Abstract
Intravenous nicotine self-administration is the most direct measure of nicotine reinforcement in laboratory animals, but this procedure has proven difficult to establish in mice. We found that stable responding for nicotine in C57BL6/J mice was facilitated by prior instrumental training for food reward, initial exposure of mice to a lower unit dose of nicotine (0.03 mg/kg/infusion) before access to higher doses, a slower rate of drug delivery (3-sec versus 1-sec infusion), consistency in schedule of daily testing, and low extraneous noise during testing. Under these conditions, we found that mice lever-pressing for nicotine (0.03–0.4 mg/kg/infusion; 60-min test sessions) under a fixed-ratio 5 time-out 20-sec (FR5TO20) reinforcement schedule consumed the drug according to an inverted ‘U’-shaped dose-response curve. Mice switched their responding onto a previously non-reinforced lever to continue earning nicotine infusions when the active/inactive lever assignment was reversed. The nicotinic acetylcholine receptor (nAChR) antagonist mecamylamine decreased responding for nicotine, but not food rewards, verifying that nAChRs regulate nicotine self-administration in mice. The cue-light paired with nicotine delivery did not support responding when delivered independently of nicotine infusions, further verifying that mice responded selectivity for the drug. Nicotine-seeking responses extinguished when nicotine infusions and the cue-light were withheld, and exposure to the cue-light reinstated responding. Finally, mice without prior instrumental food training acquired stable responding for nicotine under the FR5TO20 schedule, but required a greater number of sessions. These data demonstrate that nicotine is an effective reinforcer in mice and establish conditions under which the drug is reliably self-administered by mice.
Keywords: Nicotine, nicotinic acetylcholine receptor, addiction, animal model, mouse
Tobacco addiction exerts a tremendous negative health and economic impact on the individual and society. Approximately 5.4 million smokers die annually from smoking-related illnesses, including chronic obstructive pulmonary disorder (COPD), lung cancer and cardiovascular disease (Coe et al., 2005; Doll et al., 2004; Ezzati and Lopez, 2003; Mathers and Loncar, 2006; WHO, 2008). Smokers who quit before the onset of tobacco-related illness can largely avoid the increased mortality risk (Doll et al., 1994; Peto et al., 2000). Nevertheless, ~80% of current smokers attempting to quit relapse within the first month of abstinence (Benowitz, 2009), highlighting the pernicious nature of the habit. Nicotine is considered the primary reinforcing component of tobacco responsible for addiction in human smokers (Stolerman and Jarvis, 1995). Nicotine acts in the brain via neuronal nicotinic acetylcholine receptors (nAChRs), which are ligand-gated ion channels consisting of five membrane-spanning subunits (Lena and Changeux, 1998). Nicotine-induced activation of nAChRs engages numerous intracellular signaling cascades that likely contribute to nicotine-related neuroplasticity and ultimately the development of tobacco dependence. However, the precise identity of the nAChR subtypes and the downstream transduction mechanisms that regulate the addictive properties of nicotine remain unclear.
The availability of genetically modified mice in which expression levels of targeted genes can be constitutively or conditionally modified offers a promising approach to better understand the mechanisms of nicotine reinforcement (Changeux, 2010; Fowler et al., 2008). Genetically modified mice are particularly desirable for assessing the role of receptors or components of intracellular signaling cascades in nicotine reinforcement for which there is a lack of selective pharmacological agents. To take advantage of transgenic mice in this context, it is necessary to have a reliable measure of nicotine reinforcement in mice. To date, most studies investigating nicotine reinforcement in mice have examined oral consumption of nicotine-containing solutions (Adriani et al., 2002; Glatt et al., 2009; Grabus et al., 2005; Isiegas et al., 2009; Meliska et al., 1995; Robinson et al., 1996). While this procedure is relatively simple and does not necessitate intravenous catheter implantation, orally consumed nicotine results in slow rates of absorption and low plasma levels of the drug (Adriani et al., 2002; Meliska et al., 1995), and further, response-contingent drug delivery is not assessed, making it difficult to determine motivated to obtain the drug. Recently, nicotine reinforcement has been assessed in mice that earn intracranial nicotine infusions directly into the ventral tegmental area (VTA) (Besson et al., 2006; Maskos et al., 2005). However, repeated intra-VTA nicotine injections are likely to cause damage to this structure, and brain regions besides the VTA regulate nicotine reinforcement (Brunzell et al., 2010; Di Matteo et al., 2007; Hollander et al., 2008; Kenny et al., 2009; Salas et al., 2009).
Based on these limitations, the establishment of a self-administration procedure in which volitional and contingent nicotine infusions are delivered by intravenous (IV) infusions in mice becomes highly desirable. Indeed, the IV self-administration procedure is generally considered to be the most direct measure of drug reinforcement in animals that most closely resembles the tobacco-smoking behavior in humans (Corrigall, 1999; Rose and Corrigall, 1997). In the self-administration procedure, animals are trained to emit a response to obtain IV drug infusions. To date, the vast majority of nicotine self-administration studies in rodents have utilized rats since attempts to transfer the technique to mice have proven difficult (Le Foll and Goldberg, 2005). Nevertheless, several laboratories have tried to overcome these difficulties. In one such procedure, mice are placed into an apparatus to restrain their movement and prepared with a temporary catheter in their lateral tail vein through which nicotine infusions are delivered contingent upon nose-poke responses (Martellotta et al., 1995; Paterson et al., 2003; Pons et al., 2008; Rasmussen and Swedberg, 1998). This procedure has a number of notable confounds that limit its utility. In particular, the level of restraint under which mice are tested may impact baseline behaviors and sensitivity to nicotine reinforcement. Also, as the catheter is temporarily implanted the mice can only be tested a limited number of times (typically once). Thus, acute but not chronic responses to nicotine can only be assessed, and a large number of mice are required (Martellotta et al., 1995; Paterson et al., 2003; Pons et al., 2008; Rasmussen and Swedberg, 1998). In light of these confounding variables, other groups have sought to establish IV nicotine self-administration procedures in which freely moving mice are prepared with chronic indwelling IV catheters in the jugular vein similar to rat self-administration studies. In several of these studies, mice on a C57BL6 background were first trained to respond for IV cocaine infusions before being permitted access to nicotine (Epping-Jordan et al., 1999; Picciotto et al., 1998). Importantly, pre-exposure to cocaine can induce adaptations in brain reward circuitries that may alter subsequent responses to nicotine and can also directly impact nAChR function (Ahmed et al., 2002; Hess et al., 2000). More recently, freely moving C57BL6 mice were shown to nose-poke for nicotine infusions under a continuous reinforcement (FR1) schedule, and omission of both nicotine and the paired cue-light resulted in extinction of responding (Galeote et al., 2009; Martin-Garcia et al., 2009; Metaxas et al., 2010; Plaza-Zabala et al., 2010). This represents a major advance in the field. Nevertheless, it is unclear whether mice with stable levels of responding for nicotine in this procedure would decrease responding when nicotine is withheld, an important consideration for data interpretation (Galeote et al., 2009; Martin- Garcia et al., 2009; Metaxas et al., 2010; Plaza-Zabala et al., 2010). This point is particularly salient in light of a recent study showing that rates of responding in C57BL6 mice for nicotine infusions were no different from those detected when nicotine was substituted with saline infusions but the nicotine-paired cue light was still activated upon completion of the schedule requirements (FR3; lever-press operant) (Contet et al., 2010). The high level of responding for the cue-light in the absence of nicotine likely reflects that fact that the cue-light alone may have reinforcing properties in rodents (Contet et al., 2010; Donny et al., 2003), an effect that was particularly prominent in the mice under the conditions used in the study. Thus, it is critically important to determine if apparently high rates of responding for nicotine infusions in mice reflect the reinforcing effects of nicotine, the infusion-paired cue-light or a combination of both reinforcers. Finally, C57BL6 mice were also shown to lever-press for nicotine infusions under a FR4 reinforcement schedule at a higher (0.1 mg kg−1 per infusion) but not lower (0.075 mg kg−1 per infusion) nicotine dose (Stolerman et al., 1999). In this study, water-restricted mice were first trained to respond for water rewards before gaining access to nicotine, with the two nicotine doses presented in descending order across a limited number of sessions (Stolerman et al., 1999). Thus, it is possible that the apparently high levels of intake observed only at the higher nicotine dose could reflect water-seeking behavior in mice that gradually extinguished across sessions (Contet et al., 2010; Stolerman et al., 1999).
