Abstract
Pseudomonas aeruginosa is an opportunistic pathogen that attaches to host cells via their pili. The pilus of P. aeruginosa PAK consists of a polymer of a single subunit, pilin, which is a 144-residue polypeptide. The C-terminal end of this protein is semiconserved in a number of strains and contains a disulfide bridge. We have synthesized the C-terminal peptide PAK (128-144)-OH in both its reduced and oxidized forms and the analog PAK(A-129) (128-144)-OH, in which cysteine-129 was substituted by alanine. These three peptides were used to immunize rabbits and prepare antipeptide antisera. It was found that antipeptide antisera to reduced peptide (17-R) and to oxidized peptide (17-O) bound to native PAK pili and cross-reacted with strain PAO pili in direct enzyme-linked immunosorbent assay (ELISA) and immunoblot experiments. However, the antiserum to the peptide immunogen PAK(A-129)(128-144)-OH, which does not have the ability to form the disulfide bridge, did not bind to either PAK or PAO pili. Competitive ELISA experiments with reduced and oxidized peptides of Ac-PAK(128-144)-OH showed that there was no difference in binding between the two peptides for 17-R or 17-O immunoglobulin G. When immunoglobulin G from native PAK antipilus antiserum was used in competitive or direct ELISA experiments, there was also no preference in binding to reduced or oxidized Ac-PAK(128-144)-OH or to PAK(A-129)(128-144)-OH. This result showed that the disulfide bridge in Pseudomonas pili is not critical to the immunogenicity of this region. However, the disulfide bridge is important in the immunogenicity of the C-terminal peptide when preparing antipeptide