Summary
Disruption of reproductive function is a hallmark of abuse of anabolic androgenic steroids (AAS) in female subjects. To understand the central actions of AAS, patch clamp recordings were made in estrous, diestrous and AAS-treated mice from gonadotropin releasing hormone (GnRH) neurons, neurons in the medial preoptic area (mPOA) and neurons in the anteroventroperiventricular nucleus (AVPV); regions known to provide GABAergic and kisspeptin inputs to the GnRH cells. Action potential (AP) frequency was significantly higher in GnRH neurons of estrous mice than in AAS-treated or diestrous animals. No significant differences in AAS-treated, estrous or diestrous mice were evident in the amplitude or kinetics of spontaneous postsynaptic currents (sPCSs), miniature PSCs or tonic currents mediated by GABAA receptors or in GABAA receptor subunit expression in GnRH neurons. In contrast, the frequency of GABAA receptor-mediated sPSCs in GnRH neurons showed an inverse correlation with AP frequency across the three hormonal states. Surprisingly, AP activity in the medial preoptic area (mPOA), a likely source of GABAergic afferents to GnRH cells, did not vary in concert with the sPSCs in the GnRH neurons. Furthermore, pharmacological blockade of GABAA receptors did not alter the pattern in which there was lower AP frequency in GnRH neurons of AAS-treated and diestrous versus estrous mice. These data suggest that AAS do not impose their effects either directly on GnRH neurons or on putative GABAergic afferents in the mPOA. AP activity recorded from neurons in kisspeptin-rich regions of the anteroventroperiventricular nucleus (AVPV) and the expression of kisspeptin mRNA and peptide did vary coordinately with AP activity in GnRH neurons. Our data demonstrate that AAS treatment imposes a “diestrous-like” pattern of activity in GnRH neurons and suggest that this effect may arise from suppression of presynaptic kisspeptin-mediated excitatory drive arising from the AVPV. The actions of AAS on neuroendocrine regulatory circuits may contribute the disruption of reproductive function observed in steroid abuse.
Keywords: Anabolic steroid, GnRH, GABAA receptor, drug abuse, kisspeptin, reproduction
1. Introduction
Anabolic androgenic steroids (AAS) comprise a large class of synthetic androgens developed for therapeutic purposes, but whose predominant use is now illicit self-administration to enhance athletic performance or body image (for review, Trenton and Currier, 2005; Kanayama et al., 2008). Adult men are reported to self-administer AAS at concentrations that reflect 10–100× therapeutic doses of testosterone prescribed to restore circulating levels of testosterone in hypogonadal men (Wu, 1997; Daly et al., 2001; Matsumoto and Bremner, 2004; Trenton and Currier, 2005; Kanayama et al., 2008). Girls and women are reported to take AAS at levels equivalent to or even exceeding those administered by men (Franke and Berendonk, 1997). Thus the same doses self-administered by men may be expected to yield circulating levels of androgens, both synthetic and physiological (Ryan, 1959; Quincey and Gray, 1967; Winters, 1990), that are orders of magnitude higher still than the normal levels of physiological androgens in women and girls (Wu, 1997). Additionally, the long-term risks from AAS abuse are suggested to be greater in females than in males (Franke and Berendonk, 1997) and in teenagers of both sexes than in adults (for review, Irving et al., 2002; Kanayama et al., 2008).
Humans administer AAS in complex regimes characterized by concurrent and prolonged use of multiple AAS, and greater than 100 AAS are available that vary widely in their chemical properties and their metabolic fates (Llewellyn, 2007). Although all AAS and AAS metabolites bind to the classical androgen receptor (AR), many, upon aromatization, may also exert physiological effects via classical estrogen receptor (ER) pathways (for review, Basaria et al., 2001; Shahidi, 2001). Because of this complexity, insights into the mechanistic actions of AAS have often been gleaned from studies in which a single AAS compound is administered. Of particular relevance, C17α-alkylated AAS, such as 17α-methyltestosterone, cannot be aromatized to 17β-estradiol (Ryan, 1959; Winters, 1990, Kochakian and Yesalis, 2000) and can also inhibit aromatase activity (Mor et al., 2001; de Gooyer et al., 2003; Hong et al., 2008; Penatti et al., 2009a). Thus, AR-mediated effects of this class of AAS are believed to predominate over ER-mediated actions.
While the AAS have numerous effects on neural and endocrine systems, one of the most consistently observed repercussions from chronic AAS exposure is disruption of reproductive function. In female rodents, exposure to high doses of 17α-methyltestosterone delays the day of first vaginal estrus, suppresses estrous cyclicity (Clark et al., 2003) and decreases sexual receptivity (Blasberg et al., 1998). Signaling through the AR is necessary for the suppressive actions on receptivity since effects of this AAS are reversed by concurrent treatment with the AR antagonist, flutamide (Blasberg et al., 1998).
Final neural control of the hypothalamic-pituitary-gonadal (HPG) axis resides with a sparsely distributed population of neuroeffector cells within the mPOA/AVPV that synthesize and release the peptide GnRH (Schafer and McShan, 1974; Young et al., 1983; for review, Moenter et al., 2003). Fluctuations in gonadal steroids result in dynamic changes in the activity of GnRH neurons and in secretion of this peptide that are essential for both the onset of puberty and for the control of ovulation in females (Christian et al., 2005; Christian and Moenter, 2007; for review, Christian and Moenter, 2010; Herbison, 2008). The current consensus is that GnRH neurons do not express AR (Huang and Harlan, 1993) or ERα (Herbison and Pape, 2001; c.f. Hu et al., 2008) and in females, the ability of endogenous steroids to regulate cycle-dependent activity of GnRH neurons is believed to reflect ERα-mediated changes in the activity of forebrain afferents to these neuroeffector cells (Scott et al., 2000; Grattan et al., 2007; Herbison, 2008). Among the main afferents that provide crucial regulation of GnRH neuronal function, and thus the control of the HPG axis, reside in both the AVPV and the mPOA; regions that express high levels of AR and ER and are among the most steroid-sensitive in the mammalian brain (Simerly et al., 1990; Simerly, 2002; Dakin et al., 2008).
The mPOA is a critical site for the integration of sensory input and the regulation of neuroendocrine function and the production of sexual behaviors (Madeira and Lieberman, 1995; Theodosis and Poulain, 1993). Within the mPOA, transmission mediated by GABAA receptors has been shown to regulate gonadotropin secretion (Adler and Crowley, 1986; Moguilevsky et al., 1991) and to modulate the expression of female sexual receptivity (for review, McCarthy, 1995). Neurons in the AVPV provide GABAergic, glutamatergic and kisspeptin afferent innervation to GnRH targets (Petersen et al., 2003; Ottem et al., 2004; Smith et al., 2006; Wintermantel et al., 2006; Kauffman et al., 2007). In female mice, these neurons are the key mediators during estrogen positive feedback of the changes in GnRH neuron activity that underlie preovulatory gonadotropin surge (Le et al., 1999; Simonian et al., 1999; Christian et al., 2005; 2008; Hahn and Coen, 2006).
The goal of the present study was to characterize the normal physiological variability in neuronal activity and GABAergic transmission at different stages of the estrous cycle within these populations of neuroendocrine control neurons and to determine how chronic exposure to a suprapharmacological concentration of the synthetic steroids that characterize AAS abuse disrupts neuronal signaling in these neurons that play pivotal roles in regulating normal reproductive function.
2 Materials and Methods
2.1 Drugs and reagents
17α-Methyltestosterone (17α–MT) was purchased from Steraloids (Newport, RI, USA). All other drugs and reagents were from Sigma Chemical Corporation (St. Louis, MO) or from Thermo Fisher Scientific.
2.2 Animal care and use
Female transgenic mice in which the green fluorescent protein is driven from the gonadotropin releasing hormone promoter (GFP-GnRH mice) (Suter et al., 2000) were obtained from an in-house breeding colony at Dartmouth Medical School from founder pairs generously supplied by S.M. Moenter (University of Virginia). All animal care procedures were approved by the Institutional Animal Care and Use Committee at Dartmouth, in agreement to the guidelines and recommendations of the National Institutes of Health and American Veterinary Medical Association. All animals were housed with food and water ad libitum in a temperature controlled and 12 hrs light cycle facility with lights on starting at 0700. Estrous cycle stage was determined by daily vaginal lavage (Cooper et al., 1993). All assays from AAS-treated subjects were thus compared to control subjects in diestrus and also to control subjects in estrus, when serum levels of 17β-estradiol in the mouse are elevated in comparison to diestrus (Nothnick, 2000; Wood et al., 2007). All AAS-treated animals exhibited persistent diestrous-like vaginal cytology throughout the period of drug treatment.
2.3 Drug treatment paradigm
To determine the effects of AAS exposure during adolescence on reproductive maturation, female GFP-GnRH mice in this study were administered 7.5 mg/kg/day 17α-methyltestosterone in sesame oil 6 days a week beginning on postnatal day (PN) 25–28 for a period of 4 weeks. This treatment period spans adolescence (Laviola et al., 2003). This dosage reflects a high human abuse regime, alters the onset of puberty and inhibits reproductive behaviors in both male and female rodents (Clark et al., 2006). Control subjects were administered the same volume (10–30 μl based on body weight) of sesame oil alone.
2.4 Slice Preparation
Coronal sections (300 μm) corresponding to the rostral portion of the POA and the AVPV (0.14mm Bregma) were prepared using an Electron Microscopy Sciences OTS-4000® vibroslicer as described previously (Penatti et al., 2010). Single identified GFP-GnRH neurons within the mPOA from individual subjects were also harvested and pooled for reverse transcription coupled with quantitative real time polymerase chain reaction (qRT-PCR). All recordings and cell harvests for GnRH neurons were made from the medial population of these cells (Ottem et al, 2004; Khan et al., 2010).
