Abstract
Summary: Pneumococcal meningitis continues to be associated with high rates of mortality and long-term neurological sequelae. The most common route of infection starts by nasopharyngeal colonization by Streptococcus pneumoniae, which must avoid mucosal entrapment and evade the host immune system after local activation. During invasive disease, pneumococcal epithelial adhesion is followed by bloodstream invasion and activation of the complement and coagulation systems. The release of inflammatory mediators facilitates pneumococcal crossing of the blood-brain barrier into the brain, where the bacteria multiply freely and trigger activation of circulating antigen-presenting cells and resident microglial cells. The resulting massive inflammation leads to further neutrophil recruitment and inflammation, resulting in the well-known features of bacterial meningitis, including cerebrospinal fluid pleocytosis, cochlear damage, cerebral edema, hydrocephalus, and cerebrovascular complications. Experimental animal models continue to further our understanding of the pathophysiology of pneumococcal meningitis and provide the platform for the development of new adjuvant treatments and antimicrobial therapy. This review discusses the most recent views on the pathophysiology of pneumococcal meningitis, as well as potential targets for (adjunctive) therapy.
INTRODUCTION
Community-acquired bacterial meningitis continues to exact a heavy toll, even in developed countries, despite the implementation of childhood vaccination programs and effective antimicrobial agents (71, 497). The most common etiologic agents are Streptococcus pneumoniae and Neisseria meningitidis, with the first being responsible for two-thirds of cases in Europe and the United States (18, 70, 496). Today, despite advances in medical care, mortality from pneumococcal meningitis ranges from 16 to 37%, and neurological sequelae, including hearing loss, focal neurological deficits, and cognitive impairment, are estimated to occur in 30 to 52% of surviving patients (231, 496, 500, 526, 528).
During past decades, experimental animal models have shown that the outcome of bacterial meningitis is related to the severity of inflammation in the subarachnoid space and that the outcome can be improved by modulation of the inflammatory response, e.g., with dexamethasone (471). Many randomized clinical trials of dexamethasone in bacterial meningitis have been performed, but the results remain ambiguous (70, 115, 148, 324, 442, 494). An individual patient data meta-analysis of 5 large recent trials showed no effect of dexamethasone (499). However, a prospective cohort study showed a decrease in mortality from 30 to 20% in adults with pneumococcal meningitis after successful nationwide implementation of dexamethasone in The Netherlands (69). Nevertheless, new adjunctive therapies are needed to improve the prognosis of bacterial meningitis.
Previously, we reviewed the epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis (70). In the current review, we focus on current understandings of the pathophysiology and pathogenic mechanisms associated with pneumococcal meningitis. Finally, we discuss targets for future therapeutic strategies.
COLONIZATION
Mucosal Colonization
The human nasopharynx is the main reservoir for S. pneumoniae, where it usually leads to asymptomatic colonization. Carriage rates of S. pneumoniae are highest among young children (37%) and may rise to up to 58% in crowded situations such as day care centers (50). In adults, crowding may also lead to increased carriage rates, specifically in hospitals, long-term care facilities, shelters, and prisons, where carriage rates of up to 40% have been reported (199, 208), compared to 4% in the general adult population (410). The bacterium is transferred between people mainly by coughing and sneezing. During colonization, adherence, nutrition, and replication are the pneumococcus' main priorities. To reach these objectives, the pneumococcus is confronted with the host's natural barriers at the respiratory mucosa, the host's immune system, and other pathogens colonizing the same niche.
Natural Barrier Evasion
Two important natural barriers preventing pneumococci from binding to the respiratory mucosal surface are the respiratory mucus and lysozyme (98, 350, 449). The pneumococcus has evolved several strategies to overcome these barriers and reach the respiratory epithelial cell layer.
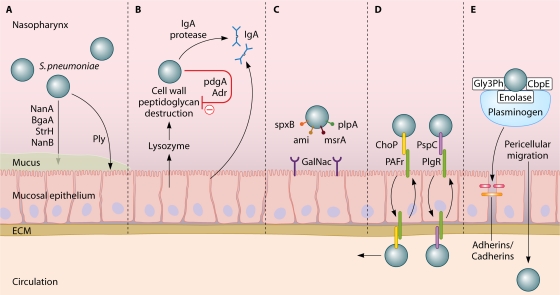
Mucus entrapment and subsequent clearing may be prevented by the pneumococcus by three ways. First, the capsule of the pneumococcus repulses the sialic acid residues of mucus by its negative charge, thereby decreasing the likelihood of entrapment (350). Second, the pneumococcus expresses several exoglycosidases, including neuraminidase A (NanA), beta-galactosidase A (BgaA), beta-N-acetylglucosaminidase (StrH), and neuraminidase B (NanB), which are capable of deglycosylating mucus glycoconjugates, thereby decreasing mucus viscosity and preventing mucus entrapment (79, 240, 480). Third, pneumolysin (Ply), a pore-forming toxin, decreases epithelial cell ciliary beating, thereby enabling the pneumococcus to bind to epithelial cells without being removed with the mucus (Fig. 1 A) (144, 145).
Fig. 1.
(A) Mucus breakdown. S. pneumoniae colonization of the nasopharynx is facilitated by mucus degradation by the enzymes NanA, BgaA, StrH, and NanB. Ply decreases epithelial cell ciliary beating, enhancing bacterial adherence. (B) Evasion of proteolytic enzymes. Pneumococcal cell wall peptidoglycans may be destroyed by lysozyme. PdgA and Adr deacetylate pneumococcal cell surface petidoglycan molecules, rendering them resistant to lysozyme. (C) Epithelial cell binding. S. pneumoniae binds host GalNac by using SpxB, Smi, MsrA, and PlpA. (D) Intracellular translocation. By binding the pIgR with PspC (or PAF receptor [PAFr] with ChoP), pneumococci can use the pIgR or PAF receptor recycling pathway to be transported through the epithelial cell layer. (E) Inter- and pericellular translocation. Plasminogen bound by Gly3Ph, CbpE, and enolase enhances epithelial cell binding and degrades interepithelial adherens junctions, allowing pericellular migration.
Lysozyme is a muramidase which cleaves peptidoglycan, a polymer of sugars and amino acids present in the cell wall of many pathogens, including S. pneumoniae (112). Acetylated peptidoglycan molecules of the pneumococcal cell wall (PCW) are specifically prone to lysozyme destruction. The pneumococcus expresses two enzymes, peptidoglycan N-acetylglucosamine-deacetylase A (PdgA) and an O-acetyltransferase (Adr), which are able to deacetylate peptidoglycan molecules on the pneumococcal surface, rendering the bacterium resistant to lysozyme (Fig. 1B) (101, 112, 515). Both enzymes have been shown to be important during colonization, as PdgA or Adr knockout pneumococci are more prone to exogenous lysozyme and are outcompeted by wild-type (WT) pneumococci in an intranasal model of pneumococcal colonization (112).
Host Mucosal Immune System
At the nasopharyngeal mucosal site, the pneumococcus is targeted by components of the host innate immune system, such as secretory IgA (sIgA), (212), lactoferrin (447), and components of the complement system (51, 390).
sIgA interferes with binding of the pneumococcus to the nasopharyngeal mucosa (223, 274) and facilitates opsonization of bacteria, which enables phagocytosis by antigen-presenting cells (APCs) and neutrophils (212). Pneumococci have several methods to limit opsonization by sIgA. First, the capsule itself prevents binding of sIgA (141). Second, capsule-bound IgA is cleaved by a pneumococcal IgA1 protease. This protease cleaves sIgA at the hinge region, inhibiting IgA-mediated opsonization and promoting binding to the respiratory mucosa (429, 523). The remaining Fab fragment of sIgA binds to the PCW, thereby exposing choline-binding proteins (Cbps) and decreasing the negative charge of the capsule, which also facilitates bacterial adhesion to the epithelial cell (Fig. 1B) (523).
Lactoferrin is an iron scavenger present in multiple human body fluids, including saliva and nasal secretions (405). Lactoferrin acts bacteriostatically by depleting iron necessary for bacterial metabolism. Unbound lactoferrin (apolactoferrin) also has direct bactericidal properties, independent of iron scavenging, toward various pathogens, including S. pneumoniae (20, 21, 447). The mechanism by which apolactoferrin destroys bacteria is not completely clear, but it appears to disrupt the bacterial cell, leading to cell lysis (446). Lactoferrin is also present in neutrophils and may enhance bacterial phagocytosis and killing (140). The peumococcus prevents apolactoferrin-mediated killing by the expression of pneumococcal surface protein A (PspA), a choline-binding protein expressed on the outer surface of the pneumococcal cell. PspA binds human apolactoferrin at its active site, thereby inhibiting apolactoferrin-mediated bacterial killing (447).
A third, important component of the mucosal innate immune system is the complement cascade. Activation of the complement pathway results in cleavage of several complement factors, leading to bacterial opsonization and phagocytosis, leukocyte recruitment, and the assembly of a membrane attack complex (MAC) which forms pores in the pathogen's membrane, inducing cell lysis (211). Complement plays an important role in the immune response against S. pneumoniae, since mice as well as humans with complement deficiencies are more susceptible to the transition of pneumococcal colonization to invasive disease (51, 390, 488).
C-reactive protein (CRP) serves as an important innate immune defense mechanism of the respiratory tract (174). CRP is a protein produced by the liver in the acute phase of an infection (211). CRP binds to phosphorylcholine on apoptotic cells (238) and several bacteria, including the pneumococcus (534). Through binding on the bacterial cell surface, CRP can activate the classical complement pathway through complement factor 1q (C1q) (465). Subsequent opsonophagocytosis by the complement system leads to more effective phagocytosis by macrophages. In addition, CRP can bind the Fcγ receptor (FcγR) on macrophages and dendritic cells, thereby enhancing phagocytosis (339, 475) and macrophage cytokine production (334).
The complement cascade is activated in three ways: the classical complement pathway, the alternative complement pathway, and the lectin-induced complement pathway. The classical complement pathway is characteristically activated by antibody-antigen complexes. Natural IgM, a part of which is directed against pneumococcal C polysaccharides (teichoic acid), contributes to the activation of the classical pathway (335). However, the classical pathway may also be activated through other mechanisms, such as by the binding of acute-phase proteins such as CRP to the pneumococcal surface and subsequent binding of complement component C1q, direct binding of C1q to the bacterium (211), and binding of C1q to the C-type lectin SIGN-R1 (224). When C1q was depleted from human serum, in vitro opsonophagocytosis of S. pneumoniae was severely affected (544). In addition, C1q-deficient mice showed a severely impaired immune response and worse outcomes in an experimental model of pneumococcal meningitis (428). Furthermore, mice deficient in the pattern recognition receptor SIGN-R1 had reduced activation of the classical complement pathway (224). In this study, C1q was directly activated upon activation of SIGN-R1 by pneumococcal polysaccharides in the spleen, leading to activation of the classical complement cascade and complement component C3 activation, with subsequent pneumococcal opsonization (224). SIGN-R1 is highly abundant on cells of the splenic red pulp and is an important factor in the spleen's function to control invasive pneumococcal disease. Another study showed that splenic macrophages of SIGN-R1 knockout mice were unable to activate splenic B cells to produce pneumococcus-specific IgM (259). Therefore, splenic SIGN-R1-mediated activation of B cells may explain, at least partially, the susceptibility of splenectomized patients to invasive pneumococcal disease.
Activation of C1q by the classical or mannose-binding lectin (MBL) pathway leads to cleavage of complement component C2. In a Swedish cohort, 40 patients with a homozygous C2 deficiency due to a deletion in the C2 gene were described (218). Invasive infections, mainly pneumococcal infections, were found in 23 (58%) of these patients (218).
The alternative pathway is also activated during infection with S. pneumoniae and occurs by the direct binding of complement component C3 to the pneumococcal surface (533). The importance of the alternative pathway in pneumococcal opsonization was shown in mice made deficient in factor D, a peptidase involved in activation of the alternative pathway (539). Opsonophagocytosis of S. pneumoniae was delayed in factor D-deficient mice compared to wild-type mice, indicating an important role for this complement pathway in the early phase of infection (539). In line with this, a recent study showed that mice deficient in complement factor B, another peptidase involved in activation of the alternative complement pathway, were more susceptible to pneumococcal otitis media (481).
The lectin-induced complement pathway appears to be less important in pneumococcal disease than the classical and alternative pathways. Polymorphisms in MBL, one of the most important activators of the lectin complement pathway, were not associated with increased risk of pneumococcal invasive disease in a genetic association study (331). A larger cohort showed a significant increase in risk for pneumococcal invasive disease, with three codon variants in the MBL locus (426). In a third study, 140 patients with invasive pneumococcal disease, defined by positive blood culture for S. pneumoniae, were assessed for three structural variant MBL alleles and one promoter allele (269). In this study, no association was found between susceptibility or outcome of invasive pneumococcal disease and any of the structural MBL variants or promoter alleles. In a subgroup analysis of the 22 patients in the cohort with pneumococcal invasive disease and meningitis, there was no association between susceptibility or outcome and the MBL genotype (269). However, a meta-analysis combining the results of the above three studies demonstrated an association between susceptibility to invasive pneumococcal disease and homozygosity for one of the three structural variants in the MBL gene, with an odds ratio (OR) of 2.57 (95% confidence interval [CI], 1.38 to 4.80) (68). In a cohort of 57 HIV-positive patients, an increased risk for invasive pneumococcal disease was found to be associated neither with MBL polymorphisms nor with polymorphisms in the downstream molecule MBL-associated serine protease 2 (MASP-2) (203). One genetic association study has been performed regarding outcome and MBL genotypes. This study included only 60 patients with community-acquired pneumococcal pneumonia and did not detect an association between MBL genotype and outcome (138). Experimental studies showed weak to no binding of MBL to S. pneumoniae compared to other bacteria (267, 352). Another experimental study showed that although MBL bound to S. pneumoniae, it did not increase opsonophagocytosis, and that complement activation by the classical pathway was much more important (73).
Another group of proteins that can activate the lectin-induced complement pathway are ficolins. Two ficolin variants, H-ficolin and L-ficolin, have been studied for the capability of binding to S. pneumoniae; only L-ficolin was found to bind some of the pneumococcal strains tested (267). However, no frequency differences were found for polymorphisms in L-ficolin among 290 patients with invasive pneumococcal disease compared to 720 controls from a similar population (89).
The pneumococcus has evolved several strategies to limit complement-mediated opsonophagocytosis. The pneumococcal capsule plays a central role by limiting the amount of complement deposited on the pneumococcal surface and impeding the access to cell-bound complement (205). Furthermore, pneumolysin has been shown to decrease complement opsonization of the pneumococcal cell (400). This is thought to result from the consumption of complement factors by released pneumolysin. In addition, several other pneumococcal outer surface proteins have been shown to affect complement deposition on the pneumococcus, including pneumococcal surface protein C (PspC), PspA, PsaA, and PhpA (111, 213, 232, 356, 399, 400, 411, 547).
PspC, also referred to as CbpA or SpsA, a choline-binding protein attached to the cell wall, is able to bind complement component C3b, thereby preventing opsonization (111, 213, 232, 399). Furthermore, PspC binds human factor H, a factor which inhibits activation of two complement components of the alternative and lectin pathways. By binding and activating factor H, the pneumococcus locally blocks the unfolding of these two complement pathways (110, 348, 399, 543). In addition, PspC binds the complement inhibitor C4b-binding protein, which blocks activation of the classical complement pathway (122). PspA has been shown to interfere with the binding of complement component C3 on the bacterial surface, thereby inhibiting complement-mediated opsonization (356, 400, 411). PhpA is a pneumococcal surface protein with C3-degrading properties (547). Since activation of the complement cascade is crucial in the defense against pneumococcal invasive disease, pneumococcal complement binding proteins are important targets for vaccine development (65, 109, 147, 336).
Binding to Epithelium
The pneumococcal capsule is advantageous in circumventing the host barriers and reaching the respiratory mucosa but covers PCW binding sites for epithelial cell binding. The pneumococcus adjusts its binding properties to its environment through a process called phase variation (106, 296, 522). In this process, the amount of polysaccharide in the capsule varies from an opaque (thick capsule) to a transparent (thin capsule) phase, either covering or exposing binding sites on the pneumococcal surface (522). During colonization, the thick capsule prevents mucus entrapment as well as immunoglobulin and complement binding, thereby preventing opsonophagocytosis (172, 206, 236, 350). Once the pneumococcus has reached the nasopharyngeal epithelium, the transparent phase becomes prominent, unveiling several adhesion molecules for binding to the host epithelium (106, 522).
At the host respiratory epithelium, the pneumococcus binds to glycoconjugates expressed on the epithelial cells of the respiratory mucosa (e.g., N-acetyl-d-galactosamine [GalNac]). Pneumococcal binding molecules interacting with the host glycoconjugates remain elusive. However, several bacterial genes involved in GalNac binding have been identified, including spxB, ami, msrA, and plpA (Fig. 1C) (104, 456, 538). Their gene products are involved either directly in binding of glycoconjugates or indirectly by inducing upregulation of their binding molecules on the epithelial lining (16, 105, 207, 268, 538). Binding of the pneumococcus to GalNac is promoted by NanA, a pneumococcal glycosidase that separates sialic acid from mucin, glycolipids, glycoproteins, and oligosaccharides, thereby enhancing the expression of N-acetylglucosamine binding sites on host epithelial cells (239, 480). Cleaved sialic acid residues serve as a carbohydrate source for bacterial metabolism (79, 240).
Pneumococcal binding is further enhanced by hydrophobic and electrostatic forces, binding of pneumococcal phosphorylcholine to the platelet activating factor (PAF) receptor, and binding of pneumococcal surface protein C (PspC) to the polymeric immunoglobulin (pIgR) receptor, all facilitating epithelial cell transcytosis (see Bloodstream Survival) (103, 137, 223). Pneumococci also display pili on their surfaces, facilitating adherence to human buccal cells in the nasopharynx; however, which components of the respiratory mucosa interact with the pili are unknown (28, 349, 454).
Cocolonization
The nasopharynx may be colonized by up to 700 different microbial species, including residential flora, transient colonizing microbes, and pathogenic species (1, 75). Microbial survival is therefore dependent on cooperative and competitive strategies, several of which were recently described in the context of pneumococcal infection (113, 372). Pneumococcal intermicrobial interactions include secondary invasive disease following viral infection, prior innate immunity activation following exposure to another pathogen, and the sharing of virulence/survival factors between pneumococcal serotypes (320).
Viral infection and subsequent bacterial infection have been investigated extensively (76, 320, 337). Prior exposure to influenza virus has been associated with secondary invasive pneumococcal disease (91, 159). The importance of preexposure to influenza virus was recently underlined during the H1N1 pandemic, in which a third of fatal H1N1 cases exhibited evidence of concurrent bacterial pneumonia (88). The underlying pathogenesis of enhanced susceptibility to invasive pneumococcal disease after influenza virus infection remains unclear but might be related to an altered expression of adhesion molecules. Prior exposure to viral infection has been demonstrated to increase the expression of epithelial cell adhesion molecules both in vitro and in vivo (25). The exposure of adhesion molecules on the epithelial lining is further aided by influenza virus neuraminidase (NA), which cleaves terminal sialic acid residues, thereby facilitating pneumococcal binding after viral exposure (321). In mice, pneumococcal binding was reduced when NA was blocked pharmacologically or when either the pneumococcus or influenza virus was mutated to be NA deficient (383). Of particular interest has been the PAF receptor, which may be used by pneumococci for adherence to and transcytosis of the epithelium. Though the PAF receptor is upregulated following viral exposure, murine PAF receptor knockout studies yielded conflicting results regarding the contribution of PAF receptor to pneumococcal adherence and subsequent invasion (322, 417, 507). These conflicting results might be explained by variations in pneumococcal serotype, dosing, and timing of coinfection. There are alternative explanations to PAF receptor upregulation for the association of viral and bacterial infections, including mechanical lung epithelium damage, overall impaired pulmonary function, and an altered immune response to secondary infection following viral exposure (320). Ex vivo studies in which the tracheal epithelium was severely damaged following viral infection did not show increased binding of S. pneumoniae but showed a decreased mucociliary velocity leading to a higher local bacterial burden after secondary infection (392).
Nasopharyngeal interactions between cocolonizing bacteria can lead to growth inhibition, synergism, and exchange of genetic material. Epidemiologic data suggested a negative association between nasopharyngeal colonization of Staphylococcus aureus and S. pneumoniae (52, 409). In vitro studies suggested that S. aureus killing was the result of pneumococcal H2O2 production, but this effect has not been reproduced invariably in vivo (316, 372). Bacteria may also compete or synergize in the nasopharynx by using the host response. Cocolonization of S. pneumoniae and Haemophilus influenzae led to rapid neutrophil-mediated clearance of S. pneumoniae (307). In vitro studies revealed that cell components of H. influenzae specifically stimulated the complement-dependent phagocytosis of S. pneumoniae; depletion of either complement or neutrophils abolished this competitive phenomenon (307).
Finally, multiple pneumococcal strains may cocolonize the nasopharynx, usually leading to intraspecies competition and competitive outgrowth of a single strain (305, 354). One proposed mechanism for this intraspecies competition involves the use of bacteriocins, so-called pneumocins in pneumococci, which are small peptides capable of killing bacteria of the same or closely related species (395). Additionally, S. pneumoniae is naturally able to integrate DNA from killed and closely related pathogens into its own genome, thus gaining a competitive advantage (305). In in vitro cocultures, pneumococci that were made bacteriocin deficient were rapidly outcompeted by parent strains or pneumococci of other serotypes (113).
INVASIVE DISEASE
Patients at Risk
Invasive pneumococcal disease may take place when two situations coincide: first, the host is colonized with a pneumococcal strain that it has not yet established immunity to, and second, an alteration of the natural barriers or host immune system has occurred (49, 312). Invasive pneumococcal disease is seen during the extremes of age (less than 2 or more than 50 years of age); in patients with underlying conditions, such as splenectomy or asplenic states, sickle cell disease, multiple myeloma, hypogammaglobulinemia, alcoholism, chronic liver or kidney disease, malignancy, malnutrition, Wiskott-Aldrich syndrome, thalassemia major, diabetes mellitus, and basilar skull fracture with leakage of cerebrospinal fluid (CSF); and in children with cochlear implants (3, 19, 42, 71, 161, 265, 329, 341, 364, 422, 497, 498, 524, 527). The use of immunosuppressive drugs, a history of splenectomy, or the presence of diabetes mellitus, alcoholism, or infection with HIV is found in 20% of adults with pneumococcal meningitis (364, 524). Furthermore, damage to the naso- and oropharyngeal mucosae may be elicited by local pneumococcal infection, such as sinusitis or otitis, by viral respiratory infections (specifically by influenza virus [see “Cocolonization”), by smoking, or by allergy (219, 355, 519, 528).
Invading Host Endothelial and Epithelial Cells
Pneumococci are relatively ineffective at invading host endothelial and epithelial cells. However, pressures of the host natural barriers, cocolonization of other micoorganisms, and an activated innate immune response drive pathogens to develop new strategies. Epithelial endo- and transcytosis is an important strategy of invasion and also allows intraepithelial bacterial reservoirs and subsequent recolonization of the nasopharynx. Two mechanisms of epithelial transmigration by S. pneumoniae have been described (Fig. 1D). First, pneumococcal phosphorylcholine (ChoP) may bind to the PAF receptor on activated epithelial and endothelial cells (103). ChoP is a component of cell wall-associated acids and lipoteichoic acids (LTAs) on the surfaces of transparent pneumococci (221). By binding the PAF receptor, the pneumococcus may enter the PAF receptor recycling pathway, which transports the bacterium to the basal membrane of the host epithelial cell, which may lead to invasive disease (103, 402). Intranasal challenge of mice deficient in the PAF receptor resulted in reduced rates of pneumococcal colonization, pneumonia, and invasive disease (417).
A second mechanism involves the binding of the pneumococcal choline-binding protein PspC (also known as CbpA or SpsA) to the extracellular portion of epithelial pIgR, refered to as “secretory component” (137, 223). Following attachment, the pneumococcus uses the pIgR recycling pathway, analogous to the PAF receptor pathway, to be transported between the apical and basal membranes of the epithelial cell (223, 546). Pneumococcal expression of PspC has been shown to be an important factor for colonization and invasive disease, although its effect on virulence may vary between pneumococcal strains (67, 190, 232, 424, 546). The PspC binding of pIg receptor is observed only in humans, not in mice, rats, or rabbits (223). In addition, PspC also binds sialic acid and lacto-N-neotetraose on respiratory epithelial cells, further facilitating colonization (424). The level of pIg receptor directly correlates with the degree of pneumococcal attachment and epithelial invasion (546). pIg receptors are expressed in a decreasing gradient from the upper to the lower respiratory tract, while the opposite pattern is observed for the PAF receptor (325, 546). Therefore, it has been suggested that where pIg receptor serves mainly as a pneumococcal receptor in the nasopharynx, the PAF receptor acts as a ligand for attachment and invasion of the pulmonary epithelium (546).
Inter- or pericellular migration is another mechanism by which bacteria may cross epithelial or endothelial cell layers (Fig. 1E) (371). Plasminogen, bound by the pneumococcal receptors enolase, Gly3Ph, and CbpE, plays a central role in this process and has been shown to serve two purposes (24, 35, 36). First, plasminogen increases adhesion of pneumococci to the epithelial surface (23). Second, bound plasmin is able to cleave proteins involved in the intercellular adherens junctions, which bind epithelial cells together to form a mechanical barrier to underlying tissues (23). This disruption is mediated by the degradation of cadherin, an essential component of interepithelial adherens junctions (23). Murine pneumococcal nasopharyngeal colonization studies demonstrated that epithelial barrier function was diminished through the downregulation of cadherins in a Toll-like receptor (TLR)-dependent manner (32). Third, epithelial permeability is also modulated by the innate immune system in a transforming growth factor beta (TGF-β)-dependent manner, possibly to allow for adequate migration of immune cells and inflammatory mediators into infected areas (31). Thus, the breakdown of the tight junctions, though necessary for an adequate immune response, may allow for pneumococcal access to the basal membrane and subsequent invasive disease.
Extracellular Matrix
At the basal side of the epithelium or endothelium lies the basement membrane, which is comprised mainly of a network of collagen type I, laminin, and proteoglygans (9). Like many bacteria, pneumococci use hyaluronan lyase to degrade major components of the extracellular matrix (ECM), hyaluronan, and certain chondroitins, thereby facilitating invasive disease (215). The importance of hyaluronan lyase for the development of invasive pneumococcal disease was demonstrated in mice, as intranasally administered hyaluronidase adjuvant enhanced the development of invasive disease after an otherwise noninvasive intranasal inoculation of pneumococci (552). Moreover, pneumococci isolated from patients with pneumococcal meningitis expressed higher levels of hyaluronidase than pneumococci isolated from asymptomatic carriers (263).
Fibronectin, a large multidomain ECM glycoprotein, is found in nearly every human tissue environment that the pneumococcus is likely to encounter and is bound by several pneumococcal adhesins, among which the most important are the pneumococcal adhesion and virulence A (PavA) and B (PavB) proteins (200, 216). In murine infection models, PavA-deficient pneumococci had impaired adherence to murine epithelium and endothelial cells and were unable to sustain long-term nasopharyngeal colonization (220, 394). Furthermore, although pneumococci lacking PavA showed similar growth to WT pneumococci in a sepsis model, PavA mutants were rapidly cleared from the central nervous system (CNS) after intracranial infections (220). Possibly, PavA not only serves to directly bind fibronectin but also plays a role in the effective adherence and virulence mediated by other, so far unknown determinants (394).
BLOODSTREAM SURVIVAL
Complement System
Once in the bloodstream, pneumococci are confronted with additional host defense mechanisms. Complement represents the first step of innate immunity against bacteremia. The classical complement pathway plays a dominant role in pneumococcal clearance, although the classical and alternative complement pathways are also activated by streptococcal species (214, 374). Pneumococci have developed two ways to minimize complement-mediated opsonization and phagocytosis. First, pneumococci undergo a second phase variation and become encapsulated. The polysaccharide capsule serves as a nonspecific barrier, significantly reducing complement deposition on the bacterial surface and limiting subsequent interaction with phagocytes (2, 221). In murine studies, systemically administered unencapsulated pneumococci were shown to be avirulent (395).
Second, pneumococcal surface proteins PspA, PspC, and pneumolysin target specific complement components, thereby reducing complement-mediated bacterial clearance. PspA, which is expressed ubiquitously among pneumococci, inhibits C1q and subsequent C3b deposition (214). PspC binds human factor H, thereby blocking the formation of C3 convertase (C3bBb), leading to lower C3b production and limiting opsonophagocytosis (292, 548). Pneumococci can also attach to erythrocytes through a process called immune adherence, which is dependent on the binding of complement components C3b, C4b, C1q, and MBL to both the pneumococcus and erythrocyte receptor CR1 (189, 292, 351). Immune complexes containing pneumococci, bound by complement to erythrocytes, are then transferred to macrophages, after which the erythrocytes are returned to the circulation (99). Recent in vitro studies showed that PspA and PspC work synergistically to limit complement-mediated adherence and transfer to phagocytes (292).
Pneumolysin, released during pneumococcal autolysis, readily binds the Fc portion of IgG, thereby potently activating the classical complement pathway, increasing bacterial virulence by independently depleting complement factors away from the bacterium, and limiting opsonophagocytosis (13). Murine bacteremia studies showed that pneumolysin-deficient pneumococci are either cleared from the bloodstream or allowed to develop into chronic bacteremia (359). Furthermore, serum complement depletion may be particularly important in circumstances of overall limited complement availability, such as liver cirrhosis (12), and may further increase pneumococcal virulence at sites of limited complement presence, such as the nasopharynx (318).
Lastly, the acute-phase CRP binds phosphorylcholine (Chop) on the PCW (4, 514) and subsequently interacts with C1q, leading to the activation of the classical complement pathway (93, 451). In mice, CRP is not an acute-phase protein, and treatment with human CRP reduced mortality following pneumococcal infection (467, 468). In vitro studies showed that CRP reduced pneumococcal binding to the epithelial cell PAF receptor (175).
