Abstract
BACKGROUND AND PURPOSE
An important role of GABAergic neurotransmission in schizophrenia was proposed a long time ago, but there is limited data to support this hypothesis. In the present study we decided to investigate GABAB receptor ligands in animal models predictive for the antipsychotic activity of drugs. The GABAB receptor antagonists CGP51176 and CGP36742, agonist CGP44532 and positive allosteric modulator GS39783 were studied.
EXPERIMENTAL APPROACH
The effects of all ligands were investigated in MK-801- and amphetamine-induced hyperactivity tests. The anti-hallucinogenic-like effect of the compounds was screened in the model of head twitches induced by (±)1-(2.5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI). Furthermore, the effect of GS39783 and CGP44532 on DOI-induced frequency of spontaneous excitatory postsynaptic currents (EPSCs) in slices from mouse brain frontal cortices was investigated. The anti-cataleptic properties of the compounds were also assessed.
KEY RESULTS
The GABAB receptor activators CGP44532 and GS39783 exhibited antipsychotic-like effects both in the MK-801- and amphetamine-induced hyperactivity tests, as well as in the head-twitch model in mice. Such effects were not observed for the GABAB receptor antagonists. DOI-induced increased frequency of spontaneous EPSCs was also decreased by the compounds. Moreover, CGP44532 and GS39783 inhibited haloperidol-induced catalepsy and EPSCs.
CONCLUSION AND IMPLICATIONS
These data suggest that selective GABAB receptor activators may be useful in the treatment of psychosis.
Keywords: GABAB receptor, GABA, schizophrenia, amphetamine, MK-801
Introduction
The majority of the research concerning psychosis has been focused on dopaminergic and glutamatergic theories, which dominated the field of neuroscience in recent decades. The dopaminergic theory of schizophrenia (Carlsson and Lindqvist, 1963; Carlsson and Carlsson, 2006) proposed that psychosis resulted from an overactivity of the dopamine function in the brain, particularly in the mesolimbic system (Kapur et al., 2005), and the glutamatergic one was grounded on the fact that NMDA receptor antagonists could induce a psychotic state in humans (Javitt, 1987).
Several lines of evidence also imply a dysfunction of the GABAergic inhibitory system in the pathophysiology of schizophrenia (Roberts, 1972; van Kammen, 1977). The post-mortem studies report decreased levels of GAD67 (the enzyme responsible for the synthesis of GABA from glutamate) in the dorsolateral prefrontal cortex, hippocampus, temporal cortex, cerebellum, caudate nuclei (Akbarian et al., 1995; Impagnatiello et al., 1998; Guidotti et al., 2000; Volk et al., 2000; Heckers et al., 2002), and hypofunction of GABAB receptors in the prefrontal cortex (Ishikawa et al., 2005) of brains from patients with schizophrenic or bipolar disorders.
The GABAB receptor (nomenclature follows Alexander et al., 2009) is one of the three receptors for GABA, and it is the only metabotropic receptor for this neurotransmitter. Two others, GABAA and GABAC, form ion channels and mediate fast synaptic current. GABAA receptor ligands, such as muscimol, 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol, diazepam or bretazenil, have not been successful in the treatment of schizophrenic patients (Guidotti et al., 2005), apart from inducing adverse effects after prolonged administration. The metabotropic GABAB receptor constitutes an alternative way to modulate the GABAergic system. Antagonists of the receptor, such as CGP51176 and CGP36742, were shown to be effective in animal models of depression (Nowak et al., 2006). Anxiolytic, but not antidepressant-like, action was shown for the positive allosteric modulator of the receptor, GS39783 (Slattery et al., 2005; Frankowska et al., 2007; Jacobson and Cryan, 2008). This compound was shown also to possess anti-addictive potential (Slattery et al., 2005; Lhuillier et al., 2007; Mombereau et al., 2007), similar to another orthosteric agonist of the receptor, CGP44532 (Paterson et al., 2004). Such compounds constitute a new generation of GABAB receptor ligands, with an advantage over the better known baclofen, which was shown to produce a number of adverse effects both in humans and animals, such as dizziness, weakness, fatigue, ataxia and somnolence (Grenier et al., 1996; Leo and Baer, 2005). However, the earlier studies with baclofen revealed that the compound exerted some antipsychotic-like effect in animal studies, restoring the phencyclidine-induced deficit in prepulse inhibition or reversing amphetamine-induced increase in dopamine release (Zhou et al., 2004; Balla et al., 2009; Fejgin et al., 2009). Therefore, in the present studies the series of experiments were aimed to determine if novel GABAB receptor agonists or positive allosteric modulators would exhibit such an activity as well. We have used GS39783, a positive allosteric modulator of the receptor, and CGP44532, an orthosteric agonist, as well as the receptor antagonists, CGP51176 and CGP36742.
Antipsychotic effects were evaluated with amphetamine- and MK-801-induced locomotion (Andinéet al., 1999; Geyer and Ellenbroek, 2003), and head twitches induced by (±)1-(2.5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) (Gewirtz and Marek, 2000), in mice. Electrophysiological studies with cortical slices demonstrated that the hallucinogen DOI induced a robust increase in spontaneous excitatory postsynaptic currents (EPSCs) in layer 5 pyramidal neurons (Aghajanian and Marek, 2000). Therefore, we performed electrophysiological studies on the effects of GS39783 and CGP44532 on DOI-induced EPSCs in slices of frontal cortex from mouse brain. Liability to cause extrapyramidal symptoms was evaluated in a model of induced catalepsy. Our results showed that the activation of GABAB receptors constitutes a promising target for the treatment of schizophrenia.
Methods
Animals and housing
All animal care and experimental procedures were performed according to the Animal Care and Use Committee at the Institute of Pharmacology, Polish Academy of Sciences in Kraków. Male Albino Swiss mice (weight range: 25–30 g) were used in all the experiments. The animals were kept under a 12:12 light-dark cycle at a room temperature of 19–21°C, with free access to food and water. Each experimental group consisted of at least eight animals, and the animals were used only once in each test. All the compounds used were injected i.p. in a volume of 10 mL·kg−1. All behavioural measurements were made by an observer unaware of the treatment.
Dose regimens
The doses and time of administration of GABAB receptor ligands used in our studies were selected according to previous experiments performed both in our laboratory as well as by other investigators. CGP44532 (0.060, 0.125 and 0.250 mg·kg−1) was previously used by Paterson et al. (2005; 2008) and Nowak et al. (2006). The doses of GS39783 (5, 10 and 30 mg·kg−1) were matched to those used by Paterson et al. (2005) and Cryan et al. (2004). The antagonists CGP51176 and CGP36742 were used at the doses of 15 and 30 mg·kg−1, following our previous study (Nowak et al., 2006). The doses of psychostimulants: d-amphetamine (3 mg·kg−1) and MK-801 (0.3 mg·kg−1) were selected following our previous study (Pałucha-Poniewiera et al., 2008) and those of others (Geyer and Ellenbroek, 2003; Leite et al., 2008).
Locomotor activity of habituated mice
The locomotor activity was recorded individually for each animal in OPTO-M3 locomotor activity cages (Columbus Instrument) linked online to a compatible PC. Each cage (13 cm × 23 cm × 15 cm) was surrounded with an array of photocell beams. Interruptions of these photobeams resulted in horizontal activity defined as ambulation scores.
The locomotor activity of habituated mice was performed as a control experiment for the studies on hyperactivity induced by MK-801. The measurements were performed as follows: mice were placed into activity cages for an acclimatization period of 30 min, then they were injected i.p. with CGP44532, GS39783 or saline (controls) and placed again into the same cages. From this point on the ambulation scores were measured every 10 min for 100 min.
Spontaneous locomotor activity
The procedure was performed as a control study for amphetamine-induced hyperactivity test. Mice were injected in their home cages with CGP44532 or GS39783 (0.250 mg·kg−1 and 30 mg·kg−1 respectively). After 30, 60 or 120 min they were placed in the activity cages and the locomotor activity was recorded every 10 min for the next 100 min.
MK-801-induced hyperactivity
The locomotor activity was recorded for each animal in locomotor activity cages (according to Rorick-Kehn et al., 2007, with small modifications). Mice were placed individually into activity cages for an acclimatization period of 30 min, then they were injected i.p. with CGP51176 (15 and 30 mg·kg−1), CGP36742 (15 and 30 mg·kg−1), CGP44532 (0.060, 0.125 and 0.250 mg·kg−1) or GS39783 (5, 10 and 30 mg·kg−1) and placed again in the same cages. The reference compounds, clozapine (5 and 10 mg·kg−1) and haloperidol (0.1 and 0.25 mg·kg−1) were also administered. After 30 min, all mice (except the control group) were injected i.p. with MK-801 in a dose of 0.3 mg·kg−1 and once again returned to the same cage. From then on, the ambulation was counted for 30 min after MK-801 administration. All groups were compared with the group given MK-801 only. The experiment also included a control group not treated with MK-801.