2.5 Electrophysiological recordings
GFP-GnRH neurons in acutely isolated coronal sections were identified by fluorescence microscopy. All recordings were made between 14:00 and 18:00 hrs at room temperature. Recordings from GnRH neurons were restricted to the medial aspect of the rostral POA and recordings from neurons in the AVPV to a region <50μm from the ventricle. Recordings of spontaneous action potential currents (AP) were made in the loose-patch on-cell configuration (Rseal = ~50 – 100MΩ). Slices were superfused with 95%O2/5%CO2-saturated artificial cerebrospinal fluid (aCSF; in mM): 125 NaCl, 1.2 CaCl2, 10 glucose, 4 KCl, 1 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, and 1 of the antioxidant, ascorbic acid (pH 7.35; 20–22°C), and aCSF was also present in the pipette. APs were recorded for a minimum of 3 min for each experimental condition, resulting in an average total time of recording of 12–20 min for GnRH neurons. Data acquisition was started only after baseline parameters (Ihold, Rseal) were stable. Frequency analysis was derived from direct assessment of inter-spike interval and patterning was determined using autocorrelation analysis (Penatti et al., 2009a,b, 2010) and classified as regular, irregular or “bursty” (Bennet and Wilson, 1999; Bar-Gad et al., 2001). Assignment of autocorrelational profiles to these three groups was found to be correlated with the coefficient of variation of AP firing: the bursty pattern was characterized by high CVs (≥ 4), the irregular pattern with CVs between 0.5 and 2.5, and the regular pattern with CVs of ≤ 0.5. The designations of patterning based upon the autocorrelogram classifications were found to be in universal agreement of classifications of firing patterns as regular, irregular or bursty that were independently made from the raw data by an independent observer.
For recordings of GABAA receptor-mediated spontaneous postsynaptic currents (sPSCs) and tonic currents, recordings were made in aCSF supplemented with 2 mM kynurenic acid to block receptors for excitatory transmission in the whole-cell configuration at a holding potential (Vhold) of −70 mV (20–22°C) (Penatti et al., 2010). Previous studies have indicated that, depending on experimental and physiological conditions, GABAA receptor activity can either excite or inhibit GnRH neurons in post-pubertal rodents (DeFazio and Moenter, 2002; Han et al. 2002, 2004; Moenter and DeFazio, 2005; Christian and Moenter, 2007; Yin et al., 2008; Chen and Moenter, 2009; Penatti et al., 2010). Since a priori the action of GABA at these receptors in GnRH neurons is not known, currents mediated by them are referred to as simply sPSCs. The pipette solution consisted of (in mM): 153 CsCl, 1 MgCl2, 5 EGTA, and 10 HEPES, to which 2 MgATP was added just prior to each experiment. The identity of synaptic currents as GABAergic was confirmed in some experiments by demonstrating blockade of events by the selective GABAA receptor competitive antagonist, bicuculline (20 μM) or strychnine (1 μM). To record miniature PSC (mPSCs), 1 μM tetrodotoxin (TTX) was added to the kynurenic acid-containing aCSF bath solution to block AP-dependent GABA release (Nusser et al., 1997; Hájos et al., 2000). Averaged PSCs were analyzed for peak current amplitude (Ipeak), frequency, and decay kinetics (biphasic and fitted with two time constants, τ1 and τ2) or with a single weighted time constant (τw) as previously described (Penatti et al., 2010). Average membrane capacitance was 20 pF, average holding current (Ihold) was 70 pA and the average series resistance (Rseries) was 20 MΩ. Recordings were only accepted for analysis if the access resistance was <25 MΩ and Ihold (in the absence of drug application) did not change more than ± ~10% during the recording. The magnitude of tonic GABAA-receptor-mediated currents (Itonic) was estimated from the difference in the amplitude of the baseline holding current before and after addition of a saturating concentration of the competitive GABAA receptor antagonist, picrotoxin (PTX; 100 μM) (Farrant and Nusser, 2005; Jones et al., 2006). Acquisition of data in the presence of 100 μM PTX was initiated ~1 min following the perfusion of this drug into the bath and data were acquired or 3 to 4 min. An average of 1–2 recordings were made per slice prepared from each animal, and no one animal within a given group disproportionately contributed cells to the group averages.
2.6 qRT-PCR
For single-cell qRT-PCR analyses, medial GFP-GnRH neurons were harvested from matched pairs of control and AAS-treated animals on the same day between 1400 and 1800 hrs with pipettes filled with aCSF containing 0.5U/μl RNase inhibitor (Ambion Inc., Austin TX). Single cell quantitative RT-PCR (qRT-PCR) was carried out for the GABAA receptor subunit mRNAs with primers and protocols previously described (Penatti et al., 2010) and Ambion’s TaqMan®PreAmp Cells-to-CT™ Kit. For real time qPCR analysis of Kiss1 mRNA, tissue sections were isolated from the mPOA of female mice during estrus and diestrus and stored in RNAlater (Ambion) at −20° C. Total RNA was extracted according to manufacturers protocol for RNAqueous (DNase treated with TURBO DNA-free), and the concentration of the RNA was determined by measuring the optical density at 260 nm. RNA was reverse transcribed using material and protocols included in the High Capacity RNA-to-cDNA kit; Ambion). Real-time PCR was performed using an AB 7500 Sequence Detection System, and all cDNAs were analyzed in triplicate with TaqMan®Gene Expression Assays (ABI) for Kiss1 (Mm03058560_m1), aromatase (Cyp19a1; (Mm00484049_m1), AR mRNA (Mm00442688_m1), and18S rRNA (Hs99999901_s1) as an internal standard.
All gene expression assays used were demonstrated to amplify with equal efficiencies. Samples with reverse transcriptase omitted were used to control for genomic DNA contamination and omission of template to control for any reagent contamination. GnRH transcripts were always detected in samples of GnRH neurons and these transcripts were not detected in single cell harvests made from non-GnRH neurons. Similarly, GnRH transcripts were not amplified from mock harvests in which the pipette was placed on the surface of the GnRH neuron or in samples of aCSF solution superfusing the slices. The 2−ΔΔCT method (Livak and Schmittgen, 2001; Peirson et al., 2003) was used for quantification of subunit mRNAs. Data are expressed normalized to internal standard as 2−ΔCT values.
2.7 Perfusions, Immunolabeling and Confocal Analysis
Mice were transcardially perfused with 4% (w/v) paraformaldehyde and post-fixed following euthanasia by ketamine/xylazine overdose (Costine et al., 2010). Coronal sections (35 μm) were prepared on a freezing sliding microtome; the sliced brain sections were divided into three sets, and stored at −20°C in an anti-freeze solution (ethylene glycol and sucrose in phosphate buffered saline; PBS). Free-floating sections were washed in PB between each step three times for 15 min on a moving platform shaker, and all reactions occurred at room temperature. Sections were reacted with 10% H2O2 (Fisher) in PBS containing 0.1% Triton X-100 (PBST). Sections were incubated for 90 minutes in blocking buffer consisting of 10% normal goat serum (NGS, Colorado Serum; Denver, CO) in 0.3% Triton X-100 in PB and subsequently with a rabbit polyclonal antiserum directed against kisspeptin-10 (Millipore AB9754, 1:1000; Billerica, MA) in PBST containing 1% NGS overnight followed by incubation with a goat anti-rabbit conjugated to Alexa-555 (Molecular Probes; 1:500 in PBT) for 2 hrs. The rabbit kisspeptin-10 polyclonal antibody (Franceschini et al., 2006), has been shown to label comparable profiles of neurons in the arcuate and the AVPV of the mouse that label with Kiss1 mRNA (Han et al., 2005, Navarro et al., 2009; Gottsch et al., 2009; Clarkson et al., 2010), to show markedly diminished immunoreactivity in Kiss1−/− mice (Lapatto et al., 2007), and to show minimal cross-reactivity with other RFamide peptides (Desroziers et al., 2010). Sections were mounted onto gelatin-covered slides and allowed to dry overnight, then cover-slipped with SlowFade Antifade (Invitrogen Corp., Gaithersburg, MD). Sections were imaged bilaterally over three anterior-posterior levels of the AVPV at 400× magnification with a confocal laser scanning microscope, digitized, subtracted for background, collected as Z-section stacks and analyzed using ImageJ (http://rsbweb.nih.gov/ij/). The AVPV was defined as the region 50μm lateral from the ventricle. The mean pixel intensity was measured in 30μm fields starting at the ventricle edge and progressing laterally on each individual image. Kisspeptin-like immunoreactive (IR) cells were counted in images taken from every third section, and the distance between the center of the nucleus (a less-labeled hollow area on the Z-stack) and the ventricle was measured for each cell. The measurements from the six images per mouse were averaged together and compared between treatment groups.
2.8 Statistical analyses
Values are presented as means ± standard error. To test for normality, Shapiro-Wilks or Kolmogorov-Smirnov tests were applied on the raw data. For qRT-PCR analysis, CT values were defined as outliers when they lay outside ± 3 standard deviations from the mean. Results were qualitatively the same whether or not outliers were included in the final analysis. Differences in the relative abundance of each mRNA between control and AAS-treated subjects were assessed using Pair-Wise Fixed Reallocation Randomization© t-test using the excel-based Relative Expression Software Tool (REST©; Pfaffl 2001; Pfaffl et al., 2002). For mRNA analysis, only positive error bars are depicted in the results; positive and negative error bars differed by ≤ 20%. For electrophysiological experiments, non-normally distributed data were log-transformed prior to statistical assessment. Significance for electrophysiological and immunohistochemical data was determined by one-way analysis of variance (ANOVA) followed by post hoc analyses using either Tukey or Fisher tests for means comparison. For all data, the alpha level was set at p < 0.05. Except where indicated to the contrary, n values indicate the number of neurons per condition.