Recognition by the Host Immune System
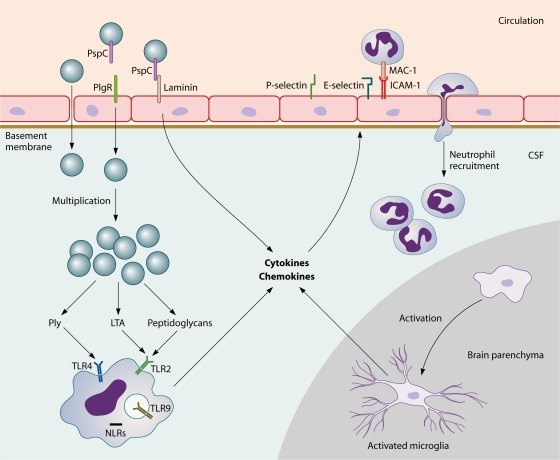
Pneumococci are recognized by APCs through the binding of pattern recognition receptors, which are specifically directed toward general motifs of molecules expressed by pathogens that are essential for pathogen survival. Pattern recognition receptors involved in sensing pneumococci include TLR2 (192, 286, 333, 441, 511, 541), TLR4 (59, 278, 311), TLR9 (10, 278, 333), and nucleotide oligomerization domain 1 (Nod1) (357, 549). Upon activation of these receptors, APCs release various cytokines, which induce a cascade of inflammatory reactions, including the recruitment of neutrophils (211). The most important cytokines released by phagocytic cells are tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-6 (419). IL-1β and TNF-α act on local vascular endothelial cells, increasing vascular permeability and vasodilatation and upregulating adhesion molecules such as E-selectin, P-selectin, and vascular cell adhesion molecule 1 (VCAM-1) to enable the influx of neutrophils and other lymphocytes from the blood to the site of infection (Fig. 2) (142, 470).
Fig. 2.
S. pneumoniae adheres to endothelial cells by using PspC, which binds laminin and pIgR, enabling transcytosis across the endothelium. Once in the CSF, pneumococci multiply freely and release bacterial products such as LTA and Ply, which are recognized by TLR2 and TLR4 on circulating APCs. The subsequent release of proinflammatory cytokines and chemokines from macrophages and microglial cells results in upregulation of endothelial cell P- and E-selectin and ICAM (which binds MAC-1 on leukocytes), leading to increased neutrophil recruitment into the CSF.
Initiation of Coagulation
Most patients with invasive pneumococcal disease show evidence of coagulation activation (288, 313). Inflammation-induced thrombin generation is not dependent on direct interaction of bacteria and the coagulation cascade but rather on the exposure of blood to tissue factor (TF) (290). TF is expressed primarily on cells outside the vasculature (128, 149) and is exposed to coagulation factors during vascular damage. Low levels of circulating TF have been detected in healthy individuals (167), in whom the role of TF in thrombin generation remains uncertain (81, 195, 407). The expression of TF in blood cells is limited to monocytes and can be elevated considerably during inflammation or sepsis (370). The upregulation of TF is largely IL-6 dependent, as studies have shown abrogation of TF-dependent thrombin generation when IL-6 is blocked (506).
Upon exposure to blood, TF forms a complex with factor VII and catalyzes the conversion of factor X into factor Xa. Factor Xa allows prothrombin conversion to thrombin, although this reaction occurs to a significant extent only after thrombin-induced feedback activation of factor VIII and factor V, nonenzymatic cofactors in the tenase and prothrombinase complexes, respectively (81, 290). The prothrombinase and tenase complexes convert prothrombin (factor II) into thrombin (factor IIa), which then leads to the conversion of fibrinogen to the clot-forming fibrin protein (289). The activity of prothrombinase and tenase complexes is markedly enhanced by the presence of activated platelets, which become activated during inflammation but may also be activated directly by thrombin itself (427).
Inflammation-mediated thrombin formation is regulated by three anticoagulant mechanisms: antithrombin (AT), the protein C system, and tissue factor pathway inhibitor (TFPI), all of which may be impaired during systemic infection (290). Antithrombin inhibits thrombin and factor Xa, though during severe infection antithrombin levels are markedly lower due to impaired synthesis, degradation, and consumption during thrombin generation (291). Circulating protein C, which upon conversion to activated protein C by the thrombin-thrombomodulin complex degrades the essential coagulation factors Va and VIIIa, is hampered during severe inflammation by enzymatic degradation by neutrophil-derived elastase and by impaired synthesis as well as decreased activation by depressed levels of thrombomodulin (135, 143). Lastly, the importance of TFPI has been demonstrated in studies in healthy human volunteers injected with endotoxin, in whom administration of TFPI induced a marked inhibition of coagulation (117). Animal studies showed that rabbits deficient in TFPI were more susceptible to severe disseminated intravascular coagulation (DIC), and primates infused with TFPI were able to survive exposure to otherwise lethal amounts of Escherichia coli (404).
The degradation of fibrin clots is mediated by plasmin, the active form of plasminogen, which is activated by tissue-type plasminogen activator (tPA) and urokinase-type plaminogen activator (uPA), both of which are stimulated by the inflammatory cytokines TNF-α and IL-1β (505). During severe infection, these cytokines subsequently induce plasminogen activator inhibitor type 1 (PAI-1), thereby limiting fibrinolysis and resulting in a net procoagulant state (505). Higher levels of PAI-1 in patients with meningococcal septicemia or disseminated intravascular coagulation have been shown to be associated with poor outcomes and mortality (326, 530).
At relatively high concentrations, thrombin forms a complex with thrombomodulin and activates thrombin-activatable fibrinolysis inhibitor (TAFI; also known as plasma carboxypeptidase B, carboxypeptidase U, and carboxypeptidase R) (48, 518). Activated TAFI inhibits fibrinolysis by limiting plasmin formation through the inhibition of plasminogen and tPA incorporation into fibrin clots (338). Furthermore, TAFI is able to inhibit several proinflammatory substrates, such as bradykinin and complement components C3 and C5a (84). The importance of TAFI and C5a was first demonstrated in a mouse model in which TAFI knockout mice showed a higher mortality when challenged with sublethal doses of lipopolysaccharide (LPS) and cobra venom factor (22).
CENTRAL NERVOUS SYSTEM INVASION
Intracellular Translocation across the Blood-Brain Barrier
Cerebral vascular endothelial cells show marked differences from their systemic counterparts. They exhibit very tight junctions, low rates of pinocytosis, and relatively large numbers of mitochondria (398). In human brain microvascular endothelial cell cultures, the pneumococcus was able to adhere to the vascular endothelial PAF receptor, allowing transmigration through the endothelial cell to the basolateral site (418). This mechanism of transcytosis is similar to that seen at the pulmonary epithelium (see Invasive Disease) and is mediated by binding of pneumococcal phosphorylcholine to the PAF receptor (103, 417). Pneumococci in the transparent phase are more efficient at invading the brain endothelial cell layer than opaque variants, which are dependent on the expression of phosphorylcholine (418). Concordantly, PAF receptor-deficient mice showed less translocation of pneumococci across the blood-brain barrier and, therefore, a decreased incidence of pneumococcal meningitis after intravenous challenge (402). Many of these studies have been performed with brain vascular endothelial cells. However, another important site of entry might be the choroid plexus epithelium, as shown for Streptococcus suis, which induces epithelial cell death and blood-brain barrier disruption in porcine choroid plexus epithelium (473) but may also translocate intracellularly across the plexus epithelium (474).
Nasopharyngeal colonization models demonstrated binding of pneumococcal PspC to pIgR on local epithelial cells, facilitating pneumococcal invasion (546). However, in a cell line of human brain microvascular endothelial cells, the pIgR was not expressed (546). In vitro and animal experiments showed that pneumococcal PspC may bind the laminin receptor on brain microvascular endothelial cells (360). This receptor, by which endothelial cells are bound to the major component of basement membranes, laminin, was also shown to be a ligand for neurotropic viruses and prions (6, 158, 360). Laminin appears to be involved in binding of bacteria that may cause meningitis, such as S. pneumoniae, N. meningitidis, and H. influenzae, to brain microvascular endothelial cells (360). Pneumococcal PspC binds to laminin, and in a mouse model of pneumococcal sepsis, a pneumococcal PspC mutant caused a decreased frequency of pneumococcal meningitis (360). These results indicate that the interaction between laminin and pneumococcal PspC plays a role in intracellular translocation of pneumococci across the blood-brain barrier.
Intercellular Translocation across the Blood-Brain Barrier
Pneumococci may translocate into the CSF intercellularly, by disruption of the interepithelial tight junctions. In an animal model of pneumococcal meningitis, tight junctions between brain microvascular endothelial cells became disrupted in the course of the disease (398). This may be due to damage caused by the pneumococcus or by factors of the host immune response (153, 448, 558). Analogous to the nasopharyngeal setting, pneumolysin was capable of disrupting an endothelial cell layer in an in vitro endothelial cell culture, which may enhance blood-brain barrier disruption in vivo (558).
After crossing the dense vascular endothelial cell lining, pneumococci have several methods of disrupting and invading the basement membrane. The first involves binding of plasminogen to the bacterial surface, which may subsequently be activated by tPA (129). In patients with bacterial meningitis, levels of uPA correlated with breakdown of the blood-brain barrier and pleocytosis (536). In vitro models showed that pneumococcus-mediated activation of plasminogen resulted in damage of extracellular matrix components and the basement membrane (129), although conversely, an in vivo mouse model failed to demonstrate an effect of tPA or uPA receptor on pneumococcal transmigration across the blood-brain barrier (379). Finally, pneumococci may bind fibronectin (502), vitronectin, and collagen in the extracellular matrix, which may enhance blood-brain barrier disruption (34, 262).
CENTRAL NERVOUS SYSTEM IMMUNE RESPONSE
Immune Activation
During multiplication, pneumococci concurrently undergo autolysis, which eventually leads to a stationary phase where multiplication and autolysis rates are similar (479). The released bacterial products are highly immunogenic and may lead to an increased inflammatory response in the host (489). Bactericidal antibiotics causing bacterial lysis may also induce a similar effect and lead to a temporarily increased host inflammatory response and increased disease severity (344, 345, 483).
A variety of pneumococcal compounds are proinflammatory. The pathophysiological aspects of the different compounds may be reproduced by intracisternal inoculation of heat-killed unencapsulated pneumococci, purified PCW, cell wall lipoteichoic acid, or cell wall peptidoglycan (490). Heat-killed encapsulated pneumococci or purified pneumococcal capsular polysaccharides inoculated intracisternally into rabbits did not cause meningitis, indicating that the pneumococcal capsule is not immunogenic in the CSF (490). Inoculation with knockout pneumococcal strains is another way to study the immunogenicity of pneumococcal compounds. In a murine model of pneumococcal meningitis, intracisternal inoculation with pneumolysin-deficient pneumococci resulted in lower bacterial loads, better clinical scores, and longer survival of the host (529). However, histological inflammatory changes in this study were similar to those induced by wild-type pneumococci (529).
Anatomical Localization of Blood-Brain Barrier Invasion by Leukocytes
Neutrophils are thought to cross the blood-brain barrier mainly at the venous side of the penetrating cerebral blood vessels (182). Here they migrate to the perivascular space, which is continuous with the subarachnoid space. However, some neutrophils penetrate the brain parenchyma. Neutrophilic infiltrates in the brain have been seen primarily in spaces adjacent to CSF, such as the corpus callosum, periventricular space, and the meninges (482). Neutrophils mediate bacterial killing by phagocytosis of opsonized bacteria (211). Phagocytosis is initiated by recognition and binding of bacteria by a neutrophil and is facilitated by opsonization of the bacteria by complement and antibody. Following binding, the neutrophil engulfs the bacteria, after which the cell membrane closes around the pathogens and is cut off, forming a free membrane-covered entity within the cell called an endosome (211). In the activated neutrophil, the endosome containing the pathogens is fused with a lysosome present in the cell, which contains several bactericidal mediators, including nitric and oxygen species, but also activated lysozymes, and the bacteria are killed. In addition to intracellular killing, neutrophils also secrete nitric and oxygen species, establishing a bactericidal milieu around the cell (211). Adversely, these nitric and oxygen species may damage the surrounding tissue when they are present in large amounts and may be responsible, at least in part, for the neuronal damage seen in pneumococcal meningitis. This topic is discussed further in Neuronal Damage and Histopathology.
Pattern Recognition Receptors
Immune activation in the cerebrospinal fluid is initiated by the recognition of different bacterial pathogen-associated molecular patterns (PAMPs) by APCs (Table 1) (7, 440). These APCs are present at low levels in the CSF, (116) or are situated in the meninges, choroid plexus, perivascular space, or brain parenchyma as astrocytes and microglial cells (92, 160). Major pattern recognition receptors involved in initial sensing of pneumococci in the CNS are TLR2 (133, 248), TLR4 (245), TLR9 (10), and Nod-like receptors (NLRs) (Fig. 3) (300).
Table 1.
Effects of pattern recognition receptor knockout or deficiency
| Model/settinga | Outcome | Reference(s) |
|---|---|---|
| TLR2 KO mice | Higher cerebellar and blood bacterial titers, increased disease severity, no difference in cytokine response | 248 |
| Significantly increased disease severity, higher CSF bacterial loads, and earlier death | 133 | |
| CD14 KO mice | Significantly increased disease severity, higher CSF bacterial loads, and earlier death | 133 |
| TLR2/CD14 double-KO mice | Significantly increased disease severity, higher CSF bacterial loads, and earlier death | 133 |
| TLR4 KO mice | No difference from WT mice | 245 |
| TLR2/TLR4 double-KO mice | Decreased inflammatory response and increased disease severity in TLR2 and TLR4 double mutants | 245 |
| TLR2/TLR4/TLR9 triple-KO mice | No differences in immune response, bacterial load, or survival compared with TLR2/TLR4 double-knockout mice | 245 |
| Nod2-deficient microglial and astroglial cell line | Reduced levels of TNF-α and IL-6 production | 300 |
| Nod2 KO mice | Decreased MIP-1α and TNF-α production and decreased cerebral demyelination and gliosis | 300 |
| SIGN-R1 on primary mouse and rat microglial cells | Involved in the uptake of pneumococcal capsular polysaccharides into the cell | 373 |
| Caspase-1 KO mice | Less severe inflammation and improved survival in a mouse model of pneumococcal meningitis | 258 |
| IRAK-4 deficiency in children | Increased susceptibility to invasive pneumococcal infections, including meningitis | 272 |
| MyD88 deficiency in children | Increased susceptibility to invasive pneumococcal infections, including meningitis | 516 |
| NEMO deficiency in patients | Increased susceptibility to invasive pneumococcal infections, including meningitis | 270, 517 |
| MyD88 KO mice | Increased mortality due to pneumococcal sepsis and meningitis, accompanied by decreased symptoms of infection and inflammatory parameters | 11, 256 |
KO, knockout.
Fig. 3.
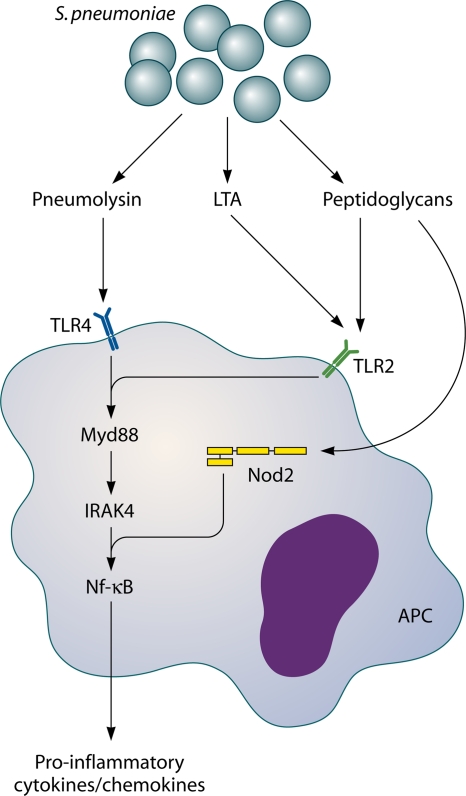
Host pattern recognition receptors involved in sensing S. pneumoniae. TLR2 is activated by pneumococcal cell wall peptidoglycan and LTA. Nod2 is activated by cell wall peptidoglycans and TLR4, which in turn is activated by Ply. TLR2 and -4 activate the transcription factor NF-κB via MyD88 and IRAK-4. Nod2 also activates NF-κB, inducing transcription of several proinflammatory cytokines.
TLR2 recognizes PCW LTA (441, 443). TLR2 signaling is enhanced by the TLR2 coreceptor, CD14, and by LPS binding protein (LBP) (441, 541). In a model of pneumococcal meningitis, TLR2-deficient mice showed increased disease severity with increased blood-brain barrier disruption and intracranial complications and increased bacterial loads (133, 248). Cytokine production was similar in TLR2-deficient and wild-type mice with pneumococcal meningitis, except for that of TNF-α, which was significantly higher in TLR2-deficient mice (133, 287). Since the phenotype of TLR2-deficient mice with pneumococcal meningitis was not as severe as that seen with mice lacking MyD88, an important general adaptor molecule for TLR signaling, it was proposed that other TLRs besides TLR2 may play a role in sensing pneumococci in the CNS (248, 256).
TLR4 recognizes pneumococcal pneumolysin (311). TLR4-deficient mice did not differ significantly from wild-type mice in their host immune response, cerebrovascular changes, or outcome during pneumococcal meningitis (245). However, in mice deficient in both TLR2 and TLR4, a marked reduction in inflammatory mediators, increased bacterial replication in the CNS, and reduced survival were seen compared to those for wild-type mice or mice with a single TLR deficiency (245). Thus, in meningitis, both TLR2 and TLR4 are important receptors in detecting the pneumococcus and initiating a robust inflammatory response to the pathogen, and one receptor may compensate for the absence of the other (245).
TLR9 is an intracellular pattern recognition receptor and is activated by CpG repeats in bacterial DNA (196). In vitro, S. pneumoniae was able to activate alveolar and peripheral macrophages through TLR9 and induced IL-8 production in TLR9-transfected human embryonic kidney cells (10, 333). In vivo, TLR9-deficient mice showed reduced resistance to S. pneumoniae after intranasal challenge (10). However, in a model of pneumococcal meningitis, triple mutant TLR2/TLR4/TLR9-deficient mice did not show significant differences in immune response, bacterial load, or survival compared with TLR2/TLR4-deficient mice (245). Therefore, TLR9 appears to play a minor role in pneumococcal meningitis, although this was assessed only in TLR triple-knockout mice.
NLRs are a second group of intracellular pattern recognition receptors involved in detecting pneumococci (357). NLRs belong to a family of receptors which, upon activation, induce activation of NF-κB or mitogen-activated protein kinase (MAPK) pathways and inflammatory caspases (357). In human embryonic kidney 293 cells, Nod2 was activated by internalized pneumococci through sensing of meso-diaminopimelic acid (meso-DAP) motifs of the bacterial peptidoglycan (151, 357). In vitro experiments showed that microglial and astroglial cells are activated by S. pneumoniae through Nod2 (300). Murine microglial and astroglial cells deficient in Nod2 showed reduced levels of TNF-α and IL-6 production (300). With in vivo experiments using a pneumococcal meningitis model, Nod2 activation of primary murine glial cells induced macrophage inflammatory protein 1α (MIP-1α) and TNF-α production and enhanced cerebral demyelination and gliosis (300). Thus, activation of Nod2 appears to be one of the contributing factors leading to cerebral damage in bacterial meningitis.
Another group of NLRs are the inflammasomes, which include a complex of various pattern recognition receptors sharing the caspase adaptor apoptosis-associated speck-like protein (ASC) and leading to caspase-1 activation when triggered (150). Cleavage and activation of caspase-1 lead to cleavage of different procytokines into their active forms, including IL-1β and IL-18 (8, 123, 408). In addition, inflammasome activation may lead to a specific form of controlled cell death, different from apoptosis, called pyroptosis (146). Inflammasomes are intracellular pattern recognition receptors and can be activated by several endogenous and exogenous ligands, including bacteria (486), bacterial DNA (237), bacterial toxins (187), endogenous reactive oxygen species (ROS) produced by macrophages in response to infection (431), and uric acid released through cell injury during inflammation (157). Little is known about the role of inflammasomes in bacterial meningitis. In patients suffering from bacterial meningitis, cerebrospinal fluid levels of caspase-1 were increased (258). In children with bacterial meningitis, as well as a rat model of pneumococcal meningitis, increased IL-1β levels were measured in the CSF (30, 86). Koedel et al. showed that mice lacking caspase-1 displayed less severe inflammation and improved survival in a pneumococcal meningitis mouse model (258). Similar results were found in a pneumococcal meningitis model with IL-18 knockout mice (554), indicating a role for inflammasome activation in the pathophysiology of pneumococcal meningitis.
A fourth group of pathogen recognition receptors involved in sensing S. pneumoniae are the C-type lectins, which are highly expressed on splenic dendritic cells and also on peritoneal macrophages (276). A member of this group, SIGN-R1, was shown to facilitate phagocytosis by recognition of the pneumococcal capsular polysaccharide (225, 276). Mice lacking functional SIGN-R1 fail to effectively phagocytose S. pneumoniae, leading to an inability to clear the infection and resulting in increased inflammatory parameters and reduced survival in both a model of pneumococcal peritoneal sepsis (276) and one of intranasally induced pneumonia (260). Furthermore, SIGN-R1 plays a role in the activation of the classical complement pathway by binding C1q (224). Park et al. showed the presence of SIGN-R1 on microglial cells in mouse and rat brains, which was functionally active in taking up pneumococcal capsular polysaccharides into the cell (373). Therefore, SIGN-R1 may be an important pathogen recognition receptor in the brain during pneumococcal meningitis.
Downstream Signaling Molecules
Upon stimulation of one of the above pattern recognition receptors, an intracellular cascade is activated and leads to the production of inflammatory molecules, usually cytokines or chemokines, which modulate the immune response by activating or attracting specialized immune cells. Deficiencies and polymorphisms in the pathogen recognition receptor downstream signaling cascade in humans have been associated with invasive pneumococcal disease, including meningitis.
The most extensively characterized TLR downstream signaling protein in pneumococcal invasive disease is IRAK-4 (Fig. 3) (391). This adaptor protein is one of the links in TLR- and IL-1 receptor (IL-1R)-induced activation of MyD88 and NF-κB, which ultimately results in cytokine production (420, 545). Specifically, children with IRAK-4 deficiency are susceptible to (recurrent) invasive pneumococcal infections, which are associated with high mortality (272). In a group of pediatric patients with normally expressed IRAK-4 but with recurrent invasive pneumococcal disease, deficiencies in the common adaptor molecule of TLR and IL-1R pathways, MyD88, were found (516). Deficiencies in IRAK-4 and MyD88 give indistinguishable phenotypes. Both patient groups are unresponsive to all TLR1, -2, -5, -6, -7, and -8 agonists (516), TLR9 agonists (323), and IL-1R agonists (271). In IRAK-4- or MyD88-deficient patients, the TLR3 signaling pathway is not affected, and the TLR4 pathway is affected only partially. Both TLR3 and -4 can still signal through the MyD88-independent TRIF pathway, leading to cytokine production (516). Stimulation of whole blood of IRAK-4- or MyD88-deficient patients with several different TLR agonists showed impaired production of IL-1β, IL-6, IL-8, IL-10, IL-12, monocyte chemoattractant protein 1 (MCP-1), MIP-1α, and MIP-1β (516). Stimulation with a TLR3 or TLR4 agonist showed impaired production of IL-6, IL-10, and IL-12, as well as that of IL-8 in the case of TLR3 stimulation and IL-1β in the case of TLR4 stimulation (516). Among patients with an IRAK-4 or MyD88 deficiency, 68% suffer from invasive pneumococcal disease, and S. pneumoniae is responsible for 53% of all episodes of infectious episodes in these patients (391). Invasive bacterial disease in these patients consists of meningitis in 41% of IRAK-4-deficient patients and 52% of MyD88-deficient patients (391). IRAK-4 and MyD88 appear to be specifically important at a young age, as no fatal disease has been reported after the age of 8 years, with no invasive infections after the age of 14 years (391). Two patients have been described as having a homozygous mutation in the gene encoding NEMO, an adaptor molecule of the MyD88-dependent TLR, IL-1R, and TNF receptor (TNF-R) signaling pathways, and this mutation is associated with invasive pneumococcal disease (270, 517).
In mice, MyD88 deficiency resulted in increased susceptibility to systemic infection after colonization and increased mortality due to pneumococcal sepsis and meningitis (11, 256). Pneumococcal infection in MyD88−/− mice was accompanied by decreased symptoms of infection and inflammatory parameters (256), similar to the phenotype seen in patients lacking functional MyD88 or IRAK-M (391, 517). Deficiencies in the TLR and IL-1R signaling pathways have been associated with recurrent pneumococcal disease (68), illustrating the importance of these pathways in controlling pneumococcal infection.
Proinflammatory Cytokines
The early response cytokines IL-1, TNF-α, and IL-6 are produced after pneumococcal recognition (472, 508). Several cells have been found to be capable of sensing pneumococci and produce proinflammatory cytokines: perivascular and meningeal macrophages (393, 557), vascular endothelial cells (153), astrocytes (154), and microglial cells (193, 413). These early-phase cytokines induce upregulation of several adhesion factors on the vascular endothelium, mediating leukocyte influx (see above) (142, 470). The majority of leukocytes recruited to the CSF are polymorphonuclear neutrophils, and influx occurs largely in the first 6 h of infection (557).
TNF-α is an important early proinflammatory response cytokine. Patients with bacterial meningitis have increased CSF TNF-α levels early in the course of disease (66, 169, 285, 448, 493). Intrathecal levels of TNF-α correlated with severity of blood-brain barrier disruption, disease severity, and neurologic sequelae in a study including 48 patients with bacterial meningitis (448). In this study, TNF-α levels decreased within 24 h after the onset of antibiotic treatment (448). In animal models of pneumococcal meningitis, TNF-α was produced mainly in the first 6 to 24 h of the immune response (29, 363). One hour after intrathecal injection of recombinant TNF-α, CSF leukocyte recruitment was observed in a rabbit model (433). Intrathecal administration of anti-TNF-α antibody together with S. pneumoniae reduced CSF leukocytosis, protein content, and brain edema in these experiments (433). TNF-α administered intravenously also mediated blood-brain barrier opening, facilitating bacterial traversal into the CSF (484). However, TNF-α production is also essential for defense, as TNF-α-deficient mice showed decreased survival in a pneumococcal meningitis model (163). Thus, TNF-α has been shown to be a marker of the acute inflammatory response and is associated with inflammation-related complications of bacterial meningitis but is also essential for an adequate host response to the infection.
IL-1β is a proinflammatory cytokine produced by, e.g., perivascular and meningeal macrophages (557). CSF IL-1β levels are increased in the first 18 h of infection (438). Pro-IL-1β is cleaved into its active form by caspase-1, which is regulated by a group of different receptors called the inflammasome (408). Reported data on the role of IL-1β in bacterial meningitis are somewhat contradictory. Levels of IL-1β were not associated with the degree of blood-brain barrier disruption in patients with bacterial meningitis (448). However, a pneumococcal model using caspase-1 knockout mice showed decreased levels of IL-1β and decreased intracranial pressure (ICP), leukocyte recruitment, and brain edema compared to those in WT mice (258). IL-1β administered intrathecally did not lead to CSF pleocytosis or brain edema in a rabbit model of pneumococcal meningitis (433). However, antibodies against IL-1β decreased leukocyte influx induced by TNF-α (433). Mice deficient in the receptor for IL-1α and IL-1β (IL-1R) showed impaired survival and decreased cytokine responses without alterations in CSF pleocytosis (551). Thus, although IL-1β did not influence CSF pleocytosis in pneumococcal meningitis, other caspase-1-cleaved cytokines may be responsible for the reduced pleocytosis observed in caspase-1 knockout mice.
IL-6 is a proinflammatory as well as anti-inflammatory cytokine and has been shown to be upregulated in the acute phase of many infection models (155). In a mouse pneumococcal meningitis model, IL-6 knockout mice displayed increased CSF pleocytosis but decreased cerebral edema, blood-brain barrier disruption, and intracranial pressure (376). This was also described for a model of pneumococcal pneumonia where IL-6 was shown to downregulate multiple proinflammatory as well as anti-inflammatory cytokines (504). Thus, in pneumococcal meningitis, IL-6 attenuates CSF leukocyte recruitment but does not inhibit complications related to fluid shift.
Gamma interferon (IFN-γ) is one of the major cytokines of the T-helper 1 (Th1) pathway. IFN-γ was increased in the CSF of patients with pneumococcal meningitis (170, 261). IFN-γ was also expressed in brain tissue of rats with pneumococcal meningitis (121). The exact role of IFN-γ in pneumococcal meningitis remains unclear. IL-12p70, an important stimulus for IFN-γ production, could be detected in patients with pneumococcal meningitis (261) and in animal models of pneumococcal meningitis (121). Macrophage inflammatory factor (MIF) was found to be increased in the CSF of patients with pneumococcal meningitis and has also been associated with disease severity (361), suggesting a role for MIF in the pathophysiology of pneumococcal meningitis (162).
Anti-Inflammatory Cytokines
Anti-inflammatory cytokines include IL-10 and TGF-β (120, 277, 466, 476). IL-6 may act partially as an anti-inflammatory cytokine and has been discussed earlier (504). IL-10 is an anti-inflammatory cytokine with multiple effects, including downregulation of proinflammatory cytokines and costimulatory molecules on macrophages (120, 476) and impairment of neutrophil phagocytosis and killing (275). IL-10 has been shown to downregulate TNF-α, IL-6, and keratinocyte-derived chemokine (KC), thereby reducing CSF pleocytosis in pneumococcal meningitis (555). Nonetheless, in experimental pneumococcal meningitis, IL-10 knockout mice did not have altered bacterial loads or survival (555). This anti-inflammatory cytokine has been described as an important repressor of sepsis-associated neuronal damage. Its pathophysiology is unclear, but it appears that inflammatory mediators as well as bacterial components cross the blood-brain barrier and induce a local inflammatory response (358, 492, 509). In mice overexpressing IL-10, the development of sepsis-associated neuronal damage as a result of pneumococcal sepsis has been shown to be decreased (358). In line with this, Koedel et al. showed that intravenously administered recombinant IL-10, as opposed to intracisternally administered IL-10, reduced the levels of CSF proinflammatory cytokines, CSF pleocytosis, cerebral edema, and intracranial pressure in a rat model of pneumococcal meningitis (249). Interestingly, intracisternally administered IL-10 had the opposite effect, as it increased CSF pleocytosis in rats with pneumococcal meningitis and induced an inflammatory response in uninfected rats (249). Thus, systemic IL-10 reduces cerebral inflammation and secondary complications in pneumococcal meningitis.
