Abstract
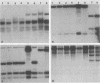
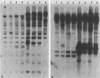
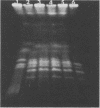
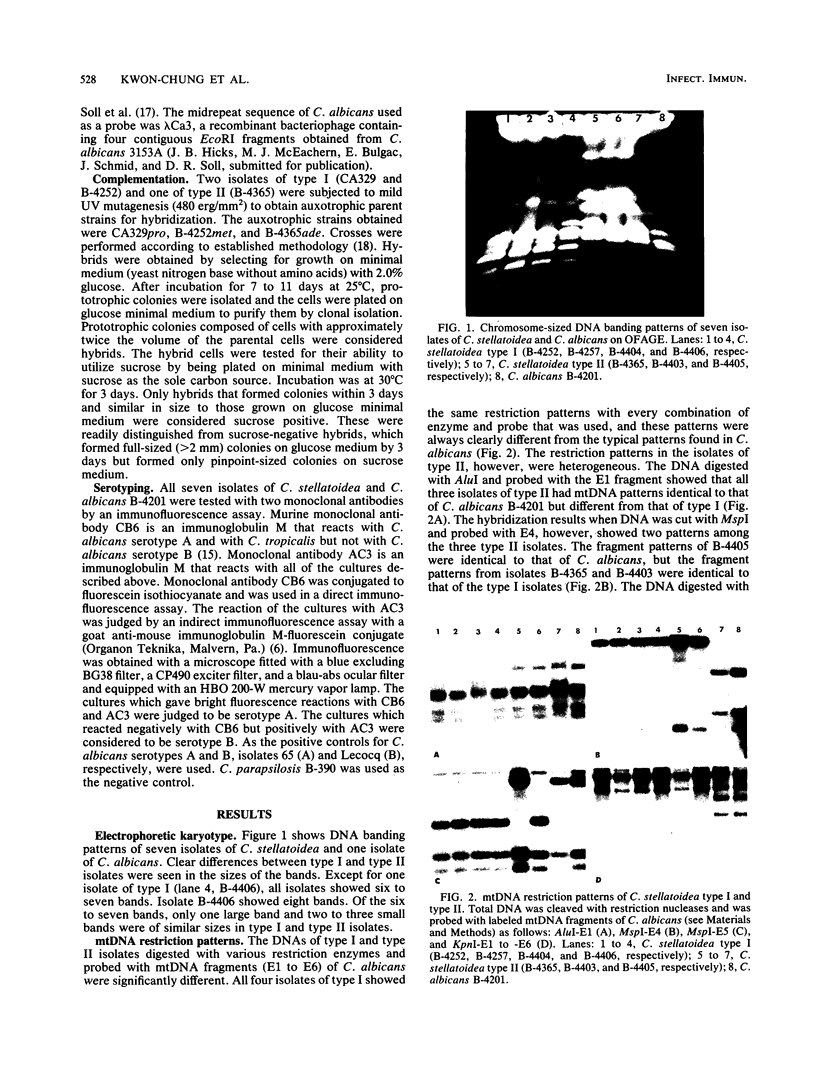
Genetic similarities and differences between type I and type II Candida stellatoidea were studied. The electrophoretic karyotype, mitochondrial DNA (mtDNA) restriction patterns, and midrepeat sequence of nuclear DNA in type I C. stellatoidea were clearly distinguishable from those of a reference culture of Candida albicans. The karyotype and the major bands of the midrepeat sequence of type II C. stellatoidea were indistinguishable from those of the reference C. albicans. The mtDNA restriction patterns of four type I isolates were homogeneous regardless of the endonucleases and probes used. The mtDNA restriction patterns of type II C. stellatoidea varied from strain to strain. Some of them were identical to that of C. albicans, while others were the same as that of type I C. stellatoidea. Immunofluorescence with C. albicans serotype A-specific monoclonal antibody indicated that the four isolates of type I C. stellatoidea were serotype B (non-A), whereas all three type II isolates studied were serotype A. Taken together, these results support the hypothesis that the isolates of C. stellatoidea type II studied are sucrose-negative mutants of serotype A C. albicans. Since C. stellatoidea type I differs from C. albicans in several major genetic characteristics, it cannot be viewed as a simple mutant derived from C. albicans. Hybrids produced by protoplast fusion of type I and type II cells were capable of assimilating sucrose, indicating that the sucrose-negative phenotypes of the parents are due to different mutations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahearn D. G., Meyer S. A., Mitchell G., Nicholson M. A., Ibrahim A. I. Sucrose-negative variants of Candida tropicalis. J Clin Microbiol. 1977 Apr;5(4):494–496. doi: 10.1128/jcm.5.4.494-496.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer D. R., Eccleshall R., Marmur J. Isolation of yeast DNA. Methods Cell Biol. 1975;12:39–44. doi: 10.1016/s0091-679x(08)60950-4. [DOI] [PubMed] [Google Scholar]
