Abstract
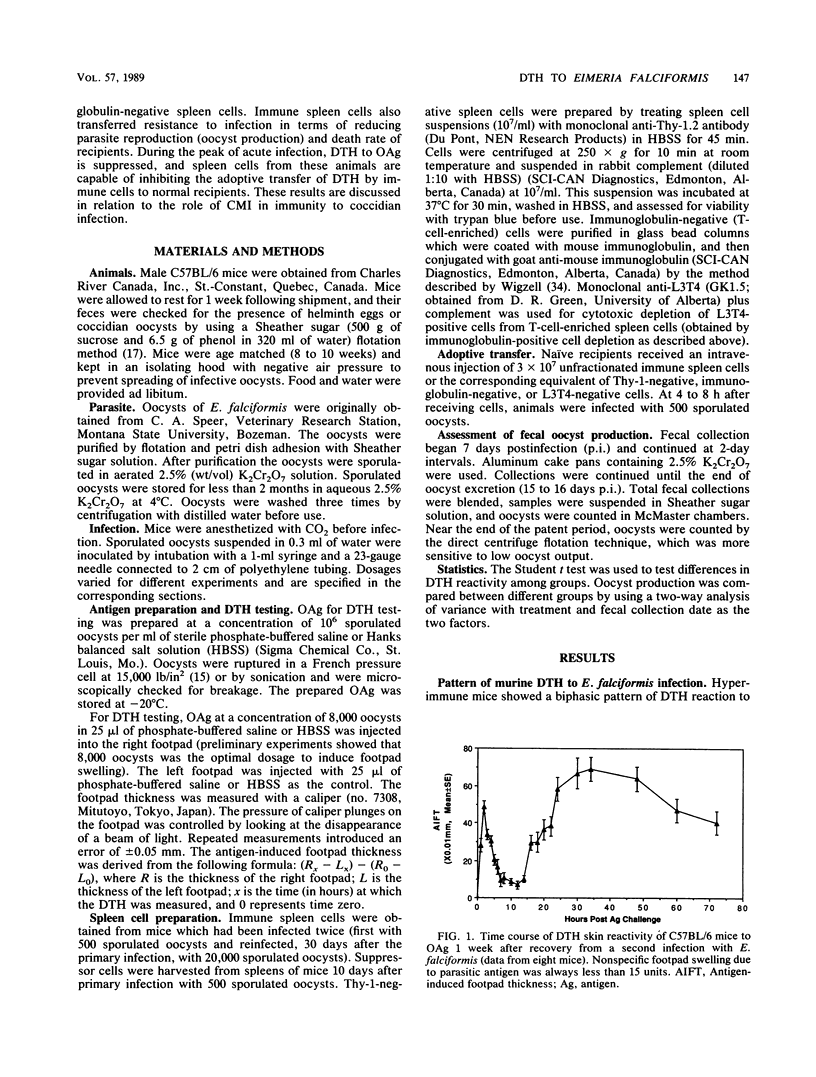
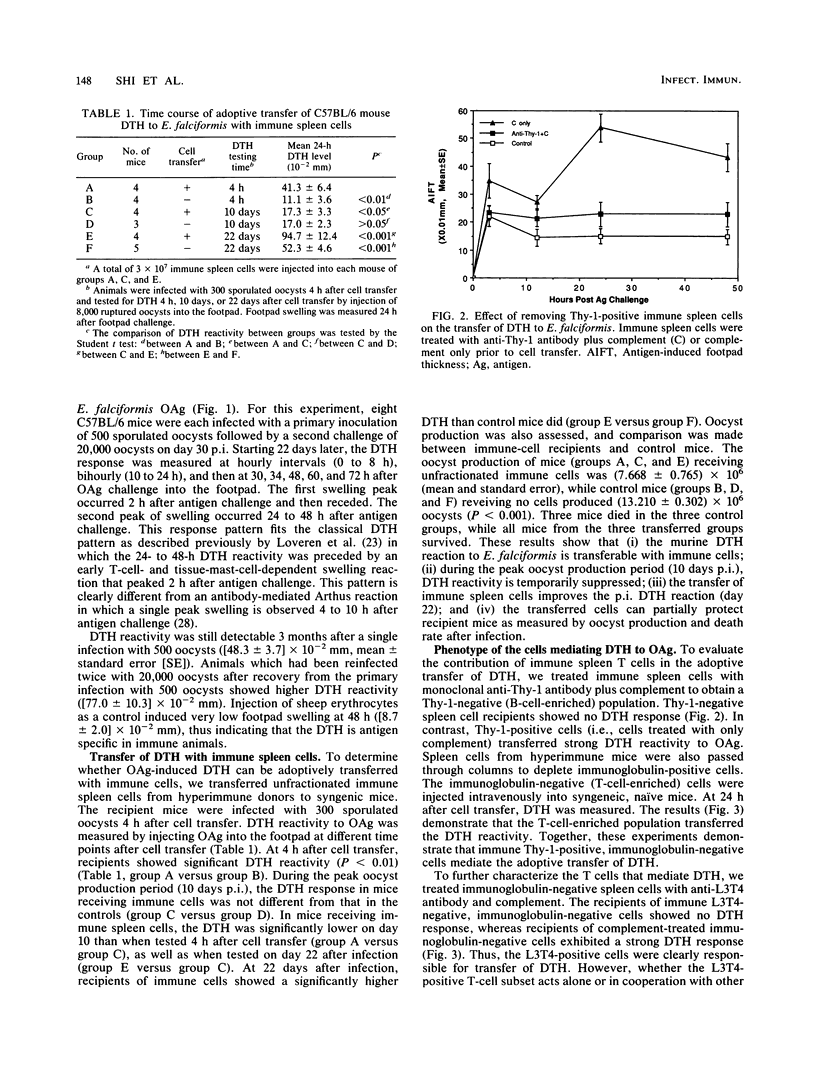
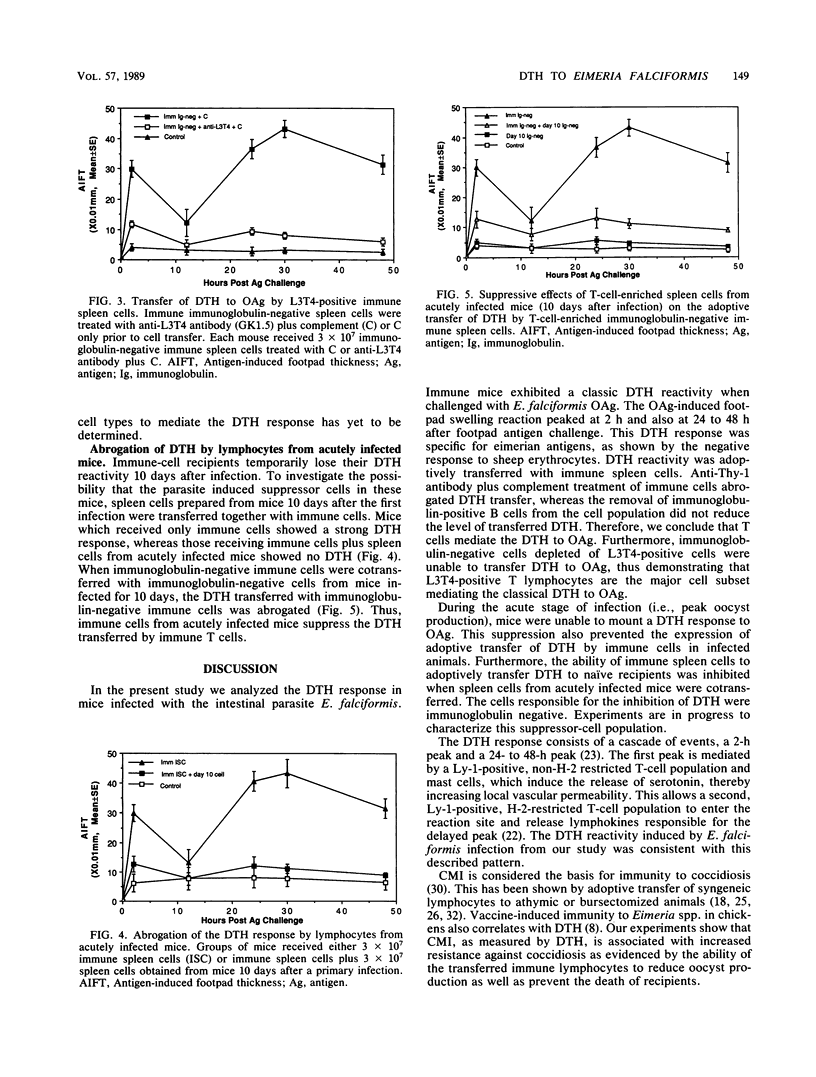
Mice recovering from a primary infection with an intestinal protozoan parasite, Eimeria falciformis (Apicomplexa: Eimeriidae), showed a classic delayed-type hypersensitivity (DTH) reaction to oocyst antigen challenge. This reaction was characterized by a biphasic pattern of footpad swelling. The first swelling peaked at 2 h after antigen challenge, whereas the second swelling peaked at 24 to 48 h after challenge. The DTH reaction was transferable with a T-cell-enriched spleen cell population from mice that had recovered from E. falciformis infection. Cytotoxic depletion of immune T cells with anti-L3T4 antibody and complement abrogated DTH transfer, indicating that L3T4-positive T cells were required. A T-cell-enriched spleen cell population from acutely infected mice suppressed the transfer of DTH with immune cells from recovered animals, implicating the existence of infection-induced immunoregulatory cells controlling the parasite-specific immune response during infection. Immune spleen cells also transferred resistance to infection as measured by oocyst production and death rate of recipients. Together, these results indicate that the DTH reaction, induced by infection with E. falciformis, is mediated by L3T4-positive T cells and is associated with resistance to infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R. Games parasites play: how parasites evade immune surveillance. Nature. 1979 May 3;279(5708):21–26. doi: 10.1038/279021a0. [DOI] [PubMed] [Google Scholar]
- Clare R. A., Strout R. G., Taylor R. L., Jr, Collins W. M., Briles W. E. Major histocompatibility (B) complex effects on acquired immunity to cecal coccidiosis. Immunogenetics. 1985;22(6):593–599. doi: 10.1007/BF00430307. [DOI] [PubMed] [Google Scholar]
