Abstract
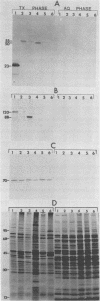
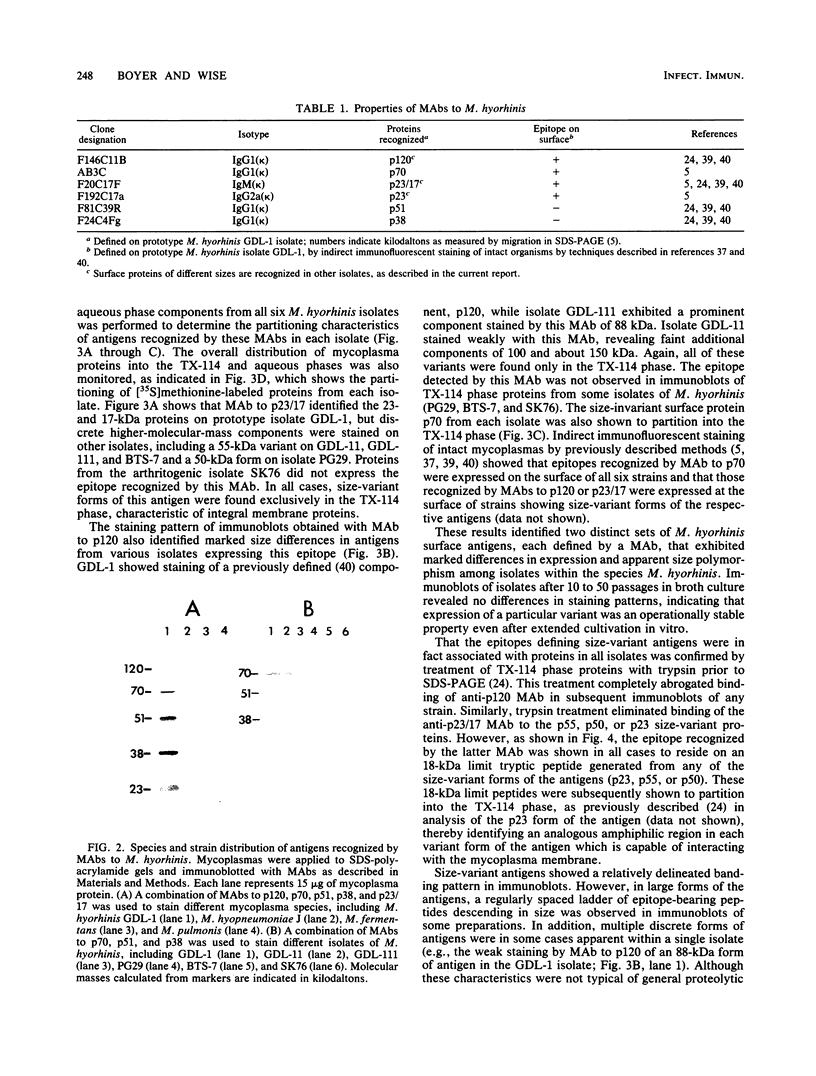
Monoclonal antibodies (MAbs) previously shown to recognize distinct epitopes selectively expressed on the surface of some Mycoplasma hyorhinis strains were used to define two discrete sets of lipid-modified membrane surface proteins showing marked size variation within this species. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis of Triton X-114 phase-fractionated proteins from six isolates of M. hyorhinis defined a set of amphiphilic integral membrane proteins of 23, 50, and 55 kilodaltons (kDa) recognized on respective isolates by one MAb and a second set of integral proteins of 88, 120, and 100 to 150 kDa recognized by another MAb. The first group of proteins all contained a common, amphiphilic 18-kDa limit tryptic polypeptide bearing the epitope. The size- and strain-variant surface antigens identified by the MAbs were shown to be lipid-modified proteins. Phase fractionation of [3H]palmitate-labeled organisms revealed numerous 3H-labeled proteins in all isolates, which partitioned exclusively into the hydrophobic phase. These proteins generally showed pronounced size variation among isolates and included the antigen variants recognized by the two MAbs, as demonstrated directly by immunoprecipitation of correspondingly sized 3H-labeled proteins from each isolate. A third MAb recognized an invariant, lipid-associated surface protein of 70 kDa on all M. hyorhinis isolates. Covalent modification of lipid-associated proteins was confirmed by identifying 3H-labeled methyl palmitate after acid methanolysis of Triton X-114 phase proteins derived from [3H]palmitate-labeled organisms. However, removal of covalently bound lipid from chloroform-methanol-extracted proteins by alkaline hydroxylamine was selective; complete removal was observed with only a few proteins, possibly including the 120-kDa form of one antigen variant. This suggested potential differences in the nature of covalent linkage among lipid-modified M. hyorhinis surface antigens. Intraspecies antigen variants described here in M. hyorhinis share some characteristics with size-variant antigens reported in phylogenetically related gram-positive eubacteria and may contribute to phenotypic diversification and differences in pathogenicity of mycoplasmas.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amikam D., Glaser G., Razin S. Mycoplasmas (Mollicutes) have a low number of rRNA genes. J Bacteriol. 1984 Apr;158(1):376–378. doi: 10.1128/jb.158.1.376-378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amikam D., Razin S., Glaser G. Ribosomal RNA genes in Mycoplasma. Nucleic Acids Res. 1982 Jul 24;10(14):4215–4222. doi: 10.1093/nar/10.14.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bricker T. M., Boyer M. J., Keith J., Watson-McKown R., Wise K. S. Association of lipids with integral membrane surface proteins of Mycoplasma hyorhinis. Infect Immun. 1988 Feb;56(2):295–301. doi: 10.1128/iai.56.2.295-301.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl C. E., Dahl J. S., Bloch K. Proteolipid formation in Mycoplasma capricolum. Influence of cholesterol on unsaturated fatty acid acylation of membrane proteins. J Biol Chem. 1983 Oct 10;258(19):11814–11818. [PubMed] [Google Scholar]
