Abstract
Human hematopoietic stem cells (hHSCs) have enormous potential for clinical use in cell-based therapies, especially as a gene delivery system. Moreover, lentiviral transduction in stem cells is very often associated with low transduction efficiency and low levels of foreign gene expression. Therefore, it is important to analyze vector and promoter systems that can generate robust foreign gene expression in these cells. In this study, we evaluated and compared the ability of different commercially available promoters to drive the expression of exogenous reporter genes in hHSCs and evaluated the effect of different doses of stem cell growth factors on the expression of transgenes. We used lentivirus based vector system carrying the following promoters: 1) Human cytomegalovirus (CMV) promoter, 2) Simian virus 40 (SV40) promoter, 3) mammalian Ubiquitin C (UBC) promoter and 4) cellular polypeptide chain elongation factor 1 alpha (EF1) promoter. EF1 and CMV promoters robustly drove the expression of green fluorescence protein (GFP) reporter gene, while SV40 and UBC promoters induced very low level of GFP expression. Lentivectors containing EF1 and CMV promoters showed high-level stable GFP expression in human cord blood stem cells for 6 weeks period after post transduction. CD133+ hHSCs stimulated with higher concentration of growth factors exhibited enhancement of transduction rate. Cord blood derived CD133+ hHSCs could be effectively transduced with lentivectors under CMV or EF-1 promoters for the expression of foreign gene
Keywords: Cord blood stem cells (AC133+), Lentiviral vectors, Promoters and Green fluorescent protein (GFP)
Introduction
Human cord blood derived hematopoietic stem cell (hHSCs) can renew it-self and can differentiate to a variety of specialized cells [1, 2]. Umbilical cord blood has been used in children with Fanconi anemia as well as in patients suffering from other diseases [3, 4]. However, there is growing interest to use hHSCs as therapeutic gene carrier or delivery systems. The gene therapy strategies were studied to correct hematopoietic and genetic disorders using several different viral delivery systems such as moloney murine leukaemia virus (MoMLV) and oncoretroviral vectors [5-7]. The advanced HIV based lentiviral-gene transfer technologies have been developed to offer better gene delivery in a variety of cells [8-11]. The luciferase and heat stable antigen (HAS) genes were transduced into HSCs using HIV-based lentivectors [12, 13]. The promoters in the lentiviral vector system are critical for maximizing and stabilizing the foreign gene expression in the HSCs. For robust transgene expression in transduced cells, a variety of cellular and viral promoters have been used [14, 15]. Among them, cytomegalovirus (CMV) has been the most widely used because of it exhibited strong activity in wide range of cells and cell lines [16, 17]. The housekeeping-elongation factor 1 (EF1) has also driven high level of expression of foreign genes in variety of cells including hematopoietic cells [18-20]. The human Ubiquitin C (UBC) promoter was used to generate stable transgenic cell lines in situations where the use of CMV promoter was impeded by different silencing mechanism of host cells [21-23]. Nevertheless, there is limited data available on the effectiveness of different promoters in human cord blood derived CD34+/AC133+ (CD34+/CD133+) HSCs. Thus, it is important to analyze the promoter strength in the hHSCs before using these cells as a gene delivery vehicle for therapeutic purposes. Very recently investigators also indicated the importance of growth factors for enhanced transduction of viral vectors and expression of transgenes [24]. However, effect of different growth factors in respect of promoters’ efficiency in transduction of exogenous genes in cord blood hHSCs has not been reported. In this study, we attempted to determine optimal promoter for transgene expression in hHSCs and to enhance the viral transduction rate in hHSCs using cell growth factors.
Materials & Methods
Human hematopoietic stem cells (hHSCs)
Freshly collected CD34+/CD133+ hHSCs were used for transduction. CD34+/CD133+ hHSCs were collected from the human cord blood with an approved IRB protocol of Henry Ford Hospital. In brief, the cord blood mononuclear cell population was generated by Ficoll gradient centrifugation and was enriched for CD34+/CD133+ cells by immunomagnetic positive selection using the MidiMACS system (Miltenyi, Auburn CA) according to the manufacturer’s protocol. Freshly prepared CD34+/CD133+ cells were incubated in stem cell basal media supplemented with 40 ng/ml of stem cell factor (SCF), 40 ng/ml of FLT3 and 10 ng/ml of thrombopoietin (TPO) (all from CellGenix, IL). Initially cells were suspended in media at 1×106 per ml and grown in 5% CO2/95% air at 37°C in humidified atmosphere containing 5% CO2, with fresh media added on every third day. The cells were propagated for 7-10 days. To confirm the purity of the culture isolated from cord blood, flow cytometry was done at day 1 to assess the levels of progenitor markers CD133, CD34, hematopoietic CD45, CD117 and CD14 markers, and CD20 and CD3 that are commonly expressed on B and T cells, respectively. Majority of the cells were CD34+/CD133+.