- Crane M. S., Murray P. K., Gnozzio M. J., MacDonald T. T. Passive protection of chickens against Eimeria tenella infection by monoclonal antibody. Infect Immun. 1988 Apr;56(4):972–976. doi: 10.1128/iai.56.4.972-976.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. J., Parry S. H., Porter P. The role of secretory IgA in anti-coccidial immunity in the chicken. Immunology. 1978 May;34(5):879–888. [PMC free article] [PubMed] [Google Scholar]
- Davis P. J., Porter P. A mechanism for secretory IgA-mediated inhibition of the cell penetration and intracellular development of Eimeria tenella. Immunology. 1979 Mar;36(3):471–477. [PMC free article] [PubMed] [Google Scholar]
- FRENKEL J. K. Dermal hypersensitivity to toxoplasma antigens. Proc Soc Exp Biol Med. 1948 JulâAug;68(3):634–639. doi: 10.3181/00379727-68-16579. [DOI] [PubMed] [Google Scholar]
- GRAY D. F., JENNINGS P. A. Allergy in experimental mouse tuberculosis. Am Rev Tuberc. 1955 Aug;72(2):171–195. doi: 10.1164/artpd.1955.72.2.171. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. III. Nature and significance of specific suppression of cell-mediated immunity in mice highly susceptible to Leishmania tropica. J Exp Med. 1980 Sep 1;152(3):594–607. doi: 10.1084/jem.152.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. IV. Prophylactic effect of sublethal irradiation as a result of abrogation of suppressor T cell generation in mice genetically susceptible to Leishmania tropica. J Exp Med. 1981 Mar 1;153(3):557–568. doi: 10.1084/jem.153.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesius P. H., Kramer T. T., Frandsen J. C. Eimeria stiedai: delayed hypersensitivity response in rabbit coccidiosis. Exp Parasitol. 1976 Feb;39(1):59–68. doi: 10.1016/0014-4894(76)90010-2. [DOI] [PubMed] [Google Scholar]
