Abstract
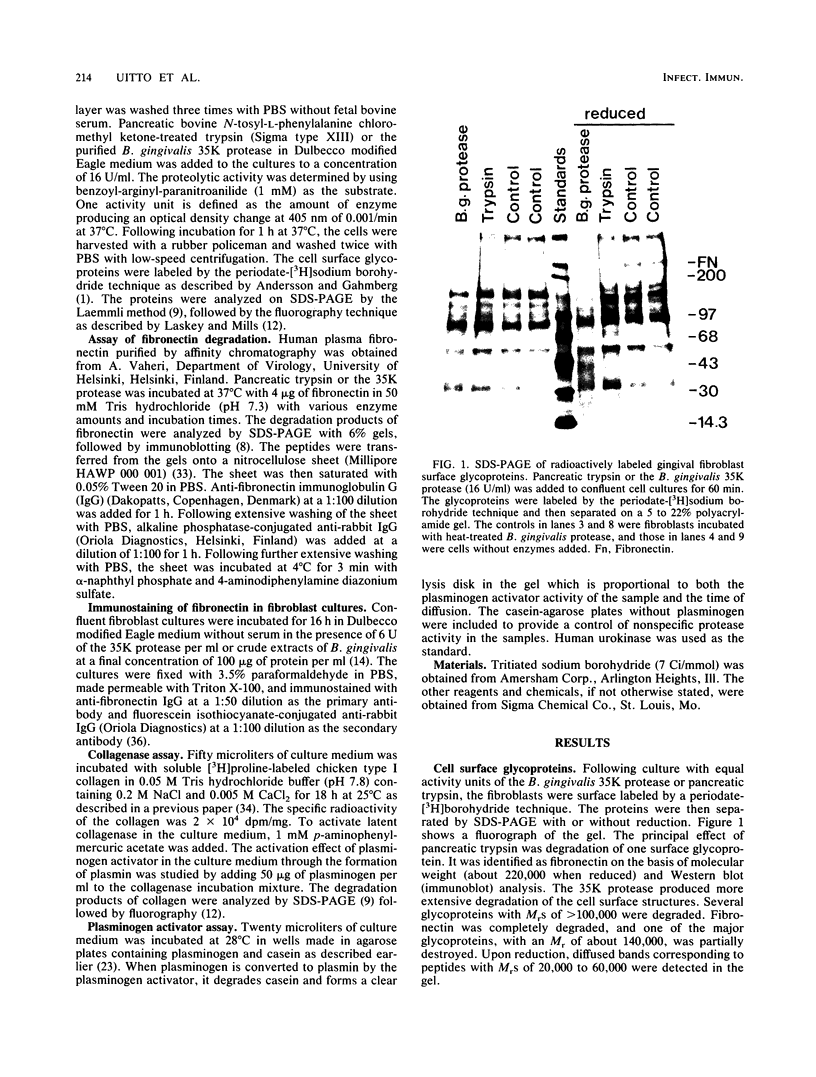
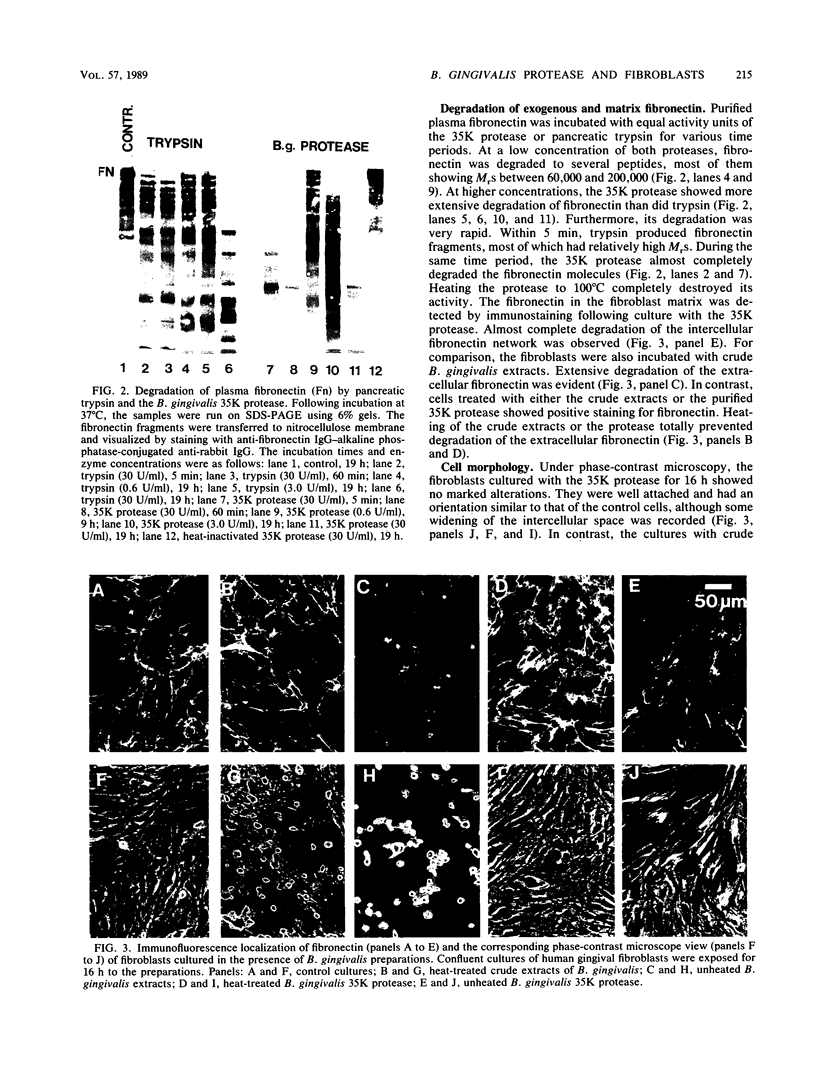
To assess the direct effects of Bacteroides gingivalis on periodontal cells, human gingival fibroblasts were cultured in the presence of B. gingivalis extracts or a trypsinlike enzyme partially purified from the bacteria by chromatography on benzamidine-Sepharose and Sephacryl S-200. Analysis of cell surface glycoproteins by the periodate-[3H]borohydride labeling technique combined with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)-fluorography demonstrated that fibronectin and some other high-molecular-weight cell surface glycoproteins were degraded by a 35,000-Mr(35K) B. gingivalis protease. Immunostaining of the fibroblast cultures showed degradation of intercellular matrix fibronectin by the 35K protease. The pattern of fibronectin degradation was monitored by examining the reaction products with the SDS-PAGE-immunoblotting technique. The protease degraded fibronectin rapidly and more extensively than did corresponding amounts of pancreatic trypsin. Collagenase secretion by the fibroblasts was assayed by incubating cell culture medium with soluble type I [3H]collagen at 25 degrees C followed by SDS-PAGE-fluorography analysis of the reaction products. The medium was also assayed for plasminogen activator activity by using a casein-agarose diffusion plate assay. The fibroblasts cultured with the 35K protease secreted increased amounts of collagenase and plasminogen activator into the medium. The results suggest that periodontal infection by B. gingivalis causes proteolytic damage of the host cell surface structures. Concomitantly, B. gingivalis may induce the cells to degrade their pericellular matrix.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. C., Gahmberg C. G. Surface glycoproteins of human white blood cells. Analysis by surface labeling. Blood. 1978 Jul;52(1):57–67. [PubMed] [Google Scholar]
- Blumberg P. M., Robbins P. W. Effect of proteases on activation of resting chick embryo fibroblasts and on cell surface proteins. Cell. 1975 Oct;6(2):137–147. doi: 10.1016/0092-8674(75)90004-5. [DOI] [PubMed] [Google Scholar]
- Heath J. K., Atkinson S. J., Hembry R. M., Reynolds J. J., Meikle M. C. Bacterial antigens induce collagenase and prostaglandin E2 synthesis in human gingival fibroblasts through a primary effect on circulating mononuclear cells. Infect Immun. 1987 Sep;55(9):2148–2154. doi: 10.1128/iai.55.9.2148-2154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Ebersole J., Felton J., Brunsvold M., Kornman K. S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988 Jan 1;239(4835):55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- Hsieh P., Chen L. B. Behavior of cells seeded in isolated fibronectin matrices. J Cell Biol. 1983 May;96(5):1208–1217. doi: 10.1083/jcb.96.