Abstract
Divalent cations and polysaccharides such as inulin and dextran reversibly inhibited hemolysis of rabbit erythrocytes caused by Vibrio metschnikovii cytolysin. On the basis of the 50% inhibitory doses, the cations were divided into two groups, group I (Cd2+, Cu2+, Ni2+, Sn2+, and Zn2+) and group II (Ba2+, Ca2+, Co2+, Mg2+, Mn2+, and Sr2+). Neither divalent cations nor polysaccharides interfered with the binding of toxins to the erythrocyte membrane. Group I cations disturbed tetramer formation of cytolysin on the cytolysin-lysed erythrocyte membrane, although group II cations and dextran did not affect the process. Erythrocytes treated with cytolysin in the presence of group II cations or dextran lysed after transfer to toxin- and inhibitor (group II cations or dextran)-free buffer at both 37 and 4 degrees C. However, erythrocytes treated in the presence of group I cations lysed at 37 degrees C but not at 4 degrees C, indicating that group I cations block the temperature-dependent lesion (tetramer)-forming step subsequent to the binding of cytolysin to the erythrocytes. The cytolysin-treated erythrocytes swelled in a colloid osmotic manner, and the swelling was preceded by the binding and the lesion-forming steps. It is also suggested that the lysis of the erythrocytes proceeds in a temperature-independent manner and that the cytolysin does not bind to the erythrocytes at 4 degrees C. These findings suggest that the sequence of V. metschnikovii cytolysin-induced hemolysis is defined by three steps: (i) a temperature-dependent binding step, (ii) a temperature-dependent lesion-forming step, and (iii) a temperature-independent lysis step.
Full text
PDF
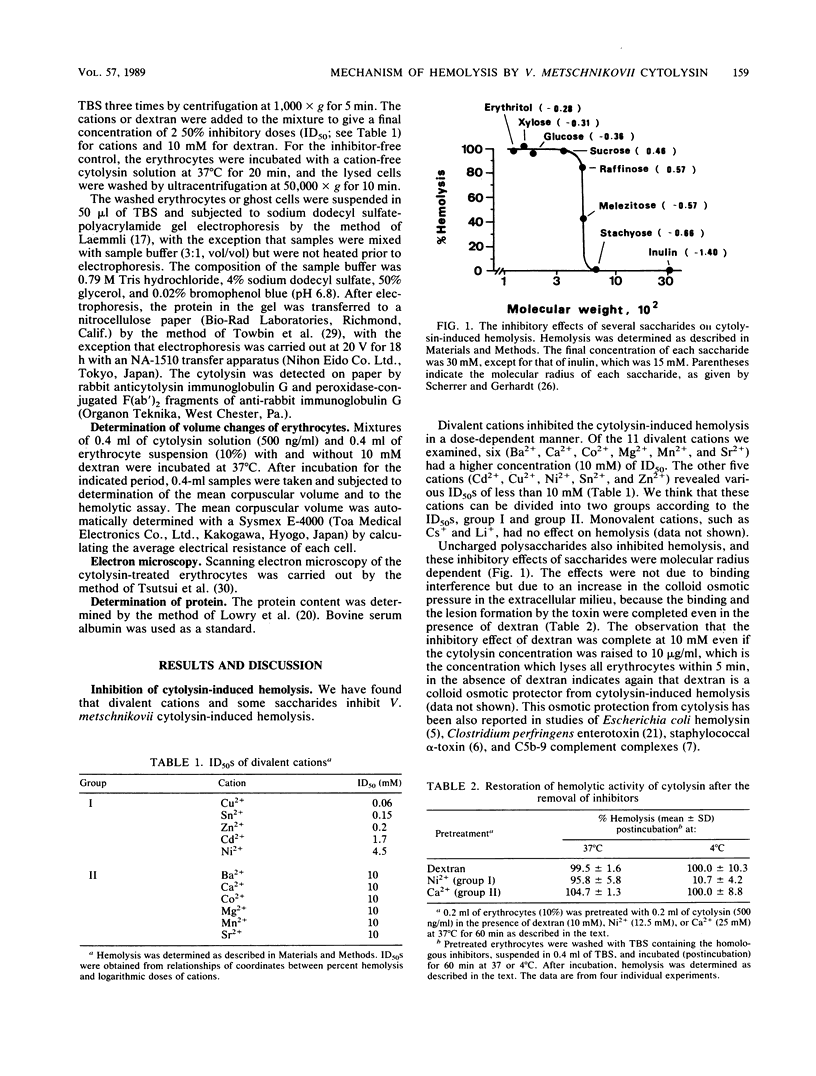
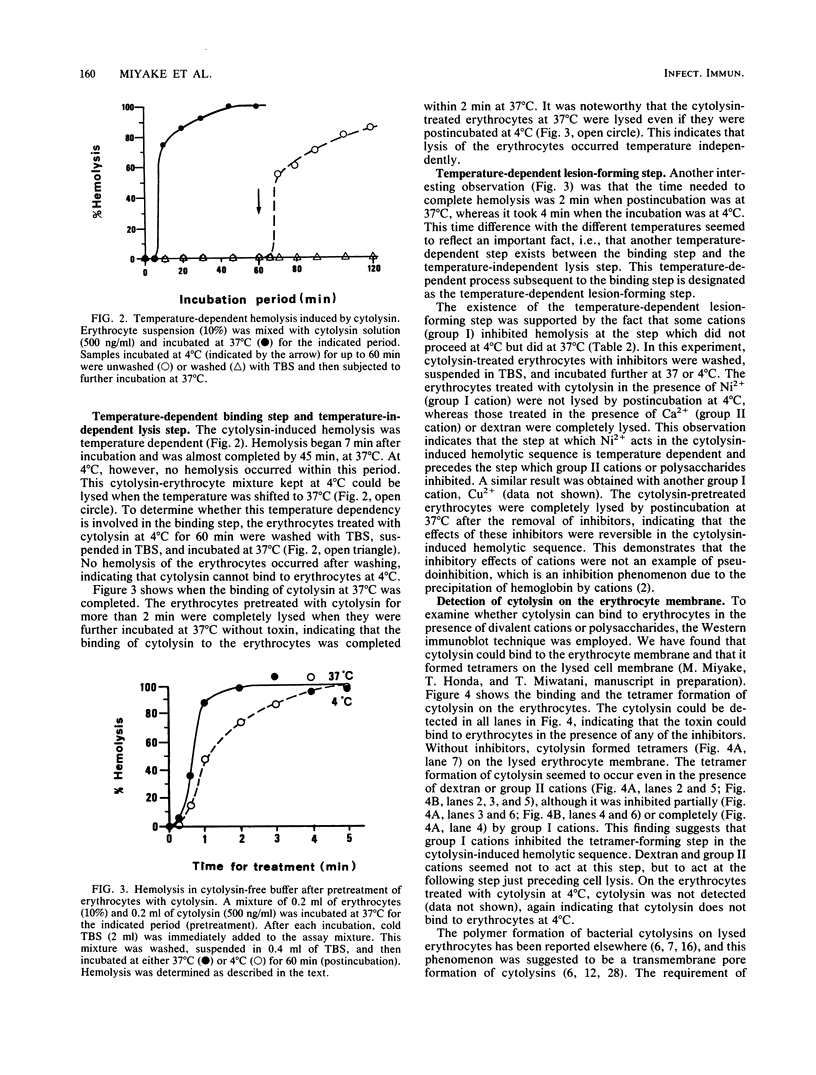
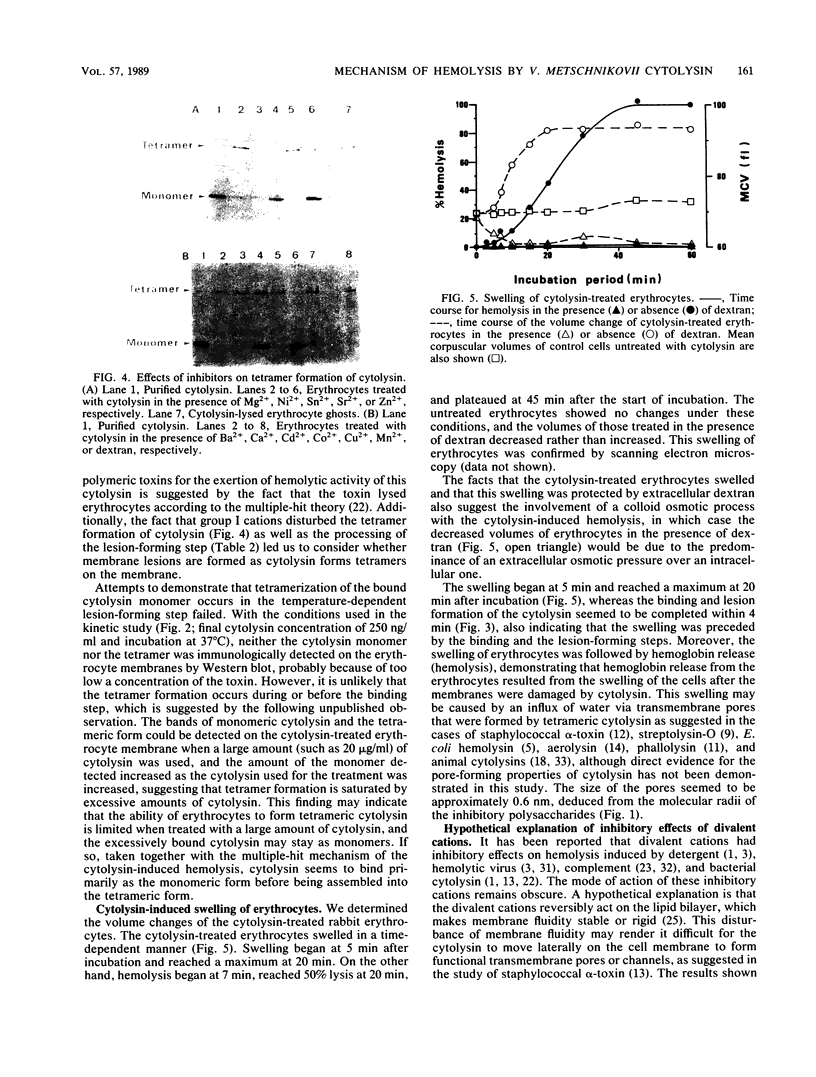