As the establishment of a reliable IV nicotine self-administration procedure for mice will likely facilitate greater mechanistic understanding of tobacco dependence, the above studies represent key milestones in this effort. The purpose of the present series of experiments was to extend this previous work by manipulating variables known to impact nicotine self-administration behavior in rats and examine nicotine intake in mice. In particular, we assessed the effects of modifying the nicotine dose available, the infusion rate of drug delivery, withholding the drug upon completion of schedule requirements, and prior instrumental food training on IV nicotine self-administration behavior in mice. The data confirm that nicotine is an effective reinforcer in mice and establish a novel procedure for the conditions under which nicotine is robustly self-administered by mice.
METHODS
Animals
Male C57BL6 mice at six weeks of age were obtained from Jackson Laboratories (Bar Harbor, ME). All mice were housed in an environmentally controlled vivarium on a 12h:12h reversed light:dark cycle. Lights off occurred at 12pm, and mice were mainly tested between the hours of 7am–12pm, during the latter portion of light phase of the cycle. During testing, the room lights were turned off. Mice were housed in groups of 2–3 unless barbering necessitated mice being housed individually. Barbering usually only occurred during food training or the acquisition period of nicotine self-administration behavior. Thus, housing conditions were only altered during these stages of testing. Food and water were provided ad libitum until behavioral training commenced. During training, mice were food-restricted to maintain ~85–90 % of their free-feeding body weight. All procedures were conducted in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute - Florida.
Drugs
(−)-Nicotine hydrogen tartrate salt (Sigma Chemical Co., St. Louis, MO) was dissolved in 0.9 % sterile saline and passed through a 0.45 μm filter. All doses of nicotine refer to the free-base form. Mecamylamine hydrochloride (Tocris, Ellisville, MO) was dissolved in 0.9% physiological saline for intraperitoneal (IP) injection and delivered in an injection volume of 10 ml kg−1 of bodyweight. The pH of all drug solutions was adjusted to ~7.4.
Surgery
Mice were anesthetized with an isoflurane (1–3%)/oxygen vapor mixture and surgically prepared with IV catheters. Briefly, the catheters consisted of a 6 cm length of silastic tubing fitted to a guide cannula (Plastics One, Wallingford, CT), bent at a curved right angle and encased in dental acrylic. Silicone sealant was applied to the tubing extending out of the base, which prolonged catheter patency. The catheter tubing was passed subcutaneously from the animals’ back to the right jugular vein, and 1 cm length of the catheter tip was inserted into the vein. A silicone bead on the tubing prevented the catheter tip from entering any further into the vein, and silk suture secured the catheter to the vein. All subjects recovered for at least 48 h prior to any further experimental manipulation. The mice were then permitted to respond for food reinforcement to reestablish instrumental responding, and access to nicotine self-administration was not provided until ≥96 h after catheter surgery. Catheters were flushed daily with 0.05 ml physiological sterile saline solution (0.9 % w/v) containing heparin (10–60 USP units per ml). Catheter integrity was tested with 1–2 % of the ultra short-acting barbiturate anesthetic Brevital (methohexital sodium, Eli Lilly, Indianapolis, IN) diluted in sterile saline solution and injected at a volume of 0.05 ml.
Apparatus
Nicotine self-administration took place in 12 mouse self-administration modular test chambers (5.5″ W × 5″ H ×, 6.25″ D; Med Associates, St. Albans, VT) housed inside standard sound attenuating chambers (22″ W × 15″ H × 16″ D). The floors of the self-administration chambers were constructed of aluminum rods. In each chamber, one wall contained two metal retractable levers, one ‘active’ and one ‘inactive’, that were mounted 0.878″ above the floor and extended 0.375″ into the chamber. A cue-light was located above each lever, but only the cue-light associated with the ‘active’ lever was activated during testing sessions. The house light remained off at all times. Plastic swivels (Plastics One Co., Roanoke, VA) connected the mice to syringes operated by Razel pumps that delivered the drug infusions. Data collection and all programming functions were controlled by an IBM-compatible microcomputer with MED-PC software (Med Associates).
Intravenous self-administration procedure
Mice were mildly food restricted to 85–90 % of their free-feeding body weight and trained in an operant chamber (Med Associates) to lever press for food pellets (20 mg pellets; TestDiet, Richmond, IN) during 1 h daily sessions until stable responding was established under a fixed-ratio 5 time out 20 sec (FR5TO20 sec) schedule of reinforcement. Briefly, mice responded on an “active” lever for food rewards under a FR1 schedule (1 lever press = 1 food reward) while the 20 sec time out period was held constant. Responding on an “inactive” lever was recorded but was without scheduled consequence. After earning ~10 food rewards per session under the FR1 schedule, mice then responded under an FR2 schedule until they again earned ~10 food rewards per session. Thereafter, they responded under the FR5 schedule until they established consistently high levels of responding (≥ 25 food pellets per session). Next, mice were provided ad libitum food access for at least 24 h and then surgically prepared with the intravenous catheter. Following a postoperative recovery period of at least 48 h, mice were again food restricted to 85–90% of their free-feeding weight and permitted to respond for food rewards under the FR5TO20 sec schedule. After stable levels of food responding were again established, mice were then permitted to respond for nicotine infusions (0.03 mg kg−1 per infusion) under the FR5TO20 sec schedule during 1 h daily sessions, 6–7 days per week. Responding on an inactive lever was again recorded but was without scheduled consequence. The mice were placed into the testing chamber at approximately the same time each day. Nicotine infusions were delivered through the intravenous catheter over a 3-sec infusion period by a syringe pump (Razel; Med Associates) in a total injection volume of 35 μl. This duration of infusion was chosen based on a pilot experiment showing that found food-trained mice that were permitted to respond for nicotine consumed only a low level of the drug when it was delivered over a 1-sec infusion period (see Results). Each time the mouse earned a nicotine infusion a cue-light located immediately above the active lever was activated signaling the delivery of the nicotine infusion. This nicotine-paired cue-light remained lit for 20 sec after each infusion, during which time responses on the active lever were recorded but had no scheduled consequence. During testing, the room lights were darkened, and no other chamber light was illuminated. Consistent with previous studies, mice received a priming infusion of nicotine at the beginning of the first few (~2–3) sessions at each unit dose of the drug available for self-administration (Contet et al., 2010; Martin-Garcia et al., 2009). Mice were first given access to a lower unit dose of nicotine (0.03 mg kg−1 per infusion; free-base) until they achieved stable levels of responding (<20% variation in number of infusions across 3 sessions). The total acquisition period was a minimum of seven sessions. Thereafter, mice were permitted to respond for a higher dose of nicotine (0.1 mg kg−1 per infusion), termed the training dose. Mice had access to the training dose for at least 5 days until stable levels of nicotine intake were achieved. IV catheter integrity was tested periodically in between different unit doses of nicotine when assessing the nicotine dose-response curve. Specifically, catheter integrity was tested if responding became unstable and was tested a maximum of 2 times during an experiment. In addition, catheter integrity was tested in all mice upon completion of a nicotine self-administration experiment. If the catheter was shown to be faulty, the mouse was no longer tested, and data prior to the negative test was excluded from analysis.
Experiment 1: Nicotine dose-response function
To characterize the nicotine dose-response (D-R) function, we varied the unit dose of nicotine available for consumption (0, 0.03, 0.1, 0.25, and 0.4 mg kg−1 per infusion) after the acquisition period and assessed responding at each dose until stable levels of responding were established. Mice had access to each nicotine dose for at least 5 consecutive days. Once stable intake on each unit dose was achieved, mean intake over the final three sessions was averaged to obtain individual subject means. To reduce the risk of accidental overdose or overconsumption with subsequent aversive reactions to the drug, unit doses of nicotine were presented in ascending order, with saline presented last. Importantly, in between each unit dose the mice responded for the training dose of nicotine (≥2 sessions) until their responding was restored to baseline levels. Of the 12 mice initially included in this experiment, 2 were excluded during acquisition due to leaking catheters, 2 were excluded during the D-R curve assessment due to failed catheter patency tests, and 2 were excluded due to health reasons.