antisera that are cross-reactive with pili from different strains, since only the disulfide bridge peptide antisera cross-reacted well with the PAO pili as shown by competitive ELISA, suggesting that this region could be an important candidate for development of a synthetic vaccine.
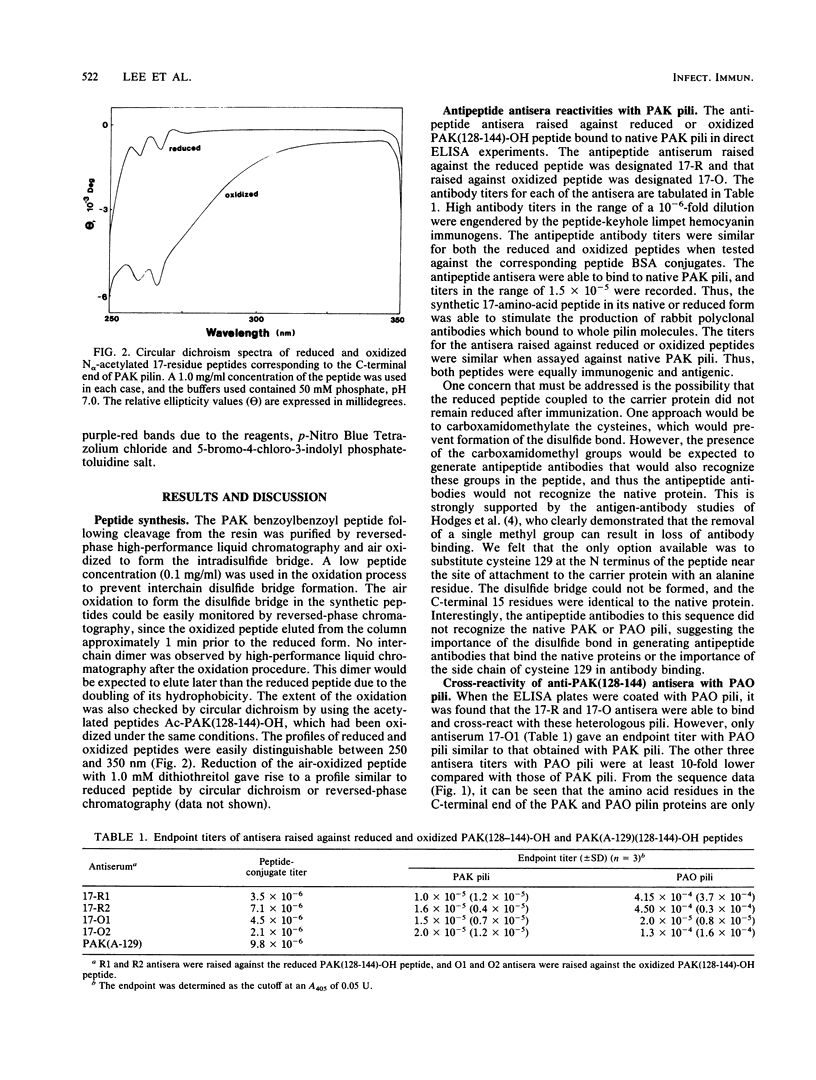
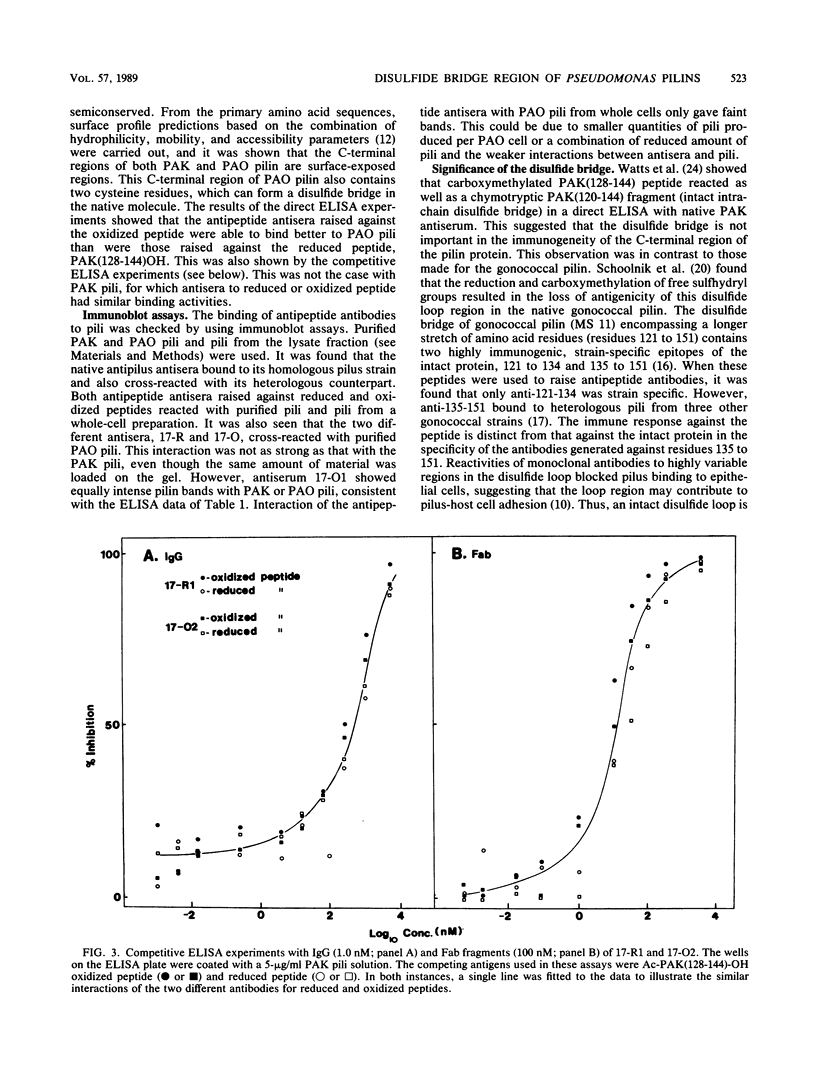
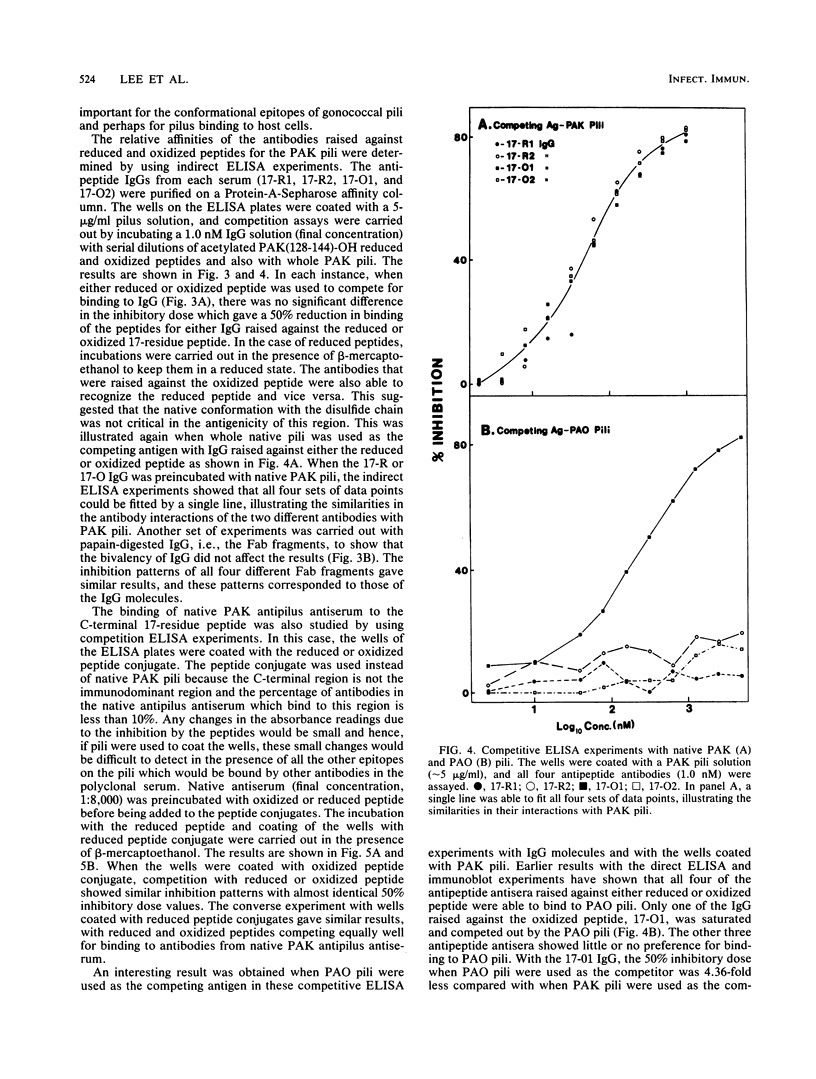
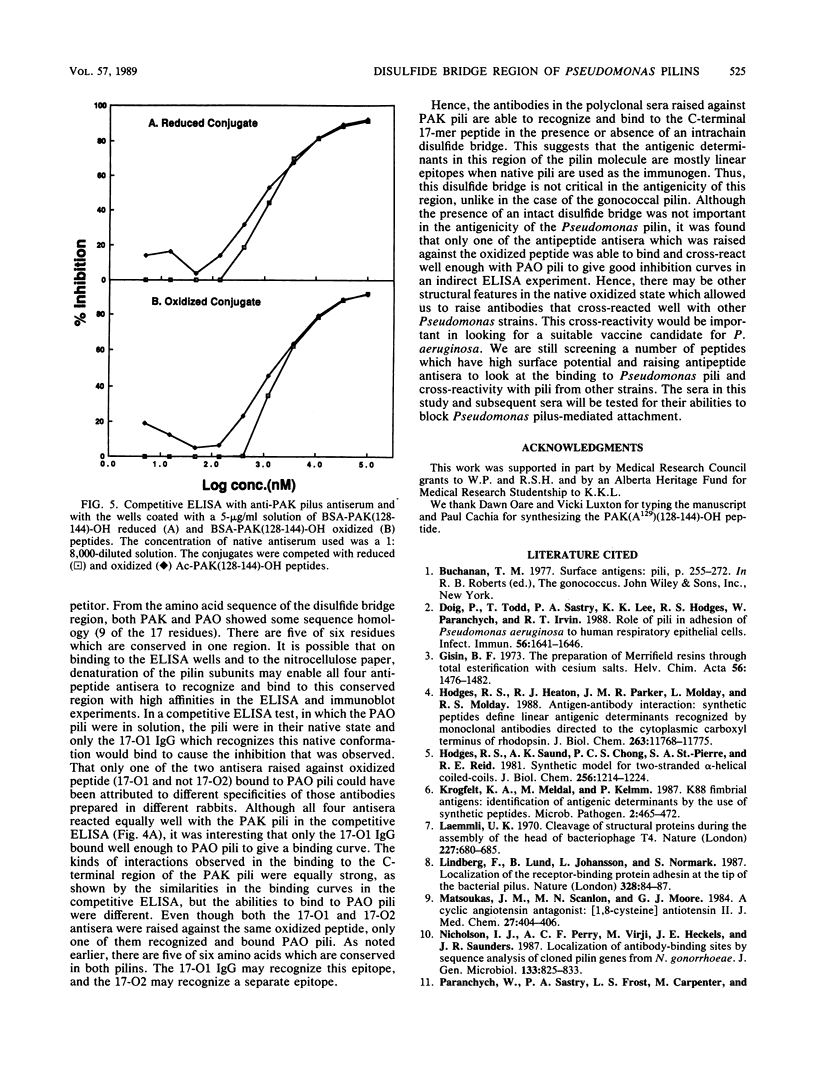
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doig P., Todd T., Sastry P. A., Lee K. K., Hodges R. S., Paranchych W., Irvin R. T. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect Immun. 1988 Jun;56(6):1641–1646. doi: 10.1128/iai.56.6.1641-1646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges R. S., Heaton R. J., Parker J. M., Molday L., Molday R. S. Antigen-antibody interaction. Synthetic peptides define linear antigenic determinants recognized by monoclonal antibodies directed to the cytoplasmic carboxyl terminus of rhodopsin. J Biol Chem. 1988 Aug 25;263(24):11768–11775. [PubMed] [Google Scholar]
- Hodges R. S., Saund A. K., Chong P. C., St-Pierre S. A., Reid R. E. Synthetic model for two-stranded alpha-helical coiled-coils. Design, synthesis, and characterization of an 86-residue analog of tropomyosin. J Biol Chem. 1981 Feb 10;256(3):1214–1224. [PubMed] [Google Scholar]
- Krogfelt K. A., Meldal M., Klemm P. K88 fimbrial antigens: identification of antigenic determinants by the use of synthetic peptides. Microb Pathog. 1987 Jun;2(6):465–472. doi: 10.1016/0882-4010(87)90053-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Matsoukas J. M., Scanlon M. N., Moore G. J. A cyclic angiotensin antagonist: [1,8-cysteine]angiotensin II. J Med Chem. 1984 Mar;27(3):404–406. doi: 10.1021/jm00369a030. [DOI] [PubMed] [Google Scholar]