HEK 293TN cell line
The HEK 293TN kidney fibroblasts (293TN cells) [System Biosciences (SBI), USA] cell line was cultured in 1XDMEM (Mediatech, USA), supplemented with 2 mmol/L L-glutamine, 100 units/mL penicillin, 100 µg/mL streptomycin, 10% fetal bovine serum (FBS) and 500 µg/mL neomycin analogue G418. All the supplements were purchased from Fisher Scientific, USA. Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Plasmid construct
Lentiviral Vectors were purchased from the SBI, USA and Invitrogen, USA. Plasmids (pCDHCMV (catalog, CD500B-1), pSIHCMV (Catalog, LV500A-1) and pLentiLacZ (catalog, K495510) were used directly for GFP expression without any modifications. For the construction of the pLentiUBC-GFP, the GFP gene was amplified by polymerase chain reaction (PCR) using oligonucleotide primers (forward primer 5’CACCATGCGGCGGCGAGCCGC3’; reverse primer 5’CACCATGGTGCTCA CTCTTATC3’) containing an EcoRI and HindIII (Fermantas, USA) site for each end. The amplified GFP gene was digested with EcoRI and HindIII and ligated at EcoRI and HindIII sites of pLentiUBC (Catalog, V499-100). Maps depicting the constructs are shown in Figure 1.
Figure 1.
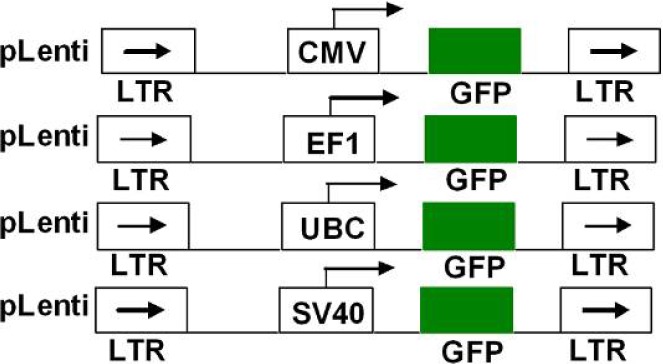
Schematic representation of promoter regions controlling GFP expression in retroviral vectors.
Lentivirus production
293TN cells (SBI, USA) were seeded at a density of 1.6 × 108 per 100 mm tissue culture dish and transiently transfected with lentivirus transfer vectors and three viral packaging plasmids (pPACKH1-GAG, pPACKH1-Rev, pVSV-G, Catalog number, LV500A-1) (SBI, USA) according to the manufacturer protocol (SBI, USA). After 16 hours, the media was replaced with Dulbecco’s modified essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 20 mM HEPES, pH 7.05 (Sigma). The supernatants were harvested at 48 and 72 hours to collect the produced lentivirus.
Concentration of produced lentivirus
Supernatants from 293T cells transfected with lentiviral vectors were cleared of cellular debris by low-speed centrifugation (1500 g, 5 min) and filtered with 0.45 µm filter (low binding filter, Millipore, USA). Then one volume of cold polyethylene glycol solution (5X PEG-it reagent, SBI, USA) was added to every four volumes of filtered viral supernatant and refrigerated over-night. Following refrigeration, the tubes containing PEG and viral containing cell culture supernatant were centrifuged at 1500g for 30 min. Then the resultant supernatant was decanted and the white viral pellet was re-suspended in the 1X PBS at 1/100 of original volume. The concentration of viral particles was determined by UV spectrometer. The concentrated lentivirus was aliquoted and stored at –80°C for further use.
Optimal viral dose determination for transduction
To determine the optimal viral dose to transduce hHSC to be able to express exogenous GFP gene efficiently, different cell to viral particles ratio were used, such as 1:1000, 1:2000 and 1:5000. Different doses of viral particles mixed with hHSCs were suspended in 100µl of stem cell media in a sterile 1.5 ml microcentrifuge tube, mixed well and incubated for 1 hour at 37°C, 5% CO2. After one hour, 400µl of fresh stem cell media was added and transferred to a 6-well plate and further incubated for 72 hours. The transfected cells were analyzed by fluorescent microscope and flowcytometer.
Determination of viability and proliferation following transduction
Following incubation of cells with different doses of viral particles for 72 hours, trypan blue dye exclusion test was performed to determine the viability of transduced cells. Transduced cells were also subjected to proliferation study (by both MTT assay and manual counting of cells) and compared with that of corresponding control cells.
Transduction of hHSCs
Based on viability and transduction efficiency, we decided to use cells to viral particle ratio of 1:2000 for subsequent studies. hHSCs were added to sterile 1.5 ml microcentrifuge tube and mixed with lentivirus at 1: 2000 ratio (based on our optimal viral dose study) and incubated for 1 hour at 37°C, 5% CO2. After one hour, 500 µl of fresh media was added and transferred to a 6-well plate and further incubated for 72 hours. The GFP expression in transduced cells was analyzed using fluorescent microscope and flow cytometer. To determine whether GFP expression was maintained over time, transduced hHSCs were further incubated and maintained for 6 weeks at 37°C, 5% CO2 and analyzed at different time points by fluorescent microscope and flow cytometer for GFP expression. To determine the effect of transduction enhancing agents, polybrene (5 µg/ml) (Millipore, USA), and lipofectamine 2000 (1µl/ml) (Invitrogen, USA) were applied during the transduction reaction.