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Jalkanen M., Jalkanen S. Immunological detection of proteins after isoelectric focusing in thin layer agarose gel: a specific application for the characterization of immunoglobulin diversity. J Clin Lab Immunol. 1983 Apr;10(4):225–228. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larjava H., Jalkanen M. Cell surface glycoconjugates of gingival fibroblasts exposed to dental plaque extract. J Periodontal Res. 1984 Sep;19(5):469–482. doi: 10.1111/j.1600-0765.1984.tb01302.x. [DOI] [PubMed] [Google Scholar]
- Larjava H., Uitto V. J., Eerola E., Haapasalo M. Inhibition of gingival fibroblast growth by Bacteroides gingivalis. Infect Immun. 1987 Jan;55(1):201–205. doi: 10.1128/iai.55.1.201-205.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Goldfarb R. H., Terranova V. P. Cleavage of laminin by thrombin and plasmin: alpha thrombin selectively cleaves the beta chain of laminin. Thromb Res. 1981 Mar 15;21(6):663–673. doi: 10.1016/0049-3848(81)90268-1. [DOI] [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins D. E., Rohrlich S. T. The role of proteinases in cellular invasiveness. Biochim Biophys Acta. 1983 Dec 29;695(3-4):177–214. doi: 10.1016/0304-419x(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Quigley J. P., Kellerman G. M., Reich E. Fibrinolysis associated with oncogenic transformation. Requirement of plasminogen for correlated changes in cellular morphology, colony formation in agar, and cell migration. J Exp Med. 1973 Nov 1;138(5):1056–1064. doi: 10.1084/jem.138.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjanpelto P. Proteases stimulate proliferation of human fibroblasts. J Cell Physiol. 1977 Jun;91(3):387–392. doi: 10.1002/jcp.1040910308. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Saksela O. Radial caseinolysis in agarose: a simple method for detection of plasminogen activator in the presence of inhibitory substances and serum. Anal Biochem. 1981 Mar 1;111(2):276–282. doi: 10.1016/0003-2697(81)90564-9. [DOI] [PubMed] [Google Scholar]
- Saksela O., Vaheri A., Schleuning W. D., Mignatti P., Barlati S. Plasminogen activators, activation inhibitors and alpha 2-macroglobulin produced by cultured normal and malignant human cells. Int J Cancer. 1984 May 15;33(5):609–616. doi: 10.1002/ijc.2910330510. [DOI] [PubMed] [Google Scholar]
