Abstract
Benign prostatic hyperplasia (BPH) is the major cause of lower urinary tract symptoms in men aged 50 or older. Symptoms are not normally life threatening, but often drastically affect the quality of life. The number of men seeking treatment for BPH is expected to grow in the next few years as a result of the ageing male population. Estimates of annual pharmaceutical sales of BPH therapies range from $US 3 to 10 billion, yet this market is dominated by two drug classes. Current drugs are only effective in treating mild to moderate symptoms, yet despite this, no emerging contenders appear to be on the horizon. This is remarkable given the increasing number of patients with severe symptoms who are required to undergo invasive and unpleasant surgery. This review provides a brief background on prostate function and the pathophysiology of BPH, followed by a brief description of BPH epidemiology, the burden it places on society, and the current surgical and pharmaceutical therapies. The recent literature on emerging contenders to current therapies and novel drug targets is then reviewed, focusing on drug targets which are able to relax prostatic smooth muscle in a similar way to the α1-adrenoceptor antagonists, as this appears to be the most effective mechanism of action. Other mechanisms which may be of benefit are also discussed. It is concluded that recent basic research has revealed a number of novel drug targets such as muscarinic receptor or P2X-purinoceptor antagonists, which have the potential to produce more effective and safer drug treatments.
Keywords: prostate contractility, adrenoceptors, 5α-reductase inhibitors, LHRH antagonists, Vitamin D3 analogues, cannabinoids, prostaglandin E2, adenosine, phosphodiesterase inhibitors, Rho kinase inhibitors
Background: normal prostate growth, development and gland function
The human prostate gland undergoes two androgen-dependent major growth stages. The first occurs during foetal development, when the gland arises from outgrowths of the urethral epithelium. The second phase occurs at puberty when the gland reaches a weight of approximately 20 g (McConnel, 1995). The adult prostate gland is a sponge-like male accessory structure which indirectly facilitates fertilization by increasing sperm motility and providing nourishment by the expulsion of fluid into the urethra. The columnar epithelial cells which line the prostatic ducts continually produce secretions. These secretions are stored in the ducts until the smooth muscle surrounding the ducts contracts under autonomic control, to expel the prostatic fluid just prior to ejaculation. The major constituents of prostatic fluid are polyamines, plasminogen, proteolytic enzymes, acid phosphatase, electrolytes, zinc, glucose and citric acid (Fair and Cordonnier, 1978; Zaichick et al., 1996; Weisser et al., 1997). Prostatic fluid, together with the fluid expelled from the seminal vesicle makes up the bulk of semen in which sperm is suspended to make up the ejaculate.
Background: benign prostatic hyperplasia (BPH)
With increasing age, alterations in hormone levels often result in the serious disorder known as benign prostatic hyperplasia (BPH) which is caused by an imbalance between cellular proliferation and apoptosis within the prostate. BPH initially manifests itself as microscopic nodules which progressively proliferate and enlarge to increase the mass of both the glandular and stromal prostatic tissue (Wilson, 1980). This abnormal third phase of prostate growth leads to an increase in smooth muscle tone and/or size of the gland. Because of the prostate's anatomical location surrounding the urethra, this growth is the major cause of bladder outlet and urethral obstruction in ageing men. Despite gross morphological and anatomical differences between prostate glands in humans and rodents, there appears to be many similarities with regard to their pharmacology and histochemistry making most laboratory animals of use as investigative models of prostate function.
Symptoms of BPH begin to appear in some men as early as age 40 and include a reduced urinary stream, nocturia, urinary retention, urgency, frequency and dribbling (Scarpa, 2001). These lower urinary tract symptoms and pathological BPH continue to worsen with age (Tsukamoto et al., 2007). Diagnosis of BPH is generally by a combination of physical examination by a physician and patient self-assessment to determine a score of urinary symptom severity.
Because of the unique capsular anatomy of the human prostate, BPH is almost exclusively unique to humans. The only experimental animals to spontaneously develop BPH are dogs (no prostatic capsule) and chimpanzees (prostatic capsule), with only the latter developing the symptoms associated with BPH (Wilson, 1980). Nearly every man at some point in his life will be affected by BPH, with 90% of men over the age of 85 affected by the disease (Gallegos and Frazee, 2008). A recent study of 5990 Australian men reported that 16% of men over the age of 40 complained of moderate to severe lower urinary tract symptoms, with 19% reporting symptoms of nocturia which necessitated two or more voids per night. Above the age of 70 years, 38% reported prostate disease and 41% reported nocturia. Twenty-nine per cent of men over the age of 70 complained of moderate to severe symptoms (Holden et al., 2005).
Background: burden on society of BPH
While BPH may be asymptomatic and undiagnosed in some men, obstruction of the urethra and deformation of the base of the bladder by the hyperplasic prostate will cause painful and debilitating lower urinary tract symptoms in most men as they age. Although rarely life threatening, the symptoms caused by BPH can have an extremely detrimental effect on the quality of life of both the men suffering from the disease as well as their partners. In the worst cases, sufferers may be forced to wake up to 10 times per night to urinate. Often, sufferers will also wake their partners as well. Such interrupted sleep patterns severely impact the ability of people to function normally during the day. Thus, BPH can reduce quality of life and drastically reduce the productivity of people affected by the disease. Because of the age-related nature of BPH progression, its prevalence will continue to increase in the short term as the population of western society ages.
Market research data for 2009 freely available on the Internet lists worldwide prostatic pharmacotherapy sales of $US 4.8 billion (Table 1). Nevertheless, pharmacological therapies are only effective for treating mild to moderate symptomatic cases of BPH. At present, patients experiencing severe symptoms can only be treated with the common but very invasive surgical procedures available such as transurethral resection of the prostate (TURP). More than 25 000 TURPs are performed in Australia each year at a cost of approximately $US 70 million (including public and private systems). The public system component alone represents 0.2% of total Medicare benefits paid (data obtained from National Hospital Morbidity Collection, Australian Institute of Health and Welfare). Similarly, in the United Kingdom, the economic burden of BPH to the National Health System (NHS) and Department of Social Security represents 0.4% of total NHS expenditure.
Table 1.
Top 4 marketed pharmacotherapy products for prostatic therapies 2009
| Annual worldwide sales $US (million) | Group share (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rank | Product | Generic name | Company | 2007 | 2008 | 2009 | 2007 | 2008 | 2009 |
| 1 | Flomax/Alna/Harnal | Tamsulosin HCl | Boehringer Ingelheim / Astellas Pharma | 2171 | 2279 | 2692 | 48 | 48 | 50 |
| 2 | Avodart/Avolve | Dutasteride | GlaxoSmithKline | 571 | 739 | 830 | 14 | 17 | 17 |
| 3 | Xatral/Uroxatral | Alfuzosin HCl | Sanofi-Aventis | 457 | 486 | 413 | 11 | 11 | 9 |
| 4 | Proscar | Finasteride | Merck & Co | 411 | 324 | 291 | 10 | 8 | 6 |
| Other | 424 | 486 | 554 | 11 | 11 | 12 | |||
| Total | 4033 | 4313 | 4779 | 100 | 100 | 100 | |||
Source: EvaluatePharma® (http://www.evaluatepharma.com).
Current therapies: surgical treatments for BPH
Current treatment options for BPH include surgical and pharmacological therapies. In severe cases, potentially life-threatening complications may occur, such as acute urinary retention, recurrent infections and renal failure. In such cases, surgery is the only truly effective treatment. As well as TURP, other surgical procedures include transurethral microwave thermotherapy (TUMT) and transurethral needle ablation (TUNA) (Miano et al., 2008). TURP is the most common surgical procedure and provides the greatest symptomatic relief, although it is a highly invasive procedure and side effects such as sexual dysfunction and incontinence are common. TUMT and TUNA are less invasive procedures and have fewer side effects, but are not as effective (Thorpe and Neal, 2003). For bothersome and enlarged prostates, open radical prostatectomy is also an option. However, nowadays this extremely invasive procedure is almost exclusively reserved for patients diagnosed with prostate cancer, which is beyond the scope of this review.
Current therapies: 5α-reductase inhibitor pharmacotherapy
Several recent reviews address the topic of future targets for the pharmacological treatment of BPH (Auffenberg et al., 2009; Stamatiou, 2009; Hashim and Abrams, 2010). This review broadens the list of potential targets by summarizing in more detail the literature on prostate function in not only humans but also laboratory animals.
Current pharmacotherapy targets either the static (an increase in the physical size of the prostate) or dynamic (an increase in the tone of the smooth muscle) component of BPH. Drugs targeting the static component of BPH act by inhibiting the proliferative action of androgens. These drugs include finasteride (Proscar®, Merck, Whitehouse Station, NJ, USA) and dutasteride (Avodart®, GlaxoSmithKline, Brentford, Middlesex, UK) which make up 23% ($US 1.1 billion) of the prostate pharmacotherapy worldwide market (Table 1). This class of drugs inhibit the action of the 5α-reductase enzyme which catalyses the conversion of testosterone to the more potent androgen dihydrotestosterone (Figure 1). Urethral obstruction is therefore relieved as the physical size of the prostate is decreased (Carson and Rittmaster, 2003; Tarter and Vaughan, 2006). The main complaints relating to patient use of 5α-reductase inhibitors are that they are slow-acting, often taking up to 6 months before effective relief of symptoms, and are associated with sexual side effects including impotence, decreased libido and abnormal ejaculation.
Figure 1.
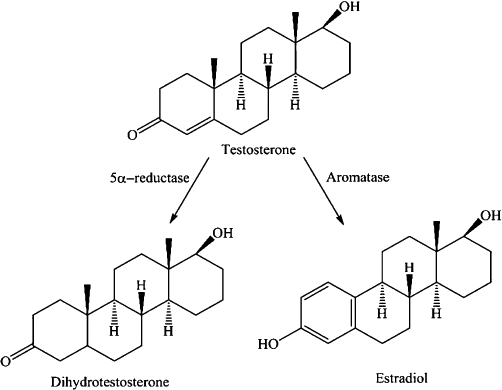
Chemical structure and metabolism pathway showing the steroid conversion of testosterone into dihydrotestosterone and estradiol, via 5α-reductase and aromatase respectively.
Current therapies: α1-adrenoceptor antagonist pharmacotherapy
The most effective and fastest acting treatments for BPH are drugs targeting the dynamic component (Miano et al., 2008). These include α1-adrenoceptor antagonists such as tamsulosin (Flomax®, Yamanouchi, Tokyo, Japan; CSL, Parkville, VIC, Australia; Alna®, Boehringer Ingelheim am Rhein, Germany; Harnal®, Astellas, Tokyo, Japan) and alfuzosin (Uroxatral®, Sanofi-Aventis, Paris, France) which make up 65% ($US 3.1 billion) of the prostate therapy worldwide market (Table 1). These drugs decrease the stromal smooth muscle tone by blocking prostatic α1-adrenoceptors (Figures 2 and 3), thus relieving urethral obstruction and the associated troublesome voiding symptoms (Lepor, 2007).
Figure 2.

Schematic diagram showing the postjunctional site of action of the α1-adrenoceptor antagonists as smooth muscle relaxants in the prostate gland.
Figure 3.