Next, we tested whether mice would respond more or less vigorously for nicotine infusions (0.03 mg kg−1 per infusion) when the drug was delivered over a 1-sec infusion period rather than the 3-sec infusion period used above. A second set of 8 mice were trained to respond for food reward and were then permitted to respond for nicotine infusions according to the standard protocol, with the exception that nicotine infusions were delivered over a 1-sec infusion period. After stable responding was established, we compared nicotine intake in the eighth self-administration session for these mice with the intake for the same session for the mice described above (3-sec infusion).
Finally, as mice appeared to prefer the 0.1 mg kg−1 per infusion dose, we tested a third set of mice of 4 mice to determine if they would acquire stable responding at this dose following food training without initial exposure to the lower unit dose of nicotine (0.03 mg kg−1 per infusion).
Experiment 2. Reassignment of the nicotine-paired lever
We examined whether mice could reliably discriminate between a nicotine-paired active lever and the inactive lever. The purpose of the experiment was to determine whether responding in mice was flexible and reflected nicotine-seeking behavior, or was inflexible and perhaps reflected habitual responding on the lever that previously delivered food rewards. Thus, a forth set of mice were trained to respond on an active lever (Lever A) for food rewards, and then switched to a lower dose of nicotine (0.03 mg kg−1 per infusion) under the FR5TO20 sec schedule of reinforcement for 7 sessions. Mice were then permitted to respond on Lever A for their preferred unit dose of nicotine (0.1 mg kg−1 per infusion). Throughout this acquisition period, responding on an inactive lever (Lever B) was without scheduled consequence. Once stable responding on Lever A for the preferred nicotine dose was established, we tested the effects of switching the lever and cue-light assignment such that responding on Lever A was now without scheduled consequence, and completion of the schedule requirements on Lever B resulted in the delivery of nicotine infusions and the nicotine-paired cue-light. Of the 9 mice that were initially included in this experiment only 1 was excluded due to health reasons.
Experiment 3: Responding for the cue-light in the absence of nicotine
To test whether mice responded primarily for nicotine infusions or whether the high levels of responding were related to cue-light activations independent of nicotine availability, we first assessed lever pressing for cue-light activations in a cohort of 12 nicotine-naïve mice that had not been previously food-trained. Mice were permitted to lever press to illuminate the cue-light in the absence of any drug infusions under increasing schedule requirements across sessions: sessions 1–2 at FR1; sessions 3–4 at FR2; and sessions 5–6 at FR5 (consistent with the schedule employed for food training). Completion of the schedule requirements resulted in cue-light activation for 20 sec, as was the case in animals responding for food or nicotine rewards. In a second experiment, we examined lever-pressing behavior in mice with stable levels of responding for nicotine (0.1 mg kg−1 per infusion) after nicotine was omitted from the infusion and only saline was delivered in conjunction with cue-light after completion of the schedule requirements. The mice used in these experiments were the same as those used above in Experiment 2 above (testing sequence experiment 3a → 2 → 3b).
Experiment 4: Effects of mecamylamine on nicotine intake
To verify that nAChRs regulate nicotine intake in mice, we assessed the effects of mecamylamine (1–2 mg/kg) on nicotine self-administration behavior in a fifth set of mice. Briefly, mice with stable levels of responding for nicotine (0.1 mg kg−1 per infusion; n=10) or food rewards (n=11) received an IP injection of either saline or mecamylamine (1 or 2 mg kg−1) immediately prior to the self-administration session, and subsequent nicotine or food intake was recorded. We found that the higher dose of mecamylamine induced long lasting decreases in nicotine self-administration behavior. Therefore, a quasi between-subjects design was necessitated for this experiment in which each mouse received a saline injection and only a single injection of mecamylamine. Data from 1 mouse were excluded due to health reasons.
Experiment 5: Extinction and cue-induced reinstatement of nicotine intake
After establishing stable responding for nicotine infusions (0.1 mg kg−1 per infusion) in a sixth set of 8 mice, we withheld the nicotine infusion and presentation of the nicotine-paired cue-light. When individual mean responding dropped below 20 active lever presses for three consecutive sessions. We then tested whether the previously nicotine-paired cue-light could reinstate nicotine-seeking responses. In this reinstatement test, completion of the FR5TO20 sec schedule resulted in activation of the cue-light previously paired with nicotine delivery but did not trigger a nicotine infusion. Responses on the inactive lever were recorded but were without scheduled consequence. No mice were excluded from this study.
Experiment 6: Acquisition of nicotine self-administration behavior without prior food training
We next investigated whether mice would acquire nicotine self-administration behavior without prior instrumental training for food rewards. A seventh set of 6 mice were prepared with IV catheters, food restricted to 85–90 % of their free-feeding weight, and then placed them into the operant chambers for 1 h acquisition sessions across days. Responses on the active lever resulted in the delivery of a nicotine infusion (0.03 mg kg−1 per infusion) in conjunction with the cue-light illumination. Mice were primed with a single nicotine infusion at the start of each session for the first week of testing and for initial sessions of each new schedule requirement. The mice responded for nicotine under the following series of schedule requirements: FR1TO20 sec (sessions 1–8), FR2TO20 sec (sessions 9–15) and FR5TO20 sec (sessions 16–20). Responding on an inactive lever during this acquisition period was recorded but was once again without any scheduled consequence. No mice were excluded from this study.
Statistical Analyses
All data were analyzed by t-test or one- or two-way analysis of variance (ANOVA; repeated measures where appropriate) using Graphpad Prism software (La Jolla, CA). Significant main or interaction effects were followed by a Newman-Keuls post-hoc test (one-way ANOVA) or Bonferroni post-hoc test (two-way ANOVA). The level for significance was set at <0.05.
RESULTS
During food training, the mice rapidly learned to respond for food rewards (Fig. 1a). Across sessions, the schedule requirements were increased, leading to a progressively increasing number of lever presses on the food-paired active lever and minimal responding on the inactive lever (Fig. 1b). When permitted access to nicotine (0.03 mg kg−1 per infusion), mice consumed ~30 nicotine rewards during their first self-administration session, but rapidly adjusted their level of responding over the next 2–3 sessions to obtain ~12 rewards during the consecutive 1 h sessions (Fig. 1c). Responding on the active lever remained higher than responses on the inactive lever (Fig. 1d).
Figure 1. Responding for food reward and intravenous nicotine infusions in mice.
(a, b) Male C57BL6 mice (n=12) were permitted to respond on an ‘active’ lever to receive food reward during 1 h daily sessions under increasing stringent schedules of reinforcement: FR1TO20 sec (sessions 1–2), FR2TO20 sec (sessions 3–4) and FR5TO20 sec (sessions 5–7). Data are presented as (a) mean ± SEM number of food pellets earned and (b) mean ± SEM number of lever presses on the ‘active’ and ‘inactive’ levers. (c,d) Food-trained mice (n=10) were permitted to respond for nicotine infusions (0.03 mg kg−1 per infusion; free-base) under the FR5TO20 sec schedule of reinforcement during 1 h daily sessions. Data are presented as (c) mean ± SEM number of infusions earned and (d) mean ± SEM number of lever presses on the ‘active’ and ‘inactive’ levers.
When the unit dose of nicotine available was varied, mice responded according to an inverted ‘U’-shaped dose-response (D-R) curve to receive nicotine infusions (Fig. 2a); one-way ANOVA, F(4,29)=12.09, p<0.0001. Maximal responding for nicotine was observed at the 0.1 mg kg−1 per infusion dose. Compared to the active lever, inactive lever responses remained low across nicotine doses (Fig. 2b); two-way ANOVA, Lever: F(1,49)=101.5, p<0.0001; Dose: F(4,49)=10.52, p<0.0001; Interaction: F(4,49)=6.32, p<0.001. Representative event records for mice at the acquisition (0.03 mg kg−1 per infusion) and preferred (0.1 mg kg−1 per infusion) doses of nicotine are shown in Fig. 2c and Fig. 2d, respectively. At the higher unit doses of nicotine (0.1–0.4 mg kg−1 per infusion), mice titrated their lever-pressing behavior to obtain ~1.5 mg kg−1 nicotine per session (Fig. 2e); one-way ANOVA, F(4,20)=20.41, p<0.0001.
