Abstract
Mango is characterized by high tocopherol and carotenoid content during ripening. From a cDNA screen of differentially expressing genes during mango ripening, a full-length p-hydroxyphenylpyruvate dioxygenase (MiHPPD) gene homologue was isolated that encodes a key enzyme in the biosynthesis of tocopherols. The gene encoded a 432-amino-acid protein. Transcript analysis during different stages of ripening revealed that the gene is ripening related and rapidly induced by ethylene. The increase in MiHPPD transcript accumulation was followed by an increase in tocopherol levels during ripening. The ripening-related increase in MiHPPD expression was also seen in response to abscisic acid and to alesser extent to indole-3-acetic acid. The expression of MiHPPD was not restricted to fruits but was also seen in other tissues such as leaves particularly during senescence. The strong ethylene induction of MiHPPD was also seen in young leaves indicating that ethylene induction of MiHPPD is tissue independent. Promoter analysis of MiHPPD gene in tomato discs and leaves of stable transgenic lines of Arabidopsis showed that the cis elements for ripening-related, ethylene-responsive, and senescence-related expression resided within the 1590 nt region upstream of the ATG codon. Functionality of the gene was demonstrated by the ability of the expressed protein in bacteria to convert p-hydroxyphenylpyruvate to homogentisate. These results provide the first evidence for HPPD expression during ripening of a climacteric fruit.
Keywords: ethylene, mango, pHPPD, promoter, ripening, senescence
Introduction
Ripening in fruits is a complex developmental process that is associated with several biochemical changes such as conversion of acids/starch to sugars, development of flavour and aroma volatiles, changes in pigment composition, changes in cell wall leading to softening of fruit tissue, etc. (Nath et al., 2006). Depending on the fruit type and texture, the metabolites undergoing changes during ripening may differ, being specific for different fruits. In climacteric fruits, several of these changes are triggered by ethylene through the expression of hundreds of genes. Regulation of pathways through which secondary metabolites that provide aroma, taste, and useful nutrients accumulate during fruit ripening have not been worked out well except in tomato. In particular, there is very little information on the metabolome in other fruits.
Mango is an important climacteric tropical fruit in India with more than a thousand varieties that differ in size, shape, aroma, and taste. It is an excellent source of nutrition and particularly rich in carotenoids and tocopherols (β-carotene 649 μg per 100g and α-tocopherol 0.9mg per 100g) according to the USDA nutrition database, 2010 (www.ars.usda.gov/nutrientdata). The biosynthetic pathways of both these phytonutrients are connected via the metabolite p-hydroxyphenylpyruvate (p-HPP). HPP is converted to homogentisate (HGA) by the enzyme p-hydroxyphenylpyruvate dioxygenase (HPPD) (EC 1.13.11.27), which in turn is used as a substrate for the formation of tocopherols (Prescott and John, 1996). HGA is also diverted towards the formation of plastoquinones in photosynthetic tissues where they act as important electron acceptors for the desaturation of phytoene to carotenoids in higher plants (Norris et al. 1995, 1998). In plants, HPPD is believed to be involved in two distinct metabolic processes, namely the catabolism of tyrosine and biosynthesis of tocopherols (Fiedler et al. 1982; Schultz et al. 1991). HPPDs have been characterized from plants such as Arabidopsis, barley, carrot, rice, etc. In barley and rice, HPPD has been shown to be senescence related (Kleber-Janke and Krupinska, 1997; Lee et al., 2001), hence has been designated a senescence-associated gene (Quirino et al., 2000).
Since tocopherols (vitamin E) are strong lipophilic antioxidants with a protective function in the preservation of cell membrane integrity during leaf development and oxidative stress (Fryer, 1992; Maeda and Della Penna, 2007; Falk and Munne-Bosch, 2010), attempts are being made to increase tocopherol levels in plants. HPPD, being a key precursor enzyme in vitamin E biosynthesis, has been studied in detail in Arabidopsis, and transgenic plants have been developed using this gene alone and in combination with other genes of the pathway in Arabidopsis, potato, mustard, etc. (Falk et al., 2003; Hofius et al., 2004; Rippert et al., 2004; Karunanandaa et al., 2005; Matringe et al., 2005; Della Penna and Pogson, 2006). Even though genes encoding enzymes for tocochromanol biosynthesis have been cloned and studied in the past decade or so (Shintani and Della Penna, 1998; van Eenennaam et al., 2003), understanding of the overall biochemical and molecular regulation of the pathway in plants remains limited. In particular, the regulation of the tocopherol biosynthesis pathway in fruits with high tocopherol levels such as mango is not known at all.
The changes that occur during fruit ripening in mango (Mangifera indica L.) are a matter of interest. In a screen for differentially expressed genes during ripening in mango, the HPPD homologue was obtained as a ripening up-regulated gene. In this article it is shown that the ripening-related expression of MiHPPD is associated with an increase in tocopherol and carotenoid content in fruits.
Materials and methods
Plant material
Mature unripe mangoes (M. indica, var. Dashehari) (12–15cm long with a hard stone) were harvested from a local mango orchard at Lucknow. Ripening was initiated by exposing the fruits to exogenous ethylene (100 μll−1) for 24 h in a closed 10-l chamber and fruits were then allowed to ripen for 6 d at 23 °C in air. Sampling of various developmental stages of fruit and other vegetative tissues was carried out as described previously (Sane et al., 2005; Chourasia et al., 2006) unless otherwise stated. For abscisic acid (ABA) and indole-3-acetic acid (IAA) treatment, fruits were dipped in a solution containing 100 μM ABA or IAA in 0.2% teepol (detergent) and vacuum infiltrated for 2 h. Control fruits were infiltrated with 0.2% teepol. For ethylene induction in leaves, young leaves were exposed to 100 μll−1 ethylene for 3 h.
RNA extraction, RT-PCR, and sequencing
RNA was extracted from various tissues using cetyl trimethyl ammonium bromide as described previously (Asha et al., 2007; Chourasia et al., 2008). RNA from day 4 ethylene-treated mango pulp (treated with RNase-free DNase) was used for cDNA synthesis. First-strand cDNA synthesis was carried out according to the manufacturer's instruction (MBI-Fermentas). A subtractive library was prepared using Clontech PCR-select cDNA subtraction kit (Clontech Laboratories, Inc.) according to the manufacturer's protocol. Subtraction was carried out between cDNAs of day 0 and day 5 after ethylene-treated mango fruit pulp taking day 0 fruit cDNA as driver and day 5 fruit cDNA as tester sample. During screening of the subtractive library for ethylene-induced ripening-related genes, a clone was obtained with a 400-bp insert that showed homology to HPPD. Based on the partial sequence, gene-specific primers (HPPDF15′-AGAGTTTACAGCGGAAGATGTTGGGAC-3′ and HPPDF25′-GTGTTGCTTCCGTTGAATGAGCCAGTG-3′) were designed for 3′ RACE and these in combination with 3′-AP primer were used to obtain the 3′end. Two gene-specific primers (F0:5′-TTGGATCCATGGGCAAACAAGACGGTTC-3′ and R0:5′- GTGGATCCTCATACAACTGAGGCTGCTC-3′) containing the initiation and termination codons, respectively, were synthesized to amplify the full-length cDNA fragment of 1299 nt containing the complete open reading frame (ORF). This was designated MiHPPD (accession no. HQ244994).