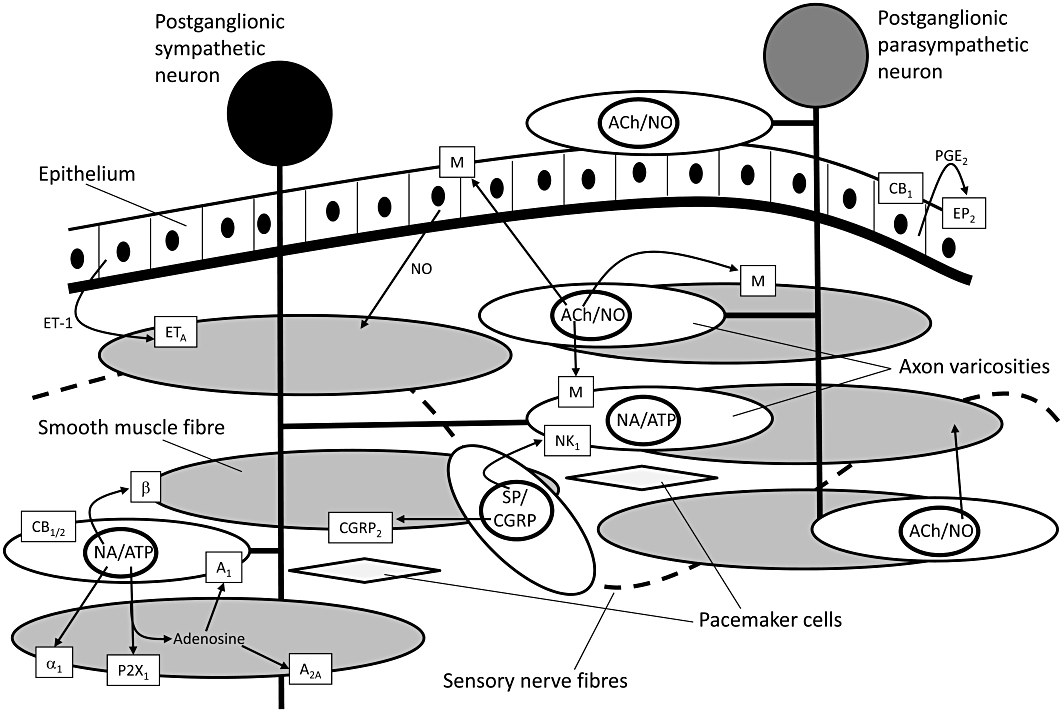
Schematic diagram showing the likely locations on various cell types of membrane bound receptors and extracellular messengers which play a role in the control of contractility of prostatic smooth muscle. The diagrammatic representation is derived from experimental data obtained in a number of species. ACh, acetylcholine; ATP, adenosine 5′-triphosphate; CGRP, calcitonin gene-related peptide; ET, endothelin; NA, noradrenaline; NO, nitric oxide; SP, substance P.
There is a direct correlation between urethral obstruction and the amount of prostatic smooth muscle (Shapiro et al., 1992). In addition, 5α-reductase inhibitors are less effective than the α1-adrenoceptor antagonists in the reduction of symptoms (Rigatti et al., 2003; Hasan et al., 2007; Lepor, 2007), indicating a greater importance for the dynamic component in the pathology of BPH. The relative importance of the dynamic component is further highlighted by observations that the severity of symptoms is not related to prostate size (Eckhardt et al., 2001a,b,c). α1-Adrenoceptor antagonists produce mainly vasodilator side effects, but selective blockade of the α1A-adrenoceptor subtype is also associated with some abnormal ejaculatory effects (Rokosh and Simpson, 2002).
Emerging contenders to current therapies
α1-Adrenoceptor antagonists and 5α-reductase inhibitors remain the major targets of new drugs for the treatment of BPH. Most pharmaceutical research centres on making new compounds which will more selectively act at these targets and reduce adverse side effects, thereby improving patient adherence. Despite the effectiveness of current BPH treatments, there still remains the opportunity for new therapies that can stop disease progression and the subsequent need for surgery. The therapeutic potential for a combination therapy of 5α-reductase inhibitors and α1-adrenoceptor antagonists is also very apparent. Combined use leads to a decreased progression of BPH, as well as mild symptomatic relief (Schulman, 2003). Indeed, in June 2010, a single-capsule formulation of dutasteride and tamsulsion (Jalyn®, GSK) was approved by the US Food and Drug Administration for use in symptomatic BPH in men with an enlarged prostate.
Gonadotrophin modulators, in particular, luteinizing hormone releasing hormone (LHRH) receptor antagonists such as cetrorelix have been extensively trialled for the treatment of BPH. The rationale for their use is based on inhibiting LHRH receptors in the pituitary, thereby blocking the signal for testosterone production in the testes and consequently inhibiting prostate growth. Clinical studies have shown that cetrorelix decreases prostate volume and improves lower urinary tract symptoms in patients suffering from BPH (Gonzalez-Barcena et al., 1994; Comaru-Schally et al., 1998; Debruyne et al., 2008, 2010). However, despite mild long-term symptomatic improvement, serum testosterone levels decrease only during dosing, and return to baseline levels in a matter of weeks (Comaru-Schally et al., 1998). This suggests that LHRH antagonists may act directly on the prostate to inhibit growth. On the contrary, LHRH agonists have been shown to have anti-proliferative effects in both androgen-dependent and independent prostate cancer cell lines (Limonta et al., 1992; 1999; Dondi et al., 1994). However, in the non-tumourgenic epithelial line BPH-1 (derived from prostate tissue of a 68-year-old BPH patient), the LHRH antagonist, cetrorelix, inhibits cell proliferation and reduces expression of mitogenic growth factors (Siejka et al., 2010). In a rat model of BPH, cetrorelix has also been shown to reduce prostate size presumably by inhibiting pro-inflammatory cytokines and growth factors (Rick et al., 2011). Because of either their direct or indirect effects on prostate growth, LHRH receptors represent an emerging drug target for the treatment of BPH. However, their efficacy appears only mild, and the amount of symptomatic relief that they can provide remains to be seen. Furthermore, they are associated with generally unacceptable anti-androgenic side effects such as impotence, which has led to a number of pharmaceutical companies abandoning this approach to treat BPH.
Novel vitamin D3 analogues such as BXL-628 have also shown efficacy in arresting prostate growth in patients with BPH in recent clinical trials (Colli et al., 2006). In this study, there was a small percentage decrease of approximately 7% in prostate size compared with placebo over 3 months of treatment. However, this decrease in prostate size did not translate to an improvement in urinary symptom score. In vitro studies have also shown BXL-628 to inhibit RhoA/Rho kinase signalling, which is an important intracellular pathway in smooth muscle cell contractility (Figure 4) (Morelli et al., 2007). However, the observed delay in carbachol-induced smooth muscle contraction was only minimal, and the lack of effect on patient urinary symptom score indicates that BXL-628 may not have the efficacy to compete with currently available treatments. On the other hand, carbachol is a non-selective muscarinic receptor agonist which suggests that it may only partially produce its effect by interaction with the RhoA/Rho kinase pathway. The merits of the RhoA/Rho kinase signalling pathway as a therapeutic target for BPH will be further discussed later in this review.
Figure 4.
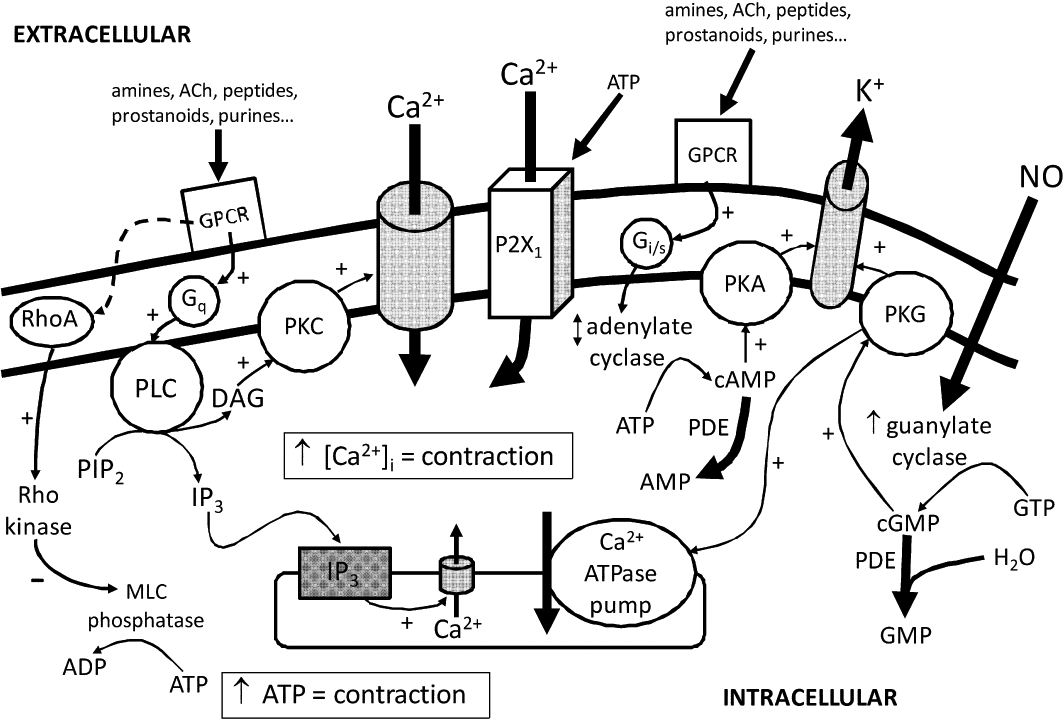
Schematic diagram of the likely intracellular signalling pathways at play in controlling contractility of prostatic smooth muscle cells. ACh, acetylcholine; Adr, adrenaline; AMP, adenosine monophosphate; ATP, adenosine 5′-triphosphate; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; DAG, diacylglycerol; GMP, guanosine monophosphate; GPCR, G-protein coupled receptor; IP3, inositol triphosphate; MLC, myosin light chain; NO, nitric oxide; PDE, phosphodiesterase; PIP2, phosphatidylinositol bisphosphate; PK, protein kinase; PL, phospholipase.
Novel targets: β-adrenoceptor agonists
There are surprisingly few studies investigating the effects of β-adrenoceptors on human prostate contractility, given their seemingly large efficacy (Table 3). Early studies, attempting to characterize β-adrenoceptor activity in human prostate tissues met with little success (Caine et al., 1975). As the pharmacological tools developed, further attempts to classify prostatic β-adrenoceptors arose. β-Adrenoceptor-mediated elevations of adenylate cyclase were seen in human prostate ‘stromal’ and ‘epithelial’ tissues (Purvis et al., 1986). Later, it was shown that the non-selective antagonist, propranolol enhanced responses to both transmural stimulation and exogenously applied noradrenaline, and that this effect was more pronounced in tissue from non-hyperplastic tissues, which also showed a reduction in dihydroalprenolol binding sites (Tsujii et al., 1992). In the mid-1990s, β3-adrenoceptor mRNA was identified in human prostatic tissue (Berkowitz et al., 1995), while more radioligand binding studies showed a dominance of the β2- over the β1-adrenoceptor subtype (Goepel et al., 1997). Subsequently, a β3-adrenoceptor antibody appeared to localize the β3-adrenoceptor to a subpopulation of prostatic stromal cells (Chamberlain et al., 1999). Functionally, the β3-adrenoceptor appears to be a better target for pharmaceutical agents as its distribution appears more restricted than that of the β1- and β2-adrenoceptors. In prostatic stromal cells derived from human prostatic tissue, β3- and possibly β1-adrenoceptor activation reduces α1-adrenoceptor-mediated contractions (Haynes and Hill, 1997), although animal studies show a predominant role for β2- and/or β1-adrenoceptors (Haynes and Hill, 1997; Kalodimos and Ventura, 2001). The most significant indication that β-adrenoceptors may prove a suitable therapeutic target is data suggesting that β blocker therapy increases the risk of developing BPH (Meigs et al., 2001).
Table 3.
Relative efficacies of various drug classes in relaxing isolated prostatic smooth muscle preparations precontracted by electrical stimulation or exogenous application of adrenoceptor agonists (where indicated by an asterisk*)
Estimates of efficacy are derived from published experimental data (often estimated from figures) in prostates taken from various species using various drugs.
CGRP, calcitonin gene-related peptide; PGE2, prostaglandin E2.
Novel targets: muscarinic receptor antagonists
Muscarinic receptor antagonists are not really novel treatments for BPH, but their use is limited by theoretical concerns that such drugs may lead to an increase in post-void residual, voiding difficulties and acute urinary retention due to detrusor inhibition. Recent reviews of clinical trials using muscarinic receptor antagonists (e.g. tolterodine, solifenacin, propiverine, oxybutynin or darifenacin) for the treatment of lower urinary tract symptoms, usually in combination with an adrenoceptor antagonist (e.g. doxazosin, tamsulosin or terazosin), show varying improvement in storage symptoms without worsening of voiding symptoms or significant development of acute urinary retention (Athanasopoulos, 2010; Chapple, 2010). This suggests that these drugs do indeed act by inhibition of detrusor instability in the bladder. Whether or not these antagonists also act in the prostate to relieve bladder outlet obstruction remains unclear.
Early studies found that compared with adrenoceptor-mediated contraction, muscarinic receptor agonists elicited only modest contractile responses from the human prostatic capsule. These responses were absent from the prostatic adenoma and did not enhance or inhibit adrenoceptor-mediated contraction (Caine et al., 1975; Gup et al., 1989; Kester et al., 2003). A more recent study observed that carbachol enhanced the contractile response to phenylephrine (Roosen et al., 2009). Furthermore, phenylephrine significantly reduced the pEC50 for carbachol-mediated contractions. The authors suggested that a significant adreno-muscarinic receptor synergy in the prostate exists which may contribute to bladder outflow resistance.
