SUMMARY
Purpose
To investigate clinical correlates and longitudinal course of interictal focal cortical glucose hypermetabolism in children with Sturge-Weber syndrome (SWS).
Methods
FDG PET scans of 60 children (age range: 3 months-15.2 years) with Sturge-Weber syndrome and epilepsy were assessed prospectively and serially for focal hypo- or hypermetabolism. Thirty-two patients had two or more consecutive PET scans. Age, seizure variables and the occurrence of epilepsy surgery were compared between patients with and without focal hypermetabolism. The severity of focal hypermetabolism was also assessed and correlated with seizure variables.
Key Findings
Interictal cortical glucose hypermetabolism, ipsilateral to the angioma, was seen in 9 patients, with the most common location in the frontal lobe. Age was lower in patients with hypermetabolism than in those without (p=0.022). In addition, time difference between the onset of first seizure and the first PET scan was much shorter in children with increased glucose metabolism than in those without (mean: 1.0 vs. 3.6 years; p=0.019). Increased metabolism was transient and switched to hypometabolism in all five children where follow-up scans were available. Focal glucose hypermetabolism occurred in 28 % of children under the age of two years. Children with transient hypermetabolism had a higher rate of subsequent epilepsy surgery as compared to those without hypermetabolism (p=0.039).
Significance
Interictal glucose hypermetabolism in young children with SWS is most often seen within a short time before or after the onset of first clinical seizures, i.e., the presumed period of epileptogenesis. Increased glucose metabolism detected by PET predicts future demise of the affected cortex based on a progressive loss of metabolism and may be an imaging marker of the most malignant cases of intractable epilepsy requiring surgery in SWS.
Keywords: Sturge-Weber syndrome, positron emission tomography, glucose metabolism, epileptogenesis
INTRODUCTION
The Sturge-Weber syndrome (SWS) is a sporadic neurocutaneous disorder characterized by facial port wine stains, glaucoma and leptomeningeal angiomatosis involving one cerebral hemisphere in most of the cases (Roach & Bodensteiner, 2010). The intracranial venous abnormalities are the result of a failed regression of the primitive embryonal vascular plexus in utero. They may undergo a proliferative process even after birth resulting in chronic hypoxia and often progressive structural and functional deterioration of the underlying and also adjacent brain tissue (Comi, 2003; Comati et al., 2007). The most common neurologic manifestations of SWS include early onset seizures, hemiparesis, peripheral visual field cut and cognitive deficit (Roach & Bodensteiner, 2010).
Functional neuroimaging in SWS using 2-deoxy-2[18F]fluorodeoxyglucose (FDG) positron emission tomography (PET) can depict metabolic dysfunction in the affected cortical regions and also in functionally connected subcortical structures (Chugani et al., 1989; Alkonyi et al., 2010). FDG PET typically demonstrates cortical hypometabolism extending beyond the structural abnormalities identified by other imaging modalities in SWS (Chugani et al., 1989; Lee et al., 2001). Previous studies have suggested that the extent of cortical hypometabolism as well as severity of thalamic hypometabolism are closely associated with cognitive function in children with SWS (Lee et al., 2001; Alkonyi et al., 2010). In addition, longitudinal metabolic changes during the early course of the disease are related, at least partly, to frequent seizures (Juhasz et al., 2007). Interestingly, a seemingly paradoxical pattern of interictal glucose hypermetabolism has been reported in three young patients; a follow-up scan of one of them showed the typical pattern of hypometabolism seen in most patients with SWS (Chugani et al., 1989). Concordantly, a high proportion (75 %) of infants showed hyperperfusion on SPECT in the affected hemisphere before seizure onset, and this phenomenon appeared to be transient regardless of the subsequent presence of epilepsy (Pinton et al., 1997).
Previous studies have not addressed the potential clinical relevance of these intriguing transient metabolic and perfusion patterns. Therefore, to understand better the significance of interictal hypermetabolism, we studied the prevalence and clinical correlates of focal increases of cerebral glucose metabolism in a prospectively collected cohort of children with SWS and associated epilepsy. In particular, the localization and longitudinal changes of these hypermetabolic regions, as well as their temporal relationship to seizure onset as well as intractable epilepsy were analyzed.
METHODS
Subjects
FDG PET scans of 60 children (age range: 3 months-15.2 years; median: 2.7 years) with the diagnosis of Sturge-Weber syndrome, acquired in our institution between 1995 and 2010, were reviewed. The inclusion criteria for the present study included age below 18 years, presence of epilepsy some time during the clinical course (onset of first seizure either before or after initial PET scanning), and the presence of at least two out of the following three features: 1. facial port-wine stain, 2. leptomeningeal angioma on contrast-enhanced MRI, 3. focal or hemispheric cortical glucose metabolic abnormality (hypo- or hypermetabolism) on interictal FDG PET. Patients with previous epilepsy surgery were excluded from the study. Children with PET scans acquired in the ictal state were also excluded. Included patients were either clinically followed in our institution (due to epilepsy and/or other neurological complications of SWS) or were prospectively involved in a longitudinal clinical/imaging research study (with or without seizures at the time of their first PET scan); thus, a subset of patients had multiple scans (n=32; two PET scans in 15 and three PET scans in the remaining 17 patients). Mean imaging follow-up time in patients with multiple PET scans was 2.8 years (5 months-11.1 years). Onset of first seizure occurred mostly before (N=57) but occasionally after (N=3) the first PET scan, and varied from a few days after birth to 9 years of age (median: 0.7 years). Altogether four patients had bilateral intracranial involvement on MRI scans.
