Abstract
Development of noninvasive techniques to discover new biomarkers in the live brain is important to further understand the underlying metabolic pathways of significance for processes such as anesthesia-induced apoptosis and cognitive dysfunction observed in the undeveloped brain. We used in vivo proton magnetic resonance spectroscopy and two different signal processing approaches to test the hypothesis that volatile (isoflurane) and intravenous (propofol) anesthetics at equipotent doses produce distinct metabolomic profiles in the hippocampus and parietal cortex of the live rodent. For both brain regions, prolonged isoflurane anesthesia was characterized by higher levels of lactate (Lac) and glutamate compared with long-lasting propofol. In contrast, propofol anesthesia was characterized by very low concentrations of Lac ([lac]) as well as glucose. Quantitative analysis revealed that the [lac] was fivefold higher with isoflurane compared with propofol anesthesia and independent of [lac] in blood. The metabolomic profiling further demonstrated that for both brain regions, Lac was the most important metabolite for the observed differences, suggesting activation of distinct metabolic pathways that may impact mechanisms of action, background cellular functions, and possible agent-specific neurotoxicity.
Keywords: anesthesia, brain, in vivo, magnetic resonance spectroscopy, metabolomics, rat
Introduction
The effects of general anesthetics on traditional measures of brain function and metabolism, including the cerebral metabolic rate of glucose utilization (CMRglu), oxygen utilization, and neurotransmission, have been well characterized in humans and animals using jugular bulb oximetry, positron emission tomography, magnetic resonance imaging, or autoradiography (Alkire et al, 1995; Newberg et al, 1983; Olsen et al, 1992; Ori et al, 1986; Sun et al, 2008). For example, cortical oxidative metabolism and glutamate (Glu) flux have been shown to be stoichiometrically related, coupled to neuronal firing, and dependent on depth of barbiturate anesthesia (Shulman et al, 2001). Further, in the human brain, isoflurane (0.5% expired) has been shown to reduce CMRglu by 46% (Alkire et al, 1997). Propofol administered in concentrations inducing unconsciousness in humans also reduced CMRglu by ∼50% (Sun et al, 2008). Although much information has been gained from positron emission tomography studies in the live brain, it is important to acknowledge, that CMRglu measured by 2-deoxy-2-(18F)fluoro-D-glucose is interpreted as an indirect measure of neuronal function, and only provides information on the rate of glycolysis under the assumption of oxidative phosphorylation (Fowler and Ido, 2002). In other words, additional metabolic processes are not assessed or quantified; and there is a lack of knowledge in regards to the effects of specific general anesthetics on other metabolic processes and pathways in the live brain.
An alternative method for characterizing the metabolic state of the brain or biofluids is by way of ‘metabolomics' (Brindle et al, 2002; Coen et al, 2008). The field of metabolomics is defined as the study of small-molecular-weight molecules involved in metabolism, with a focus on how their levels change and/or which metabolites are more involved in response to different drugs or pathologies (Ebbels and Cavill, 2009). Metabolomic profiling can be applied to spectra obtained in vitro (e.g., urine, blood, or cerebrospinal fluid) using mass spectrometry or nuclear magnetic resonance, or in vivo from the brain using magnetic resonance spectroscopy (1HMRS) (Chan et al, 2009; Griffin and Kauppinen, 2007; Hirayama et al, 2009). An advantage of metabolomic profiling using 1HMRS is its inherent ability to track multiple metabolites (known and unknown) concurrently by a noninvasive approach in the live human or animal brain. Using previous knowledge of spectral signatures of pure metabolites, several brain metabolites can be identified and quantified. To our knowledge, metabolomic profiling has not before been applied to characterize and compare effects of commonly used anesthetics on the cerebral ‘metabolome' in vivo. Such information can provide new knowledge as to metabolic patterns or biomarkers of specific importance to certain anesthetic regimes. Importantly, this approach can also be applied in future translational clinical studies in humans investigating short- and long-term effects of anesthetics. We therefore applied in vivo 1HMRS to investigate the effects of two different anesthetics (isoflurane and propofol) on the metabolomic profiles in the rat brain. Specifically, we chose to focus on the hippocampus and the parietal cortex given their role in memory formation and visual spatial processing, respectively, which are cognitive domains reported to be affected after general anesthesia and surgery exposures (Jevtovic-Todorovic et al, 2003; Zhu et al, 2010). Given the dissimilar uncoupling effects of the two anesthetics on CMRglu, cerebral blood flow, and electroenchephalographic (EEG) activity (Akrawi et al, 1996), we hypothesized that their corresponding metabolomic profiles would be distinct and reflect differences in spectral signatures related to Glu (excitatory neurotransmission) and possibly glucose (Glc) metabolites.
Materials and methods
All animal procedures were approved by the local institutional animal care and use committees (Brookhaven National Laboratory and Stony Brook University). Male Fisher-344 rats (250 to 310 g) were used for the study. Group 1 (n=12) was exposed to 1 minimal alveolar concentration (MAC) isoflurane, and group 2 (n=11) was exposed to propofol (1MAC equivalent). We chose 1MAC because isoflurane at this dose range induces less ‘luxury perfusion' when compared with >1MAC where vasodilatory and cerebral blood flow effects dominate (Maekawa et al, 1986).
Animal Preparation and 1HMRS Scanning
Group 1 (isoflurane)
The rats were initially anesthetized with 2% to 3% isoflurane, orally intubated, and mechanically ventilated. Anesthesia was maintained with 1.2% to 1.5% isoflurane delivered in oxygen. A femoral artery catheter was inserted for continuous measurement of the mean arterial blood pressure and periodic assessment of blood gases during the experiment. Additionally, blood sampling was performed during the experiment to monitor plasma levels of Glc and lactate (Lac). An intravenous femoral infusion line as well as a 24-gauge venous tail catheter was placed for administration of fluids. The femoral surgical groin site was closed with 3.0 silk, and the wound carefully infiltrated with 1% lidocaine for local analgesia.
Determination of 1 minimal alveolar concentration equivalent propofol infusion dose
For the purposes of our study, it was important to determine the 1MAC equivalent for propofol defined as the intravenous propofol infusion rate that would prevent movement in 50% of rats in response to a predefined pain stimulus (tail clamp). A separate series of rats were used for these experiments, and the procedures are described in detail elsewhere (Supplementary material); and the 1MAC equivalent propofol infusion range averaged ∼600 to 650 μL/kg/min, which is in agreement with a previous study by Todd and Weeks (1996).
Group 2 (propofol)
All rats were initially anesthetized with 2% to 3% isoflurane, orally intubated, and mechanically ventilated. Surgical procedures were the same as described for group 1 rats. The propofol infusion (1MAC equivalent to the isoflurane dosing predetermined as described above) was initiated via the femoral venous line and the isoflurane anesthesia weaned off (total isoflurane exposure time ∼20 to 30 minutes).
