Abstract
Behavioral sensitization, or augmented locomotor response to successive drug exposures, results from neuroadaptive changes contributing to addiction. Both the medial prefrontal cortex (mPFC) and ventral tegmental area (VTA) influence behavioral sensitization and display increased immediate-early gene and BDNF expression after psychostimulant administration. Here we investigate whether mPFC neurons innervating the VTA exhibit altered Fos or BDNF expression during long-term sensitization to amphetamine. Male Sprague-Dawley rats underwent unilateral intra-VTA infusion of the retrograde tracer Fluorogold (FG), followed by 5 daily injections of either amphetamine (2.5 mg/kg, i.p.) or saline vehicle. Four weeks later, rats were challenged with amphetamine (1.0 mg/kg, i.p.) or saline (1.0 mL/kg, i.p.). Repeated amphetamine treatment produced locomotor sensitization upon drug challenge. Two hours later, rats were euthanized, and mPFC sections were double-immunolabeled for either Fos-FG or Fos-BDNF. Tissue from the VTA was also double-immunolabeled for Fos-BDNF. Amphetamine challenge increased Fos and BDNF expression in the mPFC regardless of prior drug experience, and further augmented mPFC BDNF expression in sensitized rats. Similarly, more Fos-FG and Fos-BDNF double-labeling was observed in the mPFC of sensitized rats compared to drug-naïve rats after amphetamine challenge. Repeated amphetamine treatment also increased VTA BDNF, while both acute and repeated amphetamine treatment increased Fos and Fos-BDNF co-labeling, an effect enhanced in sensitized rats. These findings point to a role of cortico-tegmental BDNF in long-term amphetamine sensitization.
Keywords: BDNF, ventral tegmental area, amphetamine, sensitization, prefrontal cortex, Fluorogold
1. INTRODUCTION
Repeated exposure to psychostimulants produces neural and behavioral adaptations that may contribute to addictive behavior. Among these is sensitization, defined as augmented drug response with successive drug exposures. Behaviorally, psychomotor sensitization manifests as progressively increased locomotor response and can persist for up to a year (Paulson et al., 1991), an effect likely related to enhanced dopamine release in the ventral striatum after repeated psychostimulant administration (Boileau et al., 2006; Robinson et al., 1988).
Substantial evidence points to the medial prefrontal cortex (mPFC) as a key region in regulating sensitization, as lesions or disruption of glutamate signaling here attenuate development of sensitization (Bjijou et al., 2002; Cador et al., 1999; Li et al., 1999; Tzschentke and Schmidt, 1999; Wolf et al., 1995). The mPFC sends glutamatergic projections to the ventral tegmental area (VTA; Carr and Sesack, 2000; Geisler et al., 2007; Sesack and Pickel, 1992), a region also critical for sensitization (Kalivas and Weber, 1988; Vezina, 1996). Receptor-mediated glutamate signaling in the VTA is particularly important for sensitization (Cador et al., 1999; Kalivas and Alesdatter, 1993; Kim and Vezina, 1998), and mPFC neurons innervating the VTA are proposed to be involved (Cador et al., 1999).
Psychostimulant administration activates neurons in the mPFC, which can be measured by increased mRNA or protein expression of the immediate early gene, c-fos. Both acute and repeated administration of psychostimulants enhance mPFC Fos expression (Morshedi and Meredith, 2007, 2008; Uslaner et al., 2001), and animals exposed to repeated treatment display augmented cortical Fos response which accompanies behavioral sensitization (Hedou et al., 2002). Moreover, neurons in the mPFC specifically innervating the VTA exhibit increased Fos expression after acute amphetamine administration (Colussi-Mas et al., 2007), underlining the potential involvement of the mPFC-VTA projection in drug-induced neuroadaptations. Characterizing activated neurons during expression of sensitized behavior may elucidate its neural mechanism.
Repeated psychostimulant exposure may affect sensitization by specifically enhancing neural plasticity in the mPFC-VTA projection. Indeed, repeated exposure to psychostimulants increases mPFC spine density and dendritic branching (Morshedi et al., 2009; Robinson and Kolb, 1999) and enhances excitability of midbrain dopamine neurons when the PFC is electrically stimulated (Tong et al., 1995). The neurotrophin brain-derived neurotrophic factor (BDNF) is well-poised to influence such neuroadaptations due to its role in regulating synaptic plasticity (Horch et al., 1999; Kang and Schuman, 1995; Lessmann et al., 1994) and its expression in both the VTA and mPFC (Conner et al., 1997; Seroogy et al., 1994). Acute exposure to psychostimulants increases mPFC BDNF mRNA (Fumagalli et al., 2007; Le Foll et al., 2005; Meredith et al., 2002), an effect augmented by repeated treatment (Fumagalli et al., 2007). BDNF receptor-mediated signaling in the VTA may also be necessary for sensitization (Pu et al., 2006). These data suggest that BDNF activity within the mPFC-VTA projection may influence psychostimulant-related neuroadaptations.
In this study, we characterized neurons activated by amphetamine following expression of behavioral sensitization. We examined whether amphetamine treatment induced differential Fos and BDNF expression, and Fos-BDNF co-expression, in mPFC and VTA neurons in either drug-sensitized or drug-naïve rats. We also utilized the retrograde tracer Fluorogold to determine whether amphetamine treatment induced functional activation of mPFC neurons innervating the VTA.