Growth factor stimulation of hHSCs
To improve the transduction efficiency, the growth of hHSCs were stimulated prior to transduction using higher doses of growth factors that was 2 fold higher than the concentration used in standard hHSC growth media. Specifically, CD34+/CD133+ cells were incubated for 10 hrs in stem cell basal media (Amino Acids, Vitamins, phenol-red, L-glutamine, ß-Mercaptoethanol) supplemented with 80 ng/ml of stem cell factor (SCF), 80 ng/ml of FLT3 and 20 ng/ml of thrombopoietin (TPO). After stimulation cells were transduced with lentivirus as follows: hHSCs and lentivirus were added to sterile 1.5 ml microcentrifuge tube at 1: 2000 ratio and incubated for 1 hour at 37°C, 5% CO2. After one hour, 500 µl of fresh media was added and transferred to a 6-well plate and further incubated for 24 hours. To determine whether growth factors are at all necessary during transduction, cells were also incubated for 10 hours in stem cell basal media without any growth factor and the transduction was proceeded as described above.
Fluorescence microscopy
The transduced hHSCs were directly observed for GFP expression using a Leica pro fluorescence microscope, with FITC filter at 40X magnification. Pictures were taken using a digitalized CCD camera attached to the microscope using SPOT software (RT diagnostic instruments, MI). Corresponding bright field images were also obtained.
Flowcytometry analysis
Transduced hHSCs cells were analyzed by flow cytometry using a LSR-II flowcytometer (BD Biosciences) or C6 Accuri flowcytometer (Accuri Cytometers, Inc, MI). Cells were collected at different time points and washed with 1x PBS and then analyzed with flow cytometer to determine the GFP positive cells (FL1 channel). Non-transuded hHSCs were used as control cells.
Results
Production of lentiviral vectors
To determine the promoter strength in hHSCs, CMV-GFP, EF1-GFP, UBC-GFP and SV40-GFP plasmids were used. The lentiviral particles were produced by transiently transfecting the plasmid constructs (CMV-GFP, EF1-GFP, UBC-GFP and SV40-GFP) along with the pHDMH Gagpol, VSVG, REV plasmids into 293TN cells. The supernatant collected from the transfected cells contained relatively diluted amount of virus particles. To concentrate viral particles (VP), the supernatants containing VP were subjected to precipitation using polyethylene glycol (PEG). The concentrated suspension of VP showed a relatively high viral titer values for all four lentiviral vectors, ranging from 2 to 5X108 VP/ml. The amounts of viral vectors used for hHSCs transduction were kept identical for all four viral vectors in order to achieve similar transduction efficiencies.
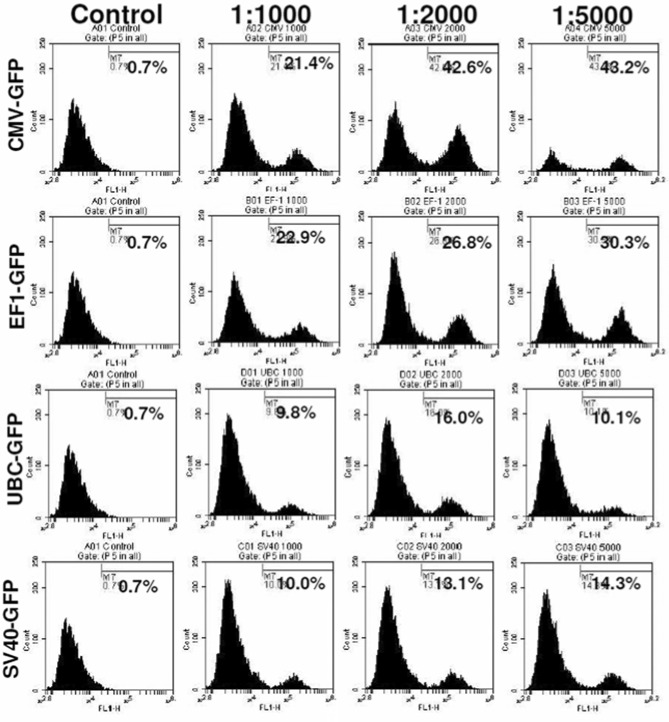
Determination of optimal viral dose for transduction
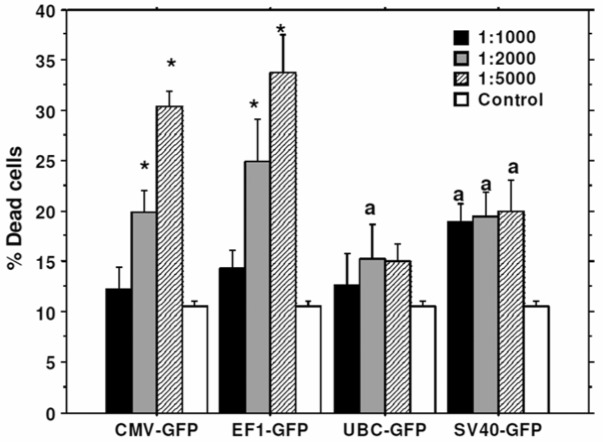
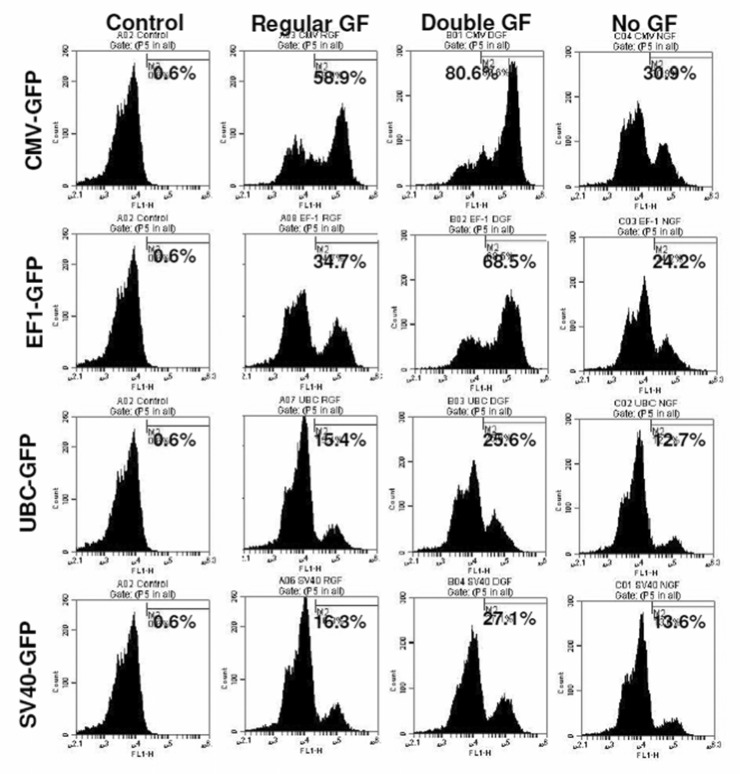