3 Results
3.1 Action potential activity in GnRH neurons
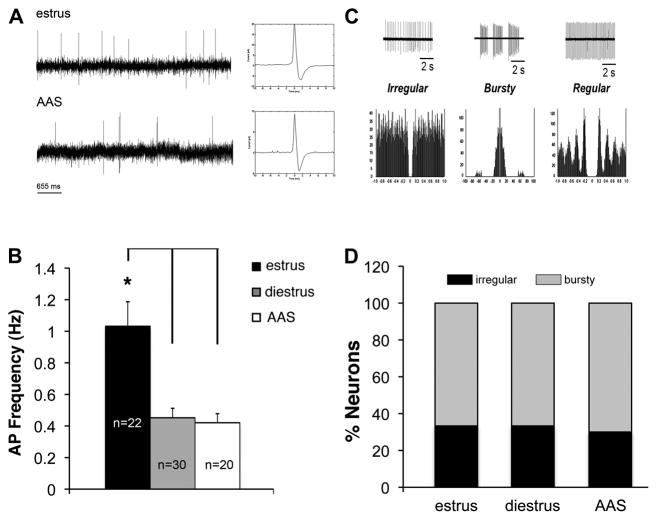
Few studies have examined changes in neural activity or synaptic inputs onto GnRH neurons in gonadally-intact mice. Therefore, to characterize the actions of chronic AAS exposure on these master control neurons of the HPG axis, we recorded on-cell AP activity from oil-injected mice in estrus and diestrus and in AAS-treated mice, all of whom showed diestrous-like vaginal lavages. Action potential frequency was found to vary with hormonal state [F(2,69) = 9.634, p < 0.0002]. The average frequency of APs in GnRH neurons was indistinguishable between diestrous (0.45 ± 0.06 Hz) and AAS-treated (0.47 ± 0.07 Hz) mice, but significantly greater in estrous mice (1.03 ± 0.16 Hz) than in either diestrous (p < 0.0001) or AAS-treated (p < 0.0009) animals (Figure 1A,B). Autocorrelation analysis indicated that AP patterning was characterized by either irregular or “bursty” profiles; no regular activity patterns were observed in GnRH neurons (Figure 1C,D). The proportion of neurons with either bursty or irregular pattern did not change significantly with cycle stage or with AAS treatment. Cells characterized by bursty firing patterns accounted for 67% of the total in estrous, 67% in diestrous and 70% in AAS-treated animals (Figure 1D). These data suggest that AAS-treatment promotes a lower-activity state in GnRH neurons that is reminiscent of a diestrous-like state, but also that there is no difference in AP patterning between the three hormonal conditions.
Figure 1. Action potential frequency and patterning in GnRH neurons of estrous, diestrous and AAS-treated mice.
(A) Representative recordings of action potentials (APs) in the on-cell configuration from GnRH neurons in estrous and AAS-treated mice. Inset shows a single representative AP on an expanded time scale. (B) Average data indicating the frequency of APs from oil-injected mice in estrus or diestrus and in mice chronically injected with 17α-methyltestosterone (AAS). Asterisk indicates that the AP firing frequency was significantly greater in estrous mice versus diestrous or AAS-treated mice; n values indicate numbers of neurons. (C) Representative APs and autocorrelational profiles for firing patterns classified as irregular, bursty and regular. (D) Relative percentage of GnRH neurons demonstrating the three different firing patterns in oil-injected animals in estrus or diestrus and in AAS-injected mice. Regular AP patterning was not observed in GnRH neurons in any of the three conditions.
3.2 GABAA receptor-mediated transmission in GnRH neurons
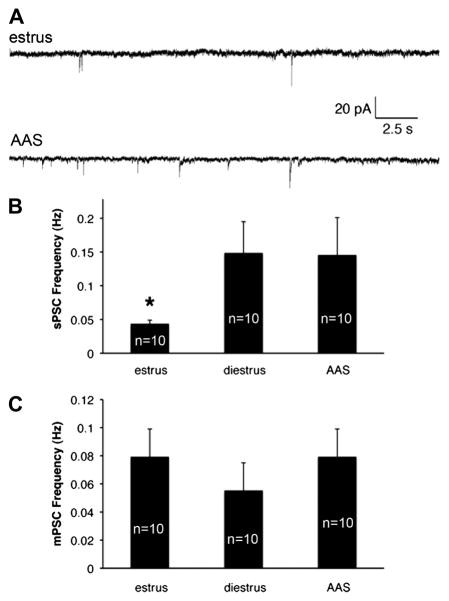
GABAergic control of GnRH pulsatility is essential for pubertal onset and regular estrous cyclicity (for review, Ojeda and Urbanski, 1994; Ojeda et al., 2006). To determine if changes in GABAA receptor-mediated inhibition played a critical role in imposing the lower level of activity observed in diestrous and AAS-treated than estrous mice, GABAA-receptor-mediated sPSCs were recorded. The frequency of sPSCs in GnRH neurons varied significantly with hormonal state [F(2,27) = 5.37, p < 0.01]. As with AP activity, the frequency of sPSCs was indistinguishable between diestrous (0.15 ± 0.05 Hz) and AAS-treated (0.15 ± 0.06 Hz) mice. In contrast, sPSC frequency in estrous mice (0.04 ± 0.01 Hz) was significantly lower than in either diestrous (p < 0.003) or AAS-treated (p < 0.02) animals (Figure 2A and B). No difference in mPSC frequency was observed (Figure 2C). These data indicate that action potential-dependent presynaptic GABAergic input varies inversely with AP frequency in GnRH neurons across the three hormonal states.
Figure 2. GABAA receptor-mediated synaptic current frequency in estrous, diestrous and AAS-treated mice.
(A) Representative sPSCs recorded from GnRH neurons of an oil-injected animal in estrus (top) and an AAS-treated (bottom) mouse. (B) Average frequency of spontaneous and (C) miniature PSCs recorded from GnRH neurons of oil-injected animals in estrus or diestrus and in AAS-treated mice; n values indicate the numbers of cells. Asterisk indicates that the frequency of GABAA receptor-mediated sPSCs in GnRH neurons was significantly lower in recordings from animals in estrus than in neurons from either diestrous or AAS-treated mice.
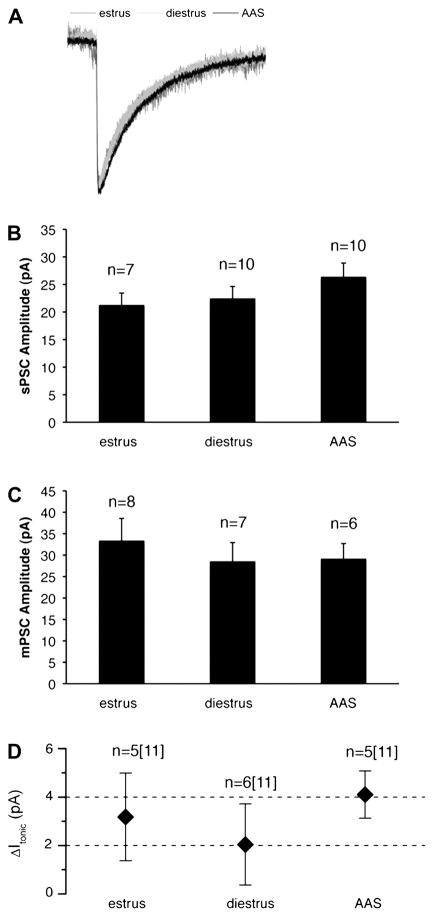
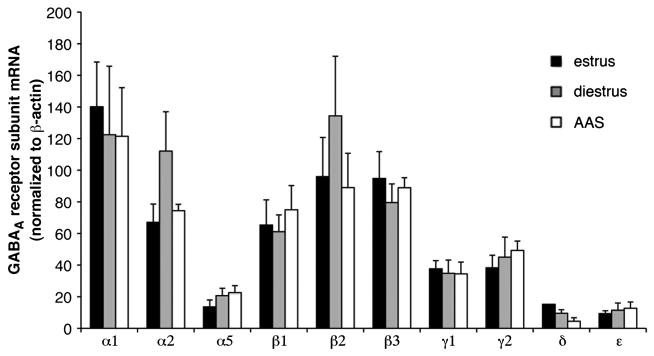
In contrast to the differences in sPSC frequency, no differences were observed in the kinetics or amplitude of sPSCs in GnRH neurons, parameters that reflect properties of postsynaptic receptors, across the three hormonal states (Figure 3A–C). Nor were there differences apparent in the frequency, amplitude or kinetics of mPSCs (Figure 2B and 3C). Similarly, while single-cell qPCR analysis of GnRH neurons indicated that these cells express a rich array of GABAA receptor subunit mRNAs, the relative expression of these subunits was not different in estrous, diestrous or AAS-treated mice (Figure 4). These data support the assertion that the predominant action of AAS exposure may be by regulating presynaptic tone to GnRH neurons rather than directly on these neurons themselves. Such indirect actions are consistent with the reported effects of AAS in male mice and consistent with the lack of AR expression in these cells (for discussion, Penatti et al., 2010).
Figure 3. Amplitudes of spontaneous, miniature and tonic GABAA receptor-mediated currents in GnRH neurons of estrous, diestrous and AAS-treated mice.
(A) Thirty or more sPSCs from each GnRH neuron from which recordings were made from 8 oil-injected animals in estrus (dark grey), 6 oil-injected animals in diestrus (light grey) and 7 AAS-treated mice (black) were average and superimposed. Average amplitude of sPSCs (B) and of mPSCs (C) recorded from GnRH neurons of mice in estrus or diestrus or from AAS-treated mice; n values indicate the number of neurons for each condition. (D) Average amplitude of picrotoxin-sensitive GABAA receptor-mediated tonic currents (Itonic-PTX) in GnRH neurons. For n values, the first number is the number of neurons that demonstrated a PTX-sensitive current; the second number in brackets indicates the total number of GnRH neurons that were assessed for Itonic.
Figure 4. GABAA receptor subunit mRNA levels in GnRH neurons of estrous, diestrous and AAS-treated mice.
Data are presented as the 2−ΔCT values, which indicate the average levels (relative to the housekeeping gene β-actin) of subunit mRNAs in 3–5 GnRH neurons isolated from estrous (n= 8) diestrous (n = 6) and AAS-treated (n = 7) mice.
