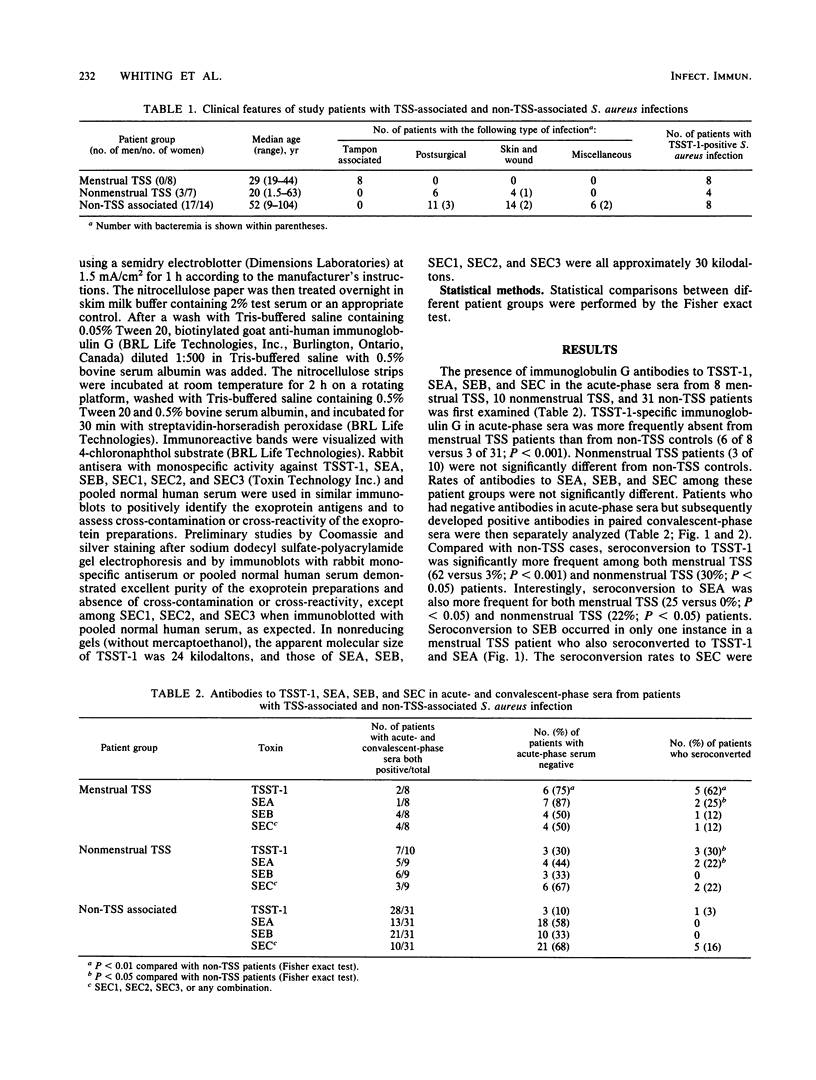
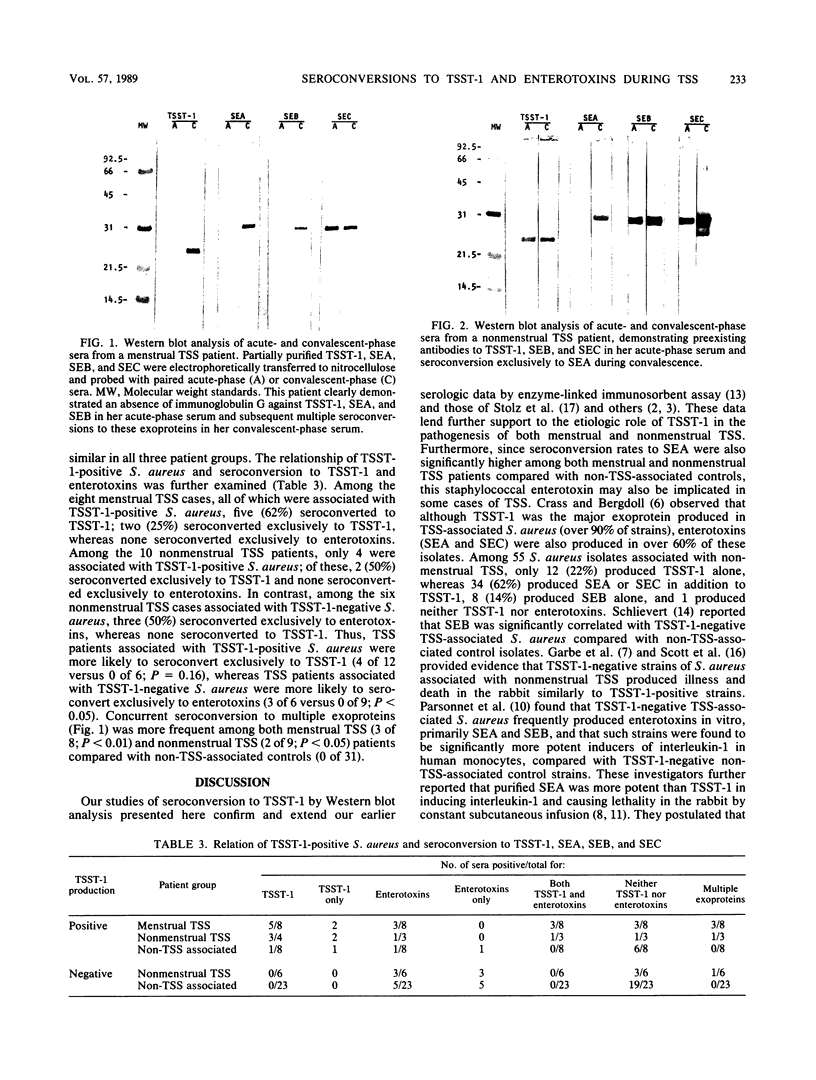
Abstract
Serum antibody responses to toxic shock syndrome (TSS) toxin 1 (TSST-1) and staphylococcal enterotoxins A, B, and C were determined by western blot (immunoblot) analysis of acute- and convalescent-phase paired sera from 18 TSS- and 31 non-TSS-associated Staphylococcus aureus infections. Compared with non-TSS cases, seroconversion to TSST-1 was significantly more frequent among both menstrual (5 of 8 versus 1 of 31; P less than 0.001) and nonmenstrual (3 of 10; P less than 0.05) patients. Seroconversion to staphylococcal enterotoxin A was also more frequent among both menstrual (2 of 8 versus 0 of 31; P less than 0.05) and nonmenstrual (2 of 9; P less than 0.05) TSS patients. In general, patients with TSS associated with TSST-1-positive S. aureus were more likely to seroconvert exclusively to TSST-1 (4 of 12 versus 0 of 6; P = 0.16), whereas those associated with TSST-1-negative S. aureus were more likely to seroconvert exclusively to enterotoxins (3 of 6 versus 0 of 11; P less than 0.05). Concurrent seroconversions to multiple exoproteins were more frequent among both menstrual (3 of 8; P less than 0.05) and nonmenstrual (2 of 9; P less than 0.05) TSS patients compared with persons without TSS (0 of 31). These data suggest but do not prove that enterotoxins (especially staphylococcal enterotoxin A) in addition to TSST-1 may be involved in both menstrual and nonmenstrual TSS. Furthermore, since exposure to multiple exoproteins is more likely to occur during TSS-associated than non-TSS-associated S. aureus infections, the possibility of additive or synergistic effects of these putative toxins in the pathogenesis of TSS should be further explored.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonventre P. F., Linnemann C., Weckbach L. S., Staneck J. L., Buncher C. R., Vigdorth E., Ritz H., Archer D., Smith B. Antibody responses to toxic-shock-syndrome (TSS) toxin by patients with TSS and by healthy staphylococcal carriers. J Infect Dis. 1984 Nov;150(5):662–666. doi: 10.1093/infdis/150.5.662. [DOI] [PubMed] [Google Scholar]
- Crass B. A., Bergdoll M. S. Involvement of staphylococcal enterotoxins in nonmenstrual toxic shock syndrome. J Clin Microbiol. 1986 Jun;23(6):1138–1139. doi: 10.1128/jcm.23.6.1138-1139.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crass B. A., Bergdoll M. S. Toxin involvement in toxic shock syndrome. J Infect Dis. 1986 May;153(5):918–926. doi: 10.1093/infdis/153.5.918. [DOI] [PubMed] [Google Scholar]
