Abstract
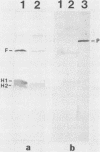
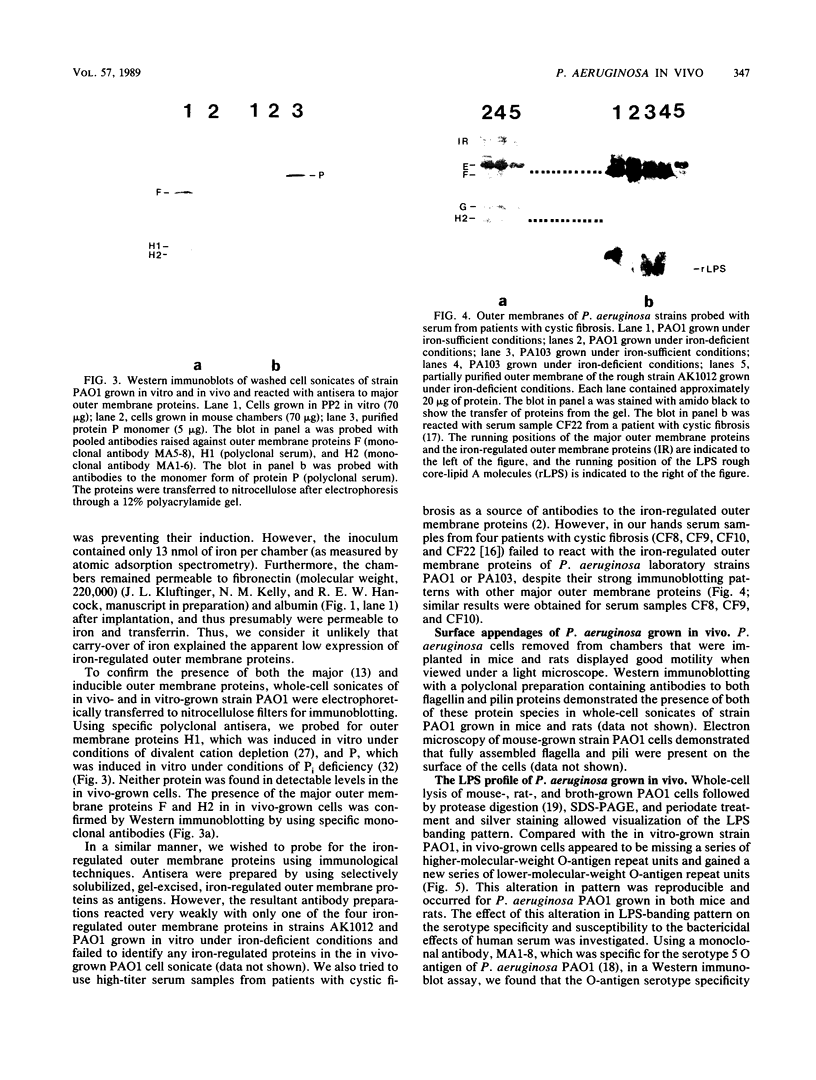
Pseudomonas aeruginosa PAO1 was grown in vivo in chambers implanted into the peritoneums of mice and rats. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of extracts of bacterial cells taken from the chambers and washed to remove loosely bound host proteins revealed the presence of the major outer membrane proteins D2, E, F, G, and H2. Western immunoblotting with specific antisera confirmed the presence of porin protein F and lipoprotein H2. However, there was no apparent induction of the phosphate starvation-inducible porin P or the divalent cation starvation-inducible protein H1. Small amounts of proteins with molecular weights similar to those of the iron-regulated outer membrane proteins were found in cells grown in vivo; however, their presence could not be confirmed immunologically. The presence of pili and flagella on the cells grown in vivo was demonstrated by electron microscopy and Western immunoblotting. A consistent alteration in the lipopolysaccharide banding pattern was observed after growth in vivo. Compared with cells of strain PAO1 grown in vitro, cells grown in vivo appeared to lack a series of high-molecular-weight O-antigen-containing lipopolysaccharide bands and gained a new series of lower-molecular-weight lipopolysaccharide bands. This alteration in the lipopolysaccharide after growth in vivo did not affect the O-antigen serotype or the resistance of the bacteria to serum.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus B. L., Fyfe J. A., Hancock R. E. Mapping and characterization of two mutations to antibiotic supersusceptibility in Pseudomonas aeruginosa. J Gen Microbiol. 1987 Oct;133(10):2905–2914. doi: 10.1099/00221287-133-10-2905. [DOI] [PubMed] [Google Scholar]
- Berk R. S., Hazlett L. D., Beisel K. W. Genetic studies on resistant and susceptibility genes controlling the mouse cornea to infection with Pseudomonas aeruginosa. Antibiot Chemother (1971) 1987;39:83–91. doi: 10.1159/000414336. [DOI] [PubMed] [Google Scholar]
- Blackwood L. L., Stone R. M., Iglewski B. H., Pennington J. E. Evaluation of Pseudomonas aeruginosa exotoxin A and elastase as virulence factors in acute lung infection. Infect Immun. 1983 Jan;39(1):198–201. doi: 10.1128/iai.39.1.198-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Simple model for the study of Pseudomonas aeruginosa infections in leukopenic mice. Infect Immun. 1983 Mar;39(3):1067–1071. doi: 10.1128/iai.39.3.1067-1071.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Pitt T. L., Fürer E., Germanier R. Role of lipopolysaccharide in virulence of Pseudomonas aeruginosa. Infect Immun. 1984 May;44(2):508–513. doi: 10.1128/iai.44.2.508-513.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day S. E., Vasli K. K., Russell R. J., Arbuthnott J. P. A simple method for the study in vivo of bacterial growth and accompanying host response. J Infect. 1980 Mar;2(1):39–51. doi: 10.1016/s0163-4453(80)91773-9. [DOI] [PubMed] [Google Scholar]
- Engels W., Endert J., Kamps M. A., van Boven C. P. Role of lipopolysaccharide in opsonization and phagocytosis of Pseudomonas aeruginosa. Infect Immun. 1985 Jul;49(1):182–189. doi: 10.1128/iai.49.1.182-189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Hantke K., Braun V. Iron transport of Escherichia coli K-12: involvement of the colicin B receptor and of a citrate-inducible protein. J Bacteriol. 1976 Sep;127(3):1370–1375. doi: 10.1128/jb.127.3.1370-1375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Irvin R. T., Costerton J. W., Carey A. M. Pseudomonas aeruginosa outer membrane: peptidoglycan-associated proteins. J Bacteriol. 1981 Jan;145(1):628–631. doi: 10.1128/jb.145.1.628-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Mouat E. C., Speert D. P. Quantitation and identification of antibodies to outer-membrane proteins of Pseudomonas aeruginosa in sera of patients with cystic fibrosis. J Infect Dis. 1984 Feb;149(2):220–226. doi: 10.1093/infdis/149.2.220. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Wieczorek A. A., Mutharia L. M., Poole K. Monoclonal antibodies against Pseudomonas aeruginosa outer membrane antigens: isolation and characterization. Infect Immun. 1982 Jul;37(1):166–171. doi: 10.1128/iai.37.1.166-171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder I. A., Wheeler R., Montie T. C. Flagellar preparations from Pseudomonas aeruginosa: animal protection studies. Infect Immun. 1982 Jan;35(1):276–280. doi: 10.1128/iai.35.1.276-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly N. M., Battershill J. L., Kuo S., Arbuthnott J. P., Hancock R. E. Colonial dissociation and susceptibility to phagocytosis of Pseudomonas aeruginosa grown in a chamber implant model in mice. Infect Immun. 1987 Nov;55(11):2841–2843. doi: 10.1128/iai.55.11.2841-2843.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutharia L. M., Hancock R. E. Surface localization of Pseudomonas aeruginosa outer membrane porin protein F by using monoclonal antibodies. Infect Immun. 1983 Dec;42(3):1027–1033. doi: 10.1128/iai.42.3.1027-1033.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutharia L. M., Nicas T. I., Hancock R. E. Outer membrane proteins of Pseudomonas aeruginosa serotype strains. J Infect Dis. 1982 Dec;146(6):770–779. doi: 10.1093/infdis/146.6.770. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Outer membrane protein H1 of Pseudomonas aeruginosa: involvement in adaptive and mutational resistance to ethylenediaminetetraacetate, polymyxin B, and gentamicin. J Bacteriol. 1980 Aug;143(2):872–878. doi: 10.1128/jb.143.2.872-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr T. R., Jr, Poole K., Crockford G. W., Hancock R. E. Lipopolysaccharide-free Escherichia coli OmpF and Pseudomonas aeruginosa protein P porins are functionally active in lipid bilayer membranes. J Bacteriol. 1986 Feb;165(2):523–526. doi: 10.1128/jb.165.2.523-526.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K., Hancock R. E. Isolation of a Tn501 insertion mutant lacking porin protein P of Pseudomonas aeruginosa. Mol Gen Genet. 1986 Mar;202(3):403–409. doi: 10.1007/BF00333269. [DOI] [PubMed] [Google Scholar]
- Rivera M., Bryan L. E., Hancock R. E., McGroarty E. J. Heterogeneity of lipopolysaccharides from Pseudomonas aeruginosa: analysis of lipopolysaccharide chain length. J Bacteriol. 1988 Feb;170(2):512–521. doi: 10.1128/jb.170.2.512-521.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandermann H., Jr, Strominger J. L. Purification and properties of C 55 -isoprenoid alcohol phosphokinase from Staphylococcus aureus. J Biol Chem. 1972 Aug 25;247(16):5123–5131. [PubMed] [Google Scholar]
- Shand G. H., Anwar H., Kadurugamuwa J., Brown M. R., Silverman S. H., Melling J. In vivo evidence that bacteria in urinary tract infection grow under iron-restricted conditions. Infect Immun. 1985 Apr;48(1):35–39. doi: 10.1128/iai.48.1.35-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P., Dimmick J. E., Pier G. B., Saunders J. M., Hancock R. E., Kelly N. An immunohistological evaluation of Pseudomonas aeruginosa pulmonary infection in two patients with cystic fibrosis. Pediatr Res. 1987 Dec;22(6):743–747. doi: 10.1203/00006450-198712000-00026. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Eftekhar F., Puterman M. L. Nonopsonic phagocytosis of strains of Pseudomonas aeruginosa from cystic fibrosis patients. Infect Immun. 1984 Mar;43(3):1006–1011. doi: 10.1128/iai.43.3.1006-1011.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieritz D. D., Holder I. A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1975 Jun;131(6):688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Cryz S. J., Friedman R. L., Iglewski B. H. Contribution of toxin A and elastase to virulence of Pseudomonas aeruginosa in chronic lung infections of rats. Infect Immun. 1982 Jun;36(3):1223–1228. doi: 10.1128/iai.36.3.1223-1228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]