2. METHODS
2.1 Animals
Subjects were male Sprague-Dawley rats (Charles River Laboratories; Hollister, CA) weighing 225–250 g upon arrival. Rats were triple-housed under a 12-h reverse light/dark cycle (lights off at 9:00 h) with ad libitum access to food (Purina Rodent Diet; Brentwood, MO) and water. Rats were allowed one week to acclimate before surgery. All experimental procedures were approved by the University of Arizona and Arizona State University Institutional Animal Care and Use Committees, and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). All efforts were made to minimize suffering and to limit the number of animals used.
2.2 Surgery
Rats were anesthetized with isoflurane gas (2–4%) and placed into a stereotaxic frame (Leica Angle Two; Richmond, IL). Fluorogold (FG, 4% in 0.1 M sodium cacodylate buffer; Fluorochrome; Denver, CO) was unilaterally infused into the VTA with a glass micropipette (20 μm tip) at a 10° angle using the following stereotaxic coordinates: AP: −5.1 mm; ML: −0.6 mm; DV: −8.8 mm (Paxinos and Watson, 2007). Infusion was performed by iontophoresis at a +6.0 μA current alternating every 7 s for 10 min. Rats were allowed one week to recover before experimental procedures.
2.3 Experimental design
Rats were randomly assigned to three groups (n = 8 each). One group received daily injections of d-amphetamine sulfate (2.5 mg/kg, i.p.; Sigma-Aldrich; St. Louis, MO) for 5 consecutive days, while the other two groups received daily saline vehicle injections (1.0 mL/kg). All injections prior to the challenge day were administered in the home cage environment. After the fifth day, injections ceased, and rats remained in their home cages for 28 days where they were handled twice weekly. On day 28, the group receiving daily amphetamine treatment was challenged with amphetamine (1.0 mg/kg, i.p.; REP group), and the daily saline groups received either an amphetamine challenge (1.0 mg/kg, i.p.; ACU group) or a saline vehicle challenge (1.0 mL/kg, i.p.; SAL group).
2.4 Locomotor tracking
On challenge day, injections were given in a separate testing room in a chamber resembling a home cage containing clean bedding where locomotor activity was tracked. Rats habituated to the testing room, testing chamber, and injection procedure for 2 days prior to the amphetamine or saline challenge day. Locomotor activity was measured using Videotrack (Viewpoint Life Sciences; Montreal, Canada), which determines a centroid point for the subject shape, and permits differentiation between small, stereotyped movements (operationally defined as centroid movements a distance of 4–10 cm), and larger ambulatory movements (defined as movements greater than 10 cm). The software calculated the number of each type of movement and total distance traveled. Rats habituated for 30 min in the testing chambers before any injections, and all rats received a saline injection prior to their drug challenge to assess baseline locomotion. Locomotor activity was recorded both 30 min before and 60 min after baseline saline injections, then for 80 min after challenge with amphetamine or saline.
2.5 Perfusion and Tissue Preparation
Two hours after amphetamine or saline challenge, animals were deeply anesthetized with an overdose of sodium pentobarbital (100 mg/kg) and perfused transcardially with 10 mL of 10% heparin in 0.1 M phosphate buffered saline (pH 7.4) followed by 200 mL of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were removed, post-fixed for 1.5 h in the same fixative at 4°C, and then placed in graded concentrations of sucrose in 0.1 M phosphate buffer at 4°C. Coronal sections (20 μm) were taken on a sliding microtome from +3.2 to +2.8 mm from bregma for the mPFC, and −5.0 to −5.4 mm from bregma for the VTA (Paxinos and Watson, 2007). Sections were collected in 0.1 M phosphate buffer (pH 7.4 at 4°C), mounted onto glass slides (Superfrost Plus; Fisher; Waltham, MA), dried, and stored at −35°C until the time of processing.
2.6 Fos-BDNF and Fos-FG double-label immunohistochemistry
Sections were washed in 0.05 M potassium phosphate-buffered saline (KPBS), then blocked for 1 h in 10% normal goat serum, 0.4% Triton X-100 in 0.05 M KPBS. Sections were incubated with polyclonal antisera for Fos (Ab-5, 1:10,000 dilution; EMD Biosciences; San Diego, CA) for 48 hours at 4°C, then washed again in KPBS and incubated for 1 h in biotin-conjugated goat anti-rabbit serum (1:40 dilution in normal goat serum solution, Vectastain ABC kit; Vector Laboratories; Burlingame, CA) at room temperature. After washing with KPBS, sections were incubated with an avidin-biotin-peroxidase complex for 45 min (ABC kit, Vector Laboratories), then washed again in KPBS and processed using the nickel-intensified DAB peroxidase substrate kit (Vector Laboratories). Sections were then washed in 0.001M biotin (Sigma-Aldrich), and incubated with anti-BDNF polyclonal antibody (AB1779SP, 1:3,000 dilution; Millipore; Temecula, CA) or anti-FG polyclonal antibody (AB153, 1:10,000 dilution; Millipore) for 48 hours at 4°C. Following this, sections were again processed with the ABC kit as described above, and counterstaining was achieved using the VIP peroxidase substrate kit (Vector Laboratories). After dehydration, coverslips were applied.