To determine the optimal dose of viral vector used for the transduction, different cell to viral ratio were tested, i.e. 1:1000, 1:2000, and 1:5000. The 1:5000 cell to viral ratio produced higher level of transduction (Figure 2), however, significantly higher (p=<0.001) cell death was also noted compared to that of 1:1000 and 1:2000 ratios (Figure 3). Similar findings were also observed on MTT and cell growth curve analysis (data not shown). Addition of transduction enhancing agent, polybrene (5µg/ml), improved transduction rate by only 4%, while lipofectamine 2000 (1µl/ml) did not show any effect on the efficiency of transduction (data not shown). Optimal viral doses were determined based on the expression of reporter protein and percentage of viable cells at different doses of viral vectors. For example, both CMV and EF-1 promoters showed higher expression of GFP at 1:5000 dose, but there were associated with significantly increased cell death at that dose. Therefore, we selected lower viral dose which produced almost comparable GFP expression with significantly lower numbers of dead cells . Similarly, 1:2000 doses also showed comparably higher expression of GFP with UBC and SV40 promoters although there was no significant increase in cell death.
Figure 2.
Viral dose effect on transudation: hHSCs transduced with lentivirus at cell to virus ratio of 1:1000, 1:2000; 1:5000. Highest level of GFP expression was found in 1:5000 cell to virus ratio and also higher level of cell death was found at this dose. 1:2000 ratio was the optimal dose for good level of transgene expression as well as low level of cell death (see Figure 3).
Figure 3.
Viral dose effect on cell viability: Cells were transduced with lenti virus at 1:1000, 1:2000, 1:5000 cell to virus ratio. Viral dose effect on cell viability was analyzed by Trypan blue assay. 1:5000 cell to viral ratio gave the highest level of GFP expression (46%), however cell death was also high, at 31-33%. On other hand, 1:2000 cell to virus ratio gave good level of GFP expression (42%) as well as low level of cell death (15-20%). * = significant differences from other viral doses and control. a = significant differences from control.
GFP expression in hHSCs
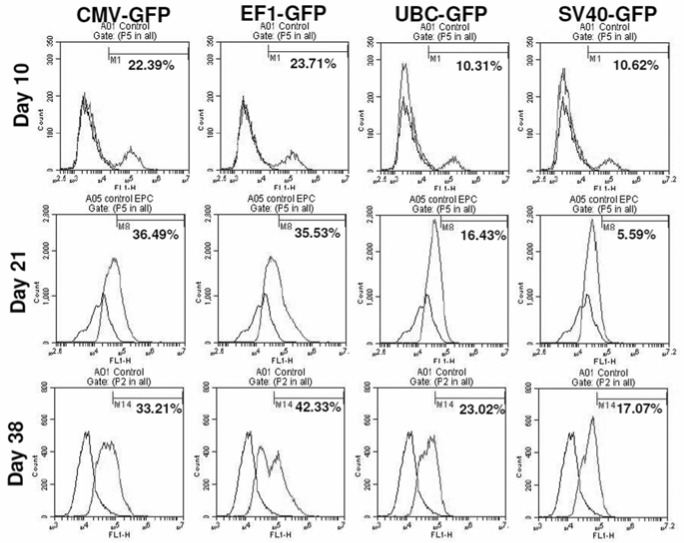
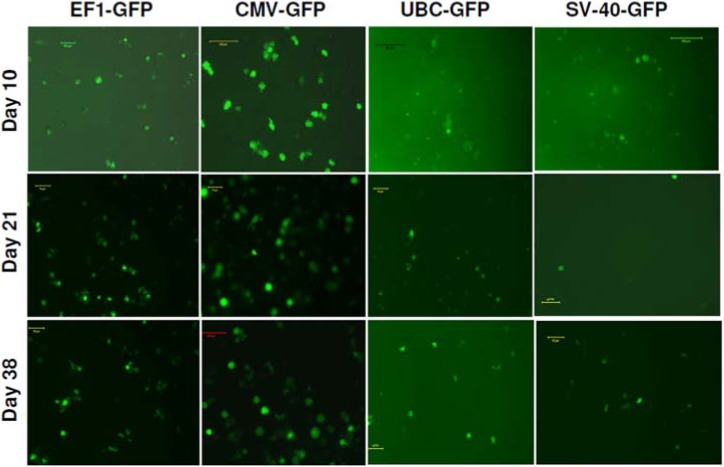
To determine the transcriptional activities of different promoters in hHSCs, we transduced hHSCs with lentiviral vectors. The GFP expression in hHSCs was analyzed at different days after transduction. The transduced cells were not subjected to antibiotic selection. The fluorescence microscopy analysis reveled that EF1 and CMV promoter drove high level of expression of GFP, while SV40 and UBC promoters drove low level expression of GFP (Figure 4). The GFP expression in transduced hHSCs was further verified using flowcytometer and the highest expression of GFP was observed under the CMV and EF1 promoters, while SV40 and UBC promoters induced the lowest levels of GFP (Figure 5). Long-term GFP expression in transduced cells was also followed. CMV promoter induced similar or higher expression level of GFP at days 10 and 21 when compared to all other promoters; however, at day 38 GFP expression under the CMV promoter dropped about 3% (Figure 5 and 6). The EF1 promoter induced expression of GFP was 24% at day 10, 36% at day 21 and 42% at day 38 (Figure 6). The UBC and SV40 promoters induced very low level of expression of GFP at days 10 and 21, however, there was slight improved GFP expression observed at day 38 but not to the level observed with CMV or EF1 promoters (Figure 5 and 6).
