Summary
Biofilm formation plays an integral role in catheter-associated bloodstream infections caused by Candida albicans. Biofilms formed on catheters placed intravenously are exposed to shear stress caused by blood flow. In this study, we investigated whether shear stress affects the ability of C. albicans to form biofilms. Candida biofilms were formed on catheter discs and exposed to physiological levels of shear stress using a rotating disc system (RDS). Control biofilms were grown under conditions of no flow. Tetrazolium (XTT) assay and dry weight (DW) measurements were used to quantify metabolic activity and biofilm mass respectively. Confocal scanning laser microscopy (CSLM) was used to evaluate architecture and biofilm thickness. After 90 min, cells attached under no-flow exhibited significantly greater XTT activity and DW than those under shear. However, by 24 h, biofilms formed under both conditions had similar XTT activities and DW. Interestingly, thickness of biofilms formed under no-flow was significantly greater after 24 h than of those formed under shear stress, demonstrating that shear exposure results in thinner, but denser biofilms. These studies suggest that biofilm architecture is modulated by shear in a phase-dependent manner.
Keywords: Candida albicans, biofilms, shear stress, catheter infections
Introduction
Central venous catheter infections caused by Candida albicans are associated with a high morbidity and mortality approaching 50%.1,2 A 2004 prospective nationwide surveillance study found that Candida species were responsible for 9.5% of nosocomial bloodstream infections.3 Biofilms (colonies of microbial cells encased in a self-produced polysaccharide matrix) formed on catheter surfaces have been previously shown to play a role in such infections.4–7 Fungal biofilms are characterised by a thick extracellular matrix and reduced susceptibility to commonly used antifungal agents.8–10
Previous work in our laboratory has focused on the development and characterisation of a C. albicans biofilm model on silicone elastomer catheter discs in order to better understand biofilm formation and antifungal agent resistance.8–10 Using this in vitro model, biofilms are formed under relatively static or ‘no-flow’ (gentle rocking) conditions. However, under in vivo conditions, biofilms formed on central venous catheters are exposed to shear conditions caused by blood flow (for an excellent review of the fluid mechanics involved in microbial adhesion under shear stress, please refer to Busscher & van der Mei [11]). Previously, Shive et al. [12, 13] and Patel et al. [14] used a rotating disc system (RDS) to study the role of shear in biomaterial-related infections caused by bacteria. In their studies, the level of shear, which is directly proportional to the distance from the centre of the disc, affected both bacterial adhesion as well as the host inflammatory response.
In this study, we examined the effect of shear stress on the formation of C. albicans biofilms on catheter discs. By developing a model that more closely represents in vivo conditions, our goal was to gain a better understanding of the role of Candida biofilms in catheter-associated infections.
Materials and methods
Yeast strain and growth conditions
The C. albicans clinical strain SC5314 was used. Cells were grown for 24 h at 37 °C in yeast nitrogen base (YNB; Sigma, St Louis, MO, USA) supplemented with 50 mmol l−1 dextrose. Cells were harvested, washed with phosphate-buffered saline (PBS; Cellgro, Media-tech, Herndon, VA, USA) and standardised to 1 × 107 cells ml−1.
Candida albicans biofilm formation
For no-flow (static) conditions, biofilms were formed on 17-mm-diameter silicone elastomer catheter discs (Bentec Medical, Inc., Wakefield, MA, USA) as previously described.9 Briefly, the discs were added to 12-well plates containing 2 ml per well of foetal bovine serum (FBS; HyClone, Logan, UT, USA) and incubated for 24 h at 37 °C. The discs were then added to fresh 12-well plates and 4 ml of standardised Candida cell suspension (in PBS) were added to each well. The cells were allowed to adhere for 90 min at 37 °C (adhesion phase). The discs were then transferred to new plates containing 4 ml per well of YNB supplemented with dextrose and incubated at 37 °C with gentle rocking for specified time periods (biofilm growth phase).