1HMRS scanning
All 1HMRS acquisitions were performed on a 9.4T/20 magnetic resonance imaging instrument interfaced to a Bruker Advance console and controlled by Paravision 5.0 software (Bruker Bio Spin, Billerica, MA, USA). To obtain the best possible 1HMRS spectra, we used a custom-built animal cradle system that allowed for accurate and consistent positioning of the rat brain in the magnetic field center with the greatest symmetry. Physiological parameters, including respiratory rate, oxygen saturation, body temperature, blood pressure, and heart rate, were continuously monitored using magnetic resonance imaging compatible optical monitors (SA Instruments, Stony Brook, NY, USA). Body temperature was kept strictly within 36.5°C to 37.5°C during imaging using a computer assisted air heating system (SA Instruments). Further, the inspiratory isoflurane concentration was continuously monitored to assure constant MAC level during the isoflurane experiments. A custom-made 3-cm surface radio-frequency coil was used as a receiver and a 16-cm diameter volume coil (Bruker) was used as a transmitter. T2-weighted images were first obtained in three orthogonal planes using a rapid acquisition refocusing echoes (RARE) sequence (repetition time (TR)=2,500 milliseconds, echo time (TE)=40 milliseconds, number of acquisitions (NA)=4, RARE factor=8, number of slices=25, in plane resolution=0.117 mm/pixel, slice thickness=0.8 mm, slice gap=0.1 mm) to assure correct positioning and accurate voxel positioning in MRS. First- and second-order shim were accomplished using Fastmap (Gruetter, 1993). For spectroscopy, rectangular single voxel volumes (1.726 × 1.2 × 4 mm3) positioned in the parietal cortex (scan 1) and subsequently in the dorsal hippocampus (scan 2) were localized using a point-resolved spectroscopy sequence. The following point-resolved spectroscopy parameters were used: TR=2,000 milliseconds, TE=12 milliseconds; number of averages=1,796; spectral width=8,012 Hz, number of acquired complex points=4,096 yielding a spectral resolution of 1.96 Hz/pts. A water unsuppressed scan was also acquired to serve as a concentration reference in quantifying metabolites' concentrations. Retro frequency lock was active during all acquisitions for monitoring of the unsuppressed navigator water signal within the same voxel volume, and frequency drift during the acquisition was corrected by in-house-developed MATLAB software. Each of the free induction decay signals was then summed before the data analysis.
Data Processing
Data analysis of 1HMRS spectra was performed using linear combination modeling LCModel (Stephen Provencher Inc., Toronto, Canada; Provencher, 2001) by fitting 15 different simulated metabolite spectra to derive metabolites concentrations, and subsequent statistical analysis included both univariate (two-tailed t-test) and multivariate (metabolomics) analyses (SIMCA-P+, version 12.0.1.0, Umetrics AB, S-907 Ume, Sweden). Metabolomics provides information on spectral differences on selected metabolites between the two conditions; and the importance of each metabolite can be quantified by discriminating between groups (i.e., propofol or isoflurane condition).
Metabolite quantification using LCModel
The raw 1HMRS spectra corrected for frequency drift and summed were used for LCModel analysis. No time-domain filtering was applied to the spectral time-domain data before LCModel analysis. The LCModel basis data set simulated for the 9.4T and TE=12 milliseconds included the following metabolites: alanine, aspartate, creatine (Cr), phosphocreatine, γ-aminobutyric acid, Glc, glutamine, Glu, glycerophosphorylcholine (GPC), phosphorylcholine (PCh), glycine, myoinositol, scyllo-inositol, Lac, N-acetylaspartate, N-acetylaspartylglutamate, and taurine. In addition, simulated spectra of lipids were included (Provencher, 2001). The LCModel analysis was performed on each of the TE 12 milliseconds spectra from the chemical shift range of 0.0 to 4.0 p.p.m. The Cramer–Rao lower bounds (CRLBs) were used to eliminate statistically unreliable values.
Incorporation of anesthesia-related macromolecules in the basis data set
Macromolecule (MM) spectra were acquired separately in vivo and were incorporated in the simulated basis data sets for accurate LCModel determination of metabolites concentrations (Behar et al, 1994; Pfeuffer et al, 1999). A single inversion pulse was applied before the point-resolved spectroscopy sequence to nullify metabolites, and MM enhanced spectra were acquired (inversion time=670 milliseconds). The in vivo MM-averaged spectra were acquired for each separate anesthetic condition. Thus, the final LCModel basis data set used for analysis of all TE=12 milliseconds spectra contained brain metabolites, simulated lipids, and MMs determined in vivo.
Statistical analysis
All data are presented as average±s.d. Statistical comparison of physiological parameters between the two anesthesia groups were performed using a two-tailed independent Student's t-test, and a P-value <0.05 was considered significant. Simple regression and Pearson's correlation analysis were performed to assess the linear relationship between corresponding concentrations of Lac in brain and blood for each animal. Metabolite concentrations for each brain region calculated by LCModel for each anesthesia condition were analyzed by a multiple t-test, controlling for multiple comparisons using the false discovery rate (QVALUE package in R software http://www.genomine.org/qvalue/). This approach is considered less conservative compared with conventional Bonferroni correction for multiple comparisons (Storey and Tibshirani, 2003).
Metabolomic analysis using the LCModel-calculated metabolite concentrations
Metabolite concentrations calculated from the LCModel were used for the metabolomic analysis, and only metabolites with CRLB ≤50% in more than 80% of the spectra were considered. Partial least square discriminative analysis was performed on these selected metabolites to quantify which of the metabolites were most involved for the separation of the two anesthesia conditions. Partial least square discriminative analysis was performed using mean centering and unit variance, and the results from the partial least square discriminative analysis were displayed using the following features:
Score plot of the first two principal components where each point in the score plot represents one subject.
The significance of each principal component as evaluated by the Q2 value that also indicates the predictive power of each component. A Q2 value >0.3 to 0.5 is considered statistically significant, and higher Q2 value means more predictive power.
Each metabolite's ‘variable importance in the projection (VIP) value' is calculated and used to assess the relative importance of the metabolite in terms of discriminating the two groups. A VIP value >1 is suggestive of a given metabolite being a better discriminatory variable.
The loading plot provides a graphical summary of the correlation of X and Y (dependent variable), and therefore in our case indicates metabolites that are most significant in separating the isoflurane anesthesia condition from that of propofol.
