Abstract
Helicobacter pylori infection and elevated expression of tissue matrix metalloproteinase 1 (MMP-1) are both associated with gastric cancer. We investigated the regulation of MMP-1 expression during H. pylori infection. Real-time reverse transcription-PCR was used to examine mucosal MMP-1 mRNA levels in 55 patients with gastric cancers and 61 control patients. Increased MMP-1 mRNA levels in the gastric mucosa and epithelial cells were observed in H. pylori infections in which both the cag pathogenicity island (PAI) and outer inflammatory protein A (OipA) were expressed. The combined induction of c-fos, c-jun, and polyoma enhancing activator-3 (pea-3) by H. pylori caused maximal increase in MMP-1 expression. Activation of the MMP-1 promoter by H. pylori involved occupation of the activator protein 1 (AP-1) sites at −72 and −181 and, surprisingly, vacancy of the −88 PEA-3 site. Electrophoretic mobility shift, supershift, and chromatin immunoprecipitation assays showed increased binding of c-Fos and c-Jun to the −72 and −181 AP-1 sites during H. pylori infection. Importantly, during wild-type H. pylori infection, we detected increased PEA-3 binding to the −72AP-1 site and decreased PEA-3 binding to the −88 PEA-3 site. However, during infection with the cag PAI and oipA mutants, PEA-3 binding to the −88 site was detected. MMP-1 and pea-3 activities are increased in gastric cancers. Maximal activation of MMP-1 transcription requires the cag PAI and OipA, which regulate AP-1 and PEA-3 binding. Thus, cag PAI and OipA provide a possible link between bacterial virulence factors and important host factors related to disease pathogenesis.
Introduction
Approximately 20% of all Helicobacter pylori infections lead to peptic ulcer disease or gastric cancer. The risk of these clinically significant outcomes is thought to vary depending on interactions between the host, the environment, and the bacterial factors. Two H. pylori virulence factors proven to be associated with increased mucosal inflammation and increased risk of peptic ulcer disease and gastric cancer are the cag pathogenicity island (PAI) and the outer inflammatory protein A (OipA; refs. 1, 2). The cag PAI is a 40-kb genome segment that encodes for ∼30 genes (1). OipA is an outer membrane protein of which the expression is transcriptionally regulated by a slipped-strand mispairing of the CT dinucleotide repeats in the 5′ region of the gene (2, 3). The majority of cag PAI-positive bacterial isolates are also OipA positive (4).
Matrix metalloproteinases (MMP) are zinc-containing endopeptidases that degrade extracellular matrix components and are involved in morphogenesis and the remodeling of organs (5, 6). MMP-1 is an interstitial collagenase able to degrade type I collagen, the main extracellular matrix component of gastric mucosa (7). MMP-1 is present in gastric epithelial cells of human gastric mucosa (8) and its expression is elevated in patients with gastric cancers (9–12). A negative correlation between the expression of MMP-1 and survival and/or metastatic potential of gastric cancer has been described (9–12). Whereas several studies have confirmed the relationship between H. pylori infection and increased gastric mucosal MMP-1 (13–15), the relationship between gastric mucosal MMP-1 expression and the cag PAI and/or OipA virulence factors has not been examined. Enhanced MMP-1 expression has also been reported in H. pylori infection of AGS cells, a gastric epithelial cancer cell line (7, 16), as well as in human gastric wall fibroblasts (14).
The mechanism(s) by which H. pylori induces MMP-1 expression in gastric cells was examined in a recent study focused on the roles of mitogen-activated protein kinase (MAPK) on MMP-1 expression in AGS cells (15). The expression of MMP-1 was shown to require the co-operation of two oncogenic transcription factors, activator protein 1 (AP-1), and polyoma enhancing activator 3 (PEA-3; refs. 17–20). However, the extent of MMP-1 expression was stimulus dependent (21). AP-1 transcription factors comprise a ubiquitously expressed family of proteins including the Jun and Fos proto-oncoproteins (22). Previous studies have shown that H. pylori induces the expression of c-jun and c-fos (23–26) and causes the transactivation of AP-1 binding sites in several promoters, including interleukin (IL)-6 and IL-8 (27, 28). Therefore, we hypothesize that H. pylori infection also leads to the transactivation of AP-1 sites in the MMP-1 promoter. Whereas the function(s) of PEA-3 is still unclear, the overexpression of PEA-3 has been reported in various cancer cells including gastric cancer (29). The role of PEA-3 in H. pylori infection has not previously been investigated.
In the present study, we examined the transcriptional regulation of MMP-1 in response to H. pylori infection. Furthermore, the role of the H. pylori virulence factors, the cag PAI and OipA, on this regulation was examined. We also investigated possible associations between H. pylori infection and MMP-1 expression levels in human gastric mucosa.
Materials and Methods
Gastric epithelial cells
Three human gastric epithelial cancer cell lines were used. MKN45 cells were obtained from Riken Cell Bank (Tsukuba, Japan). AGS cells were obtained from American Type Culture Collection, (Manassas, VA) and SNU638 cells were a gift from Dr. Antonia R. Sepulveda (Department of Pathology, University of Pittsburgh, Pittsburgh, PA). Cells were routinely maintained as previously described (27). In addition, primary gastric epithelial cells were isolated enzymatically from non-cancer-containing biopsies of adult human stomachs as previously described (27).
H. pylori preparation
H. pylori TN2GF4 (a gift from Dr. Masafumi Nakao, Takeda Chemical Industries, Ltd., Osaka, Japan), its isogenic oipA mutant, and the whole cag PAI-deleted mutant were used (2, 30). We used a multiplicity of infection (MOI) of 100 to eliminate possible confounding effects of reduced adherence by the oipA mutants (28, 29). To avoid the influence of serum, epithelial cells were serum starved for 16 hours before and during treatment with H. pylori, without H. pylori (negative control), or with phorbol 12-myristate 13-acetate (PMA; 10 ng/mL; positive control).
In some experiments, heat-killed H. pylori were used at the same MOI (27) or the same concentrations of live bacteria were added to the upper well of a transwell plate (Falcon, Lincoln Park, NJ) whereas the lower well contained subconfluent epithelial cells. In some experiments, gastric cells were pretreated (30 minutes before treatment) with U0126 [a specific inhibitor of MAPK/extracellular signal–regulated kinase (ERK)1/2 (MEK1/2)], SB203580 (a specific inhibitor of p38), or SP600125 [a specific inhibitor of c-jun NH2-terminal kinase (JNK)]. All of these inhibitors were purchased from Calbiochem (San Diego, CA). In some experiments, gastric cells were transfected with 50 nmol/L of validated SMARTpool for ERK, JNK, or p38 small interfering RNA (siRNA; Dharmacon Research, Denver, CO), c-jun, c-fos, or pea-3 siRNA (Santa Cruz Biotechnology, Santa Cruz, CA), or a scrambled siRNA negative control (Dharmacon Research) using Lipofectamine 2000 reagent (Invitrogen, San Diego, CA).
cDNA array analyses
Total RNA was extracted from infected and uninfected MKN45 cells by using TriZol reagent (Invitrogen) and 5 μg of RNA were used to convert total RNA into 32P-labeled first-strand cDNA. Labeled cDNA samples were hybridized according to the recommendations of the manufacturer to the Human Cytokine Array (GA001), which consists of 375 different cloned cDNAs (R&D Systems, Minneapolis, MN).