For shear conditions, biofilms were formed using a rotating disc system (RDS; Pine Instrument) as described previously for bacterial studies.12–14 The system was adapted for studying C. albicans biofilms as follows. All RDS components were sterilised prior to use. FBS-precoated silicone elastomer catheter discs (17 mm diameter) were attached to the rotating arm of the system using Krazy® Glue (Elmer’s Products, Columbus, OH, USA) and immersed into a beaker containing 24.1 ml of PBS, and placed in a 37 °C water bath. The disc was rotated at 350 revolutions per minute (rpm) generating shear-stress levels of 0–18 dyne cm−2, simulating physiological conditions. Four millilitres of cell suspension containing 28 × 107 cells was gently added to the beaker, with the final cell concentration of 1 × 107 cells ml−1. Cells were allowed to adhere for 90 min. Non-adherent cells were then removed using fluid exchange with 150 ml YNB supplemented with dextrose. Biofilms were then allowed to form for specified time periods.
Quantitative measurement of C. albicans biofilms
Metabolic activity of C. albicans biofilms formed under no-flow and shear conditions was assessed using a colorimetric assay, which determined mitochondrial dehydrogenase activity as previously described.15 The assay involves metabolic reduction of 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenyl amino) carbonyl]-2H-tetrazolium hydroxide (XTT) to a water-soluble XTT-formazan product.16,17 Silicone discs with biofilms were transferred to 12-well tissue culture plates containing 4 ml PBS per well. Fifty microlitres of XTT (1 mg ml−1 in PBS; Sigma) and 4 μl menadione (Sigma) solution (1 mmol l−1 in acetone) were added to each well. The plates were covered with aluminium foil to prevent light exposure and incubated at 37 °C for 5 h. The contents of the wells were aspirated and transferred into 15 ml tubes and centrifuged (5 min, 2500 g). XTT formazan in the supernatant was, determined spectrophotometrically at 492 nm. A total of three experiments were performed at each time point, and a mean activity was calculated.
Dry-weight analysis was used to determine the total biofilm mass including both fungal cells and matrix.15 After XTT analysis, the discs were rinsed in PBS, and the contents were added to the tube containing the cell/matrix pellet and remaining supernatant. The tubes were then vortexed, and the suspension was filtered through preweighed filters (0.22 μm pore size; Millipore Corporation, Billerica, MA, USA). After washing with PBS, the filters were dried at 37 °C for 48 h and then weighed. A total of three experiments were performed at each time point, and the mean dry weight was calculated.
Confocal scanning laser microscopy (CSLM)
Biofilm architecture and thickness were examined using CSLM as previously described.9 Briefly, at various time points, the silicone elastomer discs were transferred to a 12-well plate containing 2 ml of PBS and the fluorescent stains FUN-1 (2 μl) and concanavalin A-Alexa Fluor 488 conjugate (ConA; 10 μl). FUN-1 (excitation wavelength = 543 nm and emission = 560 nm long-pass filter) is converted to orange-red cylindrical intravacuolar structures by metabolically active cells, and ConA (excitation wavelength = 488 nm and emission = 505 nm long-pass filter) binds to glucose and mannose residues of fungal cell wall polysaccharides with green fluorescence. The plates were incubated at 37 °C for 30 min, and biofilms formed on the discs were viewed using a Zeiss LSM510 confocal scanning laser microscope (Carl Zeiss Inc., Thornwood, NY, USA). Images were captured at the centre, midpoint and edge of the disc to ensure that representative areas within biofilms were analysed. Biofilm thickness was measured at each location on the disc using the LSM510 Image Browser software; measurements were obtained from three separate experiments and a mean thickness was calculated.
Statistical analyses
SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA) was used to perform independent samples t-test to compare the metabolic activities, dry weight, or thickness of biofilms formed under no flow or shear stress conditions. A P-value of <0.05 was considered significant.
Results
Shear stress reduced biomass of C. albicans biofilms at early phase
To determine the effect of shear stress on biomass of biofilms, we determined the dry weight of biofilms at different phases (adhesion, early and mature) under no flow or shear stress conditions. As shown in Table 1, under no flow conditions, only a small amount of biofilm was detected at the 90 min time point (0.16 ± 0.16 mg), while no mass could be detected in biofilms growing under shear conditions. In contrast, dry weight of biofilms grown under no flow at the 6 h time point was significantly greater than those grown under shear conditions (0.870 ± 0.25 vs. 0.228 ± 0.193 mg, respectively; P = 0.003). However, by 24 h, the dry weights were similar under both conditions (3.364 ± 1.286 vs. 2.750 ± 0.957 mg; P = 0.404). These results demonstrated that shear stress reduced biomass of C. albicans biofilms at early phase.