Results
Physiological Parameters
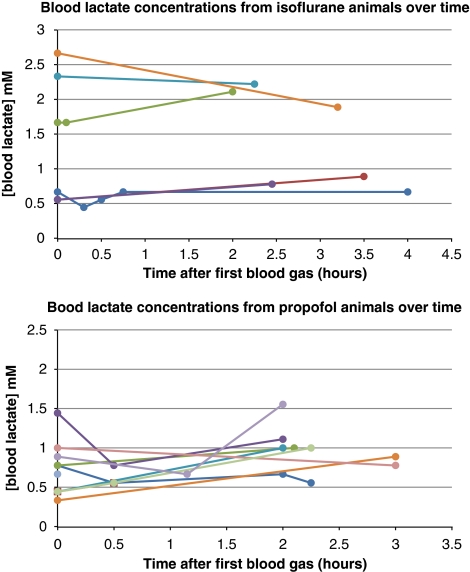
Four animals from group 1 and one animal from group 2 were excluded from the data analysis because of either physiological abnormalities or poor quality of the 1HMRS acquisitions. The hemodynamic parameters from the two groups of animals included in the analysis are presented in Table 1; and demonstrates that the average mean arterial blood pressures during the two consecutive 1HMRS scans were stable from scan to scan but significantly higher in the propofol group compared with isoflurane (∼130 mm Hg versus ∼100 mm Hg; P<0.05) albeit within normal cerebral autoregulation. Table 1 also shows that the average arterial blood gas values, including pH, PCO2, HCO3, and base excess values, were normal in both groups for the duration of the study period. In addition, in all animals, hypoxia was absent (PaO2 >300 mm Hg). The average Lac and Glc concentrations were higher in animals anesthetized with isoflurane when compared with propofol, although all of the rats were nonfasting. In the isoflurane-anesthetized rats, the blood Lac concentrations were overall more variable when compared with propofol but stable over time for a given animal (Figure 1). Importantly, the higher blood Lac levels in the isoflurane-anesthetized rats compared with propofol were not associated with metabolic acidosis.
Table 1. Physiological parameters, arterial blood gas, lactate, and glucose levels.
| Parameter | Isoflurane (n=8) | Propofol (n=10) |
|---|---|---|
| MABP (mm Hg)—1st scan (parietal cortex) | 93±9 | 129±7* |
| MABP (mm Hg)—2nd scan (hippocampus) | 94±9 | 130±8* |
| Heart rate (BPM)—1st scan (parietal cortex) | 335±13 | 367±36 |
| Heart rate (BPM)—2nd scan (hippocampus) | 341±20 | 370±23 |
| Temperature (°C)—1st scan (parietal cortex) | 36.9±0.4 | 37.3±1.0 |
| Temperature (°C)—2nd scan (hippocampus) | 36.9±0.5 | 36.8±0.4 |
| PaCO2 (mm Hg) | 34.7±3.8 | 38.5±6.3 |
| PaO2 (mm Hg) | 360±42 | 363±59 |
| pH | 7.43±0.04 | 7.41±0.05 |
| HCO3 (mmol/L) | 21.8±1.5 | 23.1±1.8 |
| BE (mmol/L) | −2.3±1.7 | −1.3±1.6 |
| Lactate (mmol/L) | 1.4±0.8 | 0.8±0.2* |
| Glucose (mmol/L) | 9.9±1.5 | 5.1±0.6* |
BE, base excess. Data are average±s.d. Temperature is rectal body temperature.
*P<0.05.
Figure 1.
The blood lactate (Lac) concentrations in 6 out of the 8 rats anesthetized with isoflurane and in 9 of the 10 propofol-anesthetized rats were measured in conjunction with the collection of blood gases from the time the animals were positioned in the scanner. As can be seen in the Figure, with isoflurane, the blood Lac concentrations were overall more variable from animal to animal when compared with propofol but stable over time for a given animal.
Quantitative LCModel 1HMRS Analysis
1HMRS spectra quality
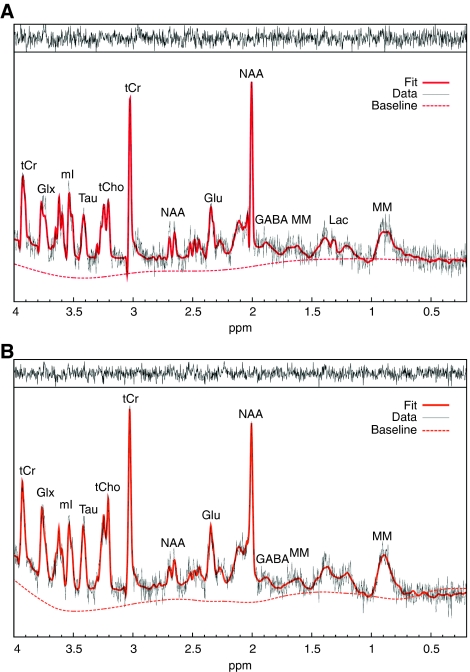
Figure 2 demonstrates typical 1HMRS spectra acquired in the hippocampus of a rat during isoflurane (A) and another rat anesthetized with propofol (B) anesthesia. Signal-to-noise ratio as evaluated by LCModel yielded average of ∼12 (the signal-to-noise ratio in LCModel is defined as the ratio of the maximum peak in the spectrum-minus-baseline over the analysis window to twice the root-mean-square; and the maximum peak occurs at N-acetylaspartate for most of the spectra); and there were no differences in spectral quality between spectra acquired in the parietal cortex or the hippocampus. Spectra with a full width half maximum >0.030 p.p.m. and/or signal-to-noise ratio <8 were considered of poor quality and were excluded from analysis. The spectral quality allowed identification of several metabolites as indicated, including Glu, glutamine, and Lac. The average CRLBs was within acceptable range (<50%) for analyzing the concentrations of the following metabolites: [Cr] (CRLB∼20%), [phosphocreatine] (CRLB∼6%), [γ-aminobutyric acid] (CRLB∼20%), [Glu] (CRLB∼5%), [glutamine] (CRLB∼15%), [myoinositol] (CRLB∼5%), [N-acetylaspartate] (CRLB∼3%), [taurine] (CRLB∼6%), [Gua] (CRLB∼10%), and [GPC+PCh] (CRLB∼7%). For all animals anesthetized with isoflurane, the CRLB for [lac] averaged 20% to 30%. For all animals where the CRLB of [lac] was >500%, the [lac] was assumed negligible and set to zero. Although the CRLB for the concentration of Glc ([glc]) was relatively high, we included it in the analysis. Specifically, the CRLB for [glc] was ∼50% to 80% for the isoflurane-anesthetized animals and for CRLB >500%, the [glc] was set to zero.
Figure 2.
Typical magnetic resonance spectroscopy spectra acquired from the hippocampus of a rat anesthetized with 1MAC isoflurane (A) and 1MAC equivalent propofol (B) analyzed using LCModel. There is sufficient water suppression and excellent spectral resolution to resolve at least 12 to 15 metabolites. The raw unsmoothed spectra are shown (black) in addition to the LCModel-fitted output (red solid line). Asp, aspartate; GABA, γ-aminobutyric acid; Glu, glutamate; Glx, glutamine+glutamate; Lac, lactate; mI, myoinositol; MAC, minimal alveolar concentration; MM, macromolecule; NAA, N-acetylaspartate; Tau, taurine; tCho, total choline; tCr, total creatine. For color figure see html version.