Quantitation of mRNA by reverse transcription-PCR
Total RNA was extracted from treated and untreated gastric cells by using TriZol reagent (Invitrogen) and converted cDNA that was then used for real-time reverse transcription-PCR (RT-PCR). The oligonucleotide primers and probes for MMP-1, pea-3, and GAPDH mRNA were as previously described (31, 32). The PCR products were cloned into the plasmid pT7Blue (Novagen, Madison, WI) and used as a standard. Absolute quantitative real-time RT-PCR using TaqMan probe was done using ABI Prism 7300 Sequence-Detection System (Applied Biosystems). The expression levels were expressed as 1,000 × target mRNA / GAPDH mRNA. The abundances of c-fos mRNA and c-jun mRNA were measured by SYBR green I–based quantitative real-time RT-PCR as previously described (33) and the expression levels normalized to the levels of GAPDH mRNA were expressed as fold induction relative to the uninfected control.
Plasmids
The full-size human MMP-1 promoter reporter gene construct −4372hMMP1luci contains the firefly luciferase gene. Various 5′ deletion constructs and site-directed mutant plasmids with mutations in three sites [AP-1 at −72 bp (−72AP-1), PEA-3 at −88 bp (−88PEA-3), and AP-1 at −181 bp (−181AP-1)] have previously been described (21). The plasmid pCMV-RafS621A, which expresses a dominant-negative mutant form of the Raf protein, was purchased from Clontech (Palo Alto, CA). The pCMV-RhoN19, pCMV-RacN17, and pCMV-RasN17 expression plasmids, which express the dominant-negative mutant forms of RhoA, Rac1, and Ras proteins, respectively, were previously described (21).
Transfection
Each luciferase reporter vector was transiently transfected into logarithmically growing gastric cancer cells using Lipofectamine 2000 reagent (Invitrogen). The luciferase assays were done using the Dual-Luciferase reporter assay system according to the instructions of the manufacturer (Promega, Madison, WI). Five, 10, and 18 hours after stimulation with H. pylori or PMA, the cells were harvested and lysed using passive lysis buffer (Promega), and the lysates were assayed for luciferase activity. Normalized luciferase activity is presented as firefly luciferase activity/Renilla luciferase activity. We also present the luciferase activity as fold increase of luciferase activity in treated cells relative to uninfected, or mock-treated, controls.
Electrophoretic mobility shift assay
Nuclear extracts of treated and untreated gastric cancer cells were prepared using hypotonic/nonionic detergent lysis (34). After extraction, equal amounts of nuclear proteins were allowed to bind to duplex oligonucleotides corresponding to the −181AP-1, −72AP-1, and −88PEA-3 sites. Electrophoretic mobility shift assay (EMSA) was done using standard methods as previously described (34). The sequences of the oligonucleotides used for the MMP-1 promoter-specific gel shift were as follows: 5′-CTTGTTTGAAGTTAATCATGACATTGCAAC-3′ for −181AP-1 site, 5′-GATCATAAAGCATGAGTCAGACACCTCT-3′ for −72AP-1 site, and 5′-TAGCTAATCAAGAGGATGTTATAAAGCA-3′ for −88PEA-3 site. The sequences of the mutated oligonucleotides used for the MMP-1 promoter-specific gel shifts were as follows: 5′-CTTGTTTGAAGTTACTAATGACATTGCAAC-3′ for −181AP-1 site (TTAATCA to TTAGTAA), 5′-GATCATAAAGCATGATTTAGACACCTCT-3′ for −72AP-1 site (TGAGTCAG to TGATTTAG), and 5′-TAGCTAATCAAGATTATGTTATAAAGCA-3′ for −88PEA-3 (GAGGATG to GATTATG); only forward primers are presented. For semiquantitation of the binding, the image was digitized and we compared the amount of radioactive probe in the protein-DNA complexes after standardization with free probe from three different experiments using a Nucleo Vision System (Nucleo Tech Co., San Carlos, CA).
For competition assays, extracts were incubated with 100-fold excess of unlabeled competitors. For supershift assays, antibodies against specific transcriptional factors (i.e., anti-c-Fos, anti-FosB, anti-Fra-1, anti-Fra-2, anti-c-Jun, anti-JunB, anti-JunD, anti-PEA-3, anti-ERM, anti-ER81, anti-Ets-1, and anti-PU.1 antibodies; Santa Cruz Biotechnology) were added to the extracts 15 minutes before addition of the probe.
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation analyses were done using an assay kit following the instructions of the manufacturer (Upstate Biotechnology, Lake Placid, NY). The input and immunoprecipitated DNA were amplified across the MMP-1 promoter region using the primers 5′-CTTGTTTGAAGTTAATCGTGACAC-3′ and 5′-AGCCTCTTGCTGCTCCAATATC-3′. PCR products were then resolved on a 1.5% agarose gel. For quantitation, we also did SYBR green I–based quantitative PCR.
In vivo studies for quantitation of mRNA by RT-PCR
Biopsy specimens from Japanese patients with histologically proven primary early gastric cancer were obtained. H. pylori infection status was defined as positive if all three tests (serology, culture, and histology) gave positive results and negative if all three tests gave negative results. Two biopsy specimens from cancer tissues and two from adjacent apparently normal mucosa were used to examine the mucosal MMP-1 and pea-3 mRNA levels. The cag PAI and the oipA status was determined by cultured H. pylori as previously described (2, 4). In addition, antral biopsy samples were selected from our tissue bank consisting of specimens from Colombian patients with chronic gastritis and whose H. pylori status as well as the cag PAI and the oipA status had been characterized (28). Informed consent was obtained from all patients and samples were taken under protocols approved by the institutional ethics committees.
Extraction of total RNA and reverse transcription was done as previously described (35). Mucosal MMP-1 and pea-3 mRNA levels were evaluated as described above.
Statistical analysis
Each in vitro experiment was done at least thrice. Statistical significance was assessed by ANOVA and differences identified were pinpointed by Student-Newman-Keul's multiple-range test (in vitro experiments) or Mann-Whitney rank sum test (in vivo experiments).