PET acquisition protocol
The details of FDG-PET acquisition and data analysis have been described previously (Lee et al., 2001). In brief, all PET scans were acquired using an EXACT/HR PET scanner (CTI/Siemens, Hoffman Estates, Ill) which provides simultaneous acquisition of 47 contiguous transaxial images with a slice thickness of 3.125 mm. The reconstructed image resolution obtained was 5.5±0.35 mm at full width at half-maximum in-plane and 6.0±0.49 mm at full width at half-maximum in the axial direction (reconstruction: filtered backprojection using Shepp-Logan filter with 0.3 cycles/pixel cutoff frequency). Scalp EEG was monitored in all children during the tracer uptake period. Initially, 0.143 mCi/kg of FDG was injected intravenously as a slow bolus followed by a 30 minutes uptake period. Forty minutes after injection, a static 20-minute emission scan was acquired parallel to the canthomeatal plane. Calculated attenuation correction was applied to the brain images using automated threshold fits to the sonogram data. Based on the EEG data, none of the scans were acquired in the ictal state (no clinical or subclinical seizures were detected during tracer uptake).
The study was approved by the Human Investigation Committee at Wayne State University, and written informed consent of the parent or legal guardian and verbal assent (between age 7-13 years) was obtained in all patients who participated in the longitudinal clinical/imaging research study.
Sedation during PET studies
Children below two years of age were sedated with chloral hydrate (50-100 mg/kg by mouth), and children aged 2-8 years were sedated with nembutal (3 mg/kg), followed by fentanyl (1 μg/kg), as necessary. Sedative medication was administered after the 30 minutes long tracer uptake period. All sedated subjects were continuously monitored by pediatric nurses, and physiological parameters (heart rate, pulse oximetry, respiration) were measured during the studies.
Qualitative and quantitative image analysis
FDG PET scans were reviewed by two investigators (C.J and H.T.C) with extensive experience in assessment of pediatric brain glucose PET. Reviewers were blinded to clinical and imaging data except for the diagnosis (SWS and, if present, epilepsy). Based on this visual assessment, all PET scans were initially classified into two groups: 1. presence of focal hypermetabolism (with or without additional hypometabolism), and 2. no focal hypermetabolism with the presence of focal hypometabolism or normal glucose metabolic pattern.
Further, quantitative analysis of glucose uptake was performed in patients with apparent focal hypermetabolism detected on visual assessment to quantitatively confirm the presence and assess the degree of hypermetabolism. None of these patients had clinical or sub-clinical seizures during tracer uptake (as determined by concurrent EEG) and none of these patients had very recent clinical seizures before the scanning procedure (see Table 1 for clinical and imaging data of these patients).
Table 1.
Clinical, EEG and imaging data of the patients
| No. | Age (mo) | Seizure onset (mo) | Brain abnormality on MRI | Last seizure before PET | EEG/PET | Loc. of hypermet. | Loc. of hypomet. (1st scan) | SUV ratio (1st scan) | SUV ratio (follow-up) |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 3 | 5 | rF,T,P | no sz.s before PET | diminished amplitude over the right hemisphere | rF,C, ant Cing, (rsupT) | (rP>lp) | 1.18 | 0.86 |
| 2. | 5 | 4 | lF,P,O | 3-4 w | decreased amplitude over the left hemisphere | lF,(lT) | lP,O | 1.16 | 0.91 |
| 3. | 7 | 1.5 | lP | 4 d | slowing over the post. quadrant of the left hemisphere | lP,O,(lT) | lF, rP | 1.21 | n.a. |
| 4. | 10 | 3 | rF,T,P,O | 7 d | background attenuation over the right hemisphere | rF | rT,P,O | 1.18 | n.a. |
| 5. | 11 | 2.5 | rF,T,P,O | 9 d | background activity slow for age, intermittent slowing over the left hemisphere | rF,T,P | rT,O,(lP) | 1.75 | 0.66 |
| 6. | 19 | 7 | rF,T,P,O | 6 mo | background attenuation over the right hemisphere | rF | rT,P,O | 1.11 | n.a. |
| 7. | 23 | 6 | rF,T,P,O,(lmedO) | 1 y | background attenuation over the right hemisphere | rF | rT,O,(rP) | 1.13 | 0.79 |
| 8. | 61 | 11 | rF,T,P,O | 1 y | background attenuation over the right hemisphere | rF | rT,P,O,(lpreF,lmedF) | 1.10 | n.a. |
| 9. | 65 | 60 | rT,P,O | 9 d | normal | rF | rT,P,O | 1.11 | <1.0* |
Note: mo: months; d: days; y: years; w: weeks; r: right; l: left; EEG/PET: EEG monitored during FDG uptake; F: frontal; preF: prefrontal; med: medial; C: central; T: temporal; sup: superior; ant: anterior; P: parietal; O: occipital; Cing: cingulate; SUV: standardized uptake value; SUV ratio: average SUV of the hypermetabolic region/average SUV of homotopic contralateral region; Follow-up SUV ratio was calculated in the same region on follow-up PET scans of the patients. n.a.: not available; Letters in parenthesis indicate possible or mild involvement of the indicated lobe.