Table 1.
Metabolic activities (XTT analysis) and dry weights of C. albicans biofilms formed under no-flow and shear conditions during adhesion phase (90 min), early phase (6 h), and intermediate/mature phase (24 h) of biofilm development
| Time | XTT OD |
Dry weight (mg) |
||||
|---|---|---|---|---|---|---|
| No flow | Shear | P-value | No flow | Shear | P-value | |
| 90 min | 0.040 ± 0.018 | 0.003 ± 0.002 | 0.007 | 0.164 ± 0.159 | 0.000 ± 0.000 | 0.114 |
| 6 h | 0.153 ± 0.020 | 0.110 ± 0.064 | 0.087 | 0.870 ± 0.257 | 0.228 ± 0.193 | 0.003 |
| 24 h | 0.182 ± 0.041 | 0.198 ± 0.046 | 0.521 | 3.364 ± 1.286 | 2.750 ± 0.957 | 0.404 |
Values for XTT analysis represent mean absorbance at 492 nm ± SD (standard deviation; n = at least 3); values for dry weight (DW) represent mean dry weight ± standard deviation (n = at least 3).
P-value was determined by comparing data between no flow and shear stress conditions at each time point, and values <0.05 was considered statistically significant.
Shear stress did not affect metabolic activity of C. albicans biofilms
To determine the effect of shear stress on metabolic activity of biofilms, XTT analysis was performed after the 90 min adhesion phase, as well as at the 6 and 24 h time points during biofilm growth, representing adhesion, early phase and intermediate/mature phase of C. albicans biofilm formation (Table 1). After 90 min, cells that adhered under no-flow conditions had significantly higher metabolic activity than those exposed to shear stress (0.04 ± 0.018 vs. 0.003 ± 0.002, respectively; P = 0.007). In contrast, the metabolic activity of cells under no-flow conditions was not significantly different from those under shear stress at the 6 and 24 h time points (P = 0.087 and 0.521, respectively, Table 1). These results demonstrated that while biofilms formed under no flow or shear stress conditions exhibit similar metabolic activities, significant differences exist in their biomass at this time point.
Effect of shear on the architecture of C. albicans biofilms
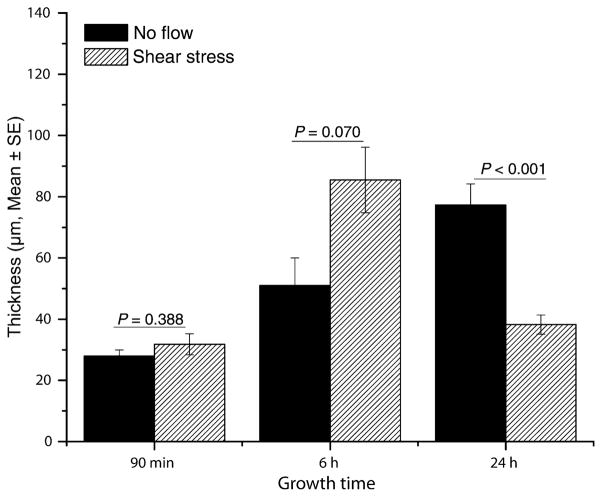
Confocal scanning laser microscopy was used to compare the gross architecture of biofilms formed under no-flow conditions with those exposed to shear stress at different time points. As can be seen in Fig. 1, after 90 min of adhesion, Candida cells had already formed a dense basal layer under no-flow conditions, while a small number of adherent cells were observed under shear stress (Fig 1a, b). By 6 h, biofilms formed under no-flow conditions produced a dense matrix (Fig. 1c), while under conditions of shear stress, only scattered clusters of Candida cells with interspersed hyphae were observed (Fig. 1d). By 24 h, biofilms formed under both no flow and shear-stress conditions exhibited the presence of extracellular matrix (Fig. 1e–f). We found that at the 90 min time point, there was no significant difference in thickness between biofilms grown under no flow or shear stress conditions (Fig. 2, P = 0.388), while biofilms grown under shear stress exhibited a non-significant trend to be thicker than those formed under no flow conditions (Fig. 2, P = 0.07). In contrast, at the 24 h time point, biofilms grown under no flow conditions were significantly thicker than those formed under shear stress conditions (77.33 ± 21.65 vs. 38.22 ± 10.00 μm, respectively; P < 0.001). Moreover, at 24 h, biofilms formed under shear conditions exhibited uniform thickness along the radius from the centre of the disc to the edge (P > 0.05). As shear changes along the radius of the discs, our results indicated that shear stress had no effect on thickness of biofilms formed under flow.