Results
H. pylori induces MMP-1 mRNA expression in gastric epithelial cells
To identify H. pylori–induced genes, we did cDNA array analysis of cells infected with wild-type, cag PAI mutant, or oipA mutant H. pylori strains. We found that expression of 33/375 genes in MKN45 cells was up-regulated 4 hours postinfection with wild-type H. pylori; the fold increase cutoff used was 2 (see Supplementary Table S1). Expression of 19 of these 33 genes was both cag PAI and OipA dependent. This set of 19 genes includes 6 of the 10 examined MMPs (MMP-1, 3, 7, 9, 12, and 14). To confirm these results, we did real-time RT-PCR analysis of MMP-1 mRNA levels in the three gastric cell lines. Wild-type H. pylori induced MMP-1 mRNA levels in a time-dependent manner with maximal levels observed at 6 hours postinfection (Fig. 1A). Importantly, this induction was restricted to cag PAI-positive/oipA-“on” wild-type strains (Fig. 1B). We also examined the protein levels of MMP-1 and confirmed that only cag PAI-positive/oipA-on H. pylori induced MMP-1 activity (see Supplementary Fig. S1).
Figure 1.
MMP-1 mRNA levels in gastric epithelial cancer cell lines (A and B) and in primary non-cancer epithelial cells (C) detected by real-time RT-PCR. Four independent coculturings were done and each was measured in triplicate for (A and B) and one coculturing was done and measured in triplicate for (C). Data expressed as 1,000 × MMP-1 mRNA / GAPDH mRNA. Points and columns, mean; bars, SE. *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared with the wild-type strain (B and C). ND, not done.
The pattern of MMP-1 expression in primary gastric epithelial cells was similar to that observed using stable cells lines. However, MMP-1 expression levels differed among the patients from which these primary cells were generated (Fig. 1C). MMP-1 mRNA levels were lower in noncancer primary cells than in cancer cell lines. Such changes are likely a result of altered signaling in the cancer tissues.
H. pylori induces expression of MMP-1 mRNA via Ras/Raf/RhoA→MAPK→c-Fos/c-Jun/PEA-3 pathways
The expression of MMP-1 is regulated by the activities of AP-1 and PEA-3 (20, 36). c-Fos/c-Jun and PEA-3 are well-known transcription factors of AP-1 and PEA-3, respectively. We measured the mRNA levels of c-fos, c-jun, and pea-3 following H. pylori infection. Real-time RT-PCR analysis showed that either infection with wild-type H. pylori or stimulation with PMA resulted in significant up-regulation of c-fos, c-jun, and pea-3 mRNA expression in MKN45 cells (Fig. 2A). The cag PAI mutant and the oipA mutant strains induced the expression of three mRNAs to a lesser extent than wild-type H. pylori did (Fig. 2B).
Figure 2.

c-fos, c-jun, and pea-3 mRNA levels in MKN45 cells detected by real-time RT-PCR. Three or four independent coculturings were done and each was measured in triplicate. No inhibitors altered mRNA levels in untreated MKN45 cells. No inhibitors altered GAPDH mRNA levels in MKN45 cells regardless of the treatment used. Expression levels normalized to the GAPDH mRNA levels are expressed as fold induction relative to uninfected controls. Points and columns, mean; bars, SE. *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared with the wild-type strain. A, time course experiments. MKN45 cells were incubated for 0 to 6 hours with wild-type H. pylori, without H. pylori (negative control), or with PMA (10 ng/mL). B, effects of cag PAI and OipA on c-fos/c-jun/pea-3 mRNA levels. MKN45 cells were incubated with wild-type H. pylori (column 1) or with mutant strains (columns 2 and 3) for 1 (c-fos and c-jun) or 4 (pea-3) hours. C, effect of inhibition of MAPK pathways on H. pylori–induced c-fos/c-jun/pea-3 mRNA levels. Each MAPK inhibitor (SB203580, U0126, or SP600125; 10 μmol/L) was added to MKN45 cells 30 minutes before infection with wild-type H. pylori, and mRNA levels were measured after 1 (c-fos and c-jun) or 4 (pea-3) hours. MKN45 cells were also transfected with 50 nmol/L of validated SMARTpool siRNA for ERK, JNK, or p38, or a scrambled siRNA negative control (Dharmacon Research). Forty hours posttransfection, the medium was changed and mRNA levels were measured following incubation with wild-type H. pylori.
The inhibition of MAPK pathways by either chemical inhibitors (SB203580, ‘U0126, and SP600125) or siRNA knockdown decreased H. pylori–induced c-fos and c-jun mRNA expression in MKN45 cells (Fig. 2C). Importantly, the inhibition of ERK and JNK also decreased the H. pylori–induced expression of pea-3 mRNA whereas the inhibition of p38 did not. AGS and SNU638 cells also showed similar results (data not shown). Therefore, unless otherwise noted, all subsequent experiments were done using MKN45 cells.
siRNA-mediated inhibition of all three MAPK pathways significantly suppresses the H. pylori–mediated induction of MMP-1 mRNA expression (Fig. 3A). Inhibition of p38 had no effect on PMA-induced MMP-1 mRNA expression. Additionally, the inhibition of c-fos and/or c-jun by siRNA knockdown significantly suppressed the H. pylori–mediated induction of MMP-1 mRNA expression whereas the knockdown of pea-3 had no effect (Fig. 3A). However, the combined inhibition of pea-3, c-fos, and c-jun more dramatically suppressed MMP-1 mRNA expression than the combined inhibition of c-fos and c-jun, suggesting that PEA-3 co-operates with c-Fos/c-Jun to achieve maximal MMP-1 induction. In contrast, the inhibition of pea-3 had no effect on PMA-induced MMP-1 mRNA expression, suggesting that PEA-3 has a unique function in the H. pylori–mediated induction of MMP-1 expression.
Figure 3.
MMP-1 mRNA levels in MKN45 cells detected by real-time RT-PCR. Three or four independent coculturings were done and each was measured in triplicate. No inhibitors altered mRNA levels in untreated MKN45 cells. No inhibitors altered GAPDH mRNA levels in MKN45 cells regardless of the treatment used. Expression levels normalized to the GAPDH mRNA levels are expressed as 1,000 × mRNA mRNAs / GAPDH mRNA. Columns, mean; bars, SE. *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared with the wild-type strain. A, effect of inhibition of MAPKs and c-fos/c-jun/pea-3 on H. pylori–induced MMP-1 mRNA levels. MKN45 cells were transfected with 50 nmol/L of validated SMARTpool siRNA for ERK, JNK, or p38 (Dharmacon Research), c-jun, c-fos, or pea-3 siRNA (Santa Cruz Biotechnology), or a scrambled siRNA negative control (Dharmacon Research). Forty hours posttransfection, the medium was changed and mRNA levels were measured following incubation with wild-type H. pylori, without H. pylori (negative control), or with PMA (10 ng/mL) for 6 hours. B, effect of dominant-negative monomeric GTP-binding protein mutants on H. pylori–induced MMP-1 mRNA levels. MKN45 cells were transfected with 0.3 μg of one of the four plasmids (pCMV-RhoN19, pCMV-RacN17, pCMV-RasN17, or pCMV) or transfected with either 50 ng of pCMV or pCMV-RafS621A. Forty hours posttransfection, the medium was changed and MMP-1 mRNA levels were measured following incubation with wild-type H. pylori, without H. pylori (negative control), or with PMA (10 ng/mL) for 6 hours.