MiHPPD promoter isolation and cloning
A mango genome walker library was constructed using the Universal genome walker kit (Clontech) according to the manufacturer's protocol. This was used to isolate the promoter of MiHPPD using gene-specific primers (HPPDPR3: 5′-TTGTTTGTTTCTAGGCGGAAA-3′; HPPDPR4: 5′-GACGTGGATGGTTTATCATGG-3′) and the genome walker adapter primers AP1 and AP2 (Clontech). A fragment of 1590 nt containing the initiation codon was cloned at the BamHI site of pBI101.1 in translational fusion with the GUS gene (HPPDpro:GUS). The plasmid was introduced into the Agrobacterium strain GV3101.
Sequence analysis
Homology searches and signal peptide predictions were performed using the Basic Local Alignment Search Tool (BLAST; NCBI, Bethesda, MD, USA) and SIGNALP version 1.1 (Center for Biological Sequence Analysis, Technical University of Denmark), respectively. Multiple alignments were carried out using the CLUSTALW program. Phylogenetic analysis of putative MiHPPD protein was carried out using the Phylip 3.5c package employing parsimony and bootstrap analysis (1000 replicates). Promoter analysis was carried out by the PLANT CARE program.
Northern analysis and real-time qRT-PCR
Total RNA was isolated from mango pulp from control (ethylene-untreated), ethylene-treated, and 1-methylcyclopropene (1-MCP)-treated fruit at different ripening stages, from different developmental stages of fruit and vegetative tissues. RNA gel blot analyses were carried out as described previously (Sane et al., 2005). The total RNA (40 μg) from each sample was resolved on a 1.2% formaldehyde–agarose gel as described by Sambrook et al. (1989) and modified by the Qiagen Oligotex handbook (2002) protocol for RNA electrophoresis. RNA was transferred to Hybond-N nylon membrane (Amersham-Pharmacia Biotech, UK). Blots were pre-hybridized at 42 °C for 6–8 h in hybridization buffer (50% formamide, 6×SSC, 5×Denhardt's solution, 0.1% SDS, and 100 μgml−1 denatured calf thymus DNA). The 1.1-kb MiHPPD cDNA fragment containing the 3′ region of the ORF and the 3′untranslated region (UTR) was labelled with [α-32P]dCTP by random priming and used as a probe for northern blots (Sambrook et al., 1989). Hybridization was carried out overnight at 42 °C in hybridization buffer containing the radiolabelled probe. The autoradiograms were developed after exposing the blots to X-ray films (Fuji) at -70 °C for 2–3d. Hybridization experiments were replicated at least twice with different RNA samples.
qRT-PCR was performed in a 20-μl reaction volume using SYBR GREEN PCR Master Mix (PE-Applied Biosystems, Foster City, CA, USA) on an ABI PRISM 7000 sequence-detection system according to the manufacturer's instructions. Relative fold differences were calculated based on the comparative Ct method using actin as an internal standard (Actf:5′-GAGAGTTTTGATGTCCCTGCCATG-3′ and Actr: 5′-CAA CGTCGCATTTCATGATGGAGT-3′). For real-time experiments, the following PCR conditions were applied: 50 °C for 2 min, followed by 95 °C for 10 min, then 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. All RT-PCR experiments were run in triplicate. To determine relative fold differences for each sample in each experiment, the Ct values for all treatments for the MiHPPD gene were normalized to the Ct value for actin and calculated relative to a calibrator that was taken as 1[control day 0unripe fruit (C) in the case of fruits and untreated leaf in experiments relating to mango leaf] using the formula 2–ΔΔCt.
Tocopherol and carotenoid estimation in fruit
Mango pulp tissue (10 g) was crushed in acetone. The homogenate was filtered through a filter paper adding acetone until the retained solids became colourless. The acetone extract was then mixed with petroleum ether and stirred vigorously. Phase separation was carried out using distilled water and sodium sulphite was added to it. The ether extract was desiccated and evaporated in a rotavapour at 42 °C. Residues were dissolved in 5 ml of the mobile phase and filtered through a 0.2-μm membrane. Sample (20 μl) was injected into the HPLC system (a Waters 2998 with PDA detector, 515 pump, automatic injector 717 plus, and Empower system data processor was used). A C-18 reverse phase column from Novapack with dimensions 3.9×150 mm and particle size of 0.4μm was used. Samples were separated with modifications in a procedure described by Gomez-Coronado and Barbas (2003) with a mobile phase comprising acetonitrile, methanol, glacial acetic acid in a 70:30:1 ratio and a flow rate of 1.5 ml min−1. Tocopherol and carotene were detected at 295 nm and 450 nm, respectively.
Heterologous expression of MiHPPD in bacteria and enzyme activity
For protein expression, the complete MiHPPD ORF was cloned in pET28a (Novagen) vector to give pET28a-MiHPPD. Escherichia coli BL21 (DE3) cells were used for isopropyl-β-thiogalactopyranoside (IPTG)-induced expression of the construct. Recombinant bacteria and E. coli BL21(DE3) cells transformed with pET28a (as a control), were grown at 37 °C in Luria–Bertani (LB) medium containing 50 μg ml−1 kanamycin and 30 μg ml−1chloramphenicol, and induced with 0.4 mM IPTG. After an induction period of 16 h at 16 °C, the cells were harvested, washed, and suspended in Tris–HCl (20 mM, pH7.2). The cells were sonicated for 3 min [5-s pulses at 10 micron amplitude; BioDigital(P) Ltd] for protein extraction. Cell debris was removed by centrifugation (12000 g, 15 min at 4 °C). Induced partially purified protein was analysed on a 10% SDS–PAGE (Laemmli, 1970). The electrophoresis was carried out in a Mini Protean III Dualslab cell system (Bio-Rad) at a constant current of 16 mA per gel. Gels were stained with Coomassie Blue R-250.