Figure 2. The dose-response function for self-administered nicotine in mice.
(a,b) After establishment of stable responding for nicotine in mice (n=6), the unit dose available for self-administration was varied. This resulted in an inverted ‘U’-shaped dose-response curve, with the highest responding detected at the 0.1 mg kg−1 per infusion dose. (a) Data are presented as mean ± SEM number of nicotine infusions earned by responses on the ‘active’ lever. *p<0.05 and ***p<0.001 compared to saline; +p<0.05 and +++p<0.001 compared to 0.1 mg kg−1 per infusion dose. (b) Data are presented as mean ± SEM number of ‘active’ and ‘inactive’ lever presses. **p<0.01 and ***p<0.001 ‘active’ versus ‘inactive’ lever at unit dose. (c) Representative event records of lever-pressing behavior (upper black lines) and rewards earned (lower grey lines) in each of the six mice at the acquisition dose (0.03 mg kg−1 per infusion). The upward ticks on the black line represent lever-press events, whereas the upward ticks on the grey line represent the delivery of nicotine infusions. (d) Representative event records of lever-pressing behavior (upper black lines) and rewards earned (lower grey lines) in each of the six mice responding for the preferred dose of nicotine (0.1 mg kg−1 per infusion). (e) The quantity of nicotine consumed at each unit dose available for self-administration was calculated (mean ± SEM). At the higher doses (≥0.1 mg kg−1 per infusion), mice displayed a titration of their responding to consume ~1.5 mg kg−1 nicotine per session. *p<0.05, ***p<0.001 compared with saline infusions; +p<0.05 compared with the acquisition dose (0.03 mg kg−1 per infusion) of nicotine.
Many previous studies in mice have used a short 1-sec infusion time to deliver IV nicotine infusions, whereas we used a 3-sec infusion time in the present experiments. We therefore compared the relative reinforcing effects of the same nicotine dose (0.03 mg kg−1 per infusion) when delivered during 1-sec or 3-sec infusion times. We found that mice responded at a lower rate when nicotine was delivered in the shorter infusion time (Fig. 3a); t-test, t(16)=2.29, p<0.05. As mice responded maximally at the 0.1 mg kg−1 per infusion dose during the D-R assessment, we next examined whether this dose could support the establishment of stable responding without prior access to the lower nicotine dose (0.03 mg kg−1 per infusion). Following food training, mice were immediately switched onto the higher unit dose of nicotine (Fig. 3b). The level of responding was lower and far more variable than that found in mice that had prior access to the 0.03 mg kg−1 per infusion dose (Fig. 1d).
Figure 3. Experimental manipulation of infusion duration and nicotine dose during acquisition.
(a) Mice were permitted to respond for nicotine infusions (0.03 mg kg−1 per infusion) following food training for 8 sessions. The duration of nicotine infusion was either 1-sec (n=8) or 3-sec (n=10). Data are presented as the mean ± number of nicotine infusions earned by responses on the ‘active’ lever in the eighth session. *p<0.05 (b) Food-trained mice (n=4) were permitted to respond for a higher dose of nicotine infusions (0.1 mg kg−1 per infusion; free-base) under the FR5TO20 sec schedule of reinforcement during 1 h daily sessions. Data are presented as number of nicotine infusions earned for each mouse across session.
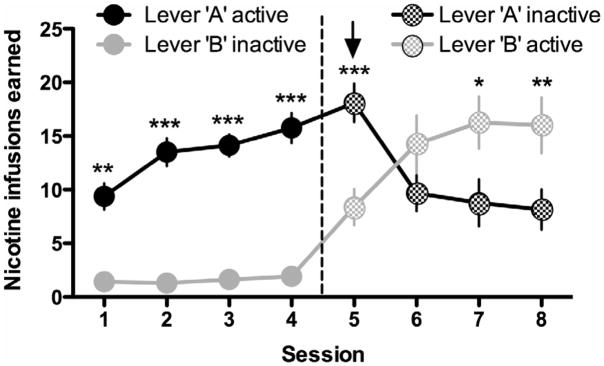
When the lever assignment was switched, we found that the mice decreased their responding on a previously drug-paired lever (Lever A), and increased responding on the previously inactive lever that now delivered nicotine infusions (Lever B) (Fig. 4); two-way ANOVA, Lever: F(1,98)=17.16, p<0.01; Session: F(7,98)=11.30, p<0.0001; Interaction: F(7,98)=18.84, p<0.0001.
Figure 4. Modified lever-pressing behavior based on availability of nicotine in mice.
Mice developed stable responding on Lever A under an FR5TO20 sec schedule of reinforcement for nicotine (0.1 mg kg−1 per infusion; n=8 mice), with low levers of responding on Lever B that was without scheduled consequence. When the lever assignments were switched after the 4th self-administration session, mice adjusted their lever-pressing behavior by increasing their responses on Lever B, permitting them to continue to attain nicotine infusions. Simultaneously, responding on the previously reinforced Lever A gradually decreased across sessions. Data are presented as mean ± SEM number of nicotine infusions earned by responses on the ‘active’ lever, and mean number of comparable bar presses (total/FR5) ± SEM for the ‘inactive’ lever. *p<0.05, **p<0.01, ***p<0.001 ‘active’ versus ‘inactive’ lever within each session.
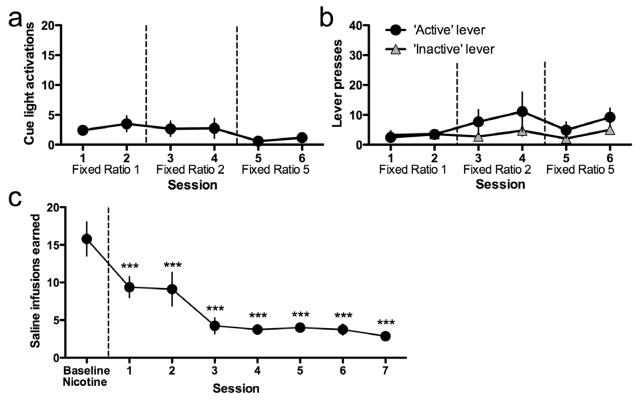
In nicotine-naïve mice, responses on the active lever were indistinguishable from responses on the inactive lever when active lever responding only resulted in the activation of the cue-light and did not trigger nicotine delivery (Fig. 5a, b); two-way ANOVA, Lever: F(1,110)=0.92, not significant (n.s.); Session: F(5,110)=2.51, p<0.05; Interaction: F(5,110)=1.18, n.s. In mice self-administering nicotine (0.1 mg kg−1 per infusion), substitution of saline infusions for nicotine resulted in a marked decline in active lever-pressing across days, even though completion of the FR5 schedule still resulted in cue-light activation (Fig. 5c); one-way ANOVA, F(7,49)=3.44, p<0.01.
Figure 5. The cue-light alone did not support lever-pressing behavior in mice.
(a, b) Nicotine- and food training-naïve mice (n=12) were permitted to respond on an active lever for the cue-light usually paired with delivery of nicotine infusions during 1 h sessions. The duration of illumination of the cue-light was 20 sec. Responding on an inactive lever was without scheduled consequence. The schedule of reinforcement for cue-light activation was increased over sessions in accordance with the same procedure usually employed for food training: FR1TO20 sec (sessions 1–2), FR2TO20 sec (sessions 3–4) and FR5TO20 sec (sessions 5–6). Data are presented as (a) mean ± SEM number of light activations earned by responses on the ‘active’ lever, and (b) mean± SEM number of lever presses on the ‘active’ and ‘inactive’ levers. (c) Stable responding for nicotine (0.1 mg kg−1 per infusion) was established in a separate cohort of mice (n=8) under an FR5TO20 sec schedule of reinforcement during 1 h sessions. The mean of the three final days of stable responding is presented as ‘Baseline Nicotine’. Thereafter, saline instead of nicotine was delivered in conjunction with cue-light activation according to the FR5TO20 sec schedule of reinforcement. Data are presented as mean number of saline infusions/cue-light activations earned ± SEM for responses on the ‘active’ lever. ***p<0.001 compared to baseline nicotine.