The generation of the GFP-GnRH has made it possible to make comparisons not only within this specific class of neurons under different conditions, but also with neighboring non-GnRH neurons in the mPOA. An interesting observation made during the course of this study was that the postsynaptic parameters of GABAA receptor-mediated sPSCs in GnRH neurons were significantly different than those observed in non-GnRH neurons. Responses in GnRH cells were both smaller (i = 22.4 ± 1.9 pA; F(9,46) =19.74, p < 0.00005) and slower (τw = 37.13 ± 3.66 ms; F(9,46) = 22.72, p < 0.00001) than what has previously been reported for sIPSCs recorded in the general non-GnRH population of mPOA neurons under identical experimental conditions from female mice of comparable age and cycle stage (i = 62.2 ± 4.1 pA and τw = 21.35 ± 1.28 ms; Penatti et al., 2009b). These data suggest that despite comparable profiles of receptor subunit expression in GnRH neurons and the mPOA as a whole, there may be appreciable differences in the subunit composition, number, or posttranslational modification of GABAA receptors in the postsynaptic sites of these two populations of cells.
It has previously been reported that expression of the ε subunit is abundant in GnRH neurons (Moragues et al., 2003) and that acutely dissociated GnRH neurons express a substantial (~70 pA) spontaneously-gated GABAA receptor-mediated tonic current. This current can be blocked by the GABAA receptor antagonist picrotoxin (PTX) and is likely to reflect ε-containing receptors (Jones et al., 2006). Despite the presence of appreciable levels of ε subunit mRNA in individual GnRH neurons (Penatti et al., 2010; this study), PTX-sensitive tonic currents of only ~2–4 pA were evident in recordings made from GnRH neurons in situ from the intact slice and did not vary among estrous, diestrous and AAS-treated conditions. The amplitude of PTX-sensitive currents in GnRH neurons from female mice is similar to that previously reported for PTX-sensitive currents in GnRH neurons from male mice (Penatti et al., 2010). Neither tonic currents in GnRH neurons of male mice (Penatti et al., 2010), nor tonic currents in non-GnRH neurons in the mPOA (Penatti et al., 2009b) were altered by AAS treatment. In this latter study, the absence of an effect of chronic AAS treatment was evident when recordings were made in aCSF alone or in aCSF supplemented with 2 μM [GABA]; an experimental condition that may unmask Itonic. Taken together, these observations indicate that neither the AAS nor endogenous steroids that vary between estrus and diestrus appear to have an appreciable effect on the expression or function of GABAA receptors in these GnRH neurons (Figure 3D).
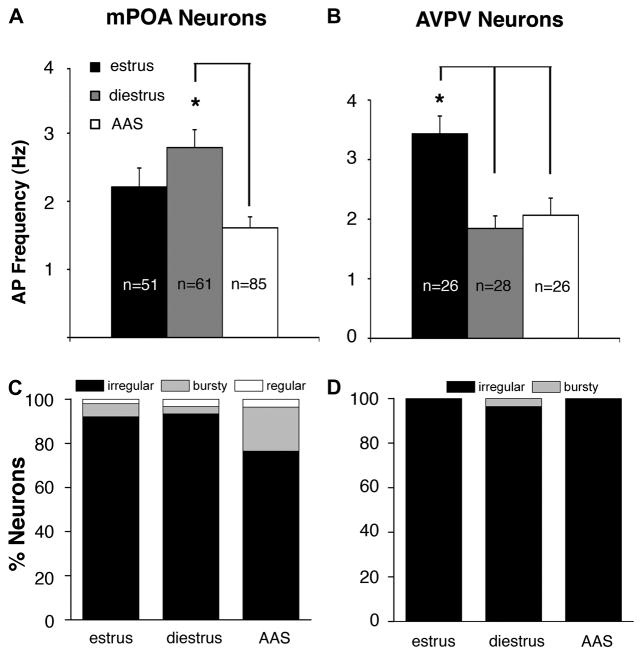
3.3 Action potential activity in mPOA neurons
The negative correlation between AP activity in GnRH neurons (low in diestrus and with AAS treatment; high in estrus) and GABAA receptor-mediated sPSC frequency (high in diestrus and with AAS treatment; low in estrus) suggested that the differences in AP activity may arise from hormonally-mediated changes in GABAergic inhibition. In male mice, it had indeed been shown that treatment through adolescence with the AAS 17α-methyltestosterone promoted a significant decrease in AP frequency in GnRH neurons and that this diminished activity was due to an increase in the frequency of GABAA receptor-mediated sPSCs arising from afferents in the more lateral regions of the mPOA (Penatti et al., 2010). The congruence in AAS actions on AP and sPSC frequencies between AAS-treated male and female mice suggested a similar mechanism for suppression of GnRH neuronal activity may exist in both sexes. Surprisingly, however, AP activity recorded in mPOA neurons of estrous, diestrous and AAS-treated female mice did not co-vary with either AP frequency or GABAA receptor-mediated sPSC frequency in GnRH neurons. Specifically, AP frequency in the mPOA was highest in diestrous mice (2.79 ± 0.26 Hz), intermediate in estrous mice (2.21 ± 0.28 Hz) and lowest in AAS-treated animals (1.61 ± 0.16 Hz) (Figure 5A). The differences in frequency between diestrous and estrous mice and between estrous and AAS-treated mice did not attain significance, but the frequency of APs in mPOA neurons from AAS-treated animals was significantly lower than that observed in diestrous mice [F(2,194) = 5.99, p < 0.003]. With respect to AP patterning, more neurons in the mPOA with bursty behavior were evident with AAS exposure than in either estrous or diestrous mice, but this difference did not attain significance (Figure 5C).
Figure 5. Action potential frequency and patterning in AVPV and mPOA neurons of estrous, diestrous and AAS-treated mice.
Average data indicating the frequency of APs from neurons in the AVPV (A) and the mPOA (B) oil-injected mice in estrus or diestrus and in mice chronically injected with AAS; n values indicate number of neurons. Asterisk in (A) indicates that the AP firing frequency in AVPV neurons was significantly higher in estrous mice than it was in diestrous or AAS-treated mice, as was also observed for GnRH neurons (see Figure 1B). Asterisk in (B) indicates that AP frequency was significantly higher in diestrous mice than either estrous or AAS-treated mice. Relative percentage of AVPV neurons (C) and mPOA neurons (D) demonstrating the three different firing patterns in oil-injected animals in estrus or diestrus and in AAS-injected mice.
The fact that AP activity in mPOA neurons did not co-vary with either sPSC frequency or AP frequency in GnRH neurons suggested that GABAergic inputs from these neurons in female mice were not the critical targets for AAS-mediated effects on GnRH neurons as they are in males. To further and more directly test this hypothesis, recordings from estrous, diestrous and AAS-treated female mice were made from GnRH cells in the presence of the GABAA receptor antagonist, PTX. Despite the antagonism of GABAA receptor-mediated responses in the presence of this blocker, the same pattern of AP firing in GnRH neurons was evident: AP frequencies were significantly (2.4×) higher in estrous than in AAS-treated mice [F(2,25) = 6.5416, p < 0.005], but not different in GnRH neurons of diestrous versus AAS-treated mice. These data indicate that, unlike AAS-treated male mice, neurons in the more lateral regions of the mPOA are not the critical presynaptic targets upon which the AAS act to alter GnRH neuron activity and that afferents that do mediated the AAS-imposed effects do so through a neurotransmitter other than GABA.
3.4 Action potential activity and kisspeptin expression in AVPV neurons
Neurons within the AVPV provide powerful kisspeptin-mediated excitatory drive to GnRH neurons (for review, Herbison, 2008; Christian and Moenter, 2010). To determine if putative kisspeptin neurons within the AVPV may mediate the AAS-dependent modulation of GnRH neuronal function, recordings were made from neurons in the AVPV within 50 μm of the 3rd ventricle, and the majority of recordings were made within 30 μm of the edge of the ventricle. AP frequency in AVPV neurons varied with hormonal state hormonal state [F(2,77) = 10.39, p < 0.0001]. The pattern of AP activity in AVPV neurons mirrored that observed in GnRH neurons, being comparable in AAS-treated and diestrous animals, but significantly lower in both diestrous (1.84 ± 0.21 Hz; p < 0.0001) and AAS-treated (2.07 ± 0.29 Hz; p < 0.002) than in estrous mice (3.43 ± 0.30 Hz) (Figure 5B). Autocorrelation analysis indicated that nearly all AVPV neurons demonstrated irregular firing patterns: 100% (estrus), 96% (diestrus), and 100% (AAS) (Figure 5D). These data are consistent with the idea that kisspeptin neurons within the AVPV may be important targets for AAS that, in turn, impose changes in activity on the GnRH neurons.
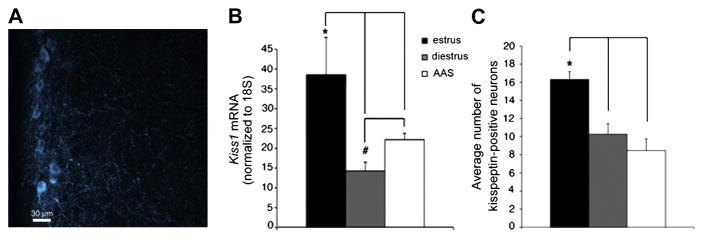
If AAS impose diminished levels of activity on putative presynaptic kisspeptin-containing afferents in the AVPV, such changes may also be reflected in the levels of kisspeptin expression within these neurons. Immunohistochemical analysis indicated that neurons demonstrating kisspeptin-like immunoreactivity (IR) predominate in the periventricular region of the AVPV from whence recordings were made (Figure 6A) and that the average distance to the ventricle of the kisspeptin population in estrous mice (29.2 ± 1.9μm), diestrous mice (26.0 ± 2.0μm), and AAS-treated mice (26.0 ± 1.9μm) was not significantly different between groups. Kisspeptin mRNA expression [F(2,22) = 9.58, p < 0.001] and the average number of kisspeptin-immunopositive neurons [F(2,13) = 12.75, p < 0.0009] in the AVPV were found to vary significantly with hormonal state in concert with the observed differences in AVPV and GnRH neuronal excitability. Specifically, steady-state levels of Kiss1 mRNA within the portion of the mPOA that includes the AVPV were significantly lower in AAS-treated (p < 0.04) and diestrous (p < 0.001) than estrous animals (Figure 6B), as were the average number of kisspeptin-positive neurons (diestrus vs. estrus, p < 0.004; AAS-treated vs. estrus, p < 0.0008) (Figure 6C). Thus, the changes in kisspeptin expression varied in a manner consistent with changes in AP activity within presumptive kisspeptin neurons.