- Dahl C. E., Dahl J. S. Phospholipids as acyl donors to membrane proteins of Mycoplasma capricolum. J Biol Chem. 1984 Sep 10;259(17):10771–10776. [PubMed] [Google Scholar]
- Dahl C. E., Sacktor N. C., Dahl J. S. Acylated proteins in Acholeplasma laidlawii. J Bacteriol. 1985 Apr;162(1):445–447. doi: 10.1128/jb.162.1.445-447.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darai G., Zöller L., Matz B., Delius H., Speck P. T., Flügel R. M. Analysis of Mycoplasma hyorhinis genome by use of restriction endonucleases and by electron microscopy. J Bacteriol. 1982 May;150(2):788–794. doi: 10.1128/jb.150.2.788-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etges R., Bouvier J., Bordier C. The major surface protein of Leishmania promastigotes is anchored in the membrane by a myristic acid-labeled phospholipid. EMBO J. 1986 Mar;5(3):597–601. doi: 10.1002/j.1460-2075.1986.tb04252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Scott J. R. Size variation of the M protein in group A streptococci. J Exp Med. 1985 Jun 1;161(6):1384–1401. doi: 10.1084/jem.161.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gois M., Kuksa F., Franz J., Taylor-Robinson D. The antigenic differentiation of seven strains of Mycoplasma hyorhinis by growth-inhibition, metabolism-inhibition, latex-agglutination, and polyacrylamide-gel-electrophoresis tests. J Med Microbiol. 1974 Feb;7(1):105–115. doi: 10.1099/00222615-7-1-105. [DOI] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Complete nucleotide sequence of type 6 M protein of the group A Streptococcus. Repetitive structure and membrane anchor. J Biol Chem. 1986 Feb 5;261(4):1677–1686. [PubMed] [Google Scholar]
- Koch F., Thiele H. G., Low M. G. Release of the rat T cell alloantigen RT-6.2 from cell membranes by phosphatidylinositol-specific phospholipase C. J Exp Med. 1986 Oct 1;164(4):1338–1343. doi: 10.1084/jem.164.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus W., Haanes-Fritz E., Cleary P. P., Seyer J. M., Dale J. B., Beachey E. H. Sequence and type-specific immunogenicity of the amino-terminal region of type 1 streptococcal M protein. J Immunol. 1987 Nov 1;139(9):3084–3090. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller L., Gray L., Beachey E., Kehoe M. Antigenic variation among group A streptococcal M proteins. Nucleotide sequence of the serotype 5 M protein gene and its relationship with genes encoding types 6 and 24 M proteins. J Biol Chem. 1988 Apr 25;263(12):5668–5673. [PubMed] [Google Scholar]
- Mouw A. R., Beachey E. H., Burdett V. Molecular evolution of streptococcal M protein: cloning and nucleotide sequence of the type 24 M protein gene and relation to other genes of Streptococcus pyogenes. J Bacteriol. 1988 Feb;170(2):676–684. doi: 10.1128/jb.170.2.676-684.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. B., Caulfield M. P., Lampen J. O. Lipoprotein nature of Bacillus licheniformis membrane penicillinase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3511–3515. doi: 10.1073/pnas.78.6.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström S., Johansson K. E., Wieslander A. Selective acylation of membrane proteins in Acholeplasma laidlawii. Eur J Biochem. 1986 Apr 1;156(1):85–94. doi: 10.1111/j.1432-1033.1986.tb09552.x. [DOI] [PubMed] [Google Scholar]