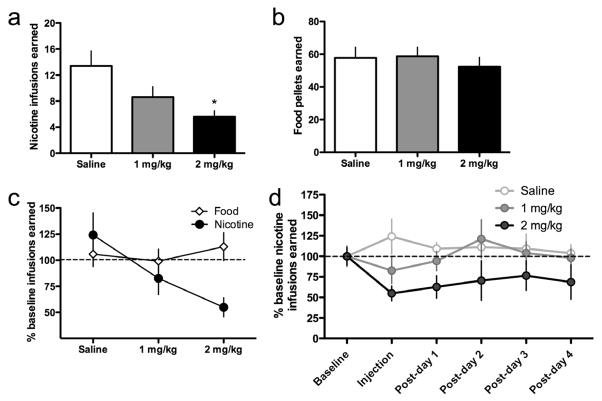
Mecamylamine dose-dependently decreased nicotine self-administration behavior in mice (Fig. 6a); one-way ANOVA, F(2,14)=5.29, p<0.05. The same doses of mecamylamine did not alter food intake (Fig. 6b, c); F(2,24)=0.24, n.s. Interestingly, the highest dose of mecamylamine (2 mg kg−1) induced long-lasting decreases in nicotine self-administration behavior that persisted for at least 4 days (Fig. 6d).
Figure 6. Nicotinic receptor antagonism decreases nicotine self-administration behavior in mice.
(a) Stable responding for nicotine (0.1 mg kg−1 per infusion) was established in mice (n=5 per group) under an FR5TO20 sec schedule of reinforcement during 1 h sessions. Mice then received an IP injection of saline or mecamylamine (1 or 2 mg kg−1) immediately prior to the self-administration session according to a betweensubjects experimental design. Data are presented as mean ± SEM number of nicotine infusions earned. *p<0.05 compared to saline. (b) The same doses of mecamylamine were assessed with responding for food reward. Data are presented as mean ± SEM number of food rewards earned. (c) Direct comparison of the effects of mecamylamine on responding for nicotine infusion or food reward. Data are expressed as mean ± SEM percent change from baseline nicotine intake. Baseline nicotine data represent the self-administration session prior to saline or mecamylamine injection. *p<0.05 compared to saline. (d) Following the mecamylamine or saline injection, the mice were permitted to respond for nicotine for an additional four sessions. Mice receiving the 2 mg kg−1 mecamylamine injection did not fully recover their prior level of baseline responding to nicotine. Data are presented as percentage of the baseline infusions earned ± SEM (0.1 mg kg−1 per infusion nicotine).
Omission of nicotine infusions and the associated cue-light resulted in an initial slight increase in lever-pressing behavior in self-administering mice, followed by a gradual decrease in responding (i.e., extinction) across sessions (Fig. 7a); two-way ANOVA, Lever: F(1,140)=34.07, p<0.0001; Session: F(10,140)=21.89, p<0.0001; Interaction: F(10,140)=18.76, p<0.0001. When the nicotine-associated cue-light was again activated upon completion of the schedule requirements in the continued absence of nicotine delivery, mice increased responding on the active lever, but not the inactive lever, thus demonstrating cue-induced reinstatement of nicotine-seeking behavior (Fig. 7b); two-way ANOVA, Lever: F(1,14)=28.33, p<0.01; Experimental condition: F(1,14)=15.46, p<0.01; Interaction: F(1,14)=13.59, p<0.01.
Figure 7. Extinction and reinstatement of nicotine-seeking behavior in mice.
(a) Stable responding for nicotine (0.1 mg kg−1 per infusion) was first established under an FR5TO20 sec schedule of reinforcement during 1 h sessions in mice (n=8 per group). Responding on the inactive lever was recorded but was without scheduled consequence. Baseline nicotine data represent the final day of nicotine self-administration. The following session, mice were permitted to respond on both the ‘active’ and ‘inactive’ levers but both levers were without scheduled consequence (e.g., no infusion or cue light). Data are presented as mean ± SEM number of responses on the previously ‘active’ or ‘inactive’ lever. **p<0.01, ***p<0.001 ‘active’ versus ‘inactive’ lever within each session. (b) After reaching the criteria of three sessions with a mean of <20 ‘active’ lever presses, the mice were permitted to respond for the cue-light on the ‘active’ lever under the previously established FR5TO20sec schedule (i.e., cue-induced reinstatement). Data are presented as mean ± SEM lever presses on ‘active’ and ‘inactive’ levers during extinction (mean of final three days) and the reinstatement session. ***p<0.001 extinction versus reinstatement on the ‘active’ lever.
Finally, mice without prior food training were permitted to lever-press for nicotine infusions under progressively increasing FR requirements across sessions (Fig. 8a). Mice preferentially responded on the active versus the inactive lever at greater levels with the increasing schedule requirements (Fig. 8b); two-way ANOVA, Lever: F(1, 190)=94.76, p<0.0001; Session: F(19,190)=10.25, p<0.0001; Interaction: F(19,190)=1.96, p<0.05. Following this acquisition period, nicotine was substituted with saline infusions in conjunction with the cue-light activation upon completion of the FR5 schedule (Fig. 8c); one-way ANOVA, F(5,35)=7.80, p<0.001. Under these conditions, mice rapidly decreased their responding on the active lever until responses were indistinguishable from those on the inactive lever (Fig. 8d); two-way ANOVA, Lever: F(1,50)=19.90, p<0.01; Session: F(5,50)=10.55, p<0.001; Interaction: F(5,50)=4.77, p<0.01. When nicotine infusions were again available for self-administration upon completion of the schedule requirements, the mean number of infusions earned subsequently increased across session (Fig. 8e); one-way ANOVA, F(3,19)=9.93, p<0.01. The mice rapidly increased responding on the nicotine-paired active lever while responses on the inactive lever remained low (Fig. 8f); two-way ANOVA, Lever: F(1,34)=40.06, p<0.0001; Session: F(3,34)=8.45, p<0.001; Interaction: F(4,34)=3.01, p<0.05.
Figure 8. Acquisition of nicotine self-administration in mice without prior food training.
(a, b) Nicotine- and food training-naïve mice (n=6) were permitted to respond for nicotine infusions during 1 h sessions under an increasing fixed ratio schedule: FR1TO20 sec (sessions 1–8), FR2TO20 sec (sessions 9–15) and FR5TO20 sec (sessions 16–20). Data are presented as (a) mean ± SEM number of nicotine infusions earned by responses on the ‘active’ lever, and (b) mean ± SEM number of lever presses on the ‘active’ and ‘inactive’ levers. *p<0.05, **p<0.01 and ***p<0.001 ‘active’ versus ‘inactive’ lever within each session. (c,d) Following the acquisition period, mice extinguished their self-administration behavior when nicotine was substituted with saline infusions. ‘Baseline nicotine’ values are from session 20 (panel a, b, respectively). (c) Data are presented as mean ± SEM number of infusions earned by responses on the ‘active’ lever. **p<0.01 and ***p<0.001 compared to baseline nicotine. (d) Data are presented as mean ± SEM number of ‘active’ and ‘inactive’ lever presses. ***p<0.001 ‘active’ versus ‘inactive’ lever within each session. (e, f) When nicotine access was reinstated, mice increased their responding under the FR5TO20 schedule of reinforcement. ‘Baseline extinction’ values are from session 25 (panel c, d, respectively). (e) Data are presented as mean ± SEM number of nicotine infusions earned by responses on the ‘active’ lever. *p<0.05, **p<0.01, and ***p<0.001 compared to baseline saline. (f) Data are presented as mean of ‘active’ and ‘inactive’ lever presses. ***p<0.001 ‘active’ versus ‘inactive’ lever within each session.