Enzymatic activity of induced recombinant MiHPPD protein was assayed to determine the functionality of the protein by quantifying HGA formed as described by Garcia et al. (1997). Enzymeactivity of IPTG-induced bacterial crude extract (100 μl) was measured in a reaction buffer (0.1 M Tris–HCl pH7.2, 5 mM ascorbate, and 0.8 μMp-HPP). After incubation at 30 °C for2 h, the reaction was stopped by the addition of 6N HCl. The reaction mixtures were extracted thrice with 0.5 ml of ethyl acetate and the combined organic phases were evaporated. The residues obtained were redissolved in 100 μl of methanol. HGA formed in the reaction after conversion of p-HPP by HPPD was detected and quantified by HPLC using pure HGA as standard. Protein was estimated by the Peterson method (Peterson, 1977).
The functionality of recombinant MiHPPD protein was also checked as a function of the accumulation of pyromelanin, which is a brown pigment formed by spontaneous oxidation and polymerization of HGA (Kim and Peterson, 2002).The pyromelanin formed was determined by spectrophotometric measurement of the cell-free media at 440 nm against LB medium according to Keon and Hargreaves (1998). For this, a culture of recombinant E. coli BL21(DE3) was grown at 37 °C, 200 rpm, until the OD600 reached 0.4–0.6. The culture was divided into two sets of 12. In one set, only the substrate (p-HPP, 0.4 mM) was added while in the other, both p-HPP and the inducer IPTG (1 mM) were added. Every 2 h (up to a duration of 24 h) one tube from each set was taken out and centrifuged at 8000 rpm, at 25 °C for 5 min to obtain a cell-free extract. Absorbance of the cell-free extracts was measured at OD440 for pyromelanin formation using LB as blank.
Transient analysis of MiHPPD promoter by agroinjection
Promoter analysis was carried out as described by Tripathi et al. (2009). Recombinant Agrobacterium containing the promoter of HPPD (in pBI101.1) was inoculated in 50ml of induction medium with gentamicin, rifampicin and kanamycin,and grown at 28 °C until the OD600 reached 1.0. The cells were centrifuged at 4000 g at room temperature and the pellet was resuspended in infiltration medium (10 mM MgCl2, 10 mM MES, 200μ M acetosyringone) to an OD of 1.0. The culture was grown at 28 °C for 3 h, 50 rpm, before infiltration.
For agro injection experiments, mature unripe and ripe tomato fruits of the same size were used. Tomato fruits were infiltrated using a 1-ml syringe with a needle (0.56×25 mm). The needle was introduced 3–4 mm deep into the fruit tissue through the styler apex, and the infiltration solution (5 ml) containing the HPPD:GUS construct was gently injected into the fruit. These fruits were incubated at 28 °C for 48 h. For negative and positive controls, pBI101.1 (no promoter), pBI121 (GUS driven by the CaMV35S promoter), respectively, were also used for agro-infiltration of independent fruits. Three fruits for each construct were injected. After 48 h, thin slices were cut from each fruit and kept in Petri dishes and washed with rinse solution (50 mM sodium phosphate buffer pH7.2, 0.5 mM potassium ferricyanide) two or three times. After washing, 100 μg ml−1X-gluc (5-bromo-4-chloro-3-indolyl β-D-glucuronic acid) was added and kept at 37 °C for 24–48 h until a blue colour appeared (Gattolin et al., 2006). The tissue was decolorized in 70% ethanol for 1 h for proper visualization.
Histochemical GUS staining of transgenic Arabidopsis plants
The plant transformation vector HPPD pro:GUS containing the MiHPPD promoter was transformed into wild-type Arabidopsisthaliana (ecotype Columbia) by the floral dip method (Clough and Bent, 1998). T1 generation seeds were selected on kanamycin-containing half-strength Murashige and Skoog (MS) agar plates. Kanamycin-resistant T1 seedlings were transferred to soilrite saturated with nutrient media and grown to maturity.
Different tissues of T1 transgenic Arabidopsis plants, namely leaves (young, mature, and senescent) were immersed individually in 1.5-ml microfuge tubes containing the GUS reagent and stained as described above. The tissue was destained in 70% alcohol at 37 °C and examined under a Leica Wild M3Z microscope (Leica, Germany). For ethylene treatment prior to GUS staining, leaves were cut fresh in water, and 10 μll−1 ethylene was given for 1 h in a desiccator. Control leaves were dipped in distilled water and kept in a desiccator.
Results
Identification, sequencing, and phylogenetic analysis of mango HPPD
A partial fragment of mango HPPD designated MiHPPD was identified from a subtractive library during screening for ethylene-induced ripening-related genes. For the subtractive library, day 0 fruit cDNA as driver and day 5 fruit cDNA as tester sample were taken. Using the RACE method and primers described in the Materials and methods section, a 3' RACE fragment of 1135bp was amplified. The 1604-bp full-length cDNA of MiHPPD (accession no. HQ244994) comprised a 1299-bp ORF encoding a 432-amino-acid protein, 41-bp 5'UTR, and 264-bp 3'UTR (Fig. 1A). The molecular weight and pI of the deduced MiHPPD protein were predicted to be 47.7 kDa and 5.48, respectively, using bioinformatics tools (www.expasy.org). The encoded protein contained a dioxygenase superfamily domain that is also present in other plant HPPDs (Fig. 1A). Protein–protein BLAST showed that MiHPPD had high sequence identity to HPPDs from other plants such as Ricinus (82% amino acid identity), Populus (79% amino acid identity), and Arabidopsis (78% amino acid identity), with the same active site metal ion motif as other non-haem Fe(II)-dependent oxygenases (Hegg and Que, 1997). A phylogenetic tree was generated from the alignment of the deduced amino acid sequences of MiHPPD with 18 HPPD homologues from other plant species and bacterial HPPD as an outgroup (Fig. 1B).
Fig. 1.
(A) Nucleotide and deduced amino acid sequenceof MiHPPD. The start and stop codons are in bold letters. 5′ and 3′ UTRs are shown in lower case letters. The underlined portion shows homology with the dioxygenase domain. (B) Phylogenetic analysis of mango HPPD amino acid sequence with other HPPD sequences. Full-length protein sequences were aligned using CLUSTAL-W and a phylogenetic tree was constructed using Phylip with the PROTPARS program. Numbers above the branches indicate bootstrap values (1000replicates).Bacillus pseudomycoides HPPD (ZP_04149396.1) was used as the outgroup. Sequences are: A. thaliana AtHPPD (NP_172144.2); Brassica rapa BrHPPD(ABI63586.1); Coptis japonica CjHPPD (BAF74636.1); Daucus carota DcHPPD (O23920.1); Glycine max GmHPPD (ABQ96868.1); Hevea brasiliensis HbHPPD (BAH10638.1); H.vulgare HvHPPD (O48604.1); Lactuca sativa LsHPPD (ACN78586.1); Mangifera indica MiHPPD (HQ244994); Medicago truncatula MtHPPD (AAX59006.1); Oryza sativa OsHPPD (BAD26248.1); Populus trichocarpa PtHPPD (XP_002300867.1); Ricinus communis RcHPPD (XP_002520369.1); Solanum lycopersicum SlHPPD (AK320166); Triticum aestivum TaHPPD (AAZ67144.1); Vitis vinifera VvHPPD (XP_002283275.1); Zea mays ZmHPPD (NP_001105782.1).