The digital format of the follow-up scan of patient #9 has been damaged; however, the printed copy shows hypometabolism in the area of initial hypermetabolism in addition to the posterior regions.
To measure the magnitude of increased glucose metabolism, a region-of-interest (ROI) approach was implemented. Cortical regions showing apparently increased glucose metabolism were outlined using the software MIPAV 4.3.0 (McAuliffe et al., 2001) by one of the investigators (B.A.). In addition, ROIs of similar size were also placed on contralateral, homotopic cortical areas. Subsequently, standardized uptake values (SUVs) were calculated as the ratio between the average radioactivity concentration obtained from each region and the injected dose per weight (mCi/kg). Finally, SUV ratios were calculated by dividing the SUV of the ipsilateral ROI by that of the contralateral homotopic ROI. An SUV ratio of ≥ 1.10 (i.e., ≥10%) was considered glucose hypermetabolism.
Four of the patients with initial glucose hypermetabolism had follow-up scans available in digital format (the storage medium containing an additional patient`s [patient #9] digital follow-up image was damaged and these images could not be retrieved for quantitative analysis). These PET images were co-registered to the corresponding initial scans using VINCI 2.50 (Cizek et al., 2004) and the original ROIs (slightly modified to account for brain growth between the two scans) were transferred to these co-registered images to evaluate interval changes of SUVs and SUV ratios.
Statistical analysis
Age and seizure variables (age at seizure onset, duration of epilepsy, as well as absolute time difference between first PET scan and age at seizure onset) of patients with and without increased glucose metabolism apparent on the first PET scan were compared using the non-parametric Mann-Whitney U test since these variables did not show normal distribution (the Kolmogorov-Smirnov test was significant). Since hypermetabolism appeared to be more common in young patients, Fisher`s exact tests (two-sided) were performed to assess the occurrence of glucose hypermetabolism in younger vs. older SWS patients using two cutoff ages (one year and two years of age). The same test was used to compare the incidence of subsequent epilepsy surgery between patients with and without initial glucose hypermetabolism. Importantly, only patients with subsequent clinical follow-ups (mean follow-up of patients with no surgery: 5.4 years [range: 1.0-14.1]) were included in the latter comparison. The age of children whose hypermetabolism was localized only to the frontal lobe (a common site of hypermetabolism) and that of children with additional/other localization of the hypermetabolism was compared also using the Mann-Whitney U test. Correlations between clinical variables and SUV ratios for patients with increased glucose metabolism were tested using non-parametric Spearman`s product moment correlations, again, due to the violation of the assumption of normal distribution. An alpha level of p=0.05 was considered as the limit of significance.
RESULTS
Focal interictal cortical hypermetabolism was visually observed in nine patients (15% of all patients), including one infant whose first clinical seizure occurred two months after the first PET scan. The other eight children already had epilepsy at the time of their first PET scan. FDG SUV ratios of apparent hypermetabolic areas in these nine patients ranged between 1.10 and 1.75 (mean: 1.21; Table 1). All 51 patients with no hypermetabolism showed at least one area of decreased cortical metabolism on visual evaluation.
Patients with foci of increased cortical glucose metabolism were significantly younger than those without increases (mean: 1.9 vs. 4.5 years; p=0.022). Five out of the 15 patients younger than one year showed glucose hypermetabolism as opposed to four out of the 45 who were older than one year (33.3% vs. 8.9%; p=0.036). The prevalence of increased glucose metabolism was 28 % in patients under the age of two years in contrast to 5.7 % above two years (p=0.027). Hypermetabolism never occurred on follow-up scans in patients whose first scan was normal or showed hypometabolism; instead, increased metabolism was transient and switched to hypometabolism (range of SUV ratio for the four analyzed follow-up scans: 0.66-0.91; mean: 0.80) in all five patients (including the one with no digital images available) who had follow-up scans (follow-up time: 0.6-2.5 years) (see Figure 1 and Figure 2). Interestingly, time difference between the onset of first seizure and the first PET scan was much shorter in children with increased glucose metabolism than in those without (mean: 1.0 vs. 3.6 years; p=0.019).