2.7 Image analysis
Tissue sections were examined for the presence of chromogen reaction product using a Zeiss Axioskop microscope with a 20× objective. Selected areas were captured and digitized using a color digital video camera (MBF Biosciences; Williston, VT) interfaced to the microscope. The number of Fos- and BDNF-immunolabeled profiles, as well as Fos-BDNF double-immunolabeled profiles, was counted bilaterally in the VTA. Likewise, the number of Fos-, BDNF-, and FG-immunolabeled profiles, as well as Fos-BDNF or Fos-FG double-immunolabeled profiles, was counted in the anterior cingulate (ACG), prelimbic (PRL), and infralimbic (IL) regions of the mPFC ipsilateral to the FG injection. Fos-labeled profiles were identified by a dark blue-gray stained nucleus, whereas FG- or BDNF-labeling exhibited a purple cytoplasmic stain. A profile was considered labeled if its pixel intensity was more than 2 standard deviations darker than the background, as calculated by Stereo Investigator software (MBF Biosciences). Neuronal labeling was quantified using a systematic random approach to achieve unbiased counts. Stereo Investigator software partitioned each image into 16 equal counting frames (100 X 75 μm each), half of which were randomly selected and analyzed. The number of single- and double-labeled neurons was counted separately for each frame, excluding any overlapping labeled profiles on the left and bottom borders. At least three adjacent sections were analyzed per animal for each brain region, and labeling density was calculated by dividing the estimated total number of labeled profiles by the total area analyzed.
2.8 Statistics
Two-way mixed ANOVA (time as within-subjects factor and drug condition as between-subjects factor) was used to compare locomotor activity across groups, and each of the 3 time frames (before and after saline injection, and during drug challenge) was treated with a separate two-way ANOVA. Statistics were performed for the 40 min time frame after the drug challenge to correspond with peak drug response time. When necessary, Tukey’s HSD post-hoc analysis was conducted to determine significant treatment effects. Immunohistochemical data were analyzed using one-way ANOVA with Tukey’s HSD post-hoc test. The results were considered significant if p ≤ 0.05. All data are expressed as mean ± SEM.
3. RESULTS
3.1 Locomotor sensitization
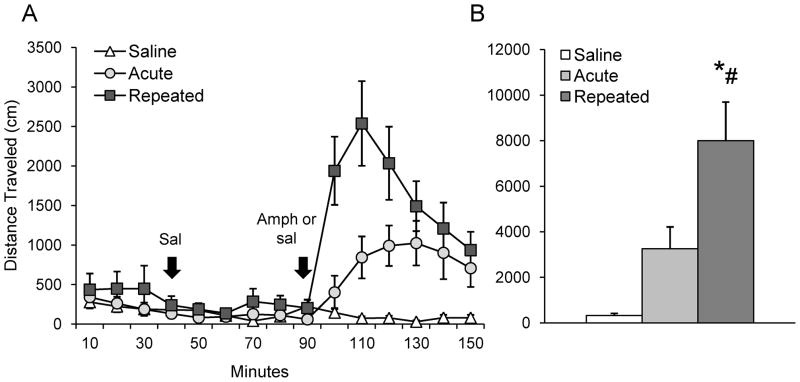
Amphetamine increased activity regardless of drug history (number of stereotyped movements, F2,21 = 29.18, p ≤ 0.05; number of ambulations, F2,21 = 15.54, p ≤ 0.05, Table 1), with repeated amphetamine treatment producing significantly greater locomotion than an acute dose (distance traveled, F2,21 = 11.90, p ≤ 0.05, Fig. 1A, B). There was a main effect of time across groups after amphetamine challenge (F3,63 = 6.18, p ≤ 0.05; Fig. 1A, B). No differences in locomotor activity were observed between treatment groups before or after baseline saline injections (Fig. 1A, B). Finally, a main effect of decreasing movement over time was observed across groups before baseline saline injections (stereotyped movements, F2, 42 = 6.1, p ≤ 0.05; number of ambulations, F2,42 = 5.7, p ≤ 0.05, data not shown), and on stereotyped movements after baseline saline injections (F5, 105 = 3.07, p ≤ 0.05, data not shown). This was likely due to locomotor habituation over time.
Table 1.
Number of stereotyped movements and ambulations on drug challenge day for SAL, ACU, and REP groups. Baseline, Baseline saline, and Amph columns reflect 30, 60, and 40 min. of tracking, respectively
| Number of stereotyped movements: | |||
|---|---|---|---|
| Baseline | Baseline saline | Amph | |
| SAL | 848.6 ± 132.3 | 1239.4 ± 160.1 | 679.5 ± 105.7 |
| ACU | 983.8 ± 134.7 | 1098.5 ± 139.4 | 2185.3* ± 283.9 |
| REP | 1131.0 ± 201.9 | 1522.8 ± 299.2 | 2771.3* ± 167.2 |
| Number of ambulations: | |||
| Baseline | Baseline saline | Amph | |
| SAL | 227.3 ± 61.8 | 280.5 ± 61.0 | 121.1 ± 29.5 |
| ACU | 284.1 ± 75.3 | 228.4 ± 60.1 | 779.3* ± 188.9 |
| REP | 358.1 ± 121.9 | 369.4 ± 120.3 | 1235.5* ± 155.0 |
p ≤ 0.05 vs. SAL
Figure 1. Locomotor activity during drug challenge.
A – Distance traveled over time before and after baseline saline injection, and following a challenge injection of amphetamine (1.0 mg/kg, i.p.) or saline. Injections indicated by arrows. B – Total distance traveled over first 40 min after drug or saline challenge; *p < 0.05 vs. saline group; #p < 0.05 vs. acute group.