Figure 1.
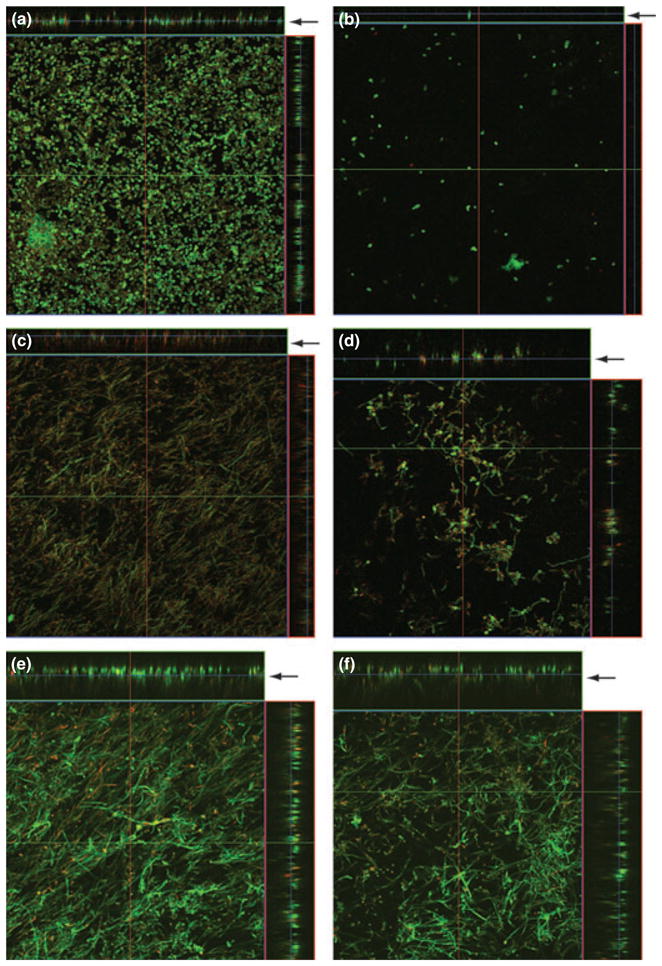
Effect of shear stress on architecture of catheter-associated Candida biofilms. Panels show orthogonal confocal images of Candida biofilms exposed to (a, c, e) no flow or (b, d, f) shear stress for (a, b) 90 min, (c, d) 6 h, or (e, f) 24 h. Magnification ×20. Arrows indicate side view for each panel, which was used to determine biofilm thickness.
Figure 2.
Effect of shear stress on thickness of Candida biofilms grown under no flow (black bars) or shear stress conditions (diagonal hatched bars). Thickness was measured at three dierent places for biofilms grown under no flow or shear conditions for 90 min, 6 or 24 h. Bars represent mean ± SE (n ≥ 3).
Discussion
In this study, we have expanded the C. albicans biofilm model developed in our laboratory to investigate the effect of shear stress on biofilm formation.15 Similar to previous studies with biofilms formed under no flow, biofilms formed under shear stress progress through an initial adhesion phase followed by a subsequent growth or maturation phase.9,15 It is during this maturation phase that fungal biofilms develop the characteristic heterogeneous structure composed of cells and extracellular matrix.
Our studies revealed that C. albicans exposed for 90 min to no-flow conditions formed a dense basal layer with measurable metabolic activity and mass. However, when exposed to shear stress for the same time, far fewer cells were able to adhere, and those that did attach, possessed significantly less metabolic activity. This was not entirely unexpected, as cells that adhere must not only do so in the face of shear, but must also overcome the force of gravity. We found that while metabolic activity of biofilms grown to early phase (6 h) was comparable under shear and no-shear conditions, dry weight of biofilms grown under shear stress was significantly less than that of biofilms grown in the absence of shear stress. Such a difference in metabolic activities or dry weights was not observed in biofilms grown to 24 h. These studies suggested that under shear stress, although there are fewer cells attached to the disc at early phase, they possess greater metabolic activity. It is possible that shear stress promotes a more rapid rate of biofilm maturation producing the comparable results seen at 24 h. Another possibility is that shear stress selects for the most healthy, robust cells that are capable of withstanding its force. Therefore, shear stress may influence the rate at which biofilms form on catheters, and may play an important role in biofilm formation in the clinical setting.