A recent study in the mouse prostate showed that after inhibition of the adrenergic contractile response, a large cholinergic component remained (White et al., 2010). Moreover, a single randomized clinical trial investigating the safety and tolerability of tolterodine for the treatment of overactive bladder in patients with BPH indicated that there was a significant reduction in the bladder outlet obstruction index; however, the study provided no discussion of this result (Abrams et al., 2006).
Finally, it should be noted that muscarinic receptor agonists can induce proliferation in a number of prostate cancer cell lines (Witte et al., 2008; Avellar et al., 2009). Therefore, as well as inhibiting detrusor overactivity, muscarinic receptor antagonists may also relieve voiding symptoms of BPH by decreasing the size of the prostate and decreasing prostatic smooth muscle tone.
Novel targets: P2X1-purinoceptor antagonists
Adenosine 5′-triphosphate (ATP) has been shown to be an excitatory co-transmitter with noradrenaline from the sympathetic nerves innervating the prostate of both the rat (Ventura et al., 2003) and guinea-pig (Buljubasich and Ventura, 2004). Despite the presence of several P2X-purinoceptor subtypes in the prostatic smooth muscle of mice (Gray and Ventura, 2005), rats (Lee et al., 2000) and guinea-pigs (Buljubasich and Ventura, 2004), both studies also used pharmacological methods to demonstrate that the P2X-purinoceptor responsible for prostatic contraction was of the P2X1-purinoceptor subtype. In both studies, blockade of P2X1-purinoceptors with either suramin or α, β-methylene ATP was able to inhibit electrically evoked nerve-mediated contractions in addition to the inhibition produced by prazosin. The relevance of these findings involving ATP to human prostate contractility is questionable, but ecto 5′-nucleotidase, an enzyme responsible for the catabolism of ATP, has been shown to be present in the human prostate (Konrad et al., 1998). Furthermore, P2X1-purinoceptors are also expressed in human prostate (Longhurst et al., 1996).
Functional studies in human prostate have shown that the contractile response to electrical field stimulation is almost completely suppressed by α-adrenoceptor antagonists (Hedlund et al., 1985; Guh et al., 1995; Chueh et al., 1996). These studies were limited in that they used only high frequency stimulation (>2 Hz). Studies using prostates from laboratory animals have shown that purinergic neurotransmission is frequency dependent and particularly important at lower concentrations (Ventura et al., 2003; Buljubasich and Ventura, 2004). Furthermore, the human studies had the limitation of tissue being solely obtained at surgery. Many prostate surgery techniques cause trauma to the nerves which causes compensatory phenotype changes. Nerves containing ATP are thought to be particularly susceptible to such plasticity of purinoceptor expression (Burnstock and Knight, 2004) which may dampen their involvement in human tissue studies.
The importance of ATP as a neurotransmitter in male genitourinary function can not be underestimated as P2X1-purinoceptor knockout mice are known to be infertile (Mulryan et al., 2000). ATP has recently been found to be involved in neurotransmission in the human vas deferens (Banks et al., 2006). If ATP is similarly involved in neurotransmission in the human prostate, the development of selective inhibitors at the P2X1-purinoceptor may provide an additional target. When used in combination with a α1A-adrenoceptor antagonist, a P2X1-purinoceptor antagonist could provide greater prostatic smooth muscle relaxation and therefore, greater relief from symptoms. There is also convincing evidence for a role of ATP at genitourinary P2X-purinoceptors in the human bladder, particularly in those displaying pathological contractile overactivity (Fry et al., 2010). Therefore, it is likely that drugs affecting this mechanism would also act at the level of the bladder.
Novel targets: adenosine receptor agonists
Evidence exists that adenosine may modulate prostate smooth muscle contractility at two sites (Figure 3). In isolated rat prostates, activation of prejunctional A1 adenosine receptors attenuates electrical field stimulation-induced contractile responses by inhibiting noradrenaline release (Preston et al., 2000). In human prostatic stromal cells, activation of post-junctional A2A adenosine receptors inhibits α1 adrenoceptor-mediated responses via stimulation of adenylate cyclase and subsequent accumulation of cyclic adenosine monophosphate (cAMP) (Preston et al., 2004). The physiological relevance of these observations is questionable, as gene disruption of the A2A adenosine receptor in mice also produces a small reduction in prostate contractility (Gray et al., 2008b). The major effects that adenosine has on the cardiovascular system also places doubt on whether adenosine receptors would be of any clinical value as a target for the treatment of BPH.
Novel targets: cannabinoids
Stimulation of CB1 cannabinoid receptors have been shown to inhibit contractions of the rat prostate gland (Tokanovic et al., 2007) (Tables 2 and 3). This action appeared to be indirect, and the authors used immunohistochemical techniques to localize the CB1 cannabinoid receptors to the prostatic epithelium. CB1 cannabinoid receptor expression has also been shown in the epithelial layer of the human prostate (Ruiz-Llorente et al., 2003). Furthermore, an anandamide uptake transporter and the fatty acid amidohydrolase enzyme, which degrades endocannabinoids, are also expressed in the human prostate (Ruiz-Llorente et al., 2004). More recently, CB1 and CB2 cannabinoid receptors have also been localized on sensory nerves innervating the human prostatic stroma and have been implicated in mediating inhibition of contraction (Gratzke et al., 2010). This indicates that cannabinoid receptors may also be a possible therapeutic target for the treatment of BPH. However, their inhibitory effects on prostate contractility appear to be only modest (Table 3) (Tokanovic et al., 2007; Gratzke et al., 2010), and the effects of cannabinoid receptor agonist treatment on the central nervous system would also need to be considered.
Table 2.
Receptor mechanisms mediating contractile and relaxant effects of prostatic stroma
Upward arrows indicate an overall excitatory effect on contractility indicated by either an increase in basal tone or an enhancement of electrically evoked contractions.
Downward arrows indicate an overall inhibitory effect on contractility indicated by a relaxation of precontracted tissue or inhibition of electrically evoked contraction.
Dashes indicate no effect on contractility elicited by electrical stimulation or exogenous addition of α-adrenoceptor agonists.
All cited studies used direct force measurements of isolated prostate tissue preparations unless indicated by an asterisk*.
Novel targets: prostaglandin E2
Prostaglandin E2 has been shown to inhibit contractions of the rat prostate gland through action at a prostanoid receptor of the EP2 subtype (Tokanovic et al., 2010) (Tables 2 and 3). Interestingly, prostaglandins were first isolated from semen and are so named because they were believed to be derived from the prostate gland (Bergstrom et al., 1968). The effects of prostaglandins on the contractility of the prostate gland are not well characterized. Early studies indicated that some of the prostaglandins caused an excitatory contractile response (Kitada and Kumazawa, 1987; Najbar-Kaszkiel et al., 1997; Sudoh et al., 1997). Nevertheless, there is now mounting evidence that inflammatory roles may play a role in the development of BPH (Kramer et al., 2007; Sciarra et al., 2007; 2008). Despite this, prostanoids have very widespread effects throughout the body (Narumiya et al., 1999) which may limit their use in the treatment of BPH.
Novel targets: histamine
Histamine has been shown to contract dog prostate (Normandin and Lodge, 1996) as well as have excitatory effects on Ca2+ mobilization in prostate cancer cells (Wasilenko et al., 1997). A later study in guinea pig prostate showed that histamine was not able to cause contraction on its own but potentiated contractions in response to electrical nerve stimulation and the exogenous application of ATP, noradrenaline or acetylcholine (Kerr, 2006). This potentiation appeared to be mediated by H1 histamine receptors. The clinical significance of these findings for the treatment of BPH is unclear as the H1 histamine receptor antagonist mepyramine on its own appeared to have no effect on contractions mediated by electrical field stimulation (Tables 2 and 3).
Novel targets: peptides
A number of peptides have been shown to have varying effects on the contractility of the prostate gland in a number of species (Table 2). Tachykinins have been demonstrated to be able to produce tonic contractions of isolated preparations of human prostate via the stimulation of NK2 tachykinin receptors (Palea et al., 1996). Similar effects on smooth muscle have not been reported in rodent models, but tachykinins potentiate electrical field stimulation induced contractile responses of prostates taken from guinea pigs through stimulation of NK1 tachykinin receptors (Buljubasich et al., 1999; Ventura et al., 2000a). In stark contrast, tachykinins have no effect on prostates taken from rats despite the presence of nerves immunoreactive for substance P and neurokinin A (Buljubasich et al., 1999).
Conversely, the sensory neuropeptide calcitonin gene-related peptide (CGRP) has been shown to inhibit contractions of the rat prostate elicited by exogenous administration of phenylephrine (Watts and Cohen, 1991) or electrical field stimulation (Ventura et al., 2000a,b) (Tables 2 and 3). In further contrast to the tachykinins, CGRP has no effect on contractility of prostates obtained from guinea-pigs despite the presence of CGRP immunoreactive nerves (Ventura et al., 2000b). The effects of CGRP are yet to be tested in human prostate despite numerous reports of CGRP immunoreactivity (Chapple et al., 1991; Jen and Dixon, 1995; Tainio, 1995; Hedlund et al., 1997). If similar smooth muscle relaxant effects were seen to CGRP, then this peptide may be potentially of some use in the treatment of BPH.
Similar to the sensory neuropeptides, endothelins also show species biodiversity in prostates taken from laboratory animals. In guinea-pigs, endothelins potentiate nerve-mediated contractions without affecting basal smooth muscle tone (Lau et al., 1999), whereas in rat prostates endothelins raise smooth muscle tone without affecting nerve mediated contractions (Salamoussa et al., 2000). In contrast to the neuropeptides, endothelins have been widely studied in human prostate. Endothelin-1 immunoreactivity has been demonstrated in the glandular epithelium (Langenstroer et al., 1993), and cultured cells derived from human prostate epithelium have been shown to secrete endothelin-1 (Walden et al., 1998). Endothelin-1 has also been shown to have contractile effects in human prostate (Langenstroer et al., 1993; Moriyama et al., 1996; Imajo et al., 1997). As in all animal species studied (Table 2), contractile effects of endothelins in the normal human prostate are mediated via ETA endothelin receptors which are found throughout the human prostatic stroma (Kobayashi et al., 1994). However, in BPH, the subtype of endothelin receptor which mediates contraction may change to ETB (Webb et al., 1995), as the number of endothelin receptors increases dramatically (Kondo et al., 1994; 1995; Moriyama et al., 1996). Endothelins also promote growth of human cultured smooth muscle prostatic cells through both ETA and ETB subtypes of endothelin receptor (Saita et al., 1998). This suggests that endothelins may play an excitatory role in proliferation as well as contractility in the human prostate. Such a combination of actions would theoretically be advantageous in a BPH drug target; however, antagonists of endothelin receptors have been shown to have little or no effect on contractility on their own (Tables 2 and 3).
Novel targets: α-adrenoceptor interacting proteins
Recent work investigating the changed pharmacology of the α1A-adrenoceptor in the prostate gland has led to the possibility that an interacting protein is associated with this receptor in this tissue. With the use of genetically modified knockout mice, it has been shown in our laboratory that the α1L-adrenoceptor pharmacological phenotype observed in the prostate arises from the α1A-adrenoceptor gene (Gray et al., 2008a) and this has since been confirmed by other researchers (Muramatsu et al., 2008). Initially, it was thought that a polymorphism or splice variant of the α1A-adrenoceptor gene may lead to the pharmacological expression of the α1L-adrenoceptor functional phenotype, but this was shown not to be the case by a number of different groups (Shibata et al., 1996; Suzuki et al., 2000; Ramsay et al., 2004). Using whole segments as well as homogenates of prostate tissue in radioligand binding experiments to support their theory, it has now been postulated that an interacting protein may be associated with the α1A-adrenoceptor in tissues where α1L-adrenoceptor pharmacology is exhibited (Muramatsu et al., 2005; Nishimune et al., 2010a). This may explain why α1L-adrenoceptor pharmacology is only seen in functional studies using intact tissue or whole cells and not in radioligand binding experiments where prostate tissue homogenates have been used (Muramatsu et al., 2005; Nishimune et al., 2010a).
A yeast two-hybrid approach to screen the human prostate cDNA library using the full open reading frame of the human α1A-adrenoceptor gene as bait was recently used to identify cysteine-rich with epidermal growth factor-like domain 1α (CRELD1α) as a protein that interacts with the α1A-adrenoceptor, and may account for the emergence of α1L-adrenoceptor pharmacology in certain cell types (Nishimune et al., 2010b). Co-expression of CRELD1α with the α1A-adrenoceptor generates a larger proportion of receptors displaying α1L pharmacology in Chinese hamster ovary cells (Nishimune et al., 2010b). However, the apparent abundance of adrenoceptors also changed markedly, so it is not clear whether CRELD1α is able to change the properties of this adrenoceptor by modifying its conformation (as seen with receptor activity modifying proteins), simply reducing its expression or changing its localization within the cell.