DISCUSSION
In the current series of experiments we have shown that mice respond robustly for IV nicotine infusions, particularly when the drug was delivered over a slower infusion time (3-sec versus 1-sec infusion), and that varying the dose of nicotine available for consumption results in an inverted ‘U’-shaped dose-response curve similar to that previously observed in other species. When challenged with reversal of the lever assignment, mice were able to modify their responding accordingly to maintain high levels of nicotine intake. Further, the nAChR antagonist mecamylamine decreased self-administration behavior in mice, confirming the involvement of nicotinic receptors in maintaining nicotine intake. Lever-pressing behavior was not supported by the cue-light alone and decreased when nicotine was omitted from the infusions and only the cue-light was available. Omission of nicotine infusions and cue-light activations upon completion of the schedule requirements resulted in extinction of responding in mice, with responding reinstated by the nicotine-paired cue-light. Finally, stable levels of nicotine self-administration behavior were established in mice without prior training to respond for food rewards, verifying that the high levels of nicotine intake we detected in mice were not secondary to food-seeking behavior. Taken together, these data demonstrate that nicotine is an effective reinforcer in mice and identity the experimental conditions under which the drug is reliably self-administered at high levels.
In the current series of experiments, we sought to address some of the drawbacks of previous IV nicotine self-administration procedures for mice, and identify those conditions that support robust levels of responding for the drug. The experimental conditions we used are very similar to the well-established conditions used by many laboratories to assess nicotine self-administration behavior in rats (Corrigall, 1992; Woolverton and Nader, 1990). We first trained mildly food-restricted mice to lever press for food rewards until they exhibited stable responding under a FR5TO20 sec reinforcement schedule. This reinforcement schedule is more stringent than those employed in previous studies (Contet et al., 2010; Martin-Garcia et al., 2009; Stolerman et al., 1999). Our intent was to increase the workload necessary for mice to obtain nicotine infusions and thereby support only purposeful responding for the drug. Mice were then permitted to respond for nicotine infusions (0.03 mg kg−1 per infusion) under the FR5TO20 sec reinforcement schedule. After approximately 8 days of responding for this dose, we increased the unit dose of nicotine to one that supported the highest levels of responding (0.1 mg kg−1 per infusion). We found that this initial exposure to a lower nicotine dose, followed by access to the preferred higher dose, was the most effective approach to achieve stable levels of responding in the mice. Indeed, in pilot experiments, permitting mice immediate access to the preferred dose of nicotine, without first becoming accustomed to the effects of the lower dose, generally resulted in low rates of responding, at least over the first week of access to the drug. Using this procedure, mice preferentially responded on the lever that delivered nicotine infusions and exhibited minimal responding on the non-reinforced inactive lever. Further, all mice acquired nicotine self-administration behavior when catheter integrity was intact; the main cause of catheter failure in our study was damage to the tubing resulting in leakage. Thus, we did not find it necessary to exclude mice because of low rates of responding as in previously studies (Contet et al., 2010; Martin-Garcia et al., 2009), even though a stringent reinforcement schedule was used in the present study. It is important to note that although the 0.1 mg kg−1 per infusion nicotine dose supported maximal responding in the current experiments, a recent study reported that the same strain of mice responded less vigorously for this dose of nicotine than for other (lower) doses (Contet et al., 2010). Interestingly, however, in the previous study mice consumed the largest amounts of nicotine per session at the 0.1 mg kg−1 per infusion dose and exhibited greater selectivity of responding on the active lever at this dose (Contet et al., 2010). This could perhaps reflect the fact that mice in the previous study had not yet developed tolerance to aversive effects of this higher dose that may initially limit intake since initial access to a lower nicotine dose (0.03 mg kg−1 per infusion) was necessary in the current study to established robust responding for this higher dose in mice. Other factors that may have contributed to this discrepancy include the differences in infusion duration (3-sec versus 1-sec), testing schedule and prior food training. Further, it is worth pointing out that Stolerman and colleagues also found that mice responded maximally for the 0.1 mg kg−1 per infusion dose of nicotine (Stolerman et al., 1999).
The experimental conditions employed in our studies also differed from those of previous studies in a number of other important regards. First, in many of the prior studies, mice were tested only on a limited number of days during the week (e.g., typically only during weekdays) and only during the dark phase of the light cycle (Contet et al., 2010; Martin-Garcia et al., 2009). Although not analyzed in a systematic manner, we found that mice performed very consistently when tested seven days per week, with daily testing times varying by no more than ± 30 min each day, but did not find any differences in responding when mice were tested during the light or dark phases of their circadian cycle, although the house light was turned off so that mice were always tested in darkness. Anecdotally, we found that environmental stimuli in the housing facility or testing rooms, which may induce stress in mice (e.g., noise, vibrations, etc.), also impacted performance and that mice performed best when few extraneous noises were present in the testing environment, irrespective of the phase of the light/dark cycle. In addition, nicotine intake was less stable if mice were subjected to injury or barbering by their cage-mates. Individual housing of these mice stabilized nicotine intake at levels that were largely similar to group-housed mice. Another difference between this and previous studies is the volume and duration of the nicotine infusion. The nicotine infusions in the present study were delivered in a total volume of 35 μl over 3 sec, whereas prior reports have delivered varying total volumes (6–8, 18, 23.5 or 50 μl) over alternate time periods (1, 2 or 7.5 sec) (Contet et al., 2010; Martin-Garcia et al., 2009; Picciotto et al., 1998; Stolerman et al., 1999). Since the rate of IV nicotine delivery is an important determinant for establishing nicotine self-administration behavior in rats (Corrigall and Coen, 1989; Sorge and Clarke, 2009), identifying the optimal volume and duration of infusion is likely to be an important factor in establishing stable responding for nicotine in mice across laboratories. Indeed, in preliminary experiments, we found that nicotine infusions delivered in a smaller volume (12.5 μl) and over a shorter duration (1-sec infusion period) did not reliably support self-administration behavior in mice.
When we varied the unit dose of nicotine available for self-administration, mice responded for the drug according to an inverted U-shaped dose-response (D-R) function similar to that seen in other species, including humans (Henningfield and Goldberg, 1983), non-human primates (Goldberg et al., 1981; Le Foll et al., 2007) and rats (Corrigall and Coen, 1989). When the total amounts of nicotine earned at each unit dose were calculated, we found that mice titrated their responding to consume ~1.5 mg kg−1 per session at higher unit doses, suggesting that this was the optimal amount of nicotine that mice seek to obtain during 1 h sessions. This observation is consistent with human smokers that increase or decrease their rate of smoking when provided cigarettes with a lower or higher nicotine yield, respectively (McMorrow and Foxx, 1983). Mice responded for far higher nicotine doses than those that support responding in rats (0.01–0.09 mg kg−1 per infusion), likely reflecting the higher rates of nicotine metabolism in mice (Matta et al., 2007). It is important to note that we characterized the nicotine D-R function only after mice had demonstrated stable levels of nicotine intake, with testing of different unit doses commencing at least eight days after the mice last responded for food rewards. Moreover, we permitted mice to self-administer each unit dose of nicotine for at least five sessions before shifting them back to the training dose for ≥2 sessions to re-establish baseline levels of responding, and then tested the next unit dose. Thus, mice were reliably responding for nicotine for ~40 days after their last food training session. Moreover, when nicotine was substituted with saline there was a marked decrease in responding even though the nicotine-paired cue-light was still activated upon completion of the schedule requirements. Further, the nAChR antagonist mecamylamine decreased nicotine intake at doses that did not influence responding for food rewards, and mice acquired stable responding for nicotine infusions without the need for prior food training. Taken together, these findings demonstrate that mice persisted in consuming nicotine across long time periods, and responding for the drug was not confounded by habit-like food seeking behavior related to the prior food-training regimen.