- Olson E. N., Towler D. A., Glaser L. Specificity of fatty acid acylation of cellular proteins. J Biol Chem. 1985 Mar 25;260(6):3784–3790. [PubMed] [Google Scholar]
- Riethman H. C., Boyer M. J., Wise K. S. Triton X-114 phase fractionation of an integral membrane surface protein mediating monoclonal antibody killing of Mycoplasma hyorhinis. Infect Immun. 1987 May;55(5):1094–1100. doi: 10.1128/iai.55.5.1094-1100.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonis S., Cullen S. E. Fatty acylation of murine Ia alpha, beta, and invariant chains. J Immunol. 1986 Apr 15;136(8):2962–2967. [PubMed] [Google Scholar]
- So M., Billyard E., Deal C., Getzoff E., Hagblom P., Meyer T. F., Segal E., Tainer J. Gonococcal pilus: genetics and structure. Curr Top Microbiol Immunol. 1985;118:13–28. doi: 10.1007/978-3-642-70586-1_2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor M. A., Ferrell R. V., Wise K. S., McIntosh M. A. Identification of a repetitive genomic sequence that is distributed among a select group of mycoplasmas. Isr J Med Sci. 1987 May;23(5):368–373. [PubMed] [Google Scholar]
- Taylor M. A., Ferrell R. V., Wise K. S., McIntosh M. A. Reiterated DNA sequences defining genomic diversity within the species Mycoplasma hyorhinis. Mol Microbiol. 1988 Sep;2(5):665–672. doi: 10.1111/j.1365-2958.1988.tb00075.x. [DOI] [PubMed] [Google Scholar]
- Taylor M. A., McIntosh M. A., Robbins J., Wise K. S. Cloned genomic DNA sequences from Mycoplasma hyorhinis encoding antigens expressed in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4154–4158. doi: 10.1073/pnas.80.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. A., Wise K. S., McIntosh M. A. Selective detection of Mycoplasma hyorhinis using cloned genomic DNA fragments. Infect Immun. 1985 Mar;47(3):827–830. doi: 10.1128/iai.47.3.827-830.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnack E., Beachey E. H. Inhibition of complement-mediated opsonization and phagocytosis of Streptococcus pyogenes by D fragments of fibrinogen and fibrin bound to cell surface M protein. J Exp Med. 1985 Dec 1;162(6):1983–1997. doi: 10.1084/jem.162.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Kim M. F. Major membrane surface proteins of Mycoplasma hyopneumoniae selectively modified by covalently bound lipid. J Bacteriol. 1987 Dec;169(12):5546–5555. doi: 10.1128/jb.169.12.5546-5555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Watson R. K. Antigenic mimicry of mammalian intermediate filaments by mycoplasmas. Infect Immun. 1985 May;48(2):587–591. doi: 10.1128/iai.48.2.587-591.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Watson R. K. Monoclonal antibodies to Mycoplasma hyorhinis surface antigens: tools for analyzing mycoplasma-lymphoid cell interactions. Yale J Biol Med. 1983 Sep-Dec;56(5-6):623–629. [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Watson R. K. Mycoplasma hyorhinis GDL surface protein antigen p120 defined by monoclonal antibody. Infect Immun. 1983 Sep;41(3):1332–1339. doi: 10.1128/iai.41.3.1332-1339.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]