Metabolite concentrations: isoflurane versus propofol
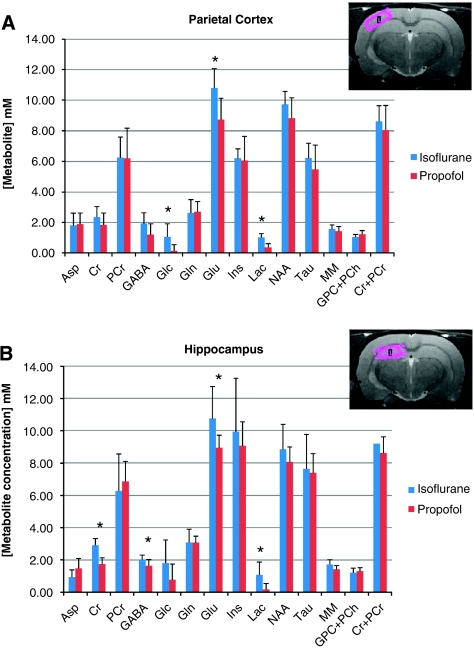
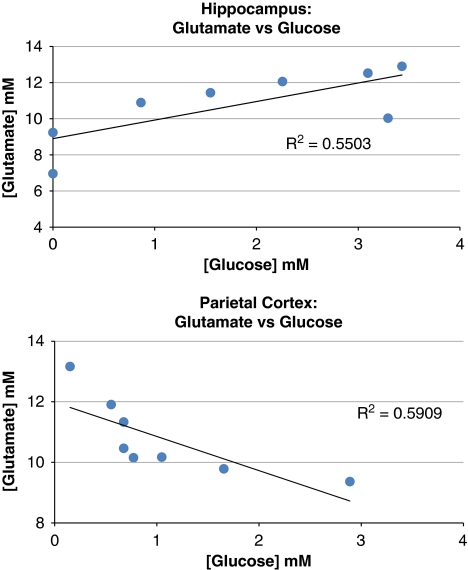
Figure 3 shows the effects of isoflurane and propofol administered at equipotent doses on the concentrations of the selected metabolites in the hippocampus and parietal cortex. In the hippocampus (Figure 3B), the [glu] and [lac] were significantly higher during isoflurane anesthesia when compared with propofol. Specifically, the [lac] during isoflurane and propofol was ∼1.1 mmol/L and ∼0.2 mmol/L, respectively (P=0.0048); and [glu] during isoflurane and propofol was 10.8 mmol/L and 8.9 mmol/L, respectively (P=0.016). Further, in the hippocampus, [Cr] was found to be 40% higher with isoflurane when compared with propofol (2.9±0.4 mmol/L versus 1.7±0.4 mmol/L, P=0.00001). Finally, the concentration of γ-aminobutyric acid was also found to be higher with isoflurane compared with propofol (P=0.029); however, concentrations estimates of γ-aminobutyric acid via LCModel is prone to error, unless it is edited because of extensive overlap with other resonances some of which are not fully described in the model spectra because of line-shape differences, susceptibility broadened water and lipids that are not eliminated by localization, and metabolites/MM resonances not in the basis set (Rothman et al, 1993). For all animals anesthetized with isoflurane, a correlation analysis of the [glu] versus [glc] for each of the animals was performed (cf. Figure 4), revealing that the two metabolites were positively correlated (Pearson's correlation between [glu] and [glc], r=0.742, P=0.035).
Figure 3.
(A) LCModel quantification of metabolites from rat parietal cortex during anesthesia with 1 minimal alveolar concentration (1MAC) isoflurane (dark blue) or 1MAC equivalent propofol (red). The anatomical magnetic resonance imaging (MRI) image in the upper right corner shows the typical location of the parietal voxel for magnetic resonance spectroscopy (1HMRS). Statistical analysis demonstrates significant higher concentrations of lactate (Lac; P=0.00003), glutamate (P=0.0049), and glucose (P=0.0085) in rats exposed to 1MAC isoflurane compared with 1MAC equivalent propofol. (B) LCModel quantification of metabolites from rat hippocampus during anesthesia with isoflurane (dark blue) or propofol (red). The anatomical MRI image in the upper right corner shows the typical location of the hippocampal voxel for 1HMRS. Statistical analysis demonstrates significant higher concentrations of Lac (P=0.0048), glutamate (P=0.016), γ-aminobutyric acid (P=0.029), and creatine (P=0.00001) in rats exposed to 1MAC isoflurane compared with 1MAC equivalent propofol. For color figure see html version.
Figure 4.
For each of isoflurane-anesthetized rats, the concentration of glutamate ([glu]) was plotted against the concentration of glucose ([glc]). Statistical analysis revealed that for the hippocampus, the Pearson's correlation between [glu] and [glc] was positively correlated (r=0.742) with a P-value of 0.035. In contrast, for the parietal cortex, [glu] and [glc] were negatively correlated (r=−0.769, P=0.026).
In the parietal cortex (Figure 3A), [lac] and [glu] were similarly observed to be higher with isoflurane compared with propofol (Figure 3). Interestingly, in the parietal cortex, [glc] was also found to be significantly higher with isoflurane and very low with propofol (P=0.0085). Finally, the concentration of total choline ([GPC]+[PCh]) trended to be higher with propofol compared with isoflurane, although this difference did not reach statistical significance (P=0.08). In contrast to the hippocampus, when plotting the parietal cortex [glu] versus [glc] for each of the isoflurane-anesthetized animals (Figure 4), the correlation analysis revealed that the two metabolites were negatively correlated (Pearson's correlation between [glu] and [glc], r=−0.769, P=0.026).
To further evaluate the concentration estimates, we combined individual spectra from each group and derived representative spectra (mean of all scans) for all animals exposed to isoflurane and propofol (results not shown). The corresponding quantitative output showed that for the hippocampus, the mean [lac] for isoflurane and propofol was 1.13 mmol/L (CRLB=18%) and 0.18 mmol/L (CRLB=68%), respectively. For the parietal cortex, the [lac] was 1.2 mmol/L (CRLB=13%) and [glc] was 2.2 mmol/L (CRLB=19%) for isoflurane; and for propofol, the [lac] and [glc] were 0.16 mmol/L and negligible (0), respectively.
Correlation Between Brain and Blood Lactate Levels
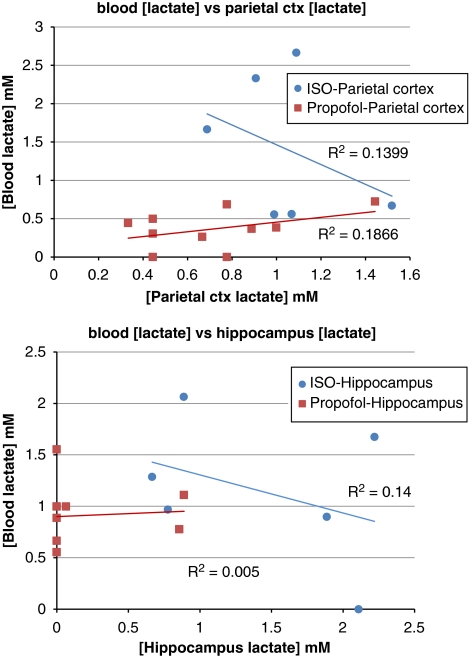
For all animals, we further assessed for each brain region the linear relationship between [lac] and the corresponding [lac] measured in blood. Figure 5 shows the data and demonstrates that the two entities are independent; suggesting that the higher [lac] observed with isoflurane was independent of blood Lac levels.
Figure 5.