TGF-β is an anti-inflammatory cytokine with multiple functions, including differentiation and maintenance of regulatory T cells (Tregs), differentiation of Th17 T cells, and inhibition of Th1 and Th2 T-cell maturation and differentiation (295), but TGF-β also suppresses macrophage activation and production of several proinflammatory cytokines, such as IL-1β, IL-6, and TNF, by microglial cells (277, 466). Activated Tregs produce TGF-β in an autocrine fashion and are thought to modulate the immune response in such a way that the host's tissues are minimally damaged while the invading pathogen is effectively eliminated, by downregulating the acute inflammatory response. In a mouse model of pneumococcal meningitis, TGF-β was associated with cerebral vasculitis, a frequent complication in patients with meningitis (231, 310). Mice with leukocytes deficient in TGF-β receptor II (TGF-βRII) showed increased neutrophil influx into the subarachnoid space, which was accompanied by increased bacterial clearance and survival of the host (310). In addition, TGF-βRII knockout mice showed decreased blood-brain barrier disruption, intracranial pressure, and cerebral vasculitis (310). However, when TGF-β2 or TGF-β1 was administered intraperitoneally in a rat model of sterile meningitis induced by a PCW lysate, cerebral edema, intracranial pressure, and cerebral blood flow (CBF) decreased (387). Thus, leukocyte TGF-βRII signaling has an unfavorable effect on the course of pneumococcal meningitis, although systemic TGF-β production appears to decrease the complications of meningitis.
Chemokines
Chemokines are a subgroup of cytokines with chemotactic activity recruiting effector immune cells to the site of infection (211). Multiple chemokines have been reported to be upregulated in the CSF of patients with pneumococcal meningitis, including MIP-1delta (CCL15), NAP-2 (CXCL7), MIF, MCP-2 (CCL8), PARC (CCL18), MIP-3α (CCL20) (226), ENA-78 (CXCL5), GRO-α (CXCL-1) (455, 553), IL-8 (CXCL-8) (201, 363, 455, 493, 553), MCP-1 (CCL2), MIP-1α (CCL3), and MIP-1β (CCL4) (455). In animal models of pneumococcal meningitis, additional chemokines have been identified by protein arrays for brain tissue, including MIP-1γ (CCL9), MIP-2 (CXCL-2), lymphotactin (XCL-1), TCA-3 (CCL1), eotaxin (CCL11), MCP-5 (CCL12), eotaxin-2 (CCL24), TECK (CCL25), PF-4 (CXCL4), CRG-2 (CXCL10), SDF-1α (CXCL12), BLC (CXCL13), and CXCL16 (246). The role in pneumococcal meningitis of many of these chemokines has not been elucidated yet.
IL-8 is one of the well-characterized chemokines involved in pneumococcal meningitis. IL-8 was found to be chemotactic for neutrophils in the CSF of patients with bacterial meningitis (455). Furthermore, CSF IL-8 levels increased as a result of blocking leukocyte recruitment in rabbits with pneumococcal meningitis, indicating local production of chemotactic cytokines (368). In patients with bacterial meningitis, no correlation was found between the CSF white blood cell (WBC) count and IL-8 (455). Ostergaard et al. showed that not intracisternal but rather systemic IL-8 levels induced CSF pleocytosis in a rabbit model of pneumococcal meningitis (369). Thus, IL-8 appears to regulate CSF pleocytosis from the systemic compartment, comparable with the proinflammatory cytokine TNF-α and the anti-inflammatory cytokines IL-10 and TGF-β.
The CCL chemokines MCP-1, MIP-1α, and MIP-2 were produced in vitro by astrocytes (193) and microglial cells in response to PCW structures (397). In vitro, antibodies against MCP-1, MIP-1α, and MIP-1β inhibited monocyte chemotactic properties of CSF from patients with pneumococcal meningitis (455). Furthermore, intracisternal inoculation of recombinant MIP-1 or MIP-2 induced blood-brain barrier disruption, CSF leukocytosis, and cerebral edema in a rabbit model of pneumococcal meningitis; blocking MIP-1 or MIP-2 delayed these inflammatory alterations by 2 h (433). Another experiment showed that blocking the receptor for MIP-1, i.e., CCR2, specifically reduced the influx of monocytes into the subarachnoid space in a mouse model of pneumococcal meningitis, while not changing bacterial clearing (328). Thus, both MIP-1 and MIP-2 are produced by immune cells resident in the brain and attract monocytes and neutrophils from the bloodstream into the CSF in the acute stage of infection. The role of MCP-1 in pneumococcal meningitis has not been studied extensively.
Of the CXCL chemokines, ENA-78 was found to be upregulated in patients with bacterial meningitis and exhibited specifically neutrophil chemotactic properties together with IL-8 (553). GRO-α was also found at high levels in the CSF of patients with bacterial meningitis (553), as well as in a rat model of pneumococcal meningitis, but it did not exert any chemotactic activity (30, 553).
In summary, multiple chemokines have been shown to be upregulated in pneumococcal meningitis. Most of them have a role in attracting leukocytes to the CSF. However, the roles of many other chemokines have not been investigated extensively.
Leukocyte Migration Adhesion Molecules
In response to proinflammatory cytokines, selectins and integrins are upregulated on the blood endothelium and leukocytes are attracted from the bloodstream (85, 182). Cerebral perivascular and meningeal macrophages play a key role in attracting leukocytes across the blood-brain barrier into the CSF (393). About 90% of the attracted leukocyte population consists of neutrophilic granulocytes, with the other 10% being predominantly monocytes (287, 328, 393). Rats depleted of perivascular and meningeal macrophages by use of clodronate showed decreased leukocyte recruitment into the CSF despite increased expression of MIP-2, IL-6, and VCAM-1 (393). Furthermore, these depleted rats showed increased bacterial outgrowth in the CSF and poorer clinical scores than those for control rats with pneumococcal meningitis (393). Thus, leukocyte attraction to the subarachnoid space seems to be crucial for efficacious clearing of S. pneumoniae from the subarachnoid space and dependent on perivascular and meningeal macrophage activation but appears to be mediated by cytokines other than IL-6 and MIP-2.
Other cytokines and chemokines attracting leukocytes to the subarachnoid space are TNF-α, IL-8 (systemic), MIP-2, and ENA-78 (see above) (433, 553). Monocytes are also attracted from the bloodstream into the CSF but appear to play a minor role in the pathogenesis of pneumococcal meningitis (328).
Leukocytes cross the blood-brain barrier by binding to selectins on the endothelium (182). Binding to P- and E-selectin promotes leukocyte rolling across the endothelium (182). Blocking L-selectin by fucoidin treatment reduced leukocytosis and disruption of the blood-brain barrier in rabbits challenged intrathecally with pneumococcal antigen (182). Integrins are also upregulated on the vascular endothelium, facilitating binding of leukocytes and subsequent blood-brain barrier migration (85). An important integrin involved in leukocyte recruitment in pneumococcal meningitis is ICAM-1, which is known to bind MAC-1 (CD11b/CD18) on the leukocyte surface (82, 491). Rabbits treated intravenously with antibodies against CD18 showed decreased CSF leukocytosis (82), blood-brain barrier permeability, and brain edema and improved survival after intracisternal challenge with PCW or S. pneumoniae (491). Interestingly, antibodies directed against CD11b did not alter CSF leukocytosis in the same rabbit model of pneumococcal meningitis (491), which may implicate a role for CD11a/CD18 or CD11c/CD18. In line with this, CD11a/CD18-deficient mice showed increased rates of meningitis and otitis media following intraperitoneal infection with S. pneumoniae (396).
The integrin ICAM-1 was shown to be expressed on brain vascular endothelial cells in response to PCW, through an autocrine loop involving TNF-α (153). In a rat model of meningitis induced by PCW, antibodies against ICAM-1 reduced the increase in CBF, increase in ICP, brain edema, and CSF leukocyte counts observed in the first hours after induction of meningitis (520). In an infant mouse model of pneumococcal peritonitis, ICAM-1 deficiency did not reduce the incidence of meningitis, and histopathologically there was no difference in the severity of inflammation (469). Thus, ICAM-1 is not solely responsible for leukocyte recruitment to the brain.
Other Chemoattractants
PAF is a protein produced by neutrophils and endothelial cells in response to inflammatory stimuli, and it facilitates adhesion of leukocytes to the vascular endothelium (43, 308, 460). In rabbits, PAF administered intrathecally induced blood-brain barrier permeability and cerebral edema at doses much lower than those at which it induced leukocytosis (82). Antibodies against CD18 blocked these effects (82).
In response to pneumococci, endothelial cells and neutrophils are stimulated to produce reactive nitrogen species (RNS), such as NO, by endothelial nitric oxide synthetase (eNOS) and inducible nitric oxide synthetase (iNOS), respectively (153, 537). The cerebral vasculature appears to be the main location where ROS are active (434), and increased levels of ROS are associated with blood-brain barrier disruption (254). In patients with meningitis, positive correlations were found between CSF derivatives of NO production and CSF leukocyte counts and protein concentrations in a group of 27 children with bacterial meningitis; however, only 2 of these children had confirmed pneumococcal meningitis (340). Mice deficient for iNOS showed decreased blood-brain barrier disruption and decreased IL-1β, IL-6, TNF-α, MIP-1α, and MIP-2 mRNA levels in the brain (537). The opposite was true for eNOS-deficient mice, which showed more profound leukocyte infiltrates, increased cytokine levels, and decreased survival due to pneumococcal meningitis (253). A third form of NOS, neuronal NOS (nNOS), appears to play a minor role in fluid balance-related complications of pneumococcal meningitis (375). In addition to RNS, ROS such as O2− are produced by the enzyme NADPH oxidase in neutrophils, macrophages, and endothelial cells in response to infection (435). In mice deficient for the subunit of NADPH oxidase in nonphagocytic cells, such as endothelial cells (p47), detrimental effects on blood-brain barrier permeability, subarachnoid space inflammation, and bacterial outgrowth were found (435). Mice deficient for the subunit of NADPH oxidase in phagocytic cells (gp91) did not show any inflammatory differences from WT mice in the course of pneumococcal meningitis (435). Thus, RNS/ROS produced specifically by cerebral endothelial cells, as opposed to granulocytes and macrophages, contribute to the blood-brain barrier damage and associated complications observed during pneumococcal meningitis.
The fibrinolysis factor uPA is also implicated in leukocyte recruitment to the brain in pneumococcal meningitis. In a group of 12 patients with bacterial meningitis (67% of cases were caused by S. pneumoniae), CSF uPA levels were associated with leukocyte recruitment and blood-brain barrier disruption (536). In this study, serum uPA levels correlated with unfavorable clinical outcomes for these patients with bacterial meningitis (536). Mice deficient in uPA showed reduced CSF leukocytosis, although blood-brain barrier permeability, ICP, expression of chemokines, bacterial killing, and clinical outcomes were not different from those for WT mice (379). Interestingly, deficiency in tPA did not have any implications in a mouse pneumococcal meningitis model (379).
The Complement System
A fourth chemoattractant factor for CSF leukocytes is the complement system. Low or undetectable CSF levels of C3, C4, and B were found in uninfected control subjects (478). In response to infection, the liver produces an array of acute-phase proteins which includes several complement components (188, 461). Circulating monocytes, macrophages (95, 461), and epithelial cells of the pulmonary and gastrointestinal tracts (96, 462) also produce substantial amounts of complement components. In addition, brain resident macrophages and monocytes recruited to the CSF during meningitis may also locally produce complement components. C3 was also found to be produced by astrocytes and neurons in response to HIV or proinflammatory cytokines (74, 314, 457). Cultured human brain pericytes from a patient with Alzheimer's disease have been shown to produce C1q (512), and activated astroglial cells can produce C1q, which has been associated with increased blood-brain barrier damage in a rat model of neurotoxicity (306). Microglial cells have been shown to upregulate C1q, C3, C4, and C5a production in response to injury (183). Thus, levels of complement components are increased in the peripheral blood but may also be produced locally in the brain during infection or inflammation.
During infection or inflammation, the immune response in the brain compartment may do more harm than good. Under normal circumstances, the brain expresses multiple inhibitory factors for complement activation. One of these, factor H, was found to be expressed constitutively in neurons, brain endothelial cells, microglial cells, and astrocytes in mice (184). In a mouse model of antibody-mediated inflammation, expression of factor H in these cells was suppressed; when recombinant factor H was administered, complement opsonization, axonal injury, and leukocyte infiltration decreased (184). Thus, in addition to monocytes and macrophages, brain resident cells may contribute to the production of complement factors leading to leukocyte influx during inflammation.
In a rabbit model of pneumococcal meningitis where cobra venom (known to consume complement factor 3) was administered systemically, treated mice showed decreased survival accompanied by increased bacterial outgrowth in the CSF (488). CSF pleocytosis was similar between the treated and untreated groups, but neutrophils were severely impaired in phagocytosis and killing of bacteria (488). In line with these results, mice deficient in C1q or C3 also showed increased bacterial outgrowth in the CSF in a pneumococcal meningitis model, which was accompanied by decreased survival (428). C1q and C3 knockout mice displayed a tempered inflammatory response which was reflected by a decreased leukocyte count in the CSF, decreased brain cytokine and chemokine levels, and fewer meningitis-associated intracranial complications. However, survival was decreased in this model as a result of more fulminant sepsis accompanied by systemic complications (428). Similar results were found in mice deficient for the C3b receptor (CR3), which is also an integrin involved in binding of leukocytes to the endothelium (378). CR3−/− mice showed increased bacterial outgrowth compared to WT mice, with decreased survival, as a result of decreased neutrophilic superoxidase production in the CR3−/− mice leading to ineffective bacterial killing, while CSF pleocytosis was not different between groups (378). In rabbits, intracisternally administered C5a resulted in rapid CSF pleocytosis and increased CSF protein levels which peaked 1 h after injection (222). Furthermore, the CSF of rabbits with pneumococcal meningitis lost its chemotactic activity to neutrophils after incubation with an antibody against C5a (139).
MMPs
Matrix metalloproteinases (MMPs) are Zn2+- and Ca2+-dependent endopeptidases capable of breaking down and remodeling extracellular matrix components such as fibronectin, laminin, proteoglycans, and type IV collagen (423, 540). MMPs are produced mainly by activated neutrophils and, to a lesser extent, also by macrophages, monocytes, and possibly TNF-α-stimulated endothelial cells (194, 233). Furthermore, constitutive MMP expression is found on microglial cells and astrocytes and may be modulated during neuroinflammation and meningitis (102, 173, 464).
The action of MMPs was initially believed to be limited to the breakdown of ECM during leukocyte migration across the subendothelial layer (319). However, experimental bacterial meningitis models revealed that inhibition of MMPs did not result in a reduction of CSF pleocytosis, although blood-brain barrier permeability disruption was attenuated (377). More recent studies have revealed a wide range of MMP substrates, such as chemokines, growth factors, and adhesion molecules, as well as cytokines and cytokine receptors, allowing MMPs to influence the course of various inflammatory conditions (319, 510).
In patients with bacterial meningitis, CSF levels of MMP-8 and MMP-9 were elevated (285). Moreover, higher levels of MMP-9 were detected in children with meningitis who developed hearing impairment or secondary epilepsy than in those who recovered without neurological deficit (285).
To avoid unwanted proteolytic activity, the activity of all MMPs is tightly regulated by binding to inhibitory proteins called tissue inhibitors of metalloproteinases (TIMPs). MMP-9, for instance, forms complexes with and is inactivated by TIMP-1, both of which are upregulated during pneumococcal meningitis (377). In a murine model of pneumococcal meningitis, the induction of TIMP-1 was delayed in relation to that of MMP-9, favoring increased collagen IV degradation and subsequent increased blood-brain barrier permeability (445). Thus, treatment options including drugs specifically targeting MMPs are being investigated (281). Interestingly, in a rat model of pneumococcal meningitis, adjuvant treatment of pneumococcal meningitis with dexamethasone resulted in lower MMP-9 mRNA expression, suggesting a possible mechanism of corticosteroids as an adjuvant treatment for bacterial meningitis (301).
Oxidative Stress
One of the characterizing features of pneumococcal meningitis is the marked recruitment of leukocytes into the CSF (364, 524). The subsequent release of large amounts of RNS and ROS has been documented for patient populations as well as in animal models and plays a central role in the development of intracranial complications and brain damage (119, 229, 283). In the past decade, ROS and RNS have been investigated extensively as potential targets for adjuvant treatment (reviewed by Klein et al. [242]).
During pneumococcal meningitis, RNS are produced by iNOS and eNOS (537). NOS inhibition studies with experimental pneumococcal meningitis models have yielded contradictory results, at least in part due to a lack of drug specificity for single isoforms of NOS (436). Moreover, iNOS and eNOS knockout studies show that the source of RNS is pivotal in determining its function during disease progression. Thus, NO derived from iNOS appears to contribute to blood-brain barrier disruption and to production of proinflammatory mediators (such as IL-1-β, TNF-α, and MIP-2), whereas eNOS-derived NO plays a largely protective role (253, 537).
Reactive oxygen species, such as superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals, are produced by brain resident immune cells as part of the host response to invasive bacterial infections (27). Pneumococci themselves are also an important source of H2O2, which not only is able to cause direct cytotoxic damage but also interacts with host NO to form the highly reactive species peroxynitrite (ONOO−) (37, 63, 198). When present in large quantities, ROS overwhelm the resident antioxidant mechanisms (such as superoxide dismutase and glutathione), leading to tissue exposure to oxidative stress (27). Interventions aimed at scavenging ROS or enhancing antioxidant activity have generally resulted in reductions of intracranial complications such as elevated ICP, increased CBF, brain edema, and neuronal injury (242, 436).
Peroxynitrite, which is formed by the combination of superoxide radicals and NO, is a very reactive, short-lived molecule whose direct detection has proven difficult; the compound nitrotyrosine (which is formed by the reaction of NOO− with tyrosine) is widely used as a marker (401). In patients with bacterial meningitis, elevated CSF levels of nitrotyrosine were associated with an unfavorable outcome and with lower CSF concentrations of the antioxidant ascorbic acid, suggesting antioxidant depletion by the RNS (229). Furthermore, in autopsy studies, nitrotyrosine was detected in the leptomeninges, subarachnoid granulocytes, and penetrating cortical and leptomeningeal vasculature.
Peroxynitrite can damage neurons and glial cells in two ways. First, it causes damage by means of lipid peroxidation and cell membrane destabilization, which occurs by peroxynitrite attack on lipid peroxidation and is consistently seen in brain homogenates of rats with pneumococcal meningitis (242, 257). Blocking of lipid peroxidation with aminosteroids limits neuronal damage (257). Alternatively, peroxynitrite can cause DNA fragmentation and subsequent poly(ADP-ribose) polymerase (PARP) activation, which leads to cell energy depletion and cell death (436). PARP knockout mice, as well as mice treated with a PARP inhibitor, demonstrated lower levels of inflammation and a better clinical course during pneumococcal meningitis (257, 436).
Klein et al. (242) have reviewed the mechanisms of oxidative damage (activation of cytokines and chemokines, neutrophil activation, lipid peroxidation, DNA and mitochondrial damage, tyrosine nitration, MMP activation/TIMP inactivation, K+ channel activity alterations, and prostaglandin synthesis) as well as the resulting pathophysiologic alterations in pneumococcal meningitis (242).
Coagulation
The importance of coagulation and fibrinolytic dysregulation during pneumococcal meningitis is illustrated by the large number of cerebrovascular complications, which occur in up to one-third of patients (524). Analysis of CSF in patients with bacterial meningitis revealed increased levels of both coagulation and fibrinolytic factors (Table 2) (535, 536). More recently, PAI-1 was shown to be associated with the occurrence of brain infarctions (525), though no causal relationship was determined. Furthermore, in a recent autopsy series of patients who died of pneumococcal meningitis, fibrin thrombi and cerebral infarctions were found in the absence of inflammatory vessel wall infiltrates, suggesting that disseminated cerebral intravascular coagulation might be an additional explanation for ischemic stroke in pneumococcal meningitis (513). The precise mechanism of cerebral infarction remains unclear but may include mechanisms such as vascular endothelial swelling, local vascular inflammation, and cerebral intravascular coagulopathy (386, 513).
Table 2.
Coagulation studies
| Setting | Material | Factor | Change in concn | Reference |
|---|---|---|---|---|
| Human studies | ||||
| Thirty-eight patients with bacterial meningitis (GCSa of <9 vs GCS of >9) | Serum | PLT/dPLT | ↑ | 264 |
| PTr | ↓ | |||
| INR | ↑ | |||
| D dimers | ↑ | |||
| Ninety-two patients with bacterial meningitis vs controls and patients with viral meningitis | CSF | sTF | ↑ | 525 |
| TaT | ↑ | |||
| pT fragment F1+2 | ↑ | |||
| tPA | ↑ | |||
| PAI-1 | ↑ | |||
| D dimers | ↑ | |||
| Twelve patients with bacterial meningitis vs 10 patients with group B streptococcus and 10 controls | Serum/CSF | uPA | ↑/↑ | 536 |
| uPAR | =/↑ | |||
| PAI-1 | =/↑ | |||
| PA-dependent platelet activation | ↑ | |||
| Twelve patients with bacterial meningitis vs 10 patients with group B streptococcus and 10 controls | Serum/CSF | tPA | ↑↑/↑ | 535 |
| Murine studies | ||||
| C57BL/6 mice | Brain | tPA/uPA | ↑/= | 379 |
| tPA−/− mice | Frozen samples | PAI-1/2 | ↑/↑ | |
| uPAR−/− mice | Sections | uPAR | ↑ | |
| MIP-2 | ↑ | |||
| KC | ↑ | |||
| Albumin | ↑ |
GCS, Glasgow coma score.
Microhemorrhages are also frequently observed in the leptomeninges, cortex, and white matter and are located mostly around congested small veins and capillaries (513). It may be hypothesized that the massive clotting process results in local depletion of clotting factors, thereby inducing the local formation of microhemorrhages. In severe cases of disseminated cerebral intravascular coagulation, these microhemorrhages might potentially lead to clinically manifested intracerebral macrohemorrhages, which are rarely observed in patients with bacterial meningitis.
NEURONAL DAMAGE AND HISTOPATHOLOGY
Neuronal Damage/Histopathology
Human observational studies have repeatedly found long-term sequelae after pneumococcal meningitis, including sensomotor deficit, hearing loss, and cognitive impairment, which may occur in up to 30% of surviving patients (202, 241, 496, 497, 500, 526). Human histopathological data showed that the parenchymal damage was caused by increased ICP, cytotoxic and vasogenic edema, herniation, and local leukocyte infiltration or abscess formation, as well as by cortical necrosis and hippocampal neuronal loss (346, 513). Experimental animal models of pneumococcal meningitis have demonstrated large variations in histopathological features (Fig. 4 and Table 3), most likely due to different combinations of bacterial strains, infected animal species, methods of inoculation, and stages of infection (64, 178).
Fig. 4.
Neuronal damage and histopathology in humans with pneumococcal meningitis. The images show the histopathology of patients with bacterial meningitis, including parenchymal and meningeal hemorrhages (A), neutrophilic infiltration and arteritis obliterans (B), abscess formation and venous thrombosis (C), recent infarctions (D and E), and meningitis without cortical infiltration (F).
Table 3.
Neuronal damage
| Process or type of damage | Outcome | References |
|---|---|---|
| Microglial activation | Microglial activation is induced by pneumococcus and mediated by TLRs 2, 4, and 9 | 130, 177, 209, 300 |
| Activin A may mediate microglial proliferation and activation | 132, 327, 532 | |
| Neuronal apoptosis | The early phase of hippocampal apoptosis is caspase dependent and mediated by H2O2, pneumolysin, and AIF | 60, 62, 63, 266, 318, 330, 521 |
| The late phase of hippocampal apoptosis is caspase independent and mediated by pneumococcal cell wall products, in a microglial TLR-2-dependent manner | 37, 279, 280, 330 | |
| Sepsis-mediated neuronal damage | Neuronal damage may result in sepsis without concurrent bacterial growth in the CNS compartment | 358, 365, 366, 380 |
| Cochlear damage | Cochlear damage is correlated with CSF inflammation and is mediated by TLR and MyD88 signaling pathways | 15, 39, 40, 247 |
| NO may contribute to cochlear damage; iNOS and eNOS are upregulated during pneumococcal meningitis, and damage can be attenuated using NO inhibitors/O2 scavengers | 14, 227, 241, 243 |
Microgial Activation
Microglial cells, a specific subset of cells related to monocytes and dendritic cells, form the initial line of defense of brain parenchyma against damage, injury, or infection and play a pivotal role in tissue repair, removal of dead debris, and recruitment of other immune cells to the site of infection (90, 353, 403). Together with meningeal and perivascular macrophages, microglia express a wide palette of TLRs and become activated during bacterial infections (343). Once activated, microglia are capable of producing large amounts of proinflammatory cytokines as well as reactive oxygen and reactive nitrogen intermediates, thus potentially playing both neuroprotective and neurotoxic roles (97, 209, 317).
In vitro studies have demonstrated that microglial cells express TLRs 2, 4, and 9, which upon activation by specific TLR agonists induce proinflammatory cytokine and NO production, as well as increased phagocytic activity (130, 413). More specifically, the neurotoxic effects of CpG DNA, which is released into the subarachnoidal space in large amounts by pneumococci during bacterial growth and following antibiotic treatment, were shown to be mediated largely via stimulation of TLR9 on microglial cells (209). Furthermore, pneumolysin, which is a ligand for TLR4, was also found to be a potent activator of microglial cells, causing microglial cytotoxicity at high concentrations (130).
Activin A, a member of the TGF-β superfamily, is a neuroprotective cytokine which has been shown to be expressed constitutively in CSF and elevated in patients during bacterial meningitis (131, 532). Elevated activin A levels in CSF have also been detected in a rabbit meningitis model and were produced by cultured microglial cells following treatment with agonists of TLRs 2, 4, and 9 (132, 327). Furthermore, in vitro-cultured murine microglial cells stimulated with LPS showed that cotreatment with activin A increased microglial proliferation and negatively regulated production of NO, IL-1β, IL-6, and TNF-α (532).
Although no studies have been performed regarding the effects of dexamethasone on microglial activation during pneumococcal meningitis, in vitro studies using LPS-stimulated glial cells demonstrated that microglial activation after bacterial exposure is limited by corticosteroid treatment, providing a possible explanation for the observed beneficial effects of dexamethasone treatment in vivo (115, 148, 197, 495).
A recently published hypothesis suggested that microglia may respond differently to a stimulus preceded by another stimulus, a phenomenon called “priming” (509). Aging itself may result in enhanced microglial activation following single or repeated stimulation (171, 385). Thus, the usually tightly controlled microglial activation may become self-amplifying and even neurotoxic (509). Whether this dysregulation of microglial function plays a role in pneumococcal meningitis seems plausible but has not yet been investigated.
Neuronal Apoptosis
Neuronal damage is caused by the dual effects of an overwhelming inflammatory reaction and direct effects of bacterial toxins (164). Though the hippocampus is not exposed to pneumococci or infiltrating leukocytes directly, it is surrounded by interstitial fluid which is contiguous with the CSF, allowing secreted bacterial toxins and immune system mediators to diffuse into the parenchyma (63, 412). Recent research with murine models has shown that pneumococcus-mediated hippocampal apoptosis occurs in at least two phases, separated by both time and mechanism (330, 521).
The early phase is initiated by the pneumococcal toxins H2O2 and pneumolysin and results in caspase-independent apoptosis-like pyknotic cell death of both mature and immature neurons throughout the dentate gyrus of the hippocampus (63, 330). This is subsequently followed by caspase-dependent apoptosis of the immature neurons in the subgranular region of the dentate gyrus, triggered primarily by bacterial cell wall stimulation of leukocytes (178, 330).
Pneumolysin, which is also implicated in apoptosis of microglial and brain microvascular endothelial cells, was shown to colocalize to dying neurons in the dentate gyrus in a rabbit model of pneumococcal meningitis (63, 318). Animals infected with either pneumolysin- or H2O2-deficient pneumococci showed only partial attenuation of early neuronal apoptosis (63). Additional blocking of H2O2 in animals infected with pneumolysin-deficient pneumococci led to a marked further reduction of cell death, suggesting that H2O2 and pneumolysin together are responsible for early hippocampal apoptosis (63).
Because pneumococci do not express catalase, they are capable of producing high levels of H2O2, which can diffuse freely through the cellular membranes of target cells to damage intracellular structures (384). H2O2 triggers the release of Ca2+ from the endoplasmic reticulum and its influx from the extracellular space. This results in a loss of mitochondrial membrane stability and in further increases of Ca2+ and ROS production. Pneumolysin possibly contributes directly to mitochondrial permeabilization through pore formation (60), after which apoptosis inducing factor (AIF) is cleaved from the mitochondrial membrane by calpein and cathespin is transported through the cytosol into the nucleus (62). There, AIF causes chromatin condensation and large-scale DNA fragmentation, leading to cell death (266). Paradoxically, AIF may also have antiapoptotic properties through the regulation of ROS through peroxide scavenging (266).
The late, caspase-dependent phase of neuronal apoptosis is PCW and TLR2 dependent (37, 330). The observation that in vitro exposure of isolated cultured neurons to PCW products does not lead to cell death led to the hypothesis that late-phase apoptosis may be dependent on the host inflammatory response (330). Neurons themselves do not express TLR2 or -4 and are not sensitive to exposure to the corresponding bacterial ligands. However, when cocultured with microglial cells, neurons revealed caspase-dependent, TLR-mediated late apoptosis when they were exposed to PCW or LPS (279, 280). Therefore, the inflammatory response of microglial cells and invading neutrophils may underlie the caspase-dependent hippocampal apoptosis during pneumococcal meningitis.
Several experimental treatments aimed at reducing hippocampal apoptosis have been studied in animal models of pneumococcal meningitis. Much attention has gone to nonbacteriolytic antibiotic therapies, such as rifampin, daptomycin, and clindamycin (see Targets for Adjunctive Therapy). In experimental rabbit models, rifampin given either alone or as a pretreatment before ceftriaxone resulted in a reduction of hippocampal apoptosis, though no reduction in mortality was observed (54, 165, 458). Likewise, both daptomycin and clindamycin treatments yielded less hippocampal neuronal damage than that with ceftriaxone treatment in rabbit and rat models, respectively (55, 177). Finally, inhibition of MMP and TNF-α converting enzyme (TACE) was also shown to reduce cortical damage and neuronal apoptosis, as well as preserving learning performance in rats with pneumococcal meningitis (281).