Figure 6. Kisspeptin expression in the AVPV of estrous, diestrous and AAS-treated mice.
Representative photomicrograph of a coronal section of the AVPV from a female GFP-GnRH transgenic mouse demonstrating the location of kisspeptin-immunopositive neurons (image has been pseudocolored). (B) Average levels of Kiss1 mRNA normalized to 18S rRNA in tissue that included the AVPV and surrounding mPOA in estrous, diestrous and AAS-treated mice. *Indicates Kiss1 mRNA levels in tissue from estrous mice (n = 10) were significantly higher than those from diestrous (n = 10) and AAS-treated (n = 15) animals; # indicates that Kiss1 mRNA levels were significantly lower in diestrous that in AAS-treated mice. (C) Average number of kisspeptin immunopositive neurons in the AVPV was significantly higher in estrous (n = 5) than in diestrous (n = 5) or AAS-treated (n = 6) mice.
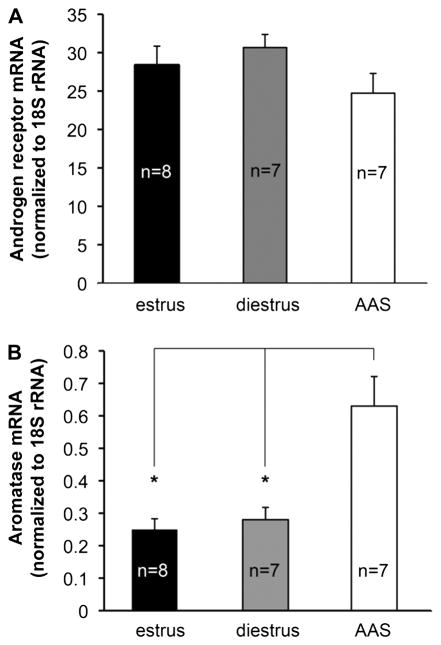
3.5 Androgen receptor and aromatase mRNA expression
Chronic AAS use has been associated with changes in expression of AR (for discussion, Kicman, 2008), and concern over the down regulation of AR influences drug use in the AAS community (e.g., see http://www.leanbulk.com/forum/lg-sciences/2940-receptor.html). To determine if effects of AAS on forebrain neuronal function were, at least in part, secondary to changes in AR expression, we measured mRNA levels for both AR itself and for the classic AR-regulated gene, aromatase (Abdelgadir et al., 1994; Foidart et al., 1995; Roselli et al., 1998; Penatti et al., 2009a). Real time PCR analysis indicated that levels of AR mRNA in the mPOA were not significantly different in estrus versus diestrus or with AAS treatment (Figure 7A). Aromatase (Cyp19a1) mRNA levels in the mPOA did vary significantly with AAS treatment [F(1,19) = 13.06, p <0.0003] and were 2.5× and 2.25× the levels observed in estrous (p < 0.001) and diestrous (p < 0.004) oil-injected female mice, respectively (Figure 7B). These data indicate that chronic AAS expression does not alter AR expression and that expression of the AR-regulated gene aromatase is significantly increased by AAS treatment in the brains of these female rodents, as has been shown previously for males (Abdelgadir et al., 1994; Foidart et al., 1995; Roselli et al., 1998; Penatti et al., 2009a).
Figure 7. Androgen receptor and aromatase mRNA levels in the mPOA of estrous, diestrous and AAS-treated mice.
Data are presented as the 2−ΔCT values for AR (A) and aromatase (Cyp19a1) mRNA levels for tissue harvested from the mPOA; n values indicate the number of animals for each condition. Levels of Cyp19 mRNA were significantly higher in AAS-treated versus either estrous or diestrous animals.
Discussion
A hallmark of illicit AAS self-administration is disruption of reproductive function and changes in sexual behaviors. In human females, early exposure to high levels of androgens alters the onset of puberty, reproductive competence, libido and sexual arousal (Strauss et al., 1985; Strauss and Yesalis, 1993; Franke and Berendonk, 1997; Elliot and Goldberg, 2000). In female rodents, AAS exposure, both prior to puberty and in adults, can alter pubertal onset, lead to irregular estrous cyclicity, diminish sexual receptivity and accelerate reproductive senescence (for review, Clark and Henderson, 2003; Clark et al., 2006). Despite these long-standing observations, the biological bases for these deleterious effects of AAS are not known, and no study to date has examined how chronic AAS exposure alters the activity of neural circuits that provide critical control of female reproduction. In order to be able to determine how AAS may alter signaling in neuroendocrine control regions, we have both characterized neuronal activity and key synaptic inputs to GnRH neurons in gonadally-intact female mice in estrus and diestrus and compared those profiles to female mice chronically administered the AAS, 17α-methyltestosterone, throughout adolescence. Our data show that AAS treatment imposed patterns of action potential firing and GABAA receptor-mediated inhibition in GnRH neurons and action potential firing and kisspeptin expression in AVPV neurons that were comparable to that observed in diestrous females, but significantly different from the patterns observed in estrous mice.
The comparable patterns of neuronal signaling in GnRH neurons in AAS-treated and diestrous mice is consistent with the observation that AAS-treated mice also demonstrate diestrous-like cytology in vaginal lavages. While at first blush the congruence in vaginal cytology and GnRH neuronal physiology suggests the conclusion that AAS exposure simply puts animals into a diestrous state, the two hormonal conditions differ in critical ways. Diestrus is a regularly occurring state that is limited in duration and characterized by relatively low levels of testosterone. The persistent anestrous state induced by AAS treatment lasts as long as the drug exposure and results in circulating levels of serum testosterone derived from the AAS that exceed endogenous levels by ~17× (Penatti et al., 2009b). Thus, the latter state is highly non-physiological with respect to the chemical composition of these synthetic androgens, as well as the concentrations and the prolonged time they are present in the brain. These sustained and nonphysiological conditions may thus be expected to impose patterns of activity in neurons that regulate both hypothalamic control of reproduction and the expression of sexual behaviors that differ from those that exist during the relatively brief physiological state of diestrus. Moreover, prolonged exposure to suprapharmacological levels of synthetic steroids during the hormone-sensitive period of adolescence may impart changes in neural organization that persist beyond the time of AAS treatment.
Previous studies highlight the divergence between AAS-imposed anestrous and the diestrous state. Specifically Pielecka et al. (2006) have shown that administration of physiological levels of the androgen, dihydrotestosterone, increased the activity of GnRH neurons in ovarectomized mice also given estrogen and progesterone (Pielecka et al., 2006) in contrast to the results from the present study which demonstrate that chronic exposure to suprapharmacological levels of the androgen, 17α-methyltestosterone, diminished AP activity in GnRH neurons compared to gonadally-intact estrous mice. In addition, it is important to note that the congruence between AAS-treatment and diestrus even in the current study was not uniform: not all neuronal populations showed comparable profiles in AAS-treated and diestrous animals. Specifically, activity of neurons within the mPOA, a region that provides critical control not only of reproduction, but also of the expression of sexual behaviors (for review, Newman, 1999; Blaustein and Erskine, 2002), was significantly lower in adolescent mice treated with 17α-methyltestosterone than in diestrous mice. Significantly lower levels of activity in mPOA neurons were also reported previously for adult female mice treated with a mixture of three commonly abused AAS (Penatti et al., 2009b). Such AAS-imposed effects in the mPOA may have repercussions beyond GnRH neuron-mediated regulation of the cycle and may underlie the changes in sexual behaviors that are observed in both rodents (for review, Clark et al., 2006) and in human subjects (Strauss et al., 1985; Franke and Berendonk, 1997; Elliot and Goldberg, 2000).
Our data suggest that AAS exposure imposes a low level of activity in GnRH neurons by modulating the activity of neurons that provide presynaptic inputs to these cells, but that postsynaptic properties, at least with respect to GABAA receptor-mediated transmission, were relatively impervious to changes in hormonal conditions. GnRH neurons do not express AR or ERα, and the current consensus is that physiological steroids regulate GnRH neuronal function indirectly through actions on upstream presynaptic partners (Huang and Harlan, 1993; Scott et al., 2000; Grattan et al., 2007; Herbison and Pape, 2001; Herbison, 2008). Data presented here as well as data from previous studies of AAS effects are also consistent with this model (Penatti et al., 2005; 2009a,b; 2010). While GnRH neurons do not express ERα or AR, they do express ERβ (Hrabovszky et al., 2001) which have the capacity to mediate signaling by androgens directly (i.e., not subsequent to aromatization) (for review, Handa et al., 2008) and they express GABAA receptors, which can be allosterically modulated by the AAS (Henderson and Jorge, 2004; Henderson, 2007). These findings suggest that further experiments should be performed to determine if the AAS may indeed have direct effects on GnRH neurons through these alternative signaling mechanisms that could influence the neural control of the HPG axis either on a briefer time frame or on other postsynaptic endpoints than were examined here.