- HASENCLEVER H. F., MITCHELL W. O., LOEWE J. Antigenic studies of Candida. II. Antigenic relation of Candida albicans group A and group B to Candida stellatoidea and Candida tropicalis. J Bacteriol. 1961 Oct;82:574–577. doi: 10.1128/jb.82.4.574-577.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Lehman D., Good C., Magee P. T. Genetic evidence for role of extracellular proteinase in virulence of Candida albicans. Infect Immun. 1985 Sep;49(3):571–575. doi: 10.1128/iai.49.3.571-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Wickes B. L., Merz W. G. Association of electrophoretic karyotype of Candida stellatoidea with virulence for mice. Infect Immun. 1988 Jul;56(7):1814–1819. doi: 10.1128/iai.56.7.1814-1819.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz W. G., Connelly C., Hieter P. Variation of electrophoretic karyotypes among clinical isolates of Candida albicans. J Clin Microbiol. 1988 May;26(5):842–845. doi: 10.1128/jcm.26.5.842-845.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C., Abbott A. B. A simple system for the presumptive identification of Candida albicans and differentiation of strains within the species. Sabouraudia. 1980 Dec;18(4):301–317. [PubMed] [Google Scholar]
- Reiss E., de Repentigny L., Kuykendall R. J., Carter A. W., Galindo R., Auger P., Bragg S. L., Kaufman L. Monoclonal antibodies against Candida tropicalis mannan: antigen detection by enzyme immunoassay and immunofluorescence. J Clin Microbiol. 1986 Nov;24(5):796–802. doi: 10.1128/jcm.24.5.796-802.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarachek A., Rhoads D. D., Schwarzhoff R. H. Hybridization of Candida albicans through fusion of protoplasts. Arch Microbiol. 1981 Mar;129(1):1–8. doi: 10.1007/BF00417169. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Langtimm C. J., McDowell J., Hicks J., Galask R. High-frequency switching in Candida strains isolated from vaginitis patients. J Clin Microbiol. 1987 Sep;25(9):1611–1622. doi: 10.1128/jcm.25.9.1611-1622.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Kwon-Chung K. J. Parasexual genetics of Torulopsis glabrata. J Bacteriol. 1987 Nov;169(11):4991–4994. doi: 10.1128/jb.169.11.4991-4994.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Markie D. M., Simpkin K. G., Poulter R. M. Instability of Candida albicans hybrids. J Bacteriol. 1985 Mar;161(3):1131–1136. doi: 10.1128/jb.161.3.1131-1136.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Markie D. UV-induced instability in Candida albicans hybrids. Curr Genet. 1985;9(2):175–177. doi: 10.1007/BF00436967. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Troutman W. B., Riggsby W. S. Circular mitochondrial genome of Candida albicans contains a large inverted duplication. J Bacteriol. 1985 Oct;164(1):7–13. doi: 10.1128/jb.164.1.7-13.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]