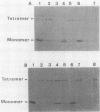
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avigad L. S., Bernheimer A. W. Inhibition by zinc of hemolysis induced by bacterial and other cytolytic agents. Infect Immun. 1976 May;13(5):1378–1381. doi: 10.1128/iai.13.5.1378-1381.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avigad L. S., Bernheimer A. W. Inhibition of hemolysis by zinc and its reversal by L-histidine. Infect Immun. 1978 Mar;19(3):1101–1103. doi: 10.1128/iai.19.3.1101-1103.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashford C. L., Alder G. M., Menestrina G., Micklem K. J., Murphy J. J., Pasternak C. A. Membrane damage by hemolytic viruses, toxins, complement, and other cytotoxic agents. A common mechanism blocked by divalent cations. J Biol Chem. 1986 Jul 15;261(20):9300–9308. [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Füssle R. Correlation between toxin binding and hemolytic activity in membrane damage by staphylococcal alpha-toxin. Infect Immun. 1984 Nov;46(2):318–323. doi: 10.1128/iai.46.2.318-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Membrane damage by complement. Biochim Biophys Acta. 1983 Aug 11;737(3-4):343–372. doi: 10.1016/0304-4157(83)90006-0. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Membrane damage by pore-forming bacterial cytolysins. Microb Pathog. 1986 Feb;1(1):5–14. doi: 10.1016/0882-4010(86)90027-6. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J., Sziegoleit A. Mechanism of membrane damage by streptolysin-O. Infect Immun. 1985 Jan;47(1):52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds D. T. Electrostatic models for ion channels and pumps. Biochem Soc Symp. 1985;50:1–10. [PubMed] [Google Scholar]