The top graph shows the parietal cortex concentration of lactate ([lac]) plotted against the blood [lac] for 6 of the 8 isoflurane rats (blood lactate (Lac) from two animals could not be measured because of malfunction of the Lac electrode in the blood gas analyzer) and the 10 propofol rats; and the linear regression analysis demonstrated that the two entities were independent (P>0.05). The lower graph shows the same parameters and results for the hippocampus. Thus, the higher concentrations of Lac observed in the parietal cortex and hippocampus with isoflurane compared with propofol are independent of blood Lac levels.
Effect of Lipids
It was important to make certain that the lower brain [lac] in animals anesthetized with propofol was not caused by higher than normal concentrations of 1HMRS-detectable lipids accumulating in the brain, given the fact that propofol is dissolved in a lipid emulsion; and further that spectral signatures of lipid (1.28 to 1.30 p.p.m.) and Lac (1.31 to 1.33 p.p.m.) potentially overlap. We therefore performed 1HMRS in three rats anesthetized with 1MAC isoflurane while receiving an intravenous intralipid infusion (∼650 μL/kg/min with 10% intralipid (10% Intralipid, Fresenius Kabi, Clayton, NY, USA)). The LCModel analysis of hippocampus and parietal cortex spectra acquired under these conditions revealed a [lac] that was within range (1.2 to 1.4 mmol/L) to that observed in isoflurane-anesthetized rats without intravenous intralipid administration (∼1.2 mmol/L). It also showed comparable levels of 1HMRS-detectable lipids in the 1.25 to 1.30 p.p.m. range as was found with propofol-infused animals, suggesting that the higher brain [lac] was isoflurane specific.
Metabolomic Analysis
Hippocampus
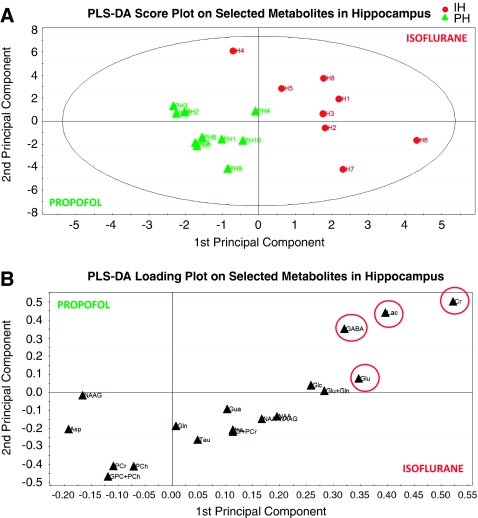
The partial least square discriminative analysis on two first principal components revealed a Q2 value of 0.44 of the first component and a cumulative Q2 value of 0.61 on the first and second, indicating a good fit of the data according to the classification (i.e., propofol versus isoflurane), and a model of high predictive power. The first component explained 69% of the variation between the two groups, and the first and second components together explained 84%. The corresponding score plot (Figure 6A) shows the clear separation of the two experimental groups (in Figure 6A, each green triangle in the score plot represents a propofol-anesthetized rat; and each of the red circles represents an isoflurane-anesthetized rat). The VIP value was calculated for each of the selected metabolites; and Cr and Lac were assigned VIP values of ∼2.2 and ∼1.7, respectively, whereas Glu's VIP was ∼1.5, indicating that Cr and Lac are more important for the metabolomic separation compared with Glu at least in the hippocampus region (cf. Figure 6B).
Figure 6.
In the hippocampus, the metabolite concentrations calculated from the LCModel were used for the metabolomic analysis; and the partial least square discriminative analysis revealed a cumulative Q2 value of 0.61 on the first and second, indicating a good fit of the data according to the classification (i.e., propofol versus isoflurane). The corresponding score plot (A) shows the separation of the two experimental groups (in A each green triangle in the score plot represents a propofol-anesthetized rat; and each of the red circles represents an isoflurane-anesthetized rat). The loading plot demonstrates that creatine ([Cr]) and lactate ([lac]) are most important for the separation compared with glutamate (cf. B). For color figure see html version.
Parietal cortex
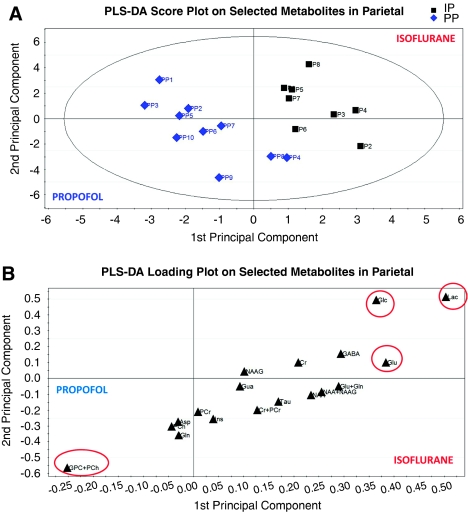
A similar analysis was performed on the spectra from the parietal cortex. First, a significant separation between isoflurane- and propofol-anesthetized animals was observed in the score plot (Figure 7A) with a Q2 value of 0.47 on the first component and a cumulative Q2 value of 0.70 on the combined first and second components, indicating an excellent fit of the data according to the classification (i.e., propofol versus isoflurane), and a model of high predictive power. The first component explained 68% of the variation between the two groups, and the first and second component together explained 86%. Lac and [glu] were assigned the VIP values of ∼2.1 and ∼1.6, respectively; whereas [glc] and [GPC+PCh] were assigned VIP values of ∼1.5 and ∼1.1, respectively. The corresponding loading plot (Figure 7B) revealed that [lac], [glu], and [glc] were relatively higher for isoflurane and that [GPC+PCh] were relatively higher for propofol.
Figure 7.
In the parietal cortex, the metabolomic analysis revealed a significant separation between the isoflurane- and propofol-anesthetized animals, which was observed in the score plot (A—each blue diamond in the score plot represents a propofol-anesthetized rat; and each of the black squares represents an isoflurane-anesthetized rat) with a cumulative Q2 value of 0.70 on the combined first and second components, indicating an excellent fit of the data according to the classification and a model of high predictive power. The corresponding loading plot (B) shows that lactate ([lac]), glutamate ([glu]), and glucose ([glc]) were relatively higher for isoflurane, and glycerophosphorylcholine ([GPC]) in combination with phosphorylcholine ([PCh]) was relatively higher for propofol. For color figure see html version.
Discussion
Using in vivo 1HMRS in combination with two different spectral signal processing approaches, we documented that the metabolomic profiles in the live rat brain during anesthesia with isoflurane differed from that acquired during propofol at equipotent doses. The quantitative LCModel analysis demonstrated that the concentration of Lac ([lac]) was approximately fivefold higher during isoflurane anesthesia compared with propofol in both the hippocampus and the parietal cortex. This increase was demonstrated to be independent of blood Lac levels, and not because of an overlap of Lac and lipid spectral signatures. Further, in both brain regions, [glu] was ∼20% higher during isoflurane anesthesia compared with propofol. In addition, the [glc] was significantly higher in the parietal cortex with isoflurane when compared with propofol. The metabolomic analysis further revealed that [lac] was one of the most important metabolites in the metabolomic separation of the two anesthesia conditions.