The possibility of a protein which interacts with the α1A-adrenoceptor to change its pharmacology is plausible and would be a logical explanation for the diverse α1A-adrenoceptor pharmacology seen in the prostate gland. CRELD1α may or may not be such a protein; however, identification of a α1A-adrenoceptor interacting protein or a α1A-adrenoceptor heteromer complex with another receptor which changed its pharmacology would be a significant advance in developing a more prostate-specific α1A-adrenoceptor antagonist.
Novel targets: phosphodiesterase (PDE) inhibitors
The major intracellular signalling pathways involved in prostatic smooth muscle cell contraction are shown in Figure 4. Of these, phosphodiesterase (PDE) would appear to be the most appropriate drug target for the treatment of BPH. PDEs play an important role in terminating signalling through the breakdown of cyclic nucleotide monophosphates [cAMP and cyclic guanosine monophosphate (cGMP); Figure 4]. Early studies showed that both direct or indirect activators of the cyclic nucleotide stimulating pathway relaxed prostate tissue (Drescher et al., 1994; Haynes and Cook, 2006; Kedia et al., 2006; Oger et al., 2009), an effect possibly mediated through the activation of potassium channels (Kurokawa et al., 1998; Cook et al., 2002; Haynes and Cook, 2006). Cyclic nucleotides and PDE inhibitors also modulate human prostatic cell proliferation (Guh et al., 1998; Adolfsson et al., 2002; Cook and Haynes, 2004; Fibbi et al., 2010) and fibroblast-to-myofibroblast transdifferentiation (Zenzmaier et al., 2010).
Prostatic tissue is reported to have at least 14 isozymes (Stacey et al., 1998; Uckert et al., 2001), but the major isozymes appear to be the PDE4A, 4B, 5A and 11A4 (Fawcett et al., 2000; Yuasa et al., 2000) which have varied, and occasionally opposing, distributions throughout the glandular and stromal compartments (Uckert et al., 2006; Fibbi et al., 2010; Waldkirch et al., 2010).
Largely, as an indirect result of current treatments for erectile dysfunction, there is clinical data suggesting that PDE inhibitors may be of benefit in treating lower urinary tract symptoms. For example, the administration of two PDE5 inhibitors, adalafil or udenafil, increases prostatic cAMP and cGMP (Zhao et al., 2011). More importantly, the PDE5 inhibitors, tadalafil, sildenafil and vardenafil improve prostate symptom scores (McVary et al., 2007a,b; 2008; Roehrborn et al., 2008; Stief et al., 2008; Dmochowski et al., 2010; Tuncel et al., 2010). Whether PDE5 inhibitor therapies provide more relief from lower urinary tract symptoms alone, than in combination with other therapies, is still equivocal (Kaplan and Gonzalez, 2007; Kaplan et al., 2007; Kaplan and Hatzichristou, 2007; Tuncel et al., 2010).
Novel targets: RhoA/Rho kinase
The RhoA/Rho kinase pathway has received much attention in recent years due to its role in regulating smooth muscle contractility. RhoA is a G-protein coupled to excitatory receptors (e.g. α1-adrenoceptors), which activates Rho kinase, which in turn maintains actin filaments in a contracted state through inhibition of myosin light chain phosphatase (Figure 4). RhoA and Rho kinase are expressed in smooth muscle cells of the human prostate, and inhibitors of Rho kinase decrease contractile responses to electrical field stimulation, noradrenaline, phenylephrine and endothelin in human prostate tissue (Takahashi et al., 2007) or cultured prostatic stromal cells (Rees et al., 2003). Interestingly, inhibitors of Rho kinase have also been found to decrease cell proliferation (Rees et al., 2003). Although studies on the efficacy of Rho kinase inhibitors are yet to be carried out in a clinical setting, the RhoA/Rho kinase pathway shows potential as a therapeutic target for controlling both the increased growth and contractility associated with BPH. Indeed, this pathway has been shown to be the mechanism of action of a number of novel compounds which show potential in BPH treatment, such as the vitamin D receptor agonist BXL-628 (Morelli et al., 2007), KMUP-1 (Liu et al., 2007) and isoflavones (Seok et al., 2008).
Novel targets: nitric oxide
The human prostate stroma receives dense nitrergic innervation which has been found to be reduced in BPH patients (Bloch et al., 1997). Studies have shown that nitric oxide donors inhibit noradrenaline-mediated contractions and induce smooth muscle relaxation in human prostate tissue (Hedlund et al., 1997) (Table 3). Similarly, inhibitors of nitric oxide synthesis have been found to decrease electrically induced relaxations (Takeda et al., 1995; Hedlund et al., 1997). Thus, nitric oxide has been postulated to play a significant role in regulating prostate smooth muscle relaxation through stimulation of cGMP production (Figure 4) and, in turn, activation of KATP channels (Cook et al., 2002). Targeting this pathway has been investigated in clinical trials which show that lower urinary tract symptoms associated with BPH improve under the influence of agents that enhance nitric oxide activity such as direct nitric oxide donors (Klotz et al., 1999) or PDE5 inhibitors such as sildenafil (Sairam et al., 2002).
Novel targets: interstitial pacemaker cells
Spontaneous contractions in the guinea pig prostate have been reported to be associated with the firing of ‘slow waves’ recorded in the smooth muscle stroma using electrophysiological techniques (Exintaris et al., 2002). This slow wave activity is myogenic in origin as it is unaffected by blockers of neural propagation or transmission, such as tetrodotoxin, guanethidine, atropine, capsaicin or indomethacin (Exintaris et al., 2002). Further characterization of this spontaneous electrical activity showed that it is dependent on an external source of Ca2+ (Exintaris et al., 2002). More recently, it was established that slow waves recorded in the guinea pig prostate are also dependent on the cycling of Ca2+ from inositol triphosphate-dependent Ca2+ stores and the buffering of Ca2+ by mitochondria (Exintaris et al., 2009). In contrast, voltage-activated 4-amino-pyridine-sensitive K+ channels, as well as iberiotoxin-sensitive large conductance Ca2+-activated K+ (BKCa) channels regulate the time-course and frequency of the spontaneous electrical events as demonstrated by patch-clamping freshly digested guinea pig prostatic cells (Oh et al., 2003; Lang et al., 2004) or human prostatic smooth muscle cells (Sui et al., 2004).
A discrete population of prostatic interstitial cells is thought to generate this electrical activity. In the guinea pig prostate gland, these cells lie between the glandular and stromal smooth muscle layers of the individual acini (Exintaris et al., 2002). Similar cells have been previously described in the rat prostate (Aumuller et al., 1987) and more recently in the human prostate (Van der Aa et al., 2003; Shafik et al., 2005). Electron microscopic examination has revealed that these prostatic interstitial cells lack myosin filaments, possess many mitochondria and have an incomplete basal lamina. In contrast, prostatic smooth muscle cells comprise a continuous basal lamina, relatively few mitochondria and an abundance of contractile filaments (Exintaris et al., 2002). By analogy with the gut, it is likely that prostatic interstitial cells provide the depolarizing pulse to neighbouring smooth muscle cells, initiating slow wave activity and subsequent contractility. It is also likely that prostatic interstitial cells are modulated by intrinsic nerves (Exintaris et al., 2006; Dey et al., 2009).
It is conceivable that pathological changes to prostatic interstitial cells or disruption to prostatic interstitial cell/nerve/smooth muscle networks may play a role in disorders of the prostate gland (Exintaris et al., 2006). An understanding of the cellular mechanisms underlying the prostatic rhythmic contractile effects of nerve-mediated agents could lead to the development of therapeutic agents to treat prostate-specific conditions such as BPH. However, whether a drug can be tailored to target this type of cell is yet to be elucidated.
Concluding remarks
It would be highly desirable to have a BPH therapy that affects the progression of disease rather than only symptom relief. Nevertheless, drugs which relax prostatic smooth muscle are faster acting, more effective and better tolerated than those which shrink or reduce prostate volume when it comes to treating BPH (Hutchison et al., 2007). Furthermore, there is poor correlation between the severity of lower urinary tract symptoms caused by BPH and prostate size (Eckhardt et al., 2001a,b,c). Thus, while an effective agent for BPH that affects disease progression remains elusive, it would seem that the best strategy to improve drugs used in the treatment of BPH would be to concentrate on finding mechanisms which can produce prostatic relaxation greater than that produced by the currently available α1-adrenoceptor antagonists.
α1-Adrenoceptor blockade is only able to block approximately half of the contractile response to nerve stimulation in most species studied (Table 3). This implies that inhibiting the nonadrenergic residual response may provide a target, which when used in combination with α1-adrenoceptor antagonists will provide greater relaxation of prostatic smooth muscle and therefore, greater relief from symptoms. In moderate to severe cases, improved efficacy of drugs may be sufficient for some patients to avoid the need for surgery. Of the possible contenders described in this review, and summarized in Figures 3 and 4, as well as other possible targets not listed here, it would appear that muscarinic receptor antagonists are the most likely candidates. Among all the rest, PDE inhibitors, nitric oxide donors and perhaps P2X1-purinoceptor antagonists also offer some hope.
Glossary
Abbreviations
- ATP
adenosine 5′-triphosphate
- BPH
benign prostatic hyperplasia
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- CRELD
cysteine-rich with epidermal growth factor (EGF)-like domain
- CGRP
calcitonin gene-related peptide
- CHO
Chinese hamster ovary
- DHT
dihydrotestosterone
- FAAH
fatty acid amidohydrolase
- FDA
Food and Drug Administration
- IP3
inositol triphosphate
- LHRH
luteinizing hormone releasing hormone
- NHS
National Health System
- PDE
phosphodiesterase
- RAMP
receptor activity modifying protein
- TUMT
transurethral microwave therapy
- TUNA
transurethral needle ablation
- TURP
transurethral resection of the prostate
Conflict of interest
The authors state no conflicts of interest.
Supporting Information
Teaching Materials; Figs 1–4 as PowerPoint slide.