3.2 Fos-BDNF Immunolabeling in mPFC
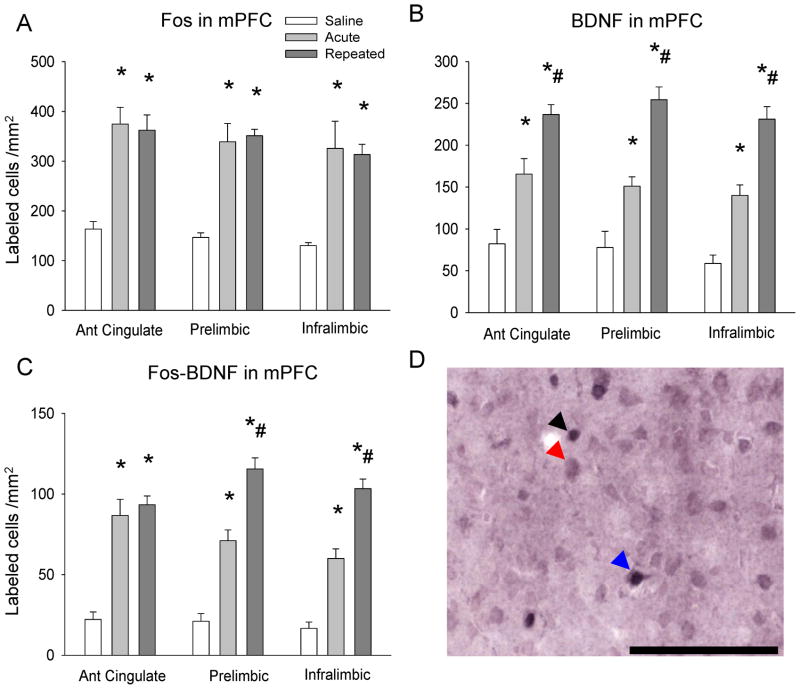
Amphetamine increased Fos labeling in both the ACU and REP groups (ACG, F2,12 = 18.27 p ≤ 0.05; PRL, F2,12 = 24.68, p ≤ 0.05; IL, F2,12 = 10.5, p ≤ 0.05), and no differences were observed between the ACU and REP groups (Fig. 2A). Amphetamine challenge also increased mPFC BDNF immunolabeling in all three subregions in both the ACU and REP groups (ACG, F2,12 = 23.06, p ≤ 0.05; PRL, F 2,12 = 32.48, p ≤ 0.05; IL, F2,12 = 45.85, p ≤ 0.05), with significantly more mPFC BDNF labeling in the REP group than in the ACU group (p ≤ 0.05; Fig. 2B). Similarly, Fos-BDNF double-labeling was enhanced by amphetamine in both ACU and REP groups (ACG, F2,12 = 30.62, p ≤ 0.05; PRL, F2,12 = 58.39, p ≤ 0.05; IL, F2,12 = 65.15, p ≤ 0.05), and significantly greater in the REP group than the ACU group in the PRL and IL (p ≤ 0.05; Fig. 2C). Finally, the percentage of Fos-labeled mPFC neurons with BDNF co-labeling was significantly greater than the SAL group only after repeated amphetamine challenge in all sub-regions examined (ACG, F2,12 = 6.52, p ≤ 0.05; PRL F2,12 = 17.57, p ≤ 0.05; IL, F2,12 = 24.41, p ≤ 0.05), and greater in comparison with the ACU group in the PRL and IL (p ≤ 0.05; Table 2).
Figure 2. Fos and Fos-BDNF immunolabeling in mPFC subregions after drug challenge.
A – Fos immunolabeling in mPFC after drug challenge; B – BDNF immunolabeling in mPFC after drug challenge; C – Fos-BDNF double-immunolabeling in mPFC after drug challenge; *p ≤ 0.05 vs. saline group; #p ≤ 0.05 vs. acute group; D – Representative photomicrograph of Fos and BDNF single and double-immunolabeling in mPFC. Black arrow indicates Fos immunolabeled cell; red arrow indicates BDNF immunolabeled cell; blue arrow indicates Fos-BDNF double-immunolabeled cell. Scale bar 100 μm.
Table 2.
Percentages of Fos-labeled neurons co-labeled for BDNF, and FG-labeled neurons co-labeled for Fos in SAL, ACU, and REP groups.
| Percent of Fos-labeled neurons with BDNF labeling | ||||
|---|---|---|---|---|
| ACG | PRL | IL | VTA | |
| SAL | 13.6 ± 3.2 | 14.3 ± 3.0 | 12.8 ± 3.2 | 2.4 ± 2.4 |
| ACU | 23.1 ± 1.8 | 21.3 ± 1.4 | 19.3 ± 1.3 | 14.7* ± 1.7 |
| REP | 26.5* ± 2.7 | 33.2*# ± 2.2 | 33.3*# ± 1.3 | 37.8*# ± 2.0 |
| Percent of FG-labeled neurons with Fos labeling | ||||
| ACG | PRL | IL | ||
| SAL | 24.1 ± 4.0 | 22.5 ± 5.0 | 18.6 ± 3.4 | |
| ACU | 48.5* ± 6.3 | 39.5* ± 2.8 | 39.3* ± 6.3 | |
| REP | 62.6* ± 4.7 | 49.2* ± 4.5 | 52.3* ± 5.0 | |
p ≤ 0.05 vs. SAL;
p ≤ 0.05 vs. ACU.