- Klesius P. H., Kristensen F., Elston A. L., Williamson O. C. Eimeria bovis: evidence for a cell-mediated immune response in bovine coccidiosis. Exp Parasitol. 1977 Apr;41(2):480–490. doi: 10.1016/0014-4894(77)90120-5. [DOI] [PubMed] [Google Scholar]
- Liburd E. M., Armstrong W. D., Mahrt J. L. Immunity to the protozoan parasite Eimeria nieschulzi in inbred CD-F rats. Cell Immunol. 1973 Jun;7(3):444–452. doi: 10.1016/0008-8749(73)90208-6. [DOI] [PubMed] [Google Scholar]
- Liew F. Y. Cell-mediated immunity in experimental cutaneous Leishmaniasis. Parasitol Today. 1986 Oct;2(10):264–270. doi: 10.1016/0169-4758(86)90135-3. [DOI] [PubMed] [Google Scholar]
- Lillehoj H. S. Effects of immunosuppression on avian coccidiosis: cyclosporin A but not hormonal bursectomy abrogates host protective immunity. Infect Immun. 1987 Jul;55(7):1616–1621. doi: 10.1128/iai.55.7.1616-1621.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj H. S., Ruff M. D. Comparison of disease susceptibility and subclass-specific antibody response in SC and FP chickens experimentally inoculated with Eimeria tenella, E. acervulina, or E. maxima. Avian Dis. 1987 Jan-Mar;31(1):112–119. [PubMed] [Google Scholar]
- Mahrt J. L., Shi Y. F. Murine major histocompatibility complex and immune response to Eimeria falciformis. Infect Immun. 1988 Jan;56(1):270–271. doi: 10.1128/iai.56.1.270-271.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesfin G. M., Bellamy J. E. Thymic dependence of immunity to Eimeria falciformis var. pragensis in mice. Infect Immun. 1979 Feb;23(2):460–464. doi: 10.1128/iai.23.2.460-464.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERCE A. E., LONG L. P., HORTON-SMITH C. Attempts to induce a passive immunity to Eimeria tenella in young fowls (Gallus domesticus). Immunology. 1963 Jan;6:37–47. [PMC free article] [PubMed] [Google Scholar]