Sepsis and Hippocampal Damage
Recent findings with experimental animal models have suggested that neuronal damage may also be caused by pneumococcal growth outside the CNS compartment (365). Mice exposed intravascularly to purified PCW developed hippocampal apoptosis at 6 h postinoculation, an effect that was not reproduced in TLR2- or NOD2-deficient mice and was limited in mice overexpressing IL-10 (358). These findings suggest a possible third, earlier, IL-10-repressible mechanism of neuronal damage, which may precede pneumococcal invasion of the CNS (358). Additionally, in the setting of experimental meningitis, bacteremia has been shown to contribute not only to increased hippocampal apoptosis but also to dysregulation of CBF autoregulation, reduced meningeal inflammation, and attenuated CSF pleocytosis (366, 380). The mechanisms involved remain largely unclear.
Cochlear Damage and Hearing Loss
Hearing loss is a common long-term complication in survivors of bacterial meningitis. Up to 30% of survivors of pneumococcal meningitis experience uni- or bilateral hearing loss, which is often permanent and may be quite severe (125, 273, 415, 524).
The underlying pathophysiology has been studied in several animal models (39, 40, 45, 244, 421, 477) Cochlear involvement may result from direct spread of pneumococcal infection from the meninges, CSF, and cochlear perilymphatic system (40, 227, 244). Alternatively, a hematogenous route may occur following bacteremia or sepsis (227, 244).
The resulting cochlear infiltration of pneumoccoci and neutrophils results in a severe granulocytic inflammation of the perilymphatic spaces and in the release of proinflammatory cytokines and cytotoxic mediators (56). Hearing loss was correlated with the level of CSF inflammation in a rabbit model of pneumococcal meningitis (40), and intrathecal administration of TNF-α alone was sufficient to induce cochlear injury similar to that observed with bacterial meningitis (15). Furthermore, TLR and MyD88 knockout mice demonstrated less hearing loss following pneumococcal meningitis (247). In a rat model, cochlear expression of iNOS and eNOS was upregulated following pneumococcal meningitis, and RNS-mediated cochlear damage could be attenuated both electrophysiologically and histopathologically by RNS scavengers (227, 241, 243). Earlier studies with guinea pigs showed that local perfusion of the scala tympani with NO donor compounds resulted in cochlear damage and could be attenuated by NO inhibitors or O2 scavengers (14).
Cerebrovascular Complications
Cerebrovascular complications are very common during pneumococcal meningitis (497, 528). Arterial stroke occurs in up to 30% of patients, cerebral venous thrombosis in 9%, and intracerebral hemorrhage in up to 9% (231, 524, 531). Autopsy studies in the 1930s through 1960s showed inflammatory infiltrations of cerebral arteries and veins (77, 83, 126). Taking these together with angiographic descriptions of segmental arterial narrowing in patients with ischemic stroke complicating pneumococcal meningitis, the general assumption has been that infarctions during bacterial meningitis are caused by vasculitis.
In a recent human histopathological analysis of patients with pneumococcal meningitis, among whom half of patients had evidence of cerebral infarctions and 67% showed microhemorrhages, there was no evidence of large-vessel vasculitis (513). Moreover, the observed small-vessel vasculitis did not colocalize with areas of infarction, and in this series, no evidence of disseminated intravascular coagulation in the systemic compartment was observed (513). These results suggest the possibility of cerebral intravascular coagulation, independent of systemic coagulopathy or cerebral vasculitis, as the cause for both cerebral infarctions and hemorrhages.
The pathogenesis of cerebral infarction remains unclear and is the subject of ongoing research, which has focused largely on two areas: first, the dysregulation of the coagulation and fibrinolytic pathways, not only systemically but also locally, as exemplified by the upregulation of PAI-1 and elevated levels of prothrombin fragments F1 and -2 and soluble tissue factor in the CSF of patients with pneumococcal meningitis (264, 525, 536); and second, endothelial cell dysfunction, which may lead to localized swelling and release of procoagulant factors and proinflammatory cytokines. Also, endothelin, which is one of several potent vasoactive peptides, has been shown to be elevated in CSF during acute stages of infection (33, 251, 389). In a rat model, treatment with bosentan (an endothelin antagonist) normalized otherwise reduced CBF. Although endothelin inhibition lowered cortical necrosis, no effect on hippocampal damage was observed (389).
TARGETS FOR ADJUNCTIVE THERAPY
Inhibition of Complement Activation
Complement activation is crucial in the early phases of host defense against pneumococcal disease. Generally, complement activation leads to formation of a membrane pore (the MAC) in the pathogen, leading to cell lysis. However, complement components C3a, C4a, and C5a (204) are cleaved in the activation of the complement cascade and serve as anaphylatoxins. They recruit leukocytes to the site of infection, enhance neutrophil survival, and inhibit neutrophil oxidative burst (107, 186, 459). In a murine sepsis model, a C1 inhibitor improved survival through complement inhibition (299). In experimental pneumococcal meningitis, inhibition of C1 resulted in reduced meningeal inflammatory responses, decreased cytokine levels, decreased bacterial outgrowth, and improved survival in rats (550). Interference in the final common complement pathway may present a promising future target for adjunctive therapy (Table 4).
Table 4.
Therapeutic/adjuvant treatments in experimental settings
| Method of treatment | Target/treatment | Outcome | Reference(s) |
|---|---|---|---|
| Inhibition of leukocyte migration | L-selectin/fucoidin | Lowers CSF pleocytosis and protein content, CBF, ICP, and cytokine production (IL-1 and TNF-α) | 17, 57, 179, 181, 182, 368, 491 |
| ICAM-1/ABs | Reduced CSF leukocyte count, CBF, ICP, and brain edema | 520 | |
| CD-18/ABs | Reduction of CSF pleocytosis, blood-brain barrier permeability, and brain edema and increased survival | 491 | |
| G-CSF | (Pre)treatment with G-CSF leads to lower levels of CSF pleocytosis and proinflammatory cytokines | 58, 118, 362 | |
| P-selectin/pertussis toxin and E-selectin | Lowers CSF pleocytosis | 432 | |
| Inhibition of pattern recognition receptors | ERK1/2/kinase inhibitor AG126 | Decreased microglial production of proinflammatory cytokines and chemokines, pleocytosis, CBF, and brain edema | 193 |
| Inhibition of proinflammatory cytokines | TNF-α/thalidomide | Decreased TNF-α levels (but not IL-1β levels), decreased CSF pleocytosis | 53 |
| TNF-α/TACE | Increased survival in murine model in both WT and TLR2 knockout mice | 134 | |
| IL-6/ABs | Reduced CSF pleocytosis and CSF protein content in rat model | 315 | |
| IL-10/ABs | Reduced CSF pleocytosis, CSF protein content, and IL-6 level | 249 | |
| Use of nonbacteriolytic antibodies | Daptomycin | Reduced levels of inflammatory cytokines and cortical damage | 100, 176, 177 |
| Rifampin | Reduced bacterial protein synthesis, lowered mortality in murine model | 463 | |
| Reduced release of PCW products, inflammation, and neuronal damage in combination with ceftriaxone | 165, 347, 458 | ||
| Moxifloxacin | Reduced bacterial cell wall components LTA and TA in CSF in rabbits | 463 | |
| No reduction in mortality over cephalosporin therapy | 124 | ||
| Radical scavenging | iNOS inhibitors/NAC | Reduction of proinflammatory cytokine production, cortical damage, CSF pleocytosis, and hearing loss | 228, 230, 242, 243, 302 |
| Inhibition of caspases | Caspase-3/BDNF | Reduction of neuronal apoptosis | 44, 293 |
| All caspases/BAF | Reduction in cognitive decline | 210 | |
| Inhibition of complement | C1 | Reduction in meningeal inflammation response | 550 |
| Inhibition of MMPs | BB-94 | Reduction of blood-brain barrier permeability, lowers ICP | 377 |
| GM6001 | Decreased TNF-α levels, reduction of neuronal apoptosis | 284 | |
| MMP and TACE/TNF484 | Reduction of cortical necrosis | ||
| No reduction of hippocampal apoptosis | |||
| MMP and TACE/BB-1101 | Reduction of both cortical necrosis and hippocampal apoptosis | 281 | |
| Preserved learning performance |
Inhibition of Proinflammatory Cytokines
TNF-α is essential for a robust inflammatory response but may also elicit inflammation-related complications (163, 433). Thalidomide is a TNF-α inhibitor which is used in the treatment of multiple myeloma (452). In a rabbit model induced by intrathecally administered heat-killed pneumococci, intraperitoneally administered thalidomide was associated with decreased CSF TNF-α levels (but not IL-1β levels) and decreased CSF pleocytosis, but there was no effect on blood-brain barrier permeability (80). When TNF-α was blocked by a TACE inhibitor in an experimental mouse model of pneumococcal meningitis, survival was increased in WT and TLR2−/− mice (134). Thus, TACE inhibition may improve survival, even in a host with deficient TLR2 signaling (134).
Blocking IL-6 intravenously in a rat model of pneumococcal meningitis reduced CSF pleocytosis and protein content (315). Similar results were found with antibodies against IL-10 administered intravenously, which also decreased CSF IL-6 levels (249). Administration of recombinant TGF-β2 intraperitoneally in the acute phase of pneumococcal meningitis in rats reduced the subarachnoid inflammatory response by inhibiting the increase in CBF and brain water content (387).
Inhibition of Pattern Recognition Receptors
Inhibiting TLR signaling with the aim of decreasing subsequent cytokine responses presents a promising strategy. The kinase inhibitor tyrphostin AG126 was shown to inhibit phosphorylation of the signaling molecule extracellular signal-regulated kinase 1/2 (ERK1/2) in microglial cells (193). ERK1/2 is activated in blood monocytes in response to LPS through activation of CD14 and TLR4 (185). In microglial cells, treatment with this kinase inhibitor resulted in decreased production of proinflammatory cytokines and chemokines (193). When the kinase inhibitor was administered intraperitoneally in a mouse pneumococcal meningitis model, leukocyte recruitment to the CSF, CBF, brain edema, and TNF-α production were reduced (193). This provided evidence that blocking the TLR signaling pathway may reduce the severity of disease in pneumococcal meningitis.
Inhibition of Leukocyte Influx into the CNS
One of the strategies to prevent brain damage is to limit leukocyte recruitment to the CSF or to increase leukocyte apoptosis. Recruitment of leukocytes (mainly polymorphonuclear leukocytes [PMNs]) to the subarachnoid space results in the clearance of bacteria, which is accompanied by the production of several toxic mediators that may induce damage not only to the bacteria but also to the brain.
The first step in extravasation of PMNs involves binding of the leukocytes to selectins on the vessel endothelium. Fucoidin is a polysaccharide that blocks the leukocyte receptor L-selectin. Intravenous treatment with fucoidin in several animal models of pneumococcal meningitis reduced CSF pleocytosis (17, 179, 182), in association with reduced CSF protein content (179, 182), modestly decreased CSF lactate (179, 182), and decreased CBF and ICP, without influencing blood-brain barrier permeability, cerebral edema, and outcome (17). Furthermore, fucoidin prevented the increase in CSF TNF-α and IL-1 levels in response to intrathecally administered PCW in rabbits (181); however, when live bacteria were administered intrathecally and the rabbits were treated with ampicillin, fucoidin had no effect on cytokine production (181). A similar study without antibiotic treatment reported a reduction in IL-1 and an increase in CSF IL-8 in fucoidin-treated rabbits with pneumococcal meningitis (368). In contrast to these studies, in a rat model of pneumococcal meningitis, fucoidin treatment led to decreased survival (57). The leukocyte concentration in CSF was lower in fucoidin-treated rats, but systemic leukocytosis was increased, as was systemic bacterial outgrowth. However, rats in this study were pretreated with fucoidin, which may have led to the differences in the systemic immune response (57).
After binding of leukocytes to selectins, firm adhesion to the vascular endothelium is mediated by ICAM-1. In a rat model of meningitis with PCW component-induced inflammation, antibodies against ICAM-1 reduced CSF leukocyte counts, CBF, ICP, and brain edema in the first 6 h (520). In experimental pneumococcal meningitis, an intravenously administered antibody against CD18, a subunit on leukocytes for binding ICAM-1, induced either by live bacteria or by PCW, reduced CSF leukocyte counts, blood-brain barrier permeability, and brain edema and increased survival (491). However, anti-CD18 antibodies tended only to inhibit CSF leukocyte counts in a rabbit model of PCW-induced inflammation (180).
Vascular endothelial growth factor (VEGF) is a peptide involved in angiogenesis, but it has also been described to function as a macrophage and granulocyte chemoattractant (485). Levels of VEGF were associated with CSF leukocyte counts in experimental meningitis induced by heat-killed pneumococci (503). However, blocking of VEGF in rabbits with pneumococcal meningitis did not reduce the extent of brain edema, leukocyte influx, or blood-brain barrier permeability (503).
A different approach involves the induction of an increased systemic proliferation of leukocytes, which would lead to better control of systemic infection and, subsequently, to enhanced control of CNS infection. In patients with meningitis, granulocyte colony-stimulating factor (G-CSF) and macrophage colony-stimulating factor (M-CSF) have been found to be elevated in the CSF (156, 450). In these studies, G-CSF and M-CSF levels correlated with CSF leukocytosis (450). In mice with pneumococcal meningitis, expression of granulocyte-macrophage colony-stimulating factor (GM-CSF) in the brain was increased (376). Rabbits pretreated with G-CSF intravenously 1 h before intrathecal inoculation with S. pneumoniae showed increased peripheral but not subarachnoid leukocytosis and increased CSF levels of TNF and IL-1 but no reduction of subarachnoid bacterial outgrowth or neuron-specific enolase, an indicator of neuronal cell damage (438). A similar experiment showed no influence on subarachnoid bacterial killing, but systemic pleocytosis and bacterial killing were increased (362). CSF leukocytosis and protein content and levels of IL-8, TNF-α, and IL-1β were decreased in G-CSF-pretreated animals, indicating a decreased subarachnoid inflammatory response (362). Similar results were found in a study with rats (58). However, late administration of G-CSF (28 h after infection) did not have any influence on disease parameters (58). In 22 patients with pneumococcal meningitis, adjunctive treatment with recombinant G-CSF was performed together with standard treatment with ceftriaxone and dexamethasone. G-CSF was continued for 4 days unless leukocyte counts exceeded 40 × 109 cells/liter. Lactate and glucose levels returned to normal more quickly in G-CSF-treated patients than in historical controls, and no adverse events were recorded. However, this was not a randomized controlled study (118).
Another approach to limit neutrophil-mediated damage in pneumococcal meningitis is to induce apoptosis in neutrophils by use of roscovitine. Mice treated with a combination of antibiotics and roscovitine showed increased resolution of inflammation, decreased cerebral hemorrhages, and faster recoveries (250).
In meningitis caused by Cryptococcus neoformans, the fungal capsular polysaccharide glucuronoxylomannan (GMX) inhibited leukocyte extravasation despite high IL-8 levels in the CSF (297). The mechanisms by which GMX inhibits leukocytosis are unknown; however, when GMX was administered intravenously in an experimental rabbit model of meningeal inflammation with heat-killed pneumococci, CSF TNF-α levels and leukocytosis decreased, in association with reduced brain edema and inflammation on brain histopathology (298).
A similar mechanism has been described for pertussis toxin, which interferes with the binding of PMNs to P-selectin and E-selectin, the first steps of diapedesis. Intravenous treatment with pertussis toxin in a rabbit model of meningeal inflammation induced by heat-killed pneumococci altered CSF pleocytosis compared to that in untreated animals (432).
Inhibition of Caspases
Caspase activation has been implicated in the activation of proinflammatory cytokines (258) as well as in the mediation of programmed cell death of cerebral endothelial cells and neurons in the hippocampal dendate gyrus (37, 330). Experimental models of pneumococcal meningitis using mice deficient in caspase-1 or after pharmacological blocking of caspase-1 demonstrated lower levels of IL-1β and subsequent diminished proinflammatory cytokine production, as well as fewer meningitis-induced complications (258). The protective effect of caspase-3 inhibition on the development of neuronal damage was demonstrated in a rat model of pneumococcal meningitis and was independent of cytokine modulation (166). Also, the broad-spectrum caspase inhibitor z-VAD-fmk was shown to reduce hippocampal neuronal apoptosis in a rabbit model of pneumococcal meningitis compared to that in untreated controls (61). Moreover, rats inoculated with group B streptococci demonstrated less cognitive decline following adjunctive treatment with the pan-caspase inhibitor bocaspartyl (OMe)-fluoromethylketone (210). An interesting observation is that exogenous administration of brain-derived neutrotrophic factor (BDNF), which has been shown to block caspase-3 (191), reduced neuronal apoptosis in both rat and murine models (44, 293). BDNF was found to be upregulated naturally during bacterial meningitis and after treatment with antibiotics with adjunctive dexamethasone yet lowered during standard antibiotic treatment, suggesting a possible mechanism of corticosteroid therapy (294). So far, no clinical trials have been performed with caspase inbitors as adjunctive therapy.
Adjunctive Dexamethasone Therapy
Dexamethasone is a widely used anti-inflammatory drug. The mechanisms by which dexamethasone inhibits inflammation are not clear, but it decreases proinflammatory cytokine production in monocytes, dendritic cells, astroglial cells, and neutrophils (108, 154, 217, 425), increases the production of anti-inflammatory cytokines such as IL-10 (108), inhibits ROS production by leukocytes (108), and decreases leukocyte adherence (153, 303). Dexamethasone acts on multiple molecules of the TLR downstream signaling cascade, including TAK-1, ERK1/2, MAPK, NF-κB, and STAT3 (41, 542). Astroglial cells stimulated with PCW components produced decreased amounts of NO and TNF-α when they were treated with dexamethasone (38, 154, 444). Brain microvascular endothelial cells also showed reduced levels of TNF-α and IL-1 and decreased expression of ICAM-1 (153). On the molecular level, in peripheral blood mononuclear cells dexamethasone was shown to inhibit S. pneumoniae-induced IκBκ phosphorylation and degradation and binding of NF-κB to DNA, both of which are downstream effector mechanisms of TLR signaling (332). Dexamethasone induced increased levels of IκBκ mRNA, which may bind and inhibit the p65 subunit of NF-κB. These effects resulted in decreased IL-8 production by peripheral blood mononuclear cells (332).
In experimental pneumococcal meningitis, adjunctive dexamethasone reduced TNF-α, lactate (304), and NO (119a) levels when it was administered together with antibiotics. Furthermore, dexamethasone decreased ICP, brain edema, and CSF pleocytosis in rats with PCW-induced meningeal inflammation (255, 388). In a rabbit model, neuron-specific enolase, a marker of overall neuronal damage, was reduced in animals treated with ceftriaxone and dexamethasone compared to those treated with ceftriaxone alone, though an increase in hippocampal apoptosis was also observed (556). Moreover, in rats treated with adjunctive dexamethasone, the observed increase in hippocampal apoptosis was accompanied by impaired learning performance (282), and uninfected rats treated with dexamethasone also demonstrated increased hippocampal damage (47). In an analysis of several prospective multicenter trials in which patients with bacterial meningitis were treated with either adjunctive dexamethasone or placebo, the use of dexamethasone was not associated with cognitive impairment (202, 526).
Adjuvant treatment with dexamethasone resulted in a reduction of hearing loss in rabbit and gerbil models of pneumococcal meningitis (235, 406) but did not have a significant effect in infant rats (94). A recent meta-analysis of human trials evaluating adjuvant dexamethasone treatment suggested that dexamethasone may reduce hearing loss among survivors (499). Clearly, further trials are necessary to assess the effects of dexamethasone on cochlear injury and hearing loss.
In children with bacterial meningitis, CSF TNF-α and IL-1 levels were decreased if patients had been treated with adjunctive dexamethasone therapy (342). In a large randomized controlled trial in Vietnam investigating the efficacy of dexamethasone addition to conventional antibiotic regimens in adults with bacterial meningitis, CSF samples from a large group of patients were examined (309). For 195 of a total of 341 patients included in this study, CSF was available at baseline (when therapy was started) and at a follow-up puncture 1 to 4 days later. Of these 195 patients, 88 had received dexamethasone along with antibiotics starting at baseline, and 107 received placebo and antibiotics. For 24% of patients, S. pneumoniae was confirmed as the causative agent by CSF culture. Other causative agents included S. suis (43%) and N. meningitidis (8%). CSF samples from these patients were analyzed for IL-6, IL-8, IL-10, IL-12, IL-1β, and TNF-α. Cytokine levels in CSF from the first lumbar puncture at baseline were similar between both groups. Dexamethasone treatment reduced the levels of IL-6 and IL-8 and increased the levels of IL-10 (median, 37 pg/ml versus 33 pg/ml; P value = 0.01) in the CSF at follow-up compared to those with placebo (309). Levels of IL-12, IL-1β, and TNF-α were similar between both groups at follow-up. In addition to the differences in anti-inflammatory cytokine profiles, opening pressure of the follow-up lumbar puncture was reduced and the CSF glucose level and CSF/plasma glucose ratio were restored sooner in patients receiving dexamethasone. CSF lactate and protein levels as well as leukocyte counts were similar at follow-up in the dexamethasone and placebo groups (309).
Many randomized clinical trials of dexamethasone for treatment of bacterial meningitis have been performed, but the results have remained somewhat ambiguous (70, 115, 494, 495, 499). An individual patient data meta-analysis of 5 large recent trials showed no effect of dexamethasone (499). A prospective cohort study showed a decrease in mortality from 30 to 20% for adults with pneumococcal meningitis after nationwide implementation of dexamethasone therapy in the Netherlands (69). Dexamethasone treatment has been implemented as routine therapy for patients with suspected or proven pneumococcal meningitis in many countries (487, 497).
Adjunctive Glycerol Therapy
Glycerol (glycerine 1-2-3-propanetriol), a hyperosmolar compound, is an essential component of cell membranes. It has been used in neurosurgery, neurology, and ophthalmology to decrease raised tissue pressures (26, 78, 87, 168, 416, 501). Toxicological data show that it is safe and is associated with few, rare, mild, mostly gastrointestinal side effects (152). Furthermore, glycerol is inexpensive and readily available, facilitating widespread implementation if it is effective. The effects of glycerol in the neurological/neurosurgical setting have been hypothesized to lie in the resulting increase of plasma osmolality, which was shown to reduce the excretion of CSF by some 20 to 30%, leading to increased cerebral blood flow and improvement of brain oxygenation (453). For acute stroke, glycerol has been shown to confer only a short-term advantage, without demonstrating benefits in the long-term outcome (416).
The first clinical trial evaluating glycerol as a potential adjuvant treatment for bacterial meningitis was conducted with 122 Finnish children with bacterial meningitis and suggested a reduction in hearing impairment as well as in long-term neurological sequelae (234). More recently, a large South American trial using adjuvant glycerol for the treatment of children with bacterial meningitis provided additional support for its efficacy in the reduction of neurological sequelae, although hearing loss and mortality were not diminished (381, 382). However, reliable interpretation of the findings was compromised by several methodological problems (430). Nevertheless, the results fueled additional trials, most recently a study in Malawi where 256 adults with bacterial meningitis were randomized to receive either placebo or glycerol as adjuvant treatment (5). The trial was halted prematurely when an interim analysis after 100 deaths showed increased death in the glycerol group (5). The discrepancies in the various studies most likely lie in the variation in study populations, such as age, comorbidity, and causative pathogen, as well as in the variation in treatment regimens and methods of assessing outcome parameters.
Relatively few animal studies have been performed with glycerol, and they have not been able to demonstrate clinical or histological benefits of adjuvant glycerol therapy (46). Moreover, in a rabbit model, pneumococcal meningitis treated with glycerol alone was associated with an increased level of hippocampal neuronal apoptosis (439). A study in which healthy mice were treated with high doses of glycerol (much higher than trial dosages) showed that treatment was associated with the occurrence of seizures (127), which were also seen more in the glycerol-treated patients in the Malawi trial (5). This finding was not in concurrence with the South American trial and may be explained by either the shorter glycerol regimen (2 days instead of 4 days) or increased disease severity in the Malawi group.
Though glycerol seemed attractive as a potential adjuvant treatment for bacterial meningitis, the lack of effectiveness in experimental models, combined with its harmful effects in the Malawi trial, question the value of additional studies on adjunctive glycerol for the treatment of adult meningitis (72).
Nonbacteriolytic Antibiotics
The massive inflammatory response following pneumococcal meningitis has been shown to play a key role in the development of brain damage and subsequent poor outcomes (252). In part, the inflammatory response is determined by bacterial lysis products, as shown in experimental pneumococcal meningitis models where inoculation with PCW led to massive inflammation and neuronal damage (489, 490). Although bacteriolytic antibiotic regimens may limit the overall amount of release of bacterial products, temporary increases in the release of bacterial components have been documented following treatment (344). These observations have fueled the study of nonbacteriolytic antimicrobials as future therapy options.
Specifically, recent research of nonbacteriolytic antimicrobials has focused on the potential use of daptomycin, rifampin, and moxifloxacin. Daptomycin, a lipopeptide antibiotic, has been shown to effectively clear S. pneumoniae in experimental rat, mouse, and rabbit meningitis models (100, 177). Treatment with daptomycin led to lower levels of inflammatory cytokines and possibly to less cortical brain damage and neuronal apoptosis than those for treatment with ceftriaxone alone (176, 177). Although adjunctive treatment with dexamethasone did not result in a reduction of overall pneumococcal clearance from the CSF, daptomycin penetration into the inflamed meninges was reduced in the presence of adjunctive corticosteroid therapy (136).
Rifampin inhibits bacterial protein synthesis and was shown to lead to diminished levels of PCW product release in vitro (463). In a murine model of pneumococcal meningitis, a decrease in mortality was observed in mice treated with rifampin versus ceftriaxone, an effect which was most notable during the first hours following antibiotic therapy (347). Moreover, recent studies with a rabbit model demonstrated that short-term pretreatment with rifampin before ceftriaxone reduced the release of PCW products, inflammation, and neuronal damage compared to those with treatment with ceftriaxone alone (54, 165, 458).
In a rabbit model of pneumococcal meningitis, treatment with moxifloxacin, a relatively novel quinolone, was shown to result in lower levels of the proinflammatory cell wall components LTA and TA in CSF than those obtained by treatment with ceftriaxone (463). Pneumococcal clearance from the CSF was comparable after treatment with either moxifloxacin or ceftriaxone, and drug levels were not reduced following adjunctive treatment with dexamethasone (367, 437). However, in a murine model, moxifloxacin failed to reduce mortality compared to standard cephalosporin therapy (124).
Radical Scavenging
Investigations of potential adjunctive antioxidant therapies have been limited to animal studies and so far lack sufficient evidence for clinical testing. A recent comprehensive review by Klein et al. summarizes the efforts and findings in this area of potential treatment options (242). Among the most promising candidates for future clinical applications are the iNOS inhibitors, the peroxynitrite scavenger Mn(III)tetrakis(4-benzoic acid)-porphyrin (MnTBAP), uric acid, alpha-pheny-tert-butyl-nitrone (PBN), and N-acetyl-l-cysteine (NAC) (242). In spite of some beneficial effects, such as reductions of proinflammatory cytokine pleocytosis (230), reductions in cortical damage (302), attenuated CSF pleocytosis (228, 230), and a diminished incidence of hearing loss (243), adverse outcomes have been reported, such as impaired bacterial killing, increased neuronal apoptosis, and impaired learning function (302). Of all potential antioxidant agents, only NAC is currently used routinely in clinical practice (for the treatment of acetaminophen intoxications), and therefore it is a likely candidate for evaluation in a clinical setting.
CONCLUSIONS
Despite significant advances in treatment and vaccinations, pneumococcal meningitis remains one of the most important infectious diseases worldwide and continues to be associated with high mortality and morbidity. The growing emergence of drug resistance as well as shifts in serotype incidence is fueling further development of novel antibiotic and adjuvant treatment strategies. In addition to the widespread introduction of dexamethasone, other options for adjuvant drugs may lie in modulating ROS/RNS-mediated damage, in caspase inhibition, or in drugs targeting specific mediators in the inflammatory, complement, or coagulation cascades. Extensive research in this area is being performed using experimental animal meningitis models, though so far no clinical treatment trials with humans have been performed. Although the limitations of animal models of meningitis lie in the great variability between species, inoculation methods, and ages of infected animals, experimental medicine continues to provide the backbone for both intervention and pathophysiology studies and will hopefully pave the way to new knowledge and treatment of this deadly disease.
ACKNOWLEDGMENT
We have no conflicts of interest.
Biographies
 Barry B. Mook-Kanamori, M.D., is a neurology resident at the Academic Medical Center, Center of Infection and Immunity Amsterdam, Amsterdam, The Netherlands. After obtaining his undergraduate degree in biology from Swarthmore College, PA, he spent 2 years as a research associate at Yale University Medical School. In 2004, he graduated from the Nijmegen School of Medicine and is currently completing the last year of his neurology residency at the Academic Medical Center in Amsterdam. In 2007 and 2008, he developed a mouse model of pneumococcal meningitis in which novel treatment strategies as well as pathophysiological mechanisms are being examined. His current research focuses on the dysregulation of coagulation during meningitis.
Barry B. Mook-Kanamori, M.D., is a neurology resident at the Academic Medical Center, Center of Infection and Immunity Amsterdam, Amsterdam, The Netherlands. After obtaining his undergraduate degree in biology from Swarthmore College, PA, he spent 2 years as a research associate at Yale University Medical School. In 2004, he graduated from the Nijmegen School of Medicine and is currently completing the last year of his neurology residency at the Academic Medical Center in Amsterdam. In 2007 and 2008, he developed a mouse model of pneumococcal meningitis in which novel treatment strategies as well as pathophysiological mechanisms are being examined. His current research focuses on the dysregulation of coagulation during meningitis.
 Madelijn Geldhoff, M.D., M.Sc., is a Ph.D. student at the Academic Medical Center, Center of Experimental and Molecular Medicine, Amsterdam, The Netherlands. She studied medicine and biomedical sciences at the University of Utrecht, Utrecht, The Netherlands, and obtained her degrees in 2007 and 2009, respectively. Currently, she is working on her Ph.D. thesis. Her research focuses on the pathophysiology of pneumococcal meningitis and the search for new adjuvant treatments in a murine meningitis model. Next year, she will start her residency in neurology at the Academic Medical Center in Amsterdam, The Netherlands.