References
- Abrams P, Kaplan S, De Koning Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol. 2006;175:999–1004. doi: 10.1016/S0022-5347(05)00483-0. [DOI] [PubMed] [Google Scholar]
- Adolfsson PI, Ahlstrand C, Varenhorst E, Svensson SP. Lysophosphatidic acid stimulates proliferation of cultured smooth muscle cells from human BPH tissue: sildenafil and papaverin generate inhibition. Prostate. 2002;51:50–58. doi: 10.1002/pros.10077. [DOI] [PubMed] [Google Scholar]
- Athanasopoulos A. Antimuscarinics and bladder outlet obstruction: from a contraindication to an indication? Neurourol Urodyn. 2010;29(Suppl. 1):S46–S50. doi: 10.1002/nau.20807. [DOI] [PubMed] [Google Scholar]
- Auffenberg GB, Helfand BT, McVary KT. Established medical therapy for benign prostatic hyperplasia. Urol Clin North Am. 2009;36:443–459. doi: 10.1016/j.ucl.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Aumuller G, Enderle-Schmitt U, Seitz J, Muntzing J, Chandler JA. Ultrastructure and immunohistochemistry of the lateral prostate in aged rats. Prostate. 1987;10:245–256. doi: 10.1002/pros.2990100307. [DOI] [PubMed] [Google Scholar]
- Avellar MC, Lazari MF, Porto CS. Expression and function of G-protein-coupled receptors in the male reproductive tract. An Acad Bras Cienc. 2009;81:321–344. doi: 10.1590/s0001-37652009000300002. [DOI] [PubMed] [Google Scholar]
- Banks FC, Knight GE, Calvert RC, Thompson CS, Morgan RJ, Burnstock G. The purinergic component of human vas deferens contraction. Fertil Steril. 2006;85:932–939. doi: 10.1016/j.fertnstert.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Bergstrom S, Carlson LA, Weeks JR. The prostaglandins: a family of biologically active lipids. Pharmacol Rev. 1968;20:1–48. [PubMed] [Google Scholar]
- Berkowitz DE, Nardone NA, Smiley RM, Price DT, Kreutter DK, Fremeau RT, et al. Distribution of beta 3-adrenoceptor mRNA in human tissues. Eur J Pharmacol. 1995;289:223–228. doi: 10.1016/0922-4106(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Bloch W, Klotz T, Loch C, Schmidt G, Engelmann U, Addicks K. Distribution of nitric oxide synthase implies a regulation of circulation, smooth muscle tone, and secretory function in the human prostate by nitric oxide. Prostate. 1997;33:1–8. doi: 10.1002/(sici)1097-0045(19970915)33:1<1::aid-pros1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Buljubasich R, Ventura S. Adenosine 5′-triphosphate and noradrenaline are excitatory cotransmitters to the fibromuscular stroma of the guinea pig prostate gland. Eur J Pharmacol. 2004;499:335–344. doi: 10.1016/j.ejphar.2004.07.080. [DOI] [PubMed] [Google Scholar]
- Buljubasich S, Lau WA, Pennefather JN, Ventura S. An immunohistochemical and pharmacological study of tachykinins in the rat and guinea-pig prostate glands. Eur J Pharmacol. 1999;380:137–144. doi: 10.1016/s0014-2999(99)00524-5. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- Caine M, Raz S, Zeigler M. Adrenergic and cholinergic receptors in the human prostate, prostatic capsule and bladder neck. Br J Urol. 1975;47:193–202. doi: 10.1111/j.1464-410x.1975.tb03947.x. [DOI] [PubMed] [Google Scholar]
- Carson C, 3rd, Rittmaster R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology. 2003;61:2–7. doi: 10.1016/s0090-4295(03)00045-1. [DOI] [PubMed] [Google Scholar]
- Chamberlain PD, Jennings KH, Paul F, Cordell J, Berry A, Holmes SD, et al. The tissue distribution of the human beta3-adrenoceptor studied using a monoclonal antibody: direct evidence of the beta3-adrenoceptor in human adipose tissue, atrium and skeletal muscle. Int J Obes Relat Metab Disord. 1999;23:1057–1065. doi: 10.1038/sj.ijo.0801039. [DOI] [PubMed] [Google Scholar]
- Chapple C. Antimuscarinics in men with lower urinary tract symptoms suggestive of bladder outlet obstruction due to benign prostatic hyperplasia. Curr Opin Urol. 2010;20:43–48. doi: 10.1097/MOU.0b013e3283330862. [DOI] [PubMed] [Google Scholar]
- Chapple CR, Crowe R, Gilpin SA, Gosling J, Burnstock G. The innervation of the human prostate gland–the changes associated with benign enlargement. J Urol. 1991;146:1637–1644. doi: 10.1016/s0022-5347(17)38203-4. [DOI] [PubMed] [Google Scholar]
- Chapple CR, Burt RP, Andersson PO, Greengrass P, Wyllie M, Marshall I. Alpha 1-adrenoceptor subtypes in the human prostate. Br J Urol. 1994;74:585–589. doi: 10.1111/j.1464-410x.1994.tb09188.x. [DOI] [PubMed] [Google Scholar]
- Chueh SC, Guh JH, Chen J, Lai MK, Ko FN, Teng CM. Inhibition by tamsulosin of tension responses of human hyperplastic prostate to electrical field stimulation. Eur J Pharmacol. 1996;305:177–180. doi: 10.1016/0014-2999(96)00197-5. [DOI] [PubMed] [Google Scholar]
- Colli E, Rigatti P, Montorsi F, Artibani W, Petta S, Mondaini N, et al. BXL628, a novel vitamin D3 analog arrests prostate growth in patients with benign prostatic hyperplasia: a randomized clinical trial. Eur Urol. 2006;49:82–86. doi: 10.1016/j.eururo.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Comaru-Schally AM, Brannan W, Schally AV, Colcolough M, Monga M. Efficacy and safety of luteinizing hormone-releasing hormone antagonist cetrorelix in the treatment of symptomatic benign prostatic hyperplasia. J Clin Endocrinol Metab. 1998;83:3826–3831. doi: 10.1210/jcem.83.11.5231. [DOI] [PubMed] [Google Scholar]
- Cook AL, Haynes JM. Protein kinase G II-mediated proliferative effects in human cultured prostatic stromal cells. Cell Signal. 2004;16:253–261. doi: 10.1016/s0898-6568(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Cook AL, Frydenberg M, Haynes JM. Protein kinase G activation of K(ATP) channels in human-cultured prostatic stromal cells. Cell Signal. 2002;14:1023–1029. doi: 10.1016/s0898-6568(02)00050-5. [DOI] [PubMed] [Google Scholar]
- Couldwell C, Jackson A, O'Brien H, Chess-Williams R. Characterization of the alpha 1-adrenoceptors of the rat prostate gland. J Pharm Pharmacol. 1993;45:922–924. doi: 10.1111/j.2042-7158.1993.tb05623.x. [DOI] [PubMed] [Google Scholar]
- Debruyne F, Gres AA, Arustamov DL. Placebo-controlled dose-ranging phase 2 study of subcutaneously administered LHRH antagonist cetrorelix in patients with symptomatic benign prostatic hyperplasia. Eur Urol. 2008;54:170–177. doi: 10.1016/j.eururo.2008.03.069. [DOI] [PubMed] [Google Scholar]
- Debruyne F, Tzvetkov M, Altarac S, Geavlete PA. Dose-ranging study of the luteinizing hormone-releasing hormone receptor antagonist cetrorelix pamoate in the treatment of patients with symptomatic benign prostatic hyperplasia. Urology. 2010;76:927–933. doi: 10.1016/j.urology.2009.09.077. [DOI] [PubMed] [Google Scholar]
- Delaflotte S, Auguet M, Chabrier PE. Pharmacological evidence that different alpha 1 adrenoceptor subtypes mediate contraction in rabbit prostate and hypogastric artery. Acta Physiol Scand. 1996;158:241–251. doi: 10.1046/j.1365-201X.1996.565310000.x. [DOI] [PubMed] [Google Scholar]
- Dey A, Nguyen DT, Lang RJ, Exintaris B. Spontaneous electrical waveforms in aging guinea pig prostates. J Urol. 2009;181:2797–2805. doi: 10.1016/j.juro.2009.01.094. [DOI] [PubMed] [Google Scholar]
- Dmochowski R, Roehrborn C, Klise S, Xu L, Kaminetsky J, Kraus S. Urodynamic effects of once daily tadalafil in men with lower urinary tract symptoms secondary to clinical benign prostatic hyperplasia: a randomized, placebo controlled 12-week clinical trial. J Urol. 2010;183:1092–1097. doi: 10.1016/j.juro.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Dondi D, Limonta P, Moretti RM, Marelli MM, Garattini E, Motta M. Antiproliferative effects of luteinizing hormone-releasing hormone (LHRH) agonists on human androgen-independent prostate cancer cell line DU 145: evidence for an autocrine-inhibitory LHRH loop. Cancer Res. 1994;54:4091–4095. [PubMed] [Google Scholar]
- Drescher P, Eckert RE, Madsen PO. Smooth muscle contractility in prostatic hyperplasia: role of cyclic adenosine monophosphate. Prostate. 1994;25:76–80. doi: 10.1002/pros.2990250204. [DOI] [PubMed] [Google Scholar]
- Eckert RE, Schreier U, Drescher P, Madsen PO, Derouet H, Becht E, et al. Regulation of prostatic smooth muscle contractility by intracellular second messengers: implications for the conservative treatment of benign prostatic hyperplasia. Urol Int. 1995;54:6–21. doi: 10.1159/000282685. [DOI] [PubMed] [Google Scholar]
- Eckhardt MD, van Venrooij GE, Boon TA. Symptoms and quality of life versus age, prostate volume, and urodynamic parameters in 565 strictly selected men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology. 2001a;57:695–700. doi: 10.1016/s0090-4295(00)01101-8. [DOI] [PubMed] [Google Scholar]
- Eckhardt MD, van Venrooij GE, Boon TA. Interactions between prostate volume, filling cystometric estimated parameters, and data from pressure-flow studies in 565 men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Neurourol Urodyn. 2001b;20:579–590. doi: 10.1002/nau.1010. [DOI] [PubMed] [Google Scholar]
- Eckhardt MD, van Venrooij GE, Boon TA. Symptoms, prostate volume, and urodynamic findings in elderly male volunteers without and with LUTS and in patients with LUTS suggestive of benign prostatic hyperplasia. Urology. 2001c;58:966–971. doi: 10.1016/s0090-4295(01)01413-3. [DOI] [PubMed] [Google Scholar]
- Exintaris B, Klemm MF, Lang RJ. Spontaneous slow wave and contractile activity of the guinea pig prostate. J Urol. 2002;168:315–322. [PubMed] [Google Scholar]
- Exintaris B, Nguyen DT, Dey A, Lang RJ. Spontaneous electrical activity in the prostate gland. Auton Neurosci. 2006;126-127:371–379. doi: 10.1016/j.autneu.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Exintaris B, Nguyen DT, Lam M, Lang RJ. Inositol trisphosphate-dependent Ca stores and mitochondria modulate slow wave activity arising from the smooth muscle cells of the guinea pig prostate gland. Br J Pharmacol. 2009;156:1098–1106. doi: 10.1111/j.1476-5381.2009.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair WR, Cordonnier JJ. The pH of prostatic fluid: a reappraisal and therapeutic implications. J Urol. 1978;120:695–698. doi: 10.1016/s0022-5347(17)57333-4. [DOI] [PubMed] [Google Scholar]
- Fawcett L, Baxendale R, Stacey P, McGrouther C, Harrow I, Soderling S, et al. Molecular cloning and characterization of a distinct human phosphodiesterase gene family: PDE11A. Proc Natl Acad Sci USA. 2000;97:3702–3707. doi: 10.1073/pnas.050585197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez JL, Rivera L, Lopez PG, Recio P, Vela-Navarrete R, Garcia-Sacristan A. Characterization of the muscarinic receptor mediating contraction of the dog prostate. J Auton Pharmacol. 1998;18:205–211. doi: 10.1046/j.1365-2680.1998.18486.x. [DOI] [PubMed] [Google Scholar]
- Fibbi B, Morelli A, Vignozzi L, Filippi S, Chavalmane A, De Vita G, et al. Characterization of phosphodiesterase type 5 expression and functional activity in the human male lower urinary tract. J Sex Med. 2010;7:59–69. doi: 10.1111/j.1743-6109.2009.01511.x. [DOI] [PubMed] [Google Scholar]
- Fry CH, Meng E, Young JS. The physiological function of lower urinary tract smooth muscle. Auton Neurosci. 2010;154:3–13. doi: 10.1016/j.autneu.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Gallegos PJ, Frazee LA. Anticholinergic therapy for lower urinary tract symptoms associated with benign prostatic hyperplasia. Pharmacotherapy. 2008;28:356–365. doi: 10.1592/phco.28.3.356. [DOI] [PubMed] [Google Scholar]