3.3 FG injection sites
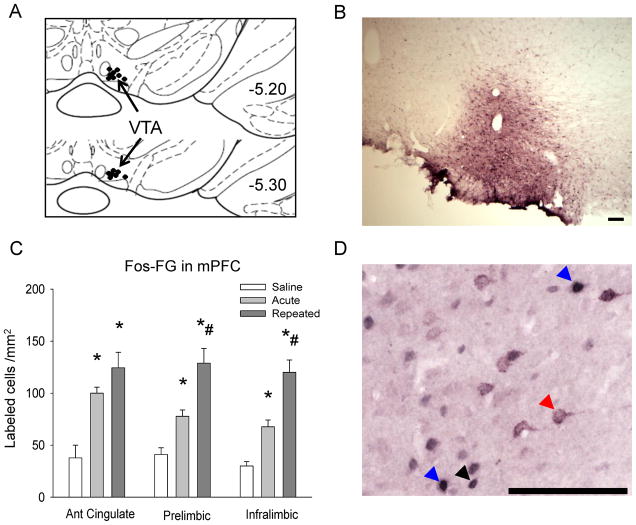
The presence of FG in the VTA was confirmed by visualization of fluorescence at the injection site and antigen labeling in the VTA and mPFC after immunohistochemistry (Fig. 3A, B). Only subjects with a FG injection centralized in the VTA were included in the analysis (n = 4–5 per group).
Figure 3. Fos and Fos-FG immunolabeling in mPFC subregions after drug challenge.
A – Schematic representation of FG microinjection sites in VTA, adapted from Paxinos and Watson (2007). Approximate injection location indicated by black dots; B – Representative photomicrograph of FG immunolabeling at injection site in VTA. Scale bar 100 μm; C – Fos-FG double-immunolabeling in mPFC after drug challenge; *p ≤ 0.05 vs. saline group; #p ≤ 0.05 vs. acute group; D – Representative photomicrograph of Fos and FG single and double-immunolabeling in mPFC. Black arrow indicates Fos immunolabeled cell; red arrow indicates FG immunolabeled cell; blue arrow indicates Fos-FG double immunolabeled cell. Scale bar 100 μm.
3.4 Fos-FG Immunolabeling in mPFC
Fos immunolabeling in the mPFC was similar to that observed in Fos-BDNF double-labeled tissue; amphetamine challenge increased Fos immunolabeling in all mPFC subregions examined in both the ACU and REP groups (ACG, F2,12 = 30.41, p ≤ 0.05; PRL, F2,12 = 10.65, p ≤ 0.05; IL, F2,12 = 21.28, p ≤ 0.05), although no differences in Fos labeling were observed between the ACU and REP groups (data not shown). Amphetamine also increased Fos-FG double-labeling in both the ACU and REP groups (ACG, F2,12 = 14.69, p ≤ 0.05; PRL, F2,12 = 21.06, p ≤ 0.05; IL, F2,12 = 30.26, p ≤ 0.05), with the REP group showing significantly more Fos-FG double-labeling than the ACU group in the PRL and IL (p ≤ 0.05), but not the ACG (p = 0.33; Fig. 3C). Total FG labeling was not statistically different across the three cortical subregions (data not shown), and the percentage of FG-labeled neurons expressing Fos was higher after amphetamine challenge regardless of drug history in all regions examined (ACG, F2,12 = 14.62, p ≤ 0.05; PRL F2,12 = 10.39, p ≤ 0.05; IL, F2,12 = 11.38, p ≤ 0.05; Table 2).
3.5 Fos-BDNF Immunolabeling in VTA
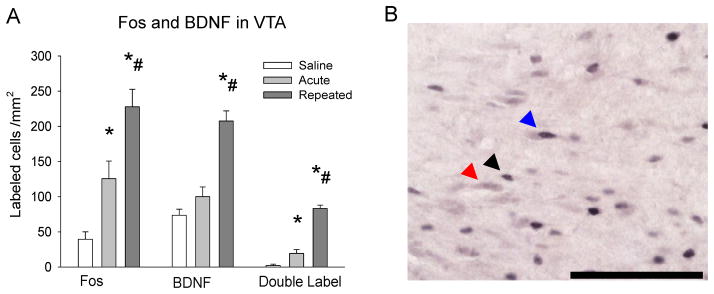
In the VTA, amphetamine challenge increased Fos labeling in both the ACU and REP groups (F2, 9 = 19.58, p ≤ 0.05), with significantly more labeling in the REP group (p ≤ 0.05; Fig. 4A). BDNF labeling, however, was enhanced only after repeated amphetamine (F2, 9 = 32.15, p ≤ 0.05; Fig. 4A). Interestingly, amphetamine challenge increased Fos-BDNF double-labeling regardless of drug history (F2, 9 = 98.80, p ≤ 0.05), but was more prominent in the REP group (p ≤ 0.05; Fig. 4A). In addition, the percentage of Fos-labeled neurons with BDNF co-labeling was significantly greater in ACU and REP groups (F2, 9 = 78.39, p ≤ 0.05), and further enhanced in the REP group (p ≤ 0.05; Table 2).
Figure 4. Fos and Fos-BDNF immunolabeling in VTA after drug challenge.
A – Fos and BDNF single-immunolabeling, and Fos-BDNF double-immunolabeling in VTA after drug challenge; *p ≤ 0.05 vs. saline group; #p ≤ 0.05 vs. acute group; B – Representative photomicrograph of Fos and double-immunolabeling in VTA. Black arrow indicates Fos immunolabeled cell; red arrow indicates BDNF immunolabeled cell; blue arrow indicates Fos-BDNF double-immunolabeled cell. Scale bar 100 μm.
4. DISCUSSION
Acute and sensitizing regimens of amphetamine differentially induced Fos and BDNF expression in the mPFC and VTA. Specifically, repeated amphetamine enhanced BDNF in both regions and activated mPFC neurons projecting to the VTA. These molecular alterations were accompanied by persisting behavioral sensitization, a phenomenon thought to contribute to addiction (Robinson and Berridge, 1993; Segal, 1975; Shuster et al., 1977).
