Abstract
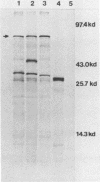
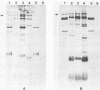
Chlamydia sp. strain TWAR is a unique Chlamydia sp. that causes acute respiratory disease. A gene bank consisting of TWAR isolate AR-39 DNA in pUC19 was screened with anti-AR-39 rabbit immune sera. Two positive clones were isolated that contained 7.3-kilobase (pLC1) and 14.9-kilobase (pLC2) plasmids. Restriction mapping and hybridization studies showed that both pLC1 and pLC2 contained a common 4.2-kilobase PstI fragment. Plasmids were used as templates of in vitro transcription-translation. All three plasmids had a novel protein product of ca. 75 kilodaltons not found in the vector alone. Western blots showed that this protein reacted with anti-TWAR rabbit immune sera and with human immune serum from an individual who had proven TWAR infection. Whole-cell lysates of TWAR demonstrated a protein having the same molecular weight and immunoreactivity as the recombinant gene product. This protein was also recognized by rabbit immune serum against Chlamydia psittaci or Chlamydia trachomatis. Southern hybridizations with the cloned fragment as a probe of digests of other Chlamydia spp. showed weakly hybridizing fragments. These results suggest that we have isolated a gene encoding a protein recognized during human TWAR infection that contains some sequences shared among Chlamydia spp.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Zhang Y. X., Joseph T., Su H., Nano F. E., Everett K. D., Caldwell H. D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger B. E., Rank R. G. Analysis of the humoral immune response to chlamydial genital infection in guinea pigs. Infect Immun. 1987 Aug;55(8):1767–1773. doi: 10.1128/iai.55.8.1767-1773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R. C., Maclean I. W., Binns B., Peeling R. W. Chlamydia trachomatis: its role in tubal infertility. J Infect Dis. 1985 Dec;152(6):1275–1282. doi: 10.1093/infdis/152.6.1275. [DOI] [PubMed] [Google Scholar]
- Brunham R. C., Peeling R., Maclean I., McDowell J., Persson K., Osser S. Postabortal Chlamydia trachomatis salpingitis: correlating risk with antigen-specific serological responses and with neutralization. J Infect Dis. 1987 Apr;155(4):749–755. doi: 10.1093/infdis/155.4.749. [DOI] [PubMed] [Google Scholar]
- Caldwell H. D., Hitchcock P. J. Monoclonal antibody against a genus-specific antigen of Chlamydia species: location of the epitope on chlamydial lipopolysaccharide. Infect Immun. 1984 May;44(2):306–314. doi: 10.1128/iai.44.2.306-314.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. A., Kuo C. C., Grayston J. T. Characterization of the new Chlamydia agent, TWAR, as a unique organism by restriction endonuclease analysis and DNA-DNA hybridization. J Clin Microbiol. 1987 Oct;25(10):1911–1916. doi: 10.1128/jcm.25.10.1911-1916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi E. Y., Kuo C. C., Grayston J. T. Unique ultrastructure in the elementary body of Chlamydia sp. strain TWAR. J Bacteriol. 1987 Aug;169(8):3757–3763. doi: 10.1128/jb.169.8.3757-3763.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir S. P., Kenny G. E., Grayston J. T. Characterization of the group antigen of Chlamydia trachomatis. Infect Immun. 1971 Dec;4(6):725–730. doi: 10.1128/iai.4.6.725-730.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON F. B., QUAN A. L. OCCURENCE OF GLYCOGEN IN INCLUSIONS OF THE PSITTACOSIS-LYMPHOGRANULOMA VENEREUM-TRACHOMA AGENTS. J Infect Dis. 1965 Apr;115:186–196. doi: 10.1093/infdis/115.2.186. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Kuo C. C., Wang S. P., Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med. 1986 Jul 17;315(3):161–168. doi: 10.1056/NEJM198607173150305. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Howard L., Orenstein N. S., King N. W. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974 Jan;27(1):102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny G. E. Heat-lability and organic solvent-solubility of mycoplasma antigens. Ann N Y Acad Sci. 1967 Jul 28;143(1):676–681. doi: 10.1111/j.1749-6632.1967.tb27713.x. [DOI] [PubMed] [Google Scholar]
- Kleemola M., Saikku P., Visakorpi R., Wang S. P., Grayston J. T. Epidemics of pneumonia caused by TWAR, a new Chlamydia organism, in military trainees in Finland. J Infect Dis. 1988 Feb;157(2):230–236. doi: 10.1093/infdis/157.2.230. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Chen H. H., Wang S. P., Grayston J. T. Identification of a new group of Chlamydia psittaci strains called TWAR. J Clin Microbiol. 1986 Dec;24(6):1034–1037. doi: 10.1128/jcm.24.6.1034-1037.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MURRAY E. S. GUINEA PIG INCLUSION CONJUNCTIVITIS VIRUS. I. ISOLATION AND IDENTIFICATION AS A MEMBER OF THE PSITTACOSIS-LYMPHOGRANULOMA-TRACHOMA GROUP. J Infect Dis. 1964 Feb;114:1–12. doi: 10.1093/infdis/114.1.1. [DOI] [PubMed] [Google Scholar]
- Maclean I. W., Peeling R. W., Brunham R. C. Characterization of Chlamydia trachomatis antigens with monoclonal and polyclonal antibodies. Can J Microbiol. 1988 Feb;34(2):141–147. doi: 10.1139/m88-028. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Tam M. R., Kuo C. C., Nowinski R. C. Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. J Immunol. 1982 Mar;128(3):1083–1089. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Immunologic relationship between genital TRIC, lymphogranuloma venereum, and related organisms in a new microtiter indirect immunofluorescence test. Am J Ophthalmol. 1970 Sep;70(3):367–374. doi: 10.1016/0002-9394(70)90096-6. [DOI] [PubMed] [Google Scholar]