- Nicolson I. J., Perry A. C., Virji M., Heckels J. E., Saunders J. R. Localization of antibody-binding sites by sequence analysis of cloned pilin genes from Neisseria gonorrhoeae. J Gen Microbiol. 1987 Apr;133(4):825–833. doi: 10.1099/00221287-133-4-825. [DOI] [PubMed] [Google Scholar]
- Paranchych W., Sastry P. A., Frost L. S., Carpenter M., Armstrong G. D., Watts T. H. Biochemical studies on pili isolated from Pseudomonas aeruginosa strain PAO. Can J Microbiol. 1979 Oct;25(10):1175–1181. doi: 10.1139/m79-182. [DOI] [PubMed] [Google Scholar]
- Parker J. M., Guo D., Hodges R. S. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry. 1986 Sep 23;25(19):5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- Pearce W. A., Buchanan T. M. Attachment role of gonococcal pili. Optimum conditions and quantitation of adherence of isolated pili to human cells in vitro. J Clin Invest. 1978 Apr;61(4):931–943. doi: 10.1172/JCI109018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Sadoff J. C., Pyle M., Silipigni J. D. Role of pili in the adherence of Pseudomonas aeruginosa to injured tracheal epithelium. Infect Immun. 1984 Apr;44(1):38–40. doi: 10.1128/iai.44.1.38-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard J. B., Fernandez R., Wang L., Teng N. N., Schoolnik G. K. Antibodies to peptides corresponding to a conserved sequence of gonococcal pilins block bacterial adhesion. Proc Natl Acad Sci U S A. 1985 Feb;82(3):915–919. doi: 10.1073/pnas.82.