Abstract
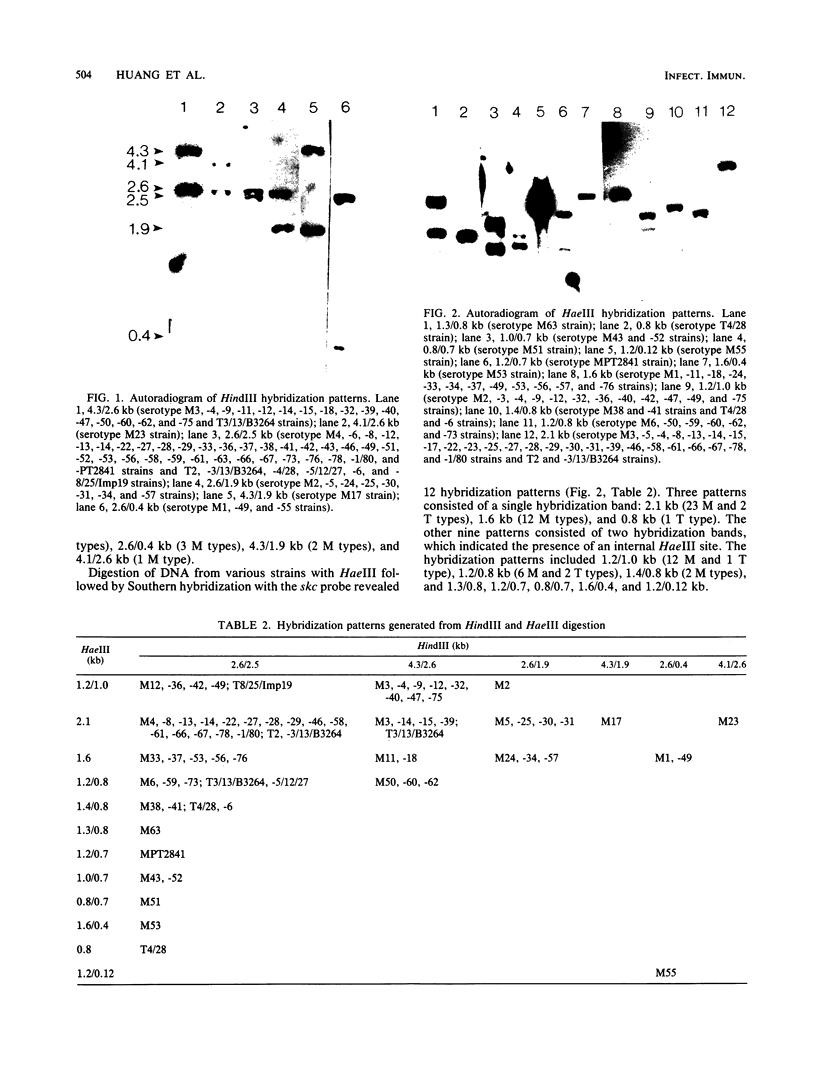
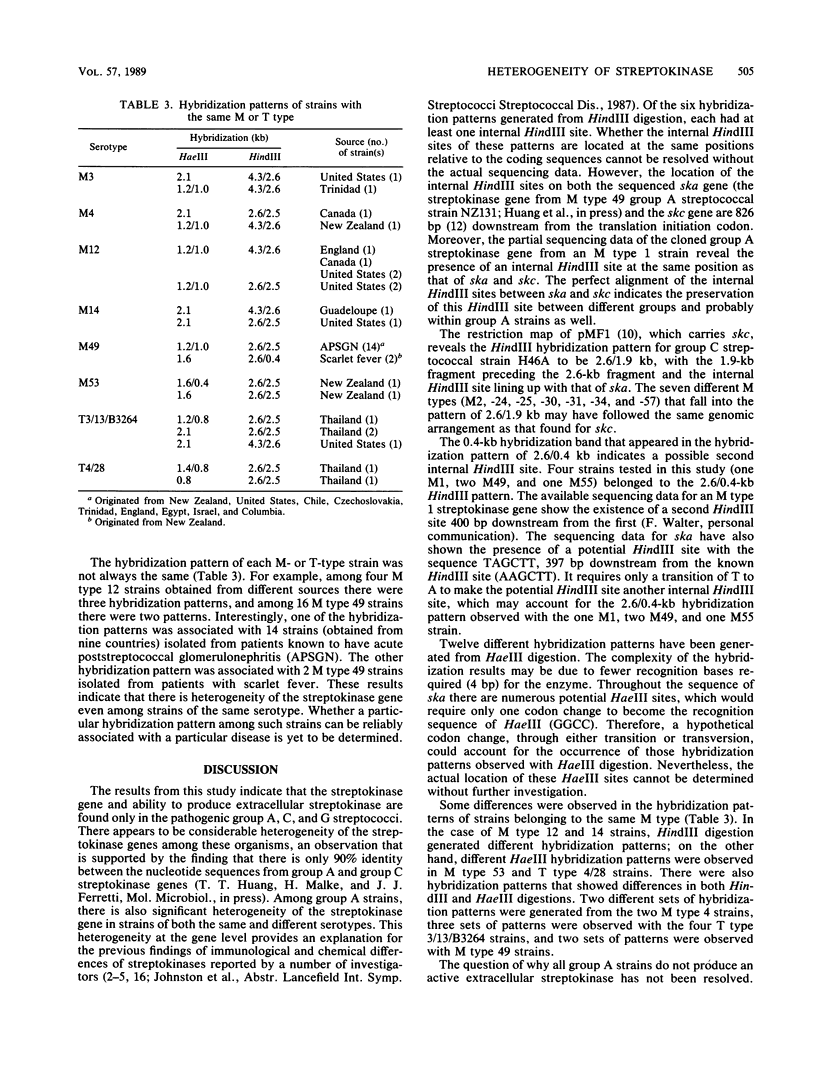
A molecular epidemiological study was conducted to determine the distribution of the streptokinase gene in group A streptococcal strains of different M types and in other streptococcal species. Plasmid pNC1, containing only the internal coding sequence of the streptokinase gene from group C streptococcal strain H46A, was used as a DNA probe in colony and Southern hybridization experiments. Only the pathogenic group A, C, and G streptococci contained a streptokinase gene; 12 other Lancefield group strains did not. A total of 134 group A strains, including 61 M types and 6 T types, were tested. Although only 62% (83 of 134) of the strains tested showed positive streptokinase activity by the casein-plasminogen overlay assay, all strains contained the streptokinase gene as evidenced by strong hybridization with the pNC1 probe. Southern blot DNA hybridizations were carried out with 101 strains of group A streptococci. The restriction enzymes HindIII and HaeIII were used to digest the genomic DNA. Six hybridization patterns were observed after HindIII digestion. Double hybridization bands appeared in all of the patterns, which indicated the existence of a highly conserved HindIII site. More complex hybridization results were obtained after HaeIII digestion. Twelve hybridization patterns were observed; three were characterized by a single hybridization band, and nine were characterized by double bands. Variations in hybridization patterns were observed in strains of both the same and different serotypes. The overall results at the gene level indicate that there is considerable heterogeneity among the streptokinases of group A streptococci, consistent with previous findings of immunological and chemical differences among streptokinases of group A streptococci.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajaj A. P., Castellino F. J. Activation of human plasminogen by equimolar levels of streptokinase. J Biol Chem. 1977 Jan 25;252(2):492–498. [PubMed] [Google Scholar]
