences in the gene expression of TREK subfamily channels between TL and LS colon DRG neurons were again observed in TNBS-treated mice; the proportion of LS colon DRG neurons expressing TREK-1 or TRAAK mRNA was greater than that of TL neurons in TNBS-treated mice (P < 0.0001 for TREK-1, P < 0.05 for TRAAK, Fisher's exact test), and the proportion of neurons devoid of any TREK subfamily channel mRNA was smaller in the LS population (P < 0.001, Fisher's exact test).
When measured in colon DRG neurons from mice 2 days after TNBS treatment, the quantity of TREK-1 mRNA (relative to the amount of reference standard) was decreased from 20.5 ± 6.5% (n = 48, N = 5) to 11.1 ± 3.0% in LS neurons (n = 45, N = 5, P < 0.05 by Holm-Sidak multiple-comparison test). The CT value of reference standard (GAPDH mRNA) in colon DRG neurons did not differ between naïve and TNBS-treated mice [F(1,24) = 1.32, P > 0.26 for TL colon DRG neurons, F(1,24) = 1.20, P > 0.28 for LS colon DRG neurons].
In electrophysiological recordings of DRG neuronal membrane patches, the observation frequencies of the TREK subfamily-like channels in colon neurons from TNBS-treated mice did not differ from their counterparts encountered in colon neurons from naïve mice except that TREK-1b-like channel activities were not observed in TL colon neurons from TNBS-treated mice (Table 2). Interestingly, the TREK-2-like channel in colon DRG neurons from TNBS-treated mice showed a significant decrease in the response to membrane stretch (negative pressure) of −4 cmH2O (n = 7 in naïve, 8 in TNBS-treated, N = 4 each, P < 0.03, Mann-Whitney U-test), and a strong tendency toward decreased response to intracellular acidosis (n = 8 in naïve, 7 in TNBS-treated, N = 4 each, P = 0.0541).
Because the findings described above in TNBS-treated mice pointed to an overall reduction in the expression and activity of mechanosensitive K2P channels in colon DRG neurons, we expected to find a decrease in the osmotic membrane stretch-induced whole cell K+ current in colon DRG neurons from TNBS-treated mice. Hypotonic cell swelling effectively activates TREK subfamily channels, resulting in an increased whole cell K+ current (5, 41).
Neuron size (area) was increased by exposure to hypotonic solution (220 mOsm). The sizes of TL colon DRG neurons from naïve and TNBS-treated mice were significantly increased to 121.4 ± 1.7% (n = 12, N = 4, P < 0.001, Wilcoxon signed-rank test) and 124.6 ± 3.8% (n = 7, N = 3, P < 0.05) of their original size, respectively, and their LS counterparts to 124.2 ± 2.0% (naïve, n = 21, N = 4, P < 0.001) and 129.3 ± 3.1% (TNBS-treated, n = 16, N = 3, P < 0.001). The degree of swelling was not significantly different either between TL and LS colon DRG neurons [F(1,52) = 1.75, two-way ANOVA; P > 0.19] or between naïve and TNBS-treated mice [F(1,52) = 2.14, two-way ANOVA; P > 0.14].
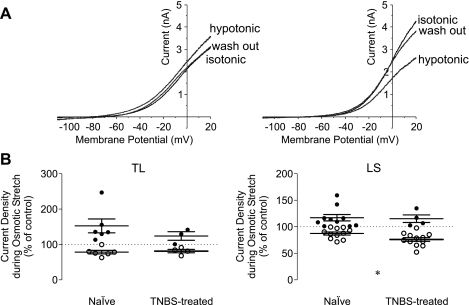
Under recording conditions where the influx of Na+ and Ca2+ was blocked, and voltage-activated K+ channels and volume-activated Cl− channels were inhibited, the osmotic stretch-induced change in outward current density was reversible and dichotomous (Fig. 5). When measured at 20-mV membrane potential, 50% (6/12) of colon TL neurons from naïve mice (N = 4) showed an increase in current density and the other 50% showed a decrease. Comparatively, 43% (3/7) of colon TL neurons from TNBS-treated mice (N = 3) showed an increase, whereas 57% (4/7) showed a decrease.
Fig. 5.