The MAPK pathway upstream signaling factors include monomeric GTP-binding proteins, such as Ras, Raf, Rac1, and RhoA. Transfection of the dominant-negative Rho (pCMV-RhoN19), Raf (pCMV-RafS621A), and Ras (pCMV-RasN17) mutants suppressed the induction of MMP-1 mRNA expression mediated by H. pylori and PMA (Fig. 3B). In contrast, the transfection of the dominant-negative Rac1 mutant (pCMV-RacN17) had no effect on the induction of MMP-1 mRNA expression.
H. pylori induces MMP-1 expression via promoter activation at AP-1 sites and inhibition at PEA-3 site
The results described above suggest that AP-1 and PEA-3 binding sites are involved in the H. pylori–mediated induction of MMP-1 expression. To better define the role(s) of these binding sites, MKN45 cells were transiently transfected with the reporter plasmid −4372hMMP1luci, which contains the full-length human MMP-1 promoter driving the expression of the luciferase gene. Luciferase activity reached maximal levels 10 hours postinfection (8.9 ± 0.4-fold increase over untreated control) and plateaued by 18 hours. In subsequent experiments, luciferase activity was assessed at 10 hours postinfection. The fold increase in MMP-1 promoter activity was similar in cells infected with the cag PAI or oipA mutant strains and in untreated controls (data not shown).
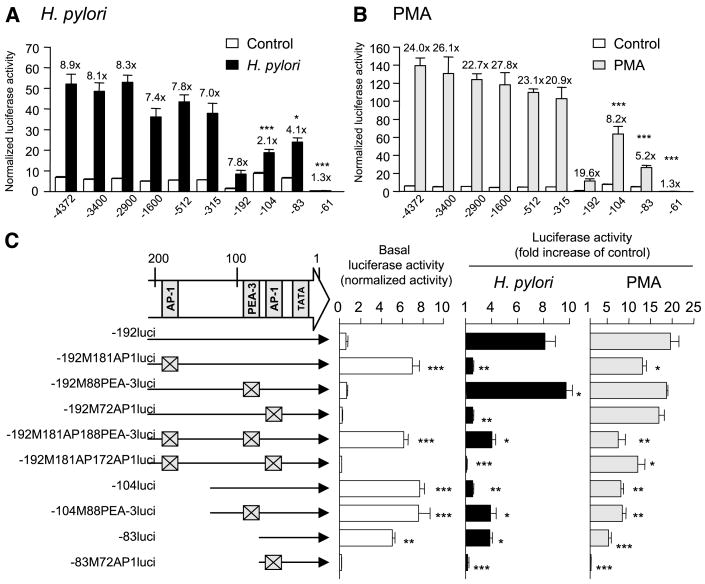
MKN45 cells were then transfected with plasmids containing serial 5′ to 3′ deletions of the MMP-1 promoter. Basal activity was decreased for the plasmid −192hMMP1luci and was restored and perhaps even slightly increased by deletion to −104 bp (Fig. 4A). This result suggests that the sequences containing the −181AP-1 site may be involved in suppressing the basal activity of the MMP-1 promoter. Basal activity was also markedly decreased for the plasmid −61hMMP1luci, suggesting that the sequences containing the −72AP-1 site have a role in increasing basal activity of the MMP-1 promoter. Wild-type H. pylori infection of cells containing these promoter deletion plasmids (down to −192 bp) resulted in a 7- to 9-fold induction of MMP-1 promoter activity (Fig. 4A), suggesting that the sequence between −192 and −61 bp is critical for the H. pylori–mediated induction of the MMP-1 promoter. Deletion to −104 bp significantly reduced the promoter induction, suggesting that the −181AP-1 site is involved in the H. pylori–mediated induction of the MMP-1 promoter. Deletion to −83 partially, but significantly, restores the induction whereas deletion to −61 bp abolishes the induction. These data suggest that the −72AP-1 site is involved in the H. pylori–mediated induction of the MMP-1 promoter and that the binding of transcription factor(s) to the −88PEA-3 site may interfere with the binding of the AP-1 transcription factor to the −72AP-1 site. Consistent with previous studies (21, 37), the sequence between −192 and −61 bp is also critical for the PMA-mediated induction of the MMP-1 promoter (Fig. 4B).
Figure 4.
MMP-1 promoter activation following H. pylori infection or PMA treatment. Four independent transfections, each done in triplicate, were done. Columns, mean; bars, SE. A and B, effect of 5′ to 3′ MMP-1 promoter deletions on activity. MKN45 cells were transiently transfected with 2 μg of the plasmids containing serial 5′ to 3′ deletions of the MMP-1 promoter and 10 ng of Renilla plasmid as an internal control. The cells were then either infected with H. pylori or stimulated with PMA for 10 hours. Untreated cells served as negative controls. For each sample, luciferase activity was normalized to the activity of the Renilla luciferase vector. Bars, fold increase in H. pylori–infected or PMA-treated cells relative to untreated controls. *, P < 0.05; ***, P < 0.001, compared with −4372hMMP1luci under each condition. The presence of nonsupercoiled DNA was not the cause of the reduction in basal expression for −192hMMP1luci and −61hMMP1luci because the five independent plasmids for each promoter construct behaved similarly. C, effect of MMP-1 promoter mutations on activity. MKN45 cells were transiently transfected with 2 μg of plasmids containing mutated MMP-1 promoters and 10 ng of Renilla plasmid as an internal control. The cells were then either infected with H. pylori or stimulated with PMA for 10 hours. Untreated cells served as controls. Basal luciferase activity is present as activity normalized to Renilla luciferase vector. Luciferase activity induced by H. pylori infection or PMA treatment is presented as normalized luciferase activity expressed as fold increase in H. pylori–infected or PMA-treated cells relative to uninfected controls. *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared with −192hMMP1luci.
To further examine the relative contributions of these three sites, the activity of a series of mutated MMP-1 promoters was examined (Fig. 4C). Mutation of the −181AP-1 site increased the basal promoter activity and mutation of the −72AP-1 site abrogated the basal promoter activity. These results confirm that the −181AP-1 site is involved in suppression of the basal promoter activity and the −72AP-1 site is involved in increasing the basal promoter activity.