In the second part of the experiment the highest effective doses of CGP44532 and GS39783 were combined with the GABAB receptor antagonists, CGP51176 or CGP36742.
Amphetamine-induced hyperactivity
The activity of mice was recorded following the method described by Costall et al. 1979 with small modifications. Mice were injected with amphetamine (3 mg·kg−1) and were immediately placed in the activity cages. CGP51176 (15 and 30 mg·kg−1), CGP36742 (15 and 30 mg·kg−1) or GS39783 (5, 10 and 30 mg·kg−1) were administered 30 min before the amphetamine administration. CGP44532 was evaluated in doses of 0.060, 0.125 and 0.250 mg·kg−1, administered 120 min before amphetamine (the times of administrations were matched according to earlier experiments concerning spontaneous locomotor activity). All groups were compared with amphetamine control group. The experiment also included a control group not treated with amphetamine. Clozapine (5 and 10 mg·kg−1) and haloperidol (0.1 and 0.25 mg·kg−1) administered 30 min before amphetamine were used as reference substances. The locomotor activity was measured as total ambulation scores over 30 min after amphetamine.
In the second part of the experiment the highest effective doses of CGP44532 or GS39783 were combined with the antagonists CGP51176 or CGP36742.
Catalepsy test
The catalepsy test was performed according to Siuciak et al. 2007, with small modifications. The catalepsy response of one mouse placed in an observation box was measured from the duration of an abnormal posture in which the forelimbs of the mouse were placed on a horizontal wire bar (0.2 mm diameter) suspended 7.5 cm above a platform. The catalepsy test ended when the forelimbs touched the bottom or the wall of the box or when the mouse climbed onto the bar. GS39783 (5, 10 and 30 mg·kg−1) or CGP44532 (0.060, 0.125 and 0.250 mg·kg−1) were administered alone and 30 min prior to haloperidol (0.1 mg·kg−1) administration. The catalepsy was measured 90 min after haloperidol administration.
Head-twitch test
The experiment was performed according to Kłodzinska et al. (2002) and Pałucha-Poniewiera et al. (2008). In order to habituate mice to the experimental environment, each animal was randomly transferred to a 12 cm (diameter) × 20 cm (height) glass cage, lined with sawdust 30 min before the treatment. The head twitches of mice were induced by DOI (2.5 mg·kg−1, i.p.). Immediately after the treatment, the number of head twitches was counted during the next 20 min. Haloperidol (0.1 and 0.25 mg·kg−1) and clozapine (5 and 10 mg·kg−1) were administered as reference compounds. CGP44532 was evaluated in 0.020, 0.060, 0.125 and 0.250 mg·kg−1, and GS39783 was administered in a doses 2.5, 5, 10 and 30 mg·kg−1. CGP51176 or CGP36742 were given in a doses of 15 and 30 mg·kg−1. All compounds were given 30 min before giving DOI.
In the second part of the experiments, the effective doses of CGP44532 (0.250 mg·kg−1) and GS39783 (30 mg·kg−1) were blocked by administration of antagonists CGP51176 and CGP36742 (15 mg·kg−1), which were given 15 min prior to the administration of the agonists.
Electrophysiological studies
Albino Swiss mice were decapitated, their frontal cortices were dissected and cut into slices (420 µm thick) in the frontal plane using a vibrating microtome. Slices were kept submerged in the artificial cerebrospinal fluid (ACSF) consisting of (in mM): 126 NaCl, 4 KCl, 2.5 CaCl2, 1.3 MgSO4, 1.25 KH2PO4, 26 NaHCO3 and 10 glucose, bubbled with 95% O2/5% CO2, pH = 7.4. A single slice was transferred to recording chamber (volume 1 mL) and superfused with warmed (32°C) ACSF at 2 mL·min−1. Individual neurons were visualized using an upright microscope (Zeiss Axioskop 2FS) equipped with a long-range water immersion objective (40×) and an infrared camera. Recording micropipettes were pulled on a Flaming-Brown puller (P-87; Sutter Instruments, Novato, CA, USA) and had resistance 6–8 MΩ. Microelectrodes were filled with (in mM): 130 K-gluconate, 5 KCl, 0.3 CaCl2, 2 MgCl2, 1 EGTA, 10 HEPES, 5 Na2-ATP, 0.4 Na-GTP, osmolarity 290 mOsm, pH = 7.2. Whole-cell recordings were made from layer II/III pyramidal cells. Neurons were voltage-clamped at −76 mV and spontaneous EPSCs were recorded. Signals were acquired using the SEC 05 L amplifier (NPI, Germany) and digitized using Digidata 1322 interface (Molecular Devices, USA). Drugs kept as concentrated stocks were diluted in ACSF just before the experiment and applied in the superfusate. After stable baseline recording for at least 15 min, DOI (10 µM) was applied for 10 min and spontaneous EPSCs were recorded (10 min). Next DOI was applied concurrently with CGP44532 or GS39783 for 15 min and again spontaneous EPSCs were recorded. The measured parameter was the frequency of spontaneous EPSCs. The data were analysed off-line, using Mini Analysis program (Synaptosoft Inc. ver.6.0.3).
Analysis of data
All the data is expressed as means ± SEM. One-way anova followed by Dunnett's post hoc comparison was used to evaluate the results of amphetamine- or MK-801-induced locomotor activity, dose–response study of compounds in haloperidol-induced catalepsy, DOI-induced head twitches and for the analysis of electrophysiological data. Two-way anova followed by the Fisher's least significant difference post hoc test was used to analyse the effect of drugs on spontaneous and habituated locomotor activity, as well as for combined antagonist/agonist administration in locomotor activity procedures and DOI-induced head twitches. For the analysis, the programs Graph Pad Prism, San Diego, CA ver. 5.0 or Statistica ver. 7 were used. Cumulative electrophysiological histograms were constructed using Clampfit 9.2 software (Molecular Devices, USA).
Materials
MK-801 (Sigma-Aldrich, St. Louis, USA), DOI (2,5-dimethoxy-4-iodo-amphetamine, Sigma-Aldrich, St. Louis, USA), d-amphetamine (Sigma-Aldrich, St. Louis, USA), CGP51176, CGP36742 (GABAB receptor antagonists, gift from Dr Froestl) and CGP44532 (GABAB receptor orthosteric agonist, gift from Dr Froestl) were dissolved in 0.9% NaCl. Clozapine (Ascent Scientific, UK) was dispersed in a 0.5% methylcellulose in physiological saline and GS39783 (GABAB positive allosteric modulator, Tocris) was dissolved in a small amount of dimethyl sulphoxide (final concentration 10%) and then diluted with physiological saline. Haloperidol (5 mg·mL−1 ampoule; Warszawskie Zakłady Farmaceutyczne, Polfa) was diluted in 0.9% saline (which was used as a solvent for haloperidol ampoules).
Results
Effect of CGP44532 and GS39783 on locomotor activity in mice habituated to activity cages
In mice adapted to activity cages for 30 min, neither compound had any significant effect on the locomotor activity measured every 10 min, over 100 min immediately after the i.p. injection (Figure 1A).
Figure 1.
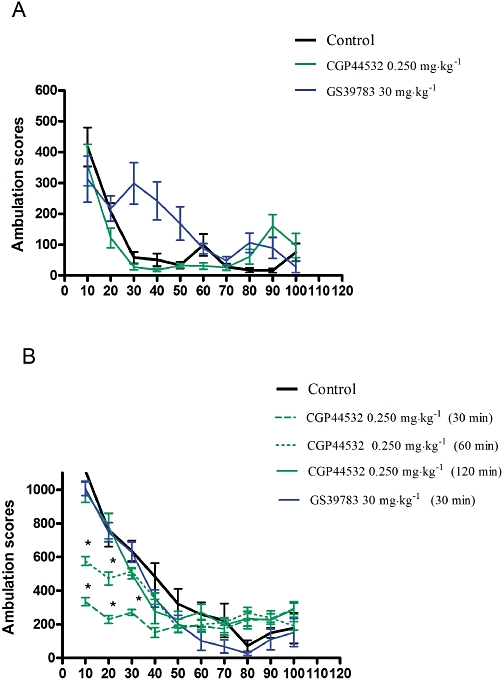
Locomotor activity. (A) effect of the GABAB receptor agonist CGP44532 and the positive modulator GS39783 on the locomotor activity in mice habituated to activity cages. Compounds were given i.p. in doses of 0.250 mg·kg−1 (CGP44532) and 30 mg·kg−1 (GS39783) after 30 min of the habituation period. Locomotor activity was monitored over 100 min after drug administration. (B) spontaneous locomotor activity after i.p. administration of the GABAB receptor agonist CGP44532 and the positive allosteric modulator, GS39783. Values represent the mean ± SEM ambulation scores during 100 min. CGP44532 was administered 120, 60 and 30 min before measurements and GS39783 was administered 30 min before the test, *P < 0.05.