Mango HPPD transcript accumulates differentially during ripening
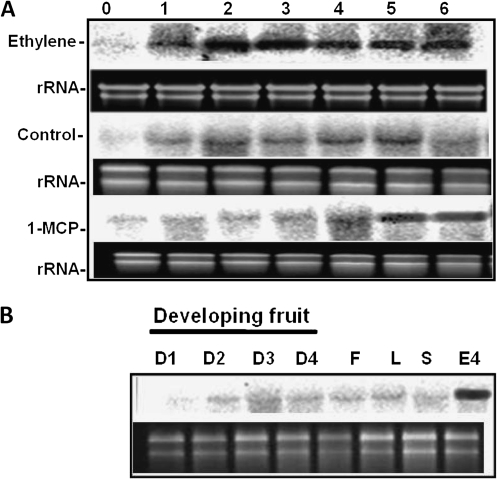
In order to get an insight into MiHPPD expression duringfruit ripening, transcript accumulation in mango was studied during the course of ethylene-induced ripening of harvested mango for 7 d. RNA was isolated from the pulp of post-harvest ethylene-treated mango on different days and probed with a 32P-labelled HPPD probe. A basal expression level of MiHPPD transcript was detected on day 0 in ethylene-untreated fruit. The transcript levels of MiHPPD increased several fold with in 24 h in ethylene-treated fruit, reaching a peak on days 2 and 3 followed by a slight decrease on subsequent days. The high levels were maintained at this level throughout the progression of ripening (Fig. 2A). MiHPPD transcript also accumulated in control and 1-MCP+ethylene-treated fruits but the levels were far lower at all stages compared with the levels in ethylene-treated fruit (Fig. 2A). Moreover, there was a delay in peak transcript accumulation of MiHPPD in these fruits with maximum levels accumulating on day 5 in control and day 5/6 in 1-MCP-treated fruits. The expression pattern of MiHPPD was also analysed in developing fruit (developmental stages D1–D4; Sane et al., 2005; Chourasia et al., 2006) and other tissues such as leaf, stem, and flower. Expression was low in developing fruit and vegetative tissues (leaf, stem) and flower similar to those found in fully mature green mango prior to onset of ripening (Fig.2B).
Fig. 2.
mRNA abundance of MiHPPD in mango. (A) MiHPPD accumulation during different stages of ethylene-induced ripening of mangoby northern blotanalysis; 0–6 indicate days after treatment. (B) Transcript abundance of MiHPPD in developing stages (D1–D4) of fruit and other tissues by northern blotanalysis. Flower; L, leaf; S, stem; E4, day 4 after ethylene treatment of fruit. The ethidium bromide-stained ribosomal RNA bands are shown to indicate RNA loading. All the treatments are described in the Materials and methods section.
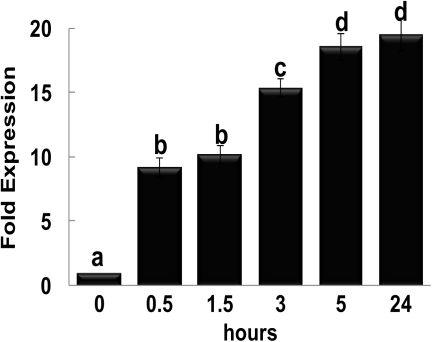
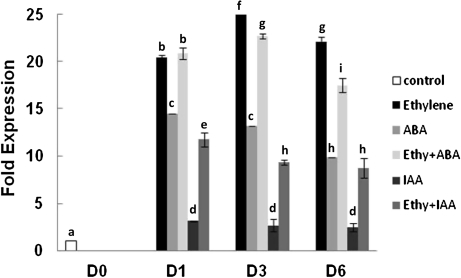
The rapid up-regulation of MiHPPD early during ethylene-induced ripening prompted us to study whether this gene belongs to the category of early ethylene-responsive genes. Mature green unripe fruits were exposed to ethylene for short periods (0.5, 1.5, 3, and 5 h of ethylene exposure) and transcript accumulation was monitored by real-time PCR. qRT-PCR results revealed a rapid increase in transcript accumulation of MiHPPD within 30 min of exposure to ethylene (Fig. 3). Transcript levels increased to 9-fold within 30min and upto 18-fold 5 h after treatment. The expression of MiHPPD in fruits was also studied in response to other hormones like ABA and IAA alone as well as in combination with ethylene. qRT-PCR results suggested that MiHPPD was induced by ABA as in the case of ethylene on day 1 although the levels of accumulation were lower than that for ethylene (Fig.4). Co-treatment of fruits with ethylene and ABA increased the levels of MiHPPD mRNA over and above those induced by ABA alone on day 1 and matched the transcript levels induced by ethylene alone. IAA treatment of fruits caused a slight increase in the levels of MiHPPD unlike in the case of ethylene and ABA (Fig.4). However, treatment of IAA in the presence of ethylene caused MiHPPD transcript levels to increase almost 5-fold over those with IAA alone. Nevertheless, the levels were almost 50% lower than the levels in fruits treated with ethylene alone.
Fig. 3.
Ethylene inducibility of MiHPPD in fruit analysed by qRT-PCR. Transcript abundance of MiHPPD mRNA in mature green fruitafter; treatment with ethylene (0–24 h). Mature green fruit were exposed to ethylene for the time intervals described in the figure and RNA isolated from the samples. Expression analysis was carried out by qRT-PCR using actin as an internal control. Five fruits were used for each treatment and RNA was isolated from pooled samples. Data from three independent experiments were analysed and expressed as mean ± standard deviation. Letters (a–d) over the bars indicate significant differences at P<0.05 (means followed by the same letter are not significantly different at P=0.05).
Fig. 4.
Fold expression of MiHPPD in Dashehari fruit after different hormonal treatments during a 6-d time course after treatments. Expression analysis was carried out by qRT-PCR. Actin was used as an internal control. Auxin and ABA (100 μM) treatments were given for 3 h as described in the Materials and methods section. Five fruits were used for each treatment and data from three independent experiments were analysed and expressed as mean ± standard deviation. Letters (a–i) over the bars indicate significant differences at P<0.05 (means followed by the same letter are not significantly different at P=0.05). D0, control fruit without treatment; D1, 1 d after treatment; D3, 3 d after treatment; D6, 6 d after treatment.