Madelijn Geldhoff, M.D., M.Sc., is a Ph.D. student at the Academic Medical Center, Center of Experimental and Molecular Medicine, Amsterdam, The Netherlands. She studied medicine and biomedical sciences at the University of Utrecht, Utrecht, The Netherlands, and obtained her degrees in 2007 and 2009, respectively. Currently, she is working on her Ph.D. thesis. Her research focuses on the pathophysiology of pneumococcal meningitis and the search for new adjuvant treatments in a murine meningitis model. Next year, she will start her residency in neurology at the Academic Medical Center in Amsterdam, The Netherlands.
 Tom van der Poll, M.D., Ph.D., is Professor of Medicine at the Academic Medical Center, University of Amsterdam, The Netherlands. He is Head of the Center of Experimental and Molecular Medicine and Chair of the Department of Infectious Diseases at this institution. Dr. van der Poll is board certified in internal medicine and infectious diseases. His training included a postdoctoral research fellowship at Cornell University Medical College in New York (1993 to 1995). Dr. van der Poll is a former Fellow of the Royal Dutch Academy of Arts and Sciences (1995 to 2000). His research focuses on the innate immune and procoagulant responses during sepsis. He has published more than 450 original articles and over 60 book chapters on this topic.
Tom van der Poll, M.D., Ph.D., is Professor of Medicine at the Academic Medical Center, University of Amsterdam, The Netherlands. He is Head of the Center of Experimental and Molecular Medicine and Chair of the Department of Infectious Diseases at this institution. Dr. van der Poll is board certified in internal medicine and infectious diseases. His training included a postdoctoral research fellowship at Cornell University Medical College in New York (1993 to 1995). Dr. van der Poll is a former Fellow of the Royal Dutch Academy of Arts and Sciences (1995 to 2000). His research focuses on the innate immune and procoagulant responses during sepsis. He has published more than 450 original articles and over 60 book chapters on this topic.
 Diederik van de Beek, M.D., Ph.D., is a neurologist at the Academic Medical Center, Center of Infection and Immunity Amsterdam, Amsterdam, The Netherlands. He qualified in medicine at the University of Amsterdam in 1999 and specialized in neurology at the Academic Medical Center (2000 to 2006). In 2004, he successfully defended his thesis on bacterial meningitis in adults. During his postdoctoral time at the Mayo Clinics, Rochester, MN, he studied neurological infections after solid organ transplantation and experimental meningitis (2006 to 2007). Currently, he holds a faculty position as a neurologist and Academic Medical Center Fellow in Amsterdam, The Netherlands. His research program focuses on bacterial meningitis but also includes projects on infections after stroke and septic encephalopathy.
Diederik van de Beek, M.D., Ph.D., is a neurologist at the Academic Medical Center, Center of Infection and Immunity Amsterdam, Amsterdam, The Netherlands. He qualified in medicine at the University of Amsterdam in 1999 and specialized in neurology at the Academic Medical Center (2000 to 2006). In 2004, he successfully defended his thesis on bacterial meningitis in adults. During his postdoctoral time at the Mayo Clinics, Rochester, MN, he studied neurological infections after solid organ transplantation and experimental meningitis (2006 to 2007). Currently, he holds a faculty position as a neurologist and Academic Medical Center Fellow in Amsterdam, The Netherlands. His research program focuses on bacterial meningitis but also includes projects on infections after stroke and septic encephalopathy.
REFERENCES
- 1. Aas J. A., Paster B. J., Stokes L. N., Olsen I., Dewhirst F. E. 2005. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 43:5721–5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abeyta M., Hardy G. G., Yother J. 2003. Genetic alteration of capsule type but not PspA type affects accessibility of surface-bound complement and surface antigens of Streptococcus pneumoniae. Infect. Immun. 71:218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adriani K. S., van de Beek D., Brouwer M. C., Spanjaard L., de Gans J. 2007. Community-acquired recurrent bacterial meningitis in adults. Clin. Infect. Dis. 45:e46–e51 [DOI] [PubMed] [Google Scholar]
- 4. Agrawal A., Simpson M. J., Black S., Carey M. P., Samols D. 2002. A C-reactive protein mutant that does not bind to phosphocholine and pneumococcal C-polysaccharide. J. Immunol. 169:3217–3222 [DOI] [PubMed] [Google Scholar]
- 5. Ajdukiewicz K. M., et al. 2011. Glycerol adjuvant therapy in adults with bacterial meningitis in a high HIV seroprevalence setting in Malawi: a double-blind, randomised controlled trial. Lancet Infect. Dis. 11:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akache B., et al. 2006. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J. Virol. 80:9831–9836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akira S., Hoshino K., Kaisho T. 2000. The role of Toll-like receptors and MyD88 in innate immune responses. J. Endotoxin Res. 6:383–387 [PubMed] [Google Scholar]
- 8. Akita K., et al. 1997. Involvement of caspase-1 and caspase-3 in the production and processing of mature human interleukin 18 in monocytic THP.1 cells. J. Biol. Chem. 272:26595–26603 [DOI] [PubMed] [Google Scholar]
- 9. Alberts B. 2009. Essential cell biology, 3rd ed Garland Science, New York, NY [Google Scholar]
- 10. Albiger B., et al. 2007. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell. Microbiol. 9:633–644 [DOI] [PubMed] [Google Scholar]
- 11. Albiger B., et al. 2005. Myeloid differentiation factor 88-dependent signalling controls bacterial growth during colonization and systemic pneumococcal disease in mice. Cell. Microbiol. 7:1603–1615 [DOI] [PubMed] [Google Scholar]
- 12. Alcantara R. B., Preheim L. C., Gentry M. J. 1999. Role of pneumolysin's complement-activating activity during pneumococcal bacteremia in cirrhotic rats. Infect. Immun. 67:2862–2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alcantara R. B., Preheim L. C., Gentry-Nielsen M. J. 2001. Pneumolysin-induced complement depletion during experimental pneumococcal bacteremia. Infect. Immun. 69:3569–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amaee F. R., Comis S. D., Osborne M. P., Drew S., Tarlow M. J. 1997. Possible involvement of nitric oxide in the sensorineural hearing loss of bacterial meningitis. Acta Otolaryngol. 117:329–336 [DOI] [PubMed] [Google Scholar]
- 15. Aminpour S., Tinling S. P., Brodie H. A. 2005. Role of tumor necrosis factor-alpha in sensorineural hearing loss after bacterial meningitis. Otol. Neurotol. 26:602–609 [DOI] [PubMed] [Google Scholar]
- 16. Andersson B., et al. 1983. Identification of an active disaccharide unit of a glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cells. J. Exp. Med. 158:559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Angstwurm K., et al. 1995. Fucoidin, a polysaccharide inhibiting leukocyte rolling, attenuates inflammatory responses in experimental pneumococcal meningitis in rats. Neurosci. Lett. 191:1–4 [DOI] [PubMed] [Google Scholar]
- 18. Arda B., Sipahi O. R., Atalay S., Ulusoy S. 2008. Pooled analysis of 2,408 cases of acute adult purulent meningitis from Turkey. Med. Princ. Pract. 17:76–79 [DOI] [PubMed] [Google Scholar]
- 19. Arditi M., et al. 1998. Three-year multicenter surveillance of pneumococcal meningitis in children: clinical characteristics, and outcome related to penicillin susceptibility and dexamethasone use. Pediatrics 102:1087–1097 [DOI] [PubMed] [Google Scholar]
- 20. Arnold R. R., Brewer M., Gauthier J. J. 1980. Bactericidal activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect. Immun. 28:893–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arnold R. R., Russell J. E., Champion W. J., Brewer M., Gauthier J. J. 1982. Bactericidal activity of human lactoferrin: differentiation from the stasis of iron deprivation. Infect. Immun. 35:792–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Asai S., et al. 2004. Absence of procarboxypeptidase R induces complement-mediated lethal inflammation in lipopolysaccharide-primed mice. J. Immunol. 173:4669–4674 [DOI] [PubMed] [Google Scholar]
- 23. Attali C., Durmort C., Vernet T., Di Guilmi A. M. 2008. The interaction of Streptococcus pneumoniae with plasmin mediates transmigration across endothelial and epithelial monolayers by intercellular junction cleavage. Infect. Immun. 76:5350–5356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Attali C., et al. 2008. Streptococcus pneumoniae choline-binding protein E interaction with plasminogen/plasmin stimulates migration across the extracellular matrix. Infect. Immun. 76:466–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Avadhanula V., et al. 2006. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J. Virol. 80:1629–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Awasthi P., Srivastava S. N. 1965. Role of oral glycerol in glaucoma. Br. J. Ophthalmol. 49:660–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aycicek A., Iscan A., Erel O., Akcali M., Ocak A. R. 2007. Oxidant and antioxidant parameters in the treatment of meningitis. Pediatr. Neurol. 37:117–120 [DOI] [PubMed] [Google Scholar]
- 28. Bagnoli F., et al. 2008. A second pilus type in Streptococcus pneumoniae is prevalent in emerging serotypes and mediates adhesion to host cells. J. Bacteriol. 190:5480–5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barichello T., et al. 2009. Tumor necrosis factor alpha (TNF-alpha) levels in the brain and cerebrospinal fluid after meningitis induced by Streptococcus pneumoniae. Neurosci. Lett. 467:217–219 [DOI] [PubMed] [Google Scholar]
- 30. Barichello T., et al. 2010. TNF-alpha, IL-1beta, IL-6, and cinc-1 levels in rat brain after meningitis induced by Streptococcus pneumoniae. J. Neuroimmunol. 221:42–45 [DOI] [PubMed] [Google Scholar]
- 31. Beisswenger C., Coyne C. B., Shchepetov M., Weiser J. N. 2007. Role of p38 MAP kinase and transforming growth factor-beta signaling in transepithelial migration of invasive bacterial pathogens. J. Biol. Chem. 282:28700–28708 [DOI] [PubMed] [Google Scholar]
- 32. Beisswenger C., Lysenko E. S., Weiser J. N. 2009. Early bacterial colonization induces Toll-like receptor-dependent transforming growth factor signaling in the epithelium. Infect. Immun. 77:2212–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berg R. M. G., et al. 2009. Circulating levels of vasoactive peptides in patients with acute bacterial meningitis. Intensive Care Med. 35:1604–1608 [DOI] [PubMed] [Google Scholar]
- 34. Bergmann S., et al. 2009. Integrin-linked kinase is required for vitronectin-mediated internalization of Streptococcus pneumoniae by host cells. J. Cell Sci. 122:256–267 [DOI] [PubMed] [Google Scholar]
- 35. Bergmann S., Rohde M., Chhatwal G. S., Hammerschmidt S. 2001. Alpha-enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 40:1273–1287 [DOI] [PubMed] [Google Scholar]
- 36. Bergmann S., Rohde M., Hammerschmidt S. 2004. Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pneumoniae is a surface-displayed plasminogen-binding protein. Infect. Immun. 72:2416–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bermpohl D., et al. 2005. Bacterial programmed cell death of cerebral endothelial cells involves dual death pathways. J. Clin. Invest. 115:1607–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bernatowicz A., Kodel U., Frei K., Fontana A., Pfister H. W. 1995. Production of nitrite by primary rat astrocytes in response to pneumococci. J. Neuroimmunol. 60:53–61 [DOI] [PubMed] [Google Scholar]
- 39. Bhatt S., et al. 1991. Hearing loss and pneumococcal meningitis: an animal model. Laryngoscope 101:1285–1292 [DOI] [PubMed] [Google Scholar]
- 40. Bhatt S. M., et al. 1993. Progression of hearing loss in experimental pneumococcal meningitis: correlation with cerebrospinal fluid cytochemistry. J. Infect. Dis. 167:675–683 [DOI] [PubMed] [Google Scholar]
- 41. Bhattacharyya S., et al. 2010. TAK1 targeting by glucocorticoids determines JNK and IkappaB regulation in Toll-like receptor-stimulated macrophages. Blood 115:1921–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Biernath K. R., et al. 2006. Bacterial meningitis among children with cochlear implants beyond 24 months after implantation. Pediatrics 117:284–289 [DOI] [PubMed] [Google Scholar]
- 43. Biffl W. L., et al. 1996. Interleukin-6 stimulates neutrophil production of platelet-activating factor. J. Leukoc. Biol. 59:569–574 [DOI] [PubMed] [Google Scholar]
- 44. Bifrare Y.-D., Kummer J., Joss P., Täuber M. G., Leib S. L. 2005. Brain-derived neurotrophic factor protects against multiple forms of brain injury in bacterial meningitis. J. Infect. Dis. 191:40–45 [DOI] [PubMed] [Google Scholar]
- 45. Blank A. L., Davis G. L., VanDeWater T. R., Ruben R. J. 1994. Acute Streptococcus pneumoniae meningogenic labyrinthitis. An experimental guinea pig model and literature review. Arch. Otolaryngol. Head Neck Surg. 120:1342–1346 [DOI] [PubMed] [Google Scholar]
- 46. Blaser C., et al. 2010. Adjuvant glycerol is not beneficial in experimental pneumococcal meningitis. BMC Infect. Dis. 10:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blaser C., Wittwer M., Grandgirard D., Leib S. L. 2011. Adjunctive dexamethasone affects the expression of genes related to inflammation, neurogenesis and apoptosis in infant rat pneumococcal meningitis. PLoS One 6:e17840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boffa M. B., Wang W., Bajzar L., Nesheim M. E. 1998. Plasma and recombinant thrombin-activable fibrinolysis inhibitor (TAFI) and activated TAFI compared with respect to glycosylation, thrombin/thrombomodulin-dependent activation, thermal stability, and enzymatic properties. J. Biol. Chem. 273:2127–2135 [DOI] [PubMed] [Google Scholar]
- 49. Bogaert D., De Groot R., Hermans P. W. M. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144–154 [DOI] [PubMed] [Google Scholar]
- 50. Bogaert D., et al. 2001. Pneumococcal carriage in children in The Netherlands: a molecular epidemiological study. J. Clin. Microbiol. 39:3316–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bogaert D., Thompson C. M., Trzcinski K., Malley R., Lipsitch M. 2010. The role of complement in innate and adaptive immunity to pneumococcal colonization and sepsis in a murine model. Vaccine 28:681–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bogaert D., et al. 2004. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 363:1871–1872 [DOI] [PubMed] [Google Scholar]
- 53. Bogdan I., Leib S. L., Bergeron M., Chow L., Tauber M. G. 1997. Tumor necrosis factor-alpha contributes to apoptosis in hippocampal neurons during experimental group B streptococcal meningitis. J. Infect. Dis. 176:693–697 [DOI] [PubMed] [Google Scholar]
- 54. Bottcher T., et al. 2000. Rifampin reduces production of reactive oxygen species of cerebrospinal fluid phagocytes and hippocampal neuronal apoptosis in experimental Streptococcus pneumoniae meningitis. J. Infect. Dis. 181:2095–2098 [DOI] [PubMed] [Google Scholar]
- 55. Bottcher T., et al. 2004. Clindamycin is neuroprotective in experimental Streptococcus pneumoniae meningitis compared with ceftriaxone. J. Neurochem. 91:1450–1460 [DOI] [PubMed] [Google Scholar]
- 56. Brandt C. T., et al. 2006. Hearing loss and cochlear damage in experimental pneumococcal meningitis, with special reference to the role of neutrophil granulocytes. Neurobiol. Dis. 23:300–311 [DOI] [PubMed] [Google Scholar]
- 57. Brandt C. T., et al. 2005. Blocking of leukocyte accumulation in the cerebrospinal fluid augments bacteremia and increases lethality in experimental pneumococcal meningitis. J. Neuroimmunol. 166:126–131 [DOI] [PubMed] [Google Scholar]
- 58. Brandt C. T., et al. 2004. Attenuation of the bacterial load in blood by pretreatment with granulocyte-colony-stimulating factor protects rats from fatal outcome and brain damage during Streptococcus pneumoniae meningitis. Infect. Immun. 72:4647–4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Branger J., et al. 2004. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect. Immun. 72:788–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Braun J. S., et al. 2007. Pneumolysin causes neuronal cell death through mitochondrial damage. Infect. Immun. 75:4245–4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Braun J. S., et al. 1999. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat. Med. 5:298–302 [DOI] [PubMed] [Google Scholar]
- 62. Braun J. S., et al. 2001. Apoptosis-inducing factor mediates microglial and neuronal apoptosis caused by pneumococcus. J. Infect. Dis. 184:1300–1309 [DOI] [PubMed] [Google Scholar]
- 63. Braun J. S., et al. 2002. Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis. J. Clin. Invest. 109:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Braun J. S., Tuomanen E. I. 1999. Molecular mechanisms of brain damage in bacterial meningitis. Adv. Pediatr. Infect. Dis. 14:49–71 [PubMed] [Google Scholar]
- 65. Briles D. E., et al. 2003. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J. Infect. Dis. 188:339–348 [DOI] [PubMed] [Google Scholar]
- 66. Brivet F. G., Jacobs F. M., Megarbane B. 2005. Cerebral output of cytokines in patients with pneumococcal meningitis. Crit. Care Med. 33:2721–2722 [DOI] [PubMed] [Google Scholar]
- 67. Brock S. C., McGraw P. A., Wright P. F., Crowe J. E., Jr 2002. The human polymeric immunoglobulin receptor facilitates invasion of epithelial cells by Streptococcus pneumoniae in a strain-specific and cell type-specific manner. Infect. Immun. 70:5091–5095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Brouwer M. C., et al. 2009. Host genetic susceptibility to pneumococcal and meningococcal disease: a systematic review and meta-analysis. Lancet Infect. Dis. 9:31–44 [DOI] [PubMed] [Google Scholar]
- 69. Brouwer M. C., et al. 2010. Nationwide implementation of adjunctive dexamethasone therapy for pneumococcal meningitis. Neurology 75:1533–1539 [DOI] [PubMed] [Google Scholar]
- 70. Brouwer M. C., McIntyre P., de Gans J., Prasad K., van de Beek D. 2010. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst. Rev. 9:CD004405. [DOI] [PubMed] [Google Scholar]
- 71. Brouwer M. C., Tunkel A. R., van de Beek D. 2010. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin. Microbiol. Rev. 23:467–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brouwer M. C., van de Beek D. 2011. Glycerol in bacterial meningitis: one strike and out? Lancet Infect. Dis. 11:257–258 [DOI] [PubMed] [Google Scholar]
- 73. Brouwer N., et al. 2008. Mannose-binding lectin (MBL) facilitates opsonophagocytosis of yeasts but not of bacteria despite MBL binding. J. Immunol. 180:4124–4132 [DOI] [PubMed] [Google Scholar]
- 74. Bruder C., et al. 2004. HIV-1 induces complement factor C3 synthesis in astrocytes and neurons by modulation of promoter activity. Mol. Immunol. 40:949–961 [DOI] [PubMed] [Google Scholar]
- 75. Brugger S. D., Hathaway L. J., Muhlemann K. 2009. Detection of Streptococcus pneumoniae strain cocolonization in the nasopharynx. J. Clin. Microbiol. 47:1750–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brundage J. F. 2006. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect. Dis. 6:303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Buchan G. C., Alvord E. C., Jr 1969. Diffuse necrosis of subcortical white matter associated with bacterial meningitis. Neurology 19:1–9 [DOI] [PubMed] [Google Scholar]
- 78. Buckell M., Walsh L. 1964. Effect of glycerol by mouth on raised intracranial pressure in man. Lancet ii:1151–1152 [DOI] [PubMed] [Google Scholar]
- 79. Burnaugh A. M., Frantz L. J., King S. J. 2008. Growth of Streptococcus pneumoniae on human glycoconjugates is dependent upon the sequential activity of bacterial exoglycosidases. J. Bacteriol. 190:221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Burroughs M. H., et al. 1995. Effect of thalidomide on the inflammatory response in cerebrospinal fluid in experimental bacterial meningitis. Microb. Pathog. 19:245–255 [DOI] [PubMed] [Google Scholar]
- 81. Butenas S., Orfeo T., Mann K. G. 2009. Tissue factor in coagulation: which? where? when? Arterioscler. Thromb. Vasc. Biol. 29:1989–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cabellos C., et al. 1992. Differing roles for platelet-activating factor during inflammation of the lung and subarachnoid space. The special case of Streptococcus pneumoniae. J. Clin. Invest. 90:612–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cairns H., Russell D. S. 1946. Cerebral arteritis and phlebitis in pneumococcal meningitis. J. Pathol. Bacteriol. 58:649–665 [DOI] [PubMed] [Google Scholar]
- 84. Campbell W. D., Lazoura E., Okada N., Okada H. 2002. Inactivation of C3a and C5a octapeptides by carboxypeptidase R and carboxypeptidase N. Microbiol. Immunol. 46:131–134 [DOI] [PubMed] [Google Scholar]
- 85. Carlos T. M., Harlan J. M. 1994. Leukocyte-endothelial adhesion molecules. Blood 84:2068–2101 [PubMed] [Google Scholar]
- 86. Carrol E. D., et al. 2007. High pneumococcal DNA loads are associated with mortality in Malawian children with invasive pneumococcal disease. Pediatr. Infect. Dis. J. 26:416–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Casey T. A., Trevor-Roper P. D. 1963. Oral glycerol in glaucoma. Br. Med. J. 2:851–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. CDC 2009. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May-August 2009. MMWR Morb. Mortal. Wkly. Rep. 58:1071–1074 [PubMed] [Google Scholar]
- 89. Chapman S. J., et al. 2007. Functional polymorphisms in the FCN2 gene are not associated with invasive pneumococcal disease. Mol. Immunol. 44:3267–3270 [DOI] [PubMed] [Google Scholar]
- 90. Chauhan V. S., Sterka D. G., Jr., Furr S. R., Young A. B., Marriott I. 2009. NOD2 plays an important role in the inflammatory responses of microglia and astrocytes to bacterial CNS pathogens. Glia 57:414–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chien Y.-W., Klugman K. P., Morens D. M. 2009. Bacterial pathogens and death during the 1918 influenza pandemic. N. Engl. J. Med. 361:2582–2583 [DOI] [PubMed] [Google Scholar]
- 92. Chinnery H. R., Ruitenberg M. J., McMenamin P. G. 2010. Novel characterization of monocyte-derived cell populations in the meninges and choroid plexus and their rates of replenishment in bone marrow chimeric mice. J. Neuropathol. Exp. Neurol. 69:896–909 [DOI] [PubMed] [Google Scholar]
- 93. Claus D. R., Siegel J., Petras K., Osmand A. P., Gewurz H. 1977. Interactions of C-reactive protein with the first component of human complement. J. Immunol. 119:187–192 [PubMed] [Google Scholar]
- 94. Coimbra R. S., Loquet G., Leib S. L. 2007. Limited efficacy of adjuvant therapy with dexamethasone in preventing hearing loss due to experimental pneumococcal meningitis in the infant rat. Pediatr. Res. 62:291–294 [DOI] [PubMed] [Google Scholar]
- 95. Colten H. R. 1984. Expression of the MHC class III genes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 306:355–366 [DOI] [PubMed] [Google Scholar]
- 96. Colten H. R., Gordon J. M., Rapp H. J., Borsos T. 1968. Synthesis of the first component of guinea pig complement by columnar epithelial cells of the small intestine. J. Immunol. 100:788–792 [PubMed] [Google Scholar]
- 97. Colton C. A., Snell J., Chernyshev O., Gilbert D. L. 1994. Induction of superoxide anion and nitric oxide production in cultured microglia. Ann. N. Y. Acad. Sci. 738:54–63 [DOI] [PubMed] [Google Scholar]
- 98. Coonrod J. D., Varble R., Yoneda K. 1991. Mechanism of killing of pneumococci by lysozyme. J. Infect. Dis. 164:527–532 [DOI] [PubMed] [Google Scholar]
- 99. Cornacoff J. B., et al. 1983. Primate erythrocyte-immune complex-clearing mechanism. J. Clin. Invest. 71:236–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cottagnoud P., et al. 2004. Daptomycin is highly efficacious against penicillin-resistant and penicillin- and quinolone-resistant pneumococci in experimental meningitis. Antimicrob. Agents Chemother. 48:3928–3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Crisóstomo M. I., et al. 2006. Attenuation of penicillin resistance in a peptidoglycan O-acetyl transferase mutant of Streptococcus pneumoniae. Mol. Microbiol. 61:1497–1509 [DOI] [PubMed] [Google Scholar]
- 102. Crocker S. J., Milner R., Pham-Mitchell N., Campbell I. L. 2006. Cell and agonist-specific regulation of genes for matrix metalloproteinases and their tissue inhibitors by primary glial cells. J. Neurochem. 98:812–823 [DOI] [PubMed] [Google Scholar]
- 103. Cundell D. R., Gerard N. P., Gerard C., Idanpaan-Heikkila I., Tuomanen E. I. 1995. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377:435–438 [DOI] [PubMed] [Google Scholar]
- 104. Cundell D. R., Pearce B. J., Sandros J., Naughton A. M., Masure H. R. 1995. Peptide permeases from Streptococcus pneumoniae affect adherence to eucaryotic cells. Infect. Immun. 63:2493–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cundell D. R., Tuomanen E. I. 1994. Receptor specificity of adherence of Streptococcus pneumoniae to human type-II pneumocytes and vascular endothelial cells in vitro. Microb. Pathog. 17:361–374 [DOI] [PubMed] [Google Scholar]
- 106. Cundell D. R., Weiser J. N., Shen J., Young A., Tuomanen E. I. 1995. Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect. Immun. 63:757–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Czermak B. J., et al. 1998. Role of complement in in vitro and in vivo lung inflammatory reactions. J. Leukoc. Biol. 64:40–48 [DOI] [PubMed] [Google Scholar]
- 108. Dandona P., et al. 1999. Effect of dexamethasone on reactive oxygen species generation by leukocytes and plasma interleukin-10 concentrations: a pharmacodynamic study. Clin. Pharmacol. Ther. 66:58–65 [DOI] [PubMed] [Google Scholar]
- 109. Daniels C. C., et al. 2010. The proline-rich region of pneumococcal surface proteins A and C contains surface-accessible epitopes common to all pneumococci and elicits antibody-mediated protection against sepsis. Infect. Immun. 78:2163–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dave S., Brooks-Walter A., Pangburn M. K., McDaniel L. S. 2001. PspC, a pneumococcal surface protein, binds human factor H. Infect. Immun. 69:3435–3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dave S., Carmicle S., Hammerschmidt S., Pangburn M. K., McDaniel L. S. 2004. Dual roles of PspC, a surface protein of Streptococcus pneumoniae, in binding human secretory IgA and factor H. J. Immunol. 173:471–477 [DOI] [PubMed] [Google Scholar]
- 112. Davis K. M., Akinbi H. T., Standish A. J., Weiser J. N. 2008. Resistance to mucosal lysozyme compensates for the fitness deficit of peptidoglycan modifications by Streptococcus pneumoniae. PLoS Pathog. 4:e1000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dawid S., Roche A. M., Weiser J. N. 2007. The blp bacteriocins of Streptococcus pneumoniae mediate intraspecies competition both in vitro and in vivo. Infect. Immun. 75:443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Reference deleted.