Effects of CGP44532 and GS39783 on the spontaneous locomotor activity in mice
The GABAB receptor agonist CGP44532 (0.250 mg·kg−1) injected i.p., altered the spontaneous locomotor activity, relative to vehicle controls. Two-way anova revealed a significant time effect [F(9,112)= 6.74, P < 0.01]. Post hoc tests indicated that relative to the vehicle group, locomotor activity, when measured 30 and 60 min after drug injection, was decreased for up to 40 min, in mice receiving 0.250 mg·kg−1 of CGP44532 (P < 0.01 and P < 0.05 respectively). The spontaneous locomotor activity was not affected when measured 120 min after of the i.p. injection (Figure 1B). According to the pharmacokinetic profile of CGP44532 (W. Froestl, pers. comm.), elimination of this compound is biphasic with a fast initial t1/2 of 0.3 h and a second, terminal t1/2 of 17 h. Therefore, some of the compound could still be in the brain 17 h after administration.
GS39783 (30 mg·kg−1) did not change spontaneous locomotor activity (Figure 1B).
MK-801-induced hyperactivity in mice
In the vehicle treated group, MK-801 given in a dose of 0.3 mg·kg−1 produced a robust increase in the ambulation scores up to 1446% of control, within 30 min of experimental session (Figure 2). One-way anova revealed a significant effect of MK-801 [F(5,55)= 33.98, P < 0.0001], which was reversed by the reference compounds, haloperidol (0.1 and 0.25 mg·kg−1; P < 0.001) or clozapine (5 and 10 mg·kg−1; P < 0.001) (Figure 2A,B).
Figure 2.
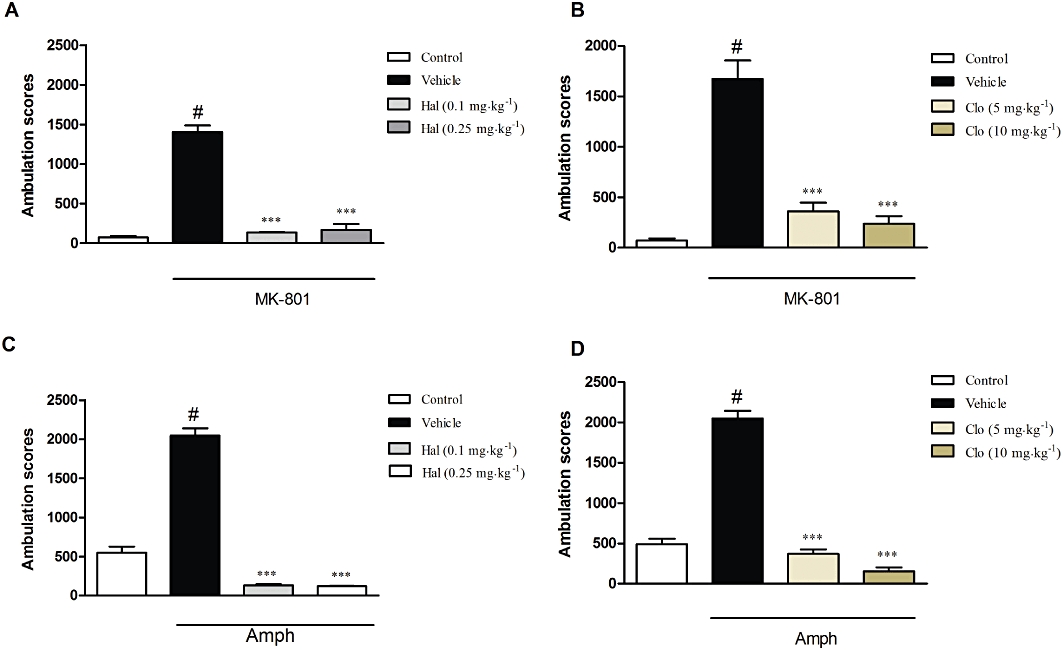
Effects of reference compounds on the MK-801-induced (A,B) and amphetamine-induced (C,D) hyperactivity test. Haloperidol (Hal: A,C) was given in doses 0.1 and 0.25 mg·kg−1 while clozapine (Clo; B,D) was given in doses of 5 and 10 mg·kg−1. Both compounds were administered 30 min before the MK-801 or amphetamine (Amph) injection. Locomotor activity was monitored over 30 min. The data presented are means ± SEM. ***P < 0.001 versus MK-801 or amphetamine only; #P < 0.001 versus control.
CGP44532 (0.125 or 0.250 mg·kg−1) significantly reduced the MK-801-induced effect [F(4,35)= 11.71, P < 0.001; one-way anova, Dunnett's post hoc test] (Figure 3A). The lowest dose (0.06 mg·kg−1) was not effective. The GABAB receptor positive allosteric modulator, GS39783 (10 or 30, but not 5 mg·kg−1), also reversed MK-801-induced locomotor activity [F(4,45)= 29.88, P < 0.001] (Figure 3B). In contrast, both the GABAB receptor antagonists, CGP51176 and CGP36742 (15 or 30 mg·kg−1) had no effect on MK-801-induced locomotor activity (P > 0.05) after 30 min of measurement (Figure 3C,D).
Figure 3.
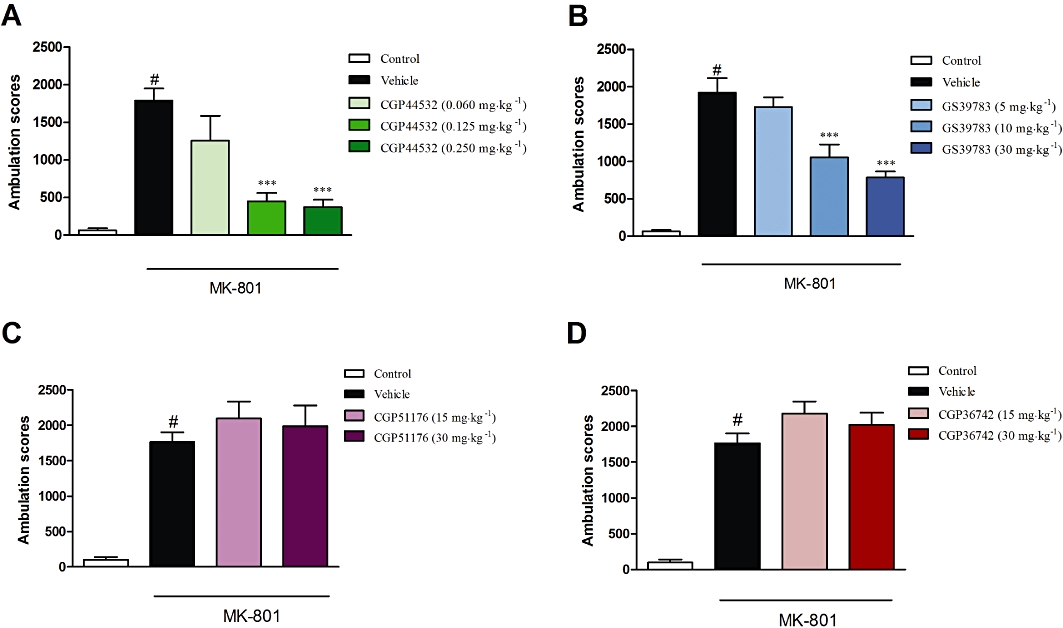
Effects of the GABAB receptor agonist CGP44532 (A), the GABAB positive allosteric modulator GS39783 (B) and the GABAB antagonists CGP51176 (C) or CGP36742 (D) in the MK-801-induced hyperactivity test in mice. CGP44532 was given i.p. in doses of 0.060, 0.125 and 0.250 mg·kg−1; GS39783 was given in doses of 5, 10 and 30 mg·kg−1, and both antagonists were given in doses of 15 and 30 mg·kg−1. All the compounds were administered 30 min before the experiments. Locomotor activity was monitored over 30 min immediately following injection of MK-801 (0.3 mg·kg−1). The data presented are means ± SEM. #P < 0.001 versus control; ***P < 0.001 versus vehicle-treated group.
Co-administration of CGP5111 (15 mg·kg−1) with CGP44532 (0.250 mg·kg−1) or GS39783 (30 mg·kg−1) completely antagonized the inhibitory effect of the latter compounds on MK-801-induced hyperactivity [F(1,30)= 4.55, P < 0.05 and F(1,32)= 18.08, P < 0.001 respectively; two-way anova] (Figure 4A,B). Similar results were observed for the other receptor antagonist used, CGP36742 (15 mg·kg−1), which reversed the effects of CGP44532 (0.250 mg·kg−1) [F(1,30)= 9.24, P < 0.01; two-way anova] or GS39783 (30 mg·kg−1) [F(1,30)= 9.65, P < 0.01; two-way anova] (Figure 4C,D).
Figure 4.