- Rose M. E. Eimeria tenella: skin hypersensitivity to injected antigen in the fowl. Exp Parasitol. 1977 Jun;42(1):129–141. doi: 10.1016/0014-4894(77)90070-4. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Hesketh P. Immunity to coccidia in chickens: adoptive transfer with peripheral blood lymphocytes and spleen cells. Parasite Immunol. 1982 May;4(3):171–185. doi: 10.1111/j.1365-3024.1982.tb00429.x. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Hesketh P. Immunity to coccidiosis: T-lymphocyte- or B-lymphocyte-deficient animals. Infect Immun. 1979 Nov;26(2):630–637. doi: 10.1128/iai.26.2.630-637.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. E., Long P. L. Immunity to coccidiosis: protective effects of transferred serum and cells investigated in chick embryos infected with Eimeria tenella. Parasitology. 1971 Oct;63(2):299–313. doi: 10.1017/s0031182000079610. [DOI] [PubMed] [Google Scholar]
- Van Loveren H., Askenase P. W. Delayed-type hypersensitivity is mediated by a sequence of two different T cell activities. J Immunol. 1984 Nov;133(5):2397–2401. [PubMed] [Google Scholar]
- Yano A., Norose K., Yamashita K., Aosai F., Sugane K., Segawa K., Hayashi S. Immune response to Toxoplasma gondii--analysis of suppressor T cells in a patient with symptomatic acute toxoplasmosis. J Parasitol. 1987 Oct;73(5):954–961. [PubMed] [Google Scholar]
- van Loveren H., Meade R., Askenase P. W. An early component of delayed-type hypersensitivity mediated by T cells and mast cells. J Exp Med. 1983 May 1;157(5):1604–1617. doi: 10.1084/jem.157.5.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]