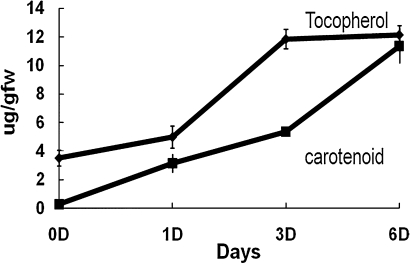
Fig. 5.
Tocopherol and carotenoid content of mango pulp during ethylene-induced ripening. Tocopherol and carotenoid content were estimated by the HPLC method described in the Materials and methods section. Five fruits were used for each treatment and data from three independent experiments were analysed and expressed as mean ± standard deviation.
Fig. 6.
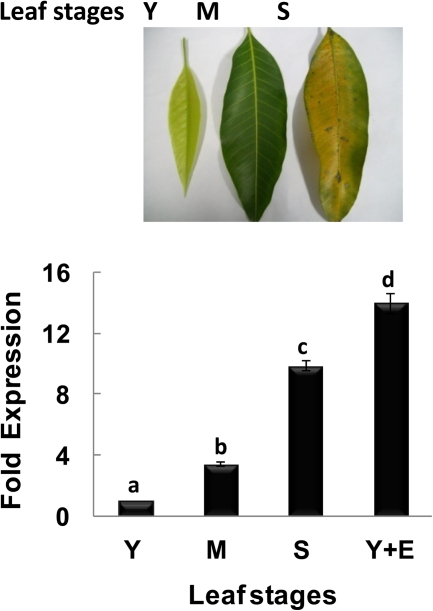
mRNA abundance of MiHPPD during different stages of mango leaf. The upper panel shows different stages of mango leaf taken for qRT-PCR. Expression analysis was carried out by qRT-PCR. Actin was used as an internal control. Pooled samples from five leaves of different stages were taken for the experiment and data from three independent experiments were analysed and expressed as mean ± standard deviation. Letters (a–d) over the bars indicate significant differences at P<0.05 (means followed by the same letter are not significantly different at P=0.05). Y, young; M, mature; S, senescent; Y+E, ethylene-treated young leaf. (This figure is available in colour at JXB online.)
Tocopherol levels increase during mango ripening
Tocopherol and carotenoid content were measured during the course of ripening in Dashehari mango. The levels of both tocopherol and carotenoids increased as the fruit ripened. The levels of tocopherol increased slowly upto day 1 followed by a rapid 3-fold increase on day 3 after ethylene treatment as compared with unripe mature fruit. The levels were maintained upto day 6. The levels of carotenoids were very low in unripe fruit but increased gradually upto day 3 followed by a rapid rise up to 12-fold from days 3 to 6 as compared with unripe fruit (Fig.5).
MiHPPD is expressed in senescent and ethylene-treated leaves
Since previous studies have reported an increase in tocopherols during senescence, the expression of MiHPPD in different leaf stages (young, mature, and senescent) was checked. qRT-PCR studies indicated that MiHPPD transcript levels were almost 8-fold higher in senescent leaves as compared with young and mature leaves (Fig.6). Interestingly, strong ethylene-inducible expression of MiHPPD was seen in leaves. Young leaves treated with ethylene for 3 h accumulated much higher levels of transcript as compared with senescent leaves.
Recombinant MiHPPD protein is expressed in E.coli
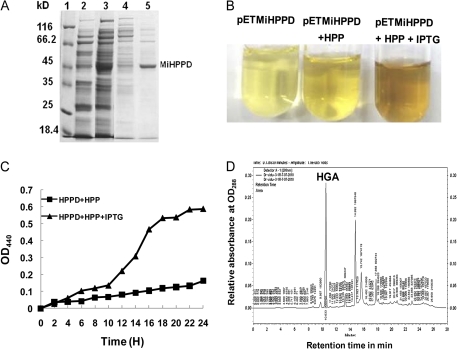
In order to test the functionality of the encoded MiHPPD protein, it was expressed in heterologous bacterial system. The recombinant His-tag–MiHPPD fusion produced by E. coli BL21 (DE3) cells expressing the pET28a-MiHPPD plasmid was purified from the soluble protein extract by affinity chromatography on an Ni-NTA column and eluted using 100 mM imidazole. SDS–PAGE analysis showed expected one intense band of fusion protein (molecular weight 50.2 kDa) and some minor bands (Fig. 7A). Bacterial cultures of E. coli BL21 (DE3) cells transformed with pET28a-MiHPPD grown in the presence of p-HPP showed brown colouration after IPTG induction due to the oxidation of HGA and the polymerization of the oxidation product to a melanin-like pigment, pyromelanin (Fig.7B). The time course of pigment formation in E. coli cultures containing the MiHPPD transgene with and without IPTG induction is shown in Fig. 7C. The brown colouration of the medium began increasing 4h after IPTG addition. The increase was slow until 10 h. Thereafter, there was a linear increase from 10 to 18 h indicating that this increase was due to induced MiHPPD protein. After 18 h there was saturation probably due to limiting p-HPP substrate (Fig. 7). Oxidation of HGA formed by HPPD from A. thaliana overexpressed in E. coli has previously been described (Garcia et al., 1999). Biochemical activity of the recombinant MiHPPD in crude bacterial extract was also estimated using HPLC (Fig.7D). AVmax of 20 nmol min−1 mg−1 crude protein was obtained.
Fig. 7.
(A) SDS–PAGE analysis of over expressed HisTag–MiHPPD fusion protein. Samples were analysed on 10% SDS–PAGE by Coomassie Blue staining. 1, protein marker (MBI-Fermentas); 2, uninduced crude extract; 3, induced crude extract; 4, unbound protein on Ni-NTA column; 5,eluted protein (MiHPPD) using 100 mM imidazol. Position and size of protein markers are shown. (B) Formation of brown pigment in the culture medium of E. coli BL21 (DE3) harbouring pET28a MiHPPD with and with out IPTG. (C) Time-dependent formation of pyromelonin (brown pigment) in cell-free media from oxidation of HGA measured at OD440. (D) Representative HPLC chromatogram showing the synthesis of HGA by E. coli BL21 (DE3) cell extract harbouring the plasmid pET28a MiHPPD. (This figure is available in colour at JXB online.)