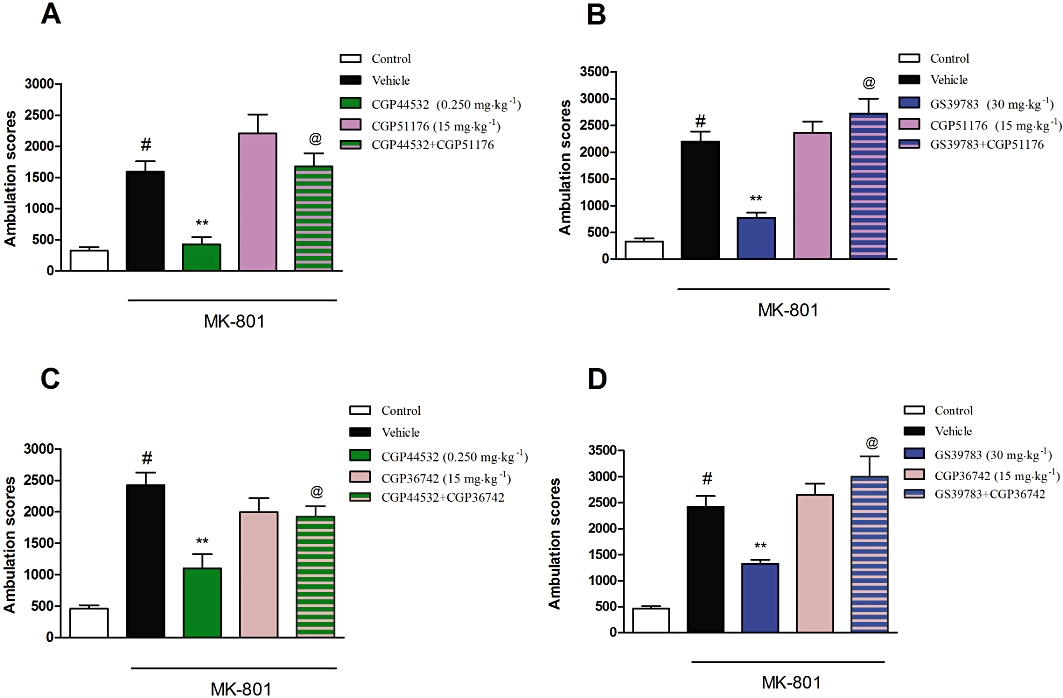
Effect of the GABAB receptor antagonists CGP51176 (15 mg·kg−1; A,B) and CGP36742 (15 mg·kg−1; C,D) on the inhibitory effects of CGP44532 (0.250 mg·kg−1; A,C) and GS39783 (30 mg·kg−1; B,D) on MK-801-induced hyperactivity. Locomotor activity was monitored over 30 min immediately following the administration of MK-801 (0.3 mg·kg−1). The data presented are means ± SEM. #P < 0.001 versus vehicle; **P < 0.001 versus vehicle-treated group; @P < 0.05 versus CGP44532 or GS39783 alone.
D-amphetamine-induced hyperactivity in mice
In the vehicle treated group, amphetamine (3 mg·kg−1) produced a robust increase in the ambulation scores (up to 417% of control) within 30 min, [F(5,50)= 201.5, P < 0.0001; one-way anova]. This effect of amphetamine was abolished by haloperidol (0.1 and 0.25 mg·kg−1) and clozapine (5 and 10 mg·kg−1), used as reference compounds (P < 0.001) (Figure 2C,D). The GABAB receptor agonist, CGP44532 (0.125 and 0.25 mg·kg−1) reversed the amphetamine-induced effect [F(4,30)= 5.08, P < 0.05; one-way anova], but the lowest dose 0.06 mg·kg−1 was not effective (Figure 5A). The GABAB receptor positive allosteric modulator GS39783 (10 and 30 mg·kg−1 but not at 5 mg·kg−1) also inhibited the amphetamine-induced hyperactivity [F(4,30)= 21.1, P < 0.001] (Figure 5B). Both the GABAB receptor antagonists, CGP51176 and CGP36742 (15 and 30 mg·kg−1) did not affect amphetamine-induced locomotor activity (Figure 5C,D) or spontaneous locomotor activity (data not shown).
Figure 5.
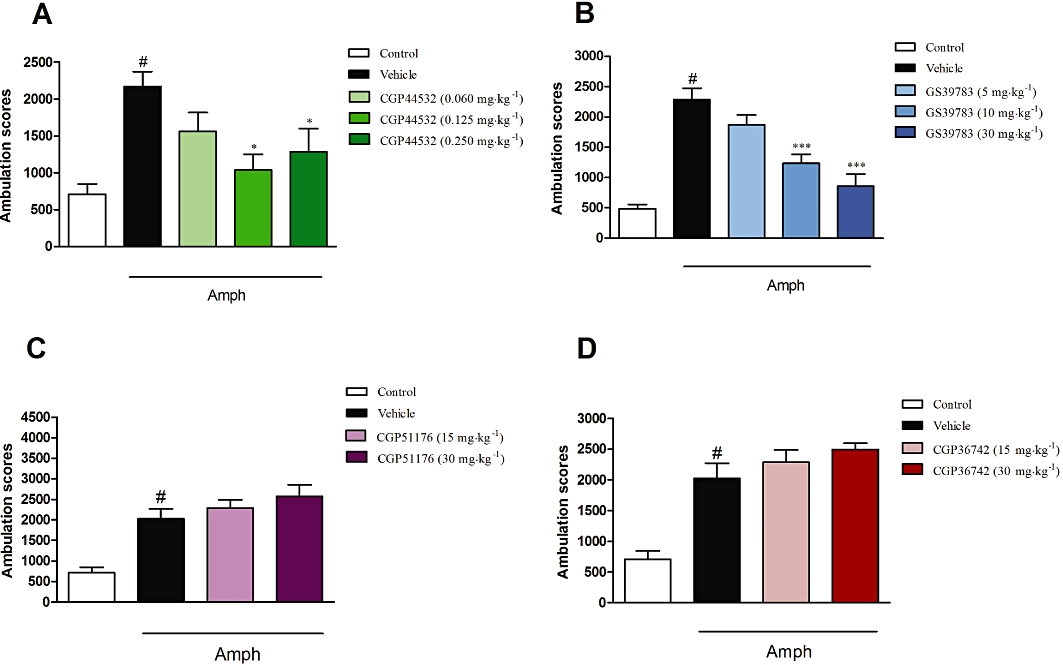
Effects of the GABAB receptor agonist CGP44532 (A), the GABAB positive allosteric modulator GS39783 (B) and the GABAB antagonists CGP51176 (C) or CGP36742 (D) in the amphetamine-induced hyperactivity test in mice. CGP44532 was given i.p. in doses of 0.060, 0.125 and 0.250 mg·kg−1; GS39783 was given in doses of 5, 10 and 30 mg·kg−1, and both antagonists were given in dose of 15 and 30 mg·kg−1. CGP44532 was administered 120 min while GS39783 and antagonists were administered 30 min before amphetamine (Amph) administration. Locomotor activity was monitored over 30 min immediately following injection of amphetamine (3 mg·kg−1). The data presented are means ± SEM. #P < 0.001 versus control; *P < 0.05 and ***P < 0.001 versus vehicle-treated group.
Co-administration of CGP51176 (15 mg·kg−1) with CGP44532 (0.25 mg·kg−1) or with GS39783 (30 mg·kg−1) completely abolished the inhibitory effect of the receptor activators on amphetamine-induced hyperactivity [F(1,31)= 4.71, P < 0.05 and F(1,32)= 9.5, P < 0.01 respectively; two-way anova] (Figure 6A,B). Similar results were observed for the combination with the second antagonist, CGP36742 (15 mg·kg−1) which decreased the effects of CGP44532 (0.25 mg·kg−1) or GS39783 (30 mg·kg−1) [F(1,30)= 8.5, P < 0.01 and F(1,30)= 8.1, P < 0.01 respectively; two-way anova] (Figure 6C,D).
Figure 6.