Analysis of gross architecture of biofilms did reveal a difference in biofilms formed under no flow or shear conditions. Biofilms formed under shear stress were significantly thinner and denser than those formed under no-flow conditions. This could be a means by which the cells avoid dislodgement in the presence of shear stress. It has been previously shown using an in vivo central venous catheter C. albicans biofilm model that the thickness of the biofilm at maturity was between 25 and 200 μm, consistent with the thickness of approximately 50 μm found for biofilms formed under shear conditions in our model.4 One possible reason for a difference in the thickness of these biofilms is the pulsatile nature of the flow present in vivo that is not present using the rotating disc system. The continuous shear stress in our model likely moulds the shape of the biofilm. In addition, the host immune response in vivo plays an additional role in biofilm formation and function.4
Several other investigators have studied the effect of shear stress on microbial growth and also reported variable response of bacterial biofilms exposed to such stress.18–26 Effect of shear stress on yeast (Saccharomyces cerevisiae) was investigated by Purevdorj-Gage et al. [22], who showed that fungal cells exposed to low-shear modelled microgravity conditions did not differ in growth rate, size, shape, or viability from the controls but did differ in the establishment of polarity as exhibited by aberrant (random) budding as well as an increased tendency of cells to aggregate. These investigators also found significant changes in the expression of genes associated with the establishment of polarity (BUD5), bipolar budding (RAX1, RAX2 and BUD25) and cell separation (DSE1, DSE2 and EGT2).
In previous studies, several investigators formed Candida biofilms under flow conditions in plate flow cells,27 modified Robbins device (MRD) or the CDC reactor (Centre for Disease Control), and investigated the effect of flow on matrix formation,28 susceptibility to catheter lock solutions,29 concomitant growth of probiotic bacteria on fungal biofilms30 and expression of adhesion-related genes (ALS1, ALS3).31 In this regard, Al Fattani et al. [28] showed that C. albicans biofilms grown under continuous flow produced more matrix than those grown statically, suggesting thicker biofilms were formed when exposed to flow. In a separate study, Li et al. [32] used a parallel plate flow chamber-based in vitro biofilm assay and showed that the adhesin Eap1p is required for biofilm formation under flow. More recently, Ramage [33] used an MRD model of denture-associated Candida biofilms and showed a high degree of heterogeneity associated with the structure of biofilms exposed to flow, complex three dimensional architecture and increased production of exopolymeric material. However, these previous studies did not evaluate the effect of such flow on biofilm architecture. In our current study, we were able to evaluate the phase-specific effects of shear stress on Candida biofilm formation and architecture. In contrast, our studies showed when grown under shear generated in our RDS model, early phase Candida biofilms were thinner than those grown under no flow conditions. This discrepancy between our results and some earlier studies suggesting thicker biofilms are formed under flow conditions may be because of the ‘rotating’ nature of the RDS model, which may result in the more compact biofilms.
Our studies showed that although we used the same concentration of cells to incubate the biomaterials in the static (no flow) and flow (shear stress) systems, early adhesion levels in the static system were higher than under flow conditions. Because cell density plays a relevant role during the different phases of biofilm formation, these early differences may have a profound influence in the subsequent phases of biofilm development. Thus, one could argue that the differences described here may not be directly related to shear vs. static, but rather they are simply a reflection of the lower number of cells that colonise the surface early on under flow conditions. In such a scenario, biofilms formed under shear stress conditions may eventually ‘catch up’ with biofilms formed statically over the length of the incubation period until maturation (24 h). Our results also showed that under flow, biofilm thickness was independent of shear stress, which may have important implication related to any type of medical device exposed to flowing blood.