Mutation of the −181AP-1 or the −72AP-1 site abrogated the promoter activity induced by wild-type H. pylori infection (Fig. 4C). This finding is consistent with both AP-1 sites playing important roles in the H. pylori–mediated induction of the MMP-1 promoter. Mutation of the −88PEA-3 site resulted in an increase in the H. pylori–mediated induction of the −192hMMP1luci plasmid, confirming that the PEA-3 site is involved in the suppression of the MMP-1 promoter. In addition, mutation of the −88PEA-3 site resulted in an increased H. pylori–mediated induction of the −104hMMP1luci and −192M181AP1luci plasmids. These results are consistent with the −88PEA-3 site having a suppressive effect on the −72AP1 site, which is capable of mediating the induction of the MMP-1 promoter.
The cag PAI and the oipA mutant H. pylori strains induced luciferase activity to very low levels regardless of the reporter plasmids used (maximum, 1.1 ± 0.1-fold). It is clear that the mechanism of the transcriptional induction of the MMP-1 promoter by H. pyloriis different from that used by PMA (Fig. 4C). For example, single mutations in both AP-1 sites only slightly suppress the MMP-1 promoter activity and mutation of the −88PEA-3 site has no effect.
Activation of transcription factors binding sites in H. pylori infection
The experiments described above show that c-Jun, c-Fos, and PEA-3 are the main transcription factors competing for binding to the AP-1 and PEA-3 sites of the MMP-1 promoter. The data also suggest that the −88PEA-3 site plays a role in suppressing MMP-1 promoter activity, although H. pylori induces pea-3 mRNA expression. One possible explanation for this discrepancy is that the PEA-3 transcription factor does not bind to the −88PEA-3 site and/or binds to a site other than the PEA-3 site. To examine the DNA binding proteins bound to the MMP-1 promoter, we did EMSA and supershift assays using the −71AP-1, −181AP-1, and −88PEA-3 sites. At each site, induction of binding was evident 1 hour after H. pylori infection; it peaked at 3 hours postinfection; and it decreased at 6 hours postinfection (data not shown).
One protein complex at −181AP-1 (C1) was weakly detected in uninfected MKN45 cells. Its presence was increased following wild-type H. pylori infection (Fig. 5A, lane 2). Infection with the cag PAI or the oipA mutant strains did not induce binding to the AP-1 site (lanes 3 and 4). Supershift assays showed that c-Jun and c-Fos were components of the −181AP-1 complexes (lanes 8-10). The other factors examined were not components of the −181AP-1 complex (data not shown).
Figure 5.

EMSA of MMP-1 binding complexes at −72AP-1 (A), −181AP-1 (B), and −88PEA-3 (C) sites in H. pylori–infected cells. Lanes 1 to 4, nuclear extracts were prepared from control and MKN45 cells infected with H. pylori for 3 hours and used for EMSA. Lanes 5 to 7, competition analysis. Nuclear extracts from infected cells were used to bind to the probe in the absence (−) or presence of 100-fold excess of unlabeled competitors [wild-type (Wt) or mutated (Mut)]. Data show that binding to each site is sequence-specific because competition was detected using unlabeled wild-type oligonucleotide but not using unlabeled mutated oligonucleotide (lanes 6 and 7). Lanes 8 to 10 (A and B) and lane 8 (C), supershift interference assay. EMSA was done using nuclear extracts of MKN45 cells infected with H. pylori for 2 hours and commercial antibodies against specific transcriptional factors. Lanes 11 and 12 (A and B) and lanes 9 and 10 (C), EMSA was done using nuclear extracts prepared from control and MKN45 cells treated with PMA for 3 hours. D, chromatin immunoprecipitation (ChIP) analysis. MKN45 cells were cocultured with wild-type H. pylori and the presence of MMP-1 promoter precipitated with anti-c-Fos, anti-c-Jun, anti-PEA-3, or without antibodies (control) was examined. One percent of the total chromatin was assayed to verify equal loading (Input). PCR products were resolved on a 1.5% agarose gel (inverse images are shown). To quantitate, we did SYBR green I–based quantitative PCR.
At least three −72AP-1 binding complexes (C1, C2, and C3) were detected in uninfected MKN45 cells. Their presence was increased following infection with wild-type H. pylori (Fig. 5B, lane 2). Infection with the cag PAI or the oipA mutant strains reduced binding to the AP-1 site. Supershift assays showed that C1 contained c-Jun, C2 contained c-Fos, and C3 contained PEA-3 (lanes 8-10). The other factors examined were not components of these −72AP-1 complexes (data not shown).
Three −88PEA-3 binding complexes (C1, C2, and C3) were detected in uninfected MKN45 cells (Fig. 5C, lane 1). The presence of the C1 and C3 complexes was significantly reduced following infection with wild-type H. pylori (lane 2). The complexes were not reduced following infection with either the cag PAI or the oipA mutant strains, suggesting that cag PAI and OipA play unique roles in the regulation of PEA-3 binding to the MMP-1 promoter. Supershift assays showed that PEA-3 was the sole component of the C3 complex and one of the components of the C2 complex. No Fos, Jun, or other Ets families were found to be component of any of these complexes (data not shown). We were unable to identify any components of the C1 complex. In contrast to findings following H. pylori infection, complex formation was not altered by PMA treatment. Overall, it is interesting that in wild-type H. pylori infections, the binding of PEA-3 to the −72AP-1 site is induced whereas the binding of PEA-3 to the −88PEA-3 site is repressed. Such selective binding of PEA-3 during H. pylori infection should result in maximal MMP-1 induction.
To further confirm these in vitro findings, the in vivo recruitment of c-Fos, c-Jun, and PEA-3 to the MMP-1 promoter following H. pylori infection was examined by chromatin immunoprecipitation assays. As expected, an increase in the binding of c-Fos and c-Jun to the promoter region −188/+18 was detected (Fig. 5D). Importantly, the binding of PEA-3 to this region was initially decreased but then increased.
H. pylori induces in vivo MMP-1 mRNA levels in the gastric mucosa
To further study the relationship between the expression of MMP-1 and PEA-3, we examined MMP-1 and pea-3 mRNA levels in early gastric cancers (all distal type) and adjacent normal-appearing mucosa (noncancer regions) from 44 H. pylori–positive patients and 11 patients without evidence of active H. pylori infection. All H. pylori cultured were cag PAI positive and oipA-on. Gastric mucosal MMP-1 mRNA levels were significantly higher in gastric cancer tissues than the in noncancer tissues (Fig. 6A; P < 0.001), a result consistent with the in vitro results described above (Fig. 1). MMP-1 mRNA levels were significantly higher in tissues from H. pylori–infected patients compared with noninfected patients regardless of biopsy location (Fig. 6A). MMP-1 mRNA levels in cancer tissues were higher in intestinal than in diffuse type gastric cancer (P = 0.01), a finding which is in agreement with previous immunochemistry or in situ hybridization studies on the localization of MMP-1 mRNA (9, 10, 38).