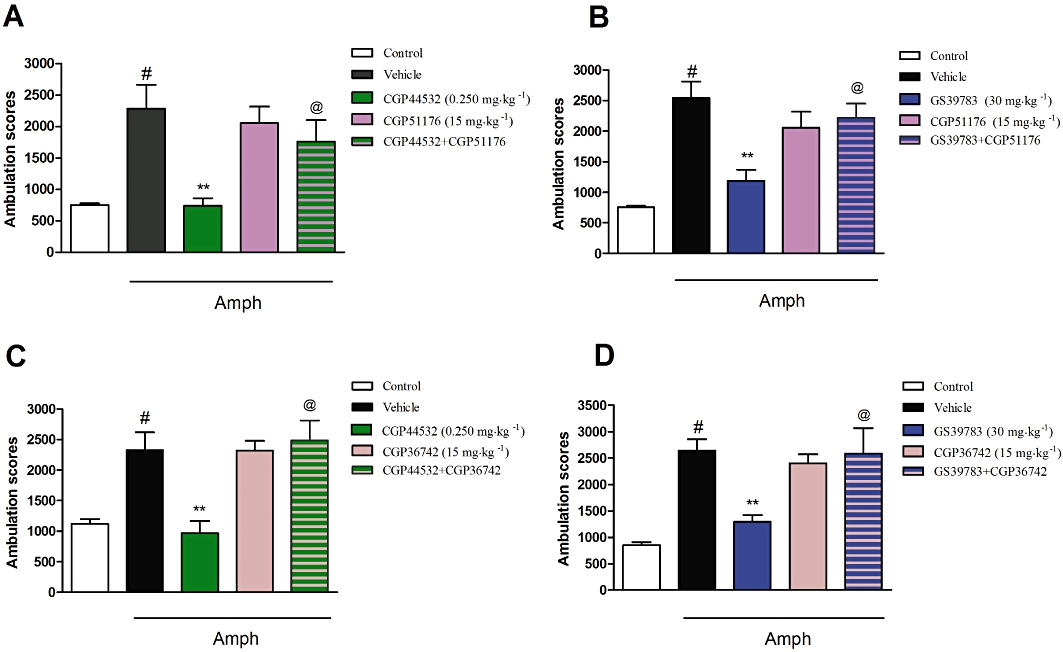
Effect of the GABAB antagonists CGP51176 (15 mg·kg−1; A,B) and CGP36742 (15 mg·kg−1; C,D) on the inhibitory effects of CGP44532 (0.250 mg·kg−1; A,C) and GS39783 (30 mg·kg−1; B,D) on amphetamine-induced hyperactivity. Locomotor activity was monitored over 30 min immediately following the injection of amphetamine (Amph) (3 mg·kg−1). The data presented are means ± SEM. #P < 0.001 versus control; **P < 0.001 versus vehicle-treated group; @P < 0.05 versus CGP44532 or GS39783 alone.
Effect on catalepsy model
Neither CGP44532 nor GS39783 induced any cataleptic-like effects when given alone (data not shown). However, CGP44532 diminished the haloperidol-induced catalepsy, by about 50%, only at one tested dose (0.125 mg·kg−1), [F(3,20)= 5.59, P < 0.01; one-way anova, Dunnett's post hoc test]. GS39783 induced dose-dependent reversal of the induced catalepsy at doses of 10 (59% control) and 30 mg·kg−1 (57% control) [F(3,29)= 5.39, P < 0.01; one-way anova, Dunnett's post hoc test] (Figure 7A,B).
Figure 7.
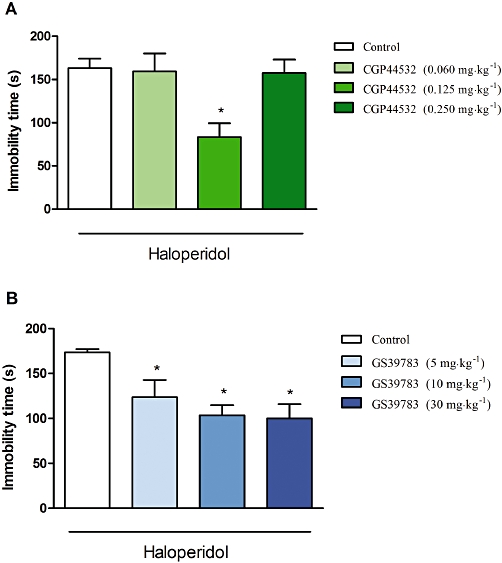
Effects of CGP44532 (A) and GS39783 (B) on haloperidol-induced catalepsy. CGP44532 was administered in doses 0.060, 0.125 and 0.250 mg·kg−1 and GS39783 was administered in doses of 5, 10 and 30 mg·kg−1. Both compounds were administered 30 min before haloperidol and the catalepsy was measured 90 min after haloperidol administration. Values represent the immobility time (mean ± SEM). *P < 0.05 and **P < 0.01 versus vehicle-treated group.
DOI-induced head twitches in mice
Both clozapine and haloperidol diminished the number of head twitches induced by DOI [F(2,21)= 9.63, P < 0.01 and F(2,21)= 14.33, P < 0.01 respectively; one-way anova, Dunnett's post hoc test] (Figure 8A). CGP44532 (0.125 and 0.25 mg·kg−1) reduced the number of head twitches, by 40% and 69%, respectively [F(4,48)= 6.85, P < 0.01], but the lower doses of this compound (0.02 and 0.06 mg·kg−1) were not effective in this test (Figure 8B).
Figure 8.

Effects of CGP44532 and GS39783 on (±)1-(2.5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-induced head twitches. In (A), dose-dependent suppression of DOI (2.5 mg·kg−1)-induced head twitches by the reference compounds haloperidol (Hal) and clozapine (Clo). In (B), the GABAB receptor agonist CGP44532 was given in doses of 0.020, 0.060, 0.125 and 0.25 mg·kg−1 and in (C), GS39783 was given in doses of 2.5, 5, 10 and 30 mg·kg−1. Values represent the number of head twitches (means ± SEM) during a 20 min session. **P < 0.01 versus vehicle-treated group.
GS39783 (2.5, 5, 10 and 30 mg·kg−1) produced a dose-dependent decrease in the number of DOI-induced head twitches in mice [F(4,46)= 22.68, P < 0.01] (Figure 8C), indicating a possible anti-hallucinogenic potential of this compound. Neither of the GABAB receptor antagonists CGP51176 nor CGP36742, given alone had any effect in the test (Figure 9A). However, treatment with CGP51176 (15 mg·kg−1) before CGP44532 (0.25 mg·kg−1) or GS39783 (30 mg·kg−1) completely reversed their anti-hallucinogenic activity [(F(1,19)= 17.3, P < 0.001 and F(1,19)= 24.39, P < 0.0001 respectively] (Figure 9B). Similar results were observed with pretreatment with CGP36742 (15 mg·kg−1) [F(1,23)= 18.52, P < 0.001 and F(1,23)= 29.4, P < 0.0001] (Figure 9C).
Figure 9.

In (A), the effect of the GABAB antagonists CGP51176 and CGP36742 given in doses of 15 and 30 mg·kg−1 on (±)1-(2.5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-induced head twitches. In (B), the effect of GABAB receptor antagonist CGP51176 (B) and in (C) of CGP36742 on the inhibitory effects on GABAB receptor activators CGP44532 (0.250 mg·kg−1) and GS39783 (30 mg·kg−1) on DOI-induced head twitches in mice. All the compounds were administered 30 min before the test. Values represent the number of head twitches (mean ± SEM) during a 20 min session. **P < 0.001 versus vehicle-treated group; #P < 0.01 versus GS39783 or CGP44532 alone.
DOI-induced spontaneous EPSCs
All recorded cells (n = 62) had electrophysiological characteristics of regular spiking pyramidal neurons (McCormick et al., 1985). Their mean (±SEM) resting membrane potential was −74.18 ± 2.42 mV. The mean (±SEM) input resistance was 176 ± 29 MΩ. The basal frequency of spontaneous synaptic activity ranged between 1.9 and 5.5 Hz (mean ± SEM: 2.9 ± 0.26 Hz). The application of DOI (10 µM) increased the mean frequency of spontaneous EPSCs to 149 ± 6% of baseline, on average (Figures 10A and 11A). Both CGP44532 (1, 5 and 10 µM) and GS39783 (1, 5 and 10 µM), when applied concurrently with DOI, dose-dependently and reversibly suppressed this DOI-induced increase in the frequency of spontaneous EPSCs (Figures 10B and 11B).
Figure 10.
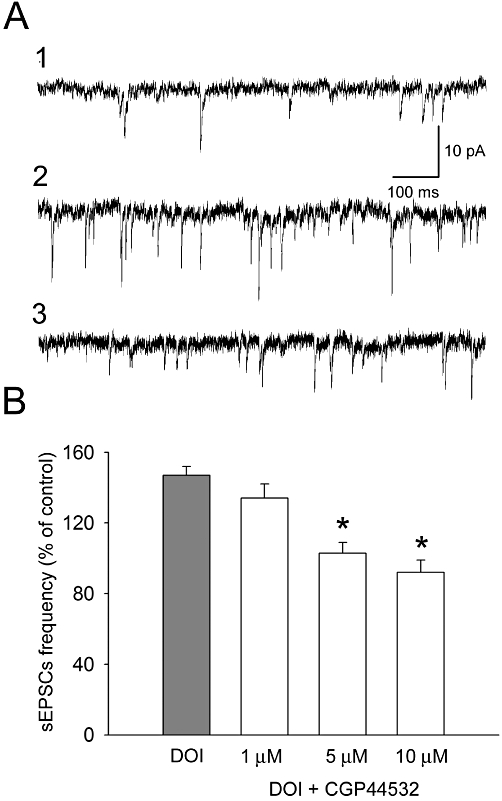
Suppression of the excitatory effect of (±)1-(2.5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) (10 µM) on the frequency of spontaneous EPSCs (sEPSCs) by CGP44532. (A) Examples of recordings from a representative neuron. (1) Baseline activity; (2) recording after 10 min incubation with DOI; (3) recording after 10 min incubation with by CGP 44532 (5 µM) in the presence of DOI. (B) Dose-dependent suppression of the effect of 10 µM DOI on the mean frequency (±SEM) of spontaneous EPSCs by CGP44532. *Significant difference between the effect of CGP44532 and that of DOI alone (5 µM: T = 2.35, P = 0.025, d.f. = 30; 10 µM: T = 4.19, P = 0.0002, d.f. = 30).