Putative promoter of MiHPPD drives GUS expression in tomato discs and transgenic Arabidopsis lines
A 1590-nt fragment for MiHPPD (upstream of the translational initiation site) containing the promoter was cloned using genome walker library for mango. In silico analysis using the PLANT CARE database revealed several elements/motifs. Besides the core cis-acting elements like TATA and CAAT, several cis-acting elements for light response were also found in the MiHPPD promoter. Two sites for ethylene-responsive element, ATTTCAAA, with one nucleotide modification were found in the promoter at positions–342 and–1416 upstream of ATG. An element homologous to the MYB binding site involved in drought induction (CAACTG) at position –547 upstream of ATG was also observed.
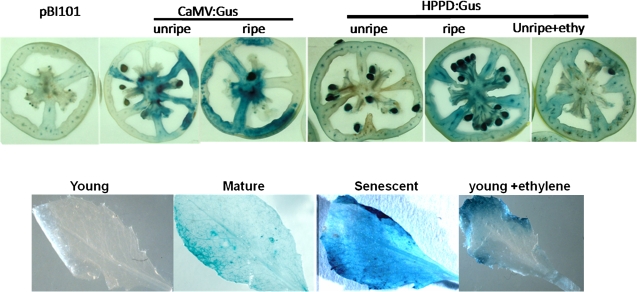
Since MiHPPD was induced during fruit ripening as well as in response to ethylene in leaves, the ability of the promoter fragment to drive expression in response to ripening-related cues was investigated by transient assay in tomato fruit using agroinjection and in stable transgenic lines of Arabidopsis. Transient assays after agroinjection of the 1590-bp MiHPPD promoter in tomato fruit clearly showed that the blue colour due to GUS expression was more prominent in discs of ripe tomato as compared with unripe tomato discs. Since the MiHPPD transcript was induced by short ethylene treatment in fruits (Fig. 8A), MiHPPD promoter-driven GUS expression was checked in unripe tomato discs after 3 h of ethylene treatment. Expression of GUS could be observed in discs treated with ethylene. The same construct was used to develop stable transgenic lines of Arabidopsis. Intense blue colour in senescent leaves of transgenic lines suggested that the MiHPPD promoter was activated in response to senescence cues (Fig. 8). No GUS expression was seen in young leaves in the absence of ethylene treatment. However, exposure to ethylene activated the expression of GUS even in young leaves (Fig.8B).
Fig. 8.
(A) Histochemical staining to test the strength of the MiHPPD promoter using a translational promoter–GUS fusion construct (MiHPPDpro:GUS) by agroinfiltration. Ripe and unripe tomato fruits were infiltrated with Agrobacteria containing different constructs using a syringe. The constructs used for the transient study were pBI101 (promoterless), pBI121 (GUS driven by the CaMV35S promoter), MiHPPD pro:GUS (GUS driven by the 1.6-kb MiHPPD promoter). (B) Histochemical staining to test the activity of the MiHPPD promoter using a translational promoter–GUS fusion construct (MiHPPD pro:GUS) in different stages of leaves of transgenic Arabidopsis line. GUS staining was carried out as described in the Materials and methods section. Transgenic lines were developed using MiHPPD pro:GUS (GUS driven by the 1.6-kb MiHPPD promoter) construct. (This figure is available in colour at JXB online.)
Discussion
Fruit ripening is associated with several physiological and biochemical changes leading to accumulation of different metabolites in different fruits. These changes are developmentally regulated through differential expression of several genes. Several fruits such as mangoes have been reported to accumulate high levels of tocopherols and carotenoids during ripening (Burns et al., 2003; Ajila et al., 2007) yet there is no information related to genes of tocopherol biosynthetic pathway and its regulation during ripening. HPPD is a key precursor enzyme in the vitamin E synthesis pathway and has been characterized from leaves and roots of plants like Arabidopsis, carrot, barley, and rice. The importance of vitamin E as an antioxidant in humans and the lack of clarity regarding the role of tocochromanols in plant developmental processes led to an interest in understanding the pathway genes and the possibility of their manipulation. There are some reports where overexpression of plant or bacterial HPPD has been shown to enhance the content of vitamin E in plants (Tsegaye et al., 2002; Van Eenennaam et al., 2003).
Here MiHPPD is identified as a differentially expressed gene during ripening in mango fruit. Structurally, it appears to be cytosolic in nature like Arabidopsis and carrot HPPDs, which also lack a signal sequence. The gene encodes functional HPPD enzyme, as evident from the ability of the overexpressed protein in E.coli to convert p-HPP to HGA. The present study shows that there is strong ripening-related control on the expression of MiHPPD. The expression is triggered with the initiation of ripening. In ethylene-untreated control fruit where the burst in ethylene is seen on day 3 (data not shown), transcription of MiHPPD begins increasing from day 4. When ripening is triggered by exogenous ethylene, MiHPPD expression begins to rise from day 1itself, reaching a peak on day 2.That the expression is ripening related and ethylene associated is evident from the fact that delay in ripening by 1-MCP treatment causes a delay in the increase inMiHPPD expression. To the best ofthe authors’ knowledge, this is the first report on a ripening-related HPPD from fruits.The increase in MiHPPD expression correlated with an increase in tocopherol and carotenoid levels during ripening in fruit. Other hormones such as ABA and IAA that are also known to enhance ripening in mango (data not shown) also increased MiHPPD expression although to a lesser extent than ethylene. A positive correlation between ABA and α-tocopherol level has also been observed in maize seedlings, Cistus creticus, and leaves of female cannabis plants (Jiang and Zhang, 2001; Munne-Bosch et al., 2009; Mansouri et al., 2009). Co-treatment with these hormones along with ethylene increased MiHPPD expression to a much higher level than was seen with either of the hormones, indicating that the gene was most responsive to ethylene. These studies provide evidence for activation of the tocopherol biosynthetic pathway in fruits, which led to a ripening-specific increase in tocopherols in fruits. The activation of MiHPPD by ethylene was rapid and high and could be seen within 30 min of ethylene treatment of green fruit. The induction by ethylene was observed not only in fruit but also in young leaves indicating that ethylene activation of MiHPPD is tissue independent. Ethylene activation of HPPD has been reported so far only for the barley HPPD where it was associated with the senescence-related expression of HPPD (Falk et al., 2002). Besides ripening, expression of MiHPPD was also found to increase with leaf age being maximum in senescent leaves. HPPD has previously been identified as a senescence-associated gene in rice and barley with enhanced levels of expression during leaf senescence under natural conditions as well as after dark induction of senescence (Kleber-Janke and Krupinska, 1997). In these plants expression of HPPD was correlated with the increase in tocopherol levels during senescence and it has been proposed that the increase in tocopherol levels may be a response to the increase in oxidative stress (Falk et al., 2002). Using mutants of the ethylene biosynthesis and signalling pathway Cela et al. (2009) showed that the levels of toccopherol in senescence might be governed by ethylene signalling.