Shear stress may impact biofilm formation in the clinical setting by influencing the rate of biofilm formation as well as its overall architecture. Further studies are necessary to examine the clinical relevance of these findings, differential molecular response of biofilms to the different shear stress conditions and their effect on antifungal susceptibility.
Acknowledgments
This work was supported by funds from NIH (1R01 DE017846) and Bristol Myers Squibb Freedom to Discover Award to MAG, NIH/NIBLB (R01 EB00279) to JMA, American Heart Association (SDG0335313N) and NIH/SDRC Pilot & Feasibility Award (P30-AR-39750) to PKM, and Flow Cytometry Core Facility of the Comprehensive Cancer Center of Case Western Reserve University and University Hospitals of Cleveland (P30CA43703).
References
- 1.Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999;29:239–44. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 2.Gudlaugsson O, Gillespie S, Lee K, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37:1172–7. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 3.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 4.Andes D, Nett J, Oschel P, Albrecht R, Marchillo K, Pitula A. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect Immun. 2004;72:6023–31. doi: 10.1128/IAI.72.10.6023-6031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schinabeck MK, Long LA, Hossain MA, et al. Rabbit model of Candida albicans biofilm infection: liposomal Amphotericin B antifungal lock therapy. Antimicrob Agents Chemother. 2004;48:1727–32. doi: 10.1128/AAC.48.5.1727-1732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee PK, Zhou G, Munyon R, Ghannoum MA. Candida biofilm: a well-designed protected environment. Med Mycol. 2005;43:191–208. doi: 10.1080/13693780500107554. [DOI] [PubMed] [Google Scholar]
- 8.Chandra J, Guangyin Z, Ghannoum MA. Fungal biofilms and antimycotics. Curr Drug Targets. 2005;6:887–94. doi: 10.2174/138945005774912762. [DOI] [PubMed] [Google Scholar]
- 9.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans–development, architecture and drug resistance. J Bacteriol. 2001;183:5385–94. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother. 2002;46:1773–80. doi: 10.1128/AAC.46.6.1773-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busscher HJ, van der Mei HC. Microbial adhesion in flow displacement systems. Clin Microbiol Rev. 2006;19:127–41. doi: 10.1128/CMR.19.1.127-141.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shive MS, Brodbeck WG, Anderson JM. Activation of caspase 3 during shear stress-induced neutrophil apoptosis on biomaterials. J Biomed Mater Res. 2002;62:163–8. doi: 10.1002/jbm.10225. [DOI] [PubMed] [Google Scholar]
- 13.Shive MS, Brodbeck WG, Colton E, Anderson JM. Shear stress and material surface effects on adherent human monocyte apoptosis. J Biomed Mater Res. 2002;60:148–58. doi: 10.1002/jbm.10035. [DOI] [PubMed] [Google Scholar]
- 14.Patel JD, Ebert M, Stokes K, Ward R, Anderson JM. Inhibition of bacterial and leukocyte adhesion under shear stress conditions by material surface chemistry. J Biomater Sci Polym Ed. 2003;14:279–95. doi: 10.1163/156856203763572725. [DOI] [PubMed] [Google Scholar]
- 15.Chandra J, Mukherjee PK, Leidich SD, et al. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J Dent Res. 2001;80:903–8. doi: 10.1177/00220345010800031101. [DOI] [PubMed] [Google Scholar]
- 16.Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev. 2005;11:127–52. doi: 10.1016/S1387-2656(05)11004-7. [DOI] [PubMed] [Google Scholar]
- 17.Meshulam T, Levitz SM, Christin L, Diamond RD. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanil ide (XTT) J Infect Dis. 1995;172:1153–6. doi: 10.1093/infdis/172.4.1153. [DOI] [PubMed] [Google Scholar]