Figure 4.
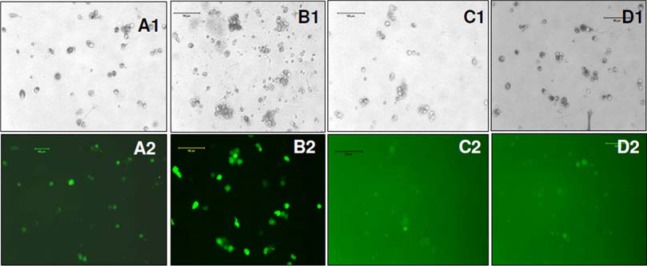
GFP expression in HSCs at day 10 of post transduction: (A1, A2) bright field and florescence images of stem cells infected with EF1-GFP, (B1, B2) bright field and fluorescence image of stem cells infected with CMV-GFP; (C1, C2) bright ield and florescence images of stem cells infected with SV40-GFP, (D1, D2) bright field and fluorescence images of stem cells infected with UBC-GFP.
Figure 5.
Promoter functionality studies for 6 weeks period using flowcytometer: Flow cytometric analysis shows the expression of transgenes (GFP) over extended period. Note the loss of expression of GFP on day 38 when CMV was used as promoter.
Figure 6.
Promoter functionality studies for 6 weeks period using florescence microscope: Same cell cultures were also photomicrographed using green fluorescent filter. Note the expression of GFP on extended culture conditions.
Improvement of transduction
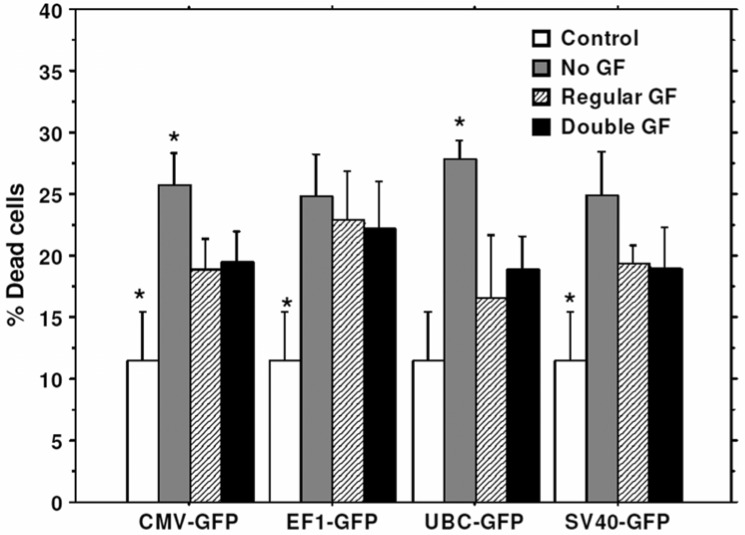
To improve the transduction rate, hHSCs were stimulated with double doses of growth factors (TPO, FLT3, SCF) for 10 hours before transduction. hHSCs stimulated with double doses of growth factors showed higher transduction efficiency for all types of lentiviral vectors (different promoters) compared to that of hHSCs cultured in regular stem cell media or cultured in stem cell basal media without growth factors (Figure 7). Cell viability was also compared in these three conditions. There was no significant difference in cell viability when compared between cells cultured with double doses of growth factors and cells cultured in regular stem cell media (Figure 8), however, cell viability was significantly reduced in condition where hHSCs were pre-incubated in stem cell media without growth factors (Figure 8).
Figure 7.
Improvement of transduction: hHSCs were stimulated with double dose of growth factors (GF) for 10 hours before the transduction. Cell to virus ratio of 1:2000 was used to for transduction. Cell stimulated with double dose of growth factors showed 33% of improved transduction.
Figure 8.
Effect of growth factor on cell viability: Lentivirus-GFP vectors were transduced with pre-stimulated stem cells with different concentrations of growth factors (GF). * = significant differences compared to other culture conditions.