- Salo T., Liotta L. A., Keski-Oja J., Turpeenniemi-Hujanen T., Tryggvason K. Secretion of basement membrane collagen degrading enzyme and plasminogen activator by transformed cells--role in metastasis. Int J Cancer. 1982 Nov 15;30(5):669–673. doi: 10.1002/ijc.2910300520. [DOI] [PubMed] [Google Scholar]
- Slots J., Bragd L., Wikström M., Dahlén G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontol. 1986 Jul;13(6):570–577. doi: 10.1111/j.1600-051x.1986.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Sorsa T., Uitto V. J., Suomalainen K., Turto H., Lindy S. A trypsin-like protease from Bacteroides gingivalis: partial purification and characterization. J Periodontal Res. 1987 Sep;22(5):375–380. doi: 10.1111/j.1600-0765.1987.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist G., Bloom G. D., Enberg K., Johansson E. Phagocytosis of Bacteroides melaninogenicus and Bacteroides gingivalis in vitro by human neutrophils. J Periodontal Res. 1982 Mar;17(2):113–121. doi: 10.1111/j.1600-0765.1982.tb01137.x. [DOI] [PubMed] [Google Scholar]
- Tarone G., Galetto G., Prat M., Comoglio P. M. Cell surface molecules and fibronectin-mediated cell adhesion: effect of proteolytic digestion of membrane proteins. J Cell Biol. 1982 Jul;94(1):179–186. doi: 10.1083/jcb.94.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng N. N., Bo Chen L. The role of surface proteins in cell proliferation as studied with thrombin and other proteases. Proc Natl Acad Sci U S A. 1975 Feb;72(2):413–417. doi: 10.1073/pnas.72.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turto H., Lindy S., Uitto V. J., Wegelius O., Uitto J. Human leukocyte collagenase: characterization of enzyme kinetics by a new method. Anal Biochem. 1977 Dec;83(2):557–569. doi: 10.1016/0003-2697(77)90059-8. [DOI] [PubMed] [Google Scholar]
- Uitto V. J., Tryggvason K., Sorsa T. Collagenolytic enzymes in periodontal diseases. Proc Finn Dent Soc. 1987;83(3):119–130. [PubMed] [Google Scholar]
- Vaheri A., Kurkinen M., Lehto V. P., Linder E., Timpl R. Codistribution of pericellular matrix proteins in cultured fibroblasts and loss in transformation: fibronectin and procollagen. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4944–4948. doi: 10.1073/pnas.75.10.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen I., Vartio T., Badley R. A., Lehto V. P. Fibronectin in adhesion, spreading and cytoskeletal organization of cultured fibroblasts. Nature. 1982 Aug 12;298(5875):660–663. doi: 10.1038/298660a0. [DOI] [PubMed] [Google Scholar]
- Werb Z., Aggeler J. Proteases induce secretion of collagenase and plasminogen activator by fibroblasts. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1839–1843. doi: 10.1073/pnas.75.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]