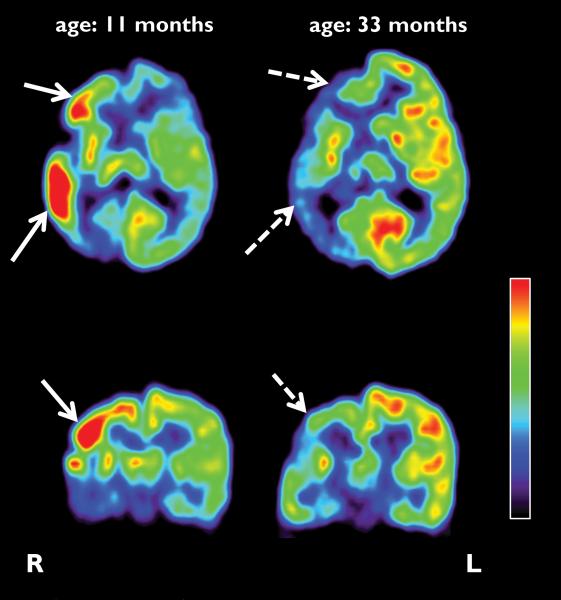
Figure 1.
Representative axial and coronal images of initial and follow-up FDG PET scans of a child (patient #5) with Sturge-Weber syndrome and epilepsy. The first scan was performed at the age of 11 months and showed increased glucose metabolism in the right frontal, temporal and parietal lobes of the right hemisphere (solid arrows), in addition to hypometabolism in the posterior parieto-temporal and occipital cortex. The second FDG PET scan, performed 2 years later, demonstrated a switch to hypometabolism in the corresponding areas (dashed arrows). L: left; R: right.
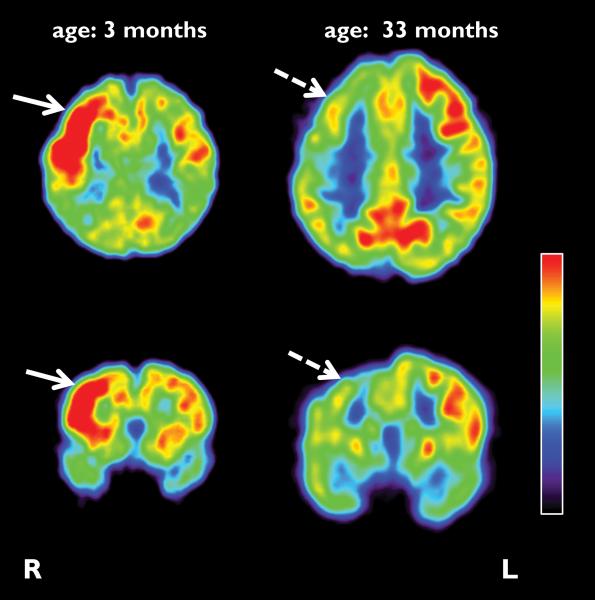
Figure 2.
Representative axial and coronal sections of initial and follow-up FDG PET scans of an infant (patient #1) with Sturge-Weber syndrome. The initial scan was done at the age of 3 months, 2 months before the onset of first seizures, and depicted increased glucose uptake in the right hemisphere, predominantly in the frontal cortex (solid arrow). The follow-up PET scan was performed 2.5 years after the initial scan, and showed an interval switch of glucose hypermetabolism to hypometabolism (dashed arrows).
In all of the cases hypermetabolic cortical regions were accompanied by hypometabolic areas, sometimes in the same lobes (see Table 1). Focal increases of glucose metabolism were localized purely to the frontal lobe in five children, while other lobes were (also) involved in four children (see Table 1). The age of patients with pure frontal lobe hypermetabolism (N=5) was significantly higher than that of children with increased metabolism involving (additionally or exclusively) other, non-frontal regions (mean: 2.9 vs. 0.5 years; p=0.019).
Within the group of patients with apparent increases of glucose metabolism the rank order of FDG SUV ratio was strongly associated with the rank order of age at seizure onset indicating more severe hypermetabolism in patients with earlier onset of epilepsy (r=-0.94; p=0.0002).
Five out of the eight children who had long-term follow up with increased metabolism on their initial PET scan eventually developed uncontrolled seizures and underwent epilepsy surgery, while the seizures of the other three became relatively controlled by medication (surgery rate: 5/8, 62.5%). In contrast, only ten out of the 43 patients (23.3%) without glucose hypermetabolism on their first scan underwent resective surgery due to intractable seizures (p=0.039).
DISCUSSION
The present study demonstrates that focal cortical increase of interictal glucose metabolism is a relatively common phenomenon in young children with SWS. In our cohort, 28% of patients younger than 2 years of age showed this seemingly paradoxical pattern of metabolism. The close relationship between the onset of first seizure and the occurrence of cortical hypermetabolism suggests that this metabolic phenomenon may be associated with processes related to epileptogenesis. Interestingly, hypermetabolism was always transient and its localization appears to be age-dependent: posterior cortex, the most common site of the primary pathology, was involved early (in younger patients), while pure frontal hypermetabolism, associated with posterior hypometabolism, was seen at older ages. From a clinical point of view, high rate of intractable epilepsy requiring epilepsy surgery in children with increased metabolism is perhaps the most interesting finding; this suggests that FDG PET hypermetabolism may be an imaging marker of drug resistant seizures in SWS.