- Goepel M, Wittmann A, Rubben H, Michel MC. Comparison of adrenoceptor subtype expression in porcine and human bladder and prostate. Urol Res. 1997;25:199–206. doi: 10.1007/BF00941983. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Barcena D, Vadillo-Buenfil M, Gomez-Orta F, Fuentes Garcia M, Cardenas-Cornejo I, Graef-Sanchez A, et al. Responses to the antagonistic analog of LH-RH (SB-75, Cetrorelix) in patients with benign prostatic hyperplasia and prostatic cancer. Prostate. 1994;24:84–92. doi: 10.1002/pros.2990240206. [DOI] [PubMed] [Google Scholar]
- Gratzke C, Weinhold P, Reich O, Seitz M, Schlenker B, Stief CG, et al. Transient receptor potential A1 and cannabinoid receptor activity in human normal and hyperplastic prostate: relation to nerves and interstitial cells. Eur Urol. 2010;57:902–910. doi: 10.1016/j.eururo.2009.08.019. [DOI] [PubMed] [Google Scholar]
- Gray KT, Ventura S. Evaluation of the mouse prostate as a suitable model for the study of human prostate function. J Pharmacol Toxicol Methods. 2005;51:41–50. doi: 10.1016/j.vascn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Gray KT, Ventura S. Alpha1L-adrenoceptors mediate contractions of the isolated mouse prostate. Eur J Pharmacol. 2006;540:155–161. doi: 10.1016/j.ejphar.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Gray K, Short J, Ventura S. The alpha1A-adrenoceptor gene is required for the alpha1L-adrenoceptor-mediated response in isolated preparations of the mouse prostate. Br J Pharmacol. 2008a;155:103–109. doi: 10.1038/bjp.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KT, Short JL, Ledent C, Ventura S. Targeted disruption of the A2A adenosine receptor reduces in-vitro prostate contractility in mature mice. Eur J Pharmacol. 2008b;592:151–157. doi: 10.1016/j.ejphar.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Guh JH, Chueh SC, Ko FN, Teng CM. Characterization of alpha 1-adrenoceptor subtypes in tension response of human prostate to electrical field stimulation. Br J Pharmacol. 1995;115:142–146. doi: 10.1111/j.1476-5381.1995.tb16331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guh JH, Chueh SC, Hwang TL, Chen J, Teng CM. Cell proliferation in human prostatic smooth muscle cells involves the modulation of protein kinase C isozymes. Eur J Pharmacol. 1998;359:281–284. doi: 10.1016/s0014-2999(98)00683-9. [DOI] [PubMed] [Google Scholar]
- Gup DI, Shapiro E, Baumann M, Lepor H. Contractile properties of human prostate adenomas and the development of infravesical obstruction. Prostate. 1989;15:105–114. doi: 10.1002/pros.2990150204. [DOI] [PubMed] [Google Scholar]
- Hasan M, Parveen F, Shamsuzzaman AK, Kibria MD. Comparison of efficacy between Tamsulosin and Finasteride on symptomatic Benign Prostatic Hyperplasia. Mymensingh Med J. 2007;16:154–159. [PubMed] [Google Scholar]
- Hashim H, Abrams P. Emerging drugs for the treatment of benign prostatic obstruction. Expert Opin Emerg Drugs. 2010;15:159–174. doi: 10.1517/14728211003716459. [DOI] [PubMed] [Google Scholar]
- Haynes JM, Cook AL. Protein kinase G-induced activation of K(ATP) channels reduces contractility of human prostate tissue. Prostate. 2006;66:377–385. doi: 10.1002/pros.20355. [DOI] [PubMed] [Google Scholar]
- Haynes JM, Hill SJ. Beta-adrenoceptor-mediated inhibition of alpha 1-adrenoceptor-mediated and field stimulation-induced contractile responses in the prostate of the guinea pig. Br J Pharmacol. 1997;122:1067–1074. doi: 10.1038/sj.bjp.0701494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund H, Andersson KE, Larsson B. Alpha-adrenoceptors and muscarinic receptors in the isolated human prostate. J Urol. 1985;134:1291–1298. doi: 10.1016/s0022-5347(17)47714-7. [DOI] [PubMed] [Google Scholar]
- Hedlund P, Ekstrom P, Larsson B, Alm P, Andersson KE. Heme oxygenase and NO-synthase in the human prostate–relation to adrenergic, cholinergic and peptide-containing nerves. J Auton Nerv Syst. 1997;63:115–126. doi: 10.1016/s0165-1838(96)00139-7. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y, Ohmura T, Sakamoto S, Hayashi H, Muramatsu I. Identification of alpha 1-adrenoceptor subtypes in the rabbit prostate. J Auton Pharmacol. 1995;15:271–278. doi: 10.1111/j.1474-8673.1995.tb00310.x. [DOI] [PubMed] [Google Scholar]
- Holden CA, McLachlan RI, Pitts M, Cumming R, Wittert G, Agius PA, et al. Men in Australia Telephone Survey (MATeS): a national survey of the reproductive health and concerns of middle-aged and older Australian men. Lancet. 2005;366:218–224. doi: 10.1016/S0140-6736(05)66911-5. [DOI] [PubMed] [Google Scholar]
- Hutchison A, Farmer R, Verhamme K, Berges R, Navarrete RV. The efficacy of drugs for the treatment of LUTS/BPH, a study in 6 European countries. Eur Urol. 2007;51:207–215. doi: 10.1016/j.eururo.2006.06.012. discussion 215–206. [DOI] [PubMed] [Google Scholar]
- Imajo C, Walden PD, Shapiro E, Doherty AM, Lepor H. Evaluation of the effect of endothelin-1 and characterization of the selective endothelin a receptor antagonist PD155080 in the prostate. J Urol. 1997;158:253–257. doi: 10.1097/00005392-199707000-00081. [DOI] [PubMed] [Google Scholar]
- Jen PY, Dixon JS. Development of peptide-containing nerves in the human fetal prostate gland. J Anat. 1995;187:169–179. [PMC free article] [PubMed] [Google Scholar]
- Kalodimos PJ, Ventura S. Beta2-adrenoceptor-mediated inhibition of field stimulation induced contractile responses of the smooth muscle of the rat prostate gland. Eur J Pharmacol. 2001;431:81–89. doi: 10.1016/s0014-2999(01)01414-5. [DOI] [PubMed] [Google Scholar]
- Kaplan SA, Gonzalez RR. Phosphodiesterase type 5 inhibitors for the treatment of male lower urinary tract symptoms. Rev Urol. 2007;9:73–77. [PMC free article] [PubMed] [Google Scholar]
- Kaplan SA, Hatzichristou D. Open to debate. The motion: PDE5 inhibitors will have a significant role in the treatment of BPH. Eur Urol. 2007;52:1523–1527. doi: 10.1016/j.eururo.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Kaplan SA, Gonzalez RR, Te AE. Combination of alfuzosin and sildenafil is superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. Eur Urol. 2007;51:1717–1723. doi: 10.1016/j.eururo.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Kedia G, Uckert S, Scheller F, Chigogidze T, Managadze L, Jonas U, et al. In vitro functional responses of isolated normal human prostatic tissue to compounds interacting with the cyclic guanosine monophosphate pathway. Urology. 2006;67:1292–1297. doi: 10.1016/j.urology.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Kerr KP. The effect of histamine on field-stimulated contractions of the guinea-pig prostate. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:237–244. doi: 10.1007/s00210-006-0061-6. [DOI] [PubMed] [Google Scholar]
- Kester RR, Mooppan UM, Gousse AE, Alver JE, Gintautas J, Gulmi FA, et al. Pharmacological characterization of isolated human prostate. J Urol. 2003;170:1032–1038. doi: 10.1097/01.ju.0000080440.74266.b1. [DOI] [PubMed] [Google Scholar]
- Kitada S, Kumazawa J. Pharmacological characteristics of smooth muscle in benign prostatic hyperplasia and normal prostatic tissue. J Urol. 1987;138:158–160. doi: 10.1016/s0022-5347(17)43034-5. [DOI] [PubMed] [Google Scholar]
- Klotz T, Mathers MJ, Bloch W, Nayal W, Engelmann U. Nitric oxide based influence of nitrates on micturition in patients with benign prostatic hyperplasia. Int Urol Nephrol. 1999;31:335–341. doi: 10.1023/a:1007174102953. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Tang R, Wang B, Opgenorth T, Langenstroer P, Shapiro E, et al. Binding and functional properties of endothelin receptor subtypes in the human prostate. Mol Pharmacol. 1994;45:306–311. [PubMed] [Google Scholar]
- Kondo S, Morita T, Tashima Y. Endothelin receptor density in human hypertrophic and non-hypertrophic prostate tissue. Tohoku J Exp Med. 1994;172:381–384. doi: 10.1620/tjem.172.381. [DOI] [PubMed] [Google Scholar]
- Kondo S, Morita T, Tashima Y. Benign prostatic hypertrophy affects the endothelin receptor density in the human urinary bladder and prostate. Urol Int. 1995;54:198–203. doi: 10.1159/000282723. [DOI] [PubMed] [Google Scholar]
- Konrad L, Schiemann P, Renneberg H, Wennemuth G, Fini C, Aumuller G. Expression and enzymic activity of ecto 5′-nucleotidase in the human male genital tract. Biol Reprod. 1998;59:190–196. doi: 10.1095/biolreprod59.1.190. [DOI] [PubMed] [Google Scholar]
- Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51:1202–1216. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Kurokawa Y, Kojima K, Kanayama H, Kagawa S, Minami K, Nakaya Y. Activation of the Ca2+-activated K+ channel via protein kinase A-dependent phosphorylation in human prostatic smooth muscle cells. Int J Urol. 1998;5:482–486. doi: 10.1111/j.1442-2042.1998.tb00394.x. [DOI] [PubMed] [Google Scholar]
- Lang RJ, Mulholland E, Exintaris B. Characterization of the ion channel currents in single myocytes of the guinea pig prostate. J Urol. 2004;172:1179–1187. doi: 10.1097/01.ju.0000135456.65892.ed. [DOI] [PubMed] [Google Scholar]
- Langenstroer P, Tang R, Shapiro E, Divish B, Opgenorth T, Lepor H. Endothelin-1 in the human prostate: tissue levels, source of production and isometric tension studies. J Urol. 1993;150:495–499. doi: 10.1016/s0022-5347(17)35534-9. [DOI] [PubMed] [Google Scholar]
- Langenstroer P, Tang R, Divish B, Opgenorth T, Shapiro E, Lepor H. Endothelins in canine genitourinary tissues. J Urol. 1997;157:1044–1048. [PubMed] [Google Scholar]
- Lau WA, Pennefather JN. Muscarinic receptor subtypes in the rat prostate gland. Eur J Pharmacol. 1998;343:151–156. doi: 10.1016/s0014-2999(97)01535-5. [DOI] [PubMed] [Google Scholar]
- Lau WA, Ventura S, Pennefather JN. Pharmacology of neurotransmission to the smooth muscle of the rat and the guinea-pig prostate glands. J Auton Pharmacol. 1998;18:349–356. doi: 10.1046/j.1365-2680.1998.1860349.x. [DOI] [PubMed] [Google Scholar]
- Lau WA, Cox SL, Pennefather JN, Mitchelson FJ. Pharmacological characterization of endothelin receptor subtypes in the guinea-pig prostate gland. Br J Pharmacol. 1999;127:1091–1098. doi: 10.1038/sj.bjp.0702644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau WA, Pennefather JN, Mitchelson FJ. Cholinergic facilitation of neurotransmission to the smooth muscle of the guinea-pig prostate gland. Br J Pharmacol. 2000;130:1013–1020. doi: 10.1038/sj.bjp.0703409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Bardini M, Burnstock G. P2X receptor immunoreactivity in the male genital organs of the rat. Cell Tissue Res. 2000;300:321–330. doi: 10.1007/s004410000207. [DOI] [PubMed] [Google Scholar]
- Lepor H. Alpha blockers for the treatment of benign prostatic hyperplasia. Rev Urol. 2007;9:181–190. [PMC free article] [PubMed] [Google Scholar]
- Limonta P, Dondi D, Moretti RM, Maggi R, Motta M. Antiproliferative effects of luteinizing hormone-releasing hormone agonists on the human prostatic cancer cell line LNCaP. J Clin Endocrinol Metab. 1992;75:207–212. doi: 10.1210/jcem.75.1.1320049. [DOI] [PubMed] [Google Scholar]
- Limonta P, Moretti RM, Marelli MM, Dondi D, Parenti M, Motta M. The luteinizing hormone-releasing hormone receptor in human prostate cancer cells: messenger ribonucleic acid expression, molecular size, and signal transduction pathway. Endocrinology. 1999;140:5250–5256. doi: 10.1210/endo.140.11.7087. [DOI] [PubMed] [Google Scholar]