Although not necessary to establish nicotine self-administration behavior, rats are often food restricted then trained to respond for food rewards before gaining access to nicotine infusions. This approach has been shown to increase nicotine intake and accelerate the acquisition of stable responding for the drug (Clemens et al., 2010; Corrigall, 1992; Donny et al., 1998; Donny et al., 2003; Shram et al., 2008; Singer et al., 1978). Importantly, prior training to respond for a natural reinforcer does not appear to alter subsequent nicotine self-administration behavior in rats (Clemens et al., 2010). There are several advantages to first training mice to respond for food in the same manner before access to nicotine. First, even though mice respond for nicotine infusions without prior food training, the time necessary to establish stable responding for the drug under these conditions is far longer. Considering the high rate of catheter attrition typically seen in mice, the accelerated establishment of stable nicotine responding in food-trained mice is a major advantage of this approach. Second, assessment of responding for food rewards under the reinforcement schedule subsequently used for nicotine self-administration is a useful control procedure to ensure that similar levels of instrumental responding can be achieved in transgenic animals and their wildtype counterparts. In this manner, alterations in nicotine intake in genetically altered mice can be more confidently attributed to differences in the underlying molecular mechanisms regulating nicotine reinforcement, rather than nonspecific deficits that impact behavioral performance. Third, as food training is often used in rat nicotine self-administration studies, its use in mice facilitates comparison of the effects of pharmacological agents or molecular manipulations across species. It is interesting to note that food-trained mice typically required 3–4 self-administration sessions to adjust their level of responding when food was replaced with nicotine infusions. In contrast, food-trained rats adjust their responding almost immediately, and their intake on the first self-administration session is very similar to those that follow. Thus, mice may be less sensitive to the initial aversive effects of nicotine compared with rats, and hence adjust their intake downward only gradually. Alternatively, alterations in instrumental performance based on post-consummatory effects of the earned reward may differ between mice and rats such that mice require more time to adjust their behavioral performance when the available reinforcer is switched.
Similar to most nicotine self-administration studies in rats, we used a discrete conditioned stimulus to signal delivery of nicotine infusions in mice. Specifically, a cue-light located immediately above the drug-paired lever was activated for 20 sec upon completion of the schedule requirements and was thereby paired with each nicotine infusion. Although nicotine was delivered directly into the jugular vein of mice, the neurobiological effects of the drug are unlikely to be experienced until several seconds later. Thus, the addition of a conditioned stimulus bridges the time gap between the lever-pressing behavior and physiological effects of the drug, thus facilitating the association between instrumental responding and nicotine’s subsequent actions. Previously, it was shown that a cue-light similar to that used in the current study supported high levels of responding in C57BL6 mice even when nicotine was omitted from infusions and the cue-light was delivered in conjunction with saline infusions (Contet et al., 2010). In fact, responding for the saline/cue-light combination was so high (~15 infusions during 60 min sessions under FR3 schedule) that it was indistinguishable from responding for the nicotine/cue-light combination (Contet et al., 2010). Similarly, high levels of responding for the cue-light were also seen in mice that had not undergone any prior training to respond for food, nicotine or any other reinforcers (Contet et al., 2010). In the current study, we verified that mice were responding vigorously for nicotine and not just the cue-light activation in three ways: First, when otherwise nicotine-naïve mice were permitted to lever press for the cue-light, we found that the cue-light alone did not support the acquisition of stable responding. Second, when we omitted nicotine from the infusions, but the cue-light was still activated by completion of the schedule requirements, we found that responding on the active lever dropped precipitously across sessions. Third, we found that mecamylamine selectively decreased responding for nicotine at a dose that did not alter responding for food rewards even though the cue-light was still activated by responses on the active lever. These data demonstrate that mice were responding specifically to obtain nicotine and not because of intrinsic reinforcing properties of the cue-light. The experimental conditions that we used in the current study likely contributed to the lower levels of responding for the cue-light compared with previously studies (Contet et al., 2010). These factors likely include a more stringent fixed ratio schedule of reinforcement, consistency of the testing schedule across days, and a slower rate of drug infusion. Further, in the previous study (Contet et al., 2010), lever-pressing criteria were imposed for both their cue-light and nicotine self-administration studies, resulting in the exclusion of ~30% of the mice responding for the cue-light alone based on low levels of lever pressing behavior. As such, the experimental design was somewhat biased toward those mice with the highest levels of responding for the cue-light. In the current lever-reversal experiment, mice exhibited flexibility in responding for nicotine and the associated cue-light by modifying their lever-pressing behavior to respond preferentially on the nicotine-associated lever after the active/inactive lever assignment had been reversed, and the cue-light alone did not support lever-pressing behavior in the current experiments. Hence, it is unlikely that mice switched their responding onto a previously inactive lever simply to activate the cue-light. Instead, this switching of behavior likely reflects the high motivation of the mice to continue to receive nicotine infusions alone or in combination with cue-light activation.
It is interesting to note that when we treated mice with the higher dose of mecamylamine (2 mg kg−1), responding for nicotine was suppressed for >4 days, an effect that contrasts with the very transient decreases in intake induced by mecamylamine in rats (Corrigall and Coen, 1989; Watkins et al., 1999). This finding is reminiscent of recent studies showing that similar concentrations of the nAChR partial agonist varenicline (Chantix®) that transiently antagonize rat and human nAChRs can induce a remarkably long-lasting inhibition of mouse nAChRs (Papke et al., 2010). This suggests that mouse nAChRs may be far more sensitive to prolonged inhibition by nAChR ligands than rat or human nAChRs through as yet unclear mechanisms. This raises a note of caution for studies proposing to use within-subject experimental designs to assess the effects of pharmacological agents on nicotine intake in mice.
Finally, we found that omission of nicotine delivery and cue-light activation upon completion of the schedule requirements resulted in a gradual extinction of nicotine-seeking responses in mice. It is interesting to note that mice exhibited more persistent responding across a greater number of extinction sessions when tested in the absence of sensory stimuli (no cue or saline infusion) compared with mice responding for cue-light/saline infusions. This apparently greater resistance to extinction appears counterintuitive, as it may have been expected that the nicotine-paired cue-light would have sustained higher levels of responding. One possible explanation is that in the absence of both the primary (nicotine) and secondary (cue-light) reinforcers, animals engage in a high degree of lever-pressing to overcome a state of “frustrative nonreward”(Gray, 1987), the magnitude of which is lower in animals exposed to the nicotine-paired cue-light and other sensory stimuli associated with nicotine delivery (i.e., IV infusion, noise of drug pump). Alternatively, the contingency between cue-light activation and reward delivery may be continuously updated in mice based on neural processing of infusion-related information. As such, withholding of the cue-light may retard extinction learning by reducing their ability to process environmental information related to the contingency between cue-light activation and reward delivery. Finally, we found that activation of the cue-light without nicotine delivery was sufficient to trigger the reinstatement of previously extinguished nicotine-seeking responses in mice. This finding is consistent with recent reports also showing that nicotine-paired cues reinstate extinguished nicotine-seeking responses in mice (Martin-Garcia et al., 2009; Plaza-Zabala et al., 2010). The cue-induced reinstatement procedure may therefore have considerable utility for assessing the neurobiological mechanisms of relapse-like behaviors for nicotine using genetically modified mice.
Taken together, our data demonstrate that C57BL6 mice readily respond for nicotine infusions with an intravenous self-administration procedure similar to that typically used in rats. Hence, under the appropriate experimental conditions, nicotine serves as an effective reinforcer in mice similar to other species. As C57BL6 is the background strain upon which many lines of genetically altered mice are bred, the nicotine self-administration procedure described herein should have considerable utility for studying the molecular mechanisms of nicotine reinforcement. Such studies may support the future development of novel therapeutics for the treatment of tobacco addiction.
Development of a robust self-administration procedure for mice has proven difficult.
We identify conditions that support nicotine self-administration in mice.
We characterize nicotine self-administration in mice across a range of conditions.
This self-administration procedure may have considerable utility to the field.