Glucose hyper metabolism in SWS
The phenomenon of increased cortical glucose metabolism in SWS along with the typical hypometabolic pattern in advanced stages of SWS was initially demonstrated by Chugani et al. (Chugani et al., 1989). In that study, interictal glucose hypermetabolism was seen in 3 patients out of the whole series of 12 cases. A subsequent multimodality imaging study also reported 2 infants with glucose hypermetabolism (Pfund et al., 2003). Similarly, interictal hyperperfusion of the affected brain region was seen on SPECT studies in infants before epilepsy onset but not in older children (Pinton et al., 1997). Interictal hypermetabolism and hyperperfusion appear to be transient phenomena, since available follow-up SPECT and PET scans (including those in the present study) invariably demonstrated an interval switch to decreased metabolism and perfusion, respectively. By studying a relatively large series of patients with this rare disorder, we have now provided further details about the prevalence, localization and longitudinal course of cortical hypermetabolism.
Interestingly, the most common location of glucose hypermetabolism in our patients was the frontal lobe. Indeed, increased glucose metabolism was solely localized to the frontal lobe in older patients, with concomitant posterior hypometabolism, while in younger children (infants) increased glucose uptake was seen in posterior cortical areas as well. The exact evolution and regression process of cortical hypermetabolism is unknown. As the majority of PET scans showed no signs of increased metabolism even in young children with SWS, it is likely that affected patients represent a distinct subgroup, although the true prevalence of this metabolic phenomenon is probably higher, as some patients may have undergone a transient hypermetbolic state before their first PET scan. It is also conceivable that a substantial portion of the affected cortex shows a transient increase in metabolic demand some time during the very early course of the disease, and then this switches to hypometabolism, as the cortical injury expands from posterior angioma-affected regions to structurally less affected frontal areas.
Glucose hyper metabolism and epilepsy
Increased metabolic activity in the region of the seizure focus, as a consequence of excessive neuronal firing and increased energy consumption, is commonly seen during seizures or even in the presence of persistent focal interictal epileptiform activity in patients with epilepsy (Engel et al., 1982; Bittar et al., 1999). In the present study, however, the PET scans of all 9 patients showing cortical hypermetabolism were acquired in the interictal state at least days after a clinical seizure and without the concomitant presence of epileptiform discharges on scalp EEG performed during the PET scan (see Table 1). Therefore, it can be argued that the pathomechanism of the detected focal hypermetabolism in SWS may not be directly associated with epilepsy, or with ongoing epileptiform activity. However, our data show that this transient phenomenon occurs mostly in young (mainly under 2 years of age) patients during a limited period before and shortly after the first clinical seizures. Transient interictal glucose hypermetabolism seems to be a unique feature of SWS associated with epilepsy, given the fact that partial epilepsy is commonly associated with focal glucose hypometabolism (in the interictal state). Furthermore, hypometabolism is relatively rare (and hypermetabolism has not been reported) in new-onset (non-lesional) partial epilepsy (Kuhl et al., 1980; Gaillard et al., 2002). Similarly, transient hypometabolism, but not hypermetabolism, has been reported in children with recent-onset West-syndrome (Maeda et al., 1994; Natsume et al., 1996).
Mechanism of increased glucose metabolism: implications for epileptogenesis
Interictal glucose hypermetabolism has been reported rarely in neurological conditions other than SWS. In the nickel-induced epilepsy model of rats, the intracortical pattern of glucose hypermetabolism and the presence of hypermetabolism in the absence of seizures indicated that not only the seizure activity itself, but also excitotoxic tissue damage may play a key role in increased glucose metabolism (Cooper et al., 2001). A recent report of two siblings with West syndrome demonstrated the atypical finding of focal glucose hypermetabolism in the interictal state (Kumada et al., 2006). Paradoxical increase in focal cortical glucose metabolism has also been reported in a few epileptic patients with malformations of cortical development (Poduri et al., 2007). Interestingly, cortical malformations have been recently identified in patients with SWS and intractable epilepsy and may be the primary cause of epilepsy, at least in some cases, although no direct evidence has been provided for this notion (Maton et al., 2010). In addition, transient glucose hypermetabolism in bilateral basal ganglia has been reported in a newborn who suffered hypoxic-ischemic encephalopathy and developed epilepsy as well as dystonic cerebral palsy (Batista et al., 2007). Based on earlier magnetic resonance spectroscopy findings showing increased glutamate concentration in the basal ganglia following perinatal hypoxia, it has been also suggested that the transient increase in glucose metabolism may be associated with hypoxia-induced excessive glutamatergic activity, a major source of excitotoxicity.