In the mPFC, repeated amphetamine sensitized BDNF response in ACG, PRL, and IL cortices above those values observed for acute amphetamine, similar to what has been reported for cocaine (Fumagalli et al., 2007). Repeated amphetamine also sensitized Fos-BDNF co-labeling in PRL and IL cells correspondingly activated after repeated amphetamine. Thus, BDNF-containing mPFC cells are more likely to be activated after repeated drug exposure. Given BDNF’s well-established role in synaptic plasticity (Lessmann et al., 1994), our results suggest that repeated amphetamine exposure induces long-term neuroadaptations in the mPFC during sensitization.
Amphetamine also increased Fos immunolabeling in mPFC-VTA projection, an effect augmented by repeated administration. Interestingly, rats undergoing short-term behavioral sensitization (3 days) display sensitized overall mPFC Fos response when compared to animals receiving drug acutely (Hedou et al., 2002). However, our results indicate mPFC Fos response to amphetamine is similar regardless of drug history. This may be explained by the finding that repeated amphetamine increases firing in not all, but only a small subpopulation of mPFC neurons which remain highly active during expression of behavioral sensitization. Acute amphetamine enhances Fos expression specifically in mPFC neurons innervating the VTA (Colussi-Mas et al., 2007; Gulley and Stanis, 2010), and we showed that repeated amphetamine sensitized Fos expression in neurons in this pathway. This suggests that prolonged enhancement of neuronal responsiveness after repeated amphetamine occurs specifically in the mPFC-VTA projection, whereas acute administration results in more general mPFC neuronal activation.
It is unknown from our findings whether repeated amphetamine induces Fos in BDNF-containing mPFC-VTA pathway neurons. However, BDNF is expressed only in PFC pyramidal neurons (Huntley et al., 1992), some of which innervate the VTA (Carr and Sesack, 2000; Sesack and Pickel, 1992). We also found similar patterns of Fos-BDNF and Fos-FG labeling after amphetamine challenge, with comparable ACG co-labeling after acute or repeated amphetamine, and sensitized co-labeling in the PRL and IL only after repeated amphetamine. The PRL and IL innervate the VTA more densely than does the ACG (Geisler and Zahm, 2005; Phillipson, 1979), but also innervate the basolateral amygdala (Sesack et al., 1989) and nucleus accumbens (NAc; Berendse et al., 1992). However, repeated amphetamine does not enhance Fos labeling in mPFC-NAc or mPFC-basolateral amygdala neurons (Morshedi and Meredith, 2008), indicating that they comprise different populations than mPFC neurons containing BDNF. It is possible that that some BDNF-containing mPFC neurons activated by repeated amphetamine originate in the lateral hypothalamus, as Fos expression in mPFC neurons innervating this region are sensitized by amphetamine (Morshedi and Meredith, 2008). However, the VTA contains substantially more BDNF-containing fibers than the lateral hypothalamus (Conner et al., 1997), and after amphetamine administration, more Fos-activated neurons innervating the VTA originate from the PFC than any other region (Colussi-Mas et al., 2007). As such, it is likely that after repeated amphetamine, BDNF-containing mPFC neurons preferentially, but not exclusively, innervate the VTA.
Because BDNF is only present in prefrontal pyramidal neurons during adulthood (Huntley et al., 1992), we infer that the observed increase in mPFC BDNF after amphetamine occurred exclusively in glutamate-containing projection neurons, and not GABA-containing interneurons. Consistent with this, the VTA receives more glutamatergic innervation from the PFC than any other region (Geisler et al., 2007). Sustained amphetamine-induced changes in BDNF-containing pyramidal neurons of the mPFC-VTA pathway may be functionally relevant given the significant role of glutamate in drug-related behaviors (Kalivas and Alesdatter, 1993; Schenk and Partridge, 1997). Behavioral sensitization is regulated by VTA glutamate (Kalivas and Alesdatter, 1993; Kim and Vezina, 1998), which might be specifically influenced by mPFC-VTA glutamate (Cador et al., 1999). Glutamate-mediated neuroadaptations are augmented by BDNF, which facilitates glutamate release (Carmignoto et al., 1997) and stimulates expression and trafficking of NMDA receptors (Caldeira et al., 2007). Anterograde transport of BDNF (Altar et al., 1997) through the mPFC-VTA projection could affect sensitization via altering VTA glutamatergic tone. An example of this is the necessity of VTA BDNF for glutamate-dependent synaptic plasticity in midbrain dopamine neurons during cocaine withdrawal (Pu et al., 2006), and BDNF-mediated facilitation of behavioral sensitization upon infusion into the VTA (Horger et al., 1999). Facilitated sensitization through glutamatergic mechanisms has been proposed for other growth factors as well (Flores and Stewart, 2000). Acute and repeated cocaine enhance fibroblast growth factor (FGF)-2 mRNA in the midbrain and PFC, lasting up to 3 days in PFC after repeated administration (Fumagalli et al., 2006), and NMDA receptor blockade attenuates amphetamine-induced increase in VTA FGF in the midbrain, which is necessary for developing behavioral sensitization (Flores et al., 2000). Altogether, these findings suggest that drug-induced alterations of neurotrophic factors may influence psychomotor sensitization by interactions with VTA glutamate transmission.