Whole cell current response of colon DRG neurons to osmotic stretch by hypotonic cell swelling. The whole cell current by a slow ramp depolarization was reversibly increased (A, left) or decreased (right) in response to osmotic stretch. The magnitude of decrease was significantly greater in LS colon DRG neurons from TNBS-treated mice than that from naïve mice. *P < 0.05, Mann-Whitney U-test.
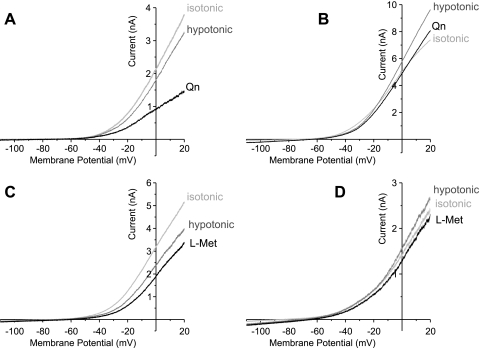
Quinine (Qn, 0.3 mM) and l-methionine (l-Met, 1 mM), both of which are known to inhibit TREK channels (4, 27) further reduced the outward current (n = 3 for Qn, n = 4 for l-Met, N = 2, Fig. 6, A and C) when the current had been decreased, or inhibited the current back to control level (n = 3 for Qn, n = 2 for l-Met, N = 2, Fig. 6, B and D) when the current had been increased by osmotic stretch.
Fig. 6.
Effect of TREK subfamily channel inhibitors on the outward current during osmotic membrane stretch. Quinine (Qn, 0.3 mM in A and B) and l-methionine (l-Met, 1 mM in C and D), nonselective but effective blockers of TREK subfamily channels, decreased the magnitude of outward current elicited under the hypotonic solution-induced membrane stretch.
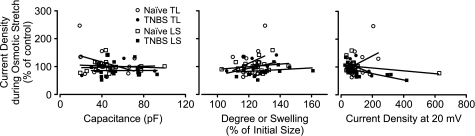
The magnitude of changes in current density upon osmotic stretch had no correlation with cell size (capacitance), degree of swelling, or current magnitude at 20 mV under isotonic recording conditions (Fig. 7). Neither the proportion of cells showing an increased outward current during osmotic stretch (P = 1, Fisher's exact test) nor the magnitude of current increase (P > 0.38, Mann-Whitney U-test) or decrease (P > 0.47) significantly differed between naïve and TNBS-treated mice.
Fig. 7.
Correlation of neuronal properties with the magnitude of changes in outward current density upon osmotic membrane stretch. No statistically significant correlation was detected between the current response to osmotic stretch and cell size (left), degree of swelling (middle), or current density at 20 mV (right) under isotonic recording condition.
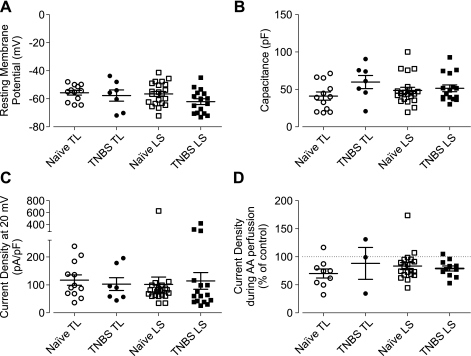
Dichotomous responses to osmotic stretch were also observed in LS colon neurons. In LS colon neurons from naïve mice (N = 4), 48% (10/21) showed an increase and the remaining 52% (11/21) showed a decrease in outward current. In contrast, the majority (75%, 12/16) of colon LS neurons from TNBS-treated mice (N = 3) showed a decrease in the outward current during osmotic stretch. Although the difference in proportion of LS colon neurons showing increased outward current did not reach statistical significance (P = 0.1908, Fisher's exact test) and the magnitude of current increase did not differ between neurons from naïve and TNBS-treated mice (P > 0.94, Mann-Whitney U-test), the magnitude of current decrease was greater in neurons from TNBS-treated mice (P < 0.029, Mann-Whitney U-test). This difference did not appear to be attributable to sampling bias because their electrophysiological properties examined (resting membrane potential, capacitance, and whole cell current density at 20 mV under isotonic recording condition) did not significantly differ (Fig. 8).
Fig. 8.
Electrophysiological properties of colon DRG neurons used for whole cell current measurement. No differences were detected in resting membrane potentials (A), cell capacitance (B), and outward current density at 20 mV (C) between samples. D: effect of AA on whole cell K+ current was predominantly inhibitory.