Discussion
Since hHSCs have potential to be used for treatment of a variety of human diseases [25, 26], the use of hHSCs as a gene delivery vehicle has been a high priority for several research groups [27]. In order to use the hHSCs as gene delivery vehicles, it is important to integrate the transgene in to chromosomal DNA of hHSCs that will successively transfer and maintain in daughter cells [28]. The lentiviral vectors are found to be better gene transduction vector for hHSCs than adenoviral or oncoretroviral gene transfer vector systems [25, 26, 28, 29]. Lentiviral vectors have been shown to transduce non-dividing cells and produce stable expression of transgene with relatively low cytotoxicity in variety of cell types including HSCs [9, 28, 29]. Our current results are also in line of the previously published reports. Our results showed efficient transduction of exogenous GFP gene in cord blood derived hHSCs by lentivectors and showed long-term expression of transgene. The transgene expression in HSCs depends on gene transfer efficiency, transduction process including promoter activity and transduction enhancing agents. [30, 31]. Although the plasmids used in this study have some slight variations in the plasmid backbone genes, which might cause a little difference in the transgene expression, however we were not expecting wide differences in expression of transgene in
HSCs because level of transgene expression is highly controlled by promoter. The potential of retroviral vector transgene delivery should be associated with the use of optimal promoter to enhance the transgene expression in HSCs. The studies by Liu et al 2006 [32] clearly showed that the expression of transgene in stem cells depends on the strength of the promoter. The promoter plays important role in gene expression, thus we investigated four different promoters and their activity in cord blood hHSCs using lentiviral vectors. For the promoter functionality studies, an efficient reporter gene system is also important. GFP has emerged as an excellent reporter gene for gene transfer studies in various cell lines including the hematopoietic cells [33] [34]. The auto-fluorescent nature of the GFP has greatly improved the gene transduction by allowing the rapid identification of transduced cells using simple microscopic examination. In addition, GFP does not require any further gene products or cofactors to generate fluorescence and its only need is oxygen and blue light for fluorescence. Our results demonstrated the promoter dependent expression of reporter gene in cord blood derived hHSCs. CMV-GFP and EF1-GFP showed the highest rate of transduction (GFP positive cells).On the other hand SV40-GFP and UBC-GFP showed very low rate of transduction. These results indicate that promoter have significant influence on transgene expression in cord blood derived hHSCs. We have also found similar results in the expression of human sodium iodide (hNIS) gene in hHSCs when two different promoters (EF-1 and CMV) were used. CMV-hNIS showed higher rate of transduction, which was determined by Tc-99m-pertechnetate (Tc-99m) uptake assay (data not shown).
Liu et al [30] showed that biological agents such as lipofectamine significantly increased the transduction in CD34+ cells (for about 10%). However, we did not find any effect of lipofectamine in transducing hHSCs. On the other hand ploybrene showed increased transduction rate (4%) in hHSCs. We also studied the effect of growth factors to improve the transduction rate in hHSCs. Growth factor stimulation prior to transduction enhanced the transduction rate for about 33% with CMV promoter. This method leads to high-level expression of transgene, which may help to improve the successful delivery of therapeutic genes.
We compared the activities of all four promoters driving the expression of GFP in hHSCs by determining the percent positive cells by flowcytometry. We observed that CMV and EF1 promoters drove the highest levels of GFP expression, while little or very low level of expression was achieved with SV40 and UBC promoters. The reason behind the low-level expression of UBC and SV40 promoters were presently unknown and it may likely be due to the fact that hHSCs commonly express small number of genes while maintaining their “stemness”. At day 10 of post transduction hHSCs may not express important co-factors required for transcriptional activities of UBC and SV40 promoters, which, therefore, lead low-level expression of GFP. On the other hand, hHSCs might be expressing necessary co-factors that are required for the optimal transcriptional activities of CMV and EF1 promoters, thus CMV and EF1 promoters drove the robust expression of GFP. A little increase in GFP expression was observed at day 21 to day 38 for the UBC and SV-40 promoters, which indicated that these promoters were up-regulated during this period, however the expression of GFP was not comparable to that of CMV and EF1 promoters.
hHSCs are attractive targets for gene therapy and have the potential to cure diseases such as immune deficiencies, hemoglobinopathies and phagocyte disorders [35]. The hHSCs as gene delivery vehicles largely depend on convenience and reliable long-term expression of transgenes. Murine leukemia virus (MLV) derived retroviral vectors were most widely used as vehicles for gene transfer into HSCs [36]. MLV based retrov transduction in HSCs is usually inefficient to maintain the transgene for long period of time probably due to the inability to integrate into non-dividing cells. The expression of transgene is often inconsistent and down regulated over long period of time [37]. In addition, efficiency of gene therapy will be further hampered due to immune responses by host cells against vectors and transgene products [38, 39]. In our studies, we showed that CMV-GFP and EF1-GFP expression was stable for over 6 weeks in continuously cultured and propagated cells, thus indicating that CMV and EF1 promoters were stably and functionally maintained in the genome of these stem cells. EF1-GFP showed similar level of expression of GFP after 6 weeks of transduction, while CMV-GFP showed a drop of 10% of expression level after 6 weeks period. This result suggests that CMV promoter was down regulated after prolonged culture, and this might be due to hHSCs differentiation during prolonged culture. The results indicate that EF1 promoter is better in driving the gene of interest in the prolonged culture system.