Acknowledgments
Supported by the National Institute on Drug Abuse (DA020686 to PJK; DA026693 to CDF). This is manuscript #20973 from Scripps Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CITED LITERATURE
- Adriani W, Macri S, Pacifici R, Laviola G. Restricted daily access to water and voluntary nicotine oral consumption in mice: methodological issues and individual differences. Behav Brain Res. 2002;134:21–30. doi: 10.1016/s0166-4328(01)00448-x. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, David V, Suarez S, Cormier A, Cazala P, Changeux JP, Granon S. Genetic dissociation of two behaviors associated with nicotine addiction: beta-2 containing nicotinic receptors are involved in nicotine reinforcement but not in withdrawal syndrome. Psychopharmacology (Berl) 2006;187:189–199. doi: 10.1007/s00213-006-0418-z. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM. alpha-Conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology. 2010;35:665–673. doi: 10.1038/npp.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci. 2010;11:389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Caille S, Cador M. The effects of response operandum and prior food training on intravenous nicotine self-administration in rats. Psychopharmacology (Berl) 2010;211:43–54. doi: 10.1007/s00213-010-1866-z. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Contet C, Whisler KN, Jarrell H, Kenny PJ, Markou A. Patterns of responding differentiate intravenous nicotine self-administration from responding for a visual stimulus in C57BL/6J mice. Psychopharmacology (Berl) 2010;212:283–299. doi: 10.1007/s00213-010-1950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA. A rodent model for nicotine self-administration. In: Boulton A, Baker G, Wu PH, editors. Animal models of drug addiction. Humana Press; New Jersey: 1992. pp. 315–344. [Google Scholar]
- Corrigall WA. Nicotine self-administration in animals as a dependence model. Nicotine Tob Res. 1999;1:11–20. doi: 10.1080/14622299050011121. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Pierucci M, Di Giovanni G, Benigno A, Esposito E. The neurobiological bases for the pharmacotherapy of nicotine addiction. Curr Pharm Des. 2007;13:1269–1284. doi: 10.2174/138161207780618920. [DOI] [PubMed] [Google Scholar]
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ. 1994;309:901–911. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl) 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Picciotto MR, Changeux JP, Pich EM. Assessment of nicotinic acetylcholine receptor subunit contributions to nicotine self-administration in mutant mice. Psychopharmacology (Berl) 1999;147:25–26. doi: 10.1007/s002130051135. [DOI] [PubMed] [Google Scholar]
- Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003;362:847–852. doi: 10.1016/S0140-6736(03)14338-3. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Arends MA, Kenny PJ. Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice. Behav Pharmacol. 2008;19:461–484. doi: 10.1097/FBP.0b013e32830c360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeote L, Berrendero F, Bura SA, Zimmer A, Maldonado R. Prodynorphin gene disruption increases the sensitivity to nicotine self-administration in mice. Int J Neuropsychopharmacol. 2009;12:615–625. doi: 10.1017/S1461145708009450. [DOI] [PubMed] [Google Scholar]
- Glatt AR, Denton K, Boughter JD., Jr Variation in nicotine consumption in inbred mice is not linked to orosensory ability. Chem Senses. 2009;34:27–35. doi: 10.1093/chemse/bjn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Imad Damaj M. Nicotine physical dependence in the mouse: involvement of the alpha7 nicotinic receptor subtype. Eur J Pharmacol. 2005;515:90–93. doi: 10.1016/j.ejphar.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Gray JA. The psychology of fear and stress. 2. Cambridge University Press; Cambridge: 1987. [Google Scholar]
- Henningfield JE, Goldberg SR. Nicotine as a reinforcer in human subjects and laboratory animals. Pharmacol Biochem Behav. 1983;19:989–992. doi: 10.1016/0091-3057(83)90405-7. [DOI] [PubMed] [Google Scholar]
- Hess GP, Ulrich H, Breitinger HG, Niu L, Gameiro AM, Grewer C, Srivastava S, Ippolito JE, Lee SM, Jayaraman V, Coombs SE. Mechanism-based discovery of ligands that counteract inhibition of the nicotinic acetylcholine receptor by cocaine and MK-801. Proc Natl Acad Sci U S A. 2000;97:13895–13900. doi: 10.1073/pnas.240459497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isiegas C, Mague SD, Blendy JA. Sex differences in response to nicotine in C57Bl/6:129SvEv mice. Nicotine Tob Res. 2009;11:851–858. doi: 10.1093/ntr/ntp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr, Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2009;34:266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Control of the reinforcing effects of nicotine by associated environmental stimuli in animals and humans. Trends Pharmacol Sci. 2005;26:287–293. doi: 10.1016/j.tips.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Wertheim C, Goldberg SR. High reinforcing efficacy of nicotine in non-human primates. PLoS ONE. 2007;2:e230. doi: 10.1371/journal.pone.0000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena C, Changeux JP. Allosteric nicotinic receptors, human pathologies. J Physiol Paris. 1998;92:63–74. doi: 10.1016/S0928-4257(98)80140-X. [DOI] [PubMed] [Google Scholar]
- Martellotta MC, Kuzmin A, Zvartau E, Cossu G, Gessa GL, Fratta W. Isradipine inhibits nicotine intravenous self-administration in drug-naive mice. Pharmacol Biochem Behav. 1995;52:271–274. doi: 10.1016/0091-3057(95)00096-f. [DOI] [PubMed] [Google Scholar]
- Martin-Garcia E, Barbano MF, Galeote L, Maldonado R. New operant model of nicotine-seeking behaviour in mice. Int J Neuropsychopharmacol. 2009;12:343–356. doi: 10.1017/S1461145708009279. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloez-Tayarani I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McMorrow MJ, Foxx RM. Nicotine’s role in smoking: an analysis of nicotine regulation. Psychol Bull. 1983;93:302–327. [PubMed] [Google Scholar]
- Meliska CJ, Bartke A, McGlacken G, Jensen RA. Ethanol, nicotine, amphetamine, and aspartame consumption and preferences in C57BL/6 and DBA/2 mice. Pharmacol Biochem Behav. 1995;50:619–626. doi: 10.1016/0091-3057(94)00354-8. [DOI] [PubMed] [Google Scholar]
- Metaxas A, Bailey A, Barbano MF, Galeote L, Maldonado R, Kitchen I. Differential region-specific regulation of alpha4beta2* nAChRs by self-administered and non-contingent nicotine in C57BL/6J mice. Addict Biol. 2010;15:464–79. doi: 10.1111/j.1369-1600.2010.00246.x. [DOI] [PubMed] [Google Scholar]
- Papke RL, Wecker L, Stitzel JA. Activation and inhibition of mouse muscle and neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2010;333:501–518. doi: 10.1124/jpet.109.164566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321:323–329. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A, Martin-Garcia E, de Lecea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J Neurosci. 2010;30:2300–2310. doi: 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T, Swedberg MD. Reinforcing effects of nicotinic compounds: intravenous self-administration in drug-naive mice. Pharmacol Biochem Behav. 1998;60:567–573. doi: 10.1016/s0091-3057(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Robinson SF, Marks MJ, Collins AC. Inbred mouse strains vary in oral self-selection of nicotine. Psychopharmacology (Berl) 1996;124:332–339. doi: 10.1007/BF02247438. [DOI] [PubMed] [Google Scholar]
- Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology (Berl) 1997;130:28–40. doi: 10.1007/s002130050209. [DOI] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology. 2008;33:739–748. doi: 10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- Singer G, Simpson F, Lang WJ. Schedule induced self injections of nicotine with recovered body weight. Pharmacol Biochem Behav. 1978;9:387–389. doi: 10.1016/0091-3057(78)90302-7. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Clarke PB. Rats self-administer intravenous nicotine delivered in a novel smoking-relevant procedure: effects of dopamine antagonists. J Pharmacol Exp Ther. 2009;330:633–640. doi: 10.1124/jpet.109.154641. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. discussion 14–20. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Naylor C, Elmer GI, Goldberg SR. Discrimination and self-administration of nicotine by inbred strains of mice. Psychopharmacology (Berl) 1999;141:297–306. doi: 10.1007/s002130050837. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- WHO. WHO Report on the Global Tobacco Epidemic, 2008: the MPOWER package. World Health Organization; Geneva: 2008. [Google Scholar]
- Woolverton WL, Nader MA. Experimental evaluation of the reinforcing effects of drugs. In: Adler MW, Cowan A, editors. Testing and evaluation of drugs of abuse, modern methods in pharmacology. Wiley-Liss; New York: 1990. pp. 165–192. [Google Scholar]