We further examined whether the whole cell K+ current response to AA was decreased in colon DRG neurons from TNBS-treated mice. Unexpectedly, the effect of AA (10 or 20 μM) on whole cell K+ current (in the isotonic recording solution) was predominantly inhibitory, suggesting complexity of its mechanisms and/or sites of action at the whole cell level. No difference was detected either between TL and LS or between naïve and TNBS-treated mice in the whole cell current response to AA (Fig. 8D).
A decrease in whole cell K+ current by hypotonic swelling or AA was also noted in hippocampal neurons (47), oligodendrocytes (46), and myocytes (45). We did not pursue elucidating its inhibitory mechanism in colon DRG neurons because it was not the immediate focus of the present study.
DISCUSSION
The present report documents the expression of mechanosensitive K2P channels, both at the gene and functional single-channel level, in DRG sensory neurons innervating mouse colon and, importantly, their downregulation after colon inflammation. Specifically, we found that the majority of colon DRG neurons (62% in TL and 83% in LS) expressed at least one gene transcript of TREK subfamily channels TREK-1, TREK-2, and TRAAK with frequent coexistence of two or more channel mRNAs in one neuron (31% in TL and 53% in LS). We observed that the proportion of TREK-1 gene-expressing cells was greater in LS than in TL DRG and, further, that the proportion of neurons devoid of any TREK subfamily channel mRNA was smaller in LS than in TL. These differences between the two pathways of innervation of the same organ, in this case the colon, add to a growing literature establishing significant functional differences between lumbar splanchnic and pelvic nerve afferent pathways innervating pelvic organs (6, 8, 11, 13, 14, 52). In addition, we confirmed electrophysiologically in colon DRG neurons activities of channels (39% of patches in TL and 40% in LS) showing characteristic biophysical and chemical properties of all TREK subfamily channels. The lower detection frequency of TREK subfamily-like channels in electrophysiological recordings than in gene transcripts assay may be accounted for by the higher detection sensitivity of the latter, the proportional difference in the quantities between mRNAs and translated channel proteins functional in the plasma membrane, or native TREK subfamily channels existing with their known channel properties modified.
The presence of mechanosensitive TREK subfamily channels in colon DRG neurons has implications relative to colon physiology; the colon regularly experiences deformation of its wall during functioning as a reservoir and peristaltic passage of luminal contents. This structural deformation has been shown to be detected by sensory neurons and encoded into changes in neuronal activities. DRG neurons cocultured with and in contact with colonic myocytes showed a rise of [Ca2+]i upon light touch of the myocyte membrane (16). In addition, Raybould et al. (43) reported that ∼40% of colon DRG neurons were responsive to direct mechanical stimulation of their soma. Channels involved in mechanotransduction, such as TRPV1, TRPV4, transient receptor potential A (TRPA)1, and acid-sensing ion channel (ASIC)3 are also found in colon DRG neurons with observations that disruption of their gene expression results in reduced mechanosensitivity of colon afferents or decreased behavioral response to colorectal distension (7, 9, 10, 12, 22, 23, 36, 40, 44). Considering the prevalent expression of these channels in colon DRG neurons (TRPV1 ∼80%, TRPV4 ∼52%, TRPA1 ∼58%, and ASIC3 ∼75%), there must be some overlap in colon DRG neurons in the expression of these nonselective cation channels and mechanosensitive K2P channels, suggesting that K+ outflow through TREK subfamily channels potentially counteracts the depolarizing effect of cation influx through TRP and ASIC channels when colon sensory neurons are mechanically stimulated. Therefore, one could speculate that a downregulation of TREK subfamily channels in colon DRG neurons would cause a decrease in hyperpolarizing drive upon mechanical stimulation, which in turn increases mechanotransduction of colon wall deformation.