- Faulstich H., Bühring H. J., Seitz J. Physical properties and function of phallolysin. Biochemistry. 1983 Sep 13;22(19):4574–4580. doi: 10.1021/bi00288a035. [DOI] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman S., Sugg N. Effect of calcium ions on staphylococcal alpha-toxin-induced hemolysis of rabbit erythrocytes. Infect Immun. 1985 Jan;47(1):37–40. doi: 10.1128/iai.47.1.37-40.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Membrane glycoprotein receptor and hole-forming properties of a cytolytic protein toxin. Biochemistry. 1982 Mar 30;21(7):1662–1667. doi: 10.1021/bi00536a029. [DOI] [PubMed] [Google Scholar]
- Jean-Jacques W., Rajashekaraiah K. R., Farmer J. J., 3rd, Hickman F. W., Morris J. G., Kallick C. A. Vibrio metschnikovii bacteremia in a patient with cholecystitis. J Clin Microbiol. 1981 Dec;14(6):711–712. doi: 10.1128/jcm.14.6.711-712.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaki S., Kato K., Asao T., Kamata Y., Sakaguchi G. Activities of Aeromonas hydrophila hemolysins and their interaction with erythrocyte membranes. Infect Immun. 1987 Jul;55(7):1594–1599. doi: 10.1128/iai.55.7.1594-1599.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarovici P., Primor N., Loew L. M. Purification and pore-forming activity of two hydrophobic polypeptides from the secretion of the Red Sea Moses sole (Pardachirus marmoratus). J Biol Chem. 1986 Dec 15;261(35):16704–16713. [PubMed] [Google Scholar]
- McClane B. A. Osmotic stabilizers differentially inhibit permeability alterations induced in Vero cells by Clostridium perfringens enterotoxin. Biochim Biophys Acta. 1984 Oct 17;777(1):99–106. doi: 10.1016/0005-2736(84)90501-7. [DOI] [PubMed] [Google Scholar]