Figure 6.
MMP-1 mRNA and pea-3 mRNA levels in gastric cancer tissues and adjuvant normal-appearing mucosa from patients with gastric cancer (A) and in the antral mucosa from patients with gastritis (B) as determined by real-time RT-PCR. Data expressed as 1,000 × target mRNA / GAPDH mRNA. Points and columns, mean; bars, SE. **, P < 0.01; ***, P < 0.001, compared with specimens infected with the cag PAI-positive/OipA-positive isolates (B).
Interestingly, only 5 of the 55 samples had measurable levels of pea-3 mRNA in the noncancer tissues whereas pea-3 mRNA levels were significantly higher in the cancer tissues, especially in tissues from patients with an H. pylori infection (Fig. 6A). These data clearly suggest that PEA-3 plays an important role in gastric cancer and its expression is H. pylori dependent. In addition, pea-3 mRNA levels correlated with MMP-1 mRNA levels (r = 0.78; Fig. 6A).
To investigate the in vivo effect of the cag PAI and OipA, samples from 61 patients with gastritis (including 41 with an H. pylori infection) were also studied (Fig. 6B). Importantly, the H. pylori–related up-regulation of MMP-1 mRNA was restricted to infections with strains that were both cag PAI and oipA-on (Fig. 6B). These data confirm the results of our in vitro studies described above (Fig. 1). None of the gastric biopsy samples had measurable levels of pea-3 mRNA.
Discussion
Our study has shown that H. pylori infection mediates an increased expression of MMP-1 in gastric mucosa. Additionally, we show that this increase in MMP-1 expression is associated with two H. pylori virulence factors, the cag PAI and OipA. The presence of only one of these factors is not enough to induce an increase in MMP-1 expression. This effect is different from that observed for the H. pylori–mediated increase in proinflammatory cytokines (e.g., IL-6 and IL-8) in which only one of these virulence factors is needed to elicit the induction of cytokines in mucosa (4, 27, 28).
We also showed that H. pylori–induced MMP-1 expression is associated with the AP-1 and PEA-3 transcription factors. Both the −181 and −72 AP-1 sites play important roles in the H. pylori–associated induction of the MMP-1 promoter, although these two sites have different effects. Specifically, mutation of the −72AP-1 site dramatically suppresses basal promoter activity and mutation of the −181AP-1 site increases basal promoter activity. Although co-operation between PEA-3 and AP-1 transcription factors is known to be required for MMP-1 promoter activity (17–19), the role(s) of PEA-3 on H. pylori–mediated MMP-1 promoter activity is unique. Luciferase reporter assays and EMSA supershift assays clearly show that wild-type H. pylori infection causes PEA-3 to bind to the −88PEA-3 site with reduced affinity and to the −72AP-1 site with increased affinity. This selective binding of PEA-3 in response to H. pylori infection is required for maximal induction of MMP-1 expression. Interestingly, the −181AP-1 site was not involved in PEA-3 binding, suggesting possible co-operation between adjacent binding sites in the promoter. This prediction was confirmed using chromatin immunoprecipitation assays that showed the binding of PEA-3 to the MMP-1 promoter initially decreased and then subsequently increased. We speculate that this initial reduction in binding could represent dissociation of PEA-3 from the −88PEA-3 site and the subsequent increase in PEA-3 binding represents the association of H. pylori–induced, newly synthesized PEA-3 with the −72AP-1 site. Our data showing that H. pylori induces pea-3 mRNA expression in the gastric cancer cells supports this prediction. Alternatively, H. pylori may induce the translocation of PEA-3 from the −88PEA-3 site to the adjacent −72AP-1 site.
Importantly, the reduction in PEA-3 binding to the −88PEA-3 site was not observed following infection with cag PAI and oipA mutant strains. This finding suggests that the cag PAI and OipA are involved in inhibiting PEA-3 binding. We also found that the H. pylori–mediated induction of pea-3 mRNA was dependent on both the cag PAI and OipA virulence factors. Therefore, it is likely that the cag PAI and OipA play major roles in pea-3 expression and both virulent factors influence the balance between inhibiting PEA-3 binding and inducing AP-1 binding.
We also investigated the upstream signaling regulating MMP-1 promoter activity. We showed that H. pylori induces c-fos, c-jun, and pea-3 mRNA expression and that these three factors cooperate to induce maximal MMP-1 expression. This finding is consistent with these three factors being the main AP-1 and PEA-3 transcription factors at the MMP-1 promoter. We confirmed that p38, ERK, and JNK mediated H. pylori–induced c-jun and c-fos expression (Fig. 2C). Inhibition of these three MAPK pathways also suppressed H. pylori–induced MMP-1 mRNA expression (Fig. 3A). However, inhibition of ERK and JNK, but not p38, affected PMA-induced MMP-1 mRNA expression. Recent studies have reported that SB203580 enhances MMP-1 secretion in AGS cells stimulated by epidermal growth factor, tumor necrosis factor, or IL-1β (39). These findings show that the mechanism involved in MMP-1 induction is stimulus dependent.
We also examined the role of the monomeric GTP-binding proteins, Ras, Raf, Rac1, and RhoA, which function upstream in the MAPK pathways. Ras was of particular interest because it can stimulate multiple signaling pathways, including the sequential activation of Raf→MEK1/2→ERK1/2 (40–42). We found that H. pylori–induced MMP-1 promoter activity was primarily through the Ras/Raf- and Rho-dependent pathways. The findings are consistent with recent studies showing that stimulation of MMP-7 by H. pylori in gastric epithelial cells is dependent on RhoA-mediated activation of AP-1 (43). Interestingly, expression of a dominant-negative Rac1 mutant did not effect on H. pylori–induced MMP-1 expression (Fig. 3B). This finding contradicts previous reports showing that H. pylori enhances the activation of Rac1 in AGS cells, a gastric epithelial cell line (44, 45). Other recent data have shown that a dominant-negative Rac1 mutant decreases the H. pylori–induced activation of the IL-6 promoter in MKN28 cells (32). Although H. pylori might enhance the activation of Rac1, the downstream signaling mediated by this Rac1 is likely to be promoter dependent.