Conclusions
In this study, we clearly demonstrated the advantages of CMV and EF1 promoters for the expression of transgene in cord blood derived hHSCs using lentiviral vectors. Based on the characteristics of hHSCs, these promoters can also be useful for any other hematopoietic stem cells and can be used in other cell types such as lymphocytes (which is under active investigation). The CMV and EF1 promoter is thus a promising tool for transgene expression in hHSCs and can be used with lentiviral vector for fundamental studies or for a gene therapy strategy targeting human hematopoietic stem cells.
Authors’ contributions
NRSV carried out the viral vector development, viral production, transduction studies, transduction improvement method, and drafted the manuscript. BJ carried out stem cell culture and designed viral production experiments. MMA participated in study design and statistical analysis. ASMI carried out stem cell isolation from Human cord blood. ASA helped overall design of the studies, coordination and helped in writing this manuscript. All authors read and approved the final manuscript.
Disclosure Statement
No competing financial interests exist
Grant support
The work is supported by NIH grants 1R21CA129801, R21NS058589, and R01CA122031.
References
- 1).Fibbe WE, Noort WA, Schipper F, Willemze R: Ex vivo expansion and engraftment potential of cord blood-derived CD34+ cells in NOD/SCID mice. Ann N Y Acad Sci 2001, 938:9-17 [DOI] [PubMed] [Google Scholar]
- 2).Hao QL, Shah AJ, Thiemann FT, Smogorzewska EM, Crooks GM: A functional comparison of CD34 + CD38- cells in cord blood and bone marrow. Blood 1995, 86:3745-3753 [PubMed] [Google Scholar]
- 3).Aker M, Varadi G, Slavin S, Nagler A: Fludarabine-based protocol for human umbilical cord blood transplantation in children with Fanconi anemia. J Pediatr Hematol Oncol 1999, 21:237-239 [DOI] [PubMed] [Google Scholar]
- 4).Bielorai B, Hughes MR, Auerbach AD, Nagler A, Loewenthal R, Rechavi G, Toren A: Successful umbilical cord blood transplantation for Fanconi anemia using preimplantation genetic diagnosis for HLA-matched donor. Am J Hematol 2004, 77:397-399 [DOI] [PubMed] [Google Scholar]
- 5).Kohn DB: Gene therapy for haematopoietic and lymphoid disorders. Clin Exp Immunol 1997, 107Suppl 1:54-57 [PubMed] [Google Scholar]
- 6).Richter J: Gene transfer to hematopoietic cells--the clinical experience. Eur J Haematol 1997, 59:67-75 [DOI] [PubMed] [Google Scholar]
- 7).Miller DG, Adam MA, Miller AD: Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol 1990, 10:4239-4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Lewis PF, Emerman M: Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol 1994, 68:510-516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D: In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996, 272:263-267 [DOI] [PubMed] [Google Scholar]
- 10).Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D: Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 1997, 15:871-875 [DOI] [PubMed] [Google Scholar]
- 11).Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L: A third-generation lentivirus vector with a conditional packaging system. J Virol 1998, 72:8463-8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Akkina RK, Walton RM, Chen ML, Li QX, Planelles V, Chen IS: High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. J Virol 1996, 70:2581-2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Reiser J, Harmison G, Kluepfel-Stahl S, Brady RO, Karlsson S, Schubert M: Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc Natl Acad Sci U S A 1996, 93:15266-15271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Najjar SM, Lewis RE: Persistent expression of foreign genes in cultured hepatocytes: expression vectors. Gene 1999, 230:41-45 [DOI] [PubMed] [Google Scholar]
- 15).Ramezani A, Hawley TS, Hawley RG: Lentiviral vectors for enhanced gene expression in human hematopoietic cells. Mol Ther 2000, 2:458-469 [DOI] [PubMed] [Google Scholar]
- 16).Keating A, Horsfall W, Hawley RG, Toneguzzo F: Effect of different promoters on expression of genes introduced hematopoietic and marrow stromal cells by electroporation. Exp Hematol 1990, 18:99-102 [PubMed] [Google Scholar]
- 17).Muller SR, Sullivan PD, Clegg DO, Feinstein SC: Efficient transfection and expression of heterologous genes in PC12 cells. DNA Cell Biol 1990, 9:221-229 [DOI] [PubMed] [Google Scholar]
- 18).Tokushige K, Moradpour D, Wakita T, Geissler M, Hayashi N, Wands JR: Comparison between cytomegalovirus promoter and elongation factor-1 alpha promoter-driven constructs in the establishment of cell lines expressing hepatitis C virus core protein. J Virol Methods 1997, 64:73-80 [DOI] [PubMed] [Google Scholar]
- 19).Nakai H, Herzog RW, Hagstrom JN, Walter J, Kung SH, Yang EY, Tai SJ, Iwaki Y, Kurtzman GJ, Fisher KJ, et al: Adeno-associated viral vector-mediated gene transfer of human blood coagulation factor IX into mouse liver. Blood 1998, 91:4600-4607 [PubMed] [Google Scholar]