- 115. de Gans J., van de Beek D. 2002. Dexamethasone in adults with bacterial meningitis. N. Engl. J. Med. 347:1549–1556 [DOI] [PubMed] [Google Scholar]
- 116. de Graaf M. T., et al. 2011. Central memory CD4(+) T cells dominate the normal cerebrospinal fluid. Cytometry B Clin. Cytom. 80:43–50 [DOI] [PubMed] [Google Scholar]
- 117. de Jonge E., et al. 2000. Tissue factor pathway inhibitor dose-dependently inhibits coagulation activation without influencing the fibrinolytic and cytokine response during human endotoxemia. Blood 95:1124–1129 [PubMed] [Google Scholar]
- 118. de Lalla F., Nicolin R., Lazzarini L. 2000. Safety and efficacy of recombinant granulocyte colony-stimulating factor as an adjunctive therapy for Streptococcus pneumoniae meningitis in non-neutropenic adult patients: a pilot study. J. Antimicrob. Chemother. 46:843–846 [DOI] [PubMed] [Google Scholar]
- 119. de Menezes C. C., et al. 2009. Oxidative stress in cerebrospinal fluid of patients with aseptic and bacterial meningitis. Neurochem. Res. 34:1255–1260 [DOI] [PubMed] [Google Scholar]
- 119a. Destache C. J., Pakiz C. B., Dash A. K., Larsen C. 1998. Nitric oxide concentrations and cerebrospinal fluid parameters in an experimental animal model of Streptococcus pneumoniae meningitis. Pharmacotherapy 18:612–619 [PubMed] [Google Scholar]
- 120. de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Diab A., et al. 1997. Haemophilus influenzae and Streptococcus pneumoniae induce different intracerebral mRNA cytokine patterns during the course of experimental bacterial meningitis. Clin. Exp. Immunol. 109:233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dieudonne-Vatran A., et al. 2009. Clinical isolates of Streptococcus pneumoniae bind the complement inhibitor C4b-binding protein in a PspC allele-dependent fashion. J. Immunol. 182:7865–7877 [DOI] [PubMed] [Google Scholar]
- 123. Dinarello C. A. 2005. An IL-1 family member requires caspase-1 processing and signals through the ST2 receptor. Immunity 23:461–462 [DOI] [PubMed] [Google Scholar]
- 124. Djukic M., et al. 2005. Moxifloxacin in experimental Streptococcus pneumoniae cerebritis and meningitis. Neurocrit. Care 2:325–329 [DOI] [PubMed] [Google Scholar]
- 125. Dodge P. R., et al. 1984. Prospective evaluation of hearing impairment as a sequela of acute bacterial meningitis. N. Engl. J. Med. 311:869–874 [DOI] [PubMed] [Google Scholar]
- 126. Dodge P. R., Swartz M. N. 1965. Bacterial meningitis—a review of selected aspects. II. Special neurologic problems, postmeningitic complications and clinicopathological correlations. N. Engl. J. Med. 272:1003–1010 [DOI] [PubMed] [Google Scholar]
- 127. Donnelly S., Loscher C., Mills K. H., Lynch M. A. 1999. Glycerol-induced seizure: involvement of IL-1beta and glutamate. Neuroreport 10:1821–1825 [DOI] [PubMed] [Google Scholar]
- 128. Drake T. A., Morrissey J. H., Edgington T. S. 1989. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am. J. Pathol. 134:1087–1097 [PMC free article] [PubMed] [Google Scholar]
- 129. Eberhard T., Kronvall G., Ullberg M. 1999. Surface bound plasmin promotes migration of Streptococcus pneumoniae through reconstituted basement membranes. Microb. Pathog. 26:175–181 [DOI] [PubMed] [Google Scholar]
- 130. Ebert S., et al. 2005. Dose-dependent activation of microglial cells by Toll-like receptor agonists alone and in combination. J. Neuroimmunol. 159:87–96 [DOI] [PubMed] [Google Scholar]
- 131. Ebert S., et al. 2006. Activin A concentrations in human cerebrospinal fluid are age-dependent and elevated in meningitis. J. Neurol. Sci. 250:50–57 [DOI] [PubMed] [Google Scholar]
- 132. Ebert S., Zeretzke M., Nau R., Michel U. 2007. Microglial cells and peritoneal macrophages release activin A upon stimulation with Toll-like receptor agonists. Neurosci. Lett. 413:241–244 [DOI] [PubMed] [Google Scholar]
- 133. Echchannaoui H., et al. 2002. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J. Infect. Dis. 186:798–806 [DOI] [PubMed] [Google Scholar]
- 134. Echchannaoui H., Leib S. L., Neumann U., Landmann R. M. 2007. Adjuvant TACE inhibitor treatment improves the outcome of TLR2−/− mice with experimental pneumococcal meningitis. BMC Infect. Dis. 7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Eckle I., Seitz R., Egbring R., Kolb G., Havemann K. 1991. Protein C degradation in vitro by neutrophil elastase. Biol. Chem. Hoppe Seyler 372:1007–1013 [DOI] [PubMed] [Google Scholar]
- 136. Egermann U., et al. 2009. Combination of daptomycin plus ceftriaxone is more active than vancomycin plus ceftriaxone in experimental meningitis after addition of dexamethasone. Antimicrob. Agents Chemother. 53:3030–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Elm C., et al. 2004. Ectodomains 3 and 4 of human polymeric immunoglobulin receptor (hpIgR) mediate invasion of Streptococcus pneumoniae into the epithelium. J. Biol. Chem. 279:6296–6304 [DOI] [PubMed] [Google Scholar]
- 138. Endeman H., et al. 2008. Mannose-binding lectin genotypes in susceptibility to community-acquired pneumonia. Chest 134:1135–1140 [DOI] [PubMed] [Google Scholar]
- 139. Ernst J. D., Hartiala K. T., Goldstein I. M., Sande M. A. 1984. Complement (C5)-derived chemotactic activity accounts for accumulation of polymorphonuclear leukocytes in cerebrospinal fluid of rabbits with pneumococcal meningitis. Infect. Immun. 46:81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Esposito A. L., Clark C. A., Poirier W. J. 1990. An assessment of the factors contributing to the killing of type 3 Streptococcus pneumoniae by human polymorphonuclear leukocytes in vitro. APMIS 98:111–121 [PubMed] [Google Scholar]
- 141. Fasching C. E., et al. 2007. Impact of the molecular form of immunoglobulin A on functional activity in defense against Streptococcus pneumoniae. Infect. Immun. 75:1801–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Fassbender K., et al. 1997. Endothelial-derived adhesion molecules in bacterial meningitis: association to cytokine release and intrathecal leukocyte-recruitment. J. Neuroimmunol. 74:130–134 [DOI] [PubMed] [Google Scholar]
- 143. Faust S. N., et al. 2001. Dysfunction of endothelial protein C activation in severe meningococcal sepsis. N. Engl. J. Med. 345:408–416 [DOI] [PubMed] [Google Scholar]
- 144. Feldman C., et al. 2002. The effects of pneumolysin and hydrogen peroxide, alone and in combination, on human ciliated epithelium in vitro. Respir. Med. 96:580–585 [DOI] [PubMed] [Google Scholar]
- 145. Feldman C., et al. 1990. The effect of Streptococcus pneumoniae pneumolysin on human respiratory epithelium in vitro. Microb. Pathog. 9:275–284 [DOI] [PubMed] [Google Scholar]
- 146. Fernandes-Alnemri T., et al. 2007. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14:1590–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ferreira D. M., et al. 2009. Characterization of protective mucosal and systemic immune responses elicited by pneumococcal surface protein PspA and PspC nasal vaccines against a respiratory pneumococcal challenge in mice. Clin. Vaccine Immunol. 16:636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Fitch M. T., van de Beek D. 2008. Drug insight: steroids in CNS infectious diseases—new indications for an old therapy. Nat. Clin. Pract. Neurol. 4:97–104 [DOI] [PubMed] [Google Scholar]
- 149. Fleck R. A., Rao L. V., Rapaport S. I., Varki N. 1990. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb. Res. 59:421–437 [DOI] [PubMed] [Google Scholar]
- 150. Franchi L., Eigenbrod T., Munoz-Planillo R., Nunez G. 2009. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10:241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Franchi L., Warner N., Viani K., Nuñez G. 2009. Function of Nod-like receptors in microbial recognition and host defense. Immunol. Rev. 227:106–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Frank M. S., Nahata M. C., Hilty M. D. 1981. Glycerol: a review of its pharmacology, pharmacokinetics, adverse reactions, and clinical use. Pharmacotherapy 1:147–160 [DOI] [PubMed] [Google Scholar]
- 153. Freyer D., et al. 1999. Cerebral endothelial cells release TNF-alpha after stimulation with cell walls of Streptococcus pneumoniae and regulate inducible nitric oxide synthase and ICAM-1 expression via autocrine loops. J. Immunol. 163:4308–4314 [PubMed] [Google Scholar]
- 154. Freyer D., et al. 1996. Pneumococcal cell wall components induce nitric oxide synthase and TNF-alpha in astroglial-enriched cultures. Glia 16:1–6 [DOI] [PubMed] [Google Scholar]
- 155. Gadient R. A., Patterson P. H. 1999. Leukemia inhibitory factor, interleukin 6, and other cytokines using the GP130 transducing receptor: roles in inflammation and injury. Stem Cells 17:127–137 [DOI] [PubMed] [Google Scholar]
- 156. Gallo P., et al. 1990. Macrophage-colony stimulating factor (M-CSF) in the cerebrospinal fluid. J. Neuroimmunol. 29:105–112 [DOI] [PubMed] [Google Scholar]
- 157. Gasse P., et al. 2009. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 179:903–913 [DOI] [PubMed] [Google Scholar]
- 158. Gauczynski S., et al. 2001. The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO J. 20:5863–5875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Geddes A. M. 2009. Influenza and bacterial pneumonia. Int. J. Antimicrob. Agents 34:293–294 [DOI] [PubMed] [Google Scholar]
- 160. Gehrmann J., Matsumoto Y., Kreutzberg G. W. 1995. Microglia: intrinsic immuneffector cell of the brain. Brain Res. Brain Res. Rev. 20:269–287 [DOI] [PubMed] [Google Scholar]
- 161. Geiseler P. J., Nelson K. E., Levin S., Reddi K. T., Moses V. K. 1980. Community-acquired purulent meningitis: a review of 1,316 cases during the antibiotic era, 1954-1976. Rev. Infect. Dis. 2:725–745 [DOI] [PubMed] [Google Scholar]
- 162. Geldhoff M., Mook-Kanamori B. B., van de Beek D. 2009. Macrophage migration inhibitory factor, infection, the brain, and corticosteroids. Crit. Care 13:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Gerber J., et al. 2004. Increased mortality and spatial memory deficits in TNF-alpha-deficient mice in ceftriaxone-treated experimental pneumococcal meningitis. Neurobiol. Dis. 16:133–138 [DOI] [PubMed] [Google Scholar]
- 164. Gerber J., Nau R. 2010. Mechanisms of injury in bacterial meningitis. Curr. Opin. Neurol. 23:312–318 [DOI] [PubMed] [Google Scholar]
- 165. Gerber J., Pohl K., Sander V., Bunkowski S., Nau R. 2003. Rifampin followed by ceftriaxone for experimental meningitis decreases lipoteichoic acid concentrations in cerebrospinal fluid and reduces neuronal damage in comparison to ceftriaxone alone. Antimicrob. Agents Chemother. 47:1313–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Gianinazzi C., et al. 2003. Caspase-3 mediates hippocampal apoptosis in pneumococcal meningitis. Acta Neuropathol. 105:499–507 [DOI] [PubMed] [Google Scholar]
- 167. Giesen P. L., et al. 1999. Blood-borne tissue factor: another view of thrombosis. Proc. Natl. Acad. Sci. U. S. A. 96:2311–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Gilsanz V., Rebollar J. L., Buencuerpo J., Chantres M. T. 1975. Controlled trial of glycerol versus dexamethasone in the treatment of cerebral oedema in acute cerebral infarction. Lancet i:1049–1051 [DOI] [PubMed] [Google Scholar]
- 169. Glimaker M., Kragsbjerg P., Forsgren M., Olcen P. 1993. Tumor necrosis factor-alpha (TNF alpha) in cerebrospinal fluid from patients with meningitis of different etiologies: high levels of TNF alpha indicate bacterial meningitis. J. Infect. Dis. 167:882–889 [DOI] [PubMed] [Google Scholar]
- 170. Glimaker M., Olcen P., Andersson B. 1994. Interferon-gamma in cerebrospinal fluid from patients with viral and bacterial meningitis. Scand. J. Infect. Dis. 26:141–147 [DOI] [PubMed] [Google Scholar]
- 171. Godbout J. P., et al. 2005. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 19:1329–1331 [DOI] [PubMed] [Google Scholar]
- 172. Goldenberg H. B., McCool T. L., Weiser J. N. 2004. Cross-reactivity of human immunoglobulin G2 recognizing phosphorylcholine and evidence for protection against major bacterial pathogens of the human respiratory tract. J. Infect. Dis. 190:1254–1263 [DOI] [PubMed] [Google Scholar]
- 173. Gottschall P. E., Deb S. 1996. Regulation of matrix metalloproteinase expressions in astrocytes, microglia and neurons. Neuroimmunomodulation 3:69–75 [DOI] [PubMed] [Google Scholar]
- 174. Gould J. M., Weiser J. N. 2001. Expression of C-reactive protein in the human respiratory tract. Infect. Immun. 69:1747–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Gould J. M., Weiser J. N. 2002. The inhibitory effect of C-reactive protein on bacterial phosphorylcholine platelet-activating factor receptor-mediated adherence is blocked by surfactant. J. Infect. Dis. 186:361–371 [DOI] [PubMed] [Google Scholar]
- 176. Grandgirard D., Oberson K., Buhlmann A., Gaumann R., Leib S. L. 2010. Attenuation of cerebrospinal fluid inflammation by the nonbacteriolytic antibiotic daptomycin versus that by ceftriaxone in experimental pneumococcal meningitis. Antimicrob. Agents Chemother. 54:1323–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Grandgirard D., Schurch C., Cottagnoud P., Leib S. L. 2007. Prevention of brain injury by the nonbacteriolytic antibiotic daptomycin in experimental pneumococcal meningitis. Antimicrob. Agents Chemother. 51:2173–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Grandgirard D., Steiner O., Täuber M. G., Leib S. L. 2007. An infant mouse model of brain damage in pneumococcal meningitis. Acta Neuropathol. 114:609–617 [DOI] [PubMed] [Google Scholar]
- 179. Granert C., Raud J., Lindquist L. 1998. The polysaccharide fucoidin inhibits the antibiotic-induced inflammatory cascade in experimental pneumococcal meningitis. Clin. Diagn. Lab. Immunol. 5:322–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Granert C., Raud J., Lindquist L., Lindbom L. 2002. Treatment with an anti-CD18 monoclonal antibody in rabbits inhibits pneumococcal-induced leukocyte recruitment in the skin, but not in the meninges. Med. Microbiol. Immunol. 191:97–100 [DOI] [PubMed] [Google Scholar]
- 181. Granert C., Raud J., Waage A., Lindquist L. 1999. Effects of polysaccharide fucoidin on cerebrospinal fluid interleukin-1 and tumor necrosis factor alpha in pneumococcal meningitis in the rabbit. Infect. Immun. 67:2071–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Granert C., Raud J., Xie X., Lindquist L., Lindbom L. 1994. Inhibition of leukocyte rolling with polysaccharide fucoidin prevents pleocytosis in experimental meningitis in the rabbit. J. Clin. Invest. 93:929–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Griffin R. S., et al. 2007. Complement induction in spinal cord microglia results in anaphylatoxin C5a-mediated pain hypersensitivity. J. Neurosci. 27:8699–8708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Griffiths M. R., Neal J. W., Fontaine M., Das T., Gasque P. 2009. Complement factor H, a marker of self protects against experimental autoimmune encephalomyelitis. J. Immunol. 182:4368–4377 [DOI] [PubMed] [Google Scholar]
- 185. Guha M., Mackman N. 2001. LPS induction of gene expression in human monocytes. Cell. Signal. 13:85–94 [DOI] [PubMed] [Google Scholar]
- 186. Guo R. F., et al. 2006. In vivo regulation of neutrophil apoptosis by C5a during sepsis. J. Leukoc. Biol. 80:1575–1583 [DOI] [PubMed] [Google Scholar]
- 187. Gurcel L., Abrami L., Girardin S., Tschopp J., van der Goot F. G. 2006. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126:1135–1145 [DOI] [PubMed] [Google Scholar]
- 188. Hall R. E., Colten H. R. 1978. Genetic defect in biosynthesis of the precursor form of the fourth component of complement. Science 199:69–70 [DOI] [PubMed] [Google Scholar]
- 189. Hament J. M., et al. 2003. Pneumococcal immune adherence to human erythrocytes. Eur. J. Clin. Invest. 33:169–175 [DOI] [PubMed] [Google Scholar]
- 190. Hammerschmidt S., Tillig M. P., Wolff S., Vaerman J. P., Chhatwal G. S. 2000. Species-specific binding of human secretory component to SpsA protein of Streptococcus pneumoniae via a hexapeptide motif. Mol. Microbiol. 36:726–736 [DOI] [PubMed] [Google Scholar]
- 191. Han B. H., et al. 2000. BDNF blocks caspase-3 activation in neonatal hypoxia-ischemia. Neurobiol. Dis. 7:38–53 [DOI] [PubMed] [Google Scholar]
- 192. Han F., et al. 2009. Role for Toll-like receptor 2 in the immune response to Streptococcus pneumoniae infection in mouse otitis media. Infect. Immun. 77:3100–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Hanisch U. K., et al. 2001. The protein tyrosine kinase inhibitor AG126 prevents the massive microglial cytokine induction by pneumococcal cell walls. Eur. J. Immunol. 31:2104–2115 [DOI] [PubMed] [Google Scholar]
- 194. Harkness K. A., et al. 2000. Dexamethasone regulation of matrix metalloproteinase expression in CNS vascular endothelium. Brain 123:698–709 [DOI] [PubMed] [Google Scholar]
- 195. Hathcock J. J., Nemerson Y. 2004. Platelet deposition inhibits tissue factor activity: in vitro clots are impermeable to factor Xa. Blood 104:123–127 [DOI] [PubMed] [Google Scholar]
- 196. Hemmi H., et al. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740–745 [DOI] [PubMed] [Google Scholar]
- 197. Hinkerohe D., et al. 2010. Dexamethasone prevents LPS-induced microglial activation and astroglial impairment in an experimental bacterial meningitis co-culture model. Brain Res. 1329:45–54 [DOI] [PubMed] [Google Scholar]
- 198. Hoffmann O., et al. 2006. Interplay of pneumococcal hydrogen peroxide and host-derived nitric oxide. Infect. Immun. 74:5058–5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Hoge C. W., et al. 1994. An epidemic of pneumococcal disease in an overcrowded, inadequately ventilated jail. N. Engl. J. Med. 331:643–648 [DOI] [PubMed] [Google Scholar]
- 200. Holmes A. R., et al. 2001. The pavA gene of Streptococcus pneumoniae encodes a fibronectin-binding protein that is essential for virulence. Mol. Microbiol. 41:1395–1408 [DOI] [PubMed] [Google Scholar]
- 201. Holub M., et al. 2007. Cortisol levels in cerebrospinal fluid correlate with severity and bacterial origin of meningitis. Crit. Care 11:R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Hoogman M., van de Beek D., Weisfelt M., de Gans J., Schmand B. 2007. Cognitive outcome in adults after bacterial meningitis. J. Neurol. Neurosurg. Psychiatry 78:1092–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Horcajada J. P., et al. 2009. Polymorphic receptors of the innate immune system (MBL/MASP-2 and TLR2/4) and susceptibility to pneumococcal bacteremia in HIV-infected patients: a case-control study. Curr. HIV Res. 7:218–223 [DOI] [PubMed] [Google Scholar]
- 204. Hugli T. E. 1986. Biochemistry and biology of anaphylatoxins. Complement 3:111–127 [DOI] [PubMed] [Google Scholar]
- 205. Hyams C., Camberlein E., Cohen J. M., Bax K., Brown J. S. 2010. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect. Immun. 78:704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Hyams C., et al. 2010. Streptococcus pneumoniae resistance to complement-mediated immunity is dependent on the capsular serotype. Infect. Immun. 78:716–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Idänpään-Heikkilä I., et al. 1997. Oligosaccharides interfere with the establishment and progression of experimental pneumococcal pneumonia. J. Infect. Dis. 176:704–712 [DOI] [PubMed] [Google Scholar]
- 208. Ihekweazu C., et al. 2010. Outbreaks of serious pneumococcal disease in closed settings in the post-antibiotic era: a systematic review. J. Infect. 61:21–27 [DOI] [PubMed] [Google Scholar]
- 209. Iliev A. I., Stringaris A. K., Nau R., Neumann H. 2004. Neuronal injury mediated via stimulation of microglial Toll-like receptor-9 (TLR9). FASEB J. 18:412–414 [DOI] [PubMed] [Google Scholar]
- 210. Irazuzta J., Pretzlaff R. K., Zingarelli B. 2008. Caspases inhibition decreases neurological sequelae in meningitis. Crit. Care Med. 36:1603–1606 [DOI] [PubMed] [Google Scholar]
- 211. Janeway C. 2005. Immunobiology: the immune system in health and disease, 6th ed. Garland Science, New York, NY [Google Scholar]
- 212. Janoff E. N., et al. 1999. Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J. Clin. Invest. 104:1139–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Jarva H., et al. 2002. Streptococcus pneumoniae evades complement attack and opsonophagocytosis by expressing the pspC locus-encoded Hic protein that binds to short consensus repeats 8-11 of factor H. J. Immunol. 168:1886–1894 [DOI] [PubMed] [Google Scholar]
- 214. Jarva H., Jokiranta T. S., Würzner R., Meri S. 2003. Complement resistance mechanisms of streptococci. Mol. Immunol. 40:95–107 [DOI] [PubMed] [Google Scholar]
- 215. Jedrzejas M. J. 2007. Unveiling molecular mechanisms of bacterial surface proteins: Streptococcus pneumoniae as a model organism for structural studies. Cell. Mol. Life Sci. 64:2799–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Jensch I., et al. 2010. PavB is a surface-exposed adhesin of Streptococcus pneumoniae contributing to nasopharyngeal colonization and airways infections. Mol. Microbiol. 77:22–43 [DOI] [PubMed] [Google Scholar]
- 217. Jensen S. S., Gad M. 2010. Differential induction of inflammatory cytokines by dendritic cells treated with novel TLR-agonist and cytokine based cocktails: targeting dendritic cells in autoimmunity. J. Inflamm. (London) 7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Jonsson G., et al. 2005. Hereditary C2 deficiency in Sweden: frequent occurrence of invasive infection, atherosclerosis, and rheumatic disease. Medicine (Baltimore) 84:23–34 [DOI] [PubMed] [Google Scholar]
- 219. Juhn Y. J., et al. 2008. Increased risk of serious pneumococcal disease in patients with asthma. J. Allergy Clin. Immunol. 122:719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Kadioglu A., et al. 2010. Pneumococcal protein PavA is important for nasopharyngeal carriage and development of sepsis. Mol. Oral Microbiol. 25:50–60 [DOI] [PubMed] [Google Scholar]
- 221. Kadioglu A., Weiser J. N., Paton J. C., Andrew P. W. 2008. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6:288–301 [DOI] [PubMed] [Google Scholar]
- 222. Kadurugamuwa J. L., Hengstler B., Bray M. A., Zak O. 1989. Inhibition of complement-factor-5a-induced inflammatory reactions by prostaglandin E2 in experimental meningitis. J. Infect. Dis. 160:715–719 [DOI] [PubMed] [Google Scholar]
- 223. Kaetzel C. S. 2001. Polymeric Ig receptor: defender of the fort or Trojan horse? Curr. Biol. 11:R35–R38 [DOI] [PubMed] [Google Scholar]
- 224. Kang Y. S., et al. 2006. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell 125:47–58 [DOI] [PubMed] [Google Scholar]
- 225. Kang Y. S., et al. 2004. The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen. Proc. Natl. Acad. Sci. U. S. A. 101:215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Kastenbauer S., Angele B., Sporer B., Pfister H.-W., Koedel U. 2005. Patterns of protein expression in infectious meningitis: a cerebrospinal fluid protein array analysis. J. Neuroimmunol. 164:134–139 [DOI] [PubMed] [Google Scholar]
- 227. Kastenbauer S., Klein M., Koedel U., Pfister H. W. 2001. Reactive nitrogen species contribute to blood-labyrinth barrier disruption in suppurative labyrinthitis complicating experimental pneumococcal meningitis in the rat. Brain Res. 904:208–217 [DOI] [PubMed] [Google Scholar]
- 228. Kastenbauer S., Koedel U., Becker B. F., Pfister H. W. 2001. Experimental meningitis in the rat: protection by uric acid at human physiological blood concentrations. Eur. J. Pharmacol. 425:149–152 [DOI] [PubMed] [Google Scholar]
- 229. Kastenbauer S., Koedel U., Becker B. F., Pfister H. W. 2002. Oxidative stress in bacterial meningitis in humans. Neurology 58:186–191 [DOI] [PubMed] [Google Scholar]
- 230. Kastenbauer S., Koedel U., Becker B. F., Pfister H. W. 2002. Pneumococcal meningitis in the rat: evaluation of peroxynitrite scavengers for adjunctive therapy. Eur. J. Pharmacol. 449:177–181 [DOI] [PubMed] [Google Scholar]
- 231. Kastenbauer S., Pfister H.-W. 2003. Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain 126:1015–1025 [DOI] [PubMed] [Google Scholar]
- 232. Kerr A. R., et al. 2006. The contribution of PspC to pneumococcal virulence varies between strains and is accomplished by both complement evasion and complement-independent mechanisms. Infect. Immun. 74:5319–5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Kieseier B. C., et al. 1999. Differential expression of matrix metalloproteinases in bacterial meningitis. Brain 122:1579–1587 [DOI] [PubMed] [Google Scholar]
- 234. Kilpi T., Peltola H., Jauhiainen T., Kallio M. J. 1995. Oral glycerol and intravenous dexamethasone in preventing neurologic and audiologic sequelae of childhood bacterial meningitis. The Finnish Study Group. Pediatr. Infect. Dis. J. 14:270–278 [DOI] [PubMed] [Google Scholar]
- 235. Kim H. H., Addison J., Suh E., Trune D. R., Richter C. P. 2007. Otoprotective effects of dexamethasone in the management of pneumococcal meningitis: an animal study. Laryngoscope 117:1209–1215 [DOI] [PubMed] [Google Scholar]
- 236. Kim J. O., et al. 1999. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect. Immun. 67:2327–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Kim S., et al. 2010. Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur. J. Immunol. 40:1545–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Kim S. J., Gershov D., Ma X., Brot N., Elkon K. B. 2003. Opsonization of apoptotic cells and its effect on macrophage and T cell immune responses. Ann. N. Y. Acad. Sci. 987:68–78 [DOI] [PubMed] [Google Scholar]
- 239. King S. J., et al. 2004. Phase variable desialylation of host proteins that bind to Streptococcus pneumoniae in vivo and protect the airway. Mol. Microbiol. 54:159–171 [DOI] [PubMed] [Google Scholar]
- 240. King S. J., Hippe K. R., Weiser J. N. 2006. Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol. Microbiol. 59:961–974 [DOI] [PubMed] [Google Scholar]
- 241. Klein M., Koedel U., Kastenbauer S., Pfister H.-W. 2008. Nitrogen and oxygen molecules in meningitis-associated labyrinthitis and hearing impairment. Infection 36:2–14 [DOI] [PubMed] [Google Scholar]
- 242. Klein M., Koedel U., Pfister H.-W. 2006. Oxidative stress in pneumococcal meningitis: a future target for adjunctive therapy? Prog. Neurobiol. 80:269–280 [DOI] [PubMed] [Google Scholar]
- 243. Klein M., Koedel U., Pfister H. W., Kastenbauer S. 2003. Meningitis-associated hearing loss: protection by adjunctive antioxidant therapy. Ann. Neurol. 54:451–458 [DOI] [PubMed] [Google Scholar]
- 244. Klein M., Koedel U., Pfister H. W., Kastenbauer S. 2003. Morphological correlates of acute and permanent hearing loss during experimental pneumococcal meningitis. Brain Pathol. 13:123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Klein M., et al. 2008. Innate immunity to pneumococcal infection of the central nervous system depends on Toll-like receptor (TLR) 2 and TLR4. J. Infect. Dis. 198:1028–1036 [DOI] [PubMed] [Google Scholar]
- 246. Klein M., et al. 2006. Protein expression pattern in experimental pneumococcal meningitis. Microbes Infect. 8:974–983 [DOI] [PubMed] [Google Scholar]
- 247. Klein M., et al. 2007. MyD88-dependent immune response contributes to hearing loss in experimental pneumococcal meningitis. J. Infect. Dis. 195:1189–1193 [DOI] [PubMed] [Google Scholar]
- 248. Koedel U., et al. 2003. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J. Immunol. 170:438–444 [DOI] [PubMed] [Google Scholar]
- 249. Koedel U., Bernatowicz A., Frei K., Fontana A., Pfister H. W. 1996. Systemically (but not intrathecally) administered IL-10 attenuates pathophysiologic alterations in experimental pneumococcal meningitis. J. Immunol. 157:5185–5191 [PubMed] [Google Scholar]
- 250. Koedel U., et al. 2009. Apoptosis is essential for neutrophil functional shutdown and determines tissue damage in experimental pneumococcal meningitis. PLoS Pathog. 5:e1000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Koedel U., Gorriz C., Lorenzl S., Pfister H. W. 1997. Increased endothelin levels in cerebrospinal fluid samples from adults with bacterial meningitis. Clin. Infect. Dis. 25:329–330 [DOI] [PubMed] [Google Scholar]
- 252. Koedel U., Klein M., Pfister H.-W. 2010. New understandings on the pathophysiology of bacterial meningitis. Curr. Opin. Infect. Dis. 23:217–223 [DOI] [PubMed] [Google Scholar]
- 253. Koedel U., et al. 2001. Lack of endothelial nitric oxide synthase aggravates murine pneumococcal meningitis. J. Neuropathol. Exp. Neurol. 60:1041–1050 [DOI] [PubMed] [Google Scholar]
- 254. Koedel U., Pfister H. W. 1999. Superoxide production by primary rat cerebral endothelial cells in response to pneumococci. J. Neuroimmunol. 96:190–200 [DOI] [PubMed] [Google Scholar]
- 255. Koedel U., Pfister H. W., Tomasz A. 1994. Methylprednisolone attenuates inflammation, increase of brain water content and intracranial pressure, but does not influence cerebral blood flow changes in experimental pneumococcal meningitis. Brain Res. 644:25–31 [DOI] [PubMed] [Google Scholar]
- 256. Koedel U., et al. 2004. MyD88 is required for mounting a robust host immune response to Streptococcus pneumoniae in the CNS. Brain 127:1437–1445 [DOI] [PubMed] [Google Scholar]
- 257. Koedel U., Scheld W. M., Pfister H.-W. 2002. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect. Dis. 2:721–736 [DOI] [PubMed] [Google Scholar]
- 258. Koedel U., et al. 2002. Role of caspase-1 in experimental pneumococcal meningitis: evidence from pharmacologic caspase inhibition and caspase-1-deficient mice. Ann. Neurol. 51:319–329 [DOI] [PubMed] [Google Scholar]
- 259. Koppel E. A., Litjens M., C. van den Berg V., van Kooyk Y., Geijtenbeek T. B. 2008. Interaction of SIGNR1 expressed by marginal zone macrophages with marginal zone B cells is essential to early IgM responses against Streptococcus pneumoniae. Mol. Immunol. 45:2881–2887 [DOI] [PubMed] [Google Scholar]
- 260. Koppel E. A., et al. 2005. Specific ICAM-3 grabbing nonintegrin-related 1 (SIGNR1) expressed by marginal zone macrophages is essential for defense against pulmonary Streptococcus pneumoniae infection. Eur. J. Immunol. 35:2962–2969 [DOI] [PubMed] [Google Scholar]
- 261. Kornelisse R. F., et al. 1997. Intrathecal production of interleukin-12 and gamma interferon in patients with bacterial meningitis. Infect. Immun. 65:877–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Kostrzynska M., Wadstrom T. 1992. Binding of laminin, type IV collagen, and vitronectin by Streptococcus pneumoniae. Zentralbl. Bakteriol. 277:80–83 [DOI] [PubMed] [Google Scholar]
- 263. Kostyukova N. N., Volkova M. O., Ivanova V. V., Kvetnaya A. S. 1995. A study of pathogenic factors of Streptococcus pneumoniae strains causing meningitis. FEMS Immunol. Med. Microbiol. 10:133–137 [DOI] [PubMed] [Google Scholar]