The lower level of activity in GnRH neurons observed in AAS-treated and diestrous versus estrous female mice may reflect augmented presynaptic inhibition or lower presynaptic excitation. Lower levels of AP activity in GnRH neurons in male mice treated with 17α-methyltestosterone has been shown to arise from an increased frequency of inhibitory GABAA receptor-mediated PSCs arising from afferents in the more lateral parts of the mPOA. In female mice, however, AP activity in the mPOA across the three different hormonal states did not correlate with AP activity in the GnRH neurons, and blockade of GABAA receptors did not eliminate the differences in AP frequency in GnRH neurons between estrous and AAS-treated/diestrous animals. In contrast, AP activity in neurons in the AVPV directly correlated with activity in the GnRH neurons across the three hormonal states. Both dual-phenotype GABAergic/glutamatergic (Ottem et al., 2004) and kisspeptin-containing neurons (Smith et al., 2006; Kauffman et al., 2007) are evident in the AVPV. Although we did not directly establish the neurochemical identity of the neurons in the AVPV from which we recorded, several findings suggest that there is a likely probability these neurons synthesize the excitatory neurotransmitter, kisspeptin. First, while GABAergic neurons are present in the AVPV, the frequency of APs in AVPV neurons did not correlate with the frequency of GABAA receptor-mediated PSCs in GnRH neurons. Second, assessment of the distribution of kisspeptin-immunopositive neurons within the periventricular region that was targeted in electrophysiological studies indicated that most of the neuronal somata in this region were immunopositive for kisspeptin. Finally, 77% (20/26) of the AVPV neurons in the current study from which recordings were made exhibited AP frequencies > 2 Hz, consistent with a previous report indicating that kisspeptin neurons in the AVPV have higher firing frequencies than do non-kisspeptin neurons (Ducret et al., 2010). Thus while in female mice both the origin and the electrophysiological effects of the GABAergic sPSCs to the GnRH neurons remain to be resolved, the simplest explanation of our data is that chronic treatment of female mice with 17α-methyltestosterone diminishes the activity in GnRH neurons by dampening the electrical activity in excitatory kisspetin-containing AVPV afferents, as well as their expression of kisspeptin, to levels significantly lower than those observed in estrous mice.
The mechanism by which AAS diminish the activity of putative AVPV afferents and thus excitatory drive to GnRH neurons is unknown. The on-cell recordings performed here do not permit determination of whether or not AAS treatment alters fundamental electrical properties or the characteristics of APs in a manner that may, in turn, alter neurotransmitter release. Little data are available on the basic biophysical properties of kisspeptin neurons. Ducret et al. (2010) have shown that AP frequencies are higher in AVPV neurons from estrous than diestrous mice and that the majority of neurons in the AVPV demonstrate irregular firing patterns during diestrus, albeit the frequency they report (58%) is lower than what we observe in this study. A recent report by Qiu et al. (2011) demonstrated that 80% of kisspeptin neurons in the arcuate nucleus of the guinea pig express both pacemaker (Ih) and T-type calcium currents, but the activity of kisspeptin neurons in this region and in this species (where no AVPV is evident) may differ appreciably for that of AVPV neurons in the mouse. AAS treatment may alter a plethora of different voltage-dependent channels via transcriptional, translational or post-translational mechanisms to change firing frequencies in these cells. One attractive candidate is the class of SK channels that have been shown to regulate the AP afterhyperpolarization and thereby AP frequency in GABAergic neurons within the mPOA (Wagner et al., 2000, 2001) and in GnRH neurons themselves (Liu and Herbison, 2008). An alternative mechanism may be that long-term treatment with AAS does not affect intrinsic excitability of AVPV neurons, but rather alters the balance of excitatory/inhibitory inputs onto these cells diminishing their firing frequency. Future experiments are needed to provide a full assessment of AAS effects on the electrophysiological properties of putative AVPV afferents and of AAS effects on synaptic drive to these GnRH afferents.
A large body of literature has firmly demonstrated the essential role of ERα in mediating estrogen-dependent positive feedback and the control of the preovulatory gonadotropin surge in female mice (for review, Herbison, 2008; Oakley et al., 2009; Christian and Moenter, 2010; d’Anglemont de Tassigny et al., 2010) and in imposing the organizational actions of aromatized androgens in establishing the sexually dimorphic nature of the AVPV (Kaufmann et al., 2007; Homma et al., 2009). Our data presented here, however, strongly support the idea that other steroid signaling pathways, in particular AR-mediated signaling, may be paramount in mediating the suppression of sexual behaviors and reproductive function in female mice subjected to chronic AAS exposure. First, the ability of chronic treatment with 17α-methyltestosterone to inhibit sexual receptivity can be antagonized by the AR antagonist, flutamide (Blasberg et al., 1998). Second, 17α-methyltestosterone cannot be aromatized to the main physiological estrogen, 17β-estradiol (Ryan, 1959; Winters, 1990; Kochakian and Yesalis, 2000). Third, while the expression of aromatase (Cyp19a1) mRNA is dramatically elevated in female AAS-treated mice, consistent with previous studies on this AR-regulated gene in males (Abdelgadir et al., 1994; Foidart et al., 1995; Roselli and Resko, 1997; Roselli et al., 1998; Penatti et al., 2009a), suprapharmacological levels of 17α-methyltestosterone may promote diminished aromatization (and thus estrogen levels) by directly inhibiting the activity of this enzyme (Mor et al., 2001; deGooyer et al, 2003; Hong et al., 2008; Penatti et al., 2009a). Finally, while ERα signaling is necessary for promoting the preovulatory gonadotropin surge, AR signaling regulates the termination of estrus (Erskine, 1983) and has been implicated in establishing the insensitivity of the GnRH pulse generator to the feedback inhibition by ovarian steroids that characterizes polycystic ovarian syndrome in humans (Eagleson et al., 2000) and in animal models of this disorder (Sullivan and Moenter, 2004; Pielecka et al., 2006). These studies illustrate that while the spotlight has been on ERα signaling with regard to physiological regulation of the cycle in females, the effects AR signaling in neuroendocrine control regions should be more fully explored with regard to both natural reproductive states and conditions of steroid abuse.
Chronic exposure to suprapharmacological levels of 17α-methyltestosterone during adolescence in both female and male mice results in diminished activity of GnRH neurons and disrupted reproductive function (Penatti et al., 2010; this study). Surprisingly, however, the neural mechanism that ultimately results in suppression of GnRH neuronal activity differs between the two sexes. In males, AAS-dependent enhanced GABAergic inhibition from more lateral regions of the mPOA promoted diminished activity in GnRH targets. In females, neither the mPOA nor the enhanced frequency of GABAergic synaptic input was necessary to generate the AAS-dependent decrease in activity observed in GnRH neurons. Our data suggest instead that excitatory kisspeptin afferents in the AVPV are likely to be the crucial corollary targets for the AAS in females. These data demonstrating differences of AAS effects on neuronal physiology in the AVPV and the mPOA in female versus male mice are consistent with the known sex-specific differences in the morphological organization of these two regions and in their roles in regulating the fundamentally different constructs that underlie relative constant reproductive function in adult males versus the phasic control of cycling females (for review, Sakuma, 2009). While important differences exist between the neural mechanisms that regulate reproduction in rodents and in human subjects, our data provide important new information to apprise adolescent female AAS users not only that the AAS have detrimental effects in the brain, but also that actions of the AAS may be critically different for them than what they may gather from information that is widely disseminated through steroid websites (e.g., http://www.mesomorphosis.com/steroid-profiles/sustanon-250.htm) and AAS users manuals (e.g., Gallaway, 1997) that is almost solely based on studies in males.
Acknowledgments
This work was supported by the National Institutes of Health R01-DA14137, R01-DA18255 and T32 DK07508.