Several investigators have quantified the neurochemical profile using 1HMRS in the rodent brain during various anesthetic regimens, and these studies are summarized in Table 2. As can be observed from Table 2, there is strong agreement that isoflurane in the 1MAC range (1.2% to 1.5%) produces a distinct neurochemical profile in the cortex with a [lac] of ∼1 mmol/L, [glu] ∼10 to 11 mmol/L, and [Cr] ∼3.0 mmol/L (Du et al, 2009; Lei et al, 2010; Pfeuffer et al, 1999; Tkac et al, 2003, 2004). For hypnotics, such as α-chloralose and thiopental (‘light'-anesthesia states), the cortical [lac], [glu], and [Cr] appear within range of that observed with 1MAC isoflurane (cf. Table 2). However, for [glc], the anesthesia condition and depth matter. First, the cerebral [glc] is known to be directly dependent on the plasma Glc concentration (Dienel et al, 1997; Savaki et al, 1980). However, for a given steady-state plasma Glc concentration, the cortical [glc] has been shown to increase under conditions where thiopental anesthesia transited from a light to a deeper state, presumably reflecting a decrease in metabolic demand (Lei et al, 2010). In our study, where isoflurane and propofol were administered at equipotent dose ranges, we observed a higher cortical [glc] in isoflurane-anesthetized rats compared with propofol, which could be explained by the corresponding difference in blood Glc concentrations between the two anesthesia regimens (Glc–isoflurane ∼9.0 mmol/L versus Glc–propofol ∼5.0 mmol/L). However, alternative explanations for the higher cortical [glc] with isoflurane compared with propofol at the same anesthetic depth could be that (a) the metabolic rate of Glc is relatively lower in the isoflurane animals in comparison with propofol and/or (b) an alternate energy source is utilized (i.e., Lac). In line with ‘a', the Glu-neurotransmitter cycling would also be expected to be reduced (Sibson et al, 1998), and the observation that [glu] and [glc] were negatively correlated in the parietal cortex suggesting that higher Glu levels were present in animals with lower Glc content is in opposition to this notion (cf. Figure 4).
Table 2. Literature regarding the effects of anesthetics on the neurochemical profile in the rodent brain investigated with 1HMRS.
| Animal species | MRI | Anesthetic | Brain region | [Lac] (mmol/L) | [Glu] (mmol/L) | [Glc] (mmol/L) | [Cr] (mmol/L) | [Cr+PCr] (mmol/L) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| SD rat | 9.4T | Isoflurane (2%) | Cortex and hippocampus | ∼1.9 | ∼8.7 | ∼3.5 | ∼3.9 | ∼8.5 | Pfeuffer et al (1999) |
| SD rat (28 days) | 9.4T | Isoflurane (1.5%–2%) | Cortex and hippocampus | ∼1.5 | ∼9–11 | ∼5.0 | ∼5.7 | Tkac et al (2003) | |
| Mouse | 9.4T | N2O and 1.2%–1.5% isoflurane | Cortex | ∼2.5 | ∼11–12 | ∼3.5 | ∼5.0 | ∼8–10 | Tkac et al (2004) |
| SD rat | 9.4T | α-Chloralose (plasma glucose ∼12 mmol/L) | Cortex | ∼1.0 | ∼10–11 | ∼3.0 | ∼3.5 | ∼8.5 | Lei et al (2010) |
| SD rat | 9.4T | Deep thiopental (plasma glucose ∼12 mmol/L) | Cortex | ∼1.0 | ∼10–11 | ∼5.0 | ∼3.0 | ∼7.5–8.0 | Lei et al (2010) |
| SD rat | 9.4T | N2O and isoflurane 2% | Cortex | ∼1.0 | ∼9.0 | ∼1.8 | ∼3.5 | ∼8.0 | Du et al (2009) |
| SD rat | 9.4T | Light thiopental | Cortex | ∼1.0 | ∼8.4 | ∼1.6 | ∼3.2 | ∼8.0 | Du et al (2009) |
| SD rat | 9.4T | Deep thiopental | Cortex | ∼1.0 | ∼8.8 | ∼0.70 | ∼4.0 | ∼8.0 | Du et al (2009) |
| Fisher rat | 9.4T | Isoflurane 1MAC | Cortex | ∼1.0 | ∼10–11 | ∼1.0 | ∼2.5 | ∼8.0–9.0 | Our study |
| Fisher rat | 9.4T | Propofol 1MAC equivalent | Cortex | ∼0.3 | ∼9.0 | <0.3 | ∼1.8 | ∼8.0–9.0 | Our study |
Cr, creatine; Glc, glucose; Glu, glutamate; Lac, lactate; MAC, minimal alveolar concentration; MRI, magnetic resonance imaging; PCr, phosphocreatine; SD, Sprague–Dawley. Italics signifies that the results originate from this study.
The neurochemical profile with propofol reported here was different from that reported for other general anesthetics and hypnotics in as much as the [lac], [glc], and [Cr] were significantly lower compared with previous reported studies of other anesthetics (cf. Table 2). The multivariate metabolomic analysis also revealed that the metabolites most involved in the spectral differences between the two anesthesia states included [lac], [glu], [glc], and [Cr] (hippocampus only). The different metabolomic profile of propofol compared with isoflurane may bear impact on future studies using more advanced spectroscopic techniques for further understanding the underlying biochemical processes behind these differences; and in particular, the significance of the lower [lac] with propofol in comparison with isoflurane needs to be investigated in more detail. For example, two-dimensional 1H homonuclear spectroscopic methods could be expected to provide better spectral resolution and more efficient identification of metabolites.
As shown in Table 2, a cerebral [lac] at ∼1 mmol/L during isoflurane anesthesia is well documented in the literature. Furthermore, more than 20 years ago, Newberg et al reported that increasing concentrations of isoflurane produced a mild dose-related cerebral lactic acidosis in dog brains (measured from cortical biopsies) while not altering normal oxidative phosphorylation (Newberg et al, 1983). Another more recent study in primates used diffusion 1HMRS and also reported higher levels of Lac with increasing isoflurane concentration, and attributed this to isoflurane-induced inhibition of mitochondrial respiration (Valette et al, 2007). Accordingly, the higher [lac] with isoflurane compared with propofol observed in our study could be contributed to differences in the two anesthetics' influence on mitochondrial respiration. Isoflurane-induced inhibition of mitochondrial tricarboxylic acid cycle and uncoupling of electron transport could direct pyruvate toward Lac production in astrocytes or reduce neuronal Lac consumption or both. However, this explanation is probably unlikely because if there is an inhibition of mitochondrial oxidative phosphorylation (by isoflurane), the cell redox balance would be expected to increase that is not in agreement with higher levels of Lac. Isoflurane and other anesthetics are also known to downregulate certain antiapoptotic proteins that cause changes in mitochondrial membrane permeability, and these processes might also have a role in the altered metabolomic patterns observed here (Yon et al, 2005). The stimulating effect of isoflurane on the activity of the GLT1/EAAT2 glial Glu transporters could also contribute to an increase in Lac production (Do et al, 2002; Zuo, 2001). Finally, higher [lac] observed with isoflurane could also, in part, be an overall increase in the rate of glycolysis under aerobic conditions (Warburg effect) independent of excess neuronal activity and Glu release.