Promoter analysis of MiHPPD showed that the cis elements for ripening-related, ethylene-responsive, and senescence-related expression reside within the 1590-nt region upstream of the ATG codon. Transient expression of MiHPPD pro:GUS in tomato through agroinjection clearly showed higher GUS expression in ripening fruit tissue than in unripe tissue as well as ethylene-responsive expression in unripe fruit treated with ethylene. Stable transgenic lines of Arabidopsis expressing the promoter showed strong leaf senescence-related expression as observed in mango leaves as well as strong ethylene-responsive expression indicating that the cis elements governing ethylene- and senescence-responsive expression are conserved between mango and Arabidopsis. Two cis elements, AATTCAAA at –1416 and TTTGAAGT at –342 (with one nucleotide change), could possibly govern expression in response to ethylene (Itzhaki et al., 1994). The functionality of these elements is currently being tested by deletion experiments.
In conclusion, evidence is here provided for the role of HPPD in tocopherol biosynthesis in fruits. The MiHPPD gene responsible for tocopherol biosynthesis is governed by ripening-related, ethylene-responsive, and senescence-associated cues. The gene in fruit is responsive majorly to ethylene; however, ABA and IAA also activate its expression though to a lesser extent.
Acknowledgments
The authors are thankful to the Central Institute for Subtropical Horticulture, Lucknow, for the mango samples. A Senior Research Fellowship provided to Rajesh K. Singh by CSIR, India is gratefully acknowledged.
Glossary
Abbreviations
- ABA
abscisic acid
- IAA
indole-3-acetic acid
- IPTG
isopropyl-β-thiogalactopyranoside
- HGA
homogentisate
- HPPD
p-hydroxyphenylpyruvate dioxygenase
- p-HPP
p-hydroxyphenylpyruvate
- 1-MCP
1-methylcyclopropene
References
- Ajila CM, Bhat SG, Prasada Rao UJS. Valuable components of raw and ripe peels from two Indian mango varieties. Food Chemistry. 2007;102:1006–1011. [Google Scholar]
- Asha SaneVA, Sane AP, Nath P. Multiple forms of banana α-expansin genes express during fruit ripening and development. Postharvest Biology and Technology. 2007;45:184–192. [Google Scholar]
- Burns J, Fraser PD, Bramley PM. Identification and quantification of carotenoids, tocoferols and chlorophyll in commonly consumed fruits and vegetables. Phytochemistry. 2003;62:939–947. doi: 10.1016/s0031-9422(02)00710-0. [DOI] [PubMed] [Google Scholar]
- Cela J, Falk J, Munné-Bosch S. Ethylene signaling may be involved in the regulation of tocopherol biosynthesis in Arabidopsis thaliana. FEBS Letters. 2009;583:992–996. doi: 10.1016/j.febslet.2009.02.036. [DOI] [PubMed] [Google Scholar]
- Chourasia A, Sane VA, Nath P. Differential expression of pectate lyase during ethylene induced post harvest softening of mango (Mangifera indica var. Dashehari) Physiologia Plantarum. 2006;128:546–555. [Google Scholar]
- Chourasia A, Sane VA, Singh RK, Nath P. Isolation and characterization of the MiCel1 gene from mango: ripening related expression and enhanced endoglucanase activity during softening. Plant Growth Regulation. 2008;56:117–127. [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Della Penna D, Pogson BJ. Vitamin synthesis in plants: tocopherols and carotenoids. Annual Review of Plant Biology. 2006;57:711–738. doi: 10.1146/annurev.arplant.56.032604.144301. [DOI] [PubMed] [Google Scholar]
- Falk J, Andersen G, Kernebeck B, Krupinska K. Constitutive over expression of barley 4-hydroxyphenylpyruvate dioxygenase in tobacco results in elevation of the vitamin E content in seeds but not in leaves. FEBS Letters. 2003;540:35–40. doi: 10.1016/s0014-5793(03)00166-2. [DOI] [PubMed] [Google Scholar]
- Falk J, Krauss N, Dahnhardt D, Krupinska K. The senescence associated gene of barley encoding 4-hydroxyphenylpyruvate dioxygenase is expressed during oxidative stress. Journal of Plant Physiology. 2002;159:1245–1253. [Google Scholar]
- Falk J Munne-Bosch S. Tocochromanol functions in plants: antioxidation and beyond. Journal of Experimental Botany. 2010;61:1549–1566. doi: 10.1093/jxb/erq030. [DOI] [PubMed] [Google Scholar]
- Fiedler E, Soll J, Schultz G. The formation of homogentisate in the biosynthesis of tocopherol and plastoquinone in spinach chloroplasts. Planta. 1982;155:511–515. doi: 10.1007/BF01607575. [DOI] [PubMed] [Google Scholar]
- Fryer MJ, Oxborough K, Mullineaux PM, Baker NR. Imaging of photo oxidative stress responses in leaves. Journal of Experimental Botany. 2002;53:1249–1254. [PubMed] [Google Scholar]
- Garcia I, Rodgers M, Lenne C, Rolland A, Sailland A, Matringe M. Subcellular localization and purification of a p-hydroxyphenylpyruvate dioxygenase from cultured carrot cells and characterization of the corresponding cDNA. Biochemistry Journal. 1997;325:761–769. doi: 10.1042/bj3250761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia I, Rodgers M, Pepin R, Hsieh TF, Matringe M. Characterization and subcellular compartmentation of recombinant 4-hydroxyphenylpyruvate dioxygenase from Arabidopsis in transgenic tobacco. Plant Physiology. 1999;119:1507–1516. doi: 10.1104/pp.119.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattolin S, Alandete-Saez M, Elliott K, Gonzalez-Carranza Z, Naomab E, Powell C, Roberts JA. Spatial and temporal expression of the response regulators ARR22 and ARR24 in Arabidopsis thaliana. Journal of Experimental Botany. 2006;57:4225–4233. doi: 10.1093/jxb/erl205. [DOI] [PubMed] [Google Scholar]
- Gomez-Coronado DJM, Barbas C. Optimized and validated HPLC method for α- and -γ tocopherol measurement in Laurus nobilis leaves. New data on tocopherol content. Journal of Agriculture and Food Chemistry. 2003;51:5196–5201. doi: 10.1021/jf030143f. [DOI] [PubMed] [Google Scholar]
- Hegg EL, Que L. The 2-His-1-carboxylate facial triad—an emerging structural motif in mononuclear non-heme iron(II) enzymes. European Journal of Biochemistry. 1997;250:625–629. doi: 10.1111/j.1432-1033.1997.t01-1-00625.x. [DOI] [PubMed] [Google Scholar]