Figure 11.
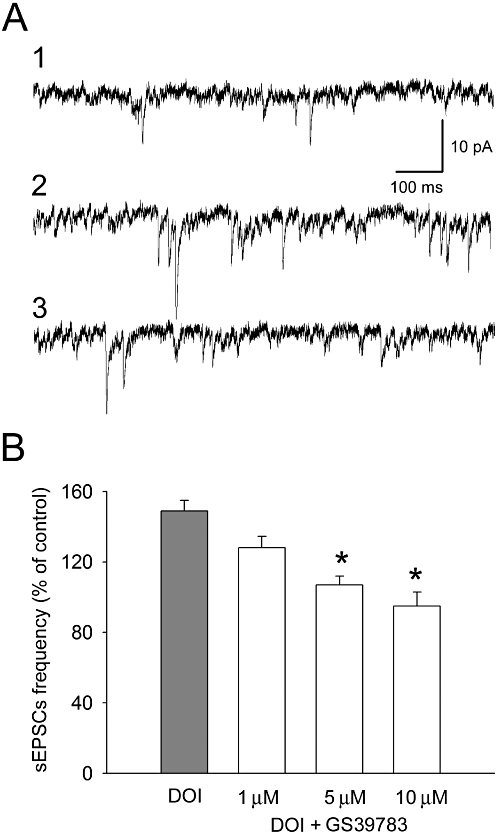
Suppression of the excitatory effect of (±)1-(2.5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) (10 µM) on the frequency of spontaneous EPSCs (sEPSCs) by GS39783. (A) Examples of recordings from a representative neuron. (1) Baseline activity; (2) recording after 10 min incubation with DOI; (3) recording after 10 min incubation with GS39783 (5 µM) in the presence of DOI. (B) Dose-dependent suppression of the effect of 10 µM DOI on the mean frequency (±SEM) of spontaneous EPSCs by GS39783. *Significant difference between the effect of GS39783 and that of DOI alone (5 µM: T = 2.22, P = 0.034, d.f. = 32; 10 µM: T = 3.63, P = 0.001, d.f. = 32).
Discussion
These studies examined the effects of selective GABAB receptor ligands in behavioural models predictive of psychosis and hallucination, such as MK-801- or amphetamine-induced hyperlocomotion and DOI-induced head twitches in mice. Both of the selective GABAB receptor stimulating agents, such as the agonist CGP44532 and the positive allosteric modulator, GS39783, were shown to be effective in those tests. Additionally, the compounds dose-dependently suppressed the DOI-induced increased frequency of spontaneous EPSCs in slices of frontal cortex.
A number of preclinical and clinical studies indicate that psychosis may be connected with disturbances in GABAergic neurotransmission (Guidotti et al., 2000; 2005; Fatemi et al., 2005; Lewis and Hashimoto, 2007). Therefore, our experiments focused on this main inhibitory system in the brain, which controls the activity of other transmitters and thus modulates various aspects of neuronal activities, such as plasticity of circuits, synchronous intermittent firing of the pyramidal neuron population assemblies and information processing. These processes were shown to be strongly involved in the pathophysiology of schizophrenia (Lewis et al., 2005; Lewis and Hashimoto, 2007).
MK-801- and amphetamine-induced hyperlocomotion are widely used models for detecting the antipsychotic-like effects of drugs in rodents (Geyer and Ellenbroek, 2003) and we adapted our procedure from previous studies (Pałucha-Poniewiera et al., 2008). The administration schedule of psychotomimetics was matched in order to obtain similar behavioural effect (e.g. hyperactivity at the level of about 2000 ambulation scores). Habituation of animals before giving MK-801 prevented the higher level of ambulation scores induced by the drug when given to animals not habituated to locomotor activity cages.
Administration of the orthosteric receptor agonist CGP44532 inhibited spontaneous locomotor activity, in a time-dependent manner, such that no reduction was observed 120 min after administration. Therefore, the same schedule was used in the amphetamine hyperactivity studies, in order to avoid any non-specific result, due to a sedative action of the drug. In parallel, the positive allosteric modulator GS39783 had no effect on spontaneous locomotor activity.
Administration of either compound inhibited hyperlocomotion induced by MK-801 and by amphetamine in a dose-dependent manner, and these inhibitory effects were antagonized by both the GABAB receptor antagonists, CGP36742 and CGP51176, which had no effect by themselves. Similar results had been observed earlier for baclofen, a well-known GABAB agonist (Arai et al., 2009). Although the mechanism of action of both these psychoactive compounds differ at the neurochemical and behavioural levels, it is commonly accepted that hyperactivity is a result of an increased dopamine release in the striatal and mesolimbic system, particularly nucleus accumbens (Segal et al., 1980; Svensson et al., 1991; Bristow et al., 1993; Adams and Moghaddam, 1998). The involvement of the dopaminergic system is supported by the fact that both typical and atypical neuroleptics, acting generally as dopamine receptor blockers, attenuate the hyperactivity induced by these psychotomimetics. Therefore, the most probable explanation of the apparent antagonism of the MK-801- and amphetamine-induced hyperlocomotion is that GS39783 and CGP44532 block dopamine release in structures responsible for that effect. In favour of this possibility are studies showing that baclofen and SKF97541 (selective GABAB receptor agonists) decreased the levels of extracellular dopamine and its metabolite 3,4-dihydroxyphenylacetic acid in the striatum (Santiago et al., 1993; Zhou et al., 2004; Sershen et al., 2008; Balla et al., 2009). Moreover, the amphetamine-induced dopamine release was significantly reduced by the drugs and the effect was reversed by the GABAB receptor antagonist, CGP52432 (Balla et al., 2009).
To further investigate the hypothesis of antipsychotic activity of the compounds, we utilize a rodent model of delusions and hallucinations, which are typical positive syndromes of schizophrenia in humans. The hallucinogenic effect can be achieved in healthy volunteers via activation of the 5-hydroxytryptaminergic system by lysergic acid diethylamide (Jacobs and Trulson, 1979). In rodents, an injection of DOI (a 5-HT2A receptor agonist) induces so-called head twitches, which are thought to reflect human hallucinations, and are reversed by both typical and atypical neuroleptics. We found that both GS39783 and CGP44532 attenuated this DOI-induced effect in a monophasic manner, similarly to recently described group III metabotropic receptor agonists (Pałucha-Poniewiera et al., 2008). This effect of the compounds was inhibited by the GABAB receptor antagonists CGP51176 and CGP36742, confirming the specificity of the effect. Neither CGP51176 nor CGP36742 had any effect by themselves. The behavioural result was further confirmed in the electrophysiological studies, in which the DOI-induced increase of spontaneous EPSCs in layer V pyramidal neurons of cortical slices was attenuated by both CGP44532 and GS39783.
Considering the role of GABAB receptor ligands as antipsychotic-like compounds it is important to note that GABA, acting as an inhibitory agent, controls the activity of the other neurotransmitter systems, restoring the physiological excitatory/inhibitory balance in the brain. Therefore, the action of GABA-mimetics may contribute to down-regulation of dopaminergic/glutamatergic overactivity through GABA receptors expressed pre- and postsynaptically (Vigot et al., 2006). Some possible mechanisms by which GABAB ligands could exert their function are outlined below.
Inhibition of dopamine release: Increased dopamine release induced by amphetamine and/or MK-801, is, to a great extent, responsible for hyperactivity observed (see above). The inhibition of this effect may be mediated directly through activation of GABAB receptors located on dopaminergic nerve terminals (Misgeld et al., 1995; Smolders et al., 1995; Wirtshafter and Sheppard, 2001). Another related possibility involves the voltage-dependent regulation of presynaptic dopamine transporters (Hoffman et al., 1999) via potentiation of GABAB (but not GABAA) presynaptic neurotransmission (Javitt et al., 2005).
Inhibition of glutamatergic neurons: GABAB heteroreceptors located on glutamatergic terminals at excitatory synapses (Isaacson et al., 1993; Dittman and Regehr, 1996; Tabata and Kano, 2006) may contribute to normalization of abnormal GABA-glutamate feedback observed in schizophrenia.