The [glc] as well as [lac] in the arterial blood of isoflurane-anesthetized rats was significantly higher than that measured in the propofol-anesthetized rats. It has been previously demonstrated that isoflurane but not propofol (when in combination with opioids) induces hyperglycemia in fed rats, in part, because of the inhibition of insulin release from the pancreas (Zuurbier et al, 2008). However, in our study, a linear regression analysis between blood and brain [lac] clearly demonstrated that the two occurred independent of each other. It has previously been shown by measuring arterio–venous differences that, at arterial concentrations of Lac below 1.5 mmol/L, there is zero or net efflux of Lac from the anesthetized rat brain (Leegsma-Vogt et al, 2001). Therefore, the higher [lac] observed with isoflurane is likely related to specific metabolic processes induced by this anesthetic and not derived from Lac crossing the blood brain barrier from the vascular compartment and accumulating in the brain. However, Lac is also known to accumulate in the brain under anaerobic (e.g., during hypoxemia, ischemia, and/or oligemia) conditions when pyruvate cannot be utilized in oxidative or gluconeogenic pathways. However, the greater [lac] during isoflurane anesthesia compared with propofol is unlikely to be caused by ischemia, hypoperfusion (oligemia), or hypoxia given the continuously normal mean arterial blood pressure (MABP) (∼100 mm Hg or higher) and oxygen and ventilation status. Thus, although different anesthetics effect the cerebral blood flow in different ways, higher than normal arterial oxygen tension as used in our study (and frequently in the clinical arena) might also have a role in the differences between the metabolomic profiles of the two anesthetic conditions; however, an additional series of animals with more physiological oxygen tensions continued to show the same pattern of differences (results not shown).
The metabolomic analysis was introduced to supplement the LCModel quantitative analysis. This approach has previously been used by other investigators to identify new spectral signatures for different disease states or experimental conditions or to understand the significance or impact of a given metabolite (Oberg et al, 2008). Univariate analysis (e.g., a t-test) of metabolites' concentrations would only provide information as to which metabolites are different between the groups, lacking information in regards to which metabolite is a better discriminatory factor. Metabolomics might therefore be valuable in regards to designing future experiments focused on further understanding biochemical pathways underlying the different metabolic patterns induced by the two anesthetics. In addition, the metabolomic analysis can also be used to assess model specificity and sensitivity that was not explored here but might be used to track long-term effects of anesthesia-induced metabolomic changes. Interestingly, Kawaguchi et al recently used extracted brain tissue from rats anesthetized from either propofol or isoflurane, and analyzed the hydrophilic fraction using in vitro nuclear magnetic resonance and multivariate statistical analysis; and reported that different metabolomic signatures (e.g., acetate, Glu, glutamine, and glycine) characterized brains exposed to isoflurane from propofol (Kawaguchi et al, 2010). The differences between metabolomic profiles between the two studies is likely because we performed the 1HMRS in vivo and not on extracted tissue free of hydrophobic substances; this would also explain why no lipid signatures were described for propofol in the study by Kawaguchi et al (2010).
Metabolomic Profiling of Anesthesia by 1HMRS—Is It Clinically Significant?
Previous work conducted in the live brain of humans and animals has documented reductions in CMRglu with isoflurane as well as propofol anesthesia, but did not elucidate other metabolic processes possibly differentiating the two states. We therefore applied in vivo 1HMRS in combination with two different signal processing approaches to investigate metabolomic consequences of isoflurane and propofol anesthesia in the brain administered at clinical relevant concentration ranges. It is clear from our data that the brain metabolome as defined by 1HMRS during isoflurane anesthesia is different than that of propofol; and that higher [lac] and [glu] characterize isoflurane at the 1MAC concentration range. It is unclear whether the different metabolomic patterns are significant from the point of view of possible anesthesia-related neurotoxicity observed in the young, immature brain or perhaps also in the senescent brain. The main advantage of in vivo 1HMRS lies in its noninvasiveness and translational abilities, and utility in all age groups, as it does not require radioactive ligands; therefore, future studies can be conducted in very young subjects. Importantly, metabolomic pattern recognition techniques can also be used to assess long-term effects of anesthetics in humans.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported in part by New York State Foundation for Science, Technology and Innovation (NYSTAR), NIH (K30 fellowship to RM), NCRR (1S10RR025515-01), and internal research funds from the Department of Anesthesiology, Stony Brook University. We thank our colleague Dr Ira J Rampil for providing expertise with the experimental design for the 1MAC equivalent of propofol determination. We also want to thank Dr Douglas L Rothman (Yale University) for spending time reading our manuscript, providing expertise including helpful and critical comments.
Supplementary Material
References
- Akrawi WP, Drummond JC, Kalkman CJ, Patel PM. A comparison of the electrophysiologic characteristics of EEG burst-suppression asproduced by isoflurane, thiopental, etomidate, and propofol. J Neurosurg Anesthesiol. 1996;8:40–46. doi: 10.1097/00008506-199601000-00010. [DOI] [PubMed] [Google Scholar]
- Alkire MT, Haier RJ, Barker SJ, Shah NK, Wu JC, Kao YJ.1995Cerebral metabolism during propofol anesthesia in humans studied with positron emission tomography Anesthesiology 82393–403.discussion 27A [DOI] [PubMed] [Google Scholar]
- Alkire MT, Haier RJ, Shah NK, Anderson CT. Positron emission tomography study of regional cerebral metabolism in humans during isoflurane anesthesia. Anesthesiology. 1997;86:549–557. doi: 10.1097/00000542-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994;32:294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- Brindle JT, Antti H, Holmes E, Tranter G, Nicholson JK, Bethell HW, Clarke S, Schofield PM, McKilligin E, Mosedale DE, Grainger DJ. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat Med. 2002;8:1439–1444. doi: 10.1038/nm1202-802. [DOI] [PubMed] [Google Scholar]
- Chan EC, Koh PK, Mal M, Cheah PY, Eu KW, Backshall A, Cavill R, Nicholson JK, Keun HC. Metabolic profiling of human colorectal cancer using high-resolution magic angle spinning nuclear magnetic resonance (HR-MAS NMR) spectroscopy and gas chromatography mass spectrometry (GC/MS) J Proteome Res. 2009;8:352–361. doi: 10.1021/pr8006232. [DOI] [PubMed] [Google Scholar]