It has been shown that a significant proportion of energy metabolism of the brain is utilized for glutamatergic synaptic activity (Sibson et al., 1998). Imbalance in ionic homeostasis as well as excitotoxicity plays a crucial role also in posttraumatic cerebral glucose hypermetabolism (Bergsneider et al., 1997). Furthermore, perinatal hypoxia can lead to excessive stimulation of glutamate receptors along with the downregulation of GABA receptor and glutamate decarboxylase genes; decreased levels of components of the GABA pathway play an important role in cell injury and may lead to high susceptibility to seizures (Johnston, 2005; Anju et al., 2010). An earlier study also showed increased regional glucose metabolism in 5 out of 6 infants with hypoxic-ischemic encephalopathy, and suggested that detection of early cerebral hypermetabolism may have clinical predictive value (Blennow et al., 1995). Indirect evidence supports the notion that hypoxia-induced excitotoxicity can be the underlying mechanism of initial hypermetabolism and hyperperfusion in infants with hypoxic-ischemic encephalopathy (Hagberg et al., 1993). As a tight coupling between glucose metabolism and glutamate cycling has been demonstrated in patients with partial epilepsy (Pfund et al., 2000), ischemic injury of the cortex in SWS may lead to glutamate excitotoxicity in conjunction with glucose hypermetabolism. In addition, the appearance of glucose hypermetabolism in young ages, within close temporal proximity to the first clinical seizures, suggests that the underlying excitotoxic damage ultimately may reach a critical level to trigger seizures. Although somewhat speculative, transient focal hypermetabolism may be also related to enhancement of GABA-ergic synaptic transmission that could transitorily compensate for the ongoing glutamate-mediated injury (Liang et al., 2009), imposing further metabolic demand to the affected brain region. Failed compensation may eventually result in excessive excitotoxic damage and seizure generation.
A recent study on cortical tissue samples obtained from infants with SWS and epilepsy (Tyzio et al., 2009) provides support that the above detailed mechanisms may indeed play a role in SWS-related epileptogenesis. In that study, cortical neurons were found to be depolarized and displayed synchronous activity driven by glutamatergic connections. This pattern was more consistent with ischemic damage than injury resulting from the epileptogenic process itself. Interestingly, the authors also found that, despite the young age of the patients, GABA exerted an inhibitory and anticonvulsive role via a shunting mechanism in SWS cortex; this was clearly different from the excitatory GABA-action described in human epileptic cortex of other etiology (such as cortical dysplasia) (Cepeda et al., 2007). Altogether, these results suggested the presence of an SWS-specific epileptogenic process (perhaps due its unique, chronic ischemia-induced cortical damage), which may explain why early metabolic patterns are different in some children with SWS as compared to other pediatric epilepsy syndromes.
The presence of glucose hypermetabolism even shortly after the first clinical seizure(s) in children with SWS is not surprising, since epileptogenesis is a dynamic process extending well beyond the onset of the first seizure (Williams et al., 2009), and progressive, age-dependent metabolic changes (hyper- as well as hypometabolism) occur in the brain of rats during epileptogenesis (Dube et al., 2000; Dube et al., 2001; Guo et al., 2009). Interestingly, chronic inflammation, angiogenesis and blood-brain barrier leakage (the latter two are also present in SWS), as potential mechanisms of epileptogenesis, appeared to be age-dependent in a rodent model (Marcon et al., 2009). Increased glucose metabolism (measured by autoradiography) was found also in non-damaged brain regions of rats after induced status epilepticus, possibly indicating processes working against or controlling seizure activity (Dube et al., 2000). Combined in vivo measurements of substrates of the glutamate and GABA pathways and glucose utilization (MR-spectroscopy/FDG PET) in infants with SWS could shed more light on the underlying mechanisms of cortical hypermetabolism.
Finally, our data suggest that epilepsy surgery due to uncontrolled seizures tend to be more frequent in patients whose FDG PET scan showed interictal hypermetabolism than in those whose early scan did not detect hypermetabolism. If further, prospective studies confirm this observation, early PET scanning in SWS using the tracer FDG could have a crucial clinical value in identifying children with higher risk for intractable seizures.
ACKNOWLEDGMENTS
This study was supported by a grant from the National Institutes of Health (R01 NS041922 to C.J.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors thank Thomas Mangner, Ph.D. and Pulak Chakraborty, Ph.D. for the reliable radiosynthesis of the PET tracers. We are also grateful to Galina Rabkin, CNMT, Angie Wigeluk, CNMT, Anrdew Mosqueda, CNMT and Carole Klapko, CNMT for their expert technical assistance in performing the PET studies and to Majid Khalaf MD, Anna Deboard RN as well as Jane Cornett RN for performing sedation. We also thank the Sturge-Weber Foundation for referring patients to us. We are grateful to the families and children who participated in the study.
Footnotes
Disclosure of Conflicts of Interest: We confirm that we have read the Journal`s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. None of the authors has any conflict of interest to disclose.