Postsynaptic effects of GABAB receptor stimulation: Postsynaptic receptors, expressed near dendritic spines of pyramidal neurons (Kulik et al., 2003), activate inwardly rectifying K channels mediating slow inhibitory postsynaptic potentials (Dutar and Nicoll, 1988). Therefore, their activation would result in decreasing abnormal glutamatergic function.
Inhibition of DOI-induced head twitches: Both GS39783 and CGP44532 suppressed frequency, but not the amplitude of the spontaneous EPSCs induced by DOI. Changes in the frequency are attributed to a presynaptic site, while changes in the amplitude are attributed to post- and presynaptic localization (Van der Kloot, 1991). Therefore, the effect of both drugs on glutamatergic neurotransmission can be considered as primarily presynaptic. As the effect of DOI occurs via induction of glutamate release caused by activation of 5-HT2A receptors placed postsynaptically on pyramidal neurons (Aghajanian and Marek, 2000); therefore, stimulation of presynaptic GABAB receptors expressed on the presynaptic site of the same neuron, may counteract the effect of DOI.
The possibilities described above show that GABAB receptors play a fundamental role in modulating the excitability of neurons and circuits through the brain, which contribute to the reduction by GS39783 and CGP44532 effects induced by amphetamine and MK-801. Functional interactions between dopaminergic, GABAergic and pyramidal neurons regulating the levels of neurotransmitters involved in the pathophysiology of schizophrenia (dopamine or glutamate) have been suggested (Benes et al., 1993) and the involvement of GABAB receptors in this process can be proposed on the basis of this study.
The findings described above concern the positive symptoms of schizophrenia. Both the GABAB receptor agonist and the positive allosteric modulator displayed beneficial antipsychotic effects, without the typical (for most antipsychotics) side-effect of inducing extrapyramidal symptoms, as shown in our results with the catalepsy model. Moreover, combining GS39783 or CGP44532 with haloperidol reversed haloperidol-induced catalepsy, indicating that the compounds may be at least considered as adjunctive therapy with other antipsychotic agents. The effect of CGP44532 was U-shaped, in contrast to the dose-dependent inhibition of haloperidol-induced catalepsy observed after GS39783. A similar relationship was observed in the case of reversal of amphetamine-induced hyperlocomotion. This difference may result from the difference in the mechanism of action of allosteric modulators and orthosteric ligands at the GABAB receptor. The agonist CGP44532 binds to the Venus flytrap module on the N-terminal extracellular domain of the receptor, as does GABA, whereas the positive allosteric modulator GS39783 binds at the sixth transmembrane domain (Froestl, 2010). This compound has no intrinsic agonistic activity of its own, but induces conformational changes in the receptor protein, which alters its interaction with the endogenous neurotransmitter (Conn et al., 2009), acting therefore in a more physiological way. Our results may indicate that positive allosteric modulators exhibit a better antipsychotic-like profile than that of orthosteric ligands.
Although we did not perform any experiments concerning negative or cognitive disturbances, the available data indicate that such activity may also result from activity of GABAB agonists. As the cortical dysfunction in schizophrenia is at least partially due to altered GABAergic neurotransmission in that structure, the role of GABAB receptors was shown to be important in synchronizing network oscillations and persistent activity, fundamental for cognitive processes (Lewis and Hashimoto, 2007; Gonzalez-Burgos et al., 2010; Kohl and Paulsen, 2010), and the effect of baclofen was shown to be positive in methamphetamine-induced cognitive deficits in mice (Arai et al., 2008). Moreover, they were shown to be efficient anxiolytic and anti-addictive agents (Slattery et al., 2005; Mombereau et al., 2007; Jacobson and Cryan, 2008).
In summary, we would propose that GABA-mimetics acting on mediating slow inhibitory current GABAB receptors, may be considered as potential antipsychotic-like agents. At the same time, these ligands also induce anxiolytic-like effects, while receptor blockade results in antidepressant-like, but not antipsychotic-like activities (Cryan et al., 2004; Nowak et al., 2006; present study), thus showing clear differences in the pathology and possible therapy of the diseases. Such a difference has also been observed for ligands of the metabotropic glutamate receptors, suggesting a common final pathway of balancing the level of both amino acids in the brain.
Acknowledgments
The study was supported by the Statutory Funds of the Institute of Pharmacology, Polish Academy of Sciences, Kraków, Poland.
Glossary
Abbreviations
- ACSF
artificial cerebrospinal fluid
- DOI
(±)1-(2.5-dimethoxy-4-iodophenyl)-2-aminopropane
- EPSCs
excitatory postsynaptic currents
- MK-801
(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate
- NMDA
N-methyl-D-aspartic acid
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
Teaching Materials; Figs 1–11 as PowerPoint slide.
References
- Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Brain Res Rev. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC). 4th Edition. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andiné P, Widermark N, Axelsson R, Nyberg G, Olofsson U, Mårtensson E, et al. Characterization of MK-801-induced behavior as a putative rat model of psychosis. J Pharmacol Exp Ther. 1999;290:1393–1408. [PubMed] [Google Scholar]
- Arai S, Takuma K, Mizoguchi H, Ibi D, Nagai T, Takahashi K, et al. Involvement of pallidotegmental neurons in methamphetamine- and MK-801-induced impairment of prepulse inhibition of the acoustic startle reflex in mice: reversal by GABAB receptor agonist baclofen. Neuropsychopharmacology. 2008;33:3164–3175. doi: 10.1038/npp.2008.41. [DOI] [PubMed] [Google Scholar]
- Arai S, Takuma K, Mizoguchi H, Ibi D, Nagai T, Kamei H, et al. GABAB receptor agonist baclofen improves methamphetamine-induced cognitive deficit in mice. Eur J Pharmacol. 2009;602:101–104. doi: 10.1016/j.ejphar.2008.10.065. [DOI] [PubMed] [Google Scholar]
- Balla A, Nattini ME, Sershen H, Lajtha A, Dunlop DS, Javitt DC. GABAB/NMDA receptor interaction in the regulation of extracellular dopamine levels in rodent prefrontal cortex and striatum. Neuropharmacology. 2009;56:915–921. doi: 10.1016/j.neuropharm.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Molloy R. Dopamine-immunoreactive axon varicosities form nonrandom contacts with GABA-immunoreactive neurons of rat medial prefrontal cortex. Synapse. 1993;15:285–295. doi: 10.1002/syn.890150405. [DOI] [PubMed] [Google Scholar]
- Bristow LJ, Hutson PH, Thorn L, Tricklebank MD. The glycine/NMDA receptor antagonist, R-(+)-HA-966, blocks activation of the mesolimbic dopaminergic system induced by phencyclidine and dizocilpine (MK-801) in rodents. Br J Pharmacol. 1993;108:1156–1163. doi: 10.1111/j.1476-5381.1993.tb13520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Carlsson ML. A dopaminergic deficit hypothesis of schizophrenia: the path to discovery. Dialogues Clin Neurosci. 2006;8:137–142. doi: 10.31887/DCNS.2006.8.1/acarlsson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Lindqvist M. Effect of chlorpromazine or haloperidol on formation of 3methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Toxicol (Copenh) 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B, Naylor RJ, Nohria V. Hyperactivity response to apomorphine and amphetamine in the mouse: the importance of the nucleus accumbens and caudate-putamen. J Pharm Pharmacol. 1979;31:259–261. doi: 10.1111/j.2042-7158.1979.tb13494.x. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, et al. Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N'-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther. 2004;310:952–963. doi: 10.1124/jpet.104.066753. [DOI] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16:1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P, Nicoll RA. A physiological role for GABAB receptors in the central nervous system. Nature. 1988;332:156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Earle JA, Araghi-Niknam M, Eagan E. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr Res. 2005;72:109–122. doi: 10.1016/j.schres.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Fejgin K, Palsson E, Wass C, Finnerty N, Lowry J, Klamer D. Prefrontal GABAB receptor activation attenuates phencyclidine-induced impairments of prepulse inhibition: involvement of nitric oxide. Neuropsychopharmacology. 2009;34:1673–1684. doi: 10.1038/npp.2008.225. [DOI] [PubMed] [Google Scholar]
- Frankowska M, Filip M, Przegaliński E. Effects of GABAB receptor ligands in animal tests of depression and anxiety. Pharmacol Rep. 2007;59:645–655. [PubMed] [Google Scholar]