- Coen M, Holmes E, Lindon JC, Nicholson JK. NMR-based metabolic profiling and metabonomic approaches to problems in molecular toxicology. Chem Res Toxicol. 2008;21:9–27. doi: 10.1021/tx700335d. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF, Adachi K, Sokoloff L, Holden JE. Determination of local brain glucose level with [14C]methylglucose: effects of glucose supply and demand. Am J Physiol. 1997;273:E839–E849. doi: 10.1152/ajpendo.1997.273.5.E839. [DOI] [PubMed] [Google Scholar]
- Do SH, Kamatchi GL, Washington JM, Zuo Z. Effects of volatile anesthetics on glutamate transporter, excitatory amino acid transporter type 3: the role of protein kinase C. Anesthesiology. 2002;96:1492–1497. doi: 10.1097/00000542-200206000-00032. [DOI] [PubMed] [Google Scholar]
- Du F, Zhang Y, Iltis I, Marjanska M, Zhu XH, Henry PG, Chen W. In vivo proton MRS to quantify anesthetic effects of pentobarbital on cerebral metabolism and brain activity in rat. Magn Reson Med. 2009;62:1385–1393. doi: 10.1002/mrm.22146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbels T, Cavill R. Bioinformatic methods in NMR-based metabolic profiling. Prog Nucl Mag Res Sp. 2009;55:361–374. [Google Scholar]
- Fowler JS, Ido T. Initial and subsequent approach for the synthesis of 18FDG. Semin Nucl Med. 2002;32:6–12. doi: 10.1053/snuc.2002.29270. [DOI] [PubMed] [Google Scholar]
- Griffin JL, Kauppinen RA. A metabolomics perspective of human brain tumours. FEBS J. 2007;274:1132–1139. doi: 10.1111/j.1742-4658.2007.05676.x. [DOI] [PubMed] [Google Scholar]
- Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M, Esumi H, Soga T. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009;69:4918–4925. doi: 10.1158/0008-5472.CAN-08-4806. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi H, Hirakawa K, Miyauchi K, Koike K, Ohno Y, Sakamoto A. Pattern recognition analysis of proton nuclear magnetic resonance spectra of brain tissue extracts from rats anesthetized with propofol or isoflurane. PLoS One. 2010;5:e11172. doi: 10.1371/journal.pone.0011172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegsma-Vogt G, Venema K, Postema F, Korf J. Monitoring arterio-venous differences of glucose and lactate in the anesthetized rat with or without brain damage with ultrafiltration and biosensor technology. J Neurosci Res. 2001;66:795–802. doi: 10.1002/jnr.10046. [DOI] [PubMed] [Google Scholar]
- Lei H, Duarte JM, Mlynarik V, Python A, Gruetter R. Deep thiopental anesthesia alters steady-state glucose homeostasis but not the neurochemical profile of rat cortex. J Neurosci Res. 2010;88:413–419. doi: 10.1002/jnr.22212. [DOI] [PubMed] [Google Scholar]
- Maekawa T, Tommasino C, Shapiro HM, Keifer-Goodman J, Kohlenberger RW. Local cerebral blood flow and glucose utilization during isoflurane anesthesia in the rat. Anesthesiology. 1986;65:144–151. doi: 10.1097/00000542-198608000-00003. [DOI] [PubMed] [Google Scholar]
- Newberg LA, Milde JH, Michenfelder JD. The cerebral metabolic effects of isoflurane at and above concentrations thatsuppress cortical electrical activity. Anesthesiology. 1983;59:23–28. doi: 10.1097/00000542-198307000-00005. [DOI] [PubMed] [Google Scholar]
- Oberg J, Spenger C, Wang FH, Andersson A, Westman E, Skoglund P, Sunnemark D, Norinder U, Klason T, Wahlund LO, Lindberg M. Age related changes in brain metabolites observed by 1H MRS in APP/PS1 mice. Neurobiol Aging. 2008;29:1423–1433. doi: 10.1016/j.neurobiolaging.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Olsen KS, Henriksen L, Dige-Petersen H, Chraemmer-Jorgensen B, Rosenorn J. Effect of ketanserin on global cerebral blood flow and cerebral oxygen metabolism during midazolam-fentanyl or isoflurane anaesthesia. Br J Anaesth. 1992;69:263–268. doi: 10.1093/bja/69.3.263. [DOI] [PubMed] [Google Scholar]
- Ori C, Dam M, Pizzolato G, Battistin L, Giron G. Effects of isoflurane anesthesia on local cerebral glucose utilization in the rat. Anesthesiology. 1986;65:152–156. doi: 10.1097/00000542-198608000-00004. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, Tkac I, Choi IY, Merkle H, Ugurbil K, Garwood M, Gruetter R. Localized in vivo 1H NMR detection of neurotransmitter labeling in rat brain during infusion of [1-13C] D-glucose. Magn Reson Med. 1999;41:1077–1083. doi: 10.1002/(sici)1522-2594(199906)41:6<1077::aid-mrm1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci USA. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaki HE, Davidsen L, Smith C, Sokoloff L. Measurement of free glucose turnover in brain. J Neurochem. 1980;35:495–502. doi: 10.1111/j.1471-4159.1980.tb06293.x. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Lactate efflux and the neuroenergetic basis of brain function. NMR Biomed. 2001;14:389–396. doi: 10.1002/nbm.741. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhang H, Gao C, Zhang G, Xu L, Lv M, Chai W. Imaging the effects of propofol on human cerebral glucose metabolism using positron emission tomography. J Int Med Res. 2008;36:1305–1310. doi: 10.1177/147323000803600618. [DOI] [PubMed] [Google Scholar]
- Tkac I, Henry PG, Andersen P, Keene CD, Low WC, Gruetter R. Highly resolved in vivo 1H NMR spectroscopy of the mouse brain at 9.4 T. Magn Reson Med. 2004;52:478–484. doi: 10.1002/mrm.20184. [DOI] [PubMed] [Google Scholar]
- Tkac I, Rao R, Georgieff MK, Gruetter R. Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo 1H NMR spectroscopy. Magn Reson Med. 2003;50:24–32. doi: 10.1002/mrm.10497. [DOI] [PubMed] [Google Scholar]
- Todd MM, Weeks J. Comparative effects of propofol, pentobarbital, and isoflurane on cerebral blood flow and blood volume. J Neurosurg Anesthesiol. 1996;8:296–303. doi: 10.1097/00008506-199610000-00007. [DOI] [PubMed] [Google Scholar]
- Valette J, Guillermier M, Besret L, Hantraye P, Bloch G, Lebon V. Isoflurane strongly affects the diffusion of intracellular metabolites, asshown by 1H nuclear magnetic resonance spectroscopy of the monkey brain. J Cereb Blood Flow Metab. 2007;27:588–596. doi: 10.1038/sj.jcbfm.9600353. [DOI] [PubMed] [Google Scholar]
- Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–827. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- Zhu C, Gao J, Karlsson N, Li Q, Zhang Y, Huang Z, Li H, Kuhn HG, Blomgren K. Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J Cereb Blood Flow Metab. 2010;30:1017–1030. doi: 10.1038/jcbfm.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z. Isoflurane enhances glutamate uptake via glutamate transporters in rat glial cells. Neuroreport. 2001;12:1077–1080. doi: 10.1097/00001756-200104170-00042. [DOI] [PubMed] [Google Scholar]
- Zuurbier CJ, Keijzers PJ, Koeman A, Van Wezel HB, Hollmann MW.2008Anesthesia's effects on plasma glucose and insulin and cardiac hexokinase at similar hemodynamics and without major surgical stress in fed rats Anesth Analg 106135–142.table of contents [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.