REFERENCES
- Alkonyi B, Chugani HT, Behen M, Halverson S, Helder E, Makki MI, Juhasz C. The role of the thalamus in neuro-cognitive dysfunction in early unilateral hemispheric injury: A multimodality imaging study of children with Sturge-Weber syndrome. Eur J Paediatr Neurol. 2010;14:425–433. doi: 10.1016/j.ejpn.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anju TR, Abraham PM, Antony S, Paulose CS. Alterations in cortical GABA(B) receptors in neonatal rats exposed to hypoxic stress: role of glucose, oxygen, and epinephrine resuscitation. Mol Cell Biochem. 2010;343:1–11. doi: 10.1007/s11010-010-0491-9. [DOI] [PubMed] [Google Scholar]
- Batista CE, Chugani HT, Juhasz C, Behen ME, Shankaran S. Transient hypermetabolism of the basal ganglia following perinatal hypoxia. Pediatr Neurol. 2007;36:330–333. doi: 10.1016/j.pediatrneurol.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Bergsneider M, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, Phelps ME, McArthur DL, Caron MJ, Kraus JF, Becker DP. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J Neurosurg. 1997;86:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- Bittar RG, Andermann F, Olivier A, Dubeau F, Dumoulin SO, Pike GB, Reutens DC. Interictal spikes increase cerebral glucose metabolism and blood flow: a PET study. Epilepsia. 1999;40:170–178. doi: 10.1111/j.1528-1157.1999.tb02071.x. [DOI] [PubMed] [Google Scholar]
- Blennow M, Ingvar M, Lagercrantz H, Stone-Elander S, Eriksson L, Forssberg H, Ericson K, Flodmark O. Early [18F]FDG positron emission tomography in infants with hypoxicischaemic encephalopathy shows hypermetabolism during the postasphyctic period. Acta Paediatr. 1995;84:1289–1295. doi: 10.1111/j.1651-2227.1995.tb13551.x. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Andre VM, Wu N, Yamazaki I, Uzgil B, Vinters HV, Levine MS, Mathern GW. Immature neurons and GABA networks may contribute to epileptogenesis in pediatric cortical dysplasia. Epilepsia. 2007;48(Suppl 5):79–85. doi: 10.1111/j.1528-1167.2007.01293.x. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Mazziotta JC, Phelps ME. Sturge-Weber syndrome: a study of cerebral glucose utilization with positron emission tomography. J Pediatr. 1989;114:244–253. doi: 10.1016/s0022-3476(89)80790-5. [DOI] [PubMed] [Google Scholar]
- Cizek J, Herholz K, Vollmar S, Schrader R, Klein J, Heiss WD. Fast and robust registration of PET and MR images of human brain. Neuroimage. 2004;22:434–442. doi: 10.1016/j.neuroimage.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Comati A, Beck H, Halliday W, Snipes GJ, Plate KH, Acker T. Upregulation of hypoxiainducible factor (HIF)-1alpha and HIF-2alpha in leptomeningeal vascular malformations of Sturge-Weber syndrome. J Neuropathol Exp Neurol. 2007;66:86–97. doi: 10.1097/nen.0b013e31802d9011. [DOI] [PubMed] [Google Scholar]
- Comi AM. Pathophysiology of Sturge-Weber syndrome. J Child Neurol. 2003;18:509–516. doi: 10.1177/08830738030180080701. [DOI] [PubMed] [Google Scholar]
- Cooper RM, Legare CE, Campbell Teskey G. Changes in (14)C-labeled 2-deoxyglucose brain uptake from nickel-induced epileptic activity. Brain Res. 2001;923:71–81. doi: 10.1016/s0006-8993(01)03034-7. [DOI] [PubMed] [Google Scholar]
- Dube C, Boyet S, Marescaux C, Nehlig A. Progressive metabolic changes underlying the chronic reorganization of brain circuits during the silent phase of the lithium-pilocarpine model of epilepsy in the immature and adult Rat. Exp Neurol. 2000;162:146–157. doi: 10.1006/exnr.2000.7324. [DOI] [PubMed] [Google Scholar]
- Dube C, da Silva Fernandes MJ, Nehlig A. Age-dependent consequences of seizures and the development of temporal lobe epilepsy in the rat. Dev Neurosci. 2001;23:219–223. doi: 10.1159/000046147. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Kuhl DE, Phelps ME. Patterns of human local cerebral glucose metabolism during epileptic seizures. Science. 1982;218:64–66. doi: 10.1126/science.6981843. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Kopylev L, Weinstein S, Conry J, Pearl PL, Spanaki MV, Fazilat S, Fazilat S, Venzina LG, Dubovsky E, Theodore WH. Low incidence of abnormal (18)FDG-PET in children with new-onset partial epilepsy: a prospective study. Neurology. 2002;58:717–722. doi: 10.1212/wnl.58.5.717. [DOI] [PubMed] [Google Scholar]