Abbreviations
- AAS
anabolic androgenic steroids
- aCSF
artificial cerebrospinal fluid
- AP
action potential
- AR
androgen receptor
- AVPV
anteroventroperiventricular nucleus
- ER
estrogen receptor
- GnRH
gonadotropin releasing hormone
- GFP
green fluorescent protein
- HPG
hypothalamic-pituitary-gonadal
- IR
immunoreactive; immunoreactivity
- mPOA
medial preoptic area
- mPSC
miniature postsynaptic current
- PBS
phosphate buffered saline
- PBST
phosphate buffered saline with Triton-X
- PN
postnatal
- PTX
picrotoxin
- qRT-PCR
quantitative real time polymerase chain reaction
- sIPSC
spontaneous inhibitory postsynaptic current
- sPSC
spontaneous postsynaptic current
- TTX
tetrodotoxin
References
- Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE. Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology. 1994;135:395–401. doi: 10.1210/endo.135.1.8013375. [DOI] [PubMed] [Google Scholar]
- Adler BA, Crowley WR. Evidence for γ-aminobutyric acid modulation of ovarian hormonal effects of luteinizing hormone secretion and hypothalamic catecholamine activity in the female rat. Endocrinol. 1986;118:91–97. doi: 10.1210/endo-118-1-91. [DOI] [PubMed] [Google Scholar]
- Bar-Gad I, Ritov Y, Bergman H. The neuronal refractory period causes a short-term peak in the autocorrelation function. J Neurosci Methods. 2001;104:155–163. doi: 10.1016/s0165-0270(00)00335-6. [DOI] [PubMed] [Google Scholar]
- Basaria S, Wahlstrom JT, Dobs AS. Clinical review 138: Anabolic-androgenic steroid therapy in the treatment of chronic diseases. J Clin Endocrinol Metab. 2001;86:5108–5117. doi: 10.1210/jcem.86.11.7983. [DOI] [PubMed] [Google Scholar]
- Blasberg ME, Robinson S, Henderson LP, Clark AS. Inhibition of estrogen-induced sexual receptivity by androgens: role of the androgen receptor. Horm Behav. 1998;34:283–293. doi: 10.1006/hbeh.1998.1484. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Wilson CJ. Spontaneous activity of neostriatal cholinergic interneurons in vitro. J Neurosci. 1999;19(13):5586–5596. doi: 10.1523/JNEUROSCI.19-13-05586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD, Erskine MS. Feminine sexual behavior: cellular integration of hormonal and afferent information in the rodent forebrain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 1. Orlando: Academic Press; 2002. pp. 139–213. [Google Scholar]
- Chen P, Moenter SM. GABAergic transmission to gonadotropin-releasing hormone (GnRH) neurons is regulated by GnRH in a concentration-dependent manner engaging multiple signaling pathways. J Neurosci. 2009;9:9809–9818. doi: 10.1523/JNEUROSCI.2509-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM. Classical estrogen receptor a signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology. 2008;149(11):5328–5334. doi: 10.1210/en.2008-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA. 2005;102(43):15682–15687. doi: 10.1073/pnas.0504270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci. 2007;27(8):1913–1921. doi: 10.1523/JNEUROSCI.4738-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocrine Revs. 2010;31:544–577. doi: 10.1210/er.2009-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AS, Costine BA, Jones BL, Kelton-Rehkopf MC, Meerts SH, Nutbrown-Greene LL, Penatti CAA, Porter DM, Yang P, Henderson LP. Sex- and age-specific effects of anabolic androgenic steroids on reproductive behaviors and on GABAergic transmission in neuroendocrine control regions. Brain Res. 2006;1126:122–138. doi: 10.1016/j.brainres.2006.08.081. [DOI] [PubMed] [Google Scholar]
- Clark AS, Kelton MC, Whitney AC. Chronic administration of anabolic steroids disrupts pubertal onset and estrous cyclicity in rats. Biol Reprod. 2003;68:465–471. doi: 10.1095/biolreprod.102.008078. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Han SK, Liu X, Lee K, Herbison AE. Neurobiological mechanisms underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Mol Cell Endocrinol. 2010;324(1–2):45–50. doi: 10.1016/j.mce.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Goldman JM, Vandenbergh JG. Monitoring of the estrous cycle in the laboratory rodent by vaginal lavage. In: Heindel JJ, Chapin RE, editors. Methods in Toxicology. Academic Press; New York: 1993. pp. 45–55. [Google Scholar]
- Costine BA, Oberlander JG, Davis MC, Penatti CAA, Porter DM, Leaton RM, Henderson LP. Chronic anabolic androgenic steroid exposure alters the central regulation and expression of anxiety in the female mouse. Psychoneuroendocrinology. 2010;35:1473–1485. doi: 10.1016/j.psyneuen.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly RC, Su TP, Schmidt PJ, Pickar D, Murphy DL, Rubinow DR. Cerebrospinal fluid and behavioral changes after methyltestosterone administration: preliminary findings. Arch Gen Psychiatry. 2001;58:172–177. doi: 10.1001/archpsyc.58.2.172. [DOI] [PubMed] [Google Scholar]
- Dakin CL, Wilson CA, Kalló I, Coen CW, Davies DC. Neonatal stimulation of 5-HT2 receptors reduces androgen receptor expression in the rat anteroventral periventricular nucleus and sexually dimorphic preoptic area. Eur J Neurosci. 2008;27:2473–2480. doi: 10.1111/j.1460-9568.2008.06216.x. [DOI] [PubMed] [Google Scholar]
- d’Anglemont de Tassigny X, Colledge WH. The role of kisspeptin signaling in reproduction. Physiology. 2010;25:207–217. doi: 10.1152/physiol.00009.2010. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Moenter SM. Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16(10):2255–2265. doi: 10.1210/me.2002-0155. [DOI] [PubMed] [Google Scholar]
- de Gooyer ME, Oppers-Tiemissen HM, Leysen D, Verheul HA, Kloosterboer HJ. Tibolone is not converted by human aromatase to 7α-methyl-17α-ethynylestradiol (7α-MEE): Analyses with sensitive bioassays for estrogens and androgens and with LC-MSMS. Steroids. 2003;68:235–243. doi: 10.1016/s0039-128x(02)00184-8. [DOI] [PubMed] [Google Scholar]
- Desroziers E, Mikkelsen J, Simonneaux V, Keller M, Tillet Y, Caraty A, Francheschini I. Mapping of kisspeptin fibres in the brain of the pro-oestrous rat. J Neuroendocrinol. 2010;22:1101–1112. doi: 10.1111/j.1365-2826.2010.02053.x. [DOI] [PubMed] [Google Scholar]
- Ducret E, Gaidamaka G, Herbison AE. Electrical and morphological characteristics of anteroventral periventricular nucleus kisspeptin and other neurons in the female mouse. Endocrinology. 2010;151(5):2223–2232. doi: 10.1210/en.2009-1480. [DOI] [PubMed] [Google Scholar]
- Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC. Polycystic ovarian syndrome: Evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85:4047–4052. doi: 10.1210/jcem.85.11.6992. [DOI] [PubMed] [Google Scholar]
- Elliot DL, Goldberg L. Women and anabolic steroids. In: Yesalis CE, editor. Anabolic steroids in sport and exercise. 2. Champaign: Human Kinetics; 2000. pp. 225–246. [Google Scholar]
- Erskine MS. Effects of an anti-androgen and 5 alpha-reductase inhibitors on estrus duration in the cycling female rat. Physiol Behav. 1983;30(4):519–524. doi: 10.1016/0031-9384(83)90214-7. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Feng Y, Weijdegård B, Wang T, Egecioglu E, Fernandez-Rodriguez J, Huhtaniemi I, Stener-Victorin E, Billig H, Shao R. Spatiotemporal expression of androgen receptors in the female rat brain during the oestrous cycle and the impact of exogenous androgen administration: A comparison with gonadally intact males. Molec Cell Endocrinol. 2010 doi: 10.1016/j.mce.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Foidart A, Harada N, Balthazart J. Aromatase-immunoreactive cells are present in mouse brain areas that are known to express high levels of aromatase activity. Cell Tissue Res. 1995;280:561–574. doi: 10.1007/BF00318360. [DOI] [PubMed] [Google Scholar]
- Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucelus co-express estrogen receptor alpha. Neurosci Lett. 2006;401:225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Franke WW, Berendonk B. Hormonal doping and androgenization of athletes: a secret program of the German Democratic Republic government. Clin Chem. 1997;43:1262–1279. [PubMed] [Google Scholar]
- Gallaway S. The Steroid Bible. 3. Sacramento: BI Press; 1997. [Google Scholar]
- Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. Neurosci. 2009;229(29):9390–9395. doi: 10.1523/JNEUROSCI.0763-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan DR, Jasoni CL, Liu X, Anderson GM, Herbison AE. Prolactin regulation of gonadotropin-releasing hormone neurons to suppress luteinizing hormone secretion in mice. Endocrinology. 2007;148(9):4344–4351. doi: 10.1210/en.2007-0403. [DOI] [PubMed] [Google Scholar]
- Hahn JD, Coen CW. Comparative study of the sources of neuronal projections to the site of gonadotrophin-releasing hormone perikarya and to the anteroventral periventricular nucleus in female rats. J Comp Neurol. 2006;494:190–214. doi: 10.1002/cne.20803. [DOI] [PubMed] [Google Scholar]
- Hájos N, Nusser Z, Rancz EA, Freund TF, Mody I. Cell type- and synapse-specific variability in synaptic GABAA receptor occupancy. Eur J Neurosci. 2000;12(3):810–818. doi: 10.1046/j.1460-9568.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- Han SK, Abraham IM, Herbison AE. Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology. 2002;143:1459–1466. doi: 10.1210/endo.143.4.8724. [DOI] [PubMed] [Google Scholar]
- Han SK, Todman MG, Herbison AE. Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology. 2004;145:495–499. doi: 10.1210/en.2003-1333. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smithe JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternative pathway for androgen regulation of brain function: Activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5α-androstane-3β,17β-diol. Horm Behav. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LP. Steroid modulation of GABAA receptor-mediated transmission in the hypothalamus: Effects on reproductive function. Neuropharmacology. 2007;52:1439–1453. doi: 10.1016/j.neuropharm.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LP, Jorge JC. Steroid modulation of GABAA receptors: CNS roles in reproduction, dysfunction and drug abuse. In: Maue RA, editor. Advances in Molecular and Cell Biology v.32: Molecular Insights into Ion Channel Biology in Health and Disease. Elsevier; 2004. pp. 217–249. [Google Scholar]
- Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: The case for the rostral periventricular area of the third ventricle (RPV3) Brain Res, Rev. 2008;57:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod. 2009;81:1216–1225. doi: 10.1095/biolreprod.109.078311. [DOI] [PubMed] [Google Scholar]
- Hong Y, Cho M, Yuan YC, Chen S. Molecular basis for the interaction of four different classes of substrates and inhibitors with human aromatase. Biochem Pharmacol. 2008;75:1161–1169. doi: 10.1016/j.bcp.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabás K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- Hu L, Gustofson RL, Feng H, Leung PK, Mores N, Krsmanovic LZ, Catt KJ. Converse regulatory functions of estrogen receptor-α and -β subtypes expressed in hypothalamic gonadotropin-releasing hormone neurons. Mol Endocrinol. 2008;22(10):2250–2259. doi: 10.1210/me.2008-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Harlan RE. Absence of androgen receptors in LHRH immunoreactive neurons. Brain Res. 1993;624:309–311. doi: 10.1016/0006-8993(93)90094-4. [DOI] [PubMed] [Google Scholar]