- 18.Nickerson CA, Ott CM, Wilson JW, Ramamurthy R, Pierson DL. Microbial responses to microgravity and other low-shear environments. Microbiol Mol Biol Rev. 2004;68:345–61. doi: 10.1128/MMBR.68.2.345-361.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crabbe A, De Boever P, Van Houdt R, Moors H, Mergeay M, Cornelis P. Use of the rotating wall vessel technology to study the effect of shear stress on growth behaviour of Pseudomonas aeruginosa PA01. Environ Microbiol. 2008;10:2098–110. doi: 10.1111/j.1462-2920.2008.01631.x. [DOI] [PubMed] [Google Scholar]
- 20.Fonseca AP, Sousa JC. Effect of shear stress on growth, adhesion and biofilm formation of Pseudomonas aeruginosa with antibiotic-induced morphological changes. Int J Antimicrob Agents. 2007;30:236–41. doi: 10.1016/j.ijantimicag.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Sahoo S, Rao KK, Suresh AK, Suraishkumar GK. Intracellular reactive oxygen species mediate suppression of sporulation in Bacillus subtilis under shear stress. Biotechnol Bioeng. 2004;87:81–9. doi: 10.1002/bit.20095. [DOI] [PubMed] [Google Scholar]
- 22.Purevdorj-Gage B, Sheehan KB, Hyman LE. Effects of lowshear modeled microgravity on cell function, gene expression, and phenotype in Saccharomyces cerevisiae. Appl Environ Microbiol. 2006;72:4569–75. doi: 10.1128/AEM.03050-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Q, Fang A, Pierson DL, Mishra SK, Demain AL. Shear stress enhances microcin B17 production in a rotating wall bioreactor, but ethanol stress does not. Appl Microbiol Biotechnol. 2001;56:384–7. doi: 10.1007/s002530100610. [DOI] [PubMed] [Google Scholar]
- 24.Arnaud JP, Lacroix C, Foussereau C, Choplin L. Shear stress effects on growth and activity of Lactobacillus delbrueckii subsp. bulgaricus. J Biotechnol. 1993;29:157–75. doi: 10.1016/0168-1656(93)90048-r. [DOI] [PubMed] [Google Scholar]
- 25.Rupp CJ, Fux CA, Stoodley P. Viscoelasticity of Staphylococcus aureus biofilms in response to fluid shear allows resistance to detachment and facilitates rolling migration. Appl Environ Microbiol. 2005;71:2175–8. doi: 10.1128/AEM.71.4.2175-2178.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azevedo NF, Pinto AR, Reis NM, Vieira MJ, Keevil CW. Shear stress, temperature, and inoculation concentration influence the adhesion of water-stressed Helicobacter pylori to stainless steel 304 and polypropylene. Appl Environ Microbiol. 2006;72:2936–41. doi: 10.1128/AEM.72.4.2936-2941.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wesenberg-Ward K, Tyler B, Sears J. Adhesion and biofilm formation of Candida albicans on native and Pluronictreated polystyrene. Biofilms. 2005;2:63–71. [Google Scholar]
- 28.Al-Fattani MA, Douglas LJ. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol. 2006;55:999–1008. doi: 10.1099/jmm.0.46569-0. [DOI] [PubMed] [Google Scholar]
- 29.Raad I, Hanna H, Dvorak T, Chaiban G, Hachem R. Optimal antimicrobial catheter lock solution, using different combinations of Minocycline, EDTA, and 25-percent ethanol, rapidly eradicates organisms embedded in biofilm. Antimicrob Agents Chemother. 2007;51:78–83. doi: 10.1128/AAC.00154-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Mei HC, Free RH, Elving GJ, van Weissenbruch R, Albers FW, Busscher HJ. Effect of probiotic bacteria on prevalence of yeasts in oropharyngeal biofilms on silicone rubber voice prostheses in vitro. J Med Microbiol. 2000;49:713–8. doi: 10.1099/0022-1317-49-8-713. [DOI] [PubMed] [Google Scholar]
- 31.Nailis H, Coenye T, Van Nieuwerburgh F, Deforce D, Nelis HJ. Development and evaluation of different normalization strategies for gene expression studies in Candida albicans biofilms by real-time PCR. BMC Mol Biol. 2006;7:25. doi: 10.1186/1471-2199-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li F, Svarovsky MJ, Karlsson AJ, et al. Eap1p, an adhesin that mediates Candida albicans biofilm formation in vitro and in vivo. Eukar Cell. 2007;6:931–9. doi: 10.1128/EC.00049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramage G, Wickes BL, Lopez-Ribot JL. A seed and feed model for the formation of Candida albicans biofilms under flow conditions using an improved modified Robbins device. Rev Iberoam Micol. 2008;25:37–40. doi: 10.1016/s1130-1406(08)70009-3. [DOI] [PubMed] [Google Scholar]