- 264. Kowalik M. M., Smiatacz T., Hlebowicz M., Pajuro R., Trocha H. 2007. Coagulation, coma, and outcome in bacterial meningitis—an observational study of 38 adult cases. J. Infect. 55:141–148 [DOI] [PubMed] [Google Scholar]
- 265. Kragsbjerg P., Kallman J., Olcen P. 1994. Pneumococcal meningitis in adults. Scand. J. Infect. Dis. 26:659–666 [DOI] [PubMed] [Google Scholar]
- 266. Krantic S., Mechawar N., Reix S., Quirion R. 2007. Apoptosis-inducing factor: a matter of neuron life and death. Prog. Neurobiol. 81:179–196 [DOI] [PubMed] [Google Scholar]
- 267. Krarup A., Sorensen U. B., Matsushita M., Jensenius J. C., Thiel S. 2005. Effect of capsulation of opportunistic pathogenic bacteria on binding of the pattern recognition molecules mannan-binding lectin, L-ficolin, and H-ficolin. Infect. Immun. 73:1052–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Krivan H. C., Roberts D. D., Ginsburg V. 1988. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc. Natl. Acad. Sci. U. S. A. 85:6157–6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Kronborg G., et al. 2002. Variant mannose-binding lectin alleles are not associated with susceptibility to or outcome of invasive pneumococcal infection in randomly included patients. J. Infect. Dis. 185:1517–1520 [DOI] [PubMed] [Google Scholar]
- 270. Ku C. L., et al. 2005. NEMO mutations in 2 unrelated boys with severe infections and conical teeth. Pediatrics 115:e615–e619 [DOI] [PubMed] [Google Scholar]
- 271. Ku C. L., et al. 2007. IRAK4 and NEMO mutations in otherwise healthy children with recurrent invasive pneumococcal disease. J. Med. Genet. 44:16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Ku C. L., et al. 2007. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J. Exp. Med. 204:2407–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Kulahli I., et al. 1997. Evaluation of hearing loss with auditory brainstem responses in the early and late period of bacterial meningitis in children. J. Laryngol. Otol. 111:223–227 [DOI] [PubMed] [Google Scholar]
- 274. Kurono Y., Shimamura K., Shigemi H., Mogi G. 1991. Inhibition of bacterial adherence by nasopharyngeal secretions. Ann. Otol. Rhinol. Laryngol. 100:455–458 [DOI] [PubMed] [Google Scholar]
- 275. Laichalk L. L., Danforth J. M., Standiford T. J. 1996. Interleukin-10 inhibits neutrophil phagocytic and bactericidal activity. FEMS Immunol. Med. Microbiol. 15:181–187 [DOI] [PubMed] [Google Scholar]
- 276. Lanoue A., et al. 2004. SIGN-R1 contributes to protection against lethal pneumococcal infection in mice. J. Exp. Med. 200:1383–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Ledeboer A., Breve J. J., Poole S., Tilders F. J., Van Dam A. M. 2000. Interleukin-10, interleukin-4, and transforming growth factor-beta differentially regulate lipopolysaccharide-induced production of pro-inflammatory cytokines and nitric oxide in co-cultures of rat astroglial and microglial cells. Glia 30:134–142 [DOI] [PubMed] [Google Scholar]
- 278. Lee K. S., Scanga C. A., Bachelder E. M., Chen Q., Snapper C. M. 2007. TLR2 synergizes with both TLR4 and TLR9 for induction of the MyD88-dependent splenic cytokine and chemokine response to Streptococcus pneumoniae. Cell. Immunol. 245:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Lehnardt S., et al. 2006. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J. Immunol. 177:583–592 [DOI] [PubMed] [Google Scholar]
- 280. Lehnardt S., et al. 2003. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. U. S. A. 100:8514–8519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Leib S. L., et al. 2001. Inhibition of matrix metalloproteinases and tumour necrosis factor alpha converting enzyme as adjuvant therapy in pneumococcal meningitis. Brain 124:1734–1742 [DOI] [PubMed] [Google Scholar]
- 282. Leib S. L., Heimgartner C., Bifrare Y.-D., Loeffler J. M., Täauber M. G. 2003. Dexamethasone aggravates hippocampal apoptosis and learning deficiency in pneumococcal meningitis in infant rats. Pediatr. Res. 54:353–357 [DOI] [PubMed] [Google Scholar]
- 283. Leib S. L., Kim Y. S., Chow L. L., Sheldon R. A., Tauber M. G. 1996. Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J. Clin. Invest. 98:2632–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Leib S. L., Leppert D., Clements J., Tauber M. G. 2000. Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect. Immun. 68:615–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Leppert D., et al. 2000. Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: association with blood-brain barrier damage and neurological sequelae. Clin. Infect. Dis. 31:80–84 [DOI] [PubMed] [Google Scholar]
- 286. Letiembre M., et al. 2005. Toll-like receptor 2 deficiency delays pneumococcal phagocytosis and impairs oxidative killing by granulocytes. Infect. Immun. 73:8397–8401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Letiembre M., Echchannaoui H., Ferracin F., Rivest S., Landmann R. 2005. Toll-like receptor-2 deficiency is associated with enhanced brain TNF gene expression during pneumococcal meningitis. J. Neuroimmunol. 168:21–33 [DOI] [PubMed] [Google Scholar]
- 288. Levi M., Opal S. M. 2006. Coagulation abnormalities in critically ill patients. Crit. Care 10:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Levi M., Ten Cate H. 1999. Disseminated intravascular coagulation. N. Engl. J. Med. 341:586–592 [DOI] [PubMed] [Google Scholar]
- 290. Levi M., van der Poll T. 2010. Inflammation and coagulation. Crit. Care Med. 38:S26–S34 [DOI] [PubMed] [Google Scholar]
- 291. Levi M., van der Poll T., Buller H. R. 2004. Bidirectional relation between inflammation and coagulation. Circulation 109:2698–2704 [DOI] [PubMed] [Google Scholar]
- 292. Li J., Glover D. T., Szalai A. J., Hollingshead S. K., Briles D. E. 2007. PspA and PspC minimize immune adherence and transfer of pneumococci from erythrocytes to macrophages through their effects on complement activation. Infect. Immun. 75:5877–5885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Li L., Shui Q. X., Liang K., Ren H. 2007. Brain-derived neurotrophic factor rescues neurons from bacterial meningitis. Pediatr. Neurol. 36:324–329 [DOI] [PubMed] [Google Scholar]
- 294. Li L., Shui Q. X., Zhao Z. Y. 2003. Regulation of brain-derived neurotrophic factor (BDNF) expression following antibiotic treatment of experimental bacterial meningitis. J. Child Neurol. 18:828–834 [DOI] [PubMed] [Google Scholar]
- 295. Li M. O., Flavell R. A. 2008. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity 28:468–476 [DOI] [PubMed] [Google Scholar]
- 296. Li-Korotky H.-S., et al. 2008. Interaction of pneumococcal phase variation and middle ear pressure/gas composition: an in vitro model of simulated otitis media. Microb. Pathog. 45:201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Lipovsky M. M., et al. 1998. Cryptococcal glucuronoxylomannan induces interleukin (IL)-8 production by human microglia but inhibits neutrophil migration toward IL-8. J. Infect. Dis. 177:260–263 [DOI] [PubMed] [Google Scholar]
- 298. Lipovsky M. M., et al. 2000. Cryptococcal glucuronoxylomannan delays translocation of leukocytes across the blood-brain barrier in an animal model of acute bacterial meningitis. J. Neuroimmunol. 111:10–14 [DOI] [PubMed] [Google Scholar]
- 299. Liu D., et al. 2007. C1 inhibitor-mediated protection from sepsis. J. Immunol. 179:3966–3972 [DOI] [PubMed] [Google Scholar]
- 300. Liu X., Chauhan V. S., Young A. B., Marriott I. 2010. NOD2 mediates inflammatory responses of primary murine glia to Streptococcus pneumoniae. Glia 58:839–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Liu X., Han Q., Sun R., Li Z. 2008. Dexamethasone regulation of matrix metalloproteinase expression in experimental pneumococcal meningitis. Brain Res. 1207:237–243 [DOI] [PubMed] [Google Scholar]
- 302. Loeffler J. M., Ringer R., Hablutzel M., Tauber M. G., Leib S. L. 2001. The free radical scavenger alpha-phenyl-tert-butyl nitrone aggravates hippocampal apoptosis and learning deficits in experimental pneumococcal meningitis. J. Infect. Dis. 183:247–252 [DOI] [PubMed] [Google Scholar]
- 303. Lorenzl S., Koedel U., Dirnagl U., Ruckdeschel G., Pfister H. W. 1993. Imaging of leukocyte-endothelium interaction using in vivo confocal laser scanning microscopy during the early phase of experimental pneumococcal meningitis. J. Infect. Dis. 168:927–933 [DOI] [PubMed] [Google Scholar]
- 304. Lutsar I., et al. 2003. Factors influencing the anti-inflammatory effect of dexamethasone therapy in experimental pneumococcal meningitis. J. Antimicrob. Chemother. 52:651–655 [DOI] [PubMed] [Google Scholar]
- 305. Lux T., Nuhn M., Hakenbeck R., Reichmann P. 2007. Diversity of bacteriocins and activity spectrum in Streptococcus pneumoniae. J. Bacteriol. 189:7741–7751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Lynch N. J., et al. 2004. Microglial activation and increased synthesis of complement component C1q precedes blood-brain barrier dysfunction in rats. Mol. Immunol. 40:709–716 [DOI] [PubMed] [Google Scholar]
- 307. Lysenko E. S., Ratner A. J., Nelson A. L., Weiser J. N. 2005. The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces. PLoS Pathog. 1:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Macconi D., et al. 1995. PAF mediates neutrophil adhesion to thrombin or TNF-stimulated endothelial cells under shear stress. Am. J. Physiol. 269:C42–C47 [DOI] [PubMed] [Google Scholar]
- 309. Mai N. T., et al. 2009. Immunological and biochemical correlates of adjunctive dexamethasone in Vietnamese adults with bacterial meningitis. Clin. Infect. Dis. 49:1387–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Malipiero U., Koedel U., Pfister W., Fontana A. 2007. Bacterial meningitis: the role of transforming growth factor-beta in innate immunity and secondary brain damage. Neurodegener. Dis. 4:43–50 [DOI] [PubMed] [Google Scholar]
- 311. Malley R., et al. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. U. S. A. 100:1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Malley R., et al. 2001. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect. Immun. 69:4870–4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Mammen E. F. 1998. The haematological manifestations of sepsis. J. Antimicrob. Chemother. 41(Suppl. A):17–24 [DOI] [PubMed] [Google Scholar]
- 314. Maranto J., Rappaport J., Datta P. K. 2008. Regulation of complement component C3 in astrocytes by IL-1beta and morphine. J. Neuroimmune Pharmacol. 3:43–51 [DOI] [PubMed] [Google Scholar]
- 315. Marby D., Lockhart G. R., Raymond R., Linakis J. G. 2001. Anti-interleukin-6 antibodies attenuate inflammation in a rat meningitis model. Acad. Emerg. Med. 8:946–949 [DOI] [PubMed] [Google Scholar]
- 316. Margolis E. 2009. Hydrogen peroxide-mediated interference competition by Streptococcus pneumoniae has no significant effect on Staphylococcus aureus nasal colonization of neonatal rats. J. Bacteriol. 191:571–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Marques C. P., Cheeran M. C., Palmquist J. M., Hu S., Lokensgard J. R. 2008. Microglia are the major cellular source of inducible nitric oxide synthase during experimental herpes encephalitis. J. Neurovirol. 14:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Marriott H. M., Mitchell T. J., Dockrell D. H. 2008. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr. Mol. Med. 8:497–509 [DOI] [PubMed] [Google Scholar]
- 319. McCawley L. J., Matrisian L. M. 2001. Matrix metalloproteinases: they're not just for matrix anymore! Curr. Opin. Cell Biol. 13:534–540 [DOI] [PubMed] [Google Scholar]
- 320. Mccullers J. A. 2006. Insights into the interaction between influenza virus and pneumococcus. Clin. Microbiol. Rev. 19:571–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Mccullers J. A., Bartmess K. C. 2003. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J. Infect. Dis. 187:1000–1009 [DOI] [PubMed] [Google Scholar]
- 322. Mccullers J. A., Iverson A. R., Mckeon R., Murray P. J. 2008. The platelet activating factor receptor is not required for exacerbation of bacterial pneumonia following influenza. Scand. J. Infect. Dis. 40:11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.McDonald D. R., Brown D., Bonilla F. A., Geha R. S. 2006. Interleukin receptor-associated kinase-4 deficiency impairs Toll-like receptor-dependent innate antiviral immune responses. J. Allergy Clin. Immunol. 118:1357–1362 [DOI] [PubMed] [Google Scholar]
- 324. McIntyre P. 2010. Adjunctive dexamethasone in meningitis: does value depend on clinical setting? Lancet Neurol. 9:229–231 [DOI] [PubMed] [Google Scholar]
- 325. Mestecky J. 2005. Mucosal immunology, 3rd ed. Elsevier Academic Press, Amsterdam, Netherlands [Google Scholar]
- 326. Mesters R. M., Florke N., Ostermann H., Kienast J. 1996. Increase of plasminogen activator inhibitor levels predicts outcome of leukocytopenic patients with sepsis. Thromb. Haemost. 75:902–907 [PubMed] [Google Scholar]
- 327. Michel U., et al. 2003. Increased activin levels in cerebrospinal fluid of rabbits with bacterial meningitis are associated with activation of microglia. J. Neurochem. 86:238–245 [DOI] [PubMed] [Google Scholar]
- 328. Mildner A., et al. 2008. Ly-6G+CCR2− myeloid cells rather than Ly-6ChighCCR2+ monocytes are required for the control of bacterial infection in the central nervous system. J. Immunol. 181:2713–2722 [DOI] [PubMed] [Google Scholar]
- 329. Miller M. L., Gao G., Pestina T., Persons D., Tuomanen E. 2007. Hypersusceptibility to invasive pneumococcal infection in experimental sickle cell disease involves platelet-activating factor receptor. J. Infect. Dis. 195:581–584 [DOI] [PubMed] [Google Scholar]
- 330. Mitchell L., et al. 2004. Dual phases of apoptosis in pneumococcal meningitis. J. Infect. Dis. 190:2039–2046 [DOI] [PubMed] [Google Scholar]
- 331. Moens L., et al. 2006. Mannose-binding lectin genotype and invasive pneumococcal infection. Hum. Immunol. 67:605–611 [DOI] [PubMed] [Google Scholar]
- 332. Mogensen T. H., Berg R. S., Paludan S. R., Ostergaard L. 2008. Mechanisms of dexamethasone-mediated inhibition of Toll-like receptor signaling induced by Neisseria meningitidis and Streptococcus pneumoniae. Infect. Immun. 76:189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Mogensen T. H., Paludan S. R., Kilian M., Ostergaard L. 2006. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J. Leukoc. Biol. 80:267–277 [DOI] [PubMed] [Google Scholar]
- 334. Mold C., Du Clos T. W. 2006. C-reactive protein increases cytokine responses to Streptococcus pneumoniae through interactions with Fc gamma receptors. J. Immunol. 176:7598–7604 [DOI] [PubMed] [Google Scholar]
- 335. Mold C., Rodic-Polic B., Du Clos T. W. 2002. Protection from Streptococcus pneumoniae infection by C-reactive protein and natural antibody requires complement but not Fc gamma receptors. J. Immunol. 168:6375–6381 [DOI] [PubMed] [Google Scholar]
- 336. Moreno A. T., et al. 2010. Immunization of mice with single PspA fragments induces antibodies capable of mediating complement deposition on different pneumococcal strains and cross-protection. Clin. Vaccine Immunol. 17:439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Morens D. M., Taubenberger J. K., Fauci A. S. 2008. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 198:962–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Morser J., Gabazza E. C., Myles T., Leung L. L. 2010. What has been learnt from the thrombin-activatable fibrinolysis inhibitor-deficient mouse? J. Thromb. Haemost. 8:868–876 [DOI] [PubMed] [Google Scholar]
- 339. Mortensen R. F., Duszkiewicz J. A. 1977. Mediation of CRP-dependent phagocytosis through mouse macrophage Fc-receptors. J. Immunol. 119:1611–1616 [PubMed] [Google Scholar]
- 340. Murawska-Cialowicz E., Szychowska Z., Trbusiewicz B. 2000. Nitric oxide production during bacterial and viral meningitis in children. Int. J. Clin. Lab. Res. 30:127–131 [DOI] [PubMed] [Google Scholar]
- 341. Musher D. M. 1992. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin. Infect. Dis. 14:801–807 [DOI] [PubMed] [Google Scholar]
- 342. Mustafa M. M., et al. 1989. Prostaglandins E2 and I2, interleukin 1-beta, and tumor necrosis factor in cerebrospinal fluid in infants and children with bacterial meningitis. Pediatr. Infect. Dis. J. 8:921–922 [DOI] [PubMed] [Google Scholar]
- 343. Nau R., Bruck W. 2002. Neuronal injury in bacterial meningitis: mechanisms and implications for therapy. Trends Neurosci. 25:38–45 [DOI] [PubMed] [Google Scholar]
- 344. Nau R., Eiffert H. 2005. Minimizing the release of proinflammatory and toxic bacterial products within the host: a promising approach to improve outcome in life-threatening infections. FEMS Immunol. Med. Microbiol. 44:1–16 [DOI] [PubMed] [Google Scholar]
- 345. Nau R., Eiffert H. 2002. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin. Microbiol. Rev. 15:95–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Nau R., Soto A., Brück W. 1999. Apoptosis of neurons in the dentate gyrus in humans suffering from bacterial meningitis. J. Neuropathol. Exp. Neurol. 58:265–274 [DOI] [PubMed] [Google Scholar]
- 347. Nau R., et al. 1999. Rifampin reduces early mortality in experimental Streptococcus pneumoniae meningitis. J. Infect. Dis. 179:1557–1560 [DOI] [PubMed] [Google Scholar]
- 348. Neeleman C., et al. 1999. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect. Immun. 67:4517–4524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Nelson A. L., et al. 2007. RrgA is a pilus-associated adhesin in Streptococcus pneumoniae. Mol. Microbiol. 66:329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Nelson A. L., et al. 2007. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect. Immun. 75:83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Nelson R. A., Jr 1953. The immune-adherence phenomenon; an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science 118:733–737 [DOI] [PubMed] [Google Scholar]
- 352. Neth O., et al. 2000. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect. Immun. 68:688–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Nimmerjahn A., Kirchhoff F., Helmchen F. 2005. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318 [DOI] [PubMed] [Google Scholar]
- 354. Nobbs A. H., Lamont R. J., Jenkinson H. F. 2009. Streptococcus adherence and colonization. Microbiol. Mol. Biol. Rev. 73:407–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Nuorti J. P., et al. 2000. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N. Engl. J. Med. 342:681–689 [DOI] [PubMed] [Google Scholar]
- 356. Ochs M. M., et al. 2008. Vaccine-induced human antibodies to PspA augment complement C3 deposition on Streptococcus pneumoniae. Microb. Pathog. 44:204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Opitz B., et al. 2004. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J. Biol. Chem. 279:36426–36432 [DOI] [PubMed] [Google Scholar]
- 358. Orihuela C. J., et al. 2006. Cell wall-mediated neuronal damage in early sepsis. Infect. Immun. 74:3783–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Orihuela C. J., Gao G., Francis K. P., Yu J., Tuomanen E. I. 2004. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J. Infect. Dis. 190:1661–1669 [DOI] [PubMed] [Google Scholar]
- 360. Orihuela C. J., et al. 2009. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J. Clin. Invest. 119:1638–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Ostergaard C., Benfield T. 2009. Macrophage migration inhibitory factor in cerebrospinal fluid from patients with central nervous system infection. Crit. Care 13:R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Ostergaard C., et al. 1999. Pretreatment with granulocyte colony-stimulating factor attenuates the inflammatory response but not the bacterial load in cerebrospinal fluid during experimental pneumococcal meningitis in rabbits. Infect. Immun. 67:3430–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Ostergaard C., Brandt C., Konradsen H. B., Samuelsson S. 2004. Differences in survival, brain damage, and cerebrospinal fluid cytokine kinetics due to meningitis caused by 3 different Streptococcus pneumoniae serotypes: evaluation in humans and in 2 experimental models. J. Infect. Dis. 190:1212–1220 [DOI] [PubMed] [Google Scholar]
- 364. Østergaard C., Konradsen H. B., Samuelsson S. 2005. Clinical presentation and prognostic factors of Streptococcus pneumoniae meningitis according to the focus of infection. BMC Infect. Dis. 5:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Østergaard C., Leib S. L., Rowland I., Brandt C. T. 2010. Bacteremia causes hippocampal apoptosis in experimental pneumococcal meningitis. BMC Infect. Dis. 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Østergaard C., O'Reilly T., Brandt C., Frimodt-Møller N., Lundgren J. D. 2006. Influence of the blood bacterial load on the meningeal inflammatory response in Streptococcus pneumoniae meningitis. BMC Infect. Dis. 6:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Ostergaard C., Sorensen T. K., Knudsen J. D., Frimodt-Moller N. 1998. Evaluation of moxifloxacin, a new 8-methoxyquinolone, for treatment of meningitis caused by a penicillin-resistant pneumococcus in rabbits. Antimicrob. Agents Chemother. 42:1706–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Ostergaard C., et al. 2000. Inhibition of leukocyte entry into the brain by the selectin blocker fucoidin decreases interleukin-1 (IL-1) levels but increases IL-8 levels in cerebrospinal fluid during experimental pneumococcal meningitis in rabbits. Infect. Immun. 68:3153–3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Ostergaard C., et al. 2000. Treatment with a monoclonal antibody to IL-8 attenuates the pleocytosis in experimental pneumococcal meningitis in rabbits when given intravenously, but not intracisternally. Clin. Exp. Immunol. 122:207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Osterud B. 2010. Tissue factor expression in blood cells. Thromb. Res. 125(Suppl. 1):S31–S34 [DOI] [PubMed] [Google Scholar]
- 371. Pancholi V., Fontan P., Jin H. 2003. Plasminogen-mediated group A streptococcal adherence to and pericellular invasion of human pharyngeal cells. Microb. Pathog. 35:293–303 [DOI] [PubMed] [Google Scholar]
- 372. Park B., Nizet V., Liu G. Y. 2008. Role of Staphylococcus aureus catalase in niche competition against Streptococcus pneumoniae. J. Bacteriol. 190:2275–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Park J. Y., et al. 2009. The C-type lectin CD209b is expressed on microglia and it mediates the uptake of capsular polysaccharides of Streptococcus pneumoniae. Neurosci. Lett. 450:246–251 [DOI] [PubMed] [Google Scholar]
- 374. Paterson G. K., Mitchell T. J. 2006. Innate immunity and the pneumococcus. Microbiology (Reading, England) 152:285–293 [DOI] [PubMed] [Google Scholar]
- 375. Paul R., Koedel U., Pfister H. W. 1997. 7-Nitroindazole inhibits pial arteriolar vasodilation in a rat model of pneumococcal meningitis. J. Cereb. Blood Flow Metab. 17:985–991 [DOI] [PubMed] [Google Scholar]
- 376. Paul R., et al. 2003. Lack of IL-6 augments inflammatory response but decreases vascular permeability in bacterial meningitis. Brain 126:1873–1882 [DOI] [PubMed] [Google Scholar]
- 377. Paul R., et al. 1998. Matrix metalloproteinases contribute to the blood-brain barrier disruption during bacterial meningitis. Ann. Neurol. 44:592–600 [DOI] [PubMed] [Google Scholar]
- 378. Paul R., et al. 2008. Myeloid Src kinases regulate phagocytosis and oxidative burst in pneumococcal meningitis by activating NADPH oxidase. J. Leukoc. Biol. 84:1141–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Paul R., et al. 2005. Urokinase-type plasminogen activator receptor regulates leukocyte recruitment during experimental pneumococcal meningitis. J. Infect. Dis. 191:776–782 [DOI] [PubMed] [Google Scholar]
- 380. Pedersen M., et al. 2008. The effect of S. pneumoniae bacteremia on cerebral blood flow autoregulation in rats. J. Cereb. Blood Flow Metab. 28:126–134 [DOI] [PubMed] [Google Scholar]
- 381. Peltola H., et al. 2010. Hearing impairment in childhood bacterial meningitis is little relieved by dexamethasone or glycerol. Pediatrics 125:e1–e8 [DOI] [PubMed] [Google Scholar]
- 382. Peltola H., et al. 2007. Adjuvant glycerol and/or dexamethasone to improve the outcomes of childhood bacterial meningitis: a prospective, randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 45:1277–1286 [DOI] [PubMed] [Google Scholar]
- 383. Peltola V. T., Mccullers J. A. 2004. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr. Infect. Dis. J. 23:S87–S97 [DOI] [PubMed] [Google Scholar]
- 384. Pericone C. D., Overweg K., Hermans P. W., Weiser J. N. 2000. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect. Immun. 68:3990–3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Perry V. H. 2007. Stress primes microglia to the presence of systemic inflammation: implications for environmental influences on the brain. Brain Behav. Immun. 21:45–46 [DOI] [PubMed] [Google Scholar]
- 386. Pfister H. W., Borasio G. D., Dirnagl U., Bauer M., Einhaupl K. M. 1992. Cerebrovascular complications of bacterial meningitis in adults. Neurology 42:1497–1504 [DOI] [PubMed] [Google Scholar]
- 387. Pfister H. W., et al. 1992. Transforming growth factor beta 2 inhibits cerebrovascular changes and brain edema formation in the tumor necrosis factor alpha-independent early phase of experimental pneumococcal meningitis. J. Exp. Med. 176:265–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Pfister H. W., et al. 1990. Superoxide dismutase inhibits brain oedema formation in experimental pneumococcal meningitis. Acta Neurochir. Suppl. (Vienna) 51:378–380 [DOI] [PubMed] [Google Scholar]
- 389. Pfister L. A., et al. 2000. Endothelin inhibition improves cerebral blood flow and is neuroprotective in pneumococcal meningitis. Ann. Neurol. 47:329–335 [PubMed] [Google Scholar]
- 390. Picard C., Puel A., Bustamante J., Ku C. L., Casanova J. L. 2003. Primary immunodeficiencies associated with pneumococcal disease. Curr. Opin. Allergy Clin. Immunol. 3:451–459 [DOI] [PubMed] [Google Scholar]
- 391. Picard C., et al. 2010. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore) 89:403–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Pittet L. A., Hall-Stoodley L., Rutkowski M. R., Harmsen A. G. 2010. Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae. Am. J. Respir. Cell Mol. Biol. 42:450–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Polfliet M. M., et al. 2001. Meningeal and perivascular macrophages of the central nervous system play a protective role during bacterial meningitis. J. Immunol. 167:4644–4650 [DOI] [PubMed] [Google Scholar]
- 394. Pracht D., et al. 2005. PavA of Streptococcus pneumoniae modulates adherence, invasion, and meningeal inflammation. Infect. Immun. 73:2680–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Preston J. A., Dockrell D. H. 2008. Virulence factors in pneumococcal respiratory pathogenesis. Future Microbiol. 3:205–221 [DOI] [PubMed] [Google Scholar]
- 396. Prince J. E., et al. 2001. The differential roles of LFA-1 and Mac-1 in host defense against systemic infection with Streptococcus pneumoniae. J. Immunol. 166:7362–7369 [DOI] [PubMed] [Google Scholar]
- 397. Prinz M., et al. 1999. Microglial activation by components of gram-positive and -negative bacteria: distinct and common routes to the induction of ion channels and cytokines. J. Neuropathol. Exp. Neurol. 58:1078–1089 [DOI] [PubMed] [Google Scholar]
- 398. Quagliarello V. J., Long W. J., Scheld W. M. 1986. Morphologic alterations of the blood-brain barrier with experimental meningitis in the rat. Temporal sequence and role of encapsulation. J. Clin. Invest. 77:1084–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Quin L. R., et al. 2005. In vivo binding of complement regulator factor H by Streptococcus pneumoniae. J. Infect. Dis. 192:1996–2003 [DOI] [PubMed] [Google Scholar]
- 400. Quin L. R., Moore III Q. C., McDaniel L. S. 2007. Pneumolysin, PspA, and PspC contribute to pneumococcal evasion of early innate immune responses during bacteremia in mice. Infect. Immun. 75:2067–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Radi R. 2004. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. U. S. A. 101:4003–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Radin J. N., et al. 2005. Beta-arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect. Immun. 73:7827–7835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Raivich G. 2005. Like cops on the beat: the active role of resting microglia. Trends Neurosci. 28:571–573 [DOI] [PubMed] [Google Scholar]
- 404. Randolph M. M., et al. 1998. Attenuation of tissue thrombosis and hemorrhage by ala-TFPI does not account for its protection against E. coli—a comparative study of treated and untreated non-surviving baboons challenged with LD100 E. coli. Thromb. Haemost. 79:1048–1053 [PubMed] [Google Scholar]
- 405. Raphael G. D., et al. 1989. Pathophysiology of rhinitis. Lactoferrin and lysozyme in nasal secretions. J. Clin. Invest. 84:1528–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Rappaport J. M., Bhatt S. M., Burkard R. F., Merchant S. N., Nadol J. B., Jr 1999. Prevention of hearing loss in experimental pneumococcal meningitis by administration of dexamethasone and ketorolac. J. Infect. Dis. 179:264–268 [DOI] [PubMed] [Google Scholar]
- 407. Rauch U., Nemerson Y. 2000. Tissue factor, the blood, and the arterial wall. Trends Cardiovasc. Med. 10:139–143 [DOI] [PubMed] [Google Scholar]
- 408. Ray C. A., et al. 1992. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell 69:597–604 [DOI] [PubMed] [Google Scholar]
- 409. Regev-Yochay G., et al. 2004. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in children. JAMA 292:716–720 [DOI] [PubMed] [Google Scholar]
- 410. Regev-Yochay G., et al. 2004. Nasopharyngeal carriage of Streptococcus pneumoniae by adults and children in community and family settings. Clin. Infect. Dis. 38:632–639 [DOI] [PubMed] [Google Scholar]
- 411. Ren B., et al. 2004. The virulence function of Streptococcus pneumoniae surface protein A involves inhibition of complement activation and impairment of complement receptor-mediated protection. J. Immunol. 173:7506–7512 [DOI] [PubMed] [Google Scholar]
- 412. Rennels M. L., Gregory T. F., Blaumanis O. R., Fujimoto K., Grady P. A. 1985. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 326:47–63 [DOI] [PubMed] [Google Scholar]
- 413. Ribes S., et al. 2010. Toll-like receptor stimulation enhances phagocytosis and intracellular killing of nonencapsulated and encapsulated Streptococcus pneumoniae by murine microglia. Infect. Immun. 78:865–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Reference deleted.