- Froestl W. Chemistry and pharmacology of GABAB receptor ligands. Adv Pharmacol. 2010;38:20–44. doi: 10.1016/S1054-3589(10)58002-5. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology. 2000;23:569–576. doi: 10.1016/S0893-133X(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier B, Mesli A, Cales J, Castel JP, Maurette P. Severe hyperthermia caused by sudden withdrawal of continuous intrathecal administration of baclofen. Ann Fr Anesth Reanim. 1996;15:659–662. doi: 10.1016/0750-7658(96)82130-7. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Giorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, et al. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology. 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM. Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch Gen Psychiatry. 2002;59:521–529. doi: 10.1001/archpsyc.59.6.521. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Zahniser NR, Lupica CR, Gerhardt GA. Voltage-dependency of the dopamine transporter in the rat substantia nigra. Neurosci Lett. 1999;260:105–108. doi: 10.1016/s0304-3940(98)00951-3. [DOI] [PubMed] [Google Scholar]
- Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, et al. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Solís JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Mizukami K, Iwakiri M, Asada T. Immunohistochemical and immunoblot analysis of gamma-aminobutyric acid B receptor in the prefrontal cortex of subjects with schizophrenia and bipolar disorder. Neurosci Lett. 2005;383:272–277. doi: 10.1016/j.neulet.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Trulson ME. Mechanisms of action of LSD. Am Sci. 1979;67:396–404. [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Evaluation of the anxiolytic-like profile of the GABAB receptor positive modulator CGP7930 in rodents. Neuropharmacology. 2008;54:854–862. doi: 10.1016/j.neuropharm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Negative schizophrenic symptomatology and the PCP (phencyclidine) model of schizophrenia. Hillside J Clin Psychiatry. 1987;9:12–35. [PubMed] [Google Scholar]
- Javitt DC, Hashim A, Sershen H. Modulation of striatal dopamine release by glycine transport inhibitors. Neuropsychopharmacology. 2005;30:649–656. doi: 10.1038/sj.npp.1300589. [DOI] [PubMed] [Google Scholar]
- van Kammen DP. gamma-Aminobutyric acid (Gaba) and the dopamine hypothesis of schizophrenia. Am J Psychiatry. 1977;134:138–143. doi: 10.1176/ajp.134.2.138. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis–linking biology, pharmacology and phenomenology of psychosis. Schizophr Res. 2005;79:59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Kłodzinska A, Bijak M, Tokarski K, Pilc A. Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol Biochem Behav. 2002;73:327–332. doi: 10.1016/s0091-3057(02)00845-6. [DOI] [PubMed] [Google Scholar]
- Kohl MM, Paulsen O. The roles of GABAB receptors in cortical network activity. Adv Pharmacol. 2010;58:205–229. doi: 10.1016/S1054-3589(10)58009-8. [DOI] [PubMed] [Google Scholar]
- Kulik A, Vida I, Luján R, Haas CA, López-Bendito G, Shigemoto R, et al. Subcellular localization of metabotropic GABA(B) receptor subunits GABA(B1a/b) and GABA(B2) in the rat hippocampus. J Neurosci. 2003;23:11026–11035. doi: 10.1523/JNEUROSCI.23-35-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite JV, Guimarães FS, Moreira FA. Aripiprazole, an atypical antipsychotic, prevents the motor hyperactivity induced by psychotomimetics and psychostimulants in mice. Eur J Pharmacol. 2008;578:222–227. doi: 10.1016/j.ejphar.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Leo RJ, Baer D. Delirium associated with baclofen withdrawal: a review of common presentations and management strategies. Psychosomatics. 2005;46:503–507. doi: 10.1176/appi.psy.46.6.503. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T. Deciphering the disease process of schizophrenia: the contribution of cortical GABA neurons. Int Rev Neurobiol. 2007;78:109–131. doi: 10.1016/S0074-7742(06)78004-7. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. Review. [DOI] [PubMed] [Google Scholar]
- Lhuillier L, Mombereau C, Cryan JF, Kaupmann K. GABA(B) receptor-positive modulation decreases selective molecular and behavioral effects of cocaine. Neuropsychopharmacology. 2007;32:388–398. doi: 10.1038/sj.npp.1301102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog Neurobiol. 1995;46:423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Lhuillier L, Kaupmann K, Cryan JF. GABAB receptor-positive modulation-induced blockade of the rewarding properties of nicotine is associated with a reduction in nucleus accumbens DeltaFosB accumulation. J Pharmacol Exp Ther. 2007;321:172–177. doi: 10.1124/jpet.106.116228. [DOI] [PubMed] [Google Scholar]
- Nowak G, Partyka A, Pałucha A, Szewczyk B, Wieronska JM, Dybala M, et al. Antidepressant-like activity of CGP36742 and CGP51176, selective GABAB receptor antagonists, in rodents. Br J Pharmacol. 2006;149:581–590. doi: 10.1038/sj.bjp.0706845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pałucha-Poniewiera A, Klodzinska A, Stachowicz K, Tokarski K, Hess G, Schann S, et al. Peripheral administration of group III mGlu receptor agonist ACPT-I exerts potential antipsychotic effects in rodents. Neuropharmacology. 2008;55:517–524. doi: 10.1016/j.neuropharm.2008.06.033. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. The GABAB receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology. 2004;172:179–186. doi: 10.1007/s00213-003-1637-1. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. Repeated administration of the GABAB receptor agonist CGP44532 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine-seeking in rats. Neuropsychopharmacology. 2005;30:119–128. doi: 10.1038/sj.npp.1300524. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Vlachou S, Guery S, Kaupmann K, Froestl W, Markou A. Positive modulation of GABA(B) receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats. J Pharmacol Exp Ther. 2008;326:306–314. doi: 10.1124/jpet.108.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E. Prospects for research on schizophrenia. An hypotheses suggesting that there is a defect in the GABA system in schizophrenia. Neurosci Res Program Bull. 1972;10:468–482. [PubMed] [Google Scholar]
- Rorick-Kehn LM, Johnson BG, Knitowski KM, Salhoff CR, Witkin JM, Perry KW, et al. In vivo pharmacological characterization of the structurally novel, potent, selective mGlu2/3 receptor agonist LY404039 in animal models of psychiatric disorders. Psychopharmacology. 2007;193:121–136. doi: 10.1007/s00213-007-0758-3. [DOI] [PubMed] [Google Scholar]
- Santiago M, Machado A, Cano J. Regulation of the prefrontal cortical dopamine release by GABAA and GABAB receptor agonist and antagonist. Brain Res. 1993;630:28–31. doi: 10.1016/0006-8993(93)90638-4. [DOI] [PubMed] [Google Scholar]
- Segal DS, Weinberger SB, Cahill J, McCunney SJ. Multiple daily amphetamine administration: behavioral and neurochemical alterations. Science. 1980;207:904–907. doi: 10.1126/science.207.4433.904. [DOI] [PubMed] [Google Scholar]
- Sershen H, Balla A, Aspromonte JM, Xie S, Cooper TB, Javitt DC. Characterization of interactions between phencyclidine and amphetamine in rodent prefrontal cortex and striatum: implications in NMDA/glycine-site mediated dopaminergic dysregulation and dopamine transporter function. Neurochem Int. 2008;52:119–129. doi: 10.1016/j.neuint.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, McCarthy SA, Martin AN. Antipsychotic profile of rolipram: efficacy in rats and reduced sensitivity in mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology. 2007;192:415–424. doi: 10.1007/s00213-007-0727-x. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Desrayaud S, Cryan JF. GABAB receptor antagonist-mediated antidepressant-like behavior is serotonin-dependent. J Pharmacol Exp Ther. 2005;312:290–296. doi: 10.1124/jpet.104.073536. [DOI] [PubMed] [Google Scholar]
- Smolders I, De Klippel N, Sarre S, Ebinger G, Michotte Y. Tonic GABA-ergic modulation of striatal dopamine release studied by in vivo microdialysis in the freely moving rat. Eur J Pharmacol. 1995;284:83–91. doi: 10.1016/0014-2999(95)00369-v. [DOI] [PubMed] [Google Scholar]
- Svensson A, Pileblad E, Carlsson M. A comparison between the non-competitive NMDA antagonist dizocilpine (MK-801) and the competitive NMDA antagonist D-CPPene with regard to dopamine turnover and locomotor-stimulatory properties in mice. J Neural Transm Gen Sect. 1991;85:117–129. doi: 10.1007/BF01244704. [DOI] [PubMed] [Google Scholar]
- Tabata T, Kano M. GABAB receptor-mediated modulation of glutamate signaling in cerebellar Purkinje cells. Cerebellum. 2006;5:127–133. doi: 10.1080/14734220600788911. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. The regulation of quantal size. Prog Neurobiol. 1991;36:93–130. doi: 10.1016/0301-0082(91)90019-w. [DOI] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, et al. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Sheppard AC. Localization of GABA(B) receptors in midbrain monoamine containing neurons in the rat. Brain Res Bull. 2001;56:1–5. doi: 10.1016/s0361-9230(01)00487-7. [DOI] [PubMed] [Google Scholar]
- Zhou W, Mailloux AW, Jung BJ, Edmunds HS, McGinty JF. GABAB receptor stimulation decreases amphetamine-induced behavior and neuropeptide gene expression in the striatum. Brain Res. 2004;1004:18–28. doi: 10.1016/j.brainres.2003.11.077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