- Liu CM, Lo YC, Wu BN, Wu WJ, Chou YH, Huang CH, et al. cGMP-enhancing- and alpha1A/alpha1D-adrenoceptor blockade-derived inhibition of Rho-kinase by KMUP-1 provides optimal prostate relaxation and epithelial cell anti-proliferation efficacy. Prostate. 2007;67:1397–1410. doi: 10.1002/pros.20634. [DOI] [PubMed] [Google Scholar]
- Longhurst PA, Schwegel T, Folander K, Swanson R. The human P2x1 receptor: molecular cloning, tissue distribution, and localization to chromosome 17. Biochim Biophys Acta. 1996;1308:185–188. doi: 10.1016/0167-4781(96)00112-1. [DOI] [PubMed] [Google Scholar]
- McConnel JD. Benign prostatic hyperplasia. J Urol. 1995;154:402–403. doi: 10.1097/00005392-199508000-00021. [DOI] [PubMed] [Google Scholar]
- McVary KT, Monnig W, Camps JL, Jr, Young JM, Tseng LJ, van den Ende G. Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial. J Urol. 2007a;177:1071–1077. doi: 10.1016/j.juro.2006.10.055. [DOI] [PubMed] [Google Scholar]
- McVary KT, Roehrborn CG, Kaminetsky JC, Auerbach SM, Wachs B, Young JM, et al. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2007b;177:1401–1407. doi: 10.1016/j.juro.2006.11.037. [DOI] [PubMed] [Google Scholar]
- McVary KT, Siegel RL, Carlsson M. Sildenafil citrate improves erectile function and lower urinary tract symptoms independent of baseline body mass index or LUTS severity. Urology. 2008;72:575–579. doi: 10.1016/j.urology.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Marshall I, Burt RP, Chapple CR. Noradrenaline contractions of human prostate mediated by alpha 1A-(alpha 1c-) adrenoceptor subtype. Br J Pharmacol. 1995;115:781–786. doi: 10.1111/j.1476-5381.1995.tb15001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigs JB, Mohr B, Barry MJ, Collins MM, McKinlay JB. Risk factors for clinical benign prostatic hyperplasia in a community-based population of healthy aging men. J Clin Epidemiol. 2001;54:935–944. doi: 10.1016/s0895-4356(01)00351-1. [DOI] [PubMed] [Google Scholar]
- Miano R, De Nunzio C, Asimakopoulos AD, Germani S, Tubaro A. Treatment options for benign prostatic hyperplasia in older men. Med Sci Monit. 2008;14:RA94–R102. [PubMed] [Google Scholar]
- Morelli A, Vignozzi L, Filippi S, Vannelli GB, Ambrosini S, Mancina R, et al. BXL-628, a vitamin D receptor agonist effective in benign prostatic hyperplasia treatment, prevents RhoA activation and inhibits RhoA/Rho kinase signaling in rat and human bladder. Prostate. 2007;67:234–247. doi: 10.1002/pros.20463. [DOI] [PubMed] [Google Scholar]
- Moriyama N, Kurimoto S, Miyata N, Yamaura H, Yamazaki R, Sudoh K, et al. Decreased contractile effect of endothelin-1 on hyperplastic prostate. Gen Pharmacol. 1996;27:1061–1065. doi: 10.1016/0306-3623(95)00117-4. [DOI] [PubMed] [Google Scholar]
- Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- Muramatsu I, Oshita M, Ohmura T, Kigoshi S, Akino H, Gobara M, et al. Pharmacological characterization of alpha 1-adrenoceptor subtypes in the human prostate: functional and binding studies. Br J Urol. 1994;74:572–578. doi: 10.1111/j.1464-410x.1994.tb09186.x. [DOI] [PubMed] [Google Scholar]
- Muramatsu I, Tanaka T, Suzuki F, Li Z, Hiraizumi-Hiraoka Y, Anisuzzaman AS, et al. Quantifying receptor properties: the tissue segment binding method – a powerful tool for the pharmacome analysis of native receptors. J Pharmacol Sci. 2005;98:331–339. doi: 10.1254/jphs.cpj05001x. [DOI] [PubMed] [Google Scholar]
- Muramatsu I, Morishima S, Suzuki F, Yoshiki H, Anisuzzaman AS, Tanaka T, et al. Identification of alpha 1L-adrenoceptor in mice and its abolition by alpha 1A-adrenoceptor gene knockout. Br J Pharmacol. 2008;155:1224–1234. doi: 10.1038/bjp.2008.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najbar-Kaszkiel AT, Di Iulio JL, Li CG, Rand MJ. Characterisation of excitatory and inhibitory transmitter systems in prostate glands of rats, guinea pigs, rabbits and pigs. Eur J Pharmacol. 1997;337:251–258. doi: 10.1016/s0014-2999(97)01270-3. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Nishimune A, Suzuki F, Yoshiki H, Morishima S, Muramatsu I. Alpha 1-adrenoceptor pharmacome: alpha 1L-adrenoceptor and alpha 1A-adrenoceptor in the lower urinary tract. Int J Urol. 2010a;17:31–37. doi: 10.1111/j.1442-2042.2009.02368.x. [DOI] [PubMed] [Google Scholar]
- Nishimune A, Suzuki F, Yoshiki H, Morishima S, Muramatsu I. Identification of cysteine-rich epidermal growth factor-like domain 1alpha (CRELD1alpha) as a novel alpha1A-adrenoceptor-down-regulating protein and establishment of an alpha1L-adrenoceptor-expressing cell line. J Pharmacol Sci. 2010b;113:169–181. doi: 10.1254/jphs.10093fp. [DOI] [PubMed] [Google Scholar]
- Normandin DE, Lodge NJ. Pharmacological characterization of the isolated canine prostate. J Urol. 1996;155:1758–1761. [PubMed] [Google Scholar]
- Oger S, Behr-Roussel D, Gorny D, Lecoz O, Lebret T, Denoux Y, et al. Combination of doxazosin and sildenafil exerts an additive relaxing effect compared with each compound alone on human cavernosal and prostatic tissue. J Sex Med. 2009;6:836–847. doi: 10.1111/j.1743-6109.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- Oh SJ, Kim KM, Chung YS, Hong EK, Shin SY, Kim SJ. Ion-channel currents of smooth muscle cells isolated from the prostate of guinea-pig. BJU Int. 2003;92:1022–1030. doi: 10.1111/j.1464-410x.2003.04510.x. [DOI] [PubMed] [Google Scholar]
- Ohmura T, Sakamoto S, Hayashi H, Kigoshi S, Muramatsu I. Identification of alpha 1-adrenoceptor subtypes in the dog prostate. Urol Res. 1993;21:211–215. doi: 10.1007/BF00590038. [DOI] [PubMed] [Google Scholar]
- Palea S, Corsi M, Artibani W, Ostardo E, Pietra C. Pharmacological characterization of tachykinin NK2 receptors on isolated human urinary bladder, prostatic urethra and prostate. J Pharmacol Exp Ther. 1996;277:700–705. [PubMed] [Google Scholar]
- Pennefather JN, Lau WA, Chin C, Story ME, Ventura S. alpha(1L)-adrenoceptors mediate noradrenaline-induced contractions of the guinea-pig prostate stroma. Eur J Pharmacol. 1999;384:25–30. doi: 10.1016/s0014-2999(99)00667-6. [DOI] [PubMed] [Google Scholar]
- Preston A, Lau WA, Pennefather JN, Ventura S. Effects of adenine nucleosides and nucleotides on neuromuscular transmission to the prostatic stroma of the rat. Br J Pharmacol. 2000;131:1073–1080. doi: 10.1038/sj.bjp.0703652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston A, Frydenberg M, Haynes JM. A1 and A2A adenosine receptor modulation of alpha 1-adrenoceptor-mediated contractility in human cultured prostatic stromal cells. Br J Pharmacol. 2004;141:302–310. doi: 10.1038/sj.bjp.0705535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis K, Rui H, Gordeladze JO, Attramadal H. Hormonal activation of the adenylyl cyclases of the rat and human prostate gland. Prostate. 1986;8:11–24. doi: 10.1002/pros.2990080104. [DOI] [PubMed] [Google Scholar]
- Ramsay D, Carr IC, Pediani J, Lopez-Gimenez JF, Thurlow R, Fidock M, et al. High-affinity interactions between human alpha1A-adrenoceptor C-terminal splice variants produce homo- and heterodimers but do not generate the alpha1L-adrenoceptor. Mol Pharmacol. 2004;66:228–239. doi: 10.1124/mol.66.2.228. [DOI] [PubMed] [Google Scholar]
- Raschack M, Gock S, Unger L, Hahn A, Amberg W, Jansen R, et al. LU 302 872 and its racemate (LU 224 332) show balanced endothelin-A/B receptor affinity, high oral activity, and inhibit human prostate tissue contractions. J Cardiovasc Pharmacol. 1998;31(Suppl. 1):S241–S244. doi: 10.1097/00005344-199800001-00068. [DOI] [PubMed] [Google Scholar]
- Rees RW, Foxwell NA, Ralph DJ, Kell PD, Moncada S, Cellek S. Y-27632, a Rho-kinase inhibitor, inhibits proliferation and adrenergic contraction of prostatic smooth muscle cells. J Urol. 2003;170:2517–2522. doi: 10.1097/01.ju.0000085024.47406.6c. [DOI] [PubMed] [Google Scholar]
- Rick FG, Schally AV, Block NL, Halmos G, Perez R, Fernandez JB, et al. LHRH antagonist Cetrorelix reduces prostate size and gene expression of proinflammatory cytokines and growth factors in a rat model of benign prostatic hyperplasia. Prostate. 2011;71:736–747. doi: 10.1002/pros.21289. [DOI] [PubMed] [Google Scholar]
- Rigatti P, Brausi M, Scarpa RM, Porru D, Schumacher H, Rizzi CA. A comparison of the efficacy and tolerability of tamsulosin and finasteride in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2003;6:315–323. doi: 10.1038/sj.pcan.4500680. [DOI] [PubMed] [Google Scholar]
- Roehrborn CG, McVary KT, Elion-Mboussa A, Viktrup L. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J Urol. 2008;180:1228–1234. doi: 10.1016/j.juro.2008.06.079. [DOI] [PubMed] [Google Scholar]
- Rokosh DG, Simpson PC. Knockout of the alpha 1A/C-adrenergic receptor subtype: the alpha 1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci USA. 2002;99:9474–9479. doi: 10.1073/pnas.132552699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosen A, Blake-James BT, Wood D, Fry CH. Clinical and experimental aspects of Adreno-muscarinic synergy in the bladder base and prostate. Neurourol Urodyn. 2009;28:938–943. doi: 10.1002/nau.20742. [DOI] [PubMed] [Google Scholar]
- Ruiz-Llorente L, Sanchez MG, Carmena MJ, Prieto JC, Sanchez-Chapado M, Izquierdo A, et al. Expression of functionally active cannabinoid receptor CB1 in the human prostate gland. Prostate. 2003;54:95–102. doi: 10.1002/pros.10165. [DOI] [PubMed] [Google Scholar]
- Ruiz-Llorente L, Ortega-Gutierrez S, Viso A, Sanchez MG, Sanchez AM, Fernandez C, et al. Characterization of an anandamide degradation system in prostate epithelial PC-3 cells: synthesis of new transporter inhibitors as tools for this study. Br J Pharmacol. 2004;141:457–467. doi: 10.1038/sj.bjp.0705628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairam K, Kulinskaya E, McNicholas TA, Boustead GB, Hanbury DC. Sildenafil influences lower urinary tract symptoms. BJU Int. 2002;90:836–839. doi: 10.1046/j.1464-410x.2002.03040.x. [DOI] [PubMed] [Google Scholar]
- Saita Y, Yazawa H, Koizumi T, Morita T, Tamura T, Takenaka T, et al. Mitogenic activity of endothelin on human cultured prostatic smooth muscle cells. Eur J Pharmacol. 1998;349:123–128. doi: 10.1016/s0014-2999(98)00183-6. [DOI] [PubMed] [Google Scholar]
- Salamoussa A, Lau WA, Pennefather JN, Ventura S. The contractile effects of endothelins on the smooth muscle of the rat prostate gland. Eur J Pharmacol. 2000;403:139–145. doi: 10.1016/s0014-2999(00)00580-x. [DOI] [PubMed] [Google Scholar]
- Scarpa RM. Lower urinary tract symptoms: what are the implications for the patients? Eur Urol. 2001;40(Suppl. 1):12–20. doi: 10.1159/000049890. [DOI] [PubMed] [Google Scholar]
- Schulman CC. Lower urinary tract symptoms/benign prostatic hyperplasia: minimizing morbidity caused by treatment. Urology. 2003;62:24–33. doi: 10.1016/s0090-4295(03)00471-0. [DOI] [PubMed] [Google Scholar]
- Sciarra A, Di Silverio F, Salciccia S, Autran Gomez AM, Gentilucci A, Gentile V. Inflammation and chronic prostatic diseases: evidence for a link? Eur Urol. 2007;52:964–972. doi: 10.1016/j.eururo.2007.06.038. [DOI] [PubMed] [Google Scholar]
- Sciarra A, Mariotti G, Salciccia S, Gomez AA, Monti S, Toscano V, et al. Prostate growth and inflammation. J Steroid Biochem Mol Biol. 2008;108:254–260. doi: 10.1016/j.jsbmb.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Seok YM, Baek I, Kim YH, Jeong YS, Lee IJ, Shin DH, et al. Isoflavone attenuates vascular contraction through inhibition of the RhoA/Rho-kinase signaling pathway. J Pharmacol Exp Ther. 2008;326:991–998. doi: 10.1124/jpet.108.138529. [DOI] [PubMed] [Google Scholar]