- Garbe P. L., Arko R. J., Reingold A. L., Graves L. M., Hayes P. S., Hightower A. W., Chandler F. W., Broome C. V. Staphylococcus aureus isolates from patients with nonmenstrual toxic shock syndrome. Evidence for additional toxins. JAMA. 1985 May 3;253(17):2538–2542. [PubMed] [Google Scholar]
- Kass E. H., Parsonnet J. On the pathogenesis of toxic shock syndrome. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S482–S489. doi: 10.1093/clinids/9.supplement_5.s482. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Gillis Z. A., Pier G. B. Induction of interleukin-1 by strains of Staphylococcus aureus from patients with nonmenstrual toxic shock syndrome. J Infect Dis. 1986 Jul;154(1):55–63. doi: 10.1093/infdis/154.1.55. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Gillis Z. A., Richter A. G., Pier G. B. A rabbit model of toxic shock syndrome that uses a constant, subcutaneous infusion of toxic shock syndrome toxin 1. Infect Immun. 1987 May;55(5):1070–1076. doi: 10.1128/iai.55.5.1070-1076.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reingold A. L., Hargrett N. T., Shands K. N., Dan B. B., Schmid G. P., Strickland B. Y., Broome C. V. Toxic shock syndrome surveillance in the United States, 1980 to 1981. Ann Intern Med. 1982 Jun;96(6 Pt 2):875–880. doi: 10.7326/0003-4819-96-6-875. [DOI] [PubMed] [Google Scholar]
- Rosten P. M., Bartlett K. H., Chow A. W. Serologic responses to toxic shock syndrome (TSS) toxin-1 in menstrual and nonmenstrual TSS. Clin Invest Med. 1988 Jun;11(3):187–192. [PubMed] [Google Scholar]
- Schlievert P. M., Shands K. N., Dan B. B., Schmid G. P., Nishimura R. D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981 Apr;143(4):509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M. Staphylococcal enterotoxin B and toxic-shock syndrome toxin-1 are significantly associated with non-menstrual TSS. Lancet. 1986 May 17;1(8490):1149–1150. doi: 10.1016/s0140-6736(86)91859-3. [DOI] [PubMed] [Google Scholar]
- Scott D. F., Kling J. M., Best G. K. Immunological protection of rabbits infected with Staphylococcus aureus isolates from patients with toxic shock syndrome. Infect Immun. 1986 Aug;53(2):441–444. doi: 10.1128/iai.53.2.441-444.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz S. J., Davis J. P., Vergeront J. M., Crass B. A., Chesney P. J., Wand P. J., Bergdoll M. S. Development of serum antibody to toxic shock toxin among individuals with toxic shock syndrome in Wisconsin. J Infect Dis. 1985 May;151(5):883–889. doi: 10.1093/infdis/151.5.883. [DOI] [PubMed] [Google Scholar]