- Irving LM, Wall M, Neumark-Sztainer D, Story M. Steroid use among adolescents: Findings from Project EAT. J Adol Health. 2002;30:243–252. doi: 10.1016/s1054-139x(01)00414-1. [DOI] [PubMed] [Google Scholar]
- Jones BL, Whiting PJ, Henderson LP. Mechanisms of anabolic androgenic steroid inhibition of mammalian ε-subunit-containing GABAA receptors. J Physiol (Lond) 2006;573(3):571–593. doi: 10.1113/jphysiol.2006.106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG., Jr Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: a looming public health concern? Drug Alcohol Depend. 2008;98:1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of KiSS1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- Khan M, De Sevilla L, Mahesh VB, Brann DW. Enhanced glutamatergic and decreased Gabaergic synaptic appositions to GnRH neurons on proestrus in the rat: Modulatory effect of aging. PLoS One. 2010;5(4):e10172. doi: 10.1371/journal.pone.0010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kicman AT. Pharmacology of anabolic steroids. Brit J Pharmacol. 2008;154:502–521. doi: 10.1038/bjp.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochakian C, Yesalis CE. Anabolic-androgenic steroids: a historical perspective and definition. In: Yesalis CE, editor. Anabolic Steroids in Sport and Exercise. Human Kinetics; Champaign: 2000. pp. 4–33. [Google Scholar]
- Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Grp54−/− mice. Endocrinology. 2007;148(10):4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Revs. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Le WW, Berghorn KA, Rassnick S, Hoffman GE. Periventricular preoptic area neurons coactivated with luteinizing hormone (LH)-releasing hormone (LHRH) neurons at the time of the LH surge are LHRH afferents. Endocrinology. 1999;140:510–519. doi: 10.1210/endo.140.1.6403. [DOI] [PubMed] [Google Scholar]
- Liu X, Herbison AE. Small-conductance calcium-activated potassium channels control excitability and firing dynamics in gonadotropin-releasing hormone (GnRH) neurons. Endocrinology. 2008;149:3598–3604. doi: 10.1210/en.2007-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 22−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Llewellyn W. Body of Science. 6. Jupiter; FL: 2007. Anabolics; pp. vii–ix. [Google Scholar]
- Madeira MD, Lieberman AR. Sexual dimorphism in the mammalian limbic system. Prog Neurobiol. 1995;45:275–333. doi: 10.1016/0301-0082(94)00052-j. [DOI] [PubMed] [Google Scholar]
- Matsumoto AM, Bremner WJ. Serum testosterone assays accuracy matters. J Clin Endocrinol Metab. 2004;89:520–524. doi: 10.1210/jc.2003-032175. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Frank A. Beach Award. Functional significance of steroid modulation of GABAergic neurotransmission: analysis at the behavioral, cellular, and molecular levels. Horm Behav. 1995;29(2):131–140. doi: 10.1006/hbeh.1995.1010. [DOI] [PubMed] [Google Scholar]
- Moenter SM, DeFazio RA. Endogenous γ-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology. 2005;146:5374–5379. doi: 10.1210/en.2005-0788. [DOI] [PubMed] [Google Scholar]
- Moenter SM, DeFazio RA, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol. 2003;24:79–93. doi: 10.1016/s0091-3022(03)00013-x. [DOI] [PubMed] [Google Scholar]
- Moguilevsky JA, Carbone S, Szwarcfarb B, Rodina D. Sexual maturation modifies the GABAergic control of gonadotropin secretion in female rats. Brain Res. 1991;563:12–16. doi: 10.1016/0006-8993(91)91508-x. [DOI] [PubMed] [Google Scholar]
- Mor G, Eliza M, Song J, Wiita B, Chen S, Naftolin F. 17α-Methyltestosterone is a competitive inhibitor of aromatase activity in Jar choriocarcinoma cells and macrophage-like THP-1 cells in culture. J Steroid Biochem Mol Biol. 2001;79:239–246. doi: 10.1016/s0960-0760(01)00162-5. [DOI] [PubMed] [Google Scholar]
- Moragues N, Ciofi P, Lafon P, Tramu G, Garret M. GABAA receptor ε subunit expression in identified peptidergic neurons of the rat hypothalamus. Brain Res. 2003;967:285–289. doi: 10.1016/s0006-8993(02)04270-1. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavioral network. Ann NY Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Nothnick WB. Disruption of the tissue inhibitor of metaloproteinase-1 gene results in altered reproductive cyclicity and uterine morphology in reproductive-age female mice. Biol Reprod. 2000;63:905–912. doi: 10.1095/biolreprod63.3.905. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABAA receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19(3):697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocrine Revs. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF. Puberty in the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. Raven Press Ltd; New York: 1994. pp. 363–410. [Google Scholar]
- Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent AS, Matagne V, Mungenast AE. Minireview: The neuroendocrine regulation of puberty: Is the time ripe for a systems biology approach? Endocrinology. 2006;147:1166–1174. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci. 2004;24(37):8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31(14):e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti CAA, Porter DM, Henderson LP. Chronic exposure to anabolic androgenic steroids alters neuronal function in the mammalian forebrain via androgen receptor- and estrogen receptor-mediated mechanisms. J Neurosci. 2009a;29(40):12484–12496. doi: 10.1523/JNEUROSCI.3108-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti CAA, Costine BA, Porter DM, Henderson LP. Effects of chronic exposure to an anabolic androgenic steroid cocktail on α5-receptor mediated GABAergic transmission and neural signaling in the forebrain of female mice. Neuroscience. 2009b;161:526–537. doi: 10.1016/j.neuroscience.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti CAA, Davis MA, Porter DM, Henderson LP. Altered GABAA receptor-mediated synaptic transmission disrupts the firing of gonadotropin-releasing hormone neurons in male mice under conditions of steroid abuse. J Neurosci. 2010;30(19):6497–6506. doi: 10.1523/JNEUROSCI.5383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod. 2003;69:1771–1778. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (RESTc) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielecka J, Quaynor SD, Moenter SM. Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology. 2006;147:1474–1479. doi: 10.1210/en.2005-1029. [DOI] [PubMed] [Google Scholar]
- Qiu J, Fang Y, Bosch MA, Rønnekleiv OK, Kelly MJ. Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology. 2011:152. doi: 10.1210/en.2010-1285. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quincey RV, Gray CH. The metabolism of [1,2-3H]17α-methyltestosterone in human subjects. J Endocrinol. 1967;37:37–55. doi: 10.1677/joe.0.0370037. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Abdelgadir SE, Rønnekleiv OK, Klosterman SA. Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol Reprod. 1998;58:79–87. doi: 10.1095/biolreprod58.1.79. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA. Sex differences in androgen-regulated expression of cytochrome P450 aromatase in the rat brain. J Steroid Biochem Mol Biol. 1997;61:365–74. [PubMed] [Google Scholar]
- Ryan KJ. Biological aromatization of steroids. J Biol Chem. 1959;234:268–272. [PubMed] [Google Scholar]
- Sakuma Y. Gonadal steroid action and brain sex differentiation in the rat. J Neuroendocrinol. 2009;21:410–414. doi: 10.1111/j.1365-2826.2009.01856.x. [DOI] [PubMed] [Google Scholar]
- Schafer SJ, McShan WH. Gonadotropic hormone release from fetal and adult rat pituitary glands after in vitro exposure to synthetic LH-FSH-RH. Neuroendocrinology. 1974;16(5–6):332–341. doi: 10.1159/000122579. [DOI] [PubMed] [Google Scholar]
- Scott CJ, Tilbrook AJ, Rawson JA, Clarke IJ. Gonadal steroid receptors in the regulation of GnRH secretion in farm animals. Anim Reprod Sci. 2000;60–61:313–326. doi: 10.1016/s0378-4320(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Shahidi NT. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin Ther. 2001;23:1355–1390. doi: 10.1016/s0149-2918(01)80114-4. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: Organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simonian SX, Spratt DP, Herbison AE. Identification and characterization of estrogen receptor α-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. J Comp Neurol. 1999;411:346–358. doi: 10.1002/(sici)1096-9861(19990823)411:2<346::aid-cne13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. KiSS1 neurons in the forebrain as central proccessors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26(25):6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss RH, Liggett MT, Lanese RR. Anabolic steroid use and perceived effects in ten weight-trained women athletes. JAMA. 1985;253:2871–2873. [PubMed] [Google Scholar]
- Strauss RH, Yesalis CE. Additional effects of anabolic steroids on women. In: Yesalis CE, editor. Anabolic steroids in sport and exercise. Human Kinetics; Champaign: 1993. pp. 151–160. [Google Scholar]
- Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: Implications for a common fertility disorder. Proc Natl Acad Sci, USA. 2004;101(18):7129–7134. doi: 10.1073/pnas.0308058101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141(1):412–419. doi: 10.1210/endo.141.1.7279. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA. Activity-dependent neuronal-glial and synaptic plasticity in the adult mammalian hypothalamus. Neuroscience. 1993;57(3):501–535. doi: 10.1016/0306-4522(93)90002-w. [DOI] [PubMed] [Google Scholar]
- Trenton AJ, Currier GW. Behavioural manifestations of anabolic steroid use. CNS Drugs. 2005;19:571–595. doi: 10.2165/00023210-200519070-00002. [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Reyes-Vazquez C, Rønnekleiv OK, Kelly MJ. The role of intrinsic and agonist-activated conductances in determining the firing patterns of preoptic area neurons in the guinea pig. Brain Res. 2000;879:29–41. doi: 10.1016/s0006-8993(00)02698-6. [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Rønnekleiv OK, Kelly MJ. The noradgrenergic inhibition of an apamine-sensitive small conductance Ca2+-activated K+ channel in hypothalamic γ-aminobutyric acid neurons: pharmacology, estrogen sensitivity and relevance to the control of the reproductive axis. J Pharmacol Exp Ther. 2001;299:21–30. [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters SJ. Androgens: endocrine physiology and pharmacology. NIDA Res Monogr. 1990;102:113–130. [PubMed] [Google Scholar]
- Wood GA, Fata JE, Watson KLM, Khokha R. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction. 2007;133:1035–1044. doi: 10.1530/REP-06-0302. [DOI] [PubMed] [Google Scholar]
- Wu FC. Endocrine aspects of anabolic steroids. Clin Chem. 1997;43:1289–1292. [PubMed] [Google Scholar]
- Yin C, Ishii H, Tanaka N, Sakuma Y, Kato M. Activation of A-type γ-aminobutyric acid receptors excites gonadotrophin-releasing hormone neurones isolated from adult rats. J Neuroendocrinol. 2008;20:566–575. doi: 10.1111/j.1365-2826.2008.01697.x. [DOI] [PubMed] [Google Scholar]
- Young LS, Speight A, Charlton HM, Clayton RN. Pituitary gonadotropin-releasing hormone receptor regulation in the hypogonadotrophic hypogonadal (hpg) mouse. Endocrinology. 1983;113(1):55–61. doi: 10.1210/endo-113-1-55. [DOI] [PubMed] [Google Scholar]