- 415. Richardson M. P., Reid A., Tarlow M. J., Rudd P. T. 1997. Hearing loss during bacterial meningitis. Arch. Dis. Child. 76:134–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Righetti E., et al. 2004. Glycerol for acute stroke. Cochrane Database Syst. Rev. 2004:CD000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Rijneveld A. W., et al. 2004. Improved host defense against pneumococcal pneumonia in platelet-activating factor receptor-deficient mice. J. Infect. Dis. 189:711–716 [DOI] [PubMed] [Google Scholar]
- 418. Ring A., Weiser J. N., Tuomanen E. I. 1998. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J. Clin. Invest. 102:347–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Roche A. M., King S. J., Weiser J. N. 2007. Live attenuated Streptococcus pneumoniae strains induce serotype-independent mucosal and systemic protection in mice. Infect. Immun. 75:2469–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Rock F. L., Hardiman G., Timans J. C., Kastelein R. A., Bazan J. F. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. U. S. A. 95:588–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Rodriguez A. F., Kaplan S. L., Hawkins E. P., Mason E. O., Jr 1991. Hematogenous pneumococcal meningitis in the infant rat: description of a model. J. Infect. Dis. 164:1207–1209 [DOI] [PubMed] [Google Scholar]
- 422. Rosch J. W., et al. 2010. Statins protect against fulminant pneumococcal infection and cytolysin toxicity in a mouse model of sickle cell disease. J. Clin. Invest. 120:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Rosenberg G. A. 2002. Matrix metalloproteinases in neuroinflammation. Glia 39:279–291 [DOI] [PubMed] [Google Scholar]
- 424. Rosenow C., et al. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25:819–829 [DOI] [PubMed] [Google Scholar]
- 425. Rowland T. L., et al. 1998. Differential regulation by thalidomide and dexamethasone of cytokine expression in human peripheral blood mononuclear cells. Immunopharmacology 40:11–20 [DOI] [PubMed] [Google Scholar]
- 426. Roy S., et al. 2002. MBL genotype and risk of invasive pneumococcal disease: a case-control study. Lancet 359:1569–1573 [DOI] [PubMed] [Google Scholar]
- 427. Ruggeri Z. M. 2002. Platelets in atherothrombosis. Nat. Med. 8:1227–1234 [DOI] [PubMed] [Google Scholar]
- 428. Rupprecht T. A., et al. 2007. Complement C1q and C3 are critical for the innate immune response to Streptococcus pneumoniae in the central nervous system. J. Immunol. 178:1861–1869 [DOI] [PubMed] [Google Scholar]
- 429. Sabharwal V., Ram S., Figueira M., Park I. H., Pelton S. I. 2009. Role of complement in host defense against pneumococcal otitis media. Infect. Immun. 77:1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Sáez-Llorens X., McCracken G. H. 2007. Glycerol and bacterial meningitis. Clin. Infect. Dis. 45:1287–1289 [DOI] [PubMed] [Google Scholar]
- 431. Said-Sadier N., Padilla E., Langsley G., Ojcius D. M. 2010. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One 5:e10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Sandros J., Rozdzinski E., Tuomanen E. 1994. Peptides from pertussis toxin interfere with neutrophil adherence in vitro and counteract inflammation in vivo. Microb. Pathog. 16:213–220 [DOI] [PubMed] [Google Scholar]
- 433. Saukkonen K., et al. 1990. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J. Exp. Med. 171:439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434. Schaper M., et al. 2002. Cerebral vasculature is the major target of oxidative protein alterations in bacterial meningitis. J. Neuropathol. Exp. Neurol. 61:605–613 [DOI] [PubMed] [Google Scholar]
- 435. Schaper M., et al. 2003. Differential effect of p47phox and gp91phox deficiency on the course of pneumococcal meningitis. Infect. Immun. 71:4087–4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436. Scheld W. M., Koedel U., Nathan B., Pfister H.-W. 2002. Pathophysiology of bacterial meningitis: mechanism(s) of neuronal injury. J. Infect. Dis. 186(Suppl. 2):S225–S233 [DOI] [PubMed] [Google Scholar]
- 437. Schmidt H., et al. 1998. Moxifloxacin in the therapy of experimental pneumococcal meningitis. Antimicrob. Agents Chemother. 42:1397–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Schmidt H., et al. 1999. Intravenous granulocyte colony-stimulating factor increases the release of tumour necrosis factor and interleukin-1beta into the cerebrospinal fluid, but does not inhibit the growth of Streptococcus pneumoniae in experimental meningitis. Scand. J. Immunol. 49:481–486 [DOI] [PubMed] [Google Scholar]
- 439. Schmidt H., et al. 1998. Glycerol does not reduce neuronal damage in experimental Streptococcus pneumoniae meningitis in rabbits. Inflammopharmacology 6:19–26 [DOI] [PubMed] [Google Scholar]
- 440. Schnare M., Holt A. C., Takeda K., Akira S., Medzhitov R. 2000. Recognition of CpG DNA is mediated by signaling pathways dependent on the adaptor protein MyD88. Curr. Biol. 10:1139–1142 [DOI] [PubMed] [Google Scholar]
- 441. Schröder N. W. J., et al. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587–15594 [DOI] [PubMed] [Google Scholar]
- 442. Schut E. S., et al. 2009. Delayed cerebral thrombosis after initial good recovery from pneumococcal meningitis. Neurology 73:1988–1995 [DOI] [PubMed] [Google Scholar]
- 443. Schwandner R., Dziarski R., Wesche H., Rothe M., Kirschning C. J. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274:17406–17409 [DOI] [PubMed] [Google Scholar]
- 444. Schwartz D., Engelhard D., Gallily R., Matoth I., Brenner T. 1998. Glial cells production of inflammatory mediators induced by Streptococcus pneumoniae: inhibition by pentoxifylline, low-molecular-weight heparin and dexamethasone. J. Neurol. Sci. 155:13–22 [DOI] [PubMed] [Google Scholar]
- 445. Sellner J., Leib S. L. 2006. In bacterial meningitis cortical brain damage is associated with changes in parenchymal MMP-9/TIMP-1 ratio and increased collagen type IV degradation. Neurobiol. Dis. 21:647–656 [DOI] [PubMed] [Google Scholar]
- 446. Senkovich O., et al. 2007. Structure of a complex of human lactoferrin N-lobe with pneumococcal surface protein A provides insight into microbial defense mechanism. J. Mol. Biol. 370:701–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. Shaper M., Hollingshead S. K., Benjamin W. H., Briles D. E. 2004. PspA protects Streptococcus pneumoniae from killing by apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin [corrected]. Infect. Immun. 72:5031–5040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448. Sharief M. K., Ciardi M., Thompson E. J. 1992. Blood-brain barrier damage in patients with bacterial meningitis: association with tumor necrosis factor-alpha but not interleukin-1 beta. J. Infect. Dis. 166:350–358 [DOI] [PubMed] [Google Scholar]
- 449. Shimada J., et al. 2008. Lysozyme M deficiency leads to an increased susceptibility to Streptococcus pneumoniae-induced otitis media. BMC Infect. Dis. 8:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450. Shimoda K., et al. 1991. Granulocyte colony-stimulating factor in cerebrospinal fluid from patients with meningitis. Blood 77:2214–2217 [PubMed] [Google Scholar]
- 451. Siegel J., Rent R., Gewurz H. 1974. Interactions of C-reactive protein with the complement system. I. Protamine-induced consumption of complement in acute phase sera. J. Exp. Med. 140:631–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452. Singhal S., et al. 1999. Antitumor activity of thalidomide in refractory multiple myeloma. N. Engl. J. Med. 341:1565–1571 [DOI] [PubMed] [Google Scholar]
- 453. Singhi S., Jarvinen A., Peltola H. 2008. Increase in serum osmolality is possible mechanism for the beneficial effects of glycerol in childhood bacterial meningitis. Pediatr. Infect. Dis. J. 27:892–896 [DOI] [PubMed] [Google Scholar]
- 454. Song X.-M., Connor W., Hokamp K., Babiuk L. A., Potter A. A. 2008. The growth phase-dependent regulation of the pilus locus genes by two-component system TCS08 in Streptococcus pneumoniae. Microb. Pathog. 46:28–35 [DOI] [PubMed] [Google Scholar]
- 455. Spanaus K. S., et al. 1997. C-X-C and C-C chemokines are expressed in the cerebrospinal fluid in bacterial meningitis and mediate chemotactic activity on peripheral blood-derived polymorphonuclear and mononuclear cells in vitro. J. Immunol. 158:1956–1964 [PubMed] [Google Scholar]
- 456. Spellerberg B., et al. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 19:803–813 [DOI] [PubMed] [Google Scholar]
- 457. Speth C., et al. 2002. Mechanism of human immunodeficiency virus-induced complement expression in astrocytes and neurons. J. Virol. 76:3179–3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458. Spreer A., et al. 2009. Short-term rifampicin pretreatment reduces inflammation and neuronal cell death in a rabbit model of bacterial meningitis. Crit. Care Med. 37:2253–2258 [DOI] [PubMed] [Google Scholar]
- 459. Sprong T., et al. 2003. Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood 102:3702–3710 [DOI] [PubMed] [Google Scholar]
- 460. Stanimirovic D., Satoh K. 2000. Inflammatory mediators of cerebral endothelium: a role in ischemic brain inflammation. Brain Pathol. 10:113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461. Stecher V. J., Morse J. H., Thorbecke G. J. 1967. Sites of production of primate serum proteins associated with complement system. Proc. Soc. Exp. Biol. Med. 124:433–438 [DOI] [PubMed] [Google Scholar]
- 462. Strunk R. C., Eidlen D. M., Mason R. J. 1988. Pulmonary alveolar type II epithelial cells synthesize and secrete proteins of the classical and alternative complement pathways. J. Clin. Invest. 81:1419–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463. Stuertz K., et al. 1999. Lower lipoteichoic and teichoic acid CSF concentrations during treatment of pneumococcal meningitis with non-bacteriolytic antibiotics than with ceftriaxone. Scand. J. Infect. Dis. 31:367–370 [DOI] [PubMed] [Google Scholar]
- 464. Sulik A., Chyczewski L. 2008. Immunohistochemical analysis of MMP-9, MMP-2 and TIMP-1, TIMP-2 expression in the central nervous system following infection with viral and bacterial meningitis. Folia Histochem. Cytobiol. 46:437–442 [DOI] [PubMed] [Google Scholar]
- 465. Suresh M. V., Singh S. K., Ferguson D. A., Agrawal A. 2006. Role of the property of C-reactive protein to activate the classical pathway of complement in protecting mice from pneumococcal infection. J. Immunol. 176:4369–4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466. Suzumura A., Sawada M., Yamamoto H., Marunouchi T. 1993. Transforming growth factor-beta suppresses activation and proliferation of microglia in vitro. J. Immunol. 151:2150–2158 [PubMed] [Google Scholar]
- 467. Szalai A. J., Briles D. E., Volanakis J. E. 1995. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in transgenic mice. J. Immunol. 155:2557–2563 [PubMed] [Google Scholar]
- 468. Szalai A. J., Briles D. E., Volanakis J. E. 1996. Role of complement in C-reactive-protein-mediated protection of mice from Streptococcus pneumoniae. Infect. Immun. 64:4850–4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469. Tan T. Q., Smith C. W., Hawkins E. P., Mason E. O., Kaplan S. L. 1995. Hematogenous bacterial meningitis in an intercellular adhesion molecule-1-deficient infant mouse model. J. Infect. Dis. 171:342–349 [DOI] [PubMed] [Google Scholar]
- 470. Tang T., Frenette P. S., Hynes R. O., Wagner D. D., Mayadas T. N. 1996. Cytokine-induced meningitis is dramatically attenuated in mice deficient in endothelial selectins. J. Clin. Invest. 97:2485–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471. Tauber M. G., Khayam-Bashi H., Sande M. A. 1985. Effects of ampicillin and corticosteroids on brain water content, cerebrospinal fluid pressure, and cerebrospinal fluid lactate levels in experimental pneumococcal meningitis. J. Infect. Dis. 151:528–534 [DOI] [PubMed] [Google Scholar]
- 472. Täuber M. G., Moser B. 1999. Cytokines and chemokines in meningeal inflammation: biology and clinical implications. Clin. Infect. Dis. 28:1–11 [DOI] [PubMed] [Google Scholar]
- 473. Tenenbaum T., et al. 2006. Cell death, caspase activation, and HMGB1 release of porcine choroid plexus epithelial cells during Streptococcus suis infection in vitro. Brain Res. 1100:1–12 [DOI] [PubMed] [Google Scholar]
- 474. Tenenbaum T., et al. 2009. Polar bacterial invasion and translocation of Streptococcus suis across the blood-cerebrospinal fluid barrier in vitro. Cell. Microbiol. 11:323–336 [DOI] [PubMed] [Google Scholar]
- 475. Thomas-Rudolph D., Du Clos T. W., Snapper C. M., Mold C. 2007. C-reactive protein enhances immunity to Streptococcus pneumoniae by targeting uptake to Fc gamma R on dendritic cells. J. Immunol. 178:7283–7291 [DOI] [PubMed] [Google Scholar]
- 476. Thomssen H., Kahan M., Londei M. 1995. Differential effects of interleukin-10 on the expression of HLA class II and CD1 molecules induced by granulocyte/macrophage colony-stimulating factor/interleukin-4. Eur. J. Immunol. 25:2465–2470 [DOI] [PubMed] [Google Scholar]
- 477. Tinling S. P., Colton J., Brodie H. A. 2004. Location and timing of initial osteoid deposition in postmeningitic labyrinthitis ossificans determined by multiple fluorescent labels. Laryngoscope 114:675–680 [DOI] [PubMed] [Google Scholar]
- 478. Tofte R. W., Peterson P. K., Kim Y., Quie P. G. 1979. Opsonic activity of normal human cerebrospinal fluid for selected bacterial species. Infect. Immun. 26:1093–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479. Tomasz A., Moreillon P., Pozzi G. 1988. Insertional inactivation of the major autolysin gene of Streptococcus pneumoniae. J. Bacteriol. 170:5931–5934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480. Tong H. H., et al. 2001. Comparison of structural changes of cell surface carbohydrates in the eustachian tube epithelium of chinchillas infected with a Streptococcus pneumoniae neuraminidase-deficient mutant or its isogenic parent strain. Microb. Pathog. 31:309–317 [DOI] [PubMed] [Google Scholar]
- 481. Tong H. H., Li Y. X., Stahl G. L., Thurman J. M. 2010. Enhanced susceptibility to acute pneumococcal otitis media in mice deficient in complement C1qa, factor B, and factor B/C2. Infect. Immun. 78:976–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482. Trostdorf F., et al. 1999. Reduction of meningeal macrophages does not decrease migration of granulocytes into the CSF and brain parenchyma in experimental pneumococcal meningitis. J. Neuroimmunol. 99:205–210 [DOI] [PubMed] [Google Scholar]
- 483. Trostdorf F., et al. 1999. Quinupristin/dalfopristin attenuates the inflammatory response and reduces the concentration of neuron-specific enolase in the cerebrospinal fluid of rabbits with experimental Streptococcus pneumoniae meningitis. J. Antimicrob. Chemother. 43:87–94 [DOI] [PubMed] [Google Scholar]
- 484. Tsao N., Chang W. W., Liu C. C., Lei H. Y. 2002. Development of hematogenous pneumococcal meningitis in adult mice: the role of TNF-alpha. FEMS Immunol. Med. Microbiol. 32:133–140 [DOI] [PubMed] [Google Scholar]
- 485. Tsuchihashi S., et al. 2006. Vascular endothelial growth factor antagonist modulates leukocyte trafficking and protects mouse livers against ischemia/reperfusion injury. Am. J. Pathol. 168:695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486. Tsuchiya K., et al. 2010. Involvement of absent in melanoma 2 in inflammasome activation in macrophages infected with Listeria monocytogenes. J. Immunol. 185:1186–1195 [DOI] [PubMed] [Google Scholar]
- 487. Tunkel A. R., et al. 2004. Practice guidelines for the management of bacterial meningitis. Clin. Infect. Dis. 39:1267–1284 [DOI] [PubMed] [Google Scholar]
- 488. Tuomanen E., Hengstler B., Zak O., Tomasz A. 1986. The role of complement in inflammation during experimental pneumococcal meningitis. Microb. Pathog. 1:15–32 [DOI] [PubMed] [Google Scholar]
- 489. Tuomanen E., Liu H., Hengstler B., Zak O., Tomasz A. 1985. The induction of meningeal inflammation by components of the pneumococcal cell wall. J. Infect. Dis. 151:859–868 [DOI] [PubMed] [Google Scholar]
- 490. Tuomanen E., Tomasz A., Hengstler B., Zak O. 1985. The relative role of bacterial cell wall and capsule in the induction of inflammation in pneumococcal meningitis. J. Infect. Dis. 151:535–540 [DOI] [PubMed] [Google Scholar]
- 491. Tuomanen E. I., Saukkonen K., Sande S., Cioffe C., Wright S. D. 1989. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J. Exp. Med. 170:959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492. Turrin N. P., et al. 2001. Pro-inflammatory and anti-inflammatory cytokine mRNA induction in the periphery and brain following intraperitoneal administration of bacterial lipopolysaccharide. Brain Res. Bull. 54:443–453 [DOI] [PubMed] [Google Scholar]
- 493. Ubenauf K. M., Krueger M., Henneke P., Berner R. 2007. Lipopolysaccharide binding protein is a potential marker for invasive bacterial infections in children. Pediatr. Infect. Dis. J. 26:159–162 [DOI] [PubMed] [Google Scholar]
- 494. van de Beek D., de Gans J. 2006. Dexamethasone in adults with community-acquired bacterial meningitis. Drugs 66:415–427 [DOI] [PubMed] [Google Scholar]
- 495. van de Beek D., de Gans J., McIntyre P., Prasad K. 2004. Steroids in adults with acute bacterial meningitis: a systematic review. Lancet Infect. Dis. 4:139–143 [DOI] [PubMed] [Google Scholar]
- 496. van de Beek D., et al. 2004. Clinical features and prognostic factors in adults with bacterial meningitis. N. Engl. J. Med. 351:1849–1859 [DOI] [PubMed] [Google Scholar]
- 497. van de Beek D., de Gans J., Tunkel A. R., Wijdicks E. F. 2006. Community-acquired bacterial meningitis in adults. N. Engl. J. Med. 354:44–53 [DOI] [PubMed] [Google Scholar]
- 498. van de Beek D., Drake J. M., Tunkel A. R. 2010. Nosocomial bacterial meningitis. N. Engl. J. Med. 362:146–154 [DOI] [PubMed] [Google Scholar]
- 499. van de Beek D., et al. 2010. Adjunctive dexamethasone in bacterial meningitis: a meta-analysis of individual patient data. Lancet Neurol. 9:254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500. van de Beek D., et al. 2002. Cognitive impairment in adults with good recovery after bacterial meningitis. J. Infect. Dis. 186:1047–1052 [DOI] [PubMed] [Google Scholar]
- 501. van de Beek D., Weisfelt M., de Gans J., Tunkel A. R., Wijdicks E. F. 2006. Drug insight: adjunctive therapies in adults with bacterial meningitis. Nat. Clin. Pract. Neurol. 2:504–516 [DOI] [PubMed] [Google Scholar]
- 502. van der Flier M., et al. 1995. Adherence of Streptococcus pneumoniae to immobilized fibronectin. Infect. Immun. 63:4317–4322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503. van der Flier M., et al. 2005. Antibody neutralization of vascular endothelial growth factor (VEGF) fails to attenuate vascular permeability and brain edema in experimental pneumococcal meningitis. J. Neuroimmunol. 160:170–177 [DOI] [PubMed] [Google Scholar]
- 504. van der Poll T., et al. 1997. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J. Infect. Dis. 176:439–444 [DOI] [PubMed] [Google Scholar]
- 505. van der Poll T., et al. 1991. Fibrinolytic response to tumor necrosis factor in healthy subjects. J. Exp. Med. 174:729–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506. van der Poll T., et al. 1994. Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J. Exp. Med. 179:1253–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507. van der Sluijs K. F., et al. 2006. Involvement of the platelet-activating factor receptor in host defense against Streptococcus pneumoniae during postinfluenza pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L194–L199 [DOI] [PubMed] [Google Scholar]
- 508. van Furth A. M., Roord J. J., van Furth R. 1996. Roles of proinflammatory and anti-inflammatory cytokines in pathophysiology of bacterial meningitis and effect of adjunctive therapy. Infect. Immun. 64:4883–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509. van Gool W. A., van de Beek D., Eikelenboom P. 2010. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 375:773–775 [DOI] [PubMed] [Google Scholar]
- 510. Van Lint P., Libert C. 2007. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J. Leukoc. Biol. 82:1375–1381 [DOI] [PubMed] [Google Scholar]
- 511. van Rossum A. M. C., Lysenko E. S., Weiser J. N. 2005. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect. Immun. 73:7718–7726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512. Verbeek M. M., Otte-Holler I., Ruiter D. J., de Waal R. M. 1999. Human brain pericytes as a model system to study the pathogenesis of cerebrovascular amyloidosis in Alzheimer's disease. Cell. Mol. Biol. (Noisy-le-Grand) 45:37–46 [PubMed] [Google Scholar]
- 513. Vergouwen M. D. I., Schut E. S., Troost D., van de Beek D. 2010. Diffuse cerebral intravascular coagulation and cerebral infarction in pneumococcal meningitis. Neurocrit. Care 13:217–227 [DOI] [PubMed] [Google Scholar]
- 514. Volanakis J. E., Kaplan M. H. 1971. Specificity of C-reactive protein for choline phosphate residues of pneumococcal C-polysaccharide. Proc. Soc. Exp. Biol. Med. 136:612–614 [DOI] [PubMed] [Google Scholar]
- 515. Vollmer W., Tomasz A. 2002. Peptidoglycan N-acetylglucosamine deacetylase, a putative virulence factor in Streptococcus pneumoniae. Infect. Immun. 70:7176–7178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516. von Bernuth H., et al. 2008. Pyogenic bacterial infections in humans with MyD88 deficiency. Science 321:691–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517. von Bernuth H., et al. 2005. Septicemia without sepsis: inherited disorders of nuclear factor-kappa B-mediated inflammation. Clin. Infect. Dis. 41(Suppl. 7):S436–S439 [DOI] [PubMed] [Google Scholar]
- 518. Von dem Borne P. A., Bajzar L., Meijers J. C., Nesheim M. E., Bouma B. N. 1997. Thrombin-mediated activation of factor XI results in a thrombin-activatable fibrinolysis inhibitor-dependent inhibition of fibrinolysis. J. Clin. Invest. 99:2323–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519. Watson M., Gilmour R., Menzies R., Ferson M., McIntyre P. 2006. The association of respiratory viruses, temperature, and other climatic parameters with the incidence of invasive pneumococcal disease in Sydney, Australia. Clin. Infect. Dis. 42:211–215 [DOI] [PubMed] [Google Scholar]
- 520. Weber J. R., Angstwurm K., Burger W., Einhaupl K. M., Dirnagl U. 1995. Anti ICAM-1 (CD 54) monoclonal antibody reduces inflammatory changes in experimental bacterial meningitis. J. Neuroimmunol. 63:63–68 [DOI] [PubMed] [Google Scholar]
- 521. Weber J. R., Tuomanen E. I. 2007. Cellular damage in bacterial meningitis: an interplay of bacterial and host driven toxicity. J. Neuroimmunol. 184:45–52 [DOI] [PubMed] [Google Scholar]
- 522. Weiser J. N., Austrian R., Sreenivasan P. K., Masure H. R. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 62:2582–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523. Weiser J. N., et al. 2003. Antibody-enhanced pneumococcal adherence requires IgA1 protease. Proc. Natl. Acad. Sci. U. S. A. 100:4215–4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524. Weisfelt M., de Gans J., van der Poll T., van de Beek D. 2006. Pneumococcal meningitis in adults: new approaches to management and prevention. Lancet Neurol. 5:332–342 [DOI] [PubMed] [Google Scholar]
- 525. Weisfelt M., et al. 2007. Procoagulant and fibrinolytic activity in cerebrospinal fluid from adults with bacterial meningitis. J. Infect. 54:545–550 [DOI] [PubMed] [Google Scholar]
- 526. Weisfelt M., et al. 2006. Dexamethasone and long-term outcome in adults with bacterial meningitis. Ann. Neurol. 60:456–468 [DOI] [PubMed] [Google Scholar]
- 527. Weisfelt M., van de Beek D., Spanjaard L., de Gans J. 2007. Nosocomial bacterial meningitis in adults: a prospective series of 50 cases. J. Hosp. Infect. 66:71–78 [DOI] [PubMed] [Google Scholar]
- 528. Weisfelt M., van de Beek D., Spanjaard L., Reitsma J. B., de Gans J. 2006. Clinical features, complications, and outcome in adults with pneumococcal meningitis: a prospective case series. Lancet Neurol. 5:123–129 [DOI] [PubMed] [Google Scholar]
- 529. Wellmer A., et al. 2002. Decreased virulence of a pneumolysin-deficient strain of Streptococcus pneumoniae in murine meningitis. Infect. Immun. 70:6504–6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530. Westendorp R. G., Hottenga J. J., Slagboom P. E. 1999. Variation in plasminogen-activator-inhibitor-1 gene and risk of meningococcal septic shock. Lancet 354:561–563 [DOI] [PubMed] [Google Scholar]
- 531. Weststrate W., Hijdra A., de Gans J. 1996. Brain infarcts in adults with bacterial meningitis. Lancet 347:399. [DOI] [PubMed] [Google Scholar]
- 532. Wilms H., et al. 2010. Regulation of activin A synthesis in microglial cells: pathophysiological implications for bacterial meningitis. J. Neurosci. Res. 88:16–23 [DOI] [PubMed] [Google Scholar]
- 533. Winkelstein J. A., Abramovitz A. S., Tomasz A. 1980. Activation of C3 via the alternative complement pathway results in fixation of C3b to the pneumococcal cell wall. J. Immunol. 124:2502–2506 [PubMed] [Google Scholar]
- 534. Winkelstein J. A., Tomasz A. 1977. Activation of the alternative pathway by pneumococcal cell walls. J. Immunol. 118:451–454 [PubMed] [Google Scholar]
- 535. Winkler F., Kastenbauer S., Koedel U., Pfister H. W. 2002. Increased serum concentrations of tissue plasminogen activator correlate with an adverse clinical outcome in patients with bacterial meningitis. J. Neurol. Neurosurg. Psychiatry 73:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536. Winkler F., Kastenbauer S., Koedel U., Pfister H. W. 2002. Role of the urokinase plasminogen activator system in patients with bacterial meningitis. Neurology 59:1350–1355 [DOI] [PubMed] [Google Scholar]
- 537. Winkler F., Koedel U., Kastenbauer S., Pfister H. W. 2001. Differential expression of nitric oxide synthases in bacterial meningitis: role of the inducible isoform for blood-brain barrier breakdown. J. Infect. Dis. 183:1749–1759 [DOI] [PubMed] [Google Scholar]
- 538. Wizemann T. M., et al. 1996. Peptide methionine sulfoxide reductase contributes to the maintenance of adhesins in three major pathogens. Proc. Natl. Acad. Sci. U. S. A. 93:7985–7990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539. Xu Y., et al. 2001. Complement activation in factor D-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 98:14577–14582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540. Yong V. W., Power C., Forsyth P., Edwards D. R. 2001. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci. 2:502–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541. Yoshimura A., et al. 1999. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163:1–5 [PubMed] [Google Scholar]
- 542. Yubero S., Ramudo L., Manso M. A., De Dios I. 2009. Mechanisms of dexamethasone-mediated chemokine down-regulation in mild and severe acute pancreatitis. Biochim. Biophys. Acta 1792:1205–1211 [DOI] [PubMed] [Google Scholar]
- 543. Yuste J., et al. 2010. The effects of PspC on complement-mediated immunity to Streptococcus pneumoniae vary with strain background and capsular serotype. Infect. Immun. 78:283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544. Yuste J., et al. 2008. Impaired opsonization with C3b and phagocytosis of Streptococcus pneumoniae in sera from subjects with defects in the classical complement pathway. Infect. Immun. 76:3761–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545. Zhang F. X., et al. 1999. Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J. Biol. Chem. 274:7611–7614 [DOI] [PubMed] [Google Scholar]
- 546. Zhang J. R., et al. 2000. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102:827–837 [DOI] [PubMed] [Google Scholar]
- 547. Zhang Y., et al. 2001. Recombinant PhpA protein, a unique histidine motif-containing protein from Streptococcus pneumoniae, protects mice against intranasal pneumococcal challenge. Infect. Immun. 69:3827–3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548. Zipfel P. F., et al. 2002. Factor H family proteins: on complement, microbes and human diseases. Biochem. Soc. Trans. 30:971–978 [DOI] [PubMed] [Google Scholar]
- 549. Zola T. A., Lysenko E. S., Weiser J. N. 2008. Mucosal clearance of capsule-expressing bacteria requires both TLR and nucleotide-binding oligomerization domain 1 signaling. J. Immunol. 181:7909–7916 [DOI] [PubMed] [Google Scholar]
- 550. Zwijnenburg P. J., et al. 2007. C1 inhibitor treatment improves host defense in pneumococcal meningitis in rats and mice. J. Infect. Dis. 196:115–123 [DOI] [PubMed] [Google Scholar]
- 551. Zwijnenburg P. J., van der Poll T., Florquin S., Roord J. J., Van Furth A. M. 2003. IL-1 receptor type 1 gene-deficient mice demonstrate an impaired host defense against pneumococcal meningitis. J. Immunol. 170:4724–4730 [DOI] [PubMed] [Google Scholar]
- 552. Zwijnenburg P. J., et al. 2001. Experimental pneumococcal meningitis in mice: a model of intranasal infection. J. Infect. Dis. 183:1143–1146 [DOI] [PubMed] [Google Scholar]
- 553. Zwijnenburg P. J. G., de Bie H. M. A., Roord J. J., van der Poll T., van Furth A. M. 2003. Chemotactic activity of CXCL5 in cerebrospinal fluid of children with bacterial meningitis. J. Neuroimmunol. 145:148–153 [DOI] [PubMed] [Google Scholar]
- 554. Zwijnenburg P. J. G., et al. 2003. Interleukin-18 gene-deficient mice show enhanced defense and reduced inflammation during pneumococcal meningitis. J. Neuroimmunol. 138:31–37 [DOI] [PubMed] [Google Scholar]
- 555. Zwijnenburg P. J. G., Van Der Poll T., Florquin S., Roord J. J., van Furth A. M. 2003. Interleukin-10 negatively regulates local cytokine and chemokine production but does not influence antibacterial host defense during murine pneumococcal meningitis. Infect. Immun. 71:2276–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556. Zysk G., et al. 1996. Anti-inflammatory treatment influences neuronal apoptotic cell death in the dentate gyrus in experimental pneumococcal meningitis. J. Neuropathol. Exp. Neurol. 55:722–728 [DOI] [PubMed] [Google Scholar]
- 557. Zysk G., et al. 1997. Elimination of blood-derived macrophages inhibits the release of interleukin-1 and the entry of leukocytes into the cerebrospinal fluid in experimental pneumococcal meningitis. J. Neuroimmunol. 73:77–80 [DOI] [PubMed] [Google Scholar]
- 558. Zysk G., et al. 2001. Pneumolysin is the main inducer of cytotoxicity to brain microvascular endothelial cells caused by Streptococcus pneumoniae. Infect. Immun. 69:845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]