Finally, we confirmed our in vitro results by examining the expression MMP-1 and pea-3 in vivo (i.e., in gastric cancer tissues). Gastric mucosal MMP-1 has been shown to be elevated in gastric cancer, and this elevated expression is related to the metastatic potential of the cancer (9–12). We also found that mucosal MMP-1 mRNA levels were significantly higher in gastric cancers with active H. pylori infections than in those cancers without an active infection. In addition, the H. pylori–associated up-regulation of MMP-1 was restricted to those infections that were both cag PAI positive and oipA-on, suggesting that the combined functions of cag PAI and OipA may play an important role(s) in the course of gastric cancer. Additionally, mucosal MMP-1 mRNA levels correlated with mucosal pea-3 mRNA levels and the up-regulation of pea-3 expression was largely restricted to H. pylori–positive cancer tissues. Pea-3 expression in gastric cancer is not well characterized and only one previous study has reported that pea-3 mRNA is up-regulated in gastric cancer tissues (29). In that study, they did not find the correlation between pea-3 expression and MMP-1 expression. This could be a result of the experimental methods they used (nonquantitative RT-PCR). In addition, they did not take account of H. pylori infection. Overall, MMP-1 and pea-3 expression is elevated in gastric cancers via H. pylori infection. Our study provides a possible link between bacterial virulence factors and important host factors involved in the pathogenesis of gastric disease.
Supplementary Material
Acknowledgments
Grant support: NIH grant DK62813 (Y. Yamaoka), Office of Research and Development Medical Research Service Department of Veterans Affairs (D.Y. Graham), and NIH grant DK56338 which funds the Texas Gulf Coast Digestive Diseases Center (D.Y. Graham).
We thank Dr. Antonia R. Sepulveda for providing SNU series cells and Dr. Masafumi Nakao for providing H. pylori TN2GF4.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Censini S, Lange C, Xiang Z, et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996;93:14648–53. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamaoka Y, Kwon DH, Graham DY. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci U S A. 2000;97:7533–8. doi: 10.1073/pnas.130079797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kudo T, Nurgalieva ZZ, Conner ME, et al. Correlation between Helicobacter pylori OipA protein expression and oipA gene switch status. J Clin Microbiol. 2004;42:2279–81. doi: 10.1128/JCM.42.5.2279-2281.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaoka Y, Kikuchi S, el-Zimaity HM, Gutierrez O, Osato MS, Graham DY. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology. 2002;123:414–24. doi: 10.1053/gast.2002.34781. [DOI] [PubMed] [Google Scholar]
- 5.Sato H, Takino T, Okada Y, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–5. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 6.Vincenti MP. The matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) genes. Transcriptional and posttranscriptional regulation, signal transduction and cell-type-specific expression. Methods Mol Biol. 2001;151:121–48. doi: 10.1385/1-59259-046-2:121. [DOI] [PubMed] [Google Scholar]
- 7.Gooz M, Gooz P, Smolka AJ. Epithelial and bacterial metalloproteinases and their inhibitors in H. pylori infection of human gastric cells. Am J Physiol Gastrointest Liver Physiol. 2001;281:G823–32. doi: 10.1152/ajpgi.2001.281.3.G823. [DOI] [PubMed] [Google Scholar]
- 8.Tatsuguchi A, Fukuda Y, Ishizaki M, Yamanaka N. Localization of matrix metalloproteinases and tissue inhibitor of metalloproteinases-2 in normal human and rabbit stomachs. Digestion. 1999;60:246–54. doi: 10.1159/000007665. [DOI] [PubMed] [Google Scholar]
- 9.Migita T, Sato E, Saito K, et al. Differing expression of MMPs-1 and -9 and urokinase receptor between diffuse- and intestinal-type gastric carcinoma. Int J Cancer. 1999;84:74–9. doi: 10.1002/(sici)1097-0215(19990219)84:1<74::aid-ijc14>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Murray GI, Duncan ME, Arbuckle E, Melvin WT, Fothergill JE. Matrix metalloproteinases and their inhibitors in gastric cancer. Gut. 1998;43:791–7. doi: 10.1136/gut.43.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nomura H, Fujimoto N, Seiki M, Mai M, Okada Y. Enhanced production of matrix metalloproteinases and activation of matrix metalloproteinase 2 (gelatinase A) in human gastric carcinomas. Int J Cancer. 1996;69:9–16. doi: 10.1002/(SICI)1097-0215(19960220)69:1<9::AID-IJC3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Sakurai Y, Otani Y, Kameyama K, et al. Expression of interstitial collagenase (matrix metalloproteinase-1) in gastric cancers. Jpn J Cancer Res. 1997;88:401–6. doi: 10.1111/j.1349-7006.1997.tb00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menges M, Chan CC, Zeitz M, Stallmach A. Higher concentration of matrix-metalloproteinase 1 (interstitial collagenase) in H. pylori- compared to NSAID-induced gastric ulcers. Z Gastroenterol. 2000;38:887–91. doi: 10.1055/s-2000-10300. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama T, Otani Y, Kurihara N, et al. Matrix metalloproteinase expression in cultured human gastric wall fibroblasts-interactions with Helicobacter pylori isolated from patients with ulcers. Aliment Pharmacol Ther. 2000;14(Suppl 1):193–8. doi: 10.1046/j.1365-2036.2000.014s1193.x. [DOI] [PubMed] [Google Scholar]
- 15.Krueger S, Hundertmark T, Kalinski T, et al. H. pylori encoding the pathogenicity island activates matrix-metalloproteinase 1 in gastric epithelial cells via JNK and ERK. J Biol Chem. 2006;281:2868–75. doi: 10.1074/jbc.M511053200. Epub 2005 Dec 1. [DOI] [PubMed] [Google Scholar]
- 16.Gooz M, Shaker M, Gooz P, Smolka AJ. Interleukin 1β induces gastric epithelial cell matrix metalloproteinase secretion and activation during Helicobacter pylori infection. Gut. 2003;52:1250–6. doi: 10.1136/gut.52.9.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutman A, Wasylyk B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990;9:2241–6. doi: 10.1002/j.1460-2075.1990.tb07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rutter JL, Benbow U, Coon CI, Brinckerhoff CE. Cell-type specific regulation of human interstitial collagenase-1 gene expression by interleukin-1β in human fibroblasts and BC-8701 breast cancer cells. J Cell Biochem. 1997;66:322–6. [PubMed] [Google Scholar]
- 19.Chapman SC, Ayala JE, Streeper RS, et al. Multiple promoter elements are required for the stimulatory effect of insulin on human collagenase-1 gene transcription. Selective effects on activator protein-1 expression may explain the quantitative difference in insulin and phorbol ester action. J Biol Chem. 1999;274:18625–34. doi: 10.1074/jbc.274.26.18625. [DOI] [PubMed] [Google Scholar]