Amphetamine increased VTA Fos and Fos-BDNF co-expression, an effect augmented by prior drug experience, and enhanced VTA BDNF only in sensitized rats. Similar sensitized VTA Fos activity is observed after repeated social defeat stress, which activates mesocorticolimbic structures and sensitizes rats to amphetamine (Covington and Miczek, 2001; Nikulina et al., 2004). Likewise, both cocaine self-administration and repeated social defeat stress also chronically increase VTA BDNF (Fanous et al., 2010; Grimm et al., 2003). Enhanced Fos activity in BDNF-expressing VTA neurons of sensitized animals may be important for sustained molecular adaptations, as signaling cascades downstream from BDNF alter dendritic morphology in the VTA-NAc projection (Russo et al., 2007; Sklair-Tavron et al., 1996). Such structural adaptations could influence drug-induced behavioral changes given the putative role of VTA BDNF in molecular and behavioral sensitization (Horger et al., 1999; Pu et al., 2006) and its proposed role in addiction (Bolanos and Nestler, 2004).
Here we show that repeated amphetamine produces long-term sensitization of drug responsiveness in mPFC neurons containing BDNF or innervating VTA, accompanied by behavioral sensitization. These neurons are likely glutamatergic and comprise, at least in part, the same population. We also show sensitized activation of VTA BDNF neurons after repeated amphetamine. These findings may be important for characterizing neurons in the mPFC-VTA pathway involved in neuroadaptations relevant to drug-related behavior.
Acknowledgments
This study was supported by NIH grants F31DA022830 (SF), DA024817 (EMN), DA026451 (EMN) and MH073930 (RPH).
Abbreviations
- ACG
anterior cingulate cortex
- ACU
acute amphetamine group
- ANOVA
analysis of variance
- AP
anterior-posterior
- BDNF
brain-derived neurotrophic factor
- DV
dorsal-ventral
- FG
Fluorogold
- FGF
fibroblast growth factor
- IL
infralimbic cortex
- KPBS
potassium phosphate-buffered saline
- ML
medial-lateral
- mPFC
medial prefrontal cortex
- PRL
prelimbic cortex
- REP
repeated amphetamine group
- SAL
saline group
- VTA
ventral tegmental area
Footnotes
Research Highlights
> Psychostimulants increase BDNF expression in the medial prefrontal cortex (mPFC)and ventral tegmental area (VTA). > Repeated amphetamine sensitized Fos and BDNFexpression in the mPFC and VTA. > Repeated amphetamine also sensitized Fos expression inmPFC-VTA neurons. > Thus, BDNF in the mPFC-VTA projection may influence chronicstimulant sensitization.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Letters to Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Bjijou Y, De Deurwaerdere P, Spampinato U, Stinus L, Cador M. D-amphetamine-induced behavioral sensitization: effect of lesioning dopaminergic terminals in the medial prefrontal cortex, the amygdala and the entorhinal cortex. Neuroscience. 2002;109:499–516. doi: 10.1016/s0306-4522(01)00508-5. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- Cador M, Bjijou Y, Cailhol S, Stinus L. D-amphetamine-induced behavioral sensitization: implication of a glutamatergic medial prefrontal cortex-ventral tegmental area innervation. Neuroscience. 1999;94:705–721. doi: 10.1016/s0306-4522(99)00361-9. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2007;35:208–219. doi: 10.1016/j.mcn.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Carmignoto G, Pizzorusso T, Tia S, Vicini S. Brain-derived neurotrophic factor and nerve growth factor potentiate excitatory synaptic transmission in the rat visual cortex. J Physiol. 1997;498 ( Pt 1):153–164. doi: 10.1113/jphysiol.1997.sp021848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colussi-Mas J, Geisler S, Zimmer L, Zahm DS, Berod A. Activation of afferents to the ventral tegmental area in response to acute amphetamine: a double-labelling study. Eur J Neurosci. 2007;26:1011–1025. doi: 10.1111/j.1460-9568.2007.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Fanous S, Hammer RP, Jr, Nikulina EM. Short- and long-term effects of intermittent social defeat stress on brain-derived neurotrophic factor expression in mesocorticolimbic brain regions. Neuroscience. 2010;167:598–607. doi: 10.1016/j.neuroscience.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C, Samaha AN, Stewart J. Requirement of endogenous basic fibroblast growth factor for sensitization to amphetamine. J Neurosci. 2000;20:RC55. doi: 10.1523/JNEUROSCI.20-02-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C, Stewart J. Basic fibroblast growth factor as a mediator of the effects of glutamate in the development of long-lasting sensitization to stimulant drugs: studies in the rat. Psychopharmacology (Berl) 2000;151:152–165. doi: 10.1007/s002130000417. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Di Pasquale L, Caffino L, Racagni G, Riva MA. Repeated exposure to cocaine differently modulates BDNF mRNA and protein levels in rat striatum and prefrontal cortex. Eur J Neurosci. 2007;26:2756–2763. doi: 10.1111/j.1460-9568.2007.05918.x. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Pasquale L, Racagni G, Riva MA. Dynamic regulation of fibroblast growth factor 2 (FGF-2) gene expression in the rat brain following single and repeated cocaine administration. J Neurochem. 2006;96:996–1004. doi: 10.1111/j.1471-4159.2005.03627.x. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley JM, Stanis JJ. Adaptations in medial prefrontal cortex function associated with amphetamine-induced behavioral sensitization. Neuroscience. 2010;166:615–624. doi: 10.1016/j.neuroscience.2009.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedou G, Jongen-Relo AL, Murphy CA, Heidbreder CA, Feldon J. Sensitized Fos expression in subterritories of the rat medial prefrontal cortex and nucleus accumbens following amphetamine sensitization as revealed by stereology. Brain Res. 2002;950:165–179. doi: 10.1016/s0006-8993(02)03034-2. [DOI] [PubMed] [Google Scholar]