- 20).Teschendorf C, Warrington KH, Jr, Siem DW, Muzyczka N: Comparison of the EF-1 alpha and the CMV promoter for engineering stable tumor cell lines using recombinant adeno-associated virus. Anticancer Res 200222:3325-3330 [PubMed] [Google Scholar]
- 21).Marinovic AC, Mitch WE, Price SR: Tools for evaluating ubiquitin (UbC) gene expression: characterization of the rat UbC promoter and use of an unique 3’ mRNA sequence. Biochem Biophys Res Commun 2000, 274:537-541 [DOI] [PubMed] [Google Scholar]
- 22).Gill DR, Smyth SE, Goddard CA, Pringle IA, Higgins CF, Colledge WH, Hyde SC: Increased persistence of lung gene expression using plasmids containing the ubiquitin C or elongation factor 1alpha promoter. Gene Ther 2001, 8:1539-1546 [DOI] [PubMed] [Google Scholar]
- 23).Yew NS, Przybylska M, Ziegler RJ, Liu D, Cheng SH: High and sustained transgene expression in vivo from plasmid vectors containing a hybrid ubiq uitin promoter. Mol Ther 2001, 4:75-82 [DOI] [PubMed] [Google Scholar]
- 24).Selden C, Mellor N, Rees M, Laurson J, Kirwan M, Escors D, Collins M, Hodgson H: Growth factors improve gene expression after lentiviral transduction in human adult and fetal hepatocytes. J Gene Med 2007, 9:67-76 [DOI] [PubMed] [Google Scholar]
- 25).Bank A: Hematopoietic stem cell gene therapy: selecting only the best. J Clin Invest 2003, 112:1478-1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Kohn DB, Sadelain M, Dunbar C, Bodine D, Kiem HP, Candotti F, Tisdale J, Riviere I, Blau CA, Richard RE, et al. : American Society of Gene Therapy (ASGT) ad hoc subcommittee on retroviral-mediated gene transfer to hematopoietic stem cells. Mol Ther 2003, 8:180-187 [DOI] [PubMed] [Google Scholar]
- 27).Rad AM, Iskander AS, Janic B, Knight RA, Arbab AS, Soltanian-Zadeh H: AC133+ progenitor cells as gene delivery vehicle and cellular probe in subcutaneous tumor models: a preliminary study. BMC Biotechnol 2009, 9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Barrette S, Douglas JL, Seidel NE, Bodine DM: Lentivirus-based vectors transduce mouse hematopoietic stem cells with similar efficiency to moloney murine leukemia virus-based vectors. Blood 2000, 96:3385-3391 [PubMed] [Google Scholar]
- 29).Blomer U, Naldini L, Kafri T, Trono D, Verma IM, Gage FH: Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol 1997, 71:6641-6649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Liu H, Hung Y, Wissink SD, Verfaillie CM: Improved retroviral transduction of hematopoietic progenitors by combining methods to enhance virus-cell interaction. Leukemia 2000, 14:307-311 [DOI] [PubMed] [Google Scholar]
- 31).Schmidt M, Hacein-Bey-Abina S, Wissler M, Carlier F, Lim A, Prinz C, Glimm H, Andre-Schmutz I, Hue C, Garrigue A, et al. : Clonal evidence for the transduction of CD34+ cells with lymphomyeloid differentiation potential and self-renewal capacity in the SCID-X1 gene therapy trial. Blood 2005, 105:2699-2706 [DOI] [PubMed] [Google Scholar]
- 32).Liu JW, Pernod G, Dunoyer-Geindre S, Fish RJ, Yang H, Bounameaux H, Kruithof EK: Promoter dependence of transgene expression by lentivirus-transduced human blood-derived endothelial progenitor cells. Stem Cells 2006, 24:199-208 [DOI] [PubMed] [Google Scholar]
- 33).Persons DA, Allay JA, Allay ER, Smeyne RJ, Ashmun RA, Sorrentino BP, Nienhuis AW: Retroviral-mediated transfer of the green fluorescent protein gene into murine hematopoietic cells facilitates scoring and selection of transduced progenitors in vitro and identification of genetically modified cells in vivo. Blood 1997, 90:1777-1786 [PubMed] [Google Scholar]
- 34).Bierhuizen MF, Westerman Y, Visser TP, Dimjati W, Wognum AW, Wagemaker G: Enhanced green fluorescent protein as selectable marker of retroviral-mediated gene transfer in immature hematopoietic bone marrow cells. Blood 1997, 90:3304-3315 [PubMed] [Google Scholar]
- 35).Karlsson S: Treatment of genetic defects in hematopoietic cell function by gene transfer. Blood 1991, 78:2481-2492 [PubMed] [Google Scholar]
- 36).Chu P, Lutzko C, Stewart AK, Dube ID: Retrovirus-mediated gene transfer into human hematopoietic stem cells. J Mol Med 1998, 76:184-192 [DOI] [PubMed] [Google Scholar]
- 37).Kohn DB, Hershfield MS, Carbonaro D, Shigeoka A, Brooks J, Smogorzewska EM, Barsky LW, Chan R, Burotto F, Annett G, et al. : T lymphocytes with a normal ADA gene accumulate after transplantation of transduced autologous umbilical cord blood CD34+ cells in ADA-deficient SCID neonates. Nat Med 1998, 4:775-780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Riddell SR, Elliott M, Lewinsohn DA, Gilbert MJ, Wilson L, Manley SA, Lupton SD, Overell RW, Reynolds TC, Corey L, Greenberg PD: T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med 1996, 2:216-223 [DOI] [PubMed] [Google Scholar]
- 39).Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, Ponzoni M, Rossini S, Mavilio F, Traversari C, Bordignon C: HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 1997, 276:1719-1724 [DOI] [PubMed] [Google Scholar]