- 20.Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–8. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Wenger L, Brinckerhoff CE, Misra RR, Cheung HS. Basic calcium phosphate crystals induce matrix metalloproteinase-1 through the Ras/mitogen-activated protein kinase/c-Fos/AP-1/metalloproteinase 1 pathway. Involvement of transcription factor binding sites AP-1 and PEA-3. J Biol Chem. 2002;277:1544–52. doi: 10.1074/jbc.M100567200. [DOI] [PubMed] [Google Scholar]
- 22.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Bio-chim Biophys Acta. 1991;1072:129–57. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 23.Meyer-ter-Vehn T, Covacci A, Kist M, Pahl HL. Helicobacter pylori activates mitogen-activated protein kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J Biol Chem. 2000;275:16064–72. doi: 10.1074/jbc.M000959200. [DOI] [PubMed] [Google Scholar]
- 24.Mitsuno Y, Maeda S, Yoshida H, et al. Helicobacter pylori activates the proto-oncogene c-fos through SRE transactivation. Biochem Biophys Res Commun. 2002;291:868–74. doi: 10.1006/bbrc.2002.6530. [DOI] [PubMed] [Google Scholar]
- 25.Naumann M, Wessler S, Bartsch C, et al. Activation of activator protein 1 and stress response kinases in epithelial cells colonized by Helicobacter pylori encoding the cag pathogenicity island. J Biol Chem. 1999;274:31655–62. doi: 10.1074/jbc.274.44.31655. [DOI] [PubMed] [Google Scholar]
- 26.Chang YJ, Wu MS, Lin JT, Chen CC. Helicobacter pylori-Induced invasion and angiogenesis of gastric cells is mediated by cyclooxygenase-2 induction through TLR2/TLR9 and promoter regulation. J Immunol. 2005;175:8242–52. doi: 10.4049/jimmunol.175.12.8242. [DOI] [PubMed] [Google Scholar]
- 27.Lu H, Wu JY, Kudo T, Ohno T, Graham DY, Yamaoka Y. Regulation of Interleukin-6 Promoter activation in gastric epithelial cells infected with Helicobacter pylori. Mol Biol Cell. 2005;16:4954–66. doi: 10.1091/mbc.E05-05-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaoka Y, Kudo T, Lu H, Casola A, Brasier AR, Graham DY. Role of interferon-stimulated responsive element-like element in interleukin-8 promoter in Helicobacter pylori infection. Gastroenterology. 2004;126:1030–43. doi: 10.1053/j.gastro.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto H, Horiuchi S, Adachi Y, et al. Expression of ets-related transcriptional factor E1AF is associated with tumor progression and overexpression of matrilysin in human gastric cancer. Carcinogenesis. 2004;25:325–32. doi: 10.1093/carcin/bgh011. [DOI] [PubMed] [Google Scholar]
- 30.Kudo T, Lu H, Wu JY, Graham DY, Casola A, Yamaoka Y. Regulation of RANTES promoter activation in gastric epithelial cells infected with Helicobacter pylori. Infect Immun. 2005;73:7602–12. doi: 10.1128/IAI.73.11.7602-7612.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boedefeld WM, II, Soong R, Weiss H, et al. E1AF is overexpressed early in human colorectal neoplasia and associated with cyclooxygenase-2 and matrix metalloproteinase-7. Mol Carcinog. 2005;43:13–7. doi: 10.1002/mc.20093. [DOI] [PubMed] [Google Scholar]
- 32.Higashikata T, Yamagishi M, Sasaki H, et al. Application of real-time RT-PCR to quantifying gene expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human abdominal aortic aneurysm. Atherosclerosis. 2004;177:353–60. doi: 10.1016/j.atherosclerosis.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Gan L, Doroudi R, Hagg U, Johansson A, Selin-Sjogren L, Jern S. Differential immediate-early gene responses to shear stress and intraluminal pressure in intact human conduit vessels. FEBS Lett. 2000;477:89–94. doi: 10.1016/s0014-5793(00)01788-9. [DOI] [PubMed] [Google Scholar]
- 34.Brasier AR, Jamaluddin M, Casola A, Duan W, Shen Q, Garofalo RP. A promoter recruitment mechanism for tumor necrosis factor-α-induced interleukin-8 transcription in type II pulmonary epithelial cells. Dependence on nuclear abundance of Rel A, NF-κB1, and c-Rel transcription factors. J Biol Chem. 1998;273:3551–61. doi: 10.1074/jbc.273.6.3551. [DOI] [PubMed] [Google Scholar]
- 35.Yamaoka Y, Kodama T, Kita M, Imanishi J, Kashima K, Graham DY. Relation between cytokines and Helicobacter pylori in gastric cancer. Helicobacter. 2001;6:116–24. doi: 10.1046/j.1523-5378.2001.00017.x. [DOI] [PubMed] [Google Scholar]
- 36.Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 37.White LA, Brinckerhoff CE. Two activator protein-1 elements in the matrix metalloproteinase-1 promoter have different effects on transcription and bind Jun D, c-Fos, and Fra-2. Matrix Biol. 1995;14:715–25. doi: 10.1016/s0945-053x(05)80014-9. [DOI] [PubMed] [Google Scholar]
- 38.Otani Y, Okazaki I, Arai M, et al. Gene expression of interstitial collagenase (matrix metalloproteinase 1) in gastrointestinal tract cancers. J Gastroenterol. 1994;29:391–7. doi: 10.1007/BF02361233. [DOI] [PubMed] [Google Scholar]
- 39.Pillinger MH, Marjanovic N, Kim SY, et al. Matrix metalloproteinase secretion by gastric epithelial cells is regulated by E prostaglandins and MAPKs. J Biol Chem. 2005;280:9973–9. doi: 10.1074/jbc.M413522200. [DOI] [PubMed] [Google Scholar]
- 40.Brunner D, Ducker K, Oellers N, Hafen E, Scholz H, Klambt C. The ETS domain protein pointed-P2 is a target of MAP kinase in the sevenless signal transduction pathway. Nature. 1994;370:386–9. doi: 10.1038/370386a0. [DOI] [PubMed] [Google Scholar]
- 41.Gille H, Sharrocks AD, Shaw PE. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992;358:414–7. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- 42.O'Neill EM, Rebay I, Tjian R, Rubin GM. The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell. 1994;78:137–47. doi: 10.1016/0092-8674(94)90580-0. [DOI] [PubMed] [Google Scholar]
- 43.Wroblewski LE, Noble PJ, Pagliocca A, et al. Stimulation of MMP-7 (matrilysin) by Helicobacter pylori in human gastric epithelial cells: role in epithelial cell migration. J Cell Sci. 2003;116:3017–26. doi: 10.1242/jcs.00518. [DOI] [PubMed] [Google Scholar]
- 44.Churin Y, Kardalinou E, Meyer TF, Naumann M. Pathogenicity island-dependent activation of Rho GTPases Rac1 and Cdc42 in Helicobacter pylori infection. Mol Microbiol. 2001;40:815–23. doi: 10.1046/j.1365-2958.2001.02443.x. [DOI] [PubMed] [Google Scholar]
- 45.Palovuori R, Perttu A, Yan Y, Karttunen R, Eskelinen S, Karttunen TJ. Helicobacter pylori induces formation of stress fibers and membrane ruffles in AGS cells by rac activation. Biochem Biophys Res Commun. 2000;269:247–53. doi: 10.1006/bbrc.2000.2276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.