- Miyake M., Honda T., Miwatani T. Purification and characterization of Vibrio metschnikovii cytolysin. Infect Immun. 1988 Apr;56(4):954–960. doi: 10.1128/iai.56.4.954-960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery D. W., Don L. K., Zukoski C. F., Chvapil M. The effect of zinc and other metals on complement hemolysis of sheep red blood cells in vitro. Proc Soc Exp Biol Med. 1974 Jan;145(1):263–267. doi: 10.3181/00379727-145-37789. [DOI] [PubMed] [Google Scholar]
- Pasternak C. A. Virus, toxin, complement: common actions and their prevention by Ca2+ or Zn2+. Bioessays. 1987 Jan;6(1):14–19. doi: 10.1002/bies.950060105. [DOI] [PubMed] [Google Scholar]
- Razin S. Reconstruction of biological membranes. Biochim Biophys Acta. 1972 Apr 18;265(2):241–296. [PubMed] [Google Scholar]
- Scherrer R., Gerhardt P. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J Bacteriol. 1971 Sep;107(3):718–735. doi: 10.1128/jb.107.3.718-735.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Tobkes N., Wallace B. A., Bayley H. Secondary structure and assembly mechanism of an oligomeric channel protein. Biochemistry. 1985 Apr 9;24(8):1915–1920. doi: 10.1021/bi00329a017. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke A. M., Impraim C. C., Knutton S., Pasternak C. A. Components involved in virally mediated membrane fusion and permeability changes. Biochem J. 1980 Sep 15;190(3):625–638. doi: 10.1042/bj1900625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Takahashi M. Inhibition of the terminal stage of complement-mediated lysis (reactive lysis) by zinc and copper ions. Int Arch Allergy Appl Immunol. 1975;48(5):653–663. doi: 10.1159/000231353. [DOI] [PubMed] [Google Scholar]
- Young J. D., Leong L. G., Liu C. C., Damiano A., Cohn Z. A. Extracellular release of lymphocyte cytolytic pore-forming protein (perforin) after ionophore stimulation. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5668–5672. doi: 10.1073/pnas.83.15.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]