- DILLON H. C., Jr, WANNAMAKER L. W. PHYSICAL AND IMMUNOLOGICAL DIFFERENCES AMONG STREPTOKINASES. J Exp Med. 1965 Mar 1;121:351–371. doi: 10.1084/jem.121.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach D., Köhler W. Untersuchungen zur Heterogenität von Streptokinasen II. Mitteilung: Aminosäurezusammensetzung und serologische Aktivitt. Zentralbl Bakteriol Orig A. 1979 Jul;244(2-3):210–221. [PubMed] [Google Scholar]
- Gerlach D., Köhler W. Untersuchungen zur Heterogenität von Streptokinasen IV. Mitteilung: Der Nachweis von Isostreptokinasen bei Streptococcus pyogenes Typ 1. Zentralbl Bakteriol A. 1981 Feb;248(4):446–454. [PubMed] [Google Scholar]
- Gerlach D., Köhler W. Untersuchungen zur Heterogenität von Streptokinasen verschiedener Herkunft. Zentralbl Bakteriol Orig A. 1977 Jul;238(3):336–349. [PubMed] [Google Scholar]
- Holm S. E. The pathogenesis of acute post-streptococcal glomerulonephritis in new lights. Review article. APMIS. 1988 Mar;96(3):189–193. doi: 10.1111/j.1699-0463.1988.tb05289.x. [DOI] [PubMed] [Google Scholar]
- Johnston K. H., Zabriskie J. B. Purification and partial characterization of the nephritis strain-associated protein from Streptococcus pyogenes, group A. J Exp Med. 1986 Mar 1;163(3):697–712. doi: 10.1084/jem.163.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malke H., Ferretti J. J. Streptokinase: cloning, expression, and excretion by Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3557–3561. doi: 10.1073/pnas.81.11.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malke H., Roe B., Ferretti J. J. Nucleotide sequence of the streptokinase gene from Streptococcus equisimilis H46A. Gene. 1985;34(2-3):357–362. doi: 10.1016/0378-1119(85)90145-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- WEINSTEIN L. Antigenic dissimilarity of streptokinases. Proc Soc Exp Biol Med. 1953 Aug-Sep;83(4):689–691. doi: 10.3181/00379727-83-20460. [DOI] [PubMed] [Google Scholar]
- Wulf R. J., Mertz E. T. Studies on plasminogen. 8. Species specificity of streptokinase. Can J Biochem. 1969 Oct;47(10):927–931. doi: 10.1139/o69-145. [DOI] [PubMed] [Google Scholar]