- Hofius D, Hajirezaei MR, Geiger M, Tschiersch H, Melzer M, Sonnewald U. RNAi-mediated tocopherol deficiency impairs photo assimilate export in transgenic potato plants. Plant Physiology. 2004;135:1256–1268. doi: 10.1104/pp.104.043927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki H, Maxson JM, Woodson WR. An ethylene-responsive enhancer element is involved in the senescence-related expression of the carnation glutathione S-transferase (GST1) gene. Proceedings of the National Academy of Sciences, USA. 1994;91:8925–8929. doi: 10.1073/pnas.91.19.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Zhang J. Effect of abscisic acid on active oxygen species, antioxidative defence system, and oxidative damage in leaves of maize seedlings. Plant and Cell Physiology. 2001;42:1265–1273. doi: 10.1093/pcp/pce162. [DOI] [PubMed] [Google Scholar]
- Karunanandaa B, Qi Q, Hao M, et al. Metabolically engineered oil seed crops with enhanced seed tocopherol. Metabolic Engineering. 2005;7:384–400. doi: 10.1016/j.ymben.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Keon J, Hargreaves J. Isolation and heterologous expression of a gene encoding 4-hydroxyphenylpyruvate dioxygenase from the wheat leaf-spot pathogen. Mycosphaerella graminicola. FEMS Microbiology Letters. 1998;161:337–343. doi: 10.1111/j.1574-6968.1998.tb12966.x. [DOI] [PubMed] [Google Scholar]
- Kim KH, Petersen M. cDNA-cloning and functional expression of hydroxyphenylpyruvate dioxygenase from cell suspension cultures of Coleus blumei. Plant Science. 2002;163:1001–1009. [Google Scholar]
- Kleber-Janke T, Krupinska K. Isolation of cDNA clones for genes showing enhanced expression in barley leaves during dark-induced senescence as well as during senescence under field conditions. Planta. 1997;203:332–340. doi: 10.1007/s004250050199. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee R-H, Wang C-H, Huang L-T, Chen S-CG. Leaf senescence in rice plants: cloning and characterization of senescence up-regulated genes. Journal of Experimental Botany. 2001;52:1117–1121. doi: 10.1093/jexbot/52.358.1117. [DOI] [PubMed] [Google Scholar]
- Maeda H, Della Penna D. Tocopherol functions in photosynthetic organisms. Current Opinion in Plant Biology. 2007;10:260–265. doi: 10.1016/j.pbi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Mansouri H, Asrar Z, Szopa J. Effects of ABA on primary terpenoids and D9-etrahydrocannabinol in Cannabis sativa L. at flowering stage. Journal of Plant Growth Regulation. 2009;58:269–277. [Google Scholar]
- Matringe M, Sailland A, Pelissier B, Rolland A, Zink O. p-Hydroxyphenylpyruvate dioxygenase inhibitor-resistant plants. Pest Management Science. 2005;61:269–276. doi: 10.1002/ps.997. [DOI] [PubMed] [Google Scholar]
- Munne-Bosch S, Falara V, Pateraki I, López-Carbonell M, Jana CJ, Kanellis AK. Physiological and molecular responses of the isoprenoid biosynthetic pathway in a drought-resistant Mediterranean shrub, Cistus creticus exposed to water deficit. Journal of Plant Physiology. 2009;166:136–145. doi: 10.1016/j.jplph.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Nath P, Trivedi PK, Sane VA, Sane AP. Role of ethylene in fruit ripening. In: Khan NA, editor. Ethylene action in plants. Berlin: Springer-Verlag; 2006. pp. 151–184. [Google Scholar]
- Norris SR, Barrette TR, DellaPenna D. Genetic dissection of carotenoid synthesis in Arabidopsis defines plastoquinone as an essential component of phytoene desaturation. The Plant Cell. 1995;7:2139–2149. doi: 10.1105/tpc.7.12.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris SR, Shen X, Della Penna D. Complementation of the Arabidopsis pds1mutation with the gene encoding p-hydroxyphenylpyruvate dioxygenase. Plant Physiology. 1998;117:1317–1323. doi: 10.1104/pp.117.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Analytical Biochemistry. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Prescott AG, John P. Dioxygenases: molecular structure and role in plant metabolism. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47:245–271. doi: 10.1146/annurev.arplant.47.1.245. [DOI] [PubMed] [Google Scholar]
- Quirino BF, Noh YS, Himelblau E, Amasino RM. Molecular aspects of leaf senescence. Trends in Plant Science. 2000;5:278–282. doi: 10.1016/s1360-1385(00)01655-1. [DOI] [PubMed] [Google Scholar]
- Rippert P, Scimemi C, Dubald M, Matringe M. Engineering plant shikimate pathway for production of tocotrienol and improving herbicide resistance. Plant Physiology. 2004;134:92–100. doi: 10.1104/pp.103.032441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook T, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sane VA, Chourasia A, Nath P. Softening in mango (Mangifera indica var Dashehari) is correlated with the expression of the early ethylene responsive, ripening related expansin gene. MiExpA1. Postharvest Biology and Technology. 2005;38:223–230. [Google Scholar]
- Shintani D, DellaPenna D. Elevating the vitamin E content of plants through metabolic engineering. Science. 1998;282:2098–2100. doi: 10.1126/science.282.5396.2098. [DOI] [PubMed] [Google Scholar]
- Schultz G, Heintze A, Hoppe P, Hagelstein P, Görlach J, Meereis K, Schwanke U, Preiss M. Tocopherol and carotenoid synthesis in chloroplasts: tight linkage to plastidic carbon metabolism in developing chloroplasts. In: Pell E, Steffen K, editors. Active oxygen/oxidative stress and plant metabolism. Rockville, MD: American Society of Plant Physiologists; 1991. pp. 156–170. [Google Scholar]
- Tripathi SK, Singh AP, Sane AP, Nath P. Transcriptional activation of a 37 kDa ethylene responsive cysteine protease gene, RbCP1, is associated with protein degradation during petal abscission in rose. Journal of Experimental Botany. 2009;60:2035–2044. doi: 10.1093/jxb/erp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsegaye J, Shintani D, DellaPenna D. Over expression of the enzyme p-hydroxyphenylpyruvate dioxygenase in Arabidopsis and its relation to tocopherol biosynthesis. Plant Physiology and Biochemistry. 2002;40:913–920. [Google Scholar]
- Van Eenennaam AL, Lincoln K, Durrett TP, et al. Engineering vitamin E content: from Arabidopsis mutant to soy oil. The Plant Cell. 2003;15:3007–3019. doi: 10.1105/tpc.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]