- Shafik A, Shafik I, el-Sibai O. Identification of c-kit-positive cells in the human prostate: the interstitial cells of Cajal. Arch Androl. 2005;51:345–351. doi: 10.1080/014850190944456. [DOI] [PubMed] [Google Scholar]
- Shapiro E, Hartanto V, Lepor H. The response to alpha blockade in benign prostatic hyperplasia is related to the percent area density of prostate smooth muscle. Prostate. 1992;21:297–307. doi: 10.1002/pros.2990210406. [DOI] [PubMed] [Google Scholar]
- Shibata K, Hirasawa A, Moriyama N, Kawabe K, Ogawa S, Tsujimoto G. Alpha 1a-adrenoceptor polymorphism: pharmacological characterization and association with benign prostatic hypertrophy. Br J Pharmacol. 1996;118:1403–1408. doi: 10.1111/j.1476-5381.1996.tb15552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siejka A, Schally AV, Block NL, Barabutis N. Mechanisms of inhibition of human benign prostatic hyperplasia in vitro by the luteinizing hormone-releasing hormone antagonist cetrorelix. BJU Int. 2010;106:1382–1388. doi: 10.1111/j.1464-410X.2010.09215.x. [DOI] [PubMed] [Google Scholar]
- Stacey P, Rulten S, Dapling A, Phillips SC. Molecular cloning and expression of human cGMP-binding cGMP-specific phosphodiesterase (PDE5) Biochem Biophys Res Commun. 1998;247:249–254. doi: 10.1006/bbrc.1998.8769. [DOI] [PubMed] [Google Scholar]
- Stamatiou K. Management of benign prostatic hypertrophy-related urinary retention: current trends and perspectives. Urol J. 2009;6:237–244. [PubMed] [Google Scholar]
- Stief CG, Porst H, Neuser D, Beneke M, Ulbrich E. A randomised, placebo-controlled study to assess the efficacy of twice-daily vardenafil in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol. 2008;53:1236–1244. doi: 10.1016/j.eururo.2008.01.075. [DOI] [PubMed] [Google Scholar]
- Sudoh K, Inagaki O, Honda K. Responsiveness of smooth muscle in the lower urinary tract of rabbits to various agonists. Gen Pharmacol. 1997;28:629–631. doi: 10.1016/s0306-3623(96)00292-3. [DOI] [PubMed] [Google Scholar]
- Sui GP, Wu C, Fry CH. Ca2+ currents in smooth muscle cells isolated from human prostate. Prostate. 2004;59:275–281. doi: 10.1002/pros.20007. [DOI] [PubMed] [Google Scholar]
- Suzuki F, Taniguchi T, Takauji R, Murata S, Muramatsu I. Splice isoforms of alpha(1a)-adrenoceptor in rabbit. Br J Pharmacol. 2000;129:1569–1576. doi: 10.1038/sj.bjp.0703242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tainio H. Peptidergic innervation of the human prostate, seminal vesicle and vas deferens. Acta Histochem. 1995;97:113–119. doi: 10.1016/S0065-1281(11)80212-6. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Nishimura J, Seki N, Yunoki T, Tomoda T, Kanaide H, et al. RhoA/Rho kinase-mediated Ca2+ sensitization in the contraction of human prostate. Neurourol Urodyn. 2007;26:547–551. doi: 10.1002/nau.20365. [DOI] [PubMed] [Google Scholar]
- Takeda M, Tang R, Shapiro E, Burnett AL, Lepor H. Effects of nitric oxide on human and canine prostates. Urology. 1995;45:440–446. doi: 10.1016/S0090-4295(99)80013-2. [DOI] [PubMed] [Google Scholar]
- Tarter TH, Vaughan ED., Jr Inhibitors of 5alpha-reductase in the treatment of benign prostatic hyperplasia. Curr Pharm Des. 2006;12:775–783. doi: 10.2174/138161206776056010. [DOI] [PubMed] [Google Scholar]
- Teng CM, Guh JH, Ko FN. Functional identification of alpha 1-adrenoceptor subtypes in human prostate: comparison with those in rat vas deferens and spleen. Eur J Pharmacol. 1994;265:61–66. doi: 10.1016/0014-2999(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Testa R, Guarneri L, Ibba M, Strada G, Poggesi E, Taddei C, et al. Characterization of alpha 1-adrenoceptor subtypes in prostate and prostatic urethra of rat, rabbit, dog and man. Eur J Pharmacol. 1993;249:307–315. doi: 10.1016/0014-2999(93)90527-o. [DOI] [PubMed] [Google Scholar]
- Testa R, Guarneri L, Taddei C, Poggesi E, Angelico P, Sartani A, et al. Functional antagonistic activity of Rec 15/2739, a novel alpha-1 antagonist selective for the lower urinary tract, on noradrenaline-induced contraction of human prostate and mesenteric artery. J Pharmacol Exp Ther. 1996;277:1237–1246. [PubMed] [Google Scholar]
- Thorpe A, Neal D. Benign prostatic hyperplasia. Lancet. 2003;361:1359–1367. doi: 10.1016/S0140-6736(03)13073-5. [DOI] [PubMed] [Google Scholar]
- Tokanovic S, Malone DT, Ventura S. Stimulation of epithelial CB1 receptors inhibits contractions of the rat prostate gland. Br J Pharmacol. 2007;150:227–234. doi: 10.1038/sj.bjp.0706952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokanovic S, White CW, Malone DT, Exintaris B, Ventura S. Characterisation of the prostanoid receptor mediating inhibition of smooth muscle contractility in the rat prostate gland. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:321–328. doi: 10.1007/s00210-010-0492-y. [DOI] [PubMed] [Google Scholar]
- Tseng-Crank J, Kost T, Goetz A, Hazum S, Roberson KM, Haizlip J, et al. The alpha 1C-adrenoceptor in human prostate: cloning, functional expression, and localization to specific prostatic cell types. Br J Pharmacol. 1995;115:1475–1485. doi: 10.1111/j.1476-5381.1995.tb16640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii T, Azuma H, Yamaguchi T, Oshima H. A possible role of decreased relaxation mediated by beta-adrenoceptors in bladder outlet obstruction by benign prostatic hyperplasia. Br J Pharmacol. 1992;107:803–807. doi: 10.1111/j.1476-5381.1992.tb14527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T, Masumori N, Rahman M, Crane MM. Change in International Prostate Symptom Score, prostrate-specific antigen and prostate volume in patients with benign prostatic hyperplasia followed longitudinally. Int J Urol. 2007;14:321–324. doi: 10.1111/j.1442-2042.2007.01596.x. discussion 325. [DOI] [PubMed] [Google Scholar]
- Tuncel A, Nalcacioglu V, Ener K, Aslan Y, Aydin O, Atan A. Sildenafil citrate and tamsulosin combination is not superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. World J Urol. 2010;28:17–22. doi: 10.1007/s00345-009-0484-z. [DOI] [PubMed] [Google Scholar]
- Uckert S, Kuthe A, Jonas U, Stief CG. Characterization and functional relevance of cyclic nucleotide phosphodiesterase isoenzymes of the human prostate. J Urol. 2001;166:2484–2490. [PubMed] [Google Scholar]
- Uckert S, Hedlund P, Andersson KE, Truss MC, Jonas U, Stief CG. Update on phosphodiesterase (PDE) isoenzymes as pharmacologic targets in urology: present and future. Eur Urol. 2006;50:1194–1207. doi: 10.1016/j.eururo.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Van der Aa F, Roskams T, Blyweert W, De Ridder D. Interstitial cells in the human prostate: a new therapeutic target? Prostate. 2003;56:250–255. doi: 10.1002/pros.10264. [DOI] [PubMed] [Google Scholar]
- Ventura S, Lau WA, Buljubasich S, Pennefather JN. Species differences in the actions of sensory neuropeptides on contractility of the smooth muscle of the rat and guinea-pig prostate. Clin Exp Pharmacol Physiol. 2000a;27:917–921. doi: 10.1046/j.1440-1681.2000.03361.x. [DOI] [PubMed] [Google Scholar]
- Ventura S, Lau WA, Buljubasich S, Pennefather JN. Calcitonin gene-related peptide (CGRP) inhibits contractions of the prostatic stroma of the rat but not the guinea-pig. Regul Pept. 2000b;91:63–73. doi: 10.1016/s0167-0115(00)00118-x. [DOI] [PubMed] [Google Scholar]
- Ventura S, Dewalagama RK, Lau LC. Adenosine 5′-triphosphate (ATP) is an excitatory cotransmitter with noradrenaline to the smooth muscle of the rat prostate gland. Br J Pharmacol. 2003;138:1277–1284. doi: 10.1038/sj.bjp.0705167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden PD, Ittmann M, Monaco ME, Lepor H. Endothelin-1 production and agonist activities in cultured prostate-derived cells: implications for regulation of endothelin bioactivity and bioavailability in prostatic hyperplasia. Prostate. 1998;34:241–250. doi: 10.1002/(sici)1097-0045(19980301)34:4<241::aid-pros1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Waldkirch E, Uckert S, Sigl K, Langnaese K, Richter K, Stief CG, et al. Expression of cAMP-dependent protein kinase isoforms in the human prostate: functional significance and relation to PDE4. Urology. 2010;76:514–518. doi: 10.1016/j.urology.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Wasilenko WJ, Cooper J, Palad AJ, Somers KD, Blackmore PF, Rhim JS, et al. Calcium signaling in prostate cancer cells: evidence for multiple receptors and enhanced sensitivity to bombesin/GRP. Prostate. 1997;30:167–173. doi: 10.1002/(sici)1097-0045(19970215)30:3<167::aid-pros4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Watts SW, Cohen ML. Effect of bombesin, bradykinin, substance P and CGRP in prostate, bladder body and neck. Peptides. 1991;12:1057–1062. doi: 10.1016/0196-9781(91)90060-3. [DOI] [PubMed] [Google Scholar]
- Webb ML, Chao CC, Rizzo M, Shapiro RA, Neubauer M, Liu EC, et al. Cloning and expression of an endothelin receptor subtype B from human prostate that mediates contraction. Mol Pharmacol. 1995;47:730–737. [PubMed] [Google Scholar]
- Weisser H, Behnke B, Helpap B, Bach D, Krieg M. Enzyme activities in tissue of human benign prostatic hyperplasia after three months' treatment with the Sabal serrulata extract IDS 89 (Strogen) or placebo. Eur Urol. 1997;31:97–101. doi: 10.1159/000474426. [DOI] [PubMed] [Google Scholar]
- White CW, Short JL, Haynes JM, Evans RJ, Ventura S. The residual nonadrenergic contractile response to nerve stimulation of the mouse prostate is mediated by acetylcholine but not ATP in a comparison with the mouse vas deferens. J Pharmacol Exp Ther. 2010;335:489–496. doi: 10.1124/jpet.110.172130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JD. The pathogenesis of benign prostatic hyperplasia. Am J Med. 1980;68:745–756. doi: 10.1016/0002-9343(80)90267-3. [DOI] [PubMed] [Google Scholar]
- Witte LP, Chapple CR, de la Rosette JJ, Michel MC. Cholinergic innervation and muscarinic receptors in the human prostate. Eur Urol. 2008;54:326–334. doi: 10.1016/j.eururo.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Yazawa H, Honda K. Alpha 1-adrenoceptor subtype in the rat prostate is preferentially the alpha 1A type. Jpn J Pharmacol. 1993;62:297–304. doi: 10.1254/jjp.62.297. [DOI] [PubMed] [Google Scholar]
- Yuasa K, Kotera J, Fujishige K, Michibata H, Sasaki T, Omori K. Isolation and characterization of two novel phosphodiesterase PDE11A variants showing unique structure and tissue-specific expression. J Biol Chem. 2000;275:31469–31479. doi: 10.1074/jbc.M003041200. [DOI] [PubMed] [Google Scholar]
- Zaichick VY, Sviridova TV, Zaichick SV. Zinc concentration in human prostatic fluid: normal, chronic prostatitis, adenoma and cancer. Int Urol Nephrol. 1996;28:687–694. doi: 10.1007/BF02552165. [DOI] [PubMed] [Google Scholar]
- Zenzmaier C, Sampson N, Pernkopf D, Plas E, Untergasser G, Berger P. Attenuated proliferation and trans-differentiation of prostatic stromal cells indicate suitability of phosphodiesterase type 5 inhibitors for prevention and treatment of benign prostatic hyperplasia. Endocrinology. 2010;151:3975–3984. doi: 10.1210/en.2009-1411. [DOI] [PubMed] [Google Scholar]
- Zhao C, Kim SH, Lee SW, Jeon JH, Kang KK, Choi SB, et al. Activity of phosphodiesterase type 5 inhibitors in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. BJU Int. 2011 doi: 10.1111/j.1464-410X.2010.09759.x. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