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry P. A., Finlay B. B., Pasloske B. L., Paranchych W., Pearlstone J. R., Smillie L. B. Comparative studies of the amino acid and nucleotide sequences of pilin derived from Pseudomonas aeruginosa PAK and PAO. J Bacteriol. 1985 Nov;164(2):571–577. doi: 10.1128/jb.164.2.571-577.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Fernandez R., Tai J. Y., Rothbard J., Gotschlich E. C. Gonococcal pili. Primary structure and receptor binding domain. J Exp Med. 1984 May 1;159(5):1351–1370. doi: 10.1084/jem.159.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Tai J. Y., Gotschlich E. C. A pilus peptide vaccine for the prevention of gonorrhea. Prog Allergy. 1983;33:314–331. [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bidwell D., Huldt G., Engvall E. A microplate method of enzyme-linked immunosorbent assay and its application to malaria. Bull World Health Organ. 1974;51(2):209–211. [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Sastry P. A., Hodges R. S., Paranchych W. Mapping of the antigenic determinants of Pseudomonas aeruginosa PAK polar pili. Infect Immun. 1983 Oct;42(1):113–121. doi: 10.1128/iai.42.1.113-121.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worobec E. A., Paranchych W., Parker J. M., Taneja A. K., Hodges R. S. Antigen-antibody interaction. The immunodominant region of EDP208 pili. J Biol Chem. 1985 Jan 25;260(2):938–943. [PubMed] [Google Scholar]
- Worobec E. A., Taneja A. K., Hodges R. S., Paranchych W. Localization of the major antigenic determinant of EDP208 pili at the N-terminus of the pilus protein. J Bacteriol. 1983 Feb;153(2):955–961. doi: 10.1128/jb.153.2.955-961.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