- Horch H, Kruttgen A, Portbury S, Katz L. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23:353–364. doi: 10.1016/s0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley GW, Benson DL, Jones EG, Isackson PJ. Developmental expression of brain derived neurotrophic factor mRNA by neurons of fetal and adult monkey prefrontal cortex. Brain Res Dev Brain Res. 1992;70:53–63. doi: 10.1016/0165-3806(92)90103-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Alesdatter JE. Involvement of N-methyl-D-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;267:486–495. [PubMed] [Google Scholar]
- Kalivas PW, Weber B. Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther. 1988;245:1095–1102. [PubMed] [Google Scholar]
- Kang HJ, Schuman EM. Neurotrophin-induced modulation of synaptic transmission in the adult hippocampus. J Physiol. 1995;89:11–22. doi: 10.1016/0928-4257(96)80547-x. [DOI] [PubMed] [Google Scholar]
- Kim JH, Vezina P. Metabotropic glutamate receptors are necessary for sensitization by amphetamine. Neuroreport. 1998;9:403–406. doi: 10.1097/00001756-199802160-00008. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. A single cocaine exposure increases BDNF and D3 receptor expression: implications for drug-conditioning. Neuroreport. 2005;16:175–178. doi: 10.1097/00001756-200502080-00022. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Heumann R. BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurones. Neuro Report. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu XT, Berney TG, Vartanian AJ, Stine CD, Wolf ME, White FJ. Both glutamate receptor antagonists and prefrontal cortex lesions prevent induction of cocaine sensitization and associated neuroadaptations. Synapse. 1999;34:169–180. doi: 10.1002/(SICI)1098-2396(19991201)34:3<169::AID-SYN1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Callen S, Scheuer DA. Brain-derived neurotrophic factor expression is increased in the rat amygdala, piriform cortex and hypothalamus following repeated amphetamine administration. Brain Res. 2002;949:218–227. doi: 10.1016/s0006-8993(02)03160-8. [DOI] [PubMed] [Google Scholar]
- Morshedi MM, Meredith GE. Differential laminar effects of amphetamine on prefrontal parvalbumin interneurons. Neuroscience. 2007;149:617–624. doi: 10.1016/j.neuroscience.2007.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedi MM, Meredith GE. Repeated amphetamine administration induces Fos in prefrontal cortical neurons that project to the lateral hypothalamus but not the nucleus accumbens or basolateral amygdala. Psychopharmacology (Berl) 2008;197:179–189. doi: 10.1007/s00213-007-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedi MM, Rademacher DJ, Meredith GE. Increased synapses in the medial prefrontal cortex are associated with repeated amphetamine administration. Synapse. 2009;63:126–135. doi: 10.1002/syn.20591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, 3rd, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos A, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier; Amsterdam: 2007. [Google Scholar]
- Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Pu L, Liu QS, Poo MM. BDNF-dependent synaptic sensitization in midbrain dopamine neurons after cocaine withdrawal. Nat Neurosci. 2006;9:605–607. doi: 10.1038/nn1687. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Jurson PA, Bennett JA, Bentgen KM. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: a microdialysis study in freely moving rats. Brain Res. 1988;462:211–222. doi: 10.1016/0006-8993(88)90549-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE, Wiley MD, Self DW, Russell DS, Neve RL, Eisch AJ, Nestler EJ. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Sensitization and tolerance in psychostimulant self-administration. Pharmacol Biochem Behav. 1997;57:543–550. doi: 10.1016/s0091-3057(96)00447-9. [DOI] [PubMed] [Google Scholar]
- Segal DS. Behavioral and neurochemical correlates of repeated d-amphetamine administration. Adv Biochem Psychopharmacol. 1975;13:247–262. [PubMed] [Google Scholar]
- Seroogy KB, Lundgren KH, Tran TM, Guthrie KM, Isackson PJ, Gall CM. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol. 1994;342:321–334. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- Sesack S, Deutch A, Roth R, Bunney B. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Shuster L, Yu G, Bates A. Sensitization to cocaine stimulation in mice. Psychopharmacology (Berl) 1977;52:185–190. doi: 10.1007/BF00439108. [DOI] [PubMed] [Google Scholar]
- Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci U S A. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Chronic administration of (+)-amphetamine alters the reactivity of midbrain dopaminergic neurons to prefrontal cortex stimulation in the rat. Brain Res. 1995;674:63–74. doi: 10.1016/0006-8993(94)01439-o. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Functional heterogeneity of the rat medial prefrontal cortex: effects of discrete subarea-specific lesions on drug-induced conditioned place preference and behavioural sensitization. Eur J Neurosci. 1999;11:4099–4109. doi: 10.1046/j.1460-9568.1999.00834.x. [DOI] [PubMed] [Google Scholar]
- Uslaner J, Badiani A, Day HE, Watson SJ, Akil H, Robinson TE. Environmental context modulates the ability of cocaine and amphetamine to induce c-fos mRNA expression in the neocortex, caudate nucleus, and nucleus accumbens. Brain Res. 2001;920:106–116. doi: 10.1016/s0006-8993(01)03040-2. [DOI] [PubMed] [Google Scholar]
- Vezina P. D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. J Neurosci. 1996;16:2411–2420. doi: 10.1523/JNEUROSCI.16-07-02411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Dahlin SL, Hu XT, Xue CJ, White K. Effects of lesions of prefrontal cortex, amygdala, or fornix on behavioral sensitization to amphetamine: comparison with N-methyl-D-aspartate antagonists. Neuroscience. 1995;69:417–439. doi: 10.1016/0306-4522(95)00248-h. [DOI] [PubMed] [Google Scholar]