- Guo Y, Gao F, Wang S, Ding Y, Zhang H, Wang J, Ding MP. In vivo mapping of temporospatial changes in glucose utilization in rat brain during epileptogenesis: an 18F-fluorodeoxyglucose-small animal positron emission tomography study. Neuroscience. 2009;162:972–979. doi: 10.1016/j.neuroscience.2009.05.041. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Thornberg E, Blennow M, Kjellmer I, Lagercrantz H, Thiringer K, Hamberger A, Sandberg M. Excitatory amino acids in the cerebrospinal fluid of asphyxiated infants: relationship to hypoxic-ischemic encephalopathy. Acta Paediatr. 1993;82:925–929. doi: 10.1111/j.1651-2227.1993.tb12601.x. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Excitotoxicity in perinatal brain injury. Brain Pathol. 2005;15:234–240. doi: 10.1111/j.1750-3639.2005.tb00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz C, Batista CE, Chugani DC, Muzik O, Chugani HT. Evolution of cortical metabolic abnormalities and their clinical correlates in Sturge-Weber syndrome. Eur J Paediatr Neurol. 2007;11:277–284. doi: 10.1016/j.ejpn.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl DE, Engel J, Jr., Phelps ME, Selin C. Epileptic patterns of local cerebral metabolism and perfusion in humans determined by emission computed tomography of 18FDG and 13NH3. Ann Neurol. 1980;8:348–360. doi: 10.1002/ana.410080403. [DOI] [PubMed] [Google Scholar]
- Kumada T, Okazawa H, Yamauchi H, Kitoh T, Ito M. Focal glucose hypermetabolism in interictal state of West syndrome. Pediatr Neurol. 2006;34:47–50. doi: 10.1016/j.pediatrneurol.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Lee JS, Asano E, Muzik O, Chugani DC, Juhasz C, Pfund Z, Philip S, Behen M, Chugani HT. Sturge-Weber syndrome: correlation between clinical course and FDG PET findings. Neurology. 2001;57:189–195. doi: 10.1212/wnl.57.2.189. [DOI] [PubMed] [Google Scholar]
- Liang R, Pang ZP, Deng P, Xu ZC. Transient enhancement of inhibitory synaptic transmission in hippocampal CA1 pyramidal neurons after cerebral ischemia. Neuroscience. 2009;160:412–418. doi: 10.1016/j.neuroscience.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, Watanabe K, Negoro T, Aso K, Ohki T, Ito K, Kato T. Evolutional changes of cortical hypometabolism in West's syndrome. Lancet. 1994;343:1620–1623. doi: 10.1016/s0140-6736(94)93065-1. [DOI] [PubMed] [Google Scholar]
- Marcon J, Gagliardi B, Balosso S, Maroso M, Noe F, Morin M, Lerner-Natoli M, Vezzani A, Ravizza T. Age-dependent vascular changes induced by status epilepticus in rat forebrain: implications for epileptogenesis. Neurobiol Dis. 2009;34:121–132. doi: 10.1016/j.nbd.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Maton B, Krsek P, Jayakar P, Resnick T, Koehn M, Morrison G, Ragheb J, Castellano-Sanchez A, Duchowny M. Medically intractable epilepsy in Sturge-Weber syndrome is associated with cortical malformation: implications for surgical therapy. Epilepsia. 2010;51:257–267. doi: 10.1111/j.1528-1167.2009.02304.x. [DOI] [PubMed] [Google Scholar]
- McAuliffe MJ, Lalonde FM, McGarry D, Gandler W, Csaky K, Trus BL. Medical image processing, analysis & visualization in clinical research. IEEE Computer-based medical systems (CBMS) 2001:381–386. [Google Scholar]
- Natsume J, Watanabe K, Maeda N, Kasai K, Negoro T, Aso K, Nakashima S, Tadokoro M. Cortical hypometabolism and delayed myelination in West syndrome. Epilepsia. 1996;37:1180–1184. doi: 10.1111/j.1528-1157.1996.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Pfund Z, Chugani DC, Juhasz C, Muzik O, Chugani HT, Wilds IB, Seraji-Bozorgzad N, Moore GJ. Evidence for coupling between glucose metabolism and glutamate cycling using FDG PET and 1H magnetic resonance spectroscopy in patients with epilepsy. J Cereb Blood Flow Metab. 2000;20:871–878. doi: 10.1097/00004647-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Pfund Z, Kagawa K, Juhasz C, Shen C, Lee JS, Chugani DC, Muzik O, Chugani HT. Quantitative analysis of gray- and white-matter volumes and glucose metabolism in Sturge-Weber syndrome. J Child Neurol. 2003;18:119–126. doi: 10.1177/08830738030180021501. [DOI] [PubMed] [Google Scholar]
- Pinton F, Chiron C, Enjolras O, Motte J, Syrota A, Dulac O. Early single photon emission computed tomography in Sturge-Weber syndrome. J Neurol Neurosurg Psychiatry. 1997;63:616–621. doi: 10.1136/jnnp.63.5.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduri A, Golja A, Takeoka M, Bourgeois BF, Connolly L, Riviello JJ., Jr Focal cortical malformations can show asymmetrically higher uptake on interictal fluorine-18 fluorodeoxyglucose positron emission tomography (PET). J Child Neurol. 2007;22:232–237. doi: 10.1177/0883073807300305. [DOI] [PubMed] [Google Scholar]
- Roach E, Bodensteiner J. Sturge-Weber syndrome. The Sturge-Weber Foundation; Mt. Freedom, NJ: 2010. [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Khalilov I, Represa A, Crepel V, Zilberter Y, Rheims S, Aniksztejn L, Cossart R, Nardou R, Mukhtarov M, Minlebaev M, Epsztein J, Milh M, Becq H, Jorquera I, Bulteau C, Fohlen M, Oliver V, Dulac O, Dorfmuller G, Delalande O, Ben-Ari Y, Khazipov R. Inhibitory actions of the gamma-aminobutyric acid in pediatric Sturge-Weber syndrome. Ann Neurol. 2009;66:209–218. doi: 10.1002/ana.21711. [DOI] [PubMed] [Google Scholar]
- Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci. 2009;29:2103–2112. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]