This idea is supported by the present findings in TNBS-treated mice. TNBS has long been used to model ulcerative colitis in rodents, which develop hypersensitivity to colorectal distension in the active inflammatory phase (17, 19, 29, 49, 50). When examined two days after intracolonic TNBS treatment in the present study, a downregulation of TREK subfamily channels was apparent in colon DRG neurons: 1) the amount of TREK-1 gene transcripts was significantly reduced in LS neurons, and the proportion of TREK-1 mRNA-expressing cells showed a tendency toward a decrease in TL neurons, indicating their gene expression is subject to plastic changes in inflammation as reported in other pathological conditions such as deafness by cochlear nerve ablation (21) and bladder hypertrophy by partial bladder outlet obstruction (3). The expression of TREK subfamily channels monitored was decreased in cochlear nucleus and in bladder smooth muscle, respectively. 2) The response of TREK-2-like channel to membrane stretch was diminished in DRG neurons from TNBS-treated mice. The mechanosensitivity of TREK-2 is regulated by its COOH terminus (28), and phosphorylation of the domain by protein kinase C decreases channel activity (25). Therefore, it seems not unlikely that, in addition to decreased channel gene expression, posttranslational modulation occurs after inflammation to regulate channel activity. The tendency of the TREK-2-like channel toward decreased sensitivity to intracellular acidosis after intracolonic TNBS treatment suggests an inhibitory modulation through changes in the COOH terminus because this domain also confers proton sensitivity on TREK-2 (28). 3) The inhibition of whole cell outward current by osmotic membrane stretch was greater in LS colon DRG neurons from TNBS-treated mice. Although caution is needed in interpretation of these results because of the nonspecific nature of osmotic stimulation (activating stretch-activated channels as well as suppressing stretch-inhibited channels), our findings suggest that a decrease in function/expression of TREK subfamily channels in colon DRG neurons augmented the inhibitory effect of hypotonic swelling on whole cell outward current and accounted for the tendency toward a decrease in the proportion of DRG neurons showing current increase upon osmotic stretch in TNBS-treated mice. Together, these outcomes suggest that the downregulation of TREK subfamily channels contributes to the heightened mechanosensitivity to colorectal distension in the presence of colon inflammation. Supporting evidence for the correlation between downregulation of these channels and visceral hypersensitivity to mechanical stimulation could be provided by experiments using a method to inhibit the channels' activities in vivo. Such efforts have been hindered largely by lack of pharmacological tools that selectively interfere with TREK subfamily channels. Alternative strategies would include silencing TREK subfamily channel gene expression to examine the effect of their absence/reduction on behavioral nociceptive responses to colorectal distension and/or analyzing the expression pattern of these channels in other models of visceral hypersensitivity in which inflammation is not present.
Another potential function of TREK subfamily channels in colon DRG neurons could be inhibition of spontaneous neuronal activity in response to colon wall deformation. When colorectal distension was used as a search stimulus to identify neurons receiving mechanosensory inputs from colorectum, a group of TL and LS spinal dorsal horn neurons were found to cease their spontaneous action potential firing during colorectal distension (37, 38). Although there is yet no evidence of spontaneously active colon DRG neurons that respond in such a fashion, it would be interesting to examine the possible link between TREK subfamily channels and neural substrates involved in inhibitory reflexes triggered by visceral organ distension.
It is noteworthy that there is both qualitative and quantitative heterogeneity of mechanosensitive colon sensory fibers. In single-fiber recordings using an in vitro colon preparation with lumbar splanchnic or pelvic nerves attached, five different fiber types were identified on the basis of their responses to mechanical stimuli (mucosal stroking, blunt probing, and circumferential stretch), including two different stretch-sensitive fiber groups on the basis of their high and low response thresholds to mechanical stimulation (8, 18). Similarly, sharp electrode recordings of colon DRG neurons also revealed two populations that are distinct in their mechanical thresholds and action potential firing frequencies upon colon distension (34). Mapping the expression pattern of the three mechanosensitive K2P channels in sensory neurons in conjunction with studies such as these would extend our understanding of the underlying mechanisms that define the neuronal heterogeneity in mechanosensitivity.
In summary, we found that all three mechanosensitive K2P channels, TREK-1, TREK-2, and TRAAK, are expressed in colon DRG neurons that represent two different visceral afferent pathways, lumbar splanchnic and pelvic nerves. Their expression (TREK-1 in LS colon neurons) and activity (TREK-2) were significantly decreased in the presence of colon inflammation. These findings suggest a potential inhibitory role for these channels in sensory coding of colon distension and in the manifestation of visceral hypermechanosensitivity in gut inflammation.
GRANTS
This work was supported by National Institutes of Health (NIH) awards R01 NS 19912 and UL1 RR024153 from the National Center for Research Resources (NCRR), a component of the NIH, NIH Roadmap for Medical Research, and by the Office of the Senior Vice Chancellor for the Health Sciences, University of Pittsburgh.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The contents of this study are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. We thank Michael Burcham for preparation of figures.
REFERENCES
- 1. Al-Taher A, Bashein A, Nolan T, Hollingsworth M, Brady G. Global cDNA amplification combined with real-time RT-PCR: accurate quantification of multiple human potassium channel genes at the single cell level. Yeast 17: 201–210, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alloui A, Zimmermann K, Mamet J, Duprat F, Noel J, Chemin J, Guy N, Blondeau N, Voilley N, Rubat-Coudert C, Borsotto M, Romey G, Heurteaux C, Reeh P, Eschalier A, Lazdunski M. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J 25: 2368–2376, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker SA, Hatton WJ, Han J, Hennig GW, Britton FC, Koh SD. Role of TREK-1 potassium channel in bladder overactivity after partial bladder outlet obstruction in mouse. J Urol 183: 793–800, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Baker SA, Hennig GW, Han J, Britton FC, Smith TK, Koh SD. Methionine and its derivatives increase bladder excitability by inhibiting stretch-dependent K(+) channels. Br J Pharmacol 153: 1259–1271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J Biol Chem 275: 17412–17419, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Brierley SM, Carter R, Jones W, III, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol 567: 267–281, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O'Donnell TA, Cooper NJ, Harrington AM, Adam B, Liebregts T, Holtmann G, Corey DP, Rychkov GY, Blackshaw LA. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology 137: 2084–2095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brierley SM, Jones RC, 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127: 166–178, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, Holtmann G, Liedtke W, Blackshaw LA. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 134: 2059–2069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms.Gastroenterology 135: 937–46, 946, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Chen X, Gebhart GF. Differential purinergic signaling in bladder sensory neurons of naive and bladder-inflamed mice. Pain 148: 462–472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christianson JA, McIlwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience 140: 247–257, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Dang K, Bielefeldt K, Gebhart GF. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci 25: 3973–3984, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dang K, Lamb K, Cohen M, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurophysiol 99: 49–59, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dulac C. Cloning of genes from single neurons. Curr Top Dev Biol 36: 245–258, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Ennes HS, Young SH, Raybould HE, Mayer EA. Intercellular communication between dorsal root ganglion cells and colonic smooth muscle cells in vitro. Neuroreport 8: 733–737, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Eutamene H, Bradesi S, Larauche M, Theodorou V, Beaufrand C, Ohning G, Fioramonti J, Cohen M, Bryant AP, Kurtz C, Currie MG, Mayer EA, Bueno L. Guanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral pain. Neurogastroenterol Motil 22: 312–e84, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Feng B, Brumovsky PR, Gebhart GF. Differential roles of stretch-sensitive pelvic nerve afferents innervating mouse distal colon and rectum. Am J Physiol Gastrointest Liver Physiol 298: G402–G409, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Friedrich AE, Gebhart GE. Effects of spinal cholecystokinin receptor antagonists on morphine antinociception in a model of visceral pain in the rat. J Pharmacol Exp Ther 292: 538–544, 2000 [PubMed] [Google Scholar]
- 20. Han J, Gnatenco C, Sladek CD, Kim D. Background and tandem-pore potassium channels in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol 546: 625–639, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holt AG, Asako M, Duncan RK, Lomax CA, Juiz JM, Altschuler RA. Deafness associated changes in expression of two-pore domain potassium channels in the rat cochlear nucleus.Hear Res 216–217: 146–153, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hughes PA, Brierley SM, Young RL, Blackshaw LA. Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. J Comp Neurol 500: 863–875, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci 25: 10981–10989, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol 564: 103–116, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kang D, Han J, Kim D. Mechanism of inhibition of TREK-2 (K2P10.1) by the Gq-coupled M3 muscarinic receptor. Am J Physiol Cell Physiol 291: C649–C656, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Kang D, Kim D. TREK2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol 291: C138–C146, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des 11: 2717–2736, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Kim Y, Gnatenco C, Bang H, Kim D. Localization of TREK-2 K+ channel domains that regulate channel kinetics and sensitivity to pressure, fatty acids and pHi. Pflügers Arch 442: 952–960, 2001 [DOI] [PubMed] [Google Scholar]
- 28a.La JH, Gebhart GF. Expression of mechanosensitive two pore-domain K+ channels in dorsal root ganglia neurons innervating mouse bladder and colon. Program No. 655.5 2009. In: Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2009 [Google Scholar]
- 29. Lamb K, Zhong F, Gebhart GF, Bielefeldt K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am J Physiol Gastrointest Liver Physiol 290: G451–G457, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Maingret F, Fosset M, Lesage F, Lazdunski M, Honore E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J Biol Chem 274: 1381–1387, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honore E. TREK-1 is a heat-activated background K(+) channel. EMBO J 19: 2483–2491, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem 274: 26691–26696, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Lysophospholipids open the two-pore domain mechano-gated K(+) channels TREK-1 and TRAAK. J Biol Chem 275: 10128–10133, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Malin SA, Christianson JA, Bielefeldt K, Davis BM. TPRV1 expression defines functionally distinct pelvic colon afferents. J Neurosci 29: 743–752, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Medhurst AD, Rennie G, Chapman CG, Meadows H, Duckworth MD, Kelsell RE, Gloger II, Pangalos MN. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Brain Res Mol Brain Res 86: 101–114, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Miranda A, Nordstrom E, Mannem A, Smith C, Banerjee B, Sengupta JN. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience 148: 1021–1032, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ness TJ, Gebhart GJ. Characterization of neuronal responses to noxious visceral and somatic stimuli in the medial lumbosacral spinal cord of the rat. J Neurophysiol 57: 1867–1892, 1987 [DOI] [PubMed] [Google Scholar]
- 38. Ness TJ, Gebhart GJ. Characterization of neurons responsive to noxious colorectal distension in the T13–L2 spinal cord of the rat. J Neurophysiol 60: 1419–1438, 1988 [DOI] [PubMed] [Google Scholar]
- 39. Noel J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J 28: 1308–1318, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut 54: 1408–1415, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J 17: 4283–4290, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peixoto A, Monteiro M, Rocha B, Veiga-Fernandes H. Quantification of multiple gene expression in individual cells. Genome Res 14: 1938–1947, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Raybould HE, Gschossman JM, Ennes H, Lembo T, Mayer EA. Involvement of stretch-sensitive calcium flux in mechanical transduction in visceral afferents. J Auton Nerv Syst 75: 1–6, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil 16: 113–124, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Smirnov SV, Aaronson PI. Modulatory effects of arachidonic acid on the delayed rectifier K+ current in rat pulmonary arterial myocytes. Structural aspects and involvement of protein kinase C. Circ Res 79: 20–31, 1996 [DOI] [PubMed] [Google Scholar]
- 46. Soliven B, Wang N. Arachidonic acid inhibits potassium conductances in cultured rat oligodendrocytes. Am J Physiol Cell Physiol 269: C341–C348, 1995 [DOI] [PubMed] [Google Scholar]
- 47. Somjen GG. Low external NaCl concentration and low osmolarity enhance voltage-gated Ca currents but depress K currents in freshly isolated rat hippocampal neurons. Brain Res 851: 189–197, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci 21: 7491–7505, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tanaka T, Shinoda M, Feng B, Albers KM, Gebhart GF. Modulation of visceral hypersensitivity by glial cell line-derived neurotrophic factor family receptor α-3 in colorectal afferents. Am J Physiol Gastrointest Liver Physiol 300: G418–G424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Toulouse M, Coelho AM, Fioramonti J, Lecci A, Maggi C, Bueno L. Role of tachykinin NK2 receptors in normal and altered rectal sensitivity in rats. Br J Pharmacol 129: 193–199, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xian TL, Dyachenko V, Zuzarte M, Putzke C, Preisig-Muller R, Isenberg G, Daut J. The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovasc Res 69: 86–97, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol 99: 244–253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yamamoto Y, Hatakeyama T, Taniguchi K. Immunohistochemical colocalization of TREK-1, TREK-2 and TRAAK with TRP channels in the trigeminal ganglion cells. Neurosci Lett 454: 129–133, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Zhao H, Sprunger LK, Simasko SM. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am J Physiol Gastrointest